Abstract
Worldwide biodiversity assessments have mainly focused on species richness but little is known about the diversity of species roles, i.e. functional diversity, while this is a key facet to understanding the consequences of global changes on the ecosystem services to human societies. Here, we report the world pattern of functional diversity of freshwater fish using a database encompassing morphological characteristics of more than 9,000 species. The Neotropical realm hosts more than 75% of global functional diversity while other realms each host less than 25%. This discrepancy is mediated by high functional uniqueness in some diversified Neotropical fish orders. Surprisingly, functional diversity patterns were weakly related to functional vulnerability. In the Neotropics the loss of threatened species will cause a limited loss of functional diversity (<10%) whereas in the Nearctic and Palearctic realms, decline of the functional diversity will reach 43% and 33%, respectively, conferring a high functional vulnerability to these realms. Conservation of the Neotropical fish diversity is a key target to maintain world fish functional diversity, but this should not hide the pressing need to conserve the vulnerable fish faunas of the rest of the world, in which functional diversity is to a large extent supported by threatened species.
Owing to the sixth mass extinction crisis1, colossal efforts have been devoted to assessing biodiversity, but to date, most worldwide biodiversity assessments have focused on the taxonomic component of biodiversity2,3,4,5. These worldwide assessments on taxonomic diversity have revealed that species richness varies strongly across the world for most clades, e.g. birds5, plants6 or fishes7, with a decreasing richness gradient from the equator to the poles2. For the freshwater fish fauna, the Neotropical realm (South and Central Americas) includes more than 4,000 fish species, while the Palearctic realm (Eurasia, Middle-East and North-Africa) contains fewer than 6008. Besides this taxonomic facet, functional diversity (FD, i.e. the range of biological traits9) is a measure of the range of functions performed by organisms, and therefore a good way to approach the role of biodiversity in sustaining ecosystem services9,10,11,12,13,14,15, as well as the detrimental effects of human disturbances on the erosion of biodiversity12. FD is expected to increase with taxonomic diversity (TD) because of the higher probability of including a larger range of biological traits when more species are present16,17, but the influence of TD on FD is still unclear, as the lack of large-scale assessments of functional traits on a large range of species limits our capacity to run global scale studies on FD18,19.
Here, we assess for the first time the FD of the native freshwater fish faunas over the 6 terrestrial biogeographic realms of the world. Functional diversity was measured as morphological diversity20,21. Although these traits are not all directly linked to the actual roles played by fish in ecosystems (e.g. nutrient recycling22, trophic control of other taxa23), they remain informative to describe features related to food acquisition and locomotion24,25,26, and remain the only traits measurable for almost all fish species with a reasonable effort. We here measured 10 morphological traits on 9,170 fish species (see Supplementary Fig. S1 for details).
We quantified the extent to which FD, calculated as the volume filled by the species in the multidimensional space defined by morphological traits, differs between the biogeographic realms. Then, we tested the contribution of each fish order to each realm’s functional diversity by measuring the functional uniqueness of each order within the realm (Funiq, i.e. proportion of the functional space of the realm filled only by this order). Finally we assessed functional vulnerability to species loss as the proportion of FD supported by species listed as ‘threatened’ by the IUCN27 (i.e. status is “critically endangered”, “endangered” or “vulnerable”), and by species endemic to a single river drainage basin and not listed as “least concern” or “near threatened” by the IUCN27,28.
Results and Discussion
Neotropics host more than three-quarters of the world fish functional diversity
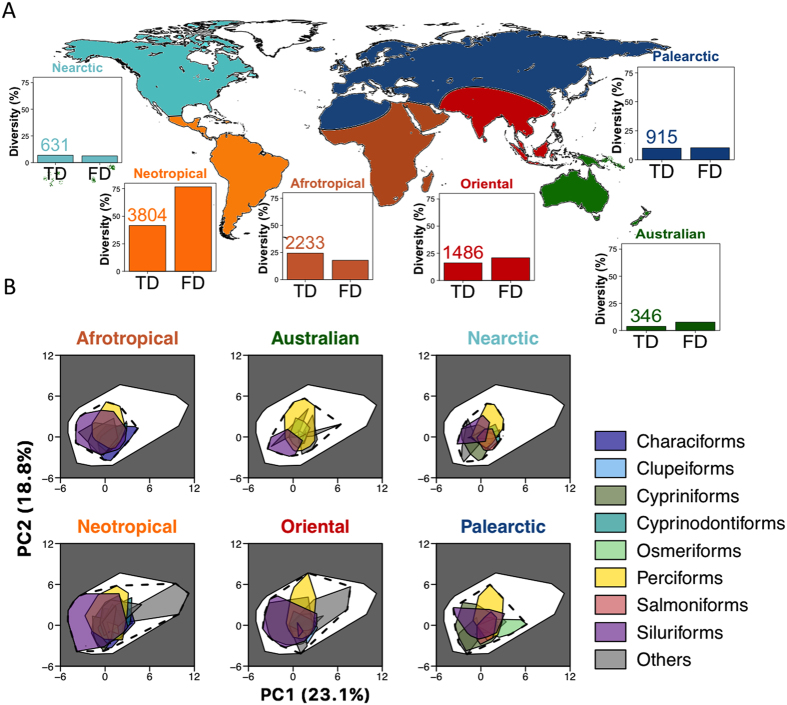
We report a threefold lower functional turnover (mean ± SD: 0.324 ± 0.206) than taxonomic turnover between realms (0.982 ± 0.022). Thus, despite strong species turnover between realms, the same core of biological attributes is shared between realms (Fig. 1B, Supplementary Fig. S2). Such a pattern of functional nestedness between realms hosting different suites of species has been reported for two other vertebrate taxa, passerine birds21 and coral reef fish19.
