Abstract
Objective
IGF1 regulates differentiation and growth of tissues and reduces stress and injury. IGF1 also in a tissue specific manner modulates the differentiation and lipid storage capacity of adipocytes in vitro, but its roles in adipose tissue development and response to stress are not known.
Methods
To study IGF1 in vivo, we identified the cellular sources of adipose tissue Igf1 expression and generated mice with targeted deletion in adipocytes and macrophages. We studied the effects of adipocyte and macrophage deficiency of IGF1 on adipose tissue development, and the response to a chronic (high fat feeding) and acute (cold challenge) stress.
Results
The expression of Igf1 by adipose tissue is derived from multiple cell types including adipocytes and macrophages. In lean animals, adipocytes are the primary source of IGF1 but in obesity expression by adipocytes is reduced and by macrophages increased, so as to maintain overall adipose tissue Igf1 expression. Genetic deletion studies reveal that adipocyte-derived IGF1 regulates perigonadal but not subcutaneous adipose tissue mass during high fat feeding and the development of obesity. Conversely, macrophage-derived IGF1 acutely modulates PGAT (PGAT) mass during thermogenic challenges.
Conclusions
Local IGF1 is not required in lean adipose tissue development but required to maintain homeostasis during both chronic and acute metabolic stresses.
Introduction
IGF1 is an anabolic factor that promotes differentiation, growth and survival during development and following injury or stress (1). In mammals homozygous null mutations of Igf1 cause severe intrauterine growth retardation, developmental defects and perinatal mortality (2–4). While IGF1 is detectable in the circulation of mammals, its primary actions are paracrine. Hence, genetic deletion of hepatic IGF1 (the primary source of circulating IGF1) reduces blood concentrations by ~ 75% but causes no gross developmental or metabolic abnormalities (5, 6).
IGF1 signaling has been implicated in the differentiation and metabolic regulation of adipocytes (7–10). In the absence of IGF1, pre-adipocytes differentiation is prevented in vitro; this can be overcome by addition of insulin, which at sufficient concentration will activate the IGF1 receptor (IGF1R) (7). During the processes of differentiation, canonical insulin signaling is required for the initial proliferation of preadipocytes and the terminal differentiation (8). Deletion of both the IGF1R and the insulin receptor (IR) in adipocytes leads to a severe lipodystrophy (11). In addition to its development roles, IGF1 also can regulate adipocyte metabolism, suppressing lipolysis in a manner analogous to insulin (12). While IGF1 can powerfully regulate adipocytes in vitro (7), its in vivo source and function are not known. Targeted deletion of IGF1R suggests that adipocytes produce IGF1 in an autocrine fashion and in a complex regulatory loop (10). Although the receptors of insulin and IGF1 are distinct, the ability of each to bind the other’s receptor (albeit at lower affinity) and common downstream signaling molecules make for partially redundant and overlapping functions (11, 13). In pathological states of overproduction, cross-activation of receptors leads to predicted phenotypes; in patients with uncontrolled gestational diabetes, hyperinsulinemia leads to macrosomy (14) consistent with activation of the IGF1 receptor in a developing fetus, and in patients with tumors that secrete IGF1, hypoglycemia is common (15). While this complex biology makes functionally isolating the role of each molecule difficult, it is important to begin to define the role of local versus systemic insulin/IGF1 action.
In addition to its role as a developmental factor, IGF1 is important in responses to stress and injury. IGF1 plays a critical role in the responses of muscle and bone to physical stress and is required for myocyte hypertrophy, osteoblast survival and elaboration of bone in response to tissue damage (16, 17). Recently it has been also recognized that immune cells, in particular macrophages, produce IGF1 and contribute to local tissue homeostasis (18–20). In muscle injury, IGF1 is required for the proliferation of satellite cells, myocyte precursors necessary for repair (21). Similarly, in an ischemic brain injury, microglia-derived IGF1 promotes neuronal survival (22).
The role of local IGF1 in adipose tissue development and function is unknown, but we hypothesized that local IGF1 promotes the differentiation and survival of adipocytes, and the accumulation of lipid. Given that obesity is associated with adipocyte stress and death, we predicted that local production of IGF1 in adipose tissue would play a critical role in the response to the development of obesity. Furthermore, given that with the onset of obesity macrophages accumulate in adipose tissue we speculated that adipose tissue macrophages may provide a critical source of IGF1 to maintain adipocyte hypertrophy and hyperplasia.
