Abstract
Background
HIV-infected patients with pulmonary TB (pTB) can have worsening of respiratory symptoms as part of TB-immune reconstitution inflammatory syndrome (TB-IRIS) following antiretroviral therapy (ART) initiation. Thus, reconstitution of immune function on ART could drive incident lung damage in HIV/TB.
Methods
We hypothesized that increases in matrix metalloproteinases (MMPs), which can degrade lung matrix, on ART are associated with TB-IRIS among a cohort of advanced, ART naïve, HIV-infected adults with pTB. Furthermore, we related early changes in immune measures and MMPs on ART to lung function in an exploratory subset of patients post-TB cure. This study was nested within a prospective cohort study. Rank sum and chi-square tests, Spearman's correlation coefficient, and logistic regression were used for analyses.
Results
Increases in MMP-8 following ART initiation were independently associated with TB-IRIS (p = 0.04; adjusted odds ratio 1.5 [95% confidence interval: 1.0–2.1]; n = 32). Increases in CD4 counts and MMP-8 on ART were also associated with reduced forced expiratory volume in one-second post-TB treatment completion (r = − 0.7, p = 0.006 and r = − 0.6, p = 0.02, respectively; n = 14).
Conclusions
ART-induced MMP increases are associated with TB-IRIS and may affect lung function post-TB cure. End-organ damage due to TB-IRIS and mechanisms whereby immune restoration impairs lung function in pTB deserve further investigation.
Keywords: Matrix metalloproteinases, Immune recovery, Lung function, HIV, TB-IRIS, ART
Highlights
-
•
Matrix metalloproteinases (MMP), capable of degrading lung collagen, can increase rapidly on ART in HIV/TB patients.
-
•
Increases in plasma MMP-8 concentrations after ART initiation are associated with the development of paradoxical TB-IRIS.
-
•
Increases in CD4 T-cells and MMP-8 concentrations after ART initiation are correlated with decreased lung function post-TB cure.
TB-associated pulmonary morbidity can persist after TB cure. However, causal mechanisms for lung damage, which may involve immune mechanisms and tissue proteases, in TB are unclear. Less is known in this regard among patients with HIV/TB, who are at risk for inflammatory reactions following ART initiation, otherwise known as TB-immune reconstitution inflammatory syndrome (IRIS). In this study, rapid ART-induced increases in certain tissue degrading proteins called matrix metalloproteinases (MMP) were associated with TB-IRIS. Furthermore, rapid recovery of CD4 T-cells and MMP-8 concentrations were associated with decreased lung function in an exploratory subset. In HIV/TB, robust increases in cellular immune function and MMPs on ART may underlie lung injury and long-term pulmonary deficits.
1. Introduction
For many patients with TB, the morbidity of the disease extends long beyond TB treatment completion (Pasipanodya et al. 2010). Indeed, approximately half of patients surviving pulmonary TB (pTB), the most common form of the disease, suffer substantial long-term pulmonary impairment despite microbiologic cure (Pasipanodya et al., 2010, Hnizdo et al., 2000, Ralph et al., 2013). Furthermore, pTB, including cured disease, is a major cause of chronic lung disease worldwide (van Zyl Smit et al. 2010).
Nearly 15% of all TB cases and 25% of global TB deaths are HIV-associated (WHO 2014). The underlying mechanisms leading to long-term pulmonary morbidity after TB treatment completion, especially among HIV-infected patients, are unclear (Pasipanodya et al., 2010, Hnizdo et al., 2000, Ralph et al., 2013). Clinically, impaired lung function after TB treatment completion is associated with greater radiographic lung involvement at initial presentation (Plit et al. 1998). Greater initial radiographic lung involvement is also associated with higher CD4 counts in individuals with HIV/TB (Kwan and Ernst, 2011, Post et al., 1995). These findings are consistent with a central role of cellular immune responses in cavity formation and structural lung damage in TB (Flynn et al., 1993, Schluger et al., 2002). Mechanistically, matrix metalloproteinases (MMPs), which can degrade lung collagen, have also been implicated in TB-associated lung tissue destruction (Greenlee et al., 2007, Ong et al., 2014, Elkington et al., 2011). However, lower MMP concentrations and reduced TB-associated lung damage assessed radiographically have been associated with HIV infection (Walker et al. 2012). Taken together, these data suggest that patients with HIV may have less lung involvement than those without HIV, but data evaluating this association are conflicting (Hnizdo et al., 2000, Ralph et al., 2013).
Antiretroviral therapy (ART) is a critical component of HIV/TB care, conferring a survival advantage to those who initiate ART during treatment of TB (Abdool Karim et al., 2011, Abdool Karim et al., 2010). While ART use in a study of HIV-infected patients was independently associated with airway obstruction (George et al. 2009), a limitation of studies relating HIV to lung damage in TB is that the effects of cellular immune restoration during TB treatment have not been assessed in detail. Rapid cellular immune restoration in the presence of a high TB antigen burden could plausibly drive incident cavity formation and worsening lung damage. Consistent with this hypothesis, case reports have described patients with advanced HIV/pTB and minimal initial radiographic lung involvement who develop massive pulmonary infiltrates, lung cavitation, and bronchiolitis obliterans soon after ART initiation (Meintjes et al., 2008, Lawn et al., 2009). Incident lung damage during immune restoration in HIV/TB may also complicate TB-immune reconstitution inflammatory syndrome (TB-IRIS), but this hypothesis has been relatively unexplored. In one study, HIV/TB co-infected adults who experienced TB-IRIS on ART were found to have higher absolute concentrations of MMPs at the time of the IRIS event compared to controls, but lung function was not assessed (Tadokera et al. 2014).
In this study, we investigated changes in MMP concentrations on ART and the association between these changes and paradoxical TB-IRIS in a well-characterized cohort of HIV-infected adults with advanced HIV/TB (Ravimohan et al., 2013, Ravimohan et al., 2015a). Furthermore, in a subset of patients, we explored the relationship between the magnitude of early immune restoration and MMP concentrations on ART and pulmonary function after TB treatment completion. We specifically selected MMP-1, -2, -3, -8, and -9 for analysis, as they have previously been found to be associated with lung tissue damage in TB (Elkington et al., 2011, Walker et al., 2012, Elkington et al., 2005, Elkington and Friedland, 2006, Ong et al., 2015).
2. Materials and Methods
2.1. Ethics
The institutional review boards of the University of Pennsylvania, Botswana Ministry of Health, and the Princess Marina Hospital approved this study. All patients provided written informed consent to participate in this study.
