Abstract
The endogenous endocannabinoid system has a crucial role in regulating appetite and feeding behavior in mammals, as well as working memory and reward mechanisms. In order to elucidate the possible role of cannabinoid type-1 receptors (CB1Rs) in the regulation of hippocampal plasticity in animals exposed to food restriction (FR), we limited the availability of food to a 2-h daily period for 3 weeks in Sprague–Dawley rats. FR rats showed a higher long-term potentiation at hippocampal CA1 excitatory synapses with a parallel increase in glutamate release when compared with animals fed ad libitum. FR rats showed a significant increase in the long-term spatial memory determined by Barnes maze. FR was also associated with a decreased inhibitory effect of the CB1R agonist win55,212-2 on glutamatergic field excitatory postsynaptic potentials, together with a decrease in hippocampal CB1R protein expression. In addition, hippocampal brain-derived neurotrophic factor protein levels and mushroom dendritic spine density were significantly enhanced in FR rats. Altogether, our data suggest that alterations of hippocampal CB1R expression and function in FR rats are associated with dendritic spine remodeling and functional potentiation of CA1 excitatory synapses, and these findings are consistent with increasing evidence supporting the idea that FR may improve cognitive functions.
Introduction
The endogenous cannabinoid (eCB) system mediates many of the psychotropic as well as the appetite-stimulating effects of cannabis (Carr et al, 2008). Exogenous (Δ9-tetrahydrocannabinol) as well as endogenous (anandamide, AEA and 2-arachidonoylglycerol, 2-AG) cannabinoid type-1 receptor (CB1R) agonists induce a state of overeating in humans and rats (Gaetani et al, 2008; Williams and Kirkham, 2002). Changes in eating motivation associated with cannabis use is further consistent with the key regulatory role of the eCB system in the physiological control of appetite, feeding behavior, energy metabolism, and body weight (Williams and Kirkham, 2002).
We recently reported that food restriction (FR) in rats is accompanied by a reduction in CB1R expression and function, as well as an increase in dopamine output in the medial prefrontal cortex (mPFC; Dazzi et al, 2014). These effects appeared to be triggered by anticipation of food intake during the daily session of food presentation, as they were no longer detectable several hours after food had been removed. Such evidences support the idea that neuronal changes induced by anticipation of feeding may be consistent with a learning process associated with FR, which, in turn, could underly synaptic modifications and plasticity in other brain regions such as the hippocampus.
The hippocampal region is characterized by a high level of CB1R expression (Mackie, 2008). Local hippocampal infusion of cannabinoid receptor agonists strongly impairs performances in the radial or T-maze test in rats (Egashira et al, 2002; Suenaga and Ichitani, 2004), suggesting that hippocampal CB1Rs also have a role in the modulation of learning and memory formation. Consistent with this hypothesis, CB1R antagonists, decrease in CB1R expression or CB1R genetic knockout, are associated with an increase in memory (Lichtman, 2000) as well as social recognition (Terranova et al, 1996). In addition, previous studies in rat hippocampal slices have indicated that CB1R agonists (Abush and Akirav, 2010) and antagonists, as well as CB1R genetic knockout (Bohme et al, 2000; Slanina et al, 2005) can impair different forms of synaptic plasticity such as long-term potentiation (LTP) and long-term depression.
eCBs in the hippocampal CA1 field act as retrograde messengers leading to a modulation of both GABAergic as well as glutamatergic synapses activity (Abush and Akirav, 2010). It is known that CB1Rs are predominantly presynaptic and their activation significantly decrease neurotransmitter release (Lovinger, 2008; Mackie, 2008). Consequently, alterations in the eCB system in animal exposed to FR might have a great impact on short as well as long-term plasticity of hippocampal synapes.
In the present study, we have further examined the effects of FR, applied for 3 weeks in Sprague–Dawley rats, on the long-term plasticity of glutamatergic synapses in the hippocampal CA1 field and analyzed the role of CB1Rs. Our results indicate that FR in rats induces a decrease of both hippocampal CB1R expression and function at CA1 glutamatergic synapses, an effect that is paralleled by an increased probability of glutamate release, enhanced LTP formation, and improved long-term spatial memory. All these effects are accompanied by marked modification of dendritic spines in CA1 hippocampal pyramidal neurons.
Materials and methods
Animals
Male Sprague–Dawley CD rats (Charles River, Como, Italy) were bred in our animal facility and maintained under a constant artificial 12-h light and 12-h dark cycle (lights on from 0800 to 2000 hours), at controlled temperature of 22±2 °C, and at relative humidity of 65%. Animal care and handling throughout the experimental procedures were in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC). The experimental protocols were also approved by the Animal Ethics Committee of the University of Cagliari.
FR Paradigm
Rats with a body mass of 200–230 g (50–60 days old) were randomly assigned either to a control group (CTRL), which received food and water ad libitum, or to the FR group, which were allowed to eat their daily meal only for 2 h (from 1100 to 1300 hours), with tap water always available. Body weight and food consumption were measured daily during the 3-week period in which the FR regimen was applied. CTRL animals consumed a constant amount of food (25.6±0.1 g per 24 h) during the whole experimental period, whereas FR animals gradually increased their daily food consumption, achieving a constant amount of 22.4±0.2 g per 2 h by day 16–18 of treatment, that results significantly less than CTRL (Supplementary Figure S1A and B). This different food consumption was no more significant when calculated respect to body weight (Supplementary Figure S1C and D). In addition, body weight was slightly reduced in FR rats during the initial 3–4 days, and FR animals reached at the end of FR period an averaged body weight that was significantly lower than that of CTRL rats (Supplementary Figure S1E and F).
Preparation of Hippocampal Coronal Slices
Coronal slices containing the hippocampal formation were prepared from both CTRL and FR animals as previously described (Sanna et al, 2011; Talani et al, 2011). Slices were obtained from FR rats that were killed at various time points with respect to the start of the feeding session. Coronal hippocampal slices (thickness of 260 and 400 μm for patch-clamp and extracellular recordings, respectively) were cut using a Leica VT1200S vibratome (Leica, Heidelberg, Germany). Slices were transferred to a nylon net submerged in standard ACSF for at least 40 min at 35 °C (for patch-clamp experiments) or at RT (for extracellular recordings). A hemi-slice was then transferred to the recording chamber and perfused with standard ACSF at a constant flow rate of ~2 ml/min. For all recordings, the temperature of the bath was maintained at 33 °C.