To understand the role of local IGF1 in adipose tissue we identified cells that express Igf1 within adipose tissue, determined the regulation of Igf1 expression during the development of obesity, and defined the effects of genetic deletion of Igf1 from ATMs and adipocytes. We found that homeostatic mechanisms maintain overall Igf1 in adipose tissue during development of obesity despite reductions in adipocyte-derived Igf1. We also discovered that while neither adipocyte nor macrophage IGF1 is required for adipose tissue development in lean animals, they respectively play a role in tissue maintenance during chronic or acute stress. In obesity, adipocyte-derived IGF1 contributes to tissue expansion while ATM-derived IGF1 maintains adipose tissue mass during an acute thermogenic challenge.
Methods
Animal and Animal Care
C57BL/6J male mice were obtained from the Jackson Laboratory at six – eight weeks of age. C57BL/6J male mice were made obese by feeding them with a high-fat diet (60% kcal high fat, Research Diet, Inc) for indicated periods. Mice carrying a Cre-recombinase inserted into the Lysozyme2 locus (Lyz2-Cre) on the C57BL/6J background were obtained from the Jackson Laboratory and C57BL/6J mice carrying alleles of the Igf1 locus in which the fourth exon was flanked by LoxP sites (Igf1 fl/fl) were provided by Derek LeRoith (Mt. Sinai School of Medicine, NY). Mice were housed in ventilated Plexiglas cages within pathogen-free barrier facility maintained in a 12-hour light/12-hour dark cycle. All protocols were approved by the Columbia University Institutional Animal Care and Use Committee.
Generation of macrophage-specific IGF1 Knockout Mouse (Mac-IKO)
Mice on the C57BL/6J background carrying Igf1 fl alleles were intercrossed with Lyz2-Cre mice to delete Igf1 in myeloid cells, but because both Lyz2 and Igf1 reside on mouse chromosome 10 we generated animals carrying a chromosome with both alleles. To achieve complete deletion of Igf1 in myeloid cells, including macrophages, we intercrossed the mice carrying the Lyz2-Cre; Igf1 fl chromosome with Igf1 fl/fl animals. Control animals for all studies were littermates carrying two floxed alleles in the absence of Cre. To assess recombination, we extracted genomic DNA from purified ATMs, as well as peritoneal macrophages, and amplified the region flanking exon 4. The amplicon product from Igf1 fl/fl (control) genomic DNA size was ~1000 base pairs. Amplification of the deleted allele yielded an amplicon of ~ 200 base pairs.
Generation of adipocyte-specific IKO mice (Adipo-Igf1 KO)
To generate adipocyte-specific deletion of Igf1 we intercrossed Igf1 fl/fl mice with mice carrying a BAC in which the expression of Cre recombinase was driven by the Adiponectin promoter. Adipo-IKO mice (C57BL/6J Igf1 fl/fl; Adiponectin-Cre) were compared to control littermate mice (C57BL/6J Igf1 fl/fl).
PGAT targeted deletion of Igf1
To delete IGF1 from adipose tissue ex vivo, PGAT from lean and obese Igf1 fl/fl mice was cultured as described previously (23). Adenoviruses that express either Green Fluorescent Protein-Cre (Ad-GFP-Cre) or Red Fluorescent Protein (Ad-RFP) under the control of the CMV promoter were obtained from Cell Bio Labs and used to infect PGAT. Five days after infection, RNA was extracted from the PGAT samples for analysis. For in vivo whole tissue Igf1 deletion, PGAT pads were identified after a laparotomy and injected with either Ad-GFP-Cre (right side) or Ad-RFP (left side). The incision was sutured and the animals treated with analgesics for 24 hours then permitted to recover. Five days after adenovirus injection, PGAT weight was measured and collected for analysis.
Adipose Tissue Explant
PGAT from lean and obese male mice was cut into ~1mm pieces (120 mg total weight) cultured in phenol-free DMEM (Invitrogen) containing 2% fatty acid–free BSA (Sigma-Aldrich) at 37°C and 5% CO2. After 4 hours of culture, the medium was replaced with fresh medium. To deplete ATMs adipose tissue explant was treated with clodronate-encapsulated liposomes (23); to delete Igf1, adipose tissue from mice carrying Igf1 fl/fl mice was infected with adenoviruses expressing either RFP (control) or GFP conjugated with Cre.