2.2. Study Design
We enrolled ART naïve, HIV-infected adults with pulmonary TB in a prospective cohort study to investigate the relationship between early immune recovery on ART and treatment outcomes, as described (Ravimohan et al., 2013, Ravimohan et al., 2015a). This cohort has previously demonstrated that early changes (measured during the first 4 weeks of ART) in CD4 counts and other immunologic parameters are differentially associated with death and paradoxical TB-IRIS (Ravimohan et al. 2015a). TB-IRIS in this cohort was symptomatically characterized primarily by worsening respiratory status (Ravimohan et al. 2015a). In this secondary analysis we therefore hypothesized that 1) concentrations of MMPs would increase after ART initiation and 2) early changes in MMPs would be associated with paradoxical TB-IRIS. Finally, in a subset of patients, we explored the hypothesis that rapid increases in CD4 counts, Mycobacterium tuberculosis (MTB)-specific cellular immune function, and MMP concentrations early after ART initiation during TB treatment, as well as TB-IRIS within 6 months after ART initiation, would be associated with impaired lung function. Lung function was assessed months after TB treatment completion to focus on stable residual effects after TB cure (Hnizdo et al. 2000).
2.3. Study Participants
Patients were enrolled into the parent study between November 2009 and July 2013 from outpatient clinics and a public tertiary care hospital in Gaborone, Botswana, as described (Ravimohan et al., 2013, Ravimohan et al., 2015a). Subjects had to have a pre-ART CD4 count ≤ 125 cells/μl and plan to initiate ART within 2 months of starting standard TB treatment for their newly diagnosed pulmonary TB (Ravimohan et al., 2013, Ravimohan et al., 2015a). Given our focus on possible effects of immune recovery on lung related parameters in those who survive TB, to be included in the primary analysis relating MMP concentrations and TB-IRIS, patients required baseline and week 4 post-ART initiation measurement of MMPs and needed to have survived to 6 months post-ART initiation (unless TB-IRIS preceded their death). For the sub-analysis of lung function, we recruited a convenience sample of patients who had completed participation in the parent cohort study, had completed their TB treatment with no relapse or recurrence of TB, were on ART, and did not have symptomatic pulmonary infection, or other signs of active pulmonary pathology at the time of the pulmonary function test (PFT).
2.4. Data Collection
We accessed clinical variables and measures of early immunologic response on ART from the parent study database (Ravimohan et al., 2015a, Ravimohan et al., 2015b). Baseline blood for MMP and immune responses assessments was collected at a median of −2 days (interquartile range [IQR]: −14 to 0 days) from day of ART initiation. The second blood draw was at median of 2 days (IQR: 0 to 5 days) from date of the week-4 post-ART initiation. In the parent study, patients who experienced TB-IRIS in the first 6 months of ART were defined as per the International Network for Study of HIV-associated IRIS and the AIDS Clinical Trials Group criteria, as described previously (Meintjes et al., 2008, Ravimohan et al., 2015a, Grant et al., 2010). In addition, we prospectively collected data on smoking as well as height, weight, and body mass index (BMI) at the time of PFTs to facilitate calculation of predictive lung function, as per American Thoracic Society (ATS) guidelines (American Thoracic Society 1995).
2.5. Luminex Assay
Previously frozen (− 80 °C) plasma received two dilutions, 1:5 and 1:50, to be within the linear range. We typically used the 1:5 dilution to determine MMP-1 (lower limit of detection [LLD]: 1.1 pg/ml) and MMP-3 (LLD: 7.3 pg/ml) and the 1:50 fold dilution to quantitate MMP-2 (LLD: 12.6 pg/ml), MMP-8 (LLD: 16.6 pg/ml), and MMP-9 (LLD: 13.7 pg/ml) concentration (R&D, Minnesota USA). Four of the total 296 (1.3%) samples tested had MMP-8 levels that were below the limit of detection and were recorded as 16.6 pg/ml for analysis. The luminex assay was carried out according to manufacturer's protocol and analyzed on the Biorad Bio-Plex2000 platform.
2.6. TB-specific Cellular Immune Responses
Briefly, peripheral blood mononuclear cells (PBMCs) were isolated from whole blood at baseline and week 4 post-ART initiation, as previously described (Ravimohan et al., 2013, Ravimohan et al., 2015a). In IFN-γ enzyme-linked immunosorbent spot (ELISPOT) assays, 2 × 105 cells were incubated overnight with and without purified protein derivative (PPD; 5 μg/ml; Statens Serum Institute), in triplicate, and developed as per manufacturer's protocol (BD Bioscience) (Ravimohan et al., 2013, Ravimohan et al., 2015a). Plates were read using an ImmunoSpot reader (Cellular Technology Limited). The average number of spot forming units (SFU) following subtraction of the negative control from the PPD stimulated wells was determined and expressed as SFU/106 PBMCs. Phorbol 12-myristate 13-acetate (50 ng/ml) and ionomycin (500 ng/ml) (Sigma-Aldrich) were used as positive control. For flow cytometry, also described previously (Ravimohan et al. 2015b), cryopreserved PBMCs were thawed in a complete RPMI 1640 medium (Gibco) containing 10% fetal bovine serum (Gemini Bio-Products) and DNase (50 μg/ml, Sigma Aldrich). Cells were stimulated for 6 h with and without PPD (5 μg/ml) and co-stimulated with CD28/CD49d (BD FastImmune). Cells were stained with LIVE/DEAD fixable aqua, anti-CD3-BV570, and anti-CD4-Qdot-705 for identification of live, CD4+ T-cells. Intracellular cytokine staining with anti-IFN-γ-Pacific blue and anti-TNF-α-AF700 was performed. Antibodies were obtained from Biolegend, eBiosciences, and Life Technologies. Stained PBMCs were analyzed on an LSR II (BD Biosciences) and using FlowJo Version 9.8.2 (TreeStar). To determine TB-specific responses, frequency of cells expressing IFN-γ or TNF-α in the negative control was deducted from the corresponding PPD-stimulated response.
