Highlights
-
•
Although it is not known which one of two influenza B lineages will circulate in any one season, only a representative virus of one of the two lineages is part of the trivalent seasonal influenza vaccine.
-
•
We describe three lineage selection strategies to choose which lineage to include in the seasonal vaccine, including the common strategy of using the last lineage that has been observed to dominate, and a new strategy which takes into account population immunity.
-
•
We show why the “hedging strategy” leads to higher expected vaccine efficacy for influenza B by describing the underlying immunity management mechanism.
-
•
We show that the hedging strategy would have lead to higher vaccine efficacy to influenza B in the seasonal vaccine in the decade 2000–2010.
-
•
We show that some of the benefit of transferring to a quadrivalent vaccine can be captured without the cost of moving to a quadrivalent vaccine with an improved trivalent vaccine lineage selection approach.
Keywords: Quadrivalent, Trivalent vaccine, Influenza B, Hedging, Vaccine strain selection, Decision tree
Abstract
Epidemics of seasonal influenza viruses cause considerable morbidity and mortality each year. Various types and subtypes of influenza circulate in humans and evolve continuously such that individuals at risk of serious complications need to be vaccinated annually to keep protection up to date with circulating viruses. The influenza vaccine in most parts of the world is a trivalent vaccine, including an antigenically representative virus of recently circulating influenza A/H3N2, A/H1N1, and influenza B viruses. However, since the 1970s influenza B has split into two antigenically distinct lineages, only one of which is represented in the annual trivalent vaccine at any time. We describe a lineage selection strategy that optimizes protection against influenza B using the standard trivalent vaccine as a potentially cost effective alternative to quadrivalent vaccines.
1. Introduction
Influenza, a highly contagious respiratory disease in humans, is associated with considerable morbidity and mortality and is estimated to affect 5–15% of the world's population annually [1]. One important characteristic of influenza viruses is antigenic drift. As population immunity builds up against circulating influenza viruses, these viruses evolve to escape the immune response rendering individuals susceptible to re-infection. Vaccination against influenza is recommended for people at risk of developing serious complications when infected in most countries, and for all persons aged ≥6 months in the United States [2], and has to be renewed annually to maintain protection.
The influenza virus vaccine in most parts of the world is a trivalent vaccine, including an antigenic representative of recently circulating influenza A/H3N2, A/H1N1, and influenza B virus strains. Twice annually, once for each hemisphere, WHO recommends the strain of each of these influenza (sub)types to be included in the vaccine, based on global surveillance data and substantial analyses and discussions of these data [3], [4], [5], [6], [7].
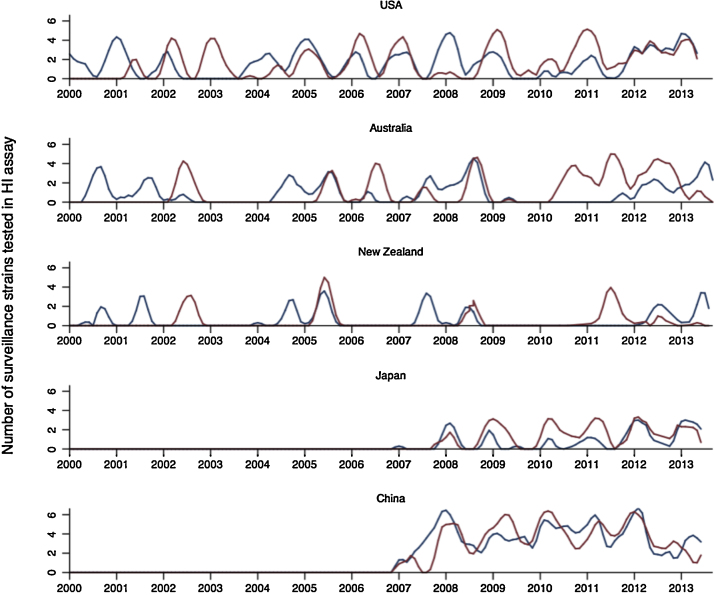
The primary challenge in vaccine strain selection is to choose a representative virus that antigenically matches the strains that will circulate in the following influenza season. Typically the viruses collected in the months immediately preceding a vaccine selection decision are considered to be the best indicators of which viruses will circulate in future influenza seasons [8]. However, influenza B viruses present an additional challenge in that two antigenically distinct lineages, B/Victoria (B/Vic) and B/Yamagata (B/Yam), coexist, evolve separately, and alternate in prevalence in a so far unpredictable pattern [9], [10] (Fig. 1). This circumstance poses a problem, because one of the two influenza B lineages is excluded from the trivalent vaccine. The vaccine thus affords limited protection against influenza B when the opposite lineage to the vaccine lineage circulates [10], [11], [12].