Figure 1. Fish taxonomic and functional diversity in the 6 biogeographic realms.
(A) The six realms are displayed on the map. For each realm, the taxonomic (TD) and the functional diversity (FD) are represented as a percentage of the world taxonomic or functional diversity. The number of species in each realm is given above the TD bar. (B) Functional space of the world freshwater fishes is the white area in the 2-dimensions space made by first and second principal component (PC) axes summarizing the 5-dimensions functional space built based on 10 morphological traits (see other projections in Supplementary Fig. S2). The percentage of variance explained by each PC axis is given in brackets (see also Supplementary Figs S2 and S3). Fish functional diversity in each realm is illustrated by the polygon delimited by the black dashed line. The coloured polygons within it show the functional diversity of the most speciose fish orders. The map was generated using ‘maps’ and ‘mapproj’ libraries, available in R 3.0 (http://www.r-project.org).
Besides this shared functional core, we show here a clear spatial discrepancy of fish FD among realms. The Neotropics host more than three-quarters of the world FD (76.7%) whereas the two other tropical speciose realms, namely the Oriental and the Afrotropical realms, each hosts only one-fifth (17.8% and 20.8%, respectively, Fig. 2). Hence, the twofold higher TD in Neotropics compared to the Afrotropics turns into a fourfold higher FD. A similar trend was observed between Neotropical and Oriental realms, confirming that the Neotropical fauna is functionally hyperdiverse (Fig. 2). Hence, the Neotropics, which represent less than 15% of the world’s continental surface, host more than 75% of the world freshwater fish FD. In contrast, it is surprising to note that the FD of the Afrotropics is close to that of the Palearctic realm, while the latter hosts less than half as many species8. Similarly, the Australian realm hosts a FD close to that of the Nearctic although it only hosts half as many species (Figs 1A and 2).
Figure 2. Taxonomic and functional facets of fish biodiversity in the 6 biogeographic realms.
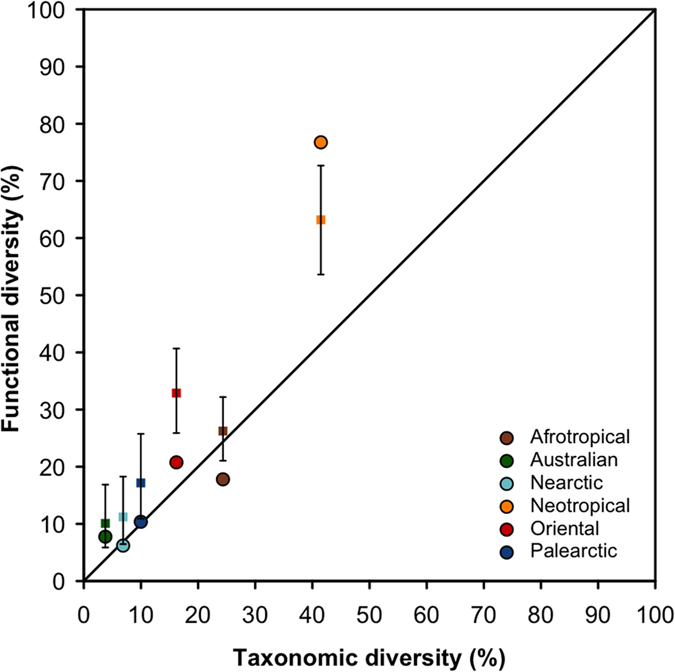
Functional diversity (FD) and taxonomic diversity (TD) are expressed as a percentage of the world functional and taxonomic diversity of freshwater fish. The effect of species identity on functional diversity is represented by the mean (colored squares) and associated 95% confidence interval (black whiskers) computed on 999 values of a null model. This null model simulated realm assemblages using random sampling in the world pool of species, while conserving the taxonomic structure of the realm fauna (i.e. number of species per order). Observed values of FD above the whiskers indicate a significant functional overdispersion, whereas values below the whiskers indicate significant functional clustering. The solid line represents the identity line FD = TD. Each color refers to one realm.
The weak association we report between TD and FD suggests that the diversity of species traits present in a realm is not a random subset of the pool of traits values at the world scale. Indeed, we found that the Neotropical fish fauna has a significantly higher FD than expected given its TD (Standardized Effect Size (SES) = 2.21, P > 0.99) whereas the FD of Afrotropical (SES = −2.46, P < 0.01) and Oriental realms (SES = −2.50, P < 0.01) were significantly lower than expected (Fig. 2). FD did not differ from random expectation for Australian fauna (SES = −0.79, P = 0.22) whereas a significantly lower FD was reported for Nearctic (SES = −1.32, P < 0.05) and Palearctic realms (SES = −1.54, P < 0.05). The functional overdispersion of the Neotropical fish fauna could be due to (i) either a few functionally diversified orders that fill most of the functional space (the remaining functionally poor orders being nested in the functionally rich orders), or (ii) a high functional dissimilarity between orders, i.e. each order filled a unique part of the realm FD. On the other hand, the functional clustering of the Afrotropical and Oriental realms could be driven by (i) a low functional diversity of all orders and/or (ii) a high functional similarity (i.e. redundancy) between orders.