2.7. Pulmonary Function Tests
PFTs were conducted by spirometry in accordance with ATS guidelines using the SpiroOne USB device (Micro Medical, U.K.) (American Thoracic Society 1995). A minimum of three and up to 8 test maneuvers per patient were carried out. The best of three maximal efforts with less than 5% variation between maneuvers was deemed “valid” and used for analysis (American Thoracic Society 1995). The flow-volume loop generated from the spirometry test was used to determine forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and FEV1/FVC ratio. As per ATS guidelines, lung function was classified as normal or abnormal, where normal lung function was defined as forced expiratory volume in one second (FEV1) ≥ 80%, forced vital capacity (FVC) ≥ 80%, and FEV1/FVC ratio ≥ 70% of predicted values (American Thoracic Society, 1991, Pellegrino et al., 2005). Impaired lung function was defined as FVC or FEV1 < 80% of the predicted value and further categorized as obstructive (FEV1/FVC < 70% and FVC > 80% of predicted values), restrictive (FEV1/FVC ≥ 70% and FVC < 80% of predicted values), or mixed defects (FEV1/FVC < 70% and FVC < 80% of predicted values) (American Thoracic Society, 1991, Pellegrino et al., 2005). The European Community of Coal and Steel (ECCS) reference values were used to determine percent predicted FEV1 and FVC (Quanjer et al. 1993), which have been suggested for general use in similar African populations (White et al. 1996).
2.8. Statistical Analysis
Continuous variables were described using medians and IQR or means and ranges based on the distribution of the variable. To describe changes in MMPs early during ART, paired measures of MMPs at baseline and week 4 post-ART initiation were compared within each subject by Wilcoxon signed rank tests. For the primary analysis of TB-IRIS, MMP concentrations (exposure) were first compared between patients with TB-IRIS (outcome) and non-IRIS survivors by Wilcoxon rank sum tests. Tests were two-sided and p values of < 0.05 were considered statistically significant. Logistic regression was used to determine the association between early change in MMP concentrations from baseline to week 4 (stratified into quartiles) and TB-IRIS, adjusting for BMI and use of nevirapine (NVP), both of which were associated with the outcome in this cohort (Ravimohan et al. 2015a). In the exploratory analyses of lung function, patients with PFTs were compared to those without lung function assessments from the primary analysis by the Wilcoxon rank sum test. Spearman's correlation coefficient (r) was used to determine the relationship between PFT measures (FEV1 and FVC) assessed after TB treatment completion and early change from baseline to week 4 of ART in 1) immunologic response (CD4 count as well as TB-specific immune response by ELISPOT assay and flow cytometry) and 2) MMP concentrations following ART initiation. The association between ATS-defined abnormal lung function post-TB treatment and change in MMPs were determined by Wilcoxon rank sum tests. Baseline factors (e.g. pre-ART CD4 count and MMP concentration) that could potentially confound this association were similarly evaluated. Lung function in those with a history of TB-IRIS was also compared to those without a history of IRIS using a chi-square test. Given the small numbers, comparisons involving lung function are unadjusted for potential confounders. In univariable analyses of the association between change in MMPs and IRIS as well as in the comparisons between change in immune function, MMPs, and PFT, uncorrected p values and p values corrected for multiple comparisons using the Benjamini–Hochberg method are presented (Benjamini and Hochberg 1995). Stata software (Version 11.2; College Station, Texas) and GraphPad Prism (Version 5.0; San Diego, California) were used for statistical analyses and to generate graphs, respectively.
2.9. Funding
This study was funded by grants from the National Institutes of Health (NIH) (grant number R01AI080337) and the Penn Center for AIDS Research (AI045008). The sponsors did not play a role in data collection, analysis, or interpretation; design of the study; or patient recruitment. We have not been paid to write this article by any agency or pharmaceutical company.
3. Results
3.1. Baseline Characteristics
One hundred and seventy patients were enrolled and initiated on ART in the parent study (Ravimohan et al. 2015a). Of these patients, 148 (74%) had paired MMP data at pre- and week 4 post-ART initiation and qualified for the current study. Patients who were excluded were not significantly different from those who were included in terms of age, sex, and CD4 count at baseline (data not shown). Thirty-two patients (22%) developed TB-IRIS, leaving 116 (78%) non-IRIS survivors. Clinical symptoms associated with TB-IRIS were primarily new or worsening respiratory complaints occurring at a median of 4 weeks post-ART initiation (IQR: 4–12 weeks) and have been previously described (Ravimohan et al. 2015a). One (3%) of the TB-IRIS patients died two weeks after the IRIS event despite initial improvement on corticosteroid therapy and was included in the primary analysis as a TB-IRIS case (Ravimohan et al. 2015a). Overall, patients had median pre-ART CD4 counts of 62 cells/μl (IQR: 32–94 cells/μl) and started ART within 28 days (IQR: 19–47 days) of TB treatment initiation (Supplemental Table 1).
3.2. Changes in MMP Concentrations After ART Initiation
There was considerable heterogeneity with respect to changes in the concentration of MMPs, measured from baseline to week 4 post-ART initiation (Fig. 1 and Supplemental Fig. 1). Median concentrations of MMP-1, -2, and -3 decreased, whereas MMP-8 and 9 concentrations increased significantly (all p < 0.01 except MMP-1, which was non-significant; Fig. 1).
Fig. 1.
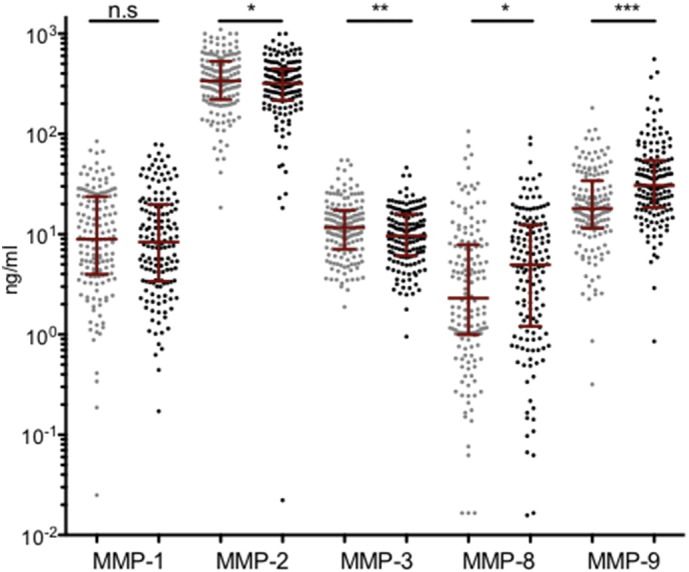
MMP concentrations change soon after ART initiation in advanced HIV/TB co-infected patients. Luminex assay was used to quantitate plasma concentrations of matrix metalloproteinase (MMP)-1, -2, -3, -8, and -9 pre- (gray) and week 4 post-ART initiation (black) in advanced HIV/TB co-infected patients (n = 148). Median MMP concentrations with interquartile ranges are shown in red. Wilcoxon sign rank test was used to compare matched pair baseline and week 4 post-ART concentration of MMPs within all patients. N.s. = non-significant, *p < 0.01, **p < 0.001, ***p < 0.0001.