Fig. 1.
Epidemic time series for B/Vic (red) and B/Yam (blue) based on virologically confirmed data from the WHO Collaborating Centers for Reference and Research on Influenza. The counts of each variant are the numbers of viruses tested antigenically by the collaborating centers. Numbers are assumed to be representative of the relative proportion of viruses that circulated. Time series were logged and smoothed with a weighted moving average to capture trends.
In case of a lineage-mismatch between the vaccine strain and the circulating strain, a certain level of protection is still expected as a result of the residual effect of prior years’ vaccination with the circulating strain, and by a degree of cross-reactivity resulting from the current vaccination event [13], [14]. For example, a randomized, placebo-controlled vaccine efficacy study by Frey et al. [11] during the 2007–2008 Northern Hemisphere influenza season reports protection levels of 50% against the circulating B/Yam lineage after vaccination with a trivalent vaccine containing a B/Vic lineage strain. In years of mismatched vaccine B lineage, vaccine efficacy against the circulating strain is therefore unlikely zero as has been assumed in a recent evaluation of the quadrivalent vaccine [7].
Table 1 shows the vaccine strain and the dominant circulating strain in the US from 2000 to 2015. In periods when influenza B lineages switch frequently vaccine efficacy due to lineage mismatch is considerable. For example, as previously observed [2], [3], the vaccine lineage and dominant circulating lineage have only coincided in 50% of the years from 2000 to 2010. When lineage changes are infrequent, such as between 2010 and the present, there is less risk of decrease in vaccine efficacy due to lineage mismatch. The last lineage switch was in the northern hemisphere season 2012/2013. At the time of this vaccine composition decision there had been steadily increasing circulation of the B Yamagata lineage in recent months and years, but B/Yamagata was not predominant. For the first time population immunity to both lineages was part of the consideration in the switch to a B/Yam strain, and an update was made to a B/Yamagata virus in an anticipated, continued increase in B/Yamagata viruses, and a recognition of solid immunity to B/Victoria viruses after four years of B/Victoria vaccination and no antigenic change in the B/Victoria viruses.
Table 1.
Comparison of vaccine B lineage and circulating B lineages in the seasons 2000/2001–2011/2012. The last two columns indicate whether there was any antigenic drift within each of the two lineages between the vaccine strain selection and the influenza season. The strains Florida/06 and Massachusetts/12 belong to the same antigenic cluster. The strain s Wisconsin/10 and Phuket/13 belong to the same antigenic cluster.
| Season | Vaccine strain | Dominant circulating strain | Secondary strain (if co-circulation) | Interim drift in Yam | Interim drift in Vic |
|---|---|---|---|---|---|
| 2000/2001 | Yam—Beijing/93 | Yam—Sichuan/99 | None | Some, SI/99 distinct from BE/93 | – |
| 2001/2002 | Yam—Sichuan/99 | Vic—Brisbane/02 | Yam—Sichuan/99 | None | – |
| 2002/2003 | Vic—Hong Kong/01 | Vic—Brisbane/02 | None | None | Some, BR/02 distinct from HK/01 |
| 2003/2004 | Vic—Hong Kong/01 | Yam—Shanghai/02 | None | Some, SH/02 distinct from SI/99 | None, very little Vic and BR/02-like |
| 2004/2005 | Yam—Shanghai/02 | Yam—Shanghai/02 | Vic—Brisbane/02 | None | None |
| 2005/2006 | Yam—Shanghai/02 | Vic—Malaysia/04 | Yam—Shanghai/02 | None | Some, ML/04 distinct from BR/02 and HK/01 |
| 2006/2007 | Vic—Malaysia/04 | Vic—Malaysia/04 | Yam—Shanghai/02 & Florida/06 | Minor, mainly genetic change, SH/02 and FL/06 antigenically similar | None |