Extracellular and Whole-Cell Patch-Clamp Recordings
Extracellular recordings of field excitatory postsynaptic potentials (fEPSPs) were performed in the stratum radiatum of the CA1 hippocampal region through stimulation of the Schaffer collateral afferents as previously described (Sanna et al, 2011; Talani et al, 2011). LTP was elicited as previously reported (Sanna et al, 2011) by a high-frequency stimulation (HFS), consisting of a single train of 100 stimuli at 250 Hz. The paired-pulse (PP) protocol consisted in delivering two different stimuli, with an interstimulus interval of 50 ms. The ratio between the slope of the second and the first fEPSP was calculated. Input–output (I–O) curves were constructed by measuring the slope of fEPSPs evoked in response to stimulation with increasing intensity (0–1.0 mA). Nonlinear regression analysis of the I–O relation was performed with Prism software (version 6, GraphPad Software Inc., San Diego, CA, USA) according to the equation:
where Imin and Imax are the minimal and maximal values of fEPSP slope, respectively, EC50 is the stimulation intensity that produced 50% of the maximal response, X is the stimulation intensity, and nH is the Hill coefficient. The value of the stimulation intensity producing half-maximal response relative to each experimental group was calculated by averaging the values from each individual curve.
Whole-cell recordings from CA1 pyramidal neurons were performed as previously described (Sanna et al, 2011; Talani et al, 2011). Recording pipettes had a resistance ranging from 2.5 to 4.5 MΩ when filled with an internal solution containing (in mM): 150 CsCl, 10 HEPES, 5 lidocaine N-ethyl bromide, 2 MgCl2, 3 Mg-ATP, 0.3 Na-GTP, and 10 BAPTA-4K, pH adjusted to 7.2 with CsOH. Spontaneous GABAA receptor-mediated miniature inhibitory postsynaptic currents (mIPSCs) were recorded in the presence of the nonselective AMPA/NMDA antagonist kynurenic acid (1 mM), whereas for AMPA/kainate receptor-mediated miniature EPSC (mEPSC) recordings, the GABAA receptor antagonist bicuculline (20 μM) was added to the extracellular ACSF. In all cases, the voltage-dependent Na+ channel blocker lidocaine (500 mM) was added. Cells were voltage clamped at −65 mV and synaptic currents were recorded with an Axopatch 200-B amplifier (Axon Instruments, Foster City, CA, USA), filtered at 2 kHz, and digitized at 5 kHz. Lidocaine-insensitive mIPSC or mEPSC amplitude, decay time, and frequency were acquired using peak and event detection software in pClamp9.2 (Union City, CA, USA) and analysis was performed with Minianalysis 60.
Other Methods
For a detailed description of the methods regarding Barnes maze test, immunoblot analysis, confocal microscopy, and eCB analysis, see Supplementary Information.
Statistical Analysis
Data are presented as mean±SEM and were compared with one-way ANOVA or Student's t-test with the use of Prism software (version 6, GraphPad). A P-value <0.05 was considered statistically significant.
Results
Effect of FR on win55-212,2-Induced Inhibition of fEPSPs in the Dendritic CA1 Field
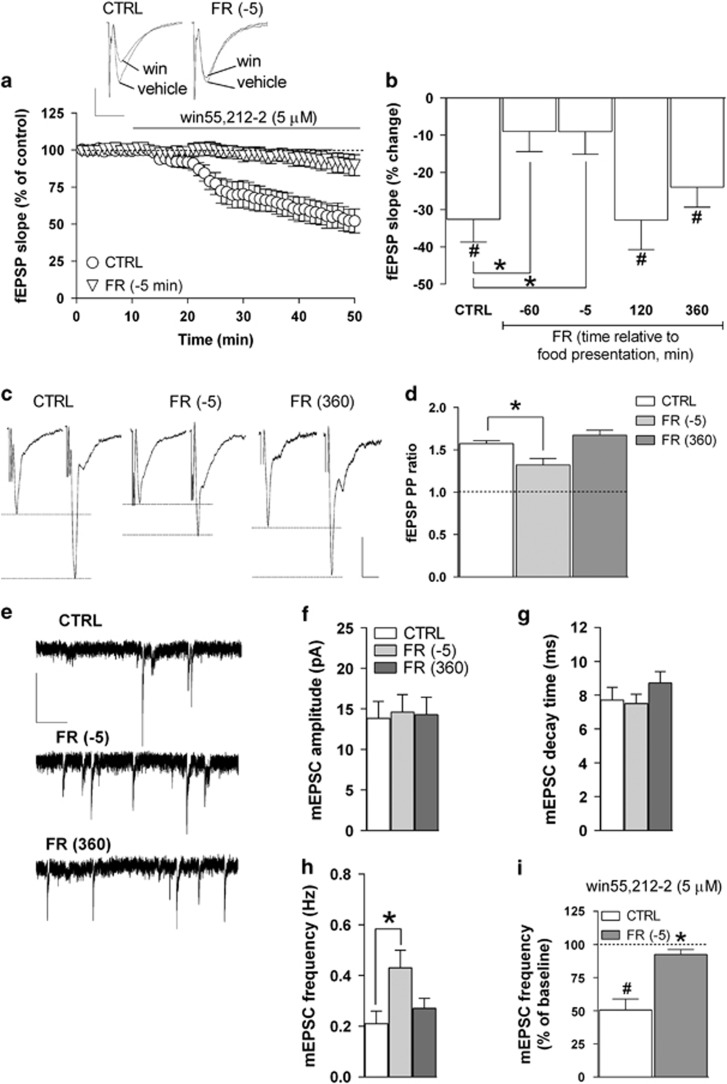
In our recent study (Dazzi et al, 2014), we showed that FR caused significant alterations in the function and expression of CB1Rs in the rat mPFC, an effect that appeared related to the anticipation of the scheduled daily food presentation. In order to determine whether FR could produce a similar pattern of changes in CB1R function also at the hippocampal level, we initially tested the effect of the CB1R agonist win55-212,2 on glutamatergic fEPSPs recorded extracellularly in the stratum radiatum of the CA1 field. Activation of CB1Rs by selective agonists, in fact, has been shown to markedly decrease glutamate release from presynaptic terminals in CA1 pyramidal neurons (Xu et al, 2010). Accordingly, we found that in slices obtained from CTRL rats, bath application of win55,212-2 (5 μM) produced a marked decrease in fEPSP slope and amplitude; in particular, the effect on slope had an apparent onset of ~10–15 min following the continuous drug perfusion and reached a value of −32.6±6.1% (P<0.05) after 30–40 min, compared with baseline (Figure 1a). The inhibitory effect of win55,212-2 was reversible upon washout and completely abolished by the coperfusion of the CB1R antagonist SR141716 (1 μM; data not shown).
Figure 1.