3.3. TB-IRIS and MMP Concentrations Following ART Initiation
Patients who experienced TB-IRIS had significantly greater increases in MMP-8 concentrations on ART compared to non-IRIS controls in unadjusted analyses (p = 0.04; pcorr = 0.13; Table 1). Every quartile increase in MMP-8 from baseline to week 4 post-ART initiation was independently associated with a 50% higher odds of TB-IRIS, after adjusting for baseline CD4 count, BMI, and NVP use (odds ratio 1.5 [95% confidence interval: 1.0–2.1]). Changes in all other MMPs were not statistically different between groups (Table 1).
Table 1.
Change from baseline to week 4 post-ART initiation in MMPs and association with TB-IRIS among advanced HIV/TB co-infected patients.
| Change from baseline median (IQR) | Overall (n = 148) |
Control (n = 116) |
TB-IRIS (n = 32) |
P value | Pcorr valuea |
|---|---|---|---|---|---|
| MMP-1 | − 92.2 (− 3685 to 4326) | − 127 (− 4186 to 4569) | − 8.9 (− 2726 to 2690) | 0.57 | 0.59 |
| MMP-2b | − 28.0 (− 122.1 to 45.4) | − 10 (− 112.8 to 62.6) | − 52.3 (− 166.2 to 6.2) | 0.07 | 0.13 |
| MMP-3 | − 1,876 (− 5196 to 1289) | − 2391 (− 6124 to 653) | − 1018 (− 3103 to 2701) | 0.08 | 0.13 |
| MMP-8 | 580 (− 1302 to 5110) | 421 (− 2070 to 4538) | 3195 (69 to 10,070) | 0.04 | 0.13 |
| MMP-9 | 9015 (504 to 26,570) | 9858 (808 to 26,506) | 5395 (− 134 to 29,239) | 0.59 | 0.59 |
Wilcoxon rank sum test was used to compare controls to TB-IRIS patients. P < 0.05 was considered statistically significant. IQR = interquartile range.
Pcorr = p value adjusted for multiple comparisons using the Benjamini–Hochberg method.
Luminex data for matrix metalloproteinase (MMP)-2 is shown as ng/ml, while all other MMPs are presented as pg/ml.
3.4. Lung Function in HIV-infected Patients Post-TB Treatment Completion
PFTs were conducted in a convenience sample of 14 patients nearly 2 years following a standard 6-month course of anti-TB therapy (Table 2), when TB-associated acute effects on lung function have been shown to have stabilized (Hnizdo et al. 2000). The subset of patients with PFTs was relatively representative of the overall cohort, as there were no major differences between those with (n = 14) and without (n = 134) PFT assessments (Supplemental Table 2). Furthermore, while changes in MMP-1 and MMP-3 concentrations as well as TB-specific IFN-γ+ and TNF-α+ CD4+ T-cell frequencies were somewhat different for this sub-group compared to the overall cohort, they were not statistically significant (Supplemental Table 2). Half of the patients with PFT analysis were female (n = 7) and two (14%) had a history of smoking. The median percent predicted FEV1 for these patients was below the cut-off for normal lung function (78% [IQR: 66–83%]). Nine (64%) patients had ATS-defined abnormal lung function, of which 5 (56%) were categorized as obstructive, 3 (33%) as restrictive, and 1 (11%) as mixed pulmonary defects (Table 2).
Table 2.
Clinical characteristics and pulmonary function post-TB treatment completion in advanced HIV/TB co-infected patients.
| Clinical variables | (n = 14) |
|---|---|
| Female, n (%) | 7 (50%) |
| Pre-ART HIV viral load, log10 copies/ml (IQR) | 5.2 (4.7–6.0) |
| Pre-ART CD4 T-cell count, median cells/μl (IQR) | 36 (24–79) |
| Age at PFT, mean (range in years) | 40 (27–61) |
| BMI at PFT, median kg/m2 (IQR); | 21.5 (19.4–23) |
| History of smoking, n (%) | 2 (14) |
| ATT initiation-PFT median years (IQR) | 1.9 (1.6–2.1) |
| ART initiation-PFT median years (IQR) | 1.8 (1.5–2.1) |
| Pulmonary function test (PFT) | |
| FEV1a, median % (IQR) | 77.5 (66–83) |
| FVCa, median % (IQR) | 83 (77–91) |
| FEV1/FVC ratioa, median % (IQR) | 97.5 (76–105) |
| Abnormal PFT, n (%) | 9 (64) |
| Obstructive, n (%) | 5 (56) |
| Restrictive, n (%) | 3 (33) |
| Mixed, n (%) | 1 (11) |
Shown are characteristics at baseline and at cross-section for patients who underwent pulmonary function testing (PFT) post-TB treatment completion. IQR = interquartile range; BMI = body mass index; ATT = anti-TB therapy; FEV1 = forced expiratory volume in 1 s; FVC = forced vital capacity.
Percent of predicted normal.
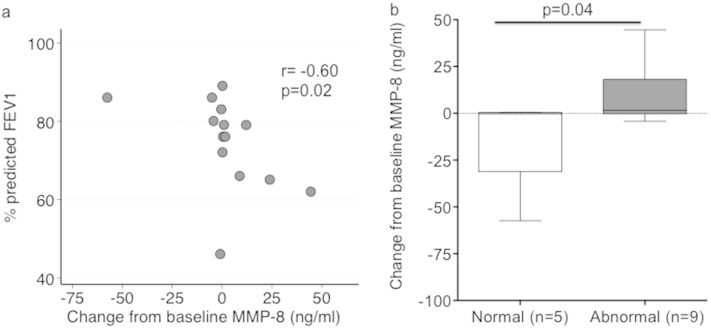
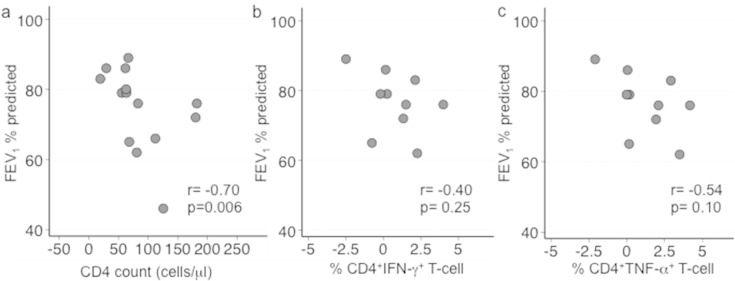
Increases in MMP-8, but not other MMPs, early after ART initiation were strongly associated with lower FEV1 after TB treatment completion (p = 0.02; pcorr = 0.09; Table 3 and Fig. 2a). Assessing the association between abnormal lung function and change in MMPs revealed that increase in MMP-8 on ART was linked to abnormal pulmonary function, while those with normal lung function generally had an early reduction in MMP-8 (p = 0.04; Fig. 2b and Supplemental Table 3). Furthermore, a strong inverse correlation was observed between the magnitude of the rise of CD4 count from baseline to week 4 of ART and the percent predicted FEV1 after TB treatment completion (p = 0.006; pcorr = 0.05; Table 3 and Fig. 3a). Greater increases in IFN-γ released by total PBMC as well as IFN-γ+ or TNF-α+ CD4+ T-cell frequency also tended to correlate with a lower percent predicted FEV1 after TB treatment completion; however, these relationships were not statistically significant (Table 3; Fig. 3b and c).