| 2007/2008 | Vic—Malaysia/04 | Yam—Florida/06 & Bangladesh/07 | None | Some, FL/06 and BA/07 different enough to warrant vaccine update | None |
| 2008/2009 | Yam—Florida/06 | Vic—Brisbane/08 | Yam—Florida/06 & Bangladesh/07 | None | Some, BR/08 distinct from ML/04 |
| 2009/2010 | Vic—Brisbane/08 | Vic—Brisbane/08 | very little Yam | None | None |
| 2010/2011 | Vic—Brisbane/08 | Vic—Brisbane/08 | Yam—Wisconsin/10 | None | None |
| 2011/2012 | Vic—Brisbane/08 | Vic—Brisbane/08 | Yam—Florida/06 & Wisconsin/10 | To previous Florida/06 like (except China Wisconsin/10 like) | None |
| 2012/2013 | Yam—Wisconsin/10 | Vic—Brisbane/08 | Yam—Florida/06 & Wisconsin/10 | None | None |
| 2013/2014 | Yam—Massachusetts/12 | Yam—Wisconsin/10 | Vic—Brisbane/08 | To Wisconsin/10 everywhere | None |
| 2014/2015 | Yam—Massachusetts/12 | Yam—Phuket/13 | Vic—Brisbane/08 | None | None |
In this paper we compare three strategies to select the vaccine B lineage in a trivalent influenza virus vaccine based on the long-term overall protection rate expected from each strategy. First, the traditional lineage selection method that was used from 2001 onwards. Second, a yearly alternation strategy where representative B/Vic and B/Yam viruses are used in alternating years to take advantage of residual protection levels. Third, a hedging strategy, which in addition to residual protection takes into account the relative effects of titer decay over time, antigenic drift, and newcomers to the annual vaccination program. We compare these strategies by estimating protection levels to vaccination with both lineages for each year between 2000 and 2010. We then present a methodology for optimal vaccine lineage selection for a trivalent vaccine based on representative serum samples.
2. Methods
2.1. Antisera and titrations
Pre- and post-vaccination antisera were obtained from 2010 influenza vaccine trials in the United States, Australia, New Zealand, Japan and China (Table 2). Individuals had been vaccinated with a trivalent vaccine containing a B/Brisbane/60/2008-like virus, an A/California/7/2009 (H1N1)-like virus, and an A/Perth/16/2009 (H3N2)-like virus. Hemagglutination inhibition (HI) titers were measured to Brisbane/60/08 (the B/Vic lineage virus), and to B/Wisconsin/1/10 (the contemporary B/Yam lineage virus).
Table 2.
Number of individuals in panel per country and age group, and age range where available.
| No. of adults | No. of elderly | No. of pediatric | ||||
|---|---|---|---|---|---|---|
| Australia | 24 | (23–58 y) | 24 | (61–83 y) | ||
| US | 24 | 24 | 21 | (8 mo–2 y) | ||
| Japan | 24 | (20–58 y) | 24 | (62–100 y) | ||
| China | 30 | (19–59 y) | 30 | (60–88 y) | 29 | (3–5 y) |
| UK | 24 | 24 | ||||
We created an additional dataset based on this vaccination data with an increased difference in pre-vaccination titers between B/Vic and B/Yam to approximate the difference one might expect in the population after repeated years of vaccination with B/Vic. In this transformation the B/Vic titers were multiplied by 1.5, and the B/Yam titers divided by 1.5.
2.2. Calculation of expected post-titer values for hypothetical vaccination with opposite lineage
To estimate the post-vaccination titers to a hypothetical vaccination with B/Yam we assumed that cross-reactivity between the representatives of the two lineages was symmetric. To determine the titer boost to the vaccine and non-vaccine lineages after vaccination, we performed a linear regression that predicts post-vaccination titers from pre-vaccination titers. The regression was done on an individual basis, by estimating the linear relationship between log2 of the pre-vaccination titer (pre-titer) and log2 of the post-vaccination titer (post-titer) for each of the lineage-match and the non-lineage-match vaccination, to estimate the corresponding post-vaccination titer for each individual.