Differences in functional diversity are driven by the functional uniqueness of a few fish taxa
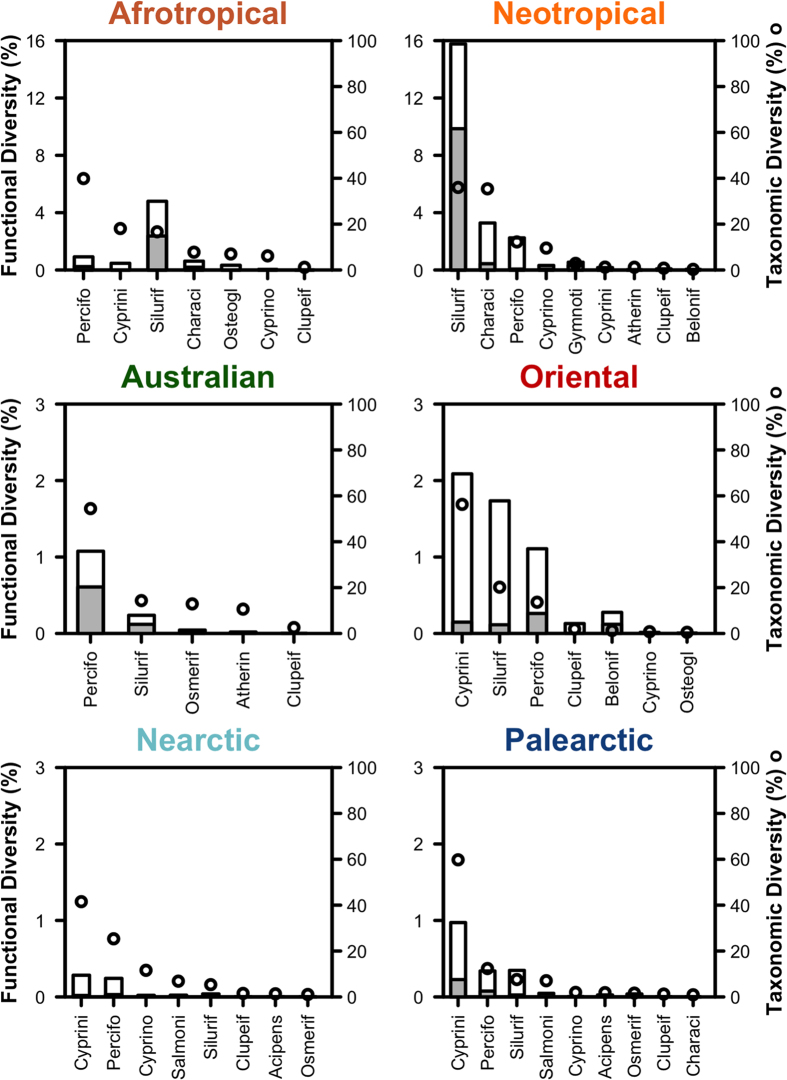
Although the FD of orders at the global scale was correlated to their TD (Spearman’s rank correlation rho = 0.31, n = 46, P < 0.05), the functional uniqueness (Funiq) of orders was independent of order TD (Spearman’s rank correlation rho = −0.04, n = 46, P = 0.76). Indeed, the realm FD results not only from the global FD of each order (Fig. 1), but also from the degree of functional uniqueness between orders (Fig. 3). The functional clustering for all realms except the Neotropics is thus due to the low functional uniqueness of their most speciose orders (Fig. 3). This is particularly striking for the Afrotropics, where the two most speciose orders (Perciforms and Cypriniforms) that together account for more than 60% of the Afrotropical species richness, contribute marginally to the realm FD (5.2 and 2.6%, respectively) because of their low Funiq (26.2 and 0.4%, respectively, Fig. 3). This was verified for the Perciforms, despite the recent and intense radiation of cichlids (the most species rich family of Perciforms in the Afrotropics) promoted by the recent opening of the rift lakes (e.g. Malawi, Tanganyika, Victoria) that produced hundreds of phylogenetically closely related species29. Although the great lakes cichlids radiations have generated diverse morphological adaptations29, the resulting morphological features did not strongly differ from those already experienced within the African fish fauna, hence explaining the low functional uniqueness of the Afrotropical fish orders.
Figure 3. Functional diversity and uniqueness of fish orders within realms.
The functional diversity (FD) of each fish order in each realm is given as the percentage of the world FD (bars). The part of FD of each order non-filled by any other order in the realm (Functional uniqueness, Funiq) is the ratio between the grey part and the FD of each order. Note that the FD scale differs between realms. The orders are sorted by decreasing TD in each realm and the black dots indicate the percentage of the realm TD represented by each order. Only the 13 most speciose orders in the world are represented (Acipens: Acipenseriforms; Atheri: Atheriforms; Belonif: Beloniforms; Characi: Characiforms; Clupei: Clupeiforms; Cyprini: Cypriniforms; Cyprino: Cyprinodontiforms; Gymnoti: Gymnotiforms; Osteogl: Osteoglossiforms; Osmerif: Osmeriforms; Percifo: Perciforms; Salmoni: Salmoniforms; Silurif: Siluriforms).