Adipose Tissue Macrophages (ATMs) Depletion
Adipose tissue explants were incubated in DMEM (Invitrogen) without serum supplementation. After 4 hours, the medium was exchanged for fresh medium containing 20% (v/v) liposomes. Control liposomes containing PBS and ATM depleting liposomes containing clodronate were prepared as previously described (23). Explants were maintained for 24 hours at 37°C in at 5% CO2. After 24 hours, tissue pieces were collected for RNA extraction or immunohistochemical (IHC) analysis. Sample analyzed by IHC were fixed in zinc-formalin fixative (Anatech Ltd.) as described previously (23).
Quantitative RT-PCR
RNA was extracted from frozen adipose tissue samples by using an acid-phenol reagent according to the manufacturer’s instructions (TRIZOL; Invitrogen). RNA was further purified using a silica-membrane technique (QIAGEN RNeasy Minikit) and used as template for cDNA synthesis (qScript cDNA SuperMix, Quanta Biosciences). Quantitative RT-PCR was performed using DNA Engine Opticon 2 system instruments (Bio-Rad) and PCR SYBR Green I QuantiTect Master Mix (Qiagen). The mRNA expression was normalized to Rps3 expression and expressed in relative units using the DDCT-2 method
Flow Cytometry
Stromal vascular cells were isolated using standard methods from PGAT and collected in FACS buffer (PBS, 0.2%, fatty acid depleted-BSA, 5 mM EDTA) (24). Information about antibodies is found in the Supplemental Methods.
Immunohistochemistry
Adipose tissue was fixed in zinc-formalin, processed and stained for macrophages as previously described (23). To quantify ATM content of adipose tissue, images of sections were analyzed using four to eight different fields per section from each of four different sections. The areas of each slide to be analyzed were identified a priori to prevent bias. The ATM content for each area analyzed was calculated by dividing the nuclei associated with F4/80 expression by the total nuclei. Adipocyte size was measured as cross-sectional area for each field using image analysis software Image-Pro Plus 7.0 (Media Cybernetics). Adipocyte number was calculated from fat pad weight and fat pad volume (25, 26).
Cold Challenge Experiment
Mice were individually housed and fed ad libitum before and during cold challenge. Mice were placed in 4 ° C for 3–4 hours to induce an adrenergic stimulus. Rectal temperatures of mice were monitored and measured using a MicroTherma Handheld Thermometer (Braintree Scientific, Inc). Mice were sacrificed immediately following cold challenge.
Results
Adipocytes and macrophages express Igf1 to maintain adipose tissue expression
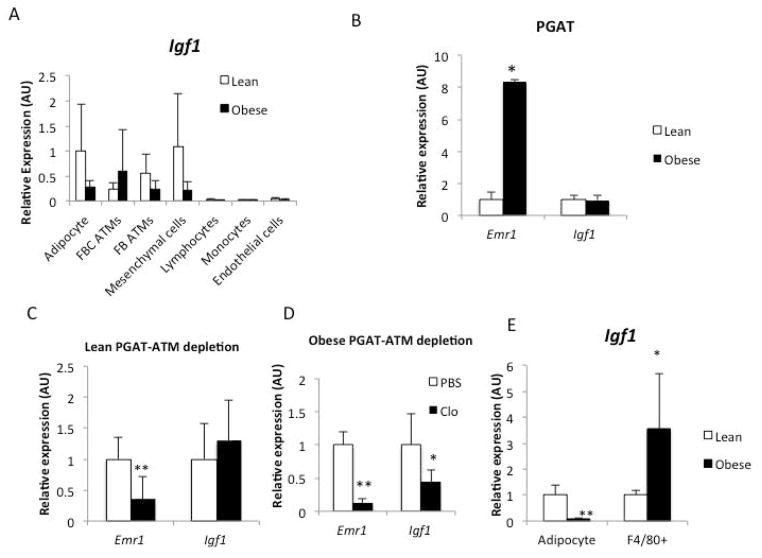
As a first step to study local production of IGF1 by adipose tissue we fractionated cells from PGAT, into adipocytes, immune cell populations, endothelial cells and non-immune mesenchymal cells. In both lean mice and high fat fed obese animals adipocytes, macrophages and mesenchymal cells expressed measurable amounts of Igf1 (Figure 1A); no other cells detectably expressed Igf1. However, with the onset of obesity, adipocyte and mesenchymal expression of Igf1 was reduced and while the expression in a CD11c+ subpopulation of adipose tissue macrophages increased (Figure 1A). This population of ATMs represents the majority of macrophages in adipose tissue in obese animals, especially within intra-abdominal fat depots (24, 27). While the expression of Igf1 was dynamically regulated among cells within adipose tissue, surprisingly the overall expression of Igf1 was not altered by obesity (Figure 1B).