We explored the relationship between pre-vaccination titer and post-vaccination titer in the lineage-match case (“Match”) and in the opposite lineage vaccination case (“NonMatch”) by running a linear regression for each subpopulation to find the cMatch and cNonMatch constants and intercepts and ßMatch and ßNonMatch coefficients
We then used the Match and NonMatch constants and coefficients to predict a hypothetical B/Vic post vaccination titer HypVicPost and a B/Yam post vaccination titer HypYamPost that would have resulted from a hypothetical vaccination with B/Yam, by inverting them as follows:
3. Results
3.1. Comparison between three lineage selection strategies
3.1.1. The incumbent lineage strategy
The current practice for vaccine composition selects the antigenically most up-to-date strain of the B lineage circulating during the most recent influenza season of the given hemisphere. This ensures good protection levels against influenza B strains when the previously dominant lineage continues to dominate.
3.1.2. The yearly alternation strategy
In the last decade of B lineage co-circulation, repeat years (in which the same B lineage dominates as in the previous season) appear to be neither more nor less frequent than years in which the lineage has alternated. Given that it is not currently possible to predict which lineage will dominate the next season [9], [10] (Table 1), the yearly alternation strategy is simply to alternate the B lineage in the vaccine on a yearly basis, one year a B/Vic lineage, the next a B/Yam lineage, and so on. This strategy exploits residual cross-reactivity to provide some protection to the lineage not included in that year's vaccine, from the previous year’ vaccination.
The yearly alternation strategy is based on the premise that any person who has been in the vaccination program for at least one year prior to the current vaccination will always have new protection against one strain, and one-year old residual protection to the other. Overall protection levels would be improved because in the 50% of year in which there will be a lineage-match protection would be at full protection level just as in the incumbent lineage strategy, but in the remaining 50% non-lineage-match years the residual immunity would be higher as will be shown.
3.1.3. The hedging strategy
Three sources of information, taken at the time of vaccine lineage choice, can inform the hedging strategy: number of years since vaccination with either lineage, whether there has been antigenic drift in either lineage during that time, and, if available, direct serological assessment of residual protection levels in the population. The hedging strategy chooses the B lineage for the vaccine that yields the highest expected vaccine efficacy. The strategy is based on a decision tree, a standard tool in operations research [15], to assist in the choice between alternative options, in this case between B lineages in a trivalent vaccine.
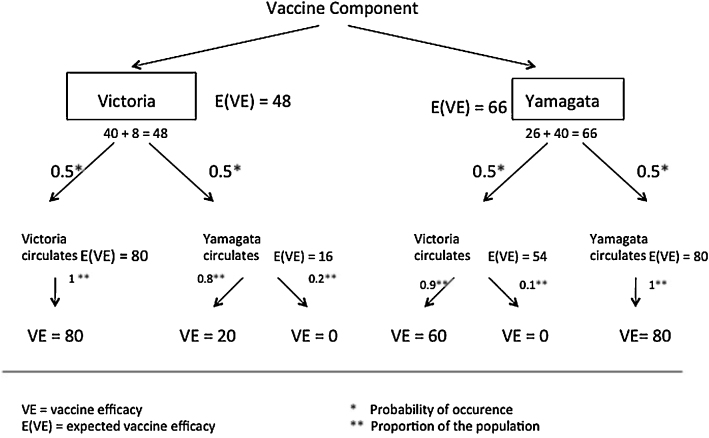
Fig. 2 shows an example decision tree based on the Northern Hemisphere influenza season 2005–2006. The previous year's vaccine included a strain from the B/Vic lineage, and the year previous to that, season 2003–2004, the vaccine included a B/Yam strain.
Fig. 2.
Example decision tree. A representative of B/Yam or B/Vic strain has to be chosen for the vaccine. Both choices lead to two possible outcomes: the vaccine lineage dominates (outermost branches of the tree), or the non-vaccine lineage dominates (innermost branches of the tree).