In contrast, the Neotropical fish fauna hosts species with unique functional attributes such as i) extremely elongated fish with a large terminal mouth and a high caudal peduncle throttling, corresponding to mobile surface predators such as some of the Beloniforms, or ii) dorso-ventraly flattened fishes, with a ventral mouth located below the head and a small caudal peduncle throttling, mainly corresponding to benthic algae browsers with limited swimming efficiency such as some Loricariid species (see Supplementary Fig. S3). The Loricariids belong to the Siluriforms that is the most speciose order in the Neotropics and they strongly contribute to the high FD of the realm due to their high Funiq (62.5%, Fig. 3). Indeed Neotropical Siluriforms have an impressive body size range (from less than 5 cm to more than 200 cm), a wide span of diet (from algae browsing in the Loricaridae, to ichthyophagy in the Pimelodidae, and even parasitism in the Trichomycteridae, see Supplementary Fig. S3), and of habitat associated with diverse body shapes from flattened to extremely elongated30. Such a high FD of Siluriforms is not observed in the other realms, explaining their low functional uniqueness (6.6% in Oriental, 7.4% in Nearctic and 7.1% in Palearctic realms, Fig. 3) despite their contribution to realm TD (e.g. 373 Siluriforms species in the Afrotropical realm).
Functional vulnerability peaks in the Nearctic and Palearctic realms, despite their low functional diversity
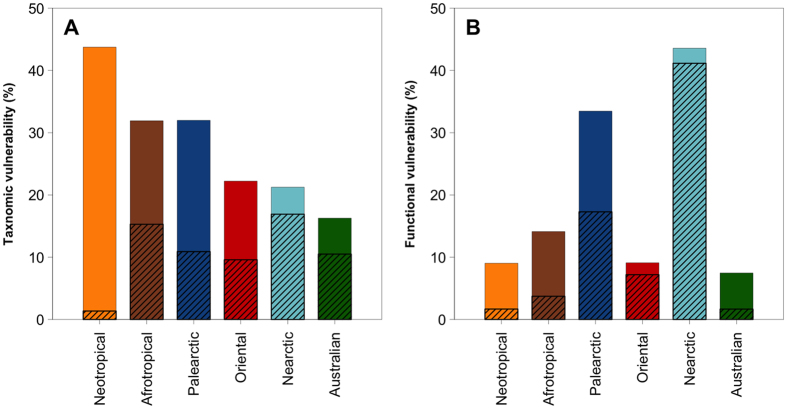
The loss of FD through species extinction is expected to be marked when threatened species support unique functions, as shown in alpine plants, tropical trees, coral reef fishes and bird regional assemblages19,31,32. For freshwater fish, the vulnerability of FD to the extinction of threatened species differed among realms by a sixfold factor (Fig. 4), and was neither correlated to the realm FD (Spearman’s rank correlation rho = −0.43, P = 0.42) nor to the number of threatened species (Spearman’s rank correlation rho = −0.08, P = 0.92). The loss of FD due to the simulated extinction of threatened species was marked in the Nearctic and Palearctic realms (43.6 and 33.5%, respectively, Fig. 4), while it was low (from 7.47 to 14.1%) in the Afrotropical, Australian, Neotropical and Oriental realms. Within this last group, the Neotropics are characterized by a vulnerability of FD to species loss lower than expected given the number of threatened species (SES = −2.32, P < 0.001, Table 1). In contrast, in the Nearctic realm the vulnerability of FD was significantly higher than expected given the number of threatened species (SES = 3.47, P > 0.999, Table 1). A similar trend although not significant was found in the Palearctic realm (SES = 0.84, P = 0.66, Table 1). While being the least functionally diverse, the Nearctic and Palearctic realms are the most vulnerable to species loss (Table 1). Although there is a large number of threatened species (743 species accounting for 31% of the Afrotropical fish fauna), the Afrotropical vulnerability is low whereas in the Nearctic and Palearctic realms, fewer species are threatened (147 and 317 species, respectively), but they differ functionally from the rest of the fauna (e.g. sturgeons and eels) and thus lead to a high vulnerability of the functional diversity of this realm. As for alpine plants, tropical trees and coral reef fishes32, the threatened species support vulnerable functions, testifying that the imminent extinction of a few endangered species will cause a marked decline of functional diversity across the world faunas.
Figure 4. Vulnerabilities of taxonomic and functional diversity to extinction of threatened species.
(A) Taxonomic vulnerability. Bars represent the proportion of threatened species (i.e. species with an IUCN status “CR”, “EN” or “VU” or species occurring in only one drainage basin across the world which IUCN status was not “Near threatened” nor “Least concern”). The hatched part of each bar represents the taxonomic vulnerability due only to the most threatened species (i.e. the species listed as “CR”, “EN” or “VU” by the IUCN). (B) Functional vulnerability. Bars represent the loss of functional diversity (FD) if threatened species become extinct. The hatched part of each bar represents the functional vulnerability due only to the most threatened species (i.e. the species listed as “CR”, “EN” or “VU” by the IUCN).