Effects of FR on CB1 receptor function and glutamatergic transmission in the hippocampal CA1 field. (a) Scatter plot relative to the effect of 5 μM win55,212-2 on fEPSP slope recorded in the stratum radiatum of the CA1 field from CTRL and FR rats killed 5 min before food presentation. Representative traces of fEPSPs showing the effect of win55,212-2 in slices from CTRL and FR killed 5 min before food presentation are shown above (scale bars: 1 mV, 10 ms). (b) Bar graph reporting the percent change of fEPSP slope value from baseline induced by win55,212-2 in slices from CTRL and FR rats that were tested at different time intervals relative to food presentation (n=5–16). (c) Representative traces of fEPSPs recorded in the CA1 field from CTRL and FR rats (killed 5 min before and 360 min after food presentation). Two stimuli (PP) were delivered with an inter-interval of 50 ms, and the ratio between the slope of the second and the first fEPSP was calculated (scale bars: 1 mV, 10 ms). (d) Bar graph illustrating the averaged PP ratio values for CTRL and FR rats (n=5–12). (e) Representative traces of mEPSCs recorded in CA1 pyramidal neurons in the presence of kynurenic acid (1 mM) and lidocaine (500 μM; scale bars: 10 pA, 1 s). (f–h) Bar graphs summarizing the basal properties (amplitude, decay time, and frequency) of mEPSCs from CA1 pyramidal neurons (n=12–16). (i) Bar graph showing the effect of 10-min bath perfusion of win55,212-2 (5 μM) on mEPSC frequency in slices from CTRL and FR rats killed 5 min before food presentation (n=5). Data are means±SEM. *P<0.05 vs CTRL. #P<0.05 vs baseline, one-way ANOVA and Bonferroni post hoc test. CTRL, control group; fEPSP, field excitatory postsynaptic potential; FR, food restriction; mEPSC, miniature EPSC; PP, paired-pulse.
As shown in Figure 1a and b, the inhibitory effect induced by win55,212-2 on fEPSP slope resulted greatly attenuated in hippocampal slices obtained from FR rats that were killed 60 or 5 min before food presentation. The effect of win55,212-2 returned to values similar to CTRL in FR animals killed 120 or 360 min after food presentation (Figure 1b). One-way ANOVA revealed a significant main effect of the treatment (FR vs CTRL; F(4,31)=4.21, P<0.01; Figure 1b).
Effects of FR on Glutamatergic Synaptic Transmission in CA1 Pyramidal Neurons
In order to evaluate whether the observed decrease in CB1R sensitivity to the selective agonist win55,212-2 found in FR rats could result in an altered control of basal glutamate release, we applied the PP protocol on the basis of the observation that changes in PP ratio are indicative of changes in the probability of neurotransmitter release (Mennerick and Zorumski, 1995). In CA1 pyramidal neurons of CTRL animals, the PP ratio had a value of 1.57±0.07, whereas in FR rats that were killed 5 min before food presentation, this value was significantly lower (1.17±0.12, P<0.05); again, the PP ratio detected in FR animals that were tested 360 min after food presentation had a value that was not statistically different from that of CTRL rats (1.67±0.05; Figure 1c and d).
To further examine the effect of FR on the probability of glutamate release in CA1 pyramidal neurons, we recorded spontaneous glutamatergic (mEPSCs) under voltage-clamp conditions (holding potential, −65 mV) in the presence of the voltage-gated Na+ channel blocker lidocaine (500 μM) and the GABAAR antagonist bicuculline (20 μM). Inward mEPSCs were completely suppressed by the glutamate receptors broad-spectrum antagonist kynurenic acid (1 mM) or CNQX (5 μM), suggesting that they were mediated by AMPA/kainate receptors (data not shown). Analysis of the kinetic properties of mEPSCs revealed that in FR animals tested 5 min before food presentation, the frequency of mEPSC frequency was significantly increased compared with CTRL (104±21%, P<0.05), with values of amplitude and decay time that were unmodified (Figure 1e–h). Furthermore, the increase in mEPSC frequency was no longer apparent in FR animals that were tested 360 min after food presentation (Figure 1e–h).
We next tested the effect of win55,212-2 on mEPSC frequency. In slices from CTRL rats, 30 min of bath perfusion with win55,212-2 (5 μM) resulted in a marked decrease (49.49±8.33%, P<0.05) in mEPSC frequency (Figure 1i), with no change in amplitude (data not shown). The inhibitory effect of win55,212-2 was greatly attenuated (7.67±3.8%, P<0.05) in hippocampal slices obtained from FR rats killed 5 min before food presentation (Figure 1i).
In a separate set of experiments, the effects of FR on basal GABAAR-mediated mIPSCs were examined in voltage-clamped (–65 mV) CA1 pyramidal neurons. We found no significant difference in mIPSC amplitude and frequency between CTRL and FR rats that were tested 5 min before as well as 360 min after food presentation (Supplementary Figure S2A and B). In addition, there was also no significant effect of FR on the modulatory action of win55,212-2 (5 μM) on mIPSC amplitude and frequency (Supplementary Figure S2C and D).
Effect of FR on Neuronal Excitability and LTP Formation in the Hippocampal CA1 Field
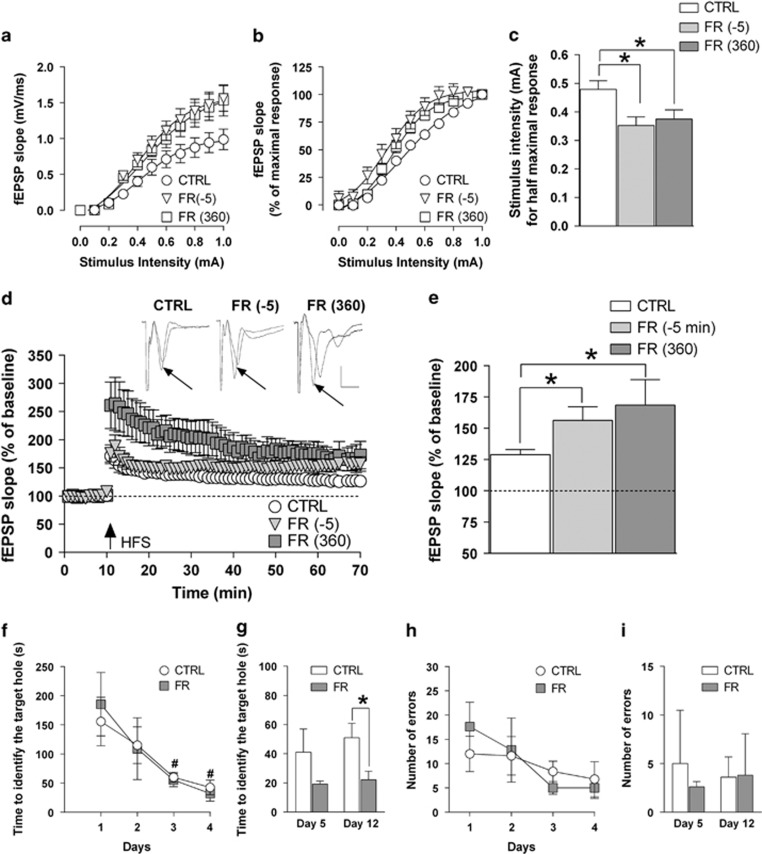
Changes related to CB1R function associated with increased probability of glutamate release may be predictive of a possible impact of FR also on neuronal excitability as well as long-term plasticity of glutamatergic synapses in the hippocampus. In order to further evaluate this hypothesis, we first recorded dendritic fEPSPs in the CA1 field and generated I–O curves by stimulating the Schaffer's collateral afferents with increasing intensity (from 0 to 1.0 mA). The slope of fEPSPs reached values that were significantly higher when recorded from FR rats that were tested either 5 min before and 360 min after food presentation compared with those obtained in the I–O curve from CTRL animals (F(2,290)=3.41, P<0.05; Figure 2a). From the normalized corresponding I–O curves, we found that the intensity of the stimulatory current that evoked a half-maximal response (quantified by analysis of the fEPSP slope) was significantly (P<0.05) decreased in hippocampal slices from FR rats killed either 5 min before (0.34±0.03 mA) and 360 min after (0.37±0.02) food presentation when compared with CTRL rats (0.47±0.03; Figure 2b and c).