In this figure we show an example parameterization of the decision tree: vaccine-induced protection is 80% following vaccination, decaying to 60% after one year, and to 40% after two years, that drift in a strain halves residual protection, and that 10% of each year's vaccinated population consists of naïve first-time vaccinees. We show in the Supplementary materials section S1 “Proof of generality of the basic model” that the hedging strategy provides higher protection in the interval 2000/2001–2011/2012 for all possible parameter values. In order to choose a vaccine lineage the expected vaccine efficacy E(VE) is calculated for the two choices, either a B/Vic or a B/Yam vaccine component. In the case of this particular parameterization, the residual protection for the B/Vic lineage is higher (54%) than for the B/Yam lineage (16%). This difference is because the B/Yam lineage had not been in the vaccine for two years, and the B/Yam lineage had undergone drift during this time. The expected overall vaccine efficacy for influenza B from the B/Vic strain selected by the incumbent lineage strategy is 48%, whereas the hedging strategy would choose a representative of B/Yam and yield an expected overall vaccine efficacy of 66%. Therefore, the hedging strategy would recommend, based on this decision tree, the use of a B/Yam virus in the vaccine.
In the case of lineage-mismatch, residual protection levels to the non-vaccine strain are relatively high compared to when a vaccinated individual has not been vaccinated or naturally infected with the circulating lineage for multiple years.
There are two sources of improvement in vaccine efficacy when the B lineage used in the vaccine changes frequently. The residual protection for the non-circulating lineage is higher, and there is also a larger group of vaccinated individuals that have been vaccinated with both strains within the last two years. This is in sharp contrast to the situation in which the last vaccination with the circulating strain is several years earlier, in which case not only might individuals’ immunity have decreased substantially, but new entrants to the vaccine program will have had no direct vaccine-induced protection to the alternate lineage.
3.2. Theoretical comparison of the three lineage selection strategies
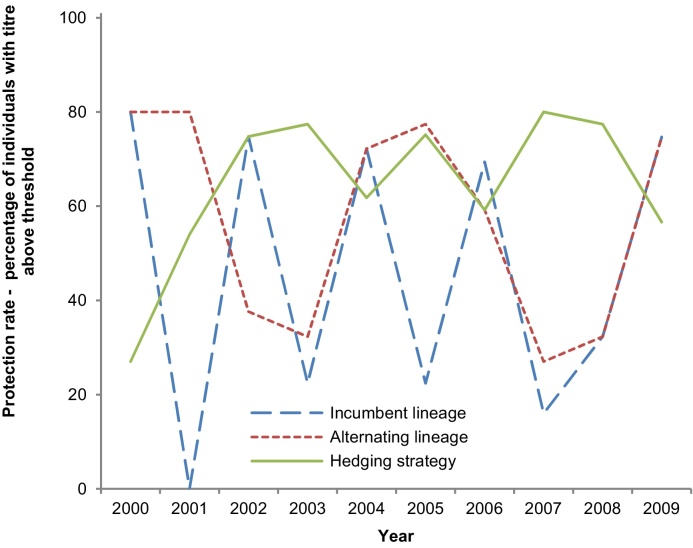
To compare the three lineage-selection strategies, we evaluated the expected protection levels over a ten-year period, from 2000 to 2010. Fig. 3 shows the expected protection levels based on the example parameterization above (parameter values and results in Table S2). For these years the hedging strategy yields overall higher levels of E(VE) than the incumbent lineage strategy and the yearly alternation strategy. The hedging strategy improves protection against influenza B not by reducing the number of lineage-mismatches, but by limiting their negative impact.
Fig. 3.
Predicted protection levels for the seasons 2000/2001 to 2009/2010 for each of the three lineage selection strategy.
The hedging strategy yields the largest gains in situations where the difference in pre-vaccination titers between the two lineages is high.
3.3. Comparison of the three lineage selection strategies based on serology data from vaccine re-registration trials
The yearly alternation strategy and the hedging strategy are based on the assumption that vaccinating with the influenza B lineage to which the population has lower antibody titers results in greater overall expected protection levels for both lineages than vaccinating with the lineage already associated with higher antibody titers. Above we have relied on estimates for pre-vaccination titers, but for greater accuracy and reliability a population sample could be titrated ahead of the vaccine composition decision.
Ideally, shortly before the vaccine composition meeting, a serologically representative population sample would be taken, measuring titers against the most evolutionarily advanced representatives of B/Vic and B/Yam, and vaccinating with the lineage that has lower protection levels.
