Abstract
At three alpine locations in Switzerland adults of Drusus melanchaetes and unknown Drusinae larvae which could not be identified with existing keys were sampled. Based on DNA association with adults, we identified the unknown larvae as D. melanchaetes. To further support the association of specimens a phylogeny was estimated with the putative closest relatives of D. melanchaetes – D. monticola and D. nigrescens – and five other species of Drusus (D. chrysotus, destitutus, discolor, muelleri and romanicus). A highly supported monophyletic clade groups unknown larvae and D. melanchaetes specimens from the central Alps and Austria (Vorarlberg), confirming the association.
Based on morphology, larvae of Drusus melanchaetes key out together with D. destitutus in existing keys. D. melanchaetes is separated from the latter species by the shape of the lateral head profile which is almost straight and shows a small step at the height of the antenna, whereas in D. destitutus the lateral head profile is evenly rounded. In addition, in frontal view, the shape of the lateral head outline is straight in D. melanchaetes and rounded in D. destititus. There are also differences in the shape of the pronotum and in the number of the posterodorsal setae at the eighth abdominal dorsum.
Keywords: Trichoptera, Drusus melanchaetes, fifth instar larva, description, identification, distribution, ecology, mitochondrial DNA
Introduction
Within the international context of the European Water Framework Directive (WFD; European Union 2000) as well as in the United States of America, Trichoptera represent an essential species group in various methododical approaches of assessing the ecological quality of water bodies (e.g. AQEM consortium 2002 (EPT-metrics); Barbour, Gerritsen, Snyder & Stribling 1999; Moog 2002; Moog & Chovanec 2000; Waringer 2003). This also fully applies to the subfamily Drusinae, where all members are restricted to water quality classes I or I-II and are used as bioindicators (sensitive species) (Moog, Graf, Janecek & Ofenböck 2002; Graf, Grasser & Waringer 2002).
In order to use the full bioindication potential of this groups, identification of larvae to species level is crucial (Goethals 2002; Margreiter-Kownacka, Pechlaner, Ritter & Saxl 1984; Pitsch 1993; Schmidt-Kloiber & Nijboer 2004). However, no comprehensive and integrated effort has been made to complete the available keys to larval Drusinae from which 24 species are reported from Austria, Germany and Switzerland (Lubini & Vicentini 2005; Malicky 1999; Robert 2001, 2004). Six of them (Drusus alpinus (Meyer-Dür), D. chapmani McL., D. franzi Schmid, D. improvisus McL., D. melanchaetes McL. and D. noricus Malicky) are still unknown in the larval stage.
In 2004-2006, however, we managed to collect larvae of D. melanchaetes at Swiss high alpine locations whose identity was confirmed by genetic association with adults (Pauls 2004). This material enabled us to work out reliable diagnostic characters permitting integration of D. melanchaetes in the identification key by Waringer & Graf (1997, 2004).
Material and methods
Larvae which obviously were not yet included in existing Drusinae keys were collected at three high alpine locations in Switzerland where the dominant adult was D. melanchaetes. To support conspecific association between larval and adult specimens we sequenced and analysed a 498bp long fragment of the mitochondrial cytochrome oxidase I gene (mtCOI) of three larvae and three adults from four localities (Table 1) following the methods outlined in Pauls (2004) and Pauls, Lumbsch, & Haase (2006). We generated uncorrected pairwise distances between individuals using the DNADist function as implemented in BioEdit 7.0.5.3 (Hall 1999). To further support the association of specimens we estimated a phylogeny using new and previously published sequence data of the putative closest relatives of D. melanchaetes – D. monticola and D. nigrescens (Pauls 2004) – and five other species of Drusus (D. chrysotus, D. destitutus, D. discolor, D. muelleri and D. romanicus). The Phylogeny was estimated using a Bayesian approach. Bayesian Markov chain Monte Carlo (B/MCMC) analysis was performed using the program MrBayes 3.1.2 (Huelsenbeck, Ronquist, Nielsen & Bollback 2001) assuming the GTR + I + G model. Two parallel MCMC samplings were performed with 4 simultaneous chains each for 5 million generations. Trees were sampled every 1000th generation for a total of 5000 trees from each sampling run. Log likelihood scores of samples were plotted against generation time using Tracer 1.3 (http://evolve.zoo.ox.ac.uk/software.html?id=tracer) to determine stationarity (Huelsenbeck, Ronquist, Nielsen & Bollback 2001). The initial 2500 trees of each run were discarded as ‘burn-in’. The phylogenetic tree was drawn in TreeView 1.6.6 (Page 1996).
Table 1.