Figure 2.
Effects of FR on neuronal excitability, LTP formation in the CA1 field, and spatial learning and memory. (a, b) Input–output (I–O) curves determined in slices from CTRL and FR rats (killed 5 min before and 360 min after food presentation) by measuring fEPSP slope in response to stimulation of Schaffer's collaterals with current steps of increasing intensity (from 0 to 1.0 mA). Data are expressed as absolute values (mV/ms) in panel a and as percentage of the corresponding maximal response in panel b (n=6–14). (c) Bar graph of the current intensity producing the half-maximal response calculated from the I–O curves shown in panels a and b. (d) Scatter plot representing the percentage change in fEPSP slope values induced by HFS with respect to baseline in rats of the different experimental groups. Above, representative traces of fEPSPs before and 1 h after HFS application (black arrow) are shown (scale bars: 1 mV, 5 ms). (e) Bar graph of the averaged fEPSP slope values obtained during the last 10 min of LTP recording, compared with the relative baseline (n=5–17). *P<0.05 vs CTRL, one-way ANOVA followed by Bonferroni post hoc test. (f–i) Performance of CTRL and FR rats in the Barnes maze. The time(s) needed to identify the target hole is shown either for the four consecutive training sessions (f) and for the test for the short- (day 5) and long-term (day 12) spatial memory (g). Number of errors during training sessions and during the test days is also shown in panels h and i, respectively (n=5). *P<0.05 vs CTRL, Student's t-test. #P<0.05 vs first training session, one-way ANOVA followed by Bonferroni test. CTRL, control group; fEPSP, field excitatory postsynaptic potential; FR, food restriction; HFS, high-frequency stimulation; LTP, long-term potentiation.
LTP was induced in the CA1 region by HFS that was delivered to the Schaffer's collateral afferents after 10 min of stable baseline. The magnitude of LTP, calculated by averaging the slope value of fEPSPs recorded during the last 10 min (ie, from 50 to 60 min post HFS), was significantly (P<0.05) increased in FR rats that were tested 5 min before (56.3±10.9%, P<0.05) and 360 min after (68.6±20.3%, P<0.05) food presentation, compared with CTRL (28.9±4% Figure 2d and e).
Effect of FR on Spatial Learning and Memory
The increased LTP formation in the hippocampal CA1 field observed in FR rats that were tested both 5 min before and 360 min after food presentation prompted us to determine whether this effect could be associated with an altered cognitive performance. We then measured spatial learning and memory in the Barnes maze test. CTRL and FR rats were trained once a day for four consecutive days during which the time needed to find the target hole decreased significantly (CTRL, F(3,16)=3.635, P<0.05; FR, F(3,16)=4.484, P<0.05) (Figure 2f). Animals were then tested on day 5 for their short-term memory. The performance of FR rats, although being slightly better, did not differ significantly (t=1.360, P=0.218) from that of CTRL animals (Figure 2g). However, when the test was repeated 7 days later (day 12), FR rats identified the target hole in a significant (t=2.470, P=0.0387) shorter time compared with CRTL animals (Figure 2g). The number of errors did not differ significantly between experimental groups during both tests (Figure 2h and i).
Expression of CB1R, eCBs, and BDNF Protein in the Hippocampus of FR Rats
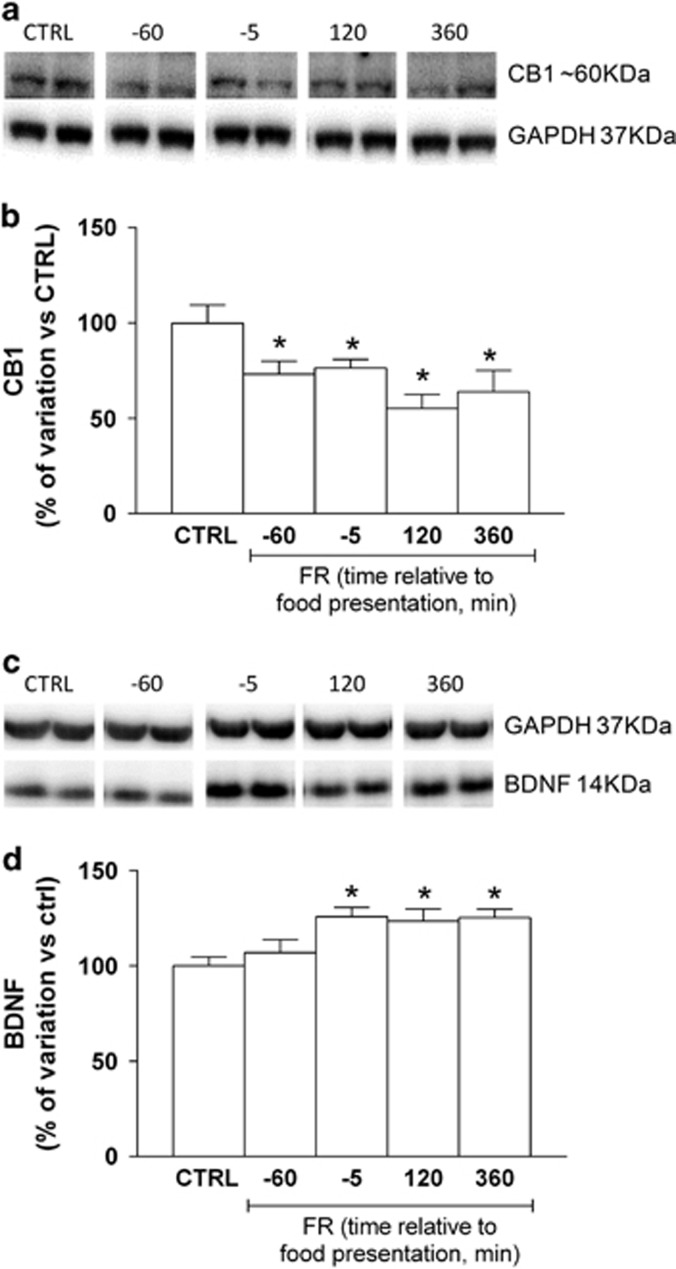
Expression of CB1R protein has been found to be markedly affected by specific dietary schedules (Dazzi et al 2014; Bello et al, 2012). In the present study, immunoblot analysis revealed that the amount of CB1R protein in the whole hippocampus was significantly decreased in FR rats killed at different time points with respect to food presentation when compared with CTRL animals (one-way ANOVA and Newman–Keuls test: F(6,50)=5.038, P<0.001; Figure 3a and b). In addition, analysis of the eCBs AEA and 2-AG in hippocampus showed no significant differences between FR and CTRL rats (AEA: CTRL=40±4.1 pmol/g tissue, FR=41.1±3.1 pmol/g tissue, P=0.8389, unpaired t-test; 2-AG: CTRL=17.5±1.8 nmol/g tissue, FR=17.1±2.4 pmol/g tissue, P=0.8926, unpaired t-test), revealing that changes in CB1R protein were not paralleled by different concentration of eCB. On the other hand, brain-derived neurotrophic factor (BDNF) protein level was increased in FR rats at all time points with respect to food presentation (one-way ANOVA and Newman–Keuls test: F(4,18)=4.82, P<0.05; Figure 3c and d).