To approximate the outcome of such a study, on data that has the sort of heterogeneity that might be expected, we next generated a sample dataset derived from an existing serological study, used at the 2010/2011 Northern Hemisphere WHO vaccine composition meeting. In this dataset, titer levels to influenza B B/Vic and B/Yam were measured for a total of 303 individuals in the United States, China, the United Kingdom, Australia, and Japan before and after vaccination with Brisbane/60/2008, a B/Vic lineage strain. From this dataset we construct three additional datasets, one in which we take the raw data and approximate what would have happened if vaccination had been against a virus in the other lineage, and then two others in which we artificially create a greater (and perhaps more likely in a random population) difference between B/Vic and B/Yam pre-titres (see Section 2), and estimate the effect of a B/Vic and B/Yam vaccination given these modified pre-titers.
A titer of 1:40 is widely used as a correlate of protection, as it is thought to correspond to 50% protection. Therefore, we measure the proportion of titers above 40 to estimate vaccine outcome (protection rate) [16]. The average pre-vaccination protection rate to B/Vic in the unadjusted 2010/2011 Northern hemisphere data set is 50%, the average pre-vaccination protection rate to B/Yam is 26% (Table 3A).
Table 3.
Average population protection rates (proportion of population with titers ≥40). Post B/Vic vaccination protection rates are the proportion of individuals in the sample with titers ≥40. Post B/Yam vaccination protection rates are estimated based on the observed vaccine response (see Section 2). Results based on 2011 sera.
| A | Pre vaccination (%) | Post B/Vic vaccination (%) | Post B/Yam vaccination (%) | Post both vaccinations (%) |
|---|---|---|---|---|
| Vic | 49 | 88 | 49 | 88 |
| Yam | 26 | 49 | 100 | 100 |
| Average | 37.5 | 68.5 | 74.5 | 94 |
| Results based on altered 2011 sera with a magnified difference in pre-vaccination titers between the two lineages | ||||
| B |
Pre vaccination (%) |
Post B/Vic vaccination (%) |
Post B/Yam vaccination (%) |
Post both vaccinations (%) |
| Vic | 49 | 74 | 62 | 74 |
| Yam | 10 | 25 | 100 | 100 |
| Average | 29.5 | 49.5 | 81 | 87 |
We calculated an overall post-vaccination protection rate as an average of the protection rates to the two lineages in Table 3, assuming a 50% chance of either lineage circulating in the 2010/2011 season. In the unadjusted 2010/2011 Northern hemisphere data set the difference in pre-vaccination titers between the two lineages is small, and the hedging strategy accordingly results in a small improvement over the incumbent lineage strategy. Given equal lineage circulation probabilities, the overall protection rate against influenza B in the 2010/2011 sample with the incumbent strategy was 69%, but would have been 75% with the hedging strategy, giving an additional 6% of protected individuals at no extra cost. The improvement of a quadrivalent vaccine is an increase in protection rate from 69% to 94% (Table 3B), i.e., an additional 25% of protected individuals.
In the dataset with a magnified difference in pre-vaccination titers, the pre-vaccination protection rate against B/Yam is 10%, and against B/Vic is 49% (Table 3B). In this case, the expected protection rate from the incumbent lineage strategy (vaccination with B/Vic) is 49.5%, and the expected protection rate from the hedging strategy (vaccination with B/Yam) is 81%. The expected protection rate from the quadrivalent vaccine is 87%. These results emphasize (given the same probabilities of either lineage circulating) that the benefit of the hedging strategy is more pronounced when the initial difference in pre-vaccination titers is larger. They also illustrate how, in any scenario, the hedging strategy provides the highest expected protection rate of the three strategies.
4. Discussion
This study was performed to evaluate three influenza B vaccine lineage selection strategies. We demonstrated that the hedging strategy provided higher overall titer levels and superior protection levels for individuals that are repeatedly vaccinated than either the current incumbent lineage selection strategy, or an annual lineage switch strategy.
A vaccination with either B lineage better for boosting titers to that particular lineage. But in the absence of knowledge of which lineage will circulate, overall a bigger improvement results from vaccinating with the lineage that has the most unprotected individuals prior to vaccination. A strong increase of low titers coupled with a weak increase of already high titers is overall more protective than a modest increase of previously high titers coupled with a weak increase of low titers. The improvement provided by the hedging strategy is expected to be larger in years when the difference between pre-vaccination titers is larger than when the difference between these titers is lower, but in any case always exceeds the protection provided by the incumbent or alternating lineage strategies.