Table 3.
Association between lung function post-TB treatment completion and change from baseline to week 4 post-ART initiation in immune parameters and MMPs in HIV-infected patients with a history of treated pTB.
| Variable | Rho# | P value | Pcorr value§ |
|---|---|---|---|
| FEV1 association with change from baseline to week 4 post-ART in | |||
| CD4 count | − 0.70 | 0.006 | 0.05 |
| TB-specific IFN-γ ELISPOT* | − 0.35 | 0.21 | 0.42 |
| CD4+ IFN-γ+ T-cell** (n = 10) | − 0.40 | 0.25 | 0.42 |
| CD4+ TNF-α+ T-cell** (n = 10) | − 0.54 | 0.10 | 0.30 |
| MMP-1 | − 0.24 | 0.42 | 0.54 |
| MMP-2 | − 0.07 | 0.81 | 0.81 |
| MMP-3 | 0.31 | 0.28 | 0.42 |
| MMP-8 | − 0.60 | 0.02 | 0.09 |
| MMP-9 | 0.16 | 0.59 | 0.66 |
| FVC association with change from baseline to week 4 post-ART in | |||
| CD4 count | − 0.26 | 0.37 | 0.55 |
| TB-specific IFN-γ ELISPOT* | − 0.18 | 0.53 | 0.60 |
| CD4+ IFN-γ+ T-cell** (n = 10) | − 0.36 | 0.31 | 0.55 |
| CD4+ TNF-α+ T-cell** (n = 10) | − 0.28 | 0.43 | 0.55 |
| MMP-1 | − 0.52 | 0.06 | 0.54 |
| MMP-2 | − 0.03 | 0.90 | 0.90 |
| MMP-3 | − 0.33 | 0.24 | 0.55 |
| MMP-8 | − 0.28 | 0.33 | 0.55 |
| MMP-9 | − 0.29 | 0.31 | 0.55 |
Shown are Spearman's correlation coefficients (rho)#. §Pcorr = p value adjusted for multiple comparisons using the Benjamini–Hochberg method. *IFN-γ produced by peripheral blood mononuclear cells (PBMC) was measured by ELISPOT assay. PBMCs were stimulated with purified protein derivative (PPD) to measure TB-specific immune responses expressed as spot forming units (SFU)/106 PBMCs. **Frequency of CD4+ T-cell expressing IFN-γ or TNF-α in response to PPD stimulation was assessed by intracellular cytokine staining and flow cytometry.
Fig. 2.
Increases in MMP-8 concentrations from baseline to week 4 post-ART initiation is associated with (a) lower forced expiratory volume in one-second (FEV1) and (b) abnormal lung function post-TB treatment in HIV-infected patients with a history of active pTB. FEV1 was measured by pulmonary function testing post-TB treatment completion in HIV-infected patients (n = 14) to determine percent predicted of normal. Normal and abnormal lung function were defined as per American Thoracic Society (ATS) guidelines. MMP-8 was measured by luminex assay. Shown is the Spearman correlation coefficient (r) and corresponding p value (a) as well as the p value from Wilcoxon rank sum test comparing patients with normal to those with abnormal lung function (b).
Fig. 3.
Lower FEV1 post-TB treatment completion is correlated with greater increases in immune function from baseline to week 4 post-ART initiation in HIV-infected patients with a history of active pTB. Forced expiratory volume in one-second (FEV1) was measured by pulmonary function testing post-TB treatment completion in HIV-infected patients to determine percent predicted of normal. Shown are Spearman's correlation coefficients (rho: r) and corresponding p values comparing percent predicted FEV1 to change from baseline to week 4 post-ART in (a) CD4 count (n = 14) and (b) frequency of IFN-γ and (c) TNF-α producing CD4+ T-cells (n = 10) upon PPD stimulation.
Furthermore, 3 (21%) of 14 patients with PFT analyses had a history of paradoxical TB-IRIS. IRIS onset among these three patients was at 4, 4, and 16 weeks post-ART initiation, and symptoms persisted between 4 and 8 weeks. These patients presented with signs and symptoms consistent with TB-IRIS, such as new onset cervical lymphadenopathy, as well as respiratory complaints, which included dyspnea and chest pains. CD4 count increase on ART among the three TB-IRIS cases was 69, 126 and 181 cells/μl and HIV viral load was decreased by median − 3.0, − 2.7 and − 2.2 log10 copies/ml. TB-IRIS patients had significantly worse FEV1 values than those without IRIS (median percent predicted FEV1 = 65%, [IQR: 46–72] versus 79% [IQR: 76–86]; p = 0.04, respectively). Additionally, TB-IRIS patients had significantly lower FEV1/FVC ratio compared to non-IRIS controls (median percent predicted FEV1/FVC = 75 [IQR: 60–76] versus 100 [IQR: 81–110], respectively, p = 0.02).
Since we did not have baseline lung function data on participants and associations between changes in MMP concentrations and CD4 counts and lung function could have influenced differences in these parameters at initial presentation, we compared baseline levels of CD4 counts and MMP concentrations among those with and without abnormal lung function (Supplemental Table 3). Patients with ATS-defined abnormal lung function tended to have lower median pre-ART CD4 counts; slightly longer interval between anti-TB therapy and ART initiation; and lower pre-ART MMP-8 concentrations compared to patients with normal lung function; however, these associations did not reach statistical significance (Supplemental Table 3).