Figure 3.
Effect of FR on CB1R receptor and BDNF expression in the hippocampus. Protein extracts, prepared from the hippocampus of CTRL and FR rats at the indicated times relative to food presentation, were subjected to immunoblot analysis with antibodies to CB1R or BDNF, and to GAPDH. (a, b) Representative blot as well as quantitation of the CB1R/GAPDH ratio (means±SEM from 5 to 10 rats) are shown. *P<0.001 vs. CTRL rats, one-way ANOVA with Bonferroni's post hoc test. (c, d) Representative blot as well as quantitation of the BDNF/GAPDH ratio (means±SEM from 5 to 10 rats) are shown. *P<0.001 vs CTRL rats, one-way ANOVA with Bonferroni's post hoc test. BDNF, brain-derived neurotrophic factor; CB1R, cannabinoid type-1 receptor; CTRL, control group; FR, food restriction.
Change in Spine Density and Dendrite Morphology in CA1 Pyramidal Neurons of FR Rats
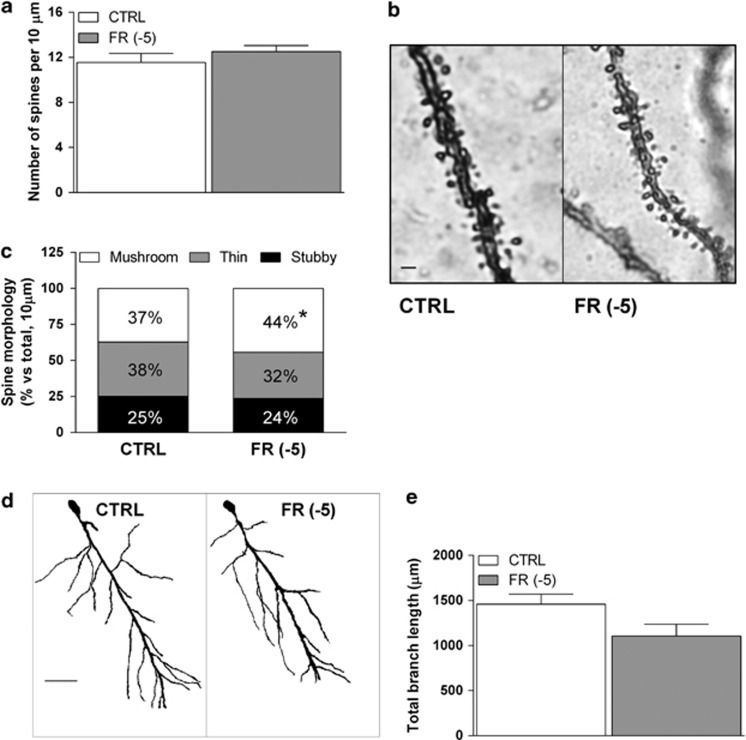
We here used a modified protocol of Golgi-Del Rio Hortega staining in order to reveal possible alterations in spine density and morphology, as well as dendritic length as a consequence of FR. We found no significant changes in total spine density, as calculated in 10 μm sections of basal dendrites in FR animals killed 5 min before food presentation (Figure 4a and b). A more detailed evaluation of spine shape, using Neuron Studio Software, revealed that FR is associated with an increase (44.3±2.1%) in the percentage of mushroom spines when compared with CTRL animals (37.2±2.7% Figure 4c). Furthermore, there was a modest reduction of thin spines, but this effect did not reach statistical significance. Moreover, FR produced no significant changes in the number of stubby spines (Figure 4c). In addition, total dendritic length was not significantly changed by FR (Figure 4d and e).
Figure 4.
Effects of FR on dendritic spine density and morphology in the rat hippocampal CA1 field. (a) Bar graph summarizing the total dendritic spine density of Golgi-impregnated pyramidal neurons of the hippocampal CA1 field. (b) Representative images of Golgi impregnated basal dendrites of CA1 pyramidal neurons (scale bar: 1 μm). (c) Bar graph summarizing the relative density of spines with different morphology as calculated in a 10-μm dendritic section of the different experimental groups. (d) Representative images of Golgi impregnated pyramidal neurons of the hippocampal CA1 field from CTRL and FR rats (scale bar: 50 μm). (e) Bar graph reporting the averaged total branch length of dendrites of CA1 pyramidal neurons from CTRL and FR rats. Data are means±SEM from 5 to 7 animals per group. CTRL, control group; FR, food restriction.