Material used in this study. Sequences were generated in A this study; B Waringer et al. 2007, or C Graf et al. 2005
| Drusus | Stage* | Locality | GenBank Accession |
|---|---|---|---|
| melanchaetes | M | CH, Meienreuss, East of Sustenpass, 16.07.2004 | EU143740A |
| F | CH, Sidelenbach, 16.07.2004 | EF464555B | |
| L° | CH, Meienreuss, East of Sustenpass, 16.07.2004 | EU143742A | |
| L° | CH, Meienreuss, East of Sustenpass, 16.07.2004 | EU143743A | |
| L° | CH, Mutt and left tributaries, 17.07.2004 | EU143744A | |
| M | AT, Vorarlberg, Klostertaler Bach, 27.07.1999 | EU143741A | |
| chrysotus | L | AT, Soboth, Krumbachquelle, 18.05.2002 | AY954395C |
| M | AT, Saualpe, Quellbäche bei Ladinger Hütte, 30.06.2006 | EU143739A | |
| destitutus | L | AT, Soboth, Krumbach, 18.05.2002 | EU143738A |
| discolor | L | F, Auvergne, nameless brook | DQ351158C |
| L | D, Ammer Mts., Kühbach, Soyermühle | DQ351160C | |
| L | D, Erzgebirge, Große Mittweida | DQ351162C | |
| L | D, Rothaargebirge, Hoppecke | DQ351165C | |
| L | F, Vosges Mts., La Meurthe | DQ351166C | |
| L | SK, Muranska Planina, Hronec at Patina | DQ351168C | |
| L | F, Pyrenees, Pec de Moli | DQ351183C | |
| M | CH, Jura Mts., Bouvier | DQ351189C | |
| L | F, Alpes maritimes, Via Ferrate l’Aguilette | DQ351193C | |
| M | RO, Rodna Mts., Lala Valley | DQ351199C | |
| M | BG, Rila Mts., Gyolska | DQ351204C | |
| M | BG, Rila Mts., Malyovishka | DQ351205C | |
| monticola | L | AT, Nockberge, St. Oswald Bach B, 01.07.2006 | EF464559B |
| F | AT, Saualpe, Ladinger Hütte, 16.06.2006 | EF464560B | |
| F | AT, Saualpe, Ladinger Hütte,16.06.2006 | EF464561B | |
| L | AT, Saualpe, Offner Hütte, 30.06.2006 | EF464558B | |
| L | AT, Soboth, Krumbach, 18.05.2002 | EF464556B | |
| L | AT, Soboth, Krumbach, 18.05.2002 | EF464557B | |
| muelleri | M | CH, Tributary to Grimselsee, Grimselpass | AY954401C |
| M | CH, Meienreuss, East of Sustenpass, 16.07.2004 | AY954400C | |
| M | CH, Mutt and left tributaries, 17.07.2004 | AY954398C | |
| nigrescens | L | CH, Furka Pass 21.7. 2006 | EF464567B |
| M | CH, Furka Pass 21.7. 2006 | EF464565B | |
| M | CH, Furka Pass 21.7. 2006 | EF464566B | |
| F | CH, Mutt and left tributaries, 17.07.2004 | EF464563B | |
| L | CH, Mutt and left tributaries, 17.07.2004 | EF464568B | |
| L | CH, Mutt and left tributaries, 17.07.2004 | EF464569B | |
| M | CH, Mutt and left tributaries, 17.07.2004 | EF464562B | |
| M | CH, Mutt and left tributaries, 17.07.2004 | EF464564B | |
| romanicus meridionalis | M | BG, Banderishka River, above Vihren Chalet, 18.08.2003 | AY954402C |
| romanicus romanicus | L | RO, Faearas Mts., Valea Buda, 07.08.2003 | AY954403C |
Larva assigned in this study
Stage: L: larva, M: male, F: female
To describe and characterise the larval morphology of D. melanchaetes and identify differentiating characters we examined three fifth instar larvae from Valetta di S. Gottardo (2270 m a.s.l.) collected by H.Vicentini on 8 September 2004; one fifth instar larva from Munt da San Franzesc, Poschiavo (2150 m a.s.l.) collected by V. Lubini on 27 June 2001; four fifth instar larvae from the Furkapaß (2386 m a.s.l.) collected by W.Graf on 13 October 2007.