Table 1. Functional Vulnerability (FV) supported by the threatened species in the six biogeographic realms.
| Threatened species |
Most threatened species |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nb. of species | FV (%) | SES | P value | Nb. of species | FV (%) | SES | P value | |||
| Afrotropical | 743 | 14.12 | −0.85 | 0.26 | ns | 356 | 3.73 | −0.91 | 0.12 | ns |
| Australian | 62 | 7.47 | −0.91 | 0.21 | ns | 40 | 1.66 | −1.03 | 0.12 | ns |
| Nearctic | 147 | 43.56 | 3.47 | >0.999 | *** | 117 | 41.15 | 4.05 | >0.999 | *** |
| Neotropical | 1680 | 9.03 | −2.32 | <0.001 | *** | 52 | 1.67 | 0.74 | 0.91 | ns |
| Oriental | 350 | 9.11 | −0.84 | 0.21 | ns | 151 | 7.2 | 0.31 | 0.71 | ns |
| Palearctic | 317 | 33.46 | 0.84 | 0.66 | ns | 108 | 17.29 | 1.02 | 0.88 | ns |
For each realm, we computed the vulnerability of FD to the loss of threatened species as the proportion of the FD of the realm that will remain if all threatened species got extinct (see details in Methods). In each realm, the number of threatened species is indicated (Nb. of species). Two levels of threat were considered. The “threatened species” are the species listed as CR, EN and VU by IUCN, and the endemic species (i.e. species occurring in only one drainage basin across the world) not listed as “near threatened” nor “least concern” by the IUCN. The “Most threatened species” are only the species listed as CR, EN and VU by IUCN. The observed vulnerability value was compared to the expected vulnerability given number of threatened (or most threatened) species in each realm, i.e. 999 values of FD loss simulated with a random extinction of the same number of species than the number actually listed as threatened (or most threatened) in the realm. Standardized Effect Size (SES) was calculated as the difference between the observed loss in FD and the mean of the random loss of FD divided by the standard error of the random loss of FD. A negative SES means that the observed loss of FD is higher (i.e. more negative) than expected if extinctions were random whereas a positive SES means that the loss of FD observed is lower than expected. The significance of the difference from null expectations was tested using a two-tailed test (α < 0.05). ns: non-significant; *p < 0.025 or p > 0.975; **p < 0.01 or p > 0.99; ***p < 0.001 or p > 0.999. The FV values significantly different from the null expectation are in bold font.
In addition, when considering only the most threatened species, i.e. those listed as critically endangered (CR), endangered (EN) or vulnerable (VU) by the IUCN, we found that these species account for most of the functional vulnerability in the Nearctic (94.4%) and Oriental (70.5%) realms and to a lower proportion in the Palearctic (51.4%) and even less than 25% in the 3 others realms. In those last realms, most of the vulnerability is supported by endemic species that are potentially threatened although being not evaluated yet (Fig. 4, Table 1). Extending the IUCN evaluation of threats to the world fish fauna is therefore a prerequisite to determine the role played by the most threatened species in the maintenance of the functional diversity over more than half of the world continental areas.
Conclusion
Assessing functional diversity and its determinants on large scale is pivotal in biodiversity mapping11. Indeed, the species number is not a good surrogate for functional diversity as illustrated by the contrasting FD observed here between the two most speciose realms, namely the Neotropics and the Afrotropics. Moreover, FD does not match with functional vulnerability because of the particular functional attributes of threatened species in the Palearctic and Nearctic realms. These mismatches between TD and FD and between FD and functional vulnerability stress the need to consider multiple biodiversity facets in efficient conservation planning. More precisely, future conservation efforts should focus on the vulnerable realms, namely the Nearctic and Palearctic, but also in the Neotropics to protect the habitats hosting the functionally most original species across the world. Such conservation actions should be taken without delay, as the vulnerable functions are overrepresented among the already-listed threatened species. In addition, as the endemic species which status are still unknown also contribute to a high proportion of functional diversity over more than half of the world continental surface, studies at the species level are urgently needed to evaluate the threats they are facing. Considering more specifically freshwater fish, that constitute an emblematic case of massive introductions of non-native species3, it is urgent to assess whether the patterns reported here for historical native fish faunas have been blurred by the biotic exchanges between continents for the last centuries. Towards effective assessments of biodiversity-ecosystem services relationships, the large-scale patterns reported here should be completed by local assessments of the key ecological roles played by fish in aquatic ecosystems.
Methods
Fish occurrence database
We considered the 6 terrestrial biogeographic realms (Afrotropical, Australian (including Oceania), Nearctic, Neotropical, Oriental and Palearctic) commonly used for freshwater fish8,33,34,35. The number and identity of the species occurring in each realm was obtained using the database on freshwater fish occurrences34 that contains 9,170 freshwater fish species out of the ca. 13,000 strictly freshwater fish species described30, and hence covers 77% of the documented world freshwater fish fauna.
Fish pictures collection
We here developed the most comprehensive fish functional database existing to date. It encompasses 9,170 freshwater fish species out of the ca. 13,000 described strictly freshwater fish species (i.e. 77% of the world freshwater fish fauna). We ran an extensive literature review to collect at least one lateral view picture of each species, which were used to measure morphological characteristics (Supplementary Fig. S1a). Some of the pictures were taken on the field by the authors. Fish captures were led in accordance with laws and guidelines concerning live animals, and all the experiments were approved by the Direction of Environment of the French ministry of environment (DEAL), the French Guiana National Park (Parc Amazonien de Guyane), and the research program LABEX CEBA (ANR-10-LABX-25-01).
For all the species present in the taxonomic database we aimed at measuring 10 functional traits describing species strategies for food acquisition and locomotion (Supplementary Fig. S1 and24). Fish size was described using the log-transformed maximum body length (values taken from FishBase36). In addition to size, 9 morphological traits were measured on side view pictures (Supplementary Fig. S1a) collected during an extensive literature review from more than 200 scientific literature sources including peer-reviewed articles, books and scientific websites. We collected at least one picture (validated photograph or scientific drawing) per species. Only good quality pictures and scientific side view drawings of entire adult animals were kept. Juveniles were not considered as morphological changes can occur during ontogeny. In the event of sexual dimorphism, we only considered male morphology, as female pictures are scarce for most species (especially for Perciforms and Cyprinodontiforms). Using lateral view pictures did not permit to collect as much external morphological information as fresh animals (e.g. oral gape surface, body transversal shape). It would nevertheless be very demanding to collect fresh or museum specimens for more than 9,000 species, and using lateral view pictures was the most efficient way to collect morphological measures for more than 70% of the world freshwater fish fauna.