The Sub-Chronic Treatment of SR141716 in CTRL non-FR Rats Mimics the Effects of FR
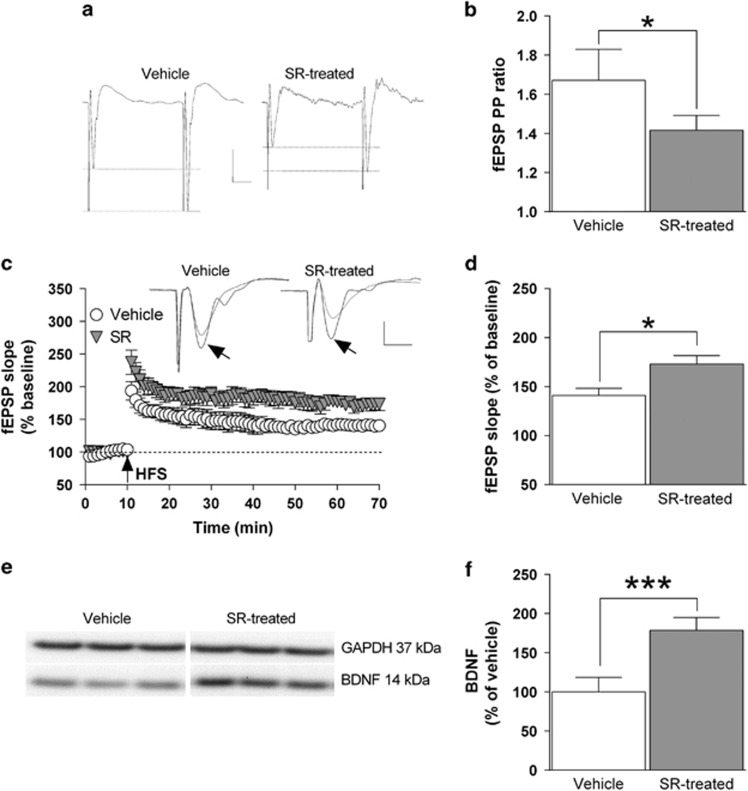
The results described in the previous paragraphs suggest that the reduced expression and function of CB1 receptors detected in the hippocampus of FR rats 5 min before food presentation could represent a crucial neurochemical alteration that may lead, in turn, to the changes in glutamatergic transmission and long-term synaptic plasticity. In order to challenge this idea, we treated CTRL non-FR rats with the selective CB1 receptor antagonist SR141716 (1 mg/kg, i.p.) for seven consecutive days. The results showed that blockade of CB1 receptors determines a significant (P<0.05) decrease of the PP ratio value for fEPSP, which is consistent with an enhanced probability of presynaptic glutamate release (Figure 5a and b). Furthermore, HFS-induced LTP formation in the CA1 field glutamatergic synapses was significantly (P<0.05) increased from 41%±7.3, in rats treated with SR141716, to 73%±8.7 in vehicle-treated animals (Figure 5c and d). Finally, treatment with the CB1 receptor antagonist was associated with a 79% increase (P<0.001) in the whole hippocampal BDNF protein levels compared with vehicle-treated animals (Figure 5e and f).
Figure 5.
Blockade of CB1 receptors mimics the effects of FR on glutamate release, LTP formation and BDNF expression in the hippocampus of non-FR rats. Control non-FR rats were treated either with SR141716 (1 mg/kg, i.p.) or vehicle for 7 days. (a) Representative traces showing paired fEPSPs from both experimental groups (scale bars: 0.5 mV, 10 ms). (b) Bar graph showing the PP ratio values for fEPSP slope obtained from vehicle and SR141716-treated rats (n=6). (c) Changes in fEPSP slope induced by HFS relative to baseline and, above, representative fEPSPs recorded before and 1 h after HFS (black arrow; scale bars: 0.5 mV, 5 ms). (d) Bar graph of the averaged fEPSP slope values obtained during the last 10 min of LTP recording, compared with the relative baseline (n=6). (e, f) Representative blot as well as quantitative analysis of the BDNF/GAPDH ratio in hippocampal protein extracts (n=5). *P<0.05; ***P<0.001 vs vehicle-treated rats, Student's t-test. BDNF, brain-derived neurotrophic factor; fEPSP, field excitatory postsynaptic potential; FR, food restriction; LTP, long-term potentiation.
Discussion
In the present work, we have examined the effects of FR on the regulatory function of CB1Rs in hippocampal CA1 glutamatergic synapses. In FR rats, trained to consume their daily food in a 2-h limited period for 3 weeks, anticipation of the scheduled food presentation is associated with a decrease in function and expression of CB1Rs with a parallel increase in the probability of glutamate release from presynaptic terminals impinging on CA1 pyramidal neurons. Such changes were also accompanied by an enhanced postsynaptic glutamatergic response (increased fEPSP slope) and LTP formation together with changes in CA1 pyramidal neuron dendritic spine pattern, with a significant increase in the mushroom type of spines. Consistent with such changes, FR rats showed an enhanced long-term spatial memory in the Barnes maze test.
It is well established that the hippocampus is directly involved in both affective and mnemonic aspects of eating, and, indirectly, in the control of food intake (Morton et al, 2006), as well as energy balance through its complex projections to the hypothalamus (Petrovic, 2013). In these brain areas, studies in rodents have shown that either CB1R agonists or pharmacological elevations of eCB levels are associated with overfeeding and enhanced rewarding properties of food intake (Cota et al, 2006). Thus, eCB system has a relevant role in the pathophysiological changes associated with altered feeding behavior and metabolic disorders (Bellocchio et al, 2010). eCBs exert their regulatory activity through the selective interaction with CB1Rs that are mainly located at presynaptic terminals with the result of inhibiting the release of different neurotransmitters, including glutamate and GABA (Ohno-Shosaku and Kano, 2014; Lovinger, 2007), in both hippocampus (Wilson and Nicoll, 2001; Abush and Akirav, 2010) as well as other brain regions (Di Marzo et al, 2001).
The lack of differences in eCB in hippocampus between FR and CTRL rats, strongly suggests that the decrease in CB1Rs protein is not due to a change in endocannabinoids concentration. On the other hand, it has been shown that hippocampal level of endocannabinoids is not related to CB1R levels (Maccarrone et al, 2001). However, an increase in the level of eCBs during acute FR in different brain areas directly connected with hippocampus has been previously demonstrated (Kirkham et al, 2002). It has also been reported that prolonged (12 days) FR in mice does reduce 2-AG levels in the hypothalamus but not in the hippocampus (Hanus et al, 2003). We may therefore speculate that adaptation to acute FR could be easily attained by modulating eCB concentrations through their prompt biosynthesis and degradation, whereas chronic adaptation could be accomplished by regulating CB1R protein expression.
The inhibition of fEPSP slope induced by bath application of win55,212-2 is likely dependent on the activation of presynaptic CB1Rs and the consequent decrease in glutamate release probability (Chevaleyre et al, 2006; Freund et al, 2003; Kano et al, 2009; Lovinger, 2008; Xu et al, 2010; Madroñal et al, 2012). However, in FR animals tested at 60 or 5 min preceding, but not 120 or 360 min after food presentation, the effect of win55,212-2 was markedly reduced. Such alteration induced by FR may depend, in turn, on the reduced surface expression of CB1Rs as indicated by western blot analysis that revealed a reduction in CB1R protein level in a hippocampal-enriched membrane preparation. These results are also in line with previous studies showing a decrease in CB1R mRNA levels in the cingulated cortex as a result of FR or palatable food consumption in rats (Bello et al, 2012). In our experimental condition, CB1R protein expression appears independent on the time point relative to food presentation, and in fact its levels were decreased at all tested times. The discrepancy with electrophysiology data is presently unclear, although it is important to note that CB1R quantitation was carried out in whole hippocampal extracts so that the possibility that the decrease protein expression occurs also in other subregions of the hippocampal formation, such as the CA3 field and the dentate gyrus where CB1Rs are highly expressed, cannot be ruled out. The reduced expression of CB1Rs in FR rats may involve a possible process of internalization. In fact, a similar mechanism of CB1R internalization has been observed in presynaptic glutamatergic terminals under several experimental conditions (Leterrier et al, 2006; Martin et al, 2004).