4. Discussion
In this study we investigated circulating concentrations of key MMPs before and soon after ART initiation among a relatively large cohort of advanced HIV-infected patients concurrently treated for active pTB. While there was substantial heterogeneity in early MMP changes, concentrations of MMP-8 and -9 increased significantly on ART and greater early MMP-8 increases were associated with both TB-IRIS and decreased lung function months after TB cure, a time when TB-associated lung damage has typically stabilized (Hnizdo et al. 2000). Similarly, rapid increases in the CD4 count early after ART were also associated with worse lung function after TB treatment completion. These findings, combined with data that MMPs and cellular immune responses are key to granuloma and cavity formation in TB (Salgame, 2011, Dutta and Karakousis, 2014), support a hypothesis where early changes in immune function and relevant tissue proteases following ART initiation contribute to long-term pulmonary function deficits post-TB cure in HIV/TB. Our data also indicate that patients with clinically apparent inflammatory events, such as those with TB-IRIS, may be especially at risk.
Previous work has indicated that MMPs degrade lung collagen matrix and mediate lung tissue destruction in TB (Ong et al., 2014, Elkington et al., 2011, Elkington and Friedland, 2006, Ong et al., 2015). Thus, our finding that systemic concentrations of MMP-8 and MMP-9 increase rapidly following ART initiation, as well as the association between increases in MMP-8 and TB-IRIS, which is frequently characterized by respiratory complaints (Meintjes et al., 2008, Naidoo et al., 2012, Luetkemeyer et al., 2014), suggest that HIV-infected patients with active pulmonary TB may be at risk for incident immune-mediated lung damage after ART initiation. While a somewhat consistent relationship emerged with MMP-8 and outcomes in this study, it is notable that concentrations of MMP-1, -2, and -3, which have also been implicated in lung tissue destruction (Ong et al., 2014, Elkington et al., 2011, Elkington and Friedland, 2006), decreased or remained unchanged on ART. One possibility is that MMP-1 and -2 have highly compartmentalized expression at the site of infection, as demonstrated in a study of pleural tuberculosis (Hoheisel et al. 2001). Furthermore, another study showed that rifampicin can downregulate MMP-3 expression in vitro, which may explain decreases in circulating concentrations of MMP-3 in our patients, all of whom were on this drug (Singh et al. 2014). Our results are broadly consistent with a cross-sectional study of MMPs in paradoxical TB-IRIS by Tadokera et al., where concentration of MMP-7 in MTB-stimulated PBMC culture supernatants and serum were significantly higher in those with TB-IRIS (n = 22) compared to non-IRIS controls (n = 22) (Tadokera et al. 2014). The Tadokera study did not find an association between MMP-8 and IRIS when assessing MTB-stimulated PBMCs (Tadokera et al. 2014); however, this may relate to the fact that MMP-8 is predominantly secreted by neutrophils (Hasty et al., 1986, Parks et al., 2004), which are lost during the PBMC isolation process. MMP concentrations are highly dynamic during TB treatment (Ugarte-Gil et al. 2013), and therefore differences between the two studies may also relate to the fact that patients in the Tadokera study had a substantially longer time to ART initiation after starting TB treatment than those included here (56 versus 28 days), whereas time to TB-IRIS was markedly shorter (14 versus 28 days) (Tadokera et al. 2014). Our relatively large population of nearly 150 patients with advanced HIV/TB and the consistent association between changes in MMP-8 concentrations and both TB-IRIS and lung function suggest a compelling role for MMP-8 recovery in inflammatory events and tissue damage on ART that should be investigated in further detail. Taken together, the two studies indicate that additional research on the pathogenesis of tissue damage in patients with HIV/TB initiating ART is needed.
An association between reconstitution of CD4 T-cells and impaired lung function, measured as oxygen saturation, has been shown in a murine model for IRIS using Mycobacterium avium (Barber et al. 2010). However, our study investigated the association between paradoxical TB-IRIS, change in MMPs on ART, and pulmonary function post-TB cure among HIV/TB co-infected patients. Our evaluation of lung function demonstrated not only lower FEV1 in TB-IRIS patients compared to non-IRIS controls, but also that the magnitude of the early CD4 cell increase and circulating MMP-8 concentrations following ART initiation were associated with impaired FEV1 post-TB cure. While the number of patients assessed using PFTs was small and this subset may not be entirely representative of the larger cohort with respect to certain MMPs (i.e., MMP-1 and -3) and TB-specific immune responses, the strength of the associations (r = − 0.7 and r = − 0.6) suggests that further research on this association is needed. Impairment of FEV1, which primarily measures airflow through large airways, fits with the endobronchial involvement characteristic of TB and with at least one anecdotal report of severe bronchiolitis obliterans after ART initiation in HIV/TB (Lawn et al. 2009). While our findings were among advanced HIV/TB patients initiating ART, they urge future studies to investigate end-organ damage in TB-IRIS and more generally among HIV/TB co-infected patients initiating ART. Central nervous system (CNS) impairment following TB-meningitis associated IRIS is also a concern, as significant localized increases in cellular immune function and MMPs have been previously noted (Marais et al. 2014).
Mechanistically, reduced lung involvement in advanced HIV-infected patients has been linked to low CD4 counts and reduced concentrations of MMPs, both of which are important to granuloma formation and cavitation (Kwan and Ernst, 2011, Post et al., 1995, Walker et al., 2012, Zhang et al., 1994, Law et al., 1996). While we did not determine the source of MMPs, neutrophils may also be involved, as discussed above. Neutrophils, which are noted to be the predominant phagocytic cell type in the airway of TB patients (Eum et al. 2010), were found to express MMP-8 and -9 in the lining of TB cavities (Ong et al. 2015), supporting a possible role in the pathogenesis of the lung impairment we observed. It is plausible that reconstituted CD4 T-cell and neutrophil activity following ART initiation in the setting of incompletely treated TB contribute, in part, to the rise in MMP-8 concentrations on ART, as seen here (Appelberg, 1992, Zhang et al., 1992). Neutrophil infiltration to the disease site soon after ART initiation has been suggested in a study of TB meningitis IRIS among HIV-infected patients (Marais et al. 2013). Furthermore, MMP-8 expression differs from other MMPs in that neutrophils contain granules with pre-synthesized MMP-8 and have the potential to secrete MMP-8 rapidly upon activation (Elkington and Friedland, 2006, Hasty et al., 1986, Weiss et al., 1985). Thus, the ensuing increase in immune function and MMPs on ART, perhaps driven by neutrophils, could potentially underlie incident lung tissue damage and worsening the airflow obstruction that frequently accompanies TB (Pasipanodya et al., 2010, Hnizdo et al., 2000, Ralph et al., 2013). Our data collection did not include neutrophil numbers, but these findings need confirmation in larger studies where longitudinal assessments of immune and pulmonary function are assessed in more detail. Furthermore, future studies should aim to identify the primary site of lung injury and the predominant form of ventilator defects in HIV/TB co-infected patients following pTB treatment completion.