Functional traits
For each specimen, 11 morphological measurements were recorded (see Supplementary Fig. S1a) using ImageJ software (http://rsb.info.nih.gov/ij/index.html) and were then used to compute 9 unitless ratios describing the morphology of the fish head (including mouth and eye), body, pectoral and caudal fins (Supplementary Fig. S1b). The 10 functional traits (9 unitless ratios and size) selected are commonly used in assessment of fish functional diversity (e.g.25,26,37). Complementary functional traits (e.g. gut length, oral gape area and shape, fecundity) were not included because they are only available for a few species. The quality of the pictures did not allow measurement of all 11 morphological traits in all species, but more than 80% of the species were functionally characterized within each taxonomic order. All nine morphological traits were measured for almost two-thirds of the resulting morphological database (6,030 species) and overall 18% of the morphological measurements were not obtained.
Some species have unusual morphologies (species without tail, flatfishes) that prevent from measuring some morphological traits. We thus defined rules for these few exceptions as in26: (i) for species with no visible caudal fin (e.g. Sternopygidae, Anguilidae, Plotosidae), Caudal Peduncle Throttling was set to 1 (assuming CFd = CPd, see Supplementary Fig. S1b), (ii) for the species with the mouth positioned under the body (e.g. Loricaridae, some Balitoridae such as Gastromyzon) mouth vertical position (Mo) was set to 0, (iii) for the species without pectoral fins (e.g. Synbranchiforms and some Anguiliforms) Pectoral fin vertical position (PFl/Bd) was set to 0.; iv) for flatfishes Bd was the body width as the fish lies on one side of its body. We hence assumed that Pleuronectiforms are functionally closer to dorso-ventrally flattened fishes (e.g. Gastromyzon) than to laterally compressed fishes (e.g. Symphysodon).
Other traits, such as ecological, behavioural or physiological traits were not considered in this study, because there is currently no database available at the world scale.
Functional space
The 10 functional traits were ordered in a multidimensional functional space by means of Principal Components Analysis using a regularized algorithm designed for ordination analysis to handle the missing values38. The first 5 axes account for 80.5% of the total variance (each selected axis had an eigenvalue >1) (Supplementary Fig. S4) and were retained to build a 5-dimensional functional space. We assessed the robustness of our findings using sensitivity procedure. We tested the effect of trait identity on the functional distance between species by rerunning all analyses using all combinations of nine functional traits out of ten. The results were hardly affected by this procedure (Mantel tests r > 0.900, P < 0.001). Thus, our findings are not affected by functional trait selection.
Threatened species
Two types of threatened species were considered, the “most threatened” and the “potentially threatened”. The “most threatened” species were identified as the species listed as critically endangered (‘CR’), endangered (‘EN’) and vulnerable (‘VU’) in the most recent IUCN Red List assessment27. The IUCN Red List of threatened species although being the most objective and authoritative system for classifying species in terms of the risk of extinction at the global scale, remains affected by spatial disparities in species evaluation. To deal with incompleteness of the IUCN assessment (52.5% of the species occurring in our database have not been IUCN evaluated, see Supplementary Table S1), strictly endemic species (species occurring in only one drainage basin across the world28,30,34) which IUCN status was not “near threatened (NT)” nor “least concern (LC)” were considered as “potentially threatened”. Those species with limited spatial distribution have been recognized as prone to extinction following human disturbance4,28,39. Thus, a total of 3,443 (37.5%) species out of the 9,170 species were considered as threatened.
Biodiversity indices
Taxonomic diversity (TD) was calculated as the number of species in each realm (or in each order). Functional diversity (FD) was assessed with the functional richness index (FRic40), computed as the volume of the minimum convex hull that includes all the species in the 5-dimensional functional space. The higher the FD, the higher the richness of combinations of functional trait values in the species pool considered. FD and TD indices were computed for all species in each realm, and for all orders within each realm.
Statistical analyses
To test whether observed functional diversity of a realm was significantly different from functional diversity of a random subset of species, we used null models based on randomization of species pools. To simulate a realistic pool of species in each realm, the number of species per taxonomic order was kept constant in the random choice process (999 iterations). The standardized effect size (SES) was used to measure the difference between observed values (obs) and null expectation (rand): SES = (FDobs − mean (FDrand))/sd (FDrand). The significance of the difference from null expectations was tested using a two-tailed test (∝ < 0.05). When FDobs is lower than 97.5% of FDrand values, the assemblage is considered as functionally clustered. When FDobs is greater than 97.5% of FDrand values, the assemblage is considered as functionally overdispersed.
The dissimilarity in FD and TD between realms was measured as taxonomic and functional turnover41. Taxonomic turnover measures the replacement of species between two assemblages independently of the difference in species richness41. Similarly, functional turnover measures the replacement of functional volume independently of the difference in functional richness. Taxonomic and functional turnover were calculated using the same formula: 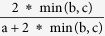 , where a is the number of species (or the volume) shared by the two assemblages and b and c the number of unique species (or the unique volume) hosted by the two assemblages41,42. Both taxonomic and functional turnover varies from 0, when the two assemblages are completely similar, to 1 when the two assemblages shared no species (taxonomic) or no portion of the functional space.