A direct consequence of the reduced CB1R function and expression in CA1 pyramidal neurons of FR rats may be an altered basal release of glutamate from presynaptic terminal. This idea is supported experimentally by the following two results: (i) the PP ratio of glutamatergic fEPSPs was reduced in FR rats tested during the anticipatory period in comparison with CTRL animals, consistent with an increased probability of presynaptic release of glutamate (Mennerick and Zorumski, 1995), and (ii) recordings of glutamatergic spontaneous mEPSCs in CA1 pyramidal neurons revealed that their frequency was significantly increased in FR animals.
In addition, we found no significant alterations in the kinetic properties of GABAergic mISPCs when recorded in the same neurons, suggesting that inhibitory GABAergic synapses are not affected by FR. Such conclusion is also further strengthened by the data showing that the inhibitory effect of win55,212-2 on mISPCs is not altered in FR compared with CTRL rats. Given that CB1Rs are expressed on both pyramidal cells as well as GABAergic interneurons, the present results indicate that the effect induced by FR on the regulation of CB1Rs is selective for glutamatergic terminals. On the other hand, this result is interesting because we previously found that, in the mPFC, FR altered the function of inhibitory GABAergic synapses, and it suggests that FR might exert a region-specific alteration of glutamatergic and GABAergic synapses.
An increase in the probability of glutamate release may have, in turn, marked consequences in terms of excitability and long-term synaptic plasticity. In fact, we observed that in slices from FR animals, the I–O curve of glutamatergic fEPSPs was significantly shifted to the left, thereby suggesting an increase of synaptic excitability. In addition, the magnitude of HFS-induced LTP in the CA1 field from FR rats was significantly greater than that measured in CTRL animals. This effect was observed in FR rats tested not only 5 min before food presentation but also 360 min after, a time point where we did not detect changes either in CB1R function or in glutamate release.
Parallel to the increase in LTP formation detected in FR rats, we found a selective increase in the density of mushroom spines in dendrites of CA1 pyramidal neurons. Mushroom spines are known to regulate the diffusion of membrane-associated ions and proteins (Hugel et al, 2009) and AMPA receptors (Ashby et al, 2006), making the glutamatergic synapse highly responsive to Ca2+ signaling involved in LTP formation (Schmidt and Eilers, 2009). Thus, mushroom spines are considered to be involved in the mechanisms of already acquired information sustaining memory storage (Bourne and Harris, 2007). Moreover, the increase in LTP formation could be related to the parallel changes in spine morphology and not to the total spine density or changes in the length of dendritic branches. In our experiments, FR was related to changes in dendritic spine morphology similarly to what observed in models of prolonged stress (Chen et al, 2008) and multiple mechanism may be involved in these changes, including alteration in neurotransmitter systems, growth factors, and hormones (Segal, 2010). Dendrite morphology as well as spine density associated with FR can be indicative of the parallel changes observed in LTP formation in FR animals. In further support of these data, we also observed an increase of BDNF in the hippocampus of FR rats, suggesting that this may be related to the gain in maturation of mushroom spines observed in these animals. The increased expression of hippocampal BDNF in FR rats may represent a further consequence of an increased presynaptic activation and a parallel enhanced glutamate release (Falkenberg et al, 1996). Our results of increased hippocampal LTP formation and mushroom spines are accompanied by an enhanced performance of FR rats in identifying the target hole 8 days after the last training session in the Barnes maze, consistent with an increase in long-term spatial memory, a result that is in line with a number of reports that have documented that rats exposed to FR perform better on learning and memory tasks with respect to animals that are fed ad libitum (Ingram et al, 1987; Rich et al, 2010).
Finally, the observation that a 7-day treatment of CTRL non-FR rats with the CB1R antagonist SR141716 recapitulates some of the changes observed in FR animals, namely, enhanced glutamate presynaptic release and LTP formation in the CA1 field, and increased protein levels of BDNF in the whole hippocampus, strongly supports our hypothesis that the reduction in expression and function of CB1Rs, consequent to adaptation of rats to the scheduled availability of food and apparent during the anticipation phase, represents a crucial neurochemical event that may be responsible in triggering the observed alterations of glutamatargic synapses.
Taken together, our results provide further evidence that prolonged exposure of rats to a limited feeding schedule represents a useful model to study the effects at hippocampal level of starvation and anticipation of food consumption. The present data underscore the important role of CB1R signaling in the regulation of glutamate release from presynaptic terminals in CA1 hippocampal field, and, in turn, also in the increase in hippocampal excitability and long-term synaptic plasticity. Our results are consistent with the hypothesis that FR rats could have ameliorating effects on hippocampal function, and support previous published data showing increased levels of neurotrophic factors and LTP formation (Duan et al, 2000).
Funding and disclosure
The present work has been funded by the Regione Autonoma della Sardegna, Progetti di Ricerca di Base, Bando 2010 (to ES) and the Sardinian Agency for R&TD (Sardegna Ricerche) (to GT). The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Abush H, Akirav I (2010). Cannabinoids modulate hippocampal memory and plasticity. Hippocampus 20: 1126–1138. [DOI] [PubMed] [Google Scholar]
- Ashby MC, Maier SR, Nishimune A, Henley JM (2006). Lateral diffusion drives constitutive exchange of AMPA receptors at dendritic spines and is regulated by spine morphology. J Neurosci 26: 7046–7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello NT, Coughlin JW, Redgrave GW, Ladenheim EE, Moran TH, Guarda AS (2012). Dietary conditions and highly palatable food access alter rat cannabinoid receptor expression and binding density. Physiol Behav 105: 720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio L, Lafenêtre P, Cannich A, Cota D, Puente N, Grandes P et al (2010). Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci 13: 281–283. [DOI] [PubMed] [Google Scholar]
- Bohme GA, Laville M, Ledent C, Parmentier M, Imperato A (2000). Enhanced long-term potentiation in mice lacking cannabinoid CB1 receptors. Neuroscience 95: 5–7. [DOI] [PubMed] [Google Scholar]
- Bourne J, Harris KM (2007). Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol 17: 381–386. [DOI] [PubMed] [Google Scholar]
- Carr TP, Jesch ED, Brown AW (2008). Endocannabinoids, metabolic regulation, and the role of diet. Nutr Res 28: 641–650. [DOI] [PubMed] [Google Scholar]
- Chen Y, Dubé CM, Rice CJ, Baram TZ (2008). Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci 28: 2903–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE (2006). Endocannabinoid-mediated synaptic plasticity in the CNS. Ann Rev Neurosci 29: 37–76. [DOI] [PubMed] [Google Scholar]