Our results demonstrate that while the B/Vic and B/Yam lineages circulate in a currently unpredictable pattern, it is best not to let protection levels to either lineage drop too low, and to vaccinate with the lineage with lowest pre-vaccination titers. The hedging strategy achieves higher overall protection than the incumbent lineage strategy by careful management of residual protection. The superiority of the hedging strategy is not that it achieves a higher proportion of lineage-match years, but that it selects the B lineage so protection is higher than the incumbent lineage strategy in non-lineage-match years.
For those vaccination programs and individuals who can afford it, a quadrivalent vaccine avoids the need for a B lineage selection strategy for the vaccine. However, the majority of the world's influenza vaccine recipients currently receive a trivalent vaccine. Analyses to compare the relative benefits and cost effectiveness of quadrivalent vaccines are ongoing [17], [18], [19]. We have shown that some of the benefit of transferring to a quadrivalent vaccine can be captured without the cost of moving to a quadrivalent vaccine with an improved trivalent vaccine lineage selection approach. Individuals who receive a trivalent vaccine should benefit from the best possible B selection strategy, and true cost-effectiveness studies for quadrivalent vaccines should compare their added value to the performance of a trivalent vaccine based on the best possible B lineage-selection strategy.
Acknowledgements
Sera used in this research were kindly provided by the following collaborators: for Australia by Dr. Ian Barr, WHO Collaborating Center, Melbourne, Australia, pediatric sera for the United States by Dr. Jackie Katz, WHO Collaborating Center, Atlanta, US, for Japan by Dr. Takato Odagiri, WHO Collaborating Center, Tokyo, Japan, for China by Dr. Yuelong Shu, WHO Collaborating Center, Beijing, China, for the United Kingdom by Dr. John Wood, NIBSC, Hertfordshire, UK. Additional thanks to Drs Nancy Cox, Michael Shaw, and Alexander Klimov. This work was supported by European Union FP7 program EMPERIE (22349) and ANTIGONE (278976), National Institute of Allergy and Infectious Diseases–NIH Centers of Excellence for Influenza Research and Surveillance contracts HHSN266200700010C and HHSN272201400008C. JMF is supported by a Fellowship in Biomedical Informatics from the Medical Research Council (UK) and a Junior Research Fellowship from Homerton College, Cambridge. Funded by: ERCPMC grant number: FP7 22349.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2016.01.042.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.WHO . WHO; 2003. Fact sheet no. 211 influenza. Available from: 〈http://www.who.int/mediacentre/factsheets/2003/fs211/en/〉. [Google Scholar]
- 2.Grohskopf L.A., Olsen S.J., Sokolow L.Z., Bresee J.S., Cox N.J., Broder K.R. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2014–15 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63(32):691–697. [PMC free article] [PubMed] [Google Scholar]
- 3.Klimov A.I., Garten R., Russell C., Barr I.G., Besselaar T.G., Daniels R. WHO recommendations for the viruses to be used in the 2012 Southern Hemisphere Influenza Vaccine: epidemiology, antigenic and genetic characteristics of influenza A(H1N1)pdm09, A(H3N2) and B influenza viruses collected from February to September 2011. Vaccine. 2012;30(45):6461–6471. doi: 10.1016/j.vaccine.2012.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barr I.G., Russell C., Besselaar T.G., Cox N.J., Daniels R.S., Donis R. WHO recommendations for the viruses used in the 2013–2014 Northern Hemisphere influenza vaccine: epidemiology, antigenic and genetic characteristics of influenza A(H1N1)pdm09, A(H3N2) and B influenza viruses collected from October 2012 to January 2013. Vaccine. 2014;32(37):4713–4725. doi: 10.1016/j.vaccine.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Barr I.G., McCauley J., Cox N., Daniels R., Engelhardt O.G., Fukuda K. Epidemiological, antigenic and genetic characteristics of seasonal influenza A(H1N1), A(H3N2) and B influenza viruses: basis for the WHO recommendation on the composition of influenza vaccines for use in the 2009–2010 northern hemisphere season. Vaccine. 2010;28(5):1156–1167. doi: 10.1016/j.vaccine.2009.11.043. [DOI] [PubMed] [Google Scholar]