, where a is the number of species (or the volume) shared by the two assemblages and b and c the number of unique species (or the unique volume) hosted by the two assemblages41,42. Both taxonomic and functional turnover varies from 0, when the two assemblages are completely similar, to 1 when the two assemblages shared no species (taxonomic) or no portion of the functional space.
The functional uniqueness of each fish order o in each realm r was computed as the proportion of the functional space filled only by the order considered: 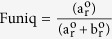 , where aro is the volume filled only by focal order o and br is the volume shared with all other orders present in realm r. Taxonomic and functional turnover, and Funiq were computed using the ‘functional.beta.core’ function from the ‘betapart’ R package43.
, where aro is the volume filled only by focal order o and br is the volume shared with all other orders present in realm r. Taxonomic and functional turnover, and Funiq were computed using the ‘functional.beta.core’ function from the ‘betapart’ R package43.
The functional vulnerability (FV) to threatened species loss in each realm was computed as the proportion of the FD of the realm that will remain if all threatened species got extinct: FVobs = (FDobs − FDnot threatened)/FDobs. Functional vulnerability is null when species not threatened contribute fully to the FD of the realm and tends to the 100% if all the most extreme combinations of traits are supported only by threatened species.
To test the significance of the observed functional vulnerability given the number of threatened species we designed a null model based on a random choice of the species expected to be lost (999 iterations). The standardized effect size (SES) was used to measure the difference between predicted FD loss values due to extinction of threatened species (FVobs) and null expectation of FD loss (rand): SES = (FVobs − mean(FVrand))/sd (FVrand). The significance of the difference from null expectations was tested using a two-tailed test (∝ < 0.05). A p-value lower than 0.025 indicates that threatened species are functionally distinct and over-contribute to FD whereas a p-value higher than 0.975 indicates that threatened species are redundant with other species of the realm FD.
We then ran the same analyses considering only the most threatened species, i.e. those listed as “critically endangered”, “endangered” or “vulnerable” by the IUCN27.
All statistical analyses were performed with R software version 3.044.
Additional Information
How to cite this article: Toussaint, A. et al. Global functional diversity of freshwater fish is concentrated in the Neotropics while functional vulnerability is widespread. Sci. Rep. 6, 22125; doi: 10.1038/srep22125 (2016).
Supplementary Material
Acknowledgments
We thank F. Leprieur and C. Violle for their comments that helped us to improve a first version of this manuscript and Peter Winterton for correcting the English. This work was supported by the EU BioFresh Project (7th Framework European Program, Contract No. 226874) and by the ‘Laboratoires d’Excellence’ (LABEX) entitled TULIP (ANR-10-LABX-41) and CEBA (ANR-10-LABX-25-01). A.T. was supported by the French National Centre of Scientific Research (CNRS, Contract No. 360776).
Footnotes
Author Contributions A.T., S.B. and S.V. designed the study, discussed the results and wrote the manuscript. N.C. and A.T. built the database and A.T. performed the analyses.
References
- Barnosky A. D. et al. Has the Earth’s sixth mass extinction already arrived? Nature 471, 51–57 (2011). [DOI] [PubMed] [Google Scholar]
- Gaston K. J. Global patterns in biodiversity. Nature 405, 220–227 (2000). [DOI] [PubMed] [Google Scholar]
- Leprieur F., Beauchard O., Blanchet S., Oberdorff T. & Brosse S. Fish invasions in the world’s river systems: when natural processes are blurred by human activities. PLoS Biol. 6, e28 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimm S. L. et al. The biodiversity of species and their rates of extinction, distribution, and protection. Science 344, 1246752 (2014). [DOI] [PubMed] [Google Scholar]
- Jetz W., Sekercioglu C. H. & Böhning-Gaese K. The Worldwide Variation in Avian Clutch Size across Species and Space. PLoS Biol. 6, e303 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devictor V. et al. Spatial mismatch and congruence between taxonomic, phylogenetic and functional diversity: the need for integrative conservation strategies in a changing world. Ecol. Lett. 13, 1030–1040 (2010). [DOI] [PubMed] [Google Scholar]
- Oberdorff T. et al. Global and Regional Patterns in Riverine Fish Species Richness: A Review. Int. J. Ecol. 2011, 1–12 (2011). [Google Scholar]
- Lévêque C., Oberdorff T., Paugy D., Stiassny M. L. J. & Tedesco P. A. Global diversity of fish (Pisces) in freshwater. Hydrobiologia 595, 545–567 (2007). [Google Scholar]
- Violle C. et al. Let the concept of trait be functional! Oikos 116, 882–892 (2007). [Google Scholar]
- Cadotte M. W., Carscadden K. & Mirotchnick N. Beyond species: functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 48, 1079–1087 (2011). [Google Scholar]
- Mouillot D., Villéger S., Scherer-Lorenzen M. & Mason N. W. H. Functional structure of biological communities predicts ecosystem multifunctionality. PLoS One 6, e17476 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale B. J. et al. Biodiversity loss and its impact on humanity. Nature 486, 59–67 (2012). [DOI] [PubMed] [Google Scholar]