Our study was limited by the relatively small number of patients with PFTs, which restricted our assessment of potential confounding. Another important limitation of our study is that we did not have baseline lung function assessments to determine if patients with pulmonary impairment post-TB treatment completion, as measured here, also started off with worse lung function before ART initiation. However, patients in the study who had abnormal lung function post-cure tended to have lower pre-ART CD4 counts and MMP-8 concentration, both of which are associated with less lung involvement in HIV/TB (Kwan and Ernst, 2011, Walker et al., 2012, Law et al., 1996). Additionally, chest radiographs, which we did not have at baseline or during follow-up, would have further enabled evaluation of lung involvement during treatment. Another limitation of our study was that we excluded patients who did not survive to 6 months post-ART initiation from our analyses. These patients may have had poor lung function. However, as the aim of our study was to investigate the determinants of lung impairment after TB treatment completion, we excluded deaths as they did not survive to this time point.
In summary, rapid changes in the concentrations of multiple MMPs were observed on ART, where rise in MMP-8 was independently associated with TB-IRIS. Increase in cellular immune function and MMP-8 following ART initiation was also linked with depressed lung function several months post-TB cure. MMPs like MMP-8 may be potential targets for host-directed therapy if found to mediate lung injury in future studies that aim to determine the mechanisms of lung damage in HIV/TB. In particular, the association between reversion of patients to a more immunocompetent phenotype, afforded by potent ART, with lung damage should be explored further.
Author contributions
Conceived and designed the study and experiments: SR DW GPB. Enrolled patients: NT. Facilitated study in Botswana: NT APS. Performed pulmonary function tests: SJK. Performed the experiments: SR KN. Analyzed the data: SR SJK GPB. Contributed reagents/materials/analysis tools: SJK DW GPB. Wrote the first draft of the manuscript: SR GPB. Contributed to the writing of the manuscript: SR NT SJK KN APS RG DW GPB. Agree with manuscript results and conclusions: SR NT SJK KN APS RG DW GPB.
Conflict of interest
The authors of this study do not have any conflict of interest to declare.
Acknowledgments
We thank the clinical care providers who referred patients, and the patients who volunteered to participate in this study.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2015.11.040.
Appendix A. Supplementary data
Supplementary material
References
- Pasipanodya J.G., McNabb S.J., Hilsenrath P. Pulmonary impairment after tuberculosis and its contribution to TB burden. BMC Public Health. 2010;10:259. doi: 10.1186/1471-2458-10-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnizdo E., Singh T., Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax. 2000;55:32–38. doi: 10.1136/thorax.55.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph A.P., Kenangalem E., Waramori G. High morbidity during treatment and residual pulmonary disability in pulmonary tuberculosis: under-recognised phenomena. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zyl Smit R.N., Pai M., Yew W.W. Global lung health: the colliding epidemics of tuberculosis, tobacco smoking, HIV and COPD. Eur. Respir. J. 2010;35:27–33. doi: 10.1183/09031936.00072909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization WHO. Global Tuberculosis Report 2014. 2014. http://www.who.int/tb/publications/global_report/gtbr14_executive_summary.pdf?ua=1 (Available: Accessed Dec 15, 2014)
- Plit M.L., Anderson R., Van Rensburg C.E. Influence of antimicrobial chemotherapy on spirometric parameters and pro-inflammatory indices in severe pulmonary tuberculosis. Eur. Respir. J. 1998;12:351–356. doi: 10.1183/09031936.98.12020351. [DOI] [PubMed] [Google Scholar]
- Kwan C.K., Ernst J.D. HIV and tuberculosis: a deadly human syndemic. Clin. Microbiol. Rev. 2011;24:351–376. doi: 10.1128/CMR.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post F.A., Wood R., Pillay G.P. Pulmonary tuberculosis in HIV infection: radiographic appearance is related to CD4 + T-lymphocyte count. Tuber. Lung Dis. 1995;76:518–521. doi: 10.1016/0962-8479(95)90527-8. [DOI] [PubMed] [Google Scholar]
- Flynn J.L., Chan J., Triebold K.J., Dalton D.K., Stewart T.A., Bloom B.R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluger N.W., Perez D., Liu Y.M. Reconstitution of immune responses to tuberculosis in patients with HIV infection who receive antiretroviral therapy. Chest. 2002;122:597–602. doi: 10.1378/chest.122.2.597. [DOI] [PubMed] [Google Scholar]
- Greenlee K.J., Werb Z., Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol. Rev. 2007;87:69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong C.W., Elkington P.T., Friedland J.S. Tuberculosis, pulmonary cavitation, and matrix metalloproteinases. Am. J. Respir. Crit. Care Med. 2014;190:9–18. doi: 10.1164/rccm.201311-2106PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkington P., Shiomi T., Breen R. MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J. Clin. Invest. 2011;121:1827–1833. doi: 10.1172/JCI45666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker N.F., Clark S.O., Oni T. Doxycycline and HIV infection suppress tuberculosis-induced matrix metalloproteinases. Am. J. Respir. Crit. Care Med. 2012;185:989–997. doi: 10.1164/rccm.201110-1769OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdool Karim S.S., Naidoo K., Grobler A. Integration of antiretroviral therapy with tuberculosis treatment. N. Engl. J. Med. 2011;365:1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdool Karim S.S., Naidoo K., Grobler A. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N. Engl. J. Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M.P., Kannass M., Huang L., Sciurba F.C., Morris A. Respiratory symptoms and airway obstruction in HIV-infected subjects in the HAART era. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintjes G., Lawn S.D., Scano F. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect. Dis. 2008;8:516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn S.D., Wainwright H., Orrell C. Fatal unmasking tuberculosis immune reconstitution disease with bronchiolitis obliterans organizing pneumonia: the role of macrophages. AIDS. 2009;23:143–145. doi: 10.1097/QAD.0b013e32831d2a98. [DOI] [PubMed] [Google Scholar]