Results
Genetic association
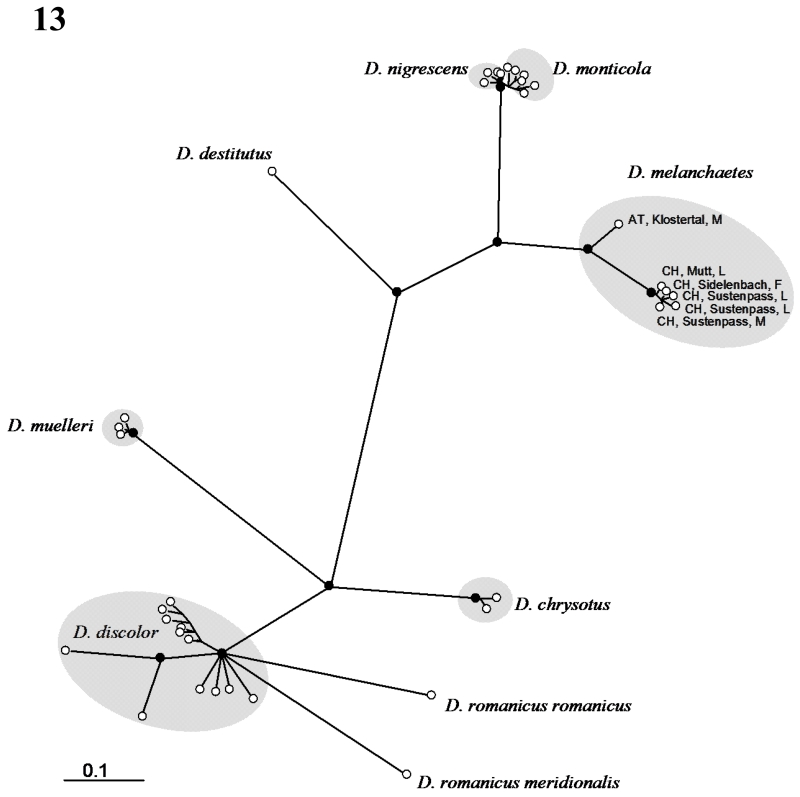
Within the central Alps, haplotypes between larvae and adults of D. melanchaetes differed by one or two base changes (p = 0.002-0.004). Maximum p within D. melanchaetes was found between a larva from the Sustenpass and an adult male from the Klostertal (p = 0.041). Minimum p between D. melanchaetes and other species was p = 0.064. The B/MCMC phylogeny of eight Drusinae species (Fig. 13) clearly shows that the unknown larvae fall within a highly supported monophyletic clade with D. melanchaetes adults from the central Alpine region (pp=1.0) and Vorarlberg (pp=0.97). D. monticola and D. nigrescens form a sister clade to D. melanchaetes (pp=1.0). The sister relationship is highly supported (pp=0.97), however the relationship between is D. monticola and nigrescens is resolved but not supported (pp=0.88). Basal to the melanchaetes-nigrescens-monticola clade is D. destitutus (pp=1.0). D. chrysotus and D. muelleri also build highly supported monospecific clades (pp=1.0). D. discolor and D. romanicus form a highly supported clade, however the relationship between these two species remains unresolved.
Fig. 13.
B/MCMC inference of haplotype phylogeny of D. melanchaetes and seven Drusus species. Unrooted 50% majority rule consensus tree based on a Bayesian sampling of 5000 trees. White circles depict terminal taxa; black circles indicate nodes with significant posterior probabilities (>0.94).
Description of the fifth instar larva of Drusus melanchaetes
The body length of final instar larvae ranges from 9.0 to 12.1 mm, the head width from 1.36 to 1.53 mm. The larval case is 8.7 – 11.8 mm in length, distinctly curved, tapering posteriorly (the width at anterior opening is 2.5 – 3.2 mm and at the posterior opening 1.6 – 1.9 mm), and consists completely of mineral particles with grain sizes increasing distinctly in anterior direction.
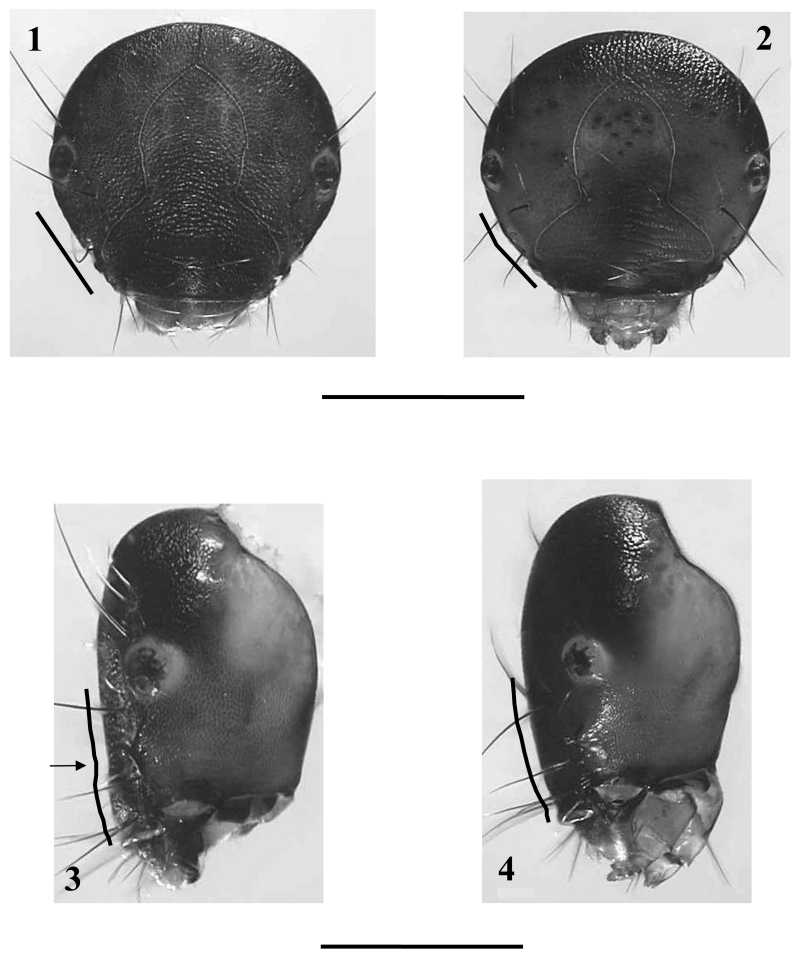
The head capsule and all body sclerites are dark brown to black brown. The head capsule (Figs. 1, 3) lacks the additional setae or spines known from other Drusinae larvae (e.g. Ecclisopteryx spp., Drusus trifidus). The mandibles lack terminal teeth along edges as well as ridges in the central concavity.