- Srivastava D. S., Cadotte M. W., MacDonald A. A. M., Marushia R. G. & Mirotchnick N. Phylogenetic diversity and the functioning of ecosystems. Ecol. Lett. 15, 637–648 (2012). [DOI] [PubMed] [Google Scholar]
- Díaz S. et al. Incorporating plant functional diversity effects in ecosystem service assessments. Proc. Natl. Acad. Sci. USA 104, 20684–20689 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’agata S. et al. Human-mediated loss of phylogenetic and functional diversity in coral reef fishes. Curr. Biol. 24, 555–560 (2014). [DOI] [PubMed] [Google Scholar]
- Tilman D., Knops J., Wedin D. & Reich P. The Influence of Functional Diversity and Composition on Ecosystem Processes. Science. 277, 1300–1302 (1997). [Google Scholar]
- Cornwell W. K., Schwilk L. D. W. & Ackerly D. D. A trait-based test for habitat filtering: convex hull volume. Ecology 87, 1465–1471 (2006). [DOI] [PubMed] [Google Scholar]
- Violle C., Reich P. B., Pacala S. W., Enquist B. J. & Kattge J. The emergence and promise of functional biogeography. Proc. Natl. Acad. Sci. USA 111, 13690–13696 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouillot D. et al. Functional over-redundancy and high functional vulnerability in global fish faunas on tropical reefs. Proc. Natl. Acad. Sci. USA 111, 13757–13762 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzurro E. et al. External morphology explains the success of biological invasions. Ecol. Lett. 17, 1455–1463 (2014). [DOI] [PubMed] [Google Scholar]
- Ricklefs R. E. Species richness and morphological diversity of passerine birds. Proc. Natl. Acad. Sci. USA 109, 14482–14487 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre P. B. et al. Fish distributions and nutrient cycling in streams: Can fish create biogeochemical hotspots? Ecology 89, 2335–2346 (2008). [DOI] [PubMed] [Google Scholar]
- Brandl S. J. & Bellwood D. R. Individual-based analyses reveal limited functional overlap in a coral reef fish community. J. Anim. Ecol. 83, 661–670 (2014). [DOI] [PubMed] [Google Scholar]
- Winemiller K. Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecol. Monogr. 61, 343–365 (1991). [Google Scholar]
- Bellwood D. R., Goatley C. H. R., Brandl S. J. & Bellwood O. Fifty million years of herbivory on coral reefs: fossils, fish and functional innovations. Proc. Biol. Sci. 281, 20133046 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villéger S., Ramos Miranda J., Flores Hernández D. & Mouillot D. Contrasting changes in taxonomic vs. functional diversity of tropical fish communities after habitat degradation. Ecol. Appl. 20, 1512–1522 (2010). [DOI] [PubMed] [Google Scholar]
- IUCN. The IUCN Red List of Threatened Species. Version 2015-4. http://www.iucnredlist.org. Downloaded on 19 November 2015 (2015).
- Tedesco P. A. et al. Patterns and processes of global riverine fish endemism. Glob. Ecol. Biogeogr. 21, 977–987 (2012). [Google Scholar]
- Albertson R. C., Markert J. A., Danley P. D. & Kocher T. D. Phylogeny of a rapidly evolving clade: the cichlid fishes of Lake Malawi, East Africa. Proc. Natl. Acad. Sci. USA 96, 5107–5110 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J. S. Fishes of the World. (Wiley, 2006). [Google Scholar]
- Thuiller W. et al. The European functional tree of bird life in the face of global change. Nat. Commun. 5, 3138 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouillot D. et al. Rare species support vulnerable functions in high-diversity ecosystems. PLoS biol. 11, e1001569 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villéger S., Blanchet S., Beauchard O., Oberdorff T. & Brosse S. Homogenization patterns of the world’s freshwater fish faunas. Proc. Natl. Acad. Sci. USA 108, 18003–18008 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosse S. et al. Fish-SPRICH: a database of freshwater fish species richness throughout the World. Hydrobiologia 700, 343–349 (2013). [Google Scholar]
- Leprieur F. et al. Partitioning global patterns of freshwater fish beta diversity reveals contrasting signatures of past climate changes. Ecol. Lett. 14, 325–334 (2011). [DOI] [PubMed] [Google Scholar]
- Froese R. & Pauly D. Fishbase. World Wilde Web electronic publication. Available at: http://www.fishbase.org (accessed on 8 May 2013) (2013).
- Pease A. A., Taylor J. M., Winemiller K. O. & King R. S. Ecoregional, catchment, and reach-scale environmental factors shape functional-trait structure of stream fish assemblages. Hydrobiologia 753, 265–283 (2015). [Google Scholar]
- Josse J. & Husson F. Handling missing values in exploratory multivariate data analysis methods. J. Soc. Fr. Statistique. 153, 79–99 (2012). [Google Scholar]
- Vitousek P. M. Human Domination of Earth’s Ecosystems. Science 277, 494–499 (1997). [Google Scholar]
- Villéger S., Mason N. W. H. & Mouillot D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89, 2290–2301 (2008). [DOI] [PubMed] [Google Scholar]
- Baselga A. The relationship between species replacement, dissimilarity derived from nestedness, and nestedness. Glob. Ecol. Biogeogr. 21, 1223–1232 (2012). [Google Scholar]
- Villéger S. & Brosse S. Measuring changes in taxonomic dissimilarity following species introductions and extirpations. Ecol. Indic. 18, 552–558 (2012). [Google Scholar]
- Baselga A. & Orme C. D. L. Betapart: an R package for the study of beta diversity. Methods Ecol. Evol. 3, 808–812 (2012). [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. (2014). at http://www.r-project.org/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.