- Tadokera R., Meintjes G.A., Wilkinson K.A. Matrix metalloproteinases and tissue damage in HIV-tuberculosis immune reconstitution inflammatory syndrome. Eur. J. Immunol. 2014;44:127–136. doi: 10.1002/eji.201343593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravimohan S., Tamuhla N., Steenhoff A.P. Early immunologic failure is associated with early mortality among advanced HIV-infected adults initiating antiretroviral therapy with active tuberculosis. J. Infect. Dis. 2013;208:1784–1793. doi: 10.1093/infdis/jit368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravimohan S., Tamuhla N., Steenhoff A.P. Immunological profiling of tuberculosis-associated immune reconstitution inflammatory syndrome and non-immune reconstitution inflammatory syndrome death in HIV-infected adults with pulmonary tuberculosis starting antiretroviral therapy: a prospective observational cohort study. Lancet Infect. Dis. 2015;15:p429–p438. doi: 10.1016/S1473-3099(15)70008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkington P.T., Emerson J.E., Lopez-Pascua L.D. Mycobacterium tuberculosis up-regulates matrix metalloproteinase-1 secretion from human airway epithelial cells via a p38 MAPK switch. J. Immunol. 2005;175:5333–5340. doi: 10.4049/jimmunol.175.8.5333. [DOI] [PubMed] [Google Scholar]
- Elkington P.T., Friedland J.S. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006;61:259–266. doi: 10.1136/thx.2005.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong C.W., Elkington P.T., Brilha S. Neutrophil-derived MMP-8 drives AMPK-dependent matrix destruction in human pulmonary tuberculosis. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1004917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravimohan S., Tamuhla N., Nfanyana K. Robust reconstitution of TB-specific polyfunctional CD4+ T-cell responses and rising systemic IL-6 in paradoxical TB-IRIS. Clinical infectious diseases : an official publicationof the Infectious Diseases Society of America. 2015 doi: 10.1093/cid/civ978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P.M., Komarow L., Andersen J. Risk factor analyses for immune reconstitution inflammatory syndrome in a randomized study of early vs. deferred ART during an opportunistic infection. PloS one. 2010;5 doi: 10.1371/journal.pone.0011416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Thoracic Society Standardization of spirometry, 1994 update. Am. J. Respir. Crit. Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society Lung function testing: selection of reference values and interpretative strategies. Am. Rev. Respir. Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- Pellegrino R., Viegi G., Brusasco V. Interpretative strategies for lung function tests. Eur. Respir. J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- Quanjer P.H., Tammeling G.J., Cotes J.E. Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur. Respir. J. Suppl. 1993;16:5–40. [PubMed] [Google Scholar]
- White N., Ehrlich R., Rees D. A guide to spirometry as applied to occupational health. S. Afr. Med. J. 1996;86:807–813. [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser. 1995;B 57:289–300. [Google Scholar]
- Salgame P. MMPs in tuberculosis: granuloma creators and tissue destroyers. J. Clin. Invest. 2011;121:1686–1688. doi: 10.1172/JCI57423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta N.K., Karakousis P.C. Latent tuberculosis infection: myths, models, and molecular mechanisms. Microbiol. Mol. Biol. Rev. 2014;78:343–371. doi: 10.1128/MMBR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo K., Yende-Zuma N., Padayatchi N. The immune reconstitution inflammatory syndrome after antiretroviral therapy initiation in patients with tuberculosis: findings from the SAPiT trial. Ann. Intern. Med. 2012;157:313–324. doi: 10.7326/0003-4819-157-5-201209040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetkemeyer A.F., Kendall M.A., Nyirenda M. Tuberculosis immune reconstitution inflammatory syndrome in A5221 STRIDE: timing, severity, and implications for HIV-TB programs. J. Acquir. Immune Defic. Syndr. 2014;65:423–428. doi: 10.1097/QAI.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoheisel G., Sack U., Hui D.S. Occurrence of matrix metalloproteinases and tissue inhibitors of metalloproteinases in tuberculous pleuritis. Tuberculosis (Edinb.) 2001;81:203–209. doi: 10.1054/tube.2000.0276. [DOI] [PubMed] [Google Scholar]
- Singh S., Kubler A., Singh U.K. Antimycobacterial drugs modulate immunopathogenic matrix metalloproteinases in a cellular model of pulmonary tuberculosis. Antimicrob. Agents Chemother. 2014;58:4657–4665. doi: 10.1128/AAC.02141-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty K.A., Hibbs M.S., Kang A.H., Mainardi C.L. Secreted forms of human neutrophil collagenase. J. Biol. Chem. 1986;261:5645–5650. [PubMed] [Google Scholar]
- Parks W.C., Wilson C.L., Lopez-Boado Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Ugarte-Gil C.A., Elkington P., Gilman R.H. Induced sputum MMP-1, -3 & -8 concentrations during treatment of tuberculosis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber D.L., Mayer-Barber K.D., Antonelli L.R. Th1-driven immune reconstitution disease in Mycobacterium avium-infected mice. Blood. 2010;116:3485–3493. doi: 10.1182/blood-2010-05-286336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais S., Wilkinson K.A., Lesosky M. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. Vol. 59. 2014. Neutrophil-associated central nervous system inflammation in tuberculous meningitis immune reconstitution inflammatory syndrome; pp. 1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Gong J., Iyer D.V. T cell cytokine responses in persons with tuberculosis and human immunodeficiency virus infection. J. Clin. Invest. 1994;94:2435–2442. doi: 10.1172/JCI117611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law K.F., Jagirdar J., Weiden M.D. Tuberculosis in HIV-positive patients: cellular response and immune activation in the lung. Am. J. Respir. Crit. Care Med. 1996;153:1377–1384. doi: 10.1164/ajrccm.153.4.8616569. [DOI] [PubMed] [Google Scholar]
- Eum S.Y., Kong J.H., Hong M.S. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest. 2010;137:122–128. doi: 10.1378/chest.09-0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg R. CD4 + T cells are required for antigen-specific recruitment of neutrophils by BCG-immune spleen cells. Immunology. 1992;75:414–419. [PMC free article] [PubMed] [Google Scholar]
- Zhang J.H., Ferrante A., Arrigo A.P., Dayer J.M. Neutrophil stimulation and priming by direct contact with activated human T lymphocytes. J. Immunol. 1992;148:177–181. [PubMed] [Google Scholar]
- Marais S., Meintjes G., Pepper D.J. Clinical infectious diseases: An Official Publication of the Infectious Diseases Society of America. Vol. 56. 2013. Frequency, severity, and prediction of tuberculous meningitis immune reconstitution inflammatory syndrome; pp. 450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S.J., Peppin G., Ortiz X. Oxidative autoactivation of latent collagenase by human neutrophils. Science. 1985;227:747–749. doi: 10.1126/science.2982211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material