Abstract
Objective
Hereditary hemorrhagic telangiectasia (HHT) is an autosomal dominant vascular dysplasia whose hallmark symptom is spontaneous recurrent epistaxis. Two major genetic sub-types of this syndrome are HHT1 and HHT2. Severity of epistaxis ranges from occasional low-volume bleeding to frequent large-volume hemorrhage. This study evaluated the severity and progression of epistaxis in HHT1 vs. HHT2.
Study Design
Retrospective cohort study
Methods
Retrospective chart review was performed for 183 genotyped HHT patients seen at our Center from 2010-2013. Data collected included epistaxis severity score (ESS), age of epistaxis onset, number and type of treatments, age at which treatments were sought, complete blood count values, ferritin, number of telangiectases, blood transfusions, iron therapy history, and patient demographics.
Results
115 subjects with HHT2 were compared to 68 with HHT1. Subjects with HHT2 had a higher ESS compared to HHT1 (p=0.043) and a later age of onset of epistaxis (p=0.005). HHT2 subjects were more likely to use oral iron (p=0.032) and were more likely to seek interventions to control their epistaxis (p= 0.029).
Conclusion
HHT2 is associated with more severe epistaxis and a subsequent higher rate of interventions, requiring more aggressive therapy as compared to HHT1.
Keywords: Hereditary Hemorrhagic Telangiectasia, HHT, HHT1, HHT2, Osler–Weber–Rendu disease, Osler–Weber–Rendu Syndrome, Laser photocoagulation, Young's procedure, Nasal closure, Epistaxis treatment
INTRODUCTION
Hereditary hemorrhagic telangiectasia (HHT), or Osler-Weber-Rendu Syndrome, is an autosomal dominant vascular dysplasia characterized by the presence of multiple arteriovenous malformations (AVMs) and telangiectases. The diagnosis of HHT is made when a patient has at least three of the four Curacao criteria, which are: recurrent spontaneous epistaxis, family history of HHT, telangiectases in characteristic sites (lips, oral cavity, nose, and fingers), and proven visceral AVM(s).1 This condition has an estimated worldwide prevalence of 1/5,000.2 Two major sub-types of this syndrome now recognized are: HHT1 caused by mutations in the endoglin (ENG) gene ([1] and HHT2 caused by mutations in the activin receptor-like kinase 1 (ACVRL1) gene.3,4
Telangiectases of the nasal mucosa are prominent in both HHT sub-types and can have a significant negative impact on quality of life as they frequently cause epistaxis. Epistaxis is the hallmark symptom of HHT and the most common complaint of patients with HHT. Severity of epistaxis ranges from nosebleeds occurring every couple of months lasting less than one minute to multiple hemorrhages daily resulting in transfusion dependence.5 Between 80-90% of diagnosed individuals report having nosebleeds by the age of 21 and nearly 95% develop recurrent epistaxis sometime in life.6,7
The overlapping phenotypes and genetic heterogeneity of HHT has stimulated evaluation and description of the clinical heterogeneity and natural history of this group of disorders. A limited number of genotype-phenotype correlation studies have been conducted comparing epistaxis in HHT1 to HHT2. Of these, it has been reported that telangiectases of the nasal and oral mucosae present earlier in life in patients with HHT1 as compared to patients with HHT2.8 It has also been suggested that patients affected by HHT1 have an earlier onset of epistaxis than those with HHT2.6,9 Yet one study indicated that adults over 30 with HHT2 may experience epistaxis at a higher frequency and also sought treatment for nosebleeds more often than those with HHT1.10 This suggests that HHT2 may be more progressive with age. Prior studies have been unsuccessful in their attempts to find a relationship between the number of telangiectases at characteristic sites and epistaxis, although a higher frequency of telangiectases in the HHT1 population has been described.9 The same group observed incomplete penetrance of epistaxis in HHT2 compared to complete penetrance in HHT1. Subsequent to these previous studies, a validated tool to quantify epistaxis severity has been described.11 The Hoag Epistaxis Severity Score is a significant improvement over previous subjective grading schemes and provides greater precision to explore modifiers of epistaxis severity.