- 6.Gerdil C. The annual production cycle for influenza vaccine. Vaccine. 2003;21(16):1776–1779. doi: 10.1016/s0264-410x(03)00071-9. [DOI] [PubMed] [Google Scholar]
- 7.Stohr K., Bucher D., Colgate T., Wood J. Influenza virus surveillance, vaccine strain selection, and manufacture. Methods Mol Biol. 2012;865:147–162. doi: 10.1007/978-1-61779-621-0_9. [DOI] [PubMed] [Google Scholar]
- 8.Russell C.A., Jones T.C., Barr I.G., Cox N.J., Garten R.J., Gregory V. Influenza vaccine strain selection and recent studies on the global migration of seasonal influenza viruses. Vaccine. 2008;26(Suppl. 4):D31–D34. doi: 10.1016/j.vaccine.2008.07.078. [DOI] [PubMed] [Google Scholar]
- 9.Ambrose C.S., Levin M.J. The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother. 2012;8(1):81–88. doi: 10.4161/hv.8.1.17623. (Epub 2012/01/19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Della Cioppa G., Vesikari T., Sokal E., Lindert K., Nicolay U. Trivalent and quadrivalent MF59((R))-adjuvanted influenza vaccine in young children: a dose- and schedule-finding study. Vaccine. 2011;29(47):8696–8704. doi: 10.1016/j.vaccine.2011.08.111. (Epub 2011/09/13) [DOI] [PubMed] [Google Scholar]
- 11.Frey S., Vesikari T., Szymczakiewicz-Multanowska A., Lattanzi M., Izu A., Groth N. Clinical efficacy of cell culture-derived and egg-derived inactivated subunit influenza vaccines in healthy adults. Clin Infect Dis. 2010;51(9):997–1004. doi: 10.1086/656578. (an official publication of the Infectious Diseases Society of America) (Epub 2010/09/28) [DOI] [PubMed] [Google Scholar]
- 12.Beran J., Wertzova V., Honegr K., Kaliskova E., Havlickova M., Havlik J. Challenge of conducting a placebo-controlled randomized efficacy study for influenza vaccine in a season with low attack rate and a mismatched vaccine B strain: a concrete example. BMC Infect Dis. 2009;9:2. doi: 10.1186/1471-2334-9-2. (Epub 2009/01/20) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skowronski D.M., Hamelin M.E., Janjua N.Z., De Serres G., Gardy J.L., Rheaume C. Cross-lineage influenza B and heterologous influenza A antibody responses in vaccinated mice: immunologic interactions and B/Yamagata dominance. PloS ONE. 2012;7(6):e38929. doi: 10.1371/journal.pone.0038929. (Epub 2012/06/30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asahi-Ozaki Y., Yoshikawa T., Iwakura Y., Suzuki Y., Tamura S., Kurata T. Secretory IgA antibodies provide cross-protection against infection with different strains of influenza B virus. J Med Virol. 2004;74(2):328–335. doi: 10.1002/jmv.20173. (Epub 2004/08/28) [DOI] [PubMed] [Google Scholar]
- 15.Savage S.L. Thomson, Brooks/Cole; Belmont, CA: 2003. Decision making with Insight: includes Insight.xla 2.0. (xxii, 360 p. p.) [Google Scholar]
- 16.Coudeville L., Bailleux F., Riche B., Megas F., Andre P., Ecochard R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC Med Res Methodol. 2010;10:18. doi: 10.1186/1471-2288-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee B.Y., Bartsch S.M., Willig A.M. The economic value of a quadrivalent versus trivalent influenza vaccine. Vaccine. 2012;30(52):7443–7446. doi: 10.1016/j.vaccine.2012.10.025. (Epub 2012/10/23) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Bellinghen L.A., Meier G., Van Vlaenderen I. The potential cost-effectiveness of quadrivalent versus trivalent influenza vaccine in elderly people and clinical risk groups in the UK: a lifetime multi-cohort model. PloS ONE. 2014;9(6):e98437. doi: 10.1371/journal.pone.0098437. (Epub 2014/06/07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clements K.M., Meier G., McGarry L.J., Pruttivarasin N., Misurski D.A. Cost-effectiveness analysis of universal influenza vaccination with quadrivalent inactivated vaccine in the United States. Hum Vaccin Immunother. 2014;10(5):1171–1180. doi: 10.4161/hv.28221. (Epub 2014/03/13) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.