- Cota D, Tschöp MH, Horvath TL, Levine AS (2006). Cannabinoids, opioids and eating behavior: the molecular face of hedonism? Brain Res Rev 51: 85–107. [DOI] [PubMed] [Google Scholar]
- Dazzi L, Talani G, Biggio F, Utzeri C, Lallai V, Licheri V et al (2014). Involvement of the cannabinoid CB1 receptor in modulation of dopamine output in the prefrontal cortex associated with food restriction in rats. PLoS One 9: e92224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Goparaju SK, Wang L, Liu J, Baktai S, Jarai Z et al (2001). Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 410: 822–825. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Mattson MP (2000). Participation of par-4 in the degeneration of striatal neurons induced by metabolic compromise with 3-nitropropionic acid. Exp Neurol 165: 1–11. [DOI] [PubMed] [Google Scholar]
- Egashira N, Mishima K, Iwasaki K, Fujiwara M (2002). Intracerebral microinjections of delta 9-tetrahydrocannabinol: search for the impairment of spatial memory in the eight-arm radial maze in rats. Brain Res 952: 239–245. [DOI] [PubMed] [Google Scholar]
- Falkenberg T, Lindefors N, Camilli F, Metsis M, Ungerstedt U (1996). Glutamate release correlates with brain-derived neurotrophic factor and trkB mRNA expression in the CA1 region of rat hippocampus. Mol Brain Res 42: 317–327. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D (2003). Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 83: 1017–1066. [DOI] [PubMed] [Google Scholar]
- Gaetani S, Kaye WH, Cuomo V, Piomelli D (2008). Role of endocannabinoids and their analogues in obesity and eating disorders. Eat Weight Disord 13: e42–e48. [PubMed] [Google Scholar]
- Hanus L, Avraham Y, Ben-Shushan D, Zolotarev O, Berry EM, Mechoulam R (2003). Short-term fasting and prolonged semistarvation have opposite effects on 2-AG levels in mouse brain. Brain Res 983: 144–151. [DOI] [PubMed] [Google Scholar]
- Hugel S, Abegg M, de Paola V, Caroni P, Gähwiler BH, McKinney RA (2009). Dendritic spine morphology determines membrane-associated protein exchange between dendritic shafts and spine heads. Cereb Cortex 19: 697–702. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Weindruch R, Spangler EL, Freeman JR, Walford RL (1987). Dietary restriction benefits learning and motor performance of aged mice. J Gerontol 42: 78–81. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M (2009). Endocannabinoid-mediated control of synaptic transmission. Physiol Rev 89: 309–380. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Williams CM, Fezza F, Di Marzo V (2002). Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br J Pharmacol 136: 550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier C, Laine J, Darmon M, Boudin H, Rossier J, Lenkei Z (2006). Constitutive activation drives compartment-selective endocytosis and axonal targeting of type 1 cannabinoid receptors. J Neurosci 26: 3141–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH (2000). SR 141716A enhances spatial memory as assessed in a radial-arm maze task in rats. Eur J Pharmacol 404: 175–179. [DOI] [PubMed] [Google Scholar]
- Lovinger DM (2007). Endocannabinoid liberation from neurons in transsynaptic signaling. J Mol Neurosci 33: 87–93. [DOI] [PubMed] [Google Scholar]
- Lovinger DM (2008). Presynaptic modulation by endocannabinoids. Handb Exp Pharmacol 184: 435–477. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Attinà M, Bari M, Cartoni A, Ledent C, Finazzi-Agrò A (2001). Anandamide degradation and N-acylethanolamines level in wild-type and CB1 cannabinoid receptor knockout mice of different ages. J Neurochem 78: 339–348. [DOI] [PubMed] [Google Scholar]
- Mackie K (2008). Cannabinoid receptors: where they are and what they do. J Neuroendocrinol 20(Suppl 1): 10–14. [DOI] [PubMed] [Google Scholar]
- Madroñal N, Gruart A, Valverde O, Espadas I, Moratalla R, Delgado-García JM (2012). Involvement of cannabinoid CB1 receptor in associative learning and in hippocampal CA3-CA1 synaptic plasticity. Cereb Cortex 22: 550–566. [DOI] [PubMed] [Google Scholar]
- Martin BR, Sim-Selley LJ, Selley DE (2004). Signaling pathways involved in the development of cannabinoid tolerance. Trends Pharmacol Sci 25: 325–330. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Zorumski CF (1995). Paired-pulse modulation of fast excitatory synaptic currents in microcultures of rat hippocampal neurons. J Physiol 488(Pt 1): 85–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW (2006). Central nervous system control of food intake and body weight. Nature 443: 289–295. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Kano M (2014). Endocannabinoid-mediated retrograde modulation of synaptic transmission. Curr Opin Neurobiol 29C: 1–8. [DOI] [PubMed] [Google Scholar]
- Petrovich GD (2013). Forebrain networks and the control of feeding by environmental learned cues. Physiol Behav 121: 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich NJ, Van Landingham JW, Figueiroa S, Seth R, Corniola RS, Levenson CW (2010). Chronic caloric restriction reduces tissue damage and improves spatial memory in a rat model of traumatic brain injury. J Neurosci Res 88: 2933–2939. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Obili N, Mascia MP, Mostallino MC, Secci PP et al (2011). Voluntary ethanol consumption induced by social isolation reverses the increase of α(4)/δ GABA(A) receptor gene expression and function in the hippocampus of C57BL/6J Mice. Front Neurosci 10: 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Eilers J (2009). Spine neck geometry determines spino-dendritic cross-talk in the presence of mobile endogenous calcium binding proteins. J Comput Neurosci 27: 229–243. [DOI] [PubMed] [Google Scholar]
- Segal M (2010). Dendritic spines, synaptic plasticity and neuronal survival: activity shapes dendritic spines to enhance neuronal viability. Eur J Neurosci 31: 2178–2184. [DOI] [PubMed] [Google Scholar]
- Slanina KA, Roberto M, Schweitzer P (2005). Endocannabinoids restrict hippocampal long term potentiation via CB1. Neuropharmacology 49: 660–668. [DOI] [PubMed] [Google Scholar]
- Suenaga T, Ichitani Y (2004). Effects of intrahippocampal WIN55,212–2 on T-maze delayed alternation in rats. Jpn J Animal Psychol 54: 143. [Google Scholar]
- Talani G, Biggio G, Sanna E (2011). Enhanced sensitivity to ethanol-induced inhibition of LTP in CA1 pyramidal neurons of socially isolated C57BL/6J mice: role of neurosteroids. Front Endocrinol 2: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova JP, Storme JJ, Lafon N, Péŕio A, Rinaldi-Carmona M, Le Fur G et al (1996). Improvement of memory in rodents by the selective CB1 cannabinoid receptor antagonist, SR 141716. Psychopharmacology 126: 165–172. [DOI] [PubMed] [Google Scholar]
- Williams CM, Kirkham TC (2002). Reversal of Δ9-THC hyperphagia by SR141716 and naloxone but not dexfenfluramine. Pharmacol Biochem Behav 71: 333–340. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll A (2001). Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410: 588–592. [DOI] [PubMed] [Google Scholar]
- Xu JY, Chen R, Zhang J, Chen C (2010). Endocannabinoids differentially modulate synaptic plasticity in rat hippocampal CA1 pyramidal neurons. PLoS One 5: e10306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.