The purpose of this study is to evaluate the severity and progression of epistaxis in HHT1 vs. HHT2 and also to examine the relationship between prevalence of characteristic telangiectases and epistaxis.
MATERIALS AND METHODS
Data Collection & Evaluation
A retrospective chart review was performed for all patients seen at the University of Utah HHT Center between January 2010 and June 2013 who had a confirmed diagnosis of HHT, and an identified family mutation in either the ENG or ACVRL1 gene. Beginning in January 2010 the epistaxis severity score (ESS) has been administered as part of each patient's clinical evaluation. The ESS is a validated tool involving a series of questions that objectively measure epistaxis severity.11 Genetic testing is performed routinely on family probands suspected to have HHT, regardless of clinical presentation or severity. We further collected all of the following: 1) age of onset of epistaxis; 2) number and type of treatments sought for epistaxis; 3) age at which treatments were performed; 4) lab values which included hemoglobin (Hb), hematocrit (Hct), mean corpuscular volume (MCV), red cell distribution width (RDW), and ferritin (Ferr); 5) number of telangiectases in characteristic sites; 6) current and past medications and supplements; 7) blood transfusion and iron infusion history; and 8) patient age and gender. Lab data was evaluated no more than four times annually. In the event that a patient had more than four labs drawn in a year, we chose the lab with the lowest Hb in each quarter to represent that quarter. The number of telangiectases was recorded for lips, mouth (included the tongue, palate, and oropharynx), face (included face, ears, and conjunctiva), and hands. We specifically documented history of oral iron, multi-vitamin with iron, and a complete medication history.
Data Analysis
This study was conducted under approval of the University of Utah Institutional Review Board. Data was collected and compiled into a database supported by Mathsoft MatLab software. Two sample two tailed t tests with pooled variants and box plots, scatter plots, and bar graphs were plotted to evaluate data.
RESULTS
Of the 183 patients in this study, there were similar percentages of males and females represented in the two disease subtypes. There were nearly twice as many HHT2 patients than there were HHT1 patients. The average age of patients did not differ significantly between the two groups (p=0.08). Males with HHT1 were slightly younger (p=0.02) but there was no difference in age for females (p=0.88) between the two groups (Table I).
Table I.
Proportion of males and females with mean age, range and respective gene status.
| Gene | # of patients | Mean age (range) | # of males (%) | Mean age (range) | # of females (%) | Mean age (range) |
|---|---|---|---|---|---|---|
| HHT1 | 68 | 36.5 (2-82) | 32 (47.1%) | 32.5 (5-78) | 36 (52.9%) | 40 (2-82) |
| HHT2 | 115 | 42.7 (1-85) | 59 (51.3%) | 44.5 (2-85) | 56 (48.7%) | 40.8 (1-81) |
Abbreviations:
• HHT- hereditary hemorrhagic telangiectasia
The profile and comparison of telangiectases at four common, characteristic sites in HHT1 and HHT2 was found to be similar. There was a trend for HHT1 patients to have more telangiectases on the face and lips relative to HHT2 patients, while HHT2 patients had more telangiectases on the mouth and hands relative to HHT1 patients. It was more common that HHT2 patients reported use of oral iron (p=0.032). It was also more common that HHT2 patients underwent either laser photocoagulation or cautery (p=0.029) as interventions for epistaxis when compared to intervention rates in HHT1 patients (Table II).
Table II.
Profile of HHT patients by gene status.