Figs.1 – 4.
Figs.1 – 2 Head, frontal view of fifth instar larvae (with frontal profile lines): 1: Drusus melanchaetes, 2: D. destitutus. Figs. 3 - 4: Head, left lateral view of fifth instar larvae (with lateral profile lines; arrow: step in profile): 3: D. melanchaetes, 4: D. destitutus. Scale bars: 1mm.
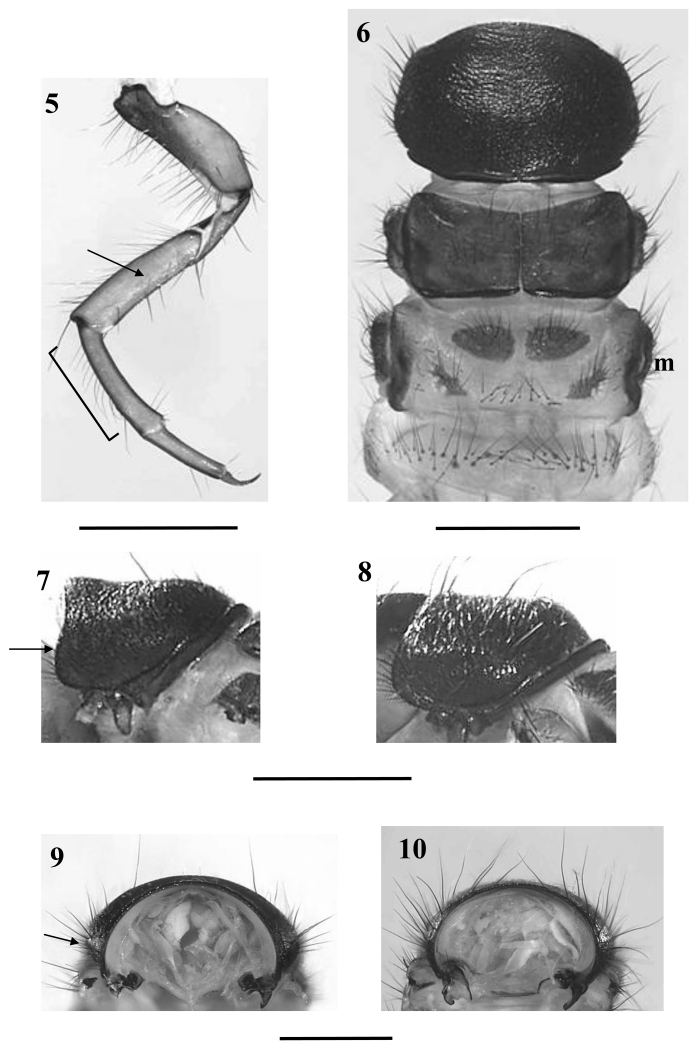
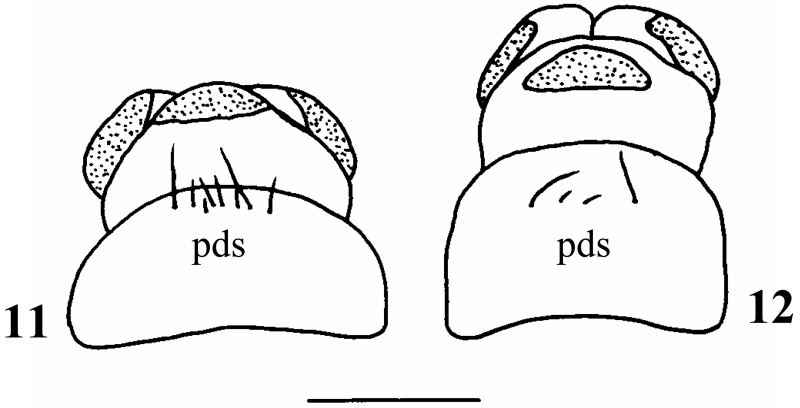
In profile, the dorsal line of the pronotum is evenly rounded, thereby creating a small dorsal hump in its posterior third (Fig. 7). In lateral and even more so in frontal view a ventrolateral bulge is clearly visible (Figs. 7, 9). The black brown pronotal surface is covered by prostrate, tiny white setae which are distinctly shorter and scarcer than in D. destitutus; in addition, larger black setae along the lateral and anterior borders are present. The prosternite is inconspicuous and a prosternal horn is present. The mesonotum is completely covered by two chestnut-brown sclerites. The metanotum is partially covered by three pairs of sclerites with the anterior metanotal sclerites being large and ovoidal; their median separation is distinctly smaller than their maximum extension along the body axis (Fig. 6). The setal bases at the central section of the first abdominal sternum are mostly small and inconspicuous except two larger bases near the midline which occasionally fuse with neighbouring smaller setal bases. However, a large sclerotized central plate as it is common in genus Metanoea (Waringer & Graf 1997, 2004; Waringer, Graf & Maier 2000) or in Drusus nigrescens (Waringer, Graf, Pauls & Lubini 2007) is lacking. At the eighth abdominal dorsum, the number of posterodorsal setae (pds) is 2-4, consisting of 2 long and 0-2 short setae (Fig. 12).
Figs. 5 - 10.
Figs. 5 - 8 Drusus melanchaetes, fifth instar larva; 5: left hindleg, anterior view; arrow=additional setae on face of femur; 6: thorax and first abdominal segment, dorsal view; m=metanotum; Fig. 7: pronotum, left lateral view; arrow: lateral bulge; Fig. 8: Drusus destitutus, pronotum, left lateral view; Fig. 9: D. melanchaetes, fifth instar larva: pronotum, frontal view; arrow: lateral bulge; Fig. 10: D. destitutus, pronotum, frontal view. Scale bars: 1mm.
Figs. 11 - 12.
Dorsal view of eighth and ninth abdominal dorsum of 11: Drusus destitutus, 12: D. melanchaetes. Setae are not shown; sclerites indicated by dotted areas; pds= posterodorsal setae at eighth abdominal dorsum. Scale bar: 1 mm.
Dorsal gills are present from the second (presegmental position) to the sixth (presegmental position). Ventral gills range from second (presegmental) to seventh segment (presegmental). Lateral gills are present on the second and third segment (postsegmental position). The lateral fringe is present on the last third of the second to the beginning of the eighth abdominal segment. Setae are present at the anterior and posterior faces of all femora. The row of dorsal setae at the mid- and hindleg tibiae extend over the whole length of the segment (Fig. 5).
Morphological separation of Drusus melanchaetes from other European Trichoptera
A summary of morphological features for the identification of limnephilid and Drusinae larvae is given in Waringer (1985). Within the framework of the limnephilid key by Waringer & Graf (1997, 2004), Drusus melanchaetes is separated from other species by the following features:
-
-
metanotum covered by three pairs of small sclerites (Fig. 6, m);
-
-
head and pronotum without a thick layer of woolly hairs (Fig. 1);
-
-
head capsule without groups of additional spines, without central concavity and rims surrounding the frontoclypeus (Fig. 1);
-
-
first abdominal sternum without a large median sclerotized patch;
-
-
pronotum without ridge; in profile, dorsal outline evenly rounded in its posterior third, thereby creating a small dorsal hump (Fig. 7);
-
-
Mandibles lacking terminal teeth along edges as well as ridges in the central concavity;
-
-
Middle and hindleg femora faces with additional setae (Fig. 5, arrow);
-
-
Anteromedian metanotal sclerites large, ovoidal, their median separation being distinctly smaller than their maximum extension along the body axis (Fig. 6);
-
-
Row of setae at anterior border of pronotum extending as far as the pronotal midline;
-
-
Row of dorsal setae at mid- and hind tibiae extending over the whole length of the segment (Fig. 5).
At this position Drusus melanchaetes keys out together with Drusus destitutus. The species are readily separated by the shape of the lateral head profile: in D. melanchaetes this profile from the anterior border of the frontoclypeus to the eyes is almost straight and shows a small step at the height of the antenna (Fig. 3, arrow), whereas in D. destitutus the lateral head profile is evenly rounded (Fig. 4). In addition, in frontal view, the shape of the lateral head outline between eyes and anterior border of the frontoclypeus is straight in D. melanchaetes (Fig. 1) and bent in D. destititus (Fig. 2). In D. melanchaetes, the pronotum has a distinct ventro-lateral bulge most easily seen in frontal view (Figs. 7, 9) which is lacking in D. destitutus (Figs. 8, 10). The number of posterodorsal setae (pds) at the eighth abdominal dorsum is 4-8 in D. destitutus (2 long, 2 (very rare) -6 short setae) and 2-4 in D. melanchaetes (2 long, 0-2 short setae) (Figs. 11, 12). In D. destitutus the pronotum is covered by prostrate white setae (Graf 1993), which are much shorter and also scarcer in D. melanchaetes.
Phenology, habitat, and distribution
Last instar larvae of D. melanchaetes were collected on 27 June 2001 at the Munt da San Franzesc, Poschiavo (2150 m a.s.l.), on 8 September 2004 at the Valetta di S. Gottardo (2270 m a.s.l.) and on 13 October 2006 at the Furkapaß (2386 m a.s.l.). D. melanchaetes is known to be on the wing from April, in higher altitudes from June to August (Schmid 1956; Graf, unpublished data). Observing last instar larvae late in the summer and early autumn suggests the species survives winter in a late larval stage, or that the species might have a semivoltine life cycle. This could result from the short growth period in high altitudes. In a study in the high altitudes of the Pyrenees, Lavandier (1992) measured head capsule widths to reconstruct the larval growth cycle of Drusus discolor and observed a two year development.
At the Furkapaß numerous adults of Allogamus mendax and Consorophylax consors as well as larvae of D. muelleri, D. nigrescens and Rhyacophila intermedia were found in October. At this location, the small, spring-fed, 50 m long, first order tributary is part of the Mutt watershed; it is a clean, fast-flowing, summer-cold mountain brook bordered by meadows. At this location, D. melanchaetes was sympatric with D. muelleri, Lithax niger and Plecoptera such as Dyctiogenus fontium, Protonemura lateralis, Leuctra ravizza, L. rosinae, Nemoura mortoni and N. sinuata. The larvae of D. melanchaetes are found also in small brooklets, fed only by snowmelt water and therefore are dry in summer; one location is an outlet of a high alpine lake. Ephemeroptera species found together at these localities with D. melanchaetes are Rhithrogena loyolaea, Baetis alpinus and Ecdyonurus helveticus.
According to Malicky (2004), D. melanchaetes is a west-alpine species; records exist from Switzerland (Lubini-Ferlin & Vicentini 2005), Italy (Piemonte, Valle d’Aosta, Lombardia and Trentino – Alto Adige; Cianficconi 2002) and France (Barnard 2005) but the species is lacking in Germany (Robert 2001, 2004). In Austria, D. melanchaetes is reported from Carinthia, the Tyrol and Vorarlberg (Malicky 1999). The altitudinal range in Switzerland is between 1960 m and 2560 m a.s.l..
Discussion
According to Schmid (1956) D. melanchaetes is an isolated species within subfamily Drusinae. The species is characterised by its black or black-brown colour, its large wings and the large lower appendices which are dinstinctly pointed upwards. The superior and intermediate appendices are medium-sized with intermediate appendices being pointed and divergent. Schmid (1956) considers D. melanchaetes an isolated species intermediate between the mixtus group (D. mixtus, D. biguttatus, D. improvisus, D. spelaeus, D. brunneus, D. trifidus, D. bolivari) and Drusus cantabricus, another isolated species which, in turn, is adjacent to the annulatus group (D. annulatus, D. rectus, D. tenellus, D. simplex). Since we are lacking the closest relatives according to Schmid (1956), we cannot explicitly test his hypotheses with the current data set. However, in a preliminary phylogeny of the group (Pauls 2004, Pauls in prep.), D. melanchaetes is situated close to the species pair D. monticola – D. nigrescens and D. destitutus. We also observed this close relationship in our morphological and genetic analyses (Fig. 13). In the larval stage, these species and their immediate relatives are characterised by spoon-shaped mandibles without teeth along the anterior edges,which identifies them as scrapers feeding mainly on epilithic algae. Generally, epilithic algal growth is much higher at lotic stream sections and midstream than in lenitic sections or near the banks (Gessner 1955). This is why scraper species within the subfamily Drusinae are forced to expose themselves much more during feeding than omnivorous generalists feeding near the banks. In addition, in order to feed effectively, last and penultimate instars of scraping Drusinae species do not fix their cases at the substrate as the filter-feeding Drusinae do. This results in a significant over-represention of scraper Drusinae species in the drift when compared with their relative abundance on the stream bed (Bacher & Waringer 1996). The grouping of D. melanchaetes and the other species of epilithic grazers with smooth mandible edges were significantly different from the group of carnivorous filtering larvae with serrated mandible edges and filtering setae and bristles, such as D. muelleri, D. chrysotus, D. discolour and D. romanicus (Fig. 13). The groupings observed in our genetic analyses reflect, therefore, mouthpart morphology and feeding ecology very well.
The current study presents the third example of a larval description based on molecular association with adult caddisflies of the Drusinae (e.g. Graf, Lubini & Pauls 2005; Waringer, Graf, Pauls & Lubini 2007). Molecular associations between sexes or life stages are becoming more commonplace in caddisflies (Shan,Yang & Wang 2004) and other insect groups (e.g. Miller, Alarie, Wolfe & Whiting 2005; Willassen 2005), exemplifying how nucleotide sequence markers can facilitate and provide supportive evidence in taxonomic and systematic research. mtCOI is often propagated as a suitable gene for “barcoding” studies (Hebert, Cywinska, Ball & deWaard 2003). Our study shows that his region is indeed suitable for designating and delimiting species, but also shows the markers’ limits for DNA based-taxonomy concerning extremely closely related species, especially if lineage sorting is still incomplete (Pamilo & Nei 1988, Morando, Avila, Baker & Sites 2004). In our study this appears to be the case between the closely related species pairs D. discolor - D. romanicus and D. nigrescens - D. monticola. The use of a single mitochondrial gene region as proposed for “DNA barcoding” may not be sufficient to resolve such situations, and the choice of the marker of utmost importance (Mueller 2006).
Summary
To support species affiliation between known adults and unknown larvae, specimens were genetically analysed by means of DNA nucleotide sequence analysis of a 498bp long fragment of the mitochondrial cytochrome oxidase I gene (mtCOI). To further elucidate the association of specimens a phylogeny was estimated with the putative closest relatives of D. melanchaetes (D. monticola and D. nigrescens) and four other species of Drusus (D. chrysotus, discolor, muelleri and romanicus), yielding a highly supported monophyletic clade with D. melanchaetes.
Based on larval morphology, D. melanchaetes is separated from the very similar D. destitutus by the shape of the lateral head profile, the shape of the lateral head outline and differences in the shape of the pronotum and in the number of posterodorsal setae at the eighth abdominal dorsum.
Acknowledgments
We wish to thank Mag. Philipp Wenzl for his assistance and Dr. W. Lechtaler for providing the photographs. DI Anne Bloch and DI Thomas Huber helped with some taxonomical remarks. This paper is part of the outcomes of a project dealing with larval taxonomy of Central European Drusinae (project number P18073-B03, PI: J.Waringer) funded by the Austrian Science Fund (FWF).
References
- AQEM consortium Manual for the application of the AQEM system. A comprehensive method to assess European streams using benthic macroinvertebrates, developed for the purpose of the Water Framework Directive. Version 1.0. 2002 www.aqem.de.
- Bacher I, Waringer J. Hydraulic microdistribution of cased caddis larvae in an Austrian mountain brook. International Revue of Hydrobiology. 1996;81:541–554. [Google Scholar]
- Barbour MT, Gerritsen J, Snyder BD, Stribling JB. Rapid bioassessment protocols for use in wadeable streams and rivers: periphyton, benthic macroinvertebrates and fish 2nd Ed. EPA 841-B-99-002. U.S. Environmental Protection Agency, Office of Water; Washington, D.C. 1999. [Google Scholar]
- Barnard P. Fauna Europaea: Trichoptera. Fauna Europaea version 1.1. 2005 http://www.faunaeur.org.
- Cianficconi F. The third list of Italian Trichoptera (1990-2000); Proceedings of the 10th International Symposium on Trichoptera; 2002.Nova Supplementa Entomologica; pp. 349–358. [Google Scholar]
- European Union . Directive 2000/60/EC. Establishing a framework for community action in the field of water policy. Luxemburg: 2000. PE-CONS 3639/1/100 Rev 1. [Google Scholar]
- Gessner F. In: Hydrobotanik. Die physiologischen Grundlagen der Pflanzenverbreitung im Wasser. I. Energiehaushalt. Hochschulbücher für Biologie. Borriss H, Gersch M, editors. VEB Deutscher Verlag der Wissenschaften; Berlin: 1955. Band 3. [Google Scholar]
- Goethals PLM, editor. Data collection concerning macrozoobenthos. European Aquatic Modelling network (EAMN); 2002. COST Action 626. [Google Scholar]
- Graf W. Beschreibung der Larven von Rhyacophila producta und Rhyacophila stigmatica und einer Larve aus der Unterfamilie Drusinae (Trichoptera: Rhyacophilidae, Limnephilidae) Braueria. 1993;20:17–18. [Google Scholar]
- Graf W, Grasser U, Waringer J. Trichoptera.- Teile IIIA, IIIB, IIIC, IIID. In: Moog O, editor. Fauna Aquatica Austriaca. second edition Wasserwirtschaftskataster, Bundesministerium für Land- und Forstwirtschaft; Wien: 2002. p. 41. [Google Scholar]
- Graf W, Lubini V, Pauls S. Larval description of Drusus muelleri McLachlan, 1868 (Trichoptera: Limnephilidae) with some notes on its ecology and systematic position within the genus Drusus. Annales de Limnologie. 2005;41:93–98. [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Hebert PDN, Cywinska A, Ball SL, deWaard JR. Biological identifications through DNA barcodes. Proc.R.Soc.Lond.B. 2003;270:313–321. doi: 10.1098/rspb.2002.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F, Nielsen R, Bollback JP. MrBayes: Bayesian inference of phylogenetic trees. Science. 2001;294:2310–2314. doi: 10.1126/science.1065889. [DOI] [PubMed] [Google Scholar]
- Lavandier P. Larval production and drift of Drusus discolor (Trichopera: Limnephilidae) in a high mountain stream in the Pyrenees (France) Archiv für Hydrobiologie. 1992;125:83–96. [Google Scholar]
- Lubini-Ferlin V, Vicentini H. To the knowledge of the Swiss caddis fly fauna (Insecta: Trichoptera) Lauterbornia. 2005;54:63–79. [Google Scholar]
- Malicky H. Eine aktualisierte Liste der österreichischen Köcherfliegen (Trichoptera) Braueria. 1999;26:31–40. [Google Scholar]
- Malicky H. Atlas of European Trichoptera. Second edition Springer; 2004. [Google Scholar]
- Margreiter-Kownacka M, Pechlaner R, Ritter H, Saxl R. Die Bodenfauna als Indikator für den Saprobitätsgrad von Fließgewässern in Tirol. Ber. nat.- med. Verein Innsbruck. 1984;71:119–135. [Google Scholar]
- Miller KB, Alarie Y, Wolfe GW, Whiting MF. Association of insect life stages using DNA sequences: the larvae of Philodytes umbrinus (Motschulsky) (Coleoptera: Dytiscidae) Systematic Entomology. 2005;30:499–509. [Google Scholar]
- Moog O, editor. Fauna Aquatica Austriaca. second edition Wasserwirtschaftskataster, Bundesministerium für Land-und Forstwirtschaft; Wien: 2002. [Google Scholar]
- Moog O, Graf W, Janecek B, Ofenböck T. Inventory of “Sensitive Taxa” of Austrian Rivers and Streams. A valuable measure among the multimetric approaches and a tool for developing a rapid field screening method to assess the ecological status of rivers and streams in Austria. In: Moog O, editor. Fauna Aquatica Austriaca. second edition Wasserwirtschaftskataster, Bundesministerium für Land-und Forstwirtschaft; Wien: 2002. [Google Scholar]
- Moog O, Chovanec A. Assessing the ecological integrity of aquatic environment: Walking the line between ecological, political and administrative interests. Hydrobiologia. 2000;422/423:99–109. [Google Scholar]
- Morando M, Avila LJ, Baker J, Sites JW. Phylogeny and phylogeography of the Liolaemus darwinii complex (Squamata: Liolaemidae): Evidence for introgression and incomplete lineage sorting. Evolution. 2004;58:842–861. doi: 10.1111/j.0014-3820.2004.tb00416.x. [DOI] [PubMed] [Google Scholar]
- Mueller RL. Evolutionary Rates, Divergence Dates, and the Performance of Mitochondrial Genes in Bayesian Phylogenetic Analysis. Systematic Biology. 2006;55:289–300. doi: 10.1080/10635150500541672. [DOI] [PubMed] [Google Scholar]
- Page RDM. Treeview: an application to display phylogenetic trees on personal computers. Computer Applications in the Biosciences. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Pamilo P, Nei M. Relationships between gene trees and species trees. Molecular Biology and Evolution. 1988;5:569–583. doi: 10.1093/oxfordjournals.molbev.a040517. [DOI] [PubMed] [Google Scholar]
- Pauls S. Phylogeny and phylogeography of the montane caddis fly Drusus discolor (Rambur, 1842) (Trichoptera: Limnephilidae, Drusinae) University of Duisburg-Essen; 2004. PhD-Thesis. [Google Scholar]
- Pauls SU, Lumbsch HT, Haase P. Phylogeography of the montane caddisfly Drusus discolor: evidence for multiple refugia and periglacial survival. Molecular Ecology. 2006;15:2153–2169. doi: 10.1111/j.1365-294X.2006.02916.x. [DOI] [PubMed] [Google Scholar]
- Pitsch T. Zur Larvaltaxonomie, Faunistik und Ökologie mitteleuropäischer Fließwasser-Köcherfliegen (Insecta: Trichoptera). 1999. Schriftenreihe des Fachbereichs Landschaftsentwicklung, Sonderheft S 81. [Google Scholar]
- Robert B. Verzeichnis der Köcherfliegen (Trichoptera) Deutschlands. Die Köcherfliegen-Fauna Deutschlands: Ein kommentiertes Verzeichnis mit Verbreitungsangaben. Entomologische Nachrichten und Berichte (Dresden), Beiheft. 2001;6:107–151. [Google Scholar]
- Robert B. Systematisches Verzeichnis der Köcherfliegen (Trichoptera) Deutschlands, Fortschreibung 02/2004. Entomologie heute. 2004;16:93–107. [Google Scholar]
- Schmidt-Kloiber A, Nijboer R. The effect of taxonomic resolution on the assessment of ecological water quality classes. Hydrobiologia. 2004;516:269–283. [Google Scholar]
- Shan L, Yang L, Wang B. Association of larval and adult stages of ecologically important caddisfly (Insecta: Trichoptera) Zoological Research. 2004;45:351–355. [Google Scholar]
- Waringer J. The larva of Metanoea rhaetica Schmid, 1955 (Trichoptera: Limnephilidae: Drusinae) from a small Austrian mountain brook. Aquatic Insects. 1985;7:243–248. [Google Scholar]
- Waringer J, Graf W. Atlas der Österreichischen Köcherfliegenlarven. Facultas Univeritätsverlag; Wien: 1997. [Google Scholar]
- Waringer J, Graf W, Maier K-J. The larva of Metanoea flavipennis Pictet, 1834 (Trichoptera: Limnephilidae: Dusinae) Aquatic Insects. 2000;22:66–70. [Google Scholar]
- Waringer J. Light-trapping of caddisflies at the Thaya (Lower Austria), a river influenced by pulsating hypolimnic water release. International Review of Hydrobiology. 2003;88:139–153. [Google Scholar]
- Waringer J, Graf W. Ergänzungen und Berichtigungen zum „Atlas der österreichischen Köcherfliegenlarven unter Einschluß der angrenzenden Gebiete“. Beilage zum 2. unveränderten Nachdruck. Facultas Universitätsverlag; Wien: 2004. [Google Scholar]
- Waringer J, Graf W, Pauls S, Lubini V. The Larva of Drusus nigrescens Meyer-Dür, 1875 (Trichoptera: Limnephilidae: Drusinae) with notes on its ecology, genetic differentiation and systematic position. Annales de Limnologie. 2007 doi: 10.1051/limn:2007010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willassen E. New species of Diamesa (Diptera: Chironomidae) from Tibet: conspecific males and females associated with mitochondrial DNA. Zootaxa. 2005;1049:19–32. [Google Scholar]