Figure 1. Regulation of local Igf1 expression is dynamically maintained during the development of obesity.
(A) Expression of Igf1 in purified cell populations from PGAT of lean (Lep +/+) and obese (Lep ob/ob) mice (n=5). (B) Gene expression of F4/80 (Emr1) and Igf1 in both lean and obese PGAT (n=3). Expression of F4/80 (Emr1) and Igf1 in PGAT explants from (C) lean mice (n=5) and (D) high fat fed obese animals (n=3). (E) Expression of Igf1 in purified adipocytes and purified adipose tissue macrophages (ATMs) population from lean and obese PGAT. Fluorescence activated cell sorting was used to purify cells from adipose tissue (n=5). All data are presented as mean ± SD *p-value <0.05 **p-value <0.01.
To confirm ATMs’ contribution to local Igf1 expression directly, we took advantage of liposome encapsulated clodronate’s ability to acutely deplete macrophages from adipose tissue. Treatment of PGAT ex vivo with clodronate liposomes effectively reduced macrophages in adipose tissue (Supplemental Figure 1). Consistent with our prediction, depletion had no consistent effect on adipose tissue Igf1 expression from lean animals but reduced by ~75% Igf1 expression in adipose tissue from obese animals (Figure 1C and 1D). These data argued that with the onset of obesity and adipocyte hypertrophy, Igf1 expression is reduce by adipocytes, but an influx of ATMs serves in part to maintain overall adipose tissue Igf1 expression.
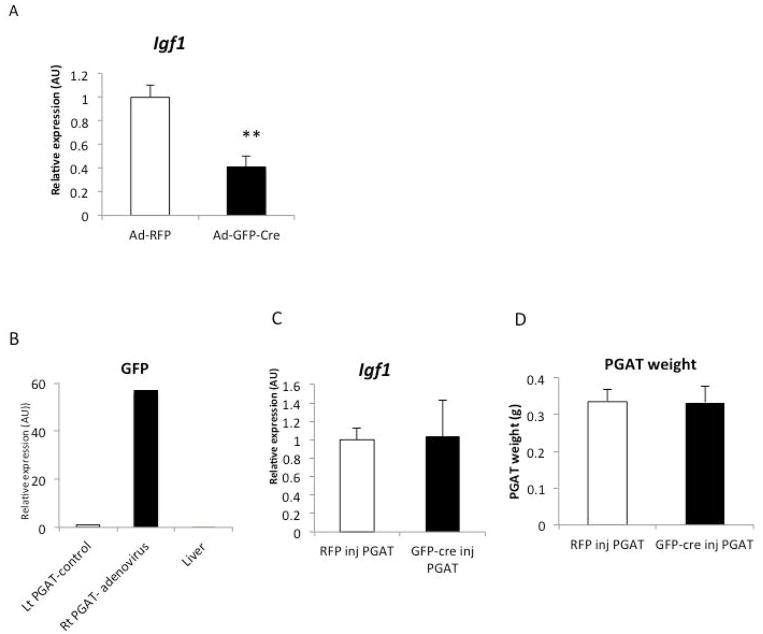
To cause acute deletion of Igf1 in whole adipose tissue either ex vivo or in vivo we injected PGAT from C57BL/6 Igf1 fl/fl with adenovirus expressing the Cre-recombinase and GFP under a CMV-promoter (Ad-Cre). As a control we injected the contralateral PGAT from the same animals with a control virus expressing RFP (Ad-RFP). When fat pads were transduced ex vivo with Ad-GFP-Cre virus there was a 70% reduction in Igf1 expression as compared to control fat pads transduced with Ad-RFP (Figure 2A). Consistent with the role of IGF1 regulation of adipocytes differentiation and survival, a 70% reduction in Igf1 expression was associated with reductions in expression of adipogenic genes, including Cebpa and Dgat (Data not shown). However, when PGAT depots were transduced in vivo there was no consistent reduction in Igf1 expression, again suggesting the existence of a system which maintains local IGF1 in adipose tissue in vivo through recruitment of Igf1 expressing cells and upregulation by resident cell expression (Figure 2B and C).
Figure 2. Compensation in vivo but not ex vivo of adipose tissue IGF1 deletion.
(A) Igf1 expression in