| HHT2 | HHT1 | p | |
|---|---|---|---|
| Number of telangiectases | avg (range) | avg (range) | |
| Face | 2.7 (0-24) | 2.8 (0-56) | 0.9618 |
| Lips | 4.2 (0-36) | 5.7 (0-36) | 0.1561 |
| Mouth | 4.5 (0-30) | 4.9 (0-50) | 0.7459 |
| Hands | 18.8 (0-165) | 13.6 (0-129) | 0.2306 |
| Age of onset for epistaxis | 12.2 (1-48) | 9.4 (1-47) | 0.0047 |
| Age at first intervention | 43.8 | 41.5 | 0.5845 |
| Treatments/Interventions | |||
| Oral iron | 39.0% | 23.9% | 0.0321 |
| Iron infusion | 19.5% | 12.7% | 0.2222 |
| RBC transfusion | 22.8% | 12.7% | 0.0849 |
| Laser or cautery (electric or chemical) | 52.8 | 36.6 | 0.0291 |
| Young's procedure or septal dermoplasty | 10.6 | 7 | 0.4147 |
| ESS range | 3.94 (0-10) | 3.18 (0-9.1) | 0.0434 |
| Mild (1-3) | 50.8% | 52.6% | 0.8162 |
| Moderate (3-7) | 38.3% | 40.4% | 0.7898 |
| Severe (7-10) | 10.9% | 7.0% | 0.4062 |
Abbreviations:
• ESS- epistaxis severity score
• HHT- hereditary hemorrhagic telangiectasia
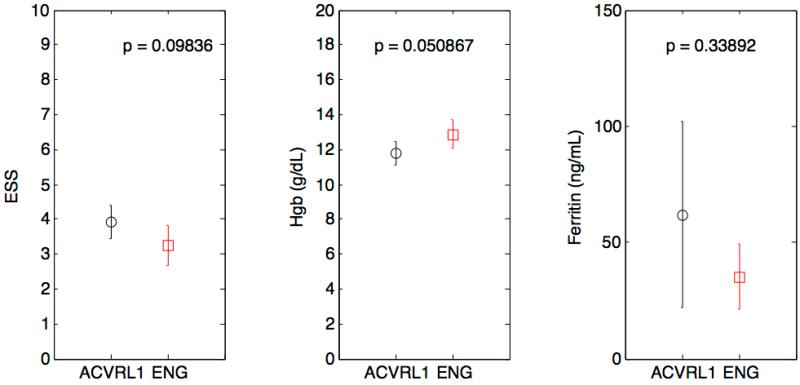
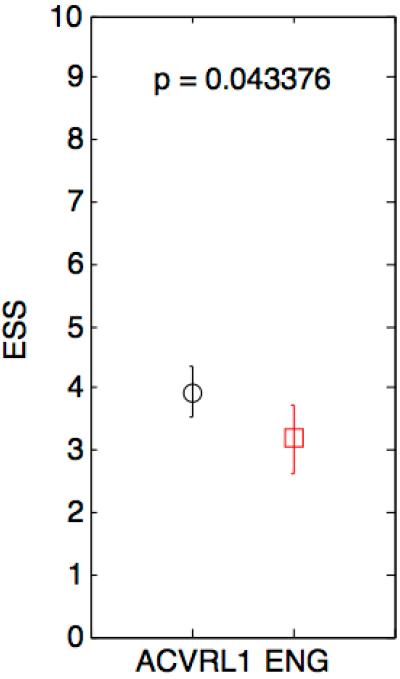
We found no statistically significant differences between groups when comparing the worst recorded ESS, hemoglobin and ferritin values (Figure 1a). However, patients with HHT2 were found to have a more severe average ESS value over time when comparing all reported ESS assessments (p<0.05) (Figure 1b).
Figure 1.
1a—Box plots depicting comparison of worst recorded ESS, Hgb, and Ferritin between HHT subtypes.
1b—Box plot depicting comparison of total reported ESS’ among HHT subtypes
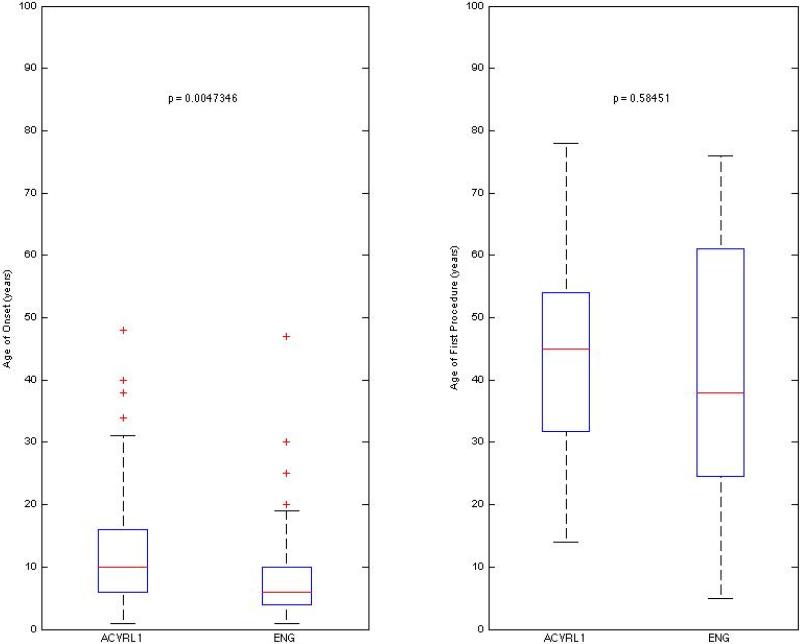
Age of onset of epistaxis was earlier in HHT1, (p=0.005). In the HHT1 group, there was an outlier who reported an age of onset of epistaxis of 68. The patient was 5.44 standard deviations from the mean and thus was excluded from this plot. There was no difference when comparing age of first procedure (p=0.585) (Figure 2).
Figure 2.
Box plots comparing age of onset for epistaxis, and age of first procedure in HHT subtypes. Procedures included cautery, laser photocoagulation, septal dermoplasty, and Young's procedure.
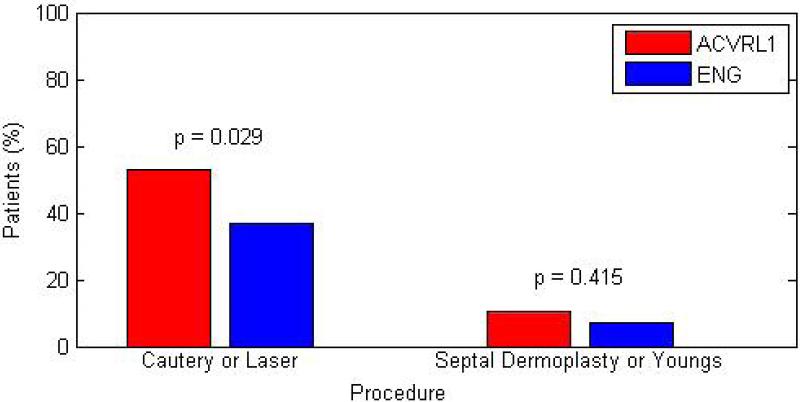
HHT2 patients were more likely to undergo intranasal cautery or laser photocoagulation (p=0.029) to control epistaxis than HHT1 patients. Because there were more HHT2 than HHT1 patients, we controlled for group size when comparing these frequencies. Under the same conditions, there was no significant difference in either the number of septal dermoplasties or Young's nasal closure surgeries between the two groups (Figure 3).
Figure 3.
Bar graph depicting frequency at which major interventions were performed among HHT subtypes.
DISCUSSION
This study examined data relating to the severity of epistaxis and oral/dermal telangiectases in a large cohort of HHT patients with the two common types of HHT (HHT1 and HHT2). The cohort includes all patients seen at our multi-disciplinary center for HHT, not just patients presenting for management of epistaxis. In fact, many patients had not been seen by an otolaryngologist. It suggests that patients with HHT1 mutations have an earlier age of onset of epistaxis than HHT2; however, patients with HHT1 were not found to have more severe lifelong complications of epistaxis. In fact, the opposite may be true; patients with HHT2 may have more severe lifelong complications of epistaxis. This is suggested in part by the inclusion of all reported ESS’ by patients of both types (Figure 1b), which indicates that HHT2 has a more severe epistaxis phenotype. This may be biased because the “severe” patients included in this calculation are likely overrepresented since patients with more severe epistaxis are seen more often in clinic and thus have ESS’ recorded more often. However, this observation may be interpreted to mean that there are more severe patients in the HHT2 group than in the HHT1 group. For the purpose of analysis, we divided ESS’ into three ranges of severity: <3 (mild), 3-7 (moderate), and >7 (severe). In Hoag's study that originally described ESS, three ranges were used to correlate the ESS with intensity of intervention. The ranges used were: <4 (mild), 4-7 (moderate), and >7 (severe). In this study, the ranges were slightly modified with a lower threshold separating mild from moderate. This lower threshold was chosen to create relatively equal intervals, given this 1-10 severity scale; but also reflects our groups’ experience having seen more than 1100 unique HHT patients over two decades, and is in line with the threshold for inclusion into clinical trials for epistaxis.12 There were similar percentages of patients who fell within each group and no notable differences were observed when comparing subgroups. Thus it cannot be assumed that HHT2 is associated with a benign epistaxis phenotype.
Over a lifetime, patients with HHT2 have equally severe or perhaps even worse anemia than those with HHT1. Patients with HHT2 were more likely to use oral iron supplements (p=0.0321) yet this did not translate into improved anemia status. There was no difference in hemoglobin or ferritin levels between the two groups. While not significant, there was a trend for hemoglobin (Figure 1a) to be lower in HHT2 patients. It is possible that without increased utilization of supplemental oral iron, HHT2 patients would be more often anemic than HHT1 patients. Patient history of RBC transfusion and/or iron infusion was often based on patient report, since many patients received these therapies at outside institutions, making quantification of the number of required treatments difficult.
This is a retrospective study. With the exception of the data regarding iron infusions/blood transfusions, all other data collected is part of the routine, systematic evaluation of all patients who present to our multi-disciplinary clinic for HHT, and is captured in patients’ medical record as such. Our HHT center may be unique in that genetic testing to identify a disease causing genetic mutation is recommended to all patients (or at least one family proband) with a clinical diagnosis of HHT, regardless of symptoms or clinical presentation. This is routinely recommended primarily to allow for diagnostic testing in at risk, but clinically undeclared, family members. Thus, in general, this study population represents the spectrum of HHT that presents in the medical arena.
CONCLUSION
For anticipatory guidance of HHT patients, it is helpful to know that although HHT2 has a later average age of onset of epistaxis, it cannot be assumed to be a “milder” form of HHT. In fact it may progress to a more severe disease state with regards to epistaxis, leading patients to seek surgical management more often than patients with HHT1. However, from a clinical management standpoint the difference is not striking. Given the variability of epistaxis, even between patients with the same type of HHT, counseling regarding medical and surgical therapies for epistaxis should be based on individual history and current presentation.
The suggestion from this study that HHT2 may be a more progressive disorder than HHT1 with regards to epistaxis raises the question as to whether the natural history of other symptoms and manifestations of HHT behave similarly. Future investigation of potential differences in progression over time between the HHT subtypes may have interesting implications with regards to the underlying pathogenesis of this group of vascular disorders.
ACKNOLEDGEMENTS
Medical Student Research Program at the University of Utah School of Medicine
Grant Title: Short-Term Training: Students in Health Professional Schools
Funding Source: NIH/NHLBI
Grant #: T35 HL007744
PI: Jerry Kaplan
This work was supported by grants from the National Institutes of Health: R01NS075168
Footnotes
Conflict of Interest Statement
No authors of this study have any conflicts of interest.
Associated Presentations
Results of this study were presented on May 16, 2014 at the poster session of the American Rhinologic Society as part of the annual Combined Otolaryngology Spring Meeting held in Las Vegas.
Contributor Information
Benjamin N. Hunter, University of Utah School of Medicine, SLC, UT, USA.
Benjamin H. Timmins, University of Utah School of Medicine, SLC, UT, USA.
Jamie McDonald, University of Utah School Departments of Pathology and Radiology, SLC, UT, USA.
Kevin J. Whitehead, University of Utah Division of Cardiovascular Medicine, Pediatric Cardiology, Molecular Medicine Program, SLC, UT, USA.
P. Daniel Ward, University of Utah Division of Otolaryngology--Head & Neck Surgery, SLC, UT, USA.
Kevin F. Wilson, University of Utah Division of Otolaryngology--Head & Neck Surgery, SLC, UT, USA
REFERENCES
- 1.Shovlin CL, Guttmacher AE, Buscarini E, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). [April 22, 2014];Am J Med Genet. 2000 91(1):66–67. doi: 10.1002/(sici)1096-8628(20000306)91:1<66::aid-ajmg12>3.0.co;2-p. http://www.ncbi.nlm.nih.gov/pubmed/10751092. [DOI] [PubMed] [Google Scholar]
- 2.Faughnan ME, Palda VA, Garcia-Tsao G, et al. International Guidelines for the Diagnosis and Management of Hereditary Hemorrhagic Telangiectasia. J Med Genet. 2009 doi: 10.1136/jmg.2009.069013. doi:jmg.2009.069013 [pii]\n10.1136/jmg.2009.069013. [DOI] [PubMed] [Google Scholar]
- 3.Johnson DW, Berg JN, Baldwin MA, et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet. 1996;13(2):189–195. doi: 10.1038/ng0696-189. doi:10.1038/ng0696-189. [DOI] [PubMed] [Google Scholar]
- 4.McAllister KA, Grogg KM, Johnson DW, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. [April 22, 2014];Nat Genet. 1994 8(4):345–351. doi: 10.1038/ng1294-345. http://www.ncbi.nlm.nih.gov/pubmed/7894484. [DOI] [PubMed] [Google Scholar]
- 5.Bayrak-Toydemir P, Mao R, Lewin S, McDonald J. Hereditary hemorrhagic telangiectasia: an overview of diagnosis and management in the molecular era for clinicians. [April 22, 2014];Genet Med. 6(4):175–191. doi: 10.1097/01.gim.0000132689.25644.7c. http://www.ncbi.nlm.nih.gov/pubmed/15266205. [DOI] [PubMed] [Google Scholar]
- 6.Berg J, Porteous M, Reinhardt D, et al. Hereditary haemorrhagic telangiectasia: a questionnaire based study to delineate the different phenotypes caused by endoglin and ALK1 mutations. J Med Genet. 2003;40(8):585–590. doi: 10.1136/jmg.40.8.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.AAssar OS, Friedman CM, White RI. The natural history of epistaxis in hereditary hemorrhagic telangiectasia. [April 22, 2014];Laryngoscope. 1991 101(9):977–980. doi: 10.1288/00005537-199109000-00008. http://www.ncbi.nlm.nih.gov/pubmed/1886446. [DOI] [PubMed] [Google Scholar]
- 8.Letteboer TGW, Mager H-J, Snijder RJ, et al. Genotype-phenotype relationship for localization and age distribution of telangiectases in hereditary hemorrhagic telangiectasia. Am J Med Genet A. 2008;146A(21):2733–2739. doi: 10.1002/ajmg.a.32243. doi:10.1002/ajmg.a.32243. [DOI] [PubMed] [Google Scholar]
- 9.Lesca G, Olivieri C, Burnichon N, et al. Genotype-phenotype correlations in hereditary hemorrhagic telangiectasia: data from the French-Italian HHT network. Genet Med. 2007;9(1):14–22. doi: 10.1097/gim.0b013e31802d8373. doi:10.1097/GIM.0b013e31802d8373. [DOI] [PubMed] [Google Scholar]
- 10.Bayrak-Toydemir P, McDonald J, Markewitz B, et al. Genotype-phenotype correlation in hereditary hemorrhagic telangiectasia: mutations and manifestations. Am J Med Genet A. 2006;140(5):463–470. doi: 10.1002/ajmg.a.31101. doi:10.1002/ajmg.a. [DOI] [PubMed] [Google Scholar]
- 11.Hoag JB, Terry P, Mitchell S, Reh D, Merlo C a. An epistaxis severity score for hereditary hemorrhagic telangiectasia. Laryngoscope. 2010;120(4):838–843. doi: 10.1002/lary.20818. doi:10.1002/lary.20818. [DOI] [PubMed] [Google Scholar]
- 12.Gossage J, Hereditary Hemorrhagic Telangiectasia Foundation International . ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2011-2014. North American Study of Epistaxis in HHT. Available from: https://clinicaltrials.gov/ct2/show/NCT01408030 NLM Identifier: NCT01408030. [Google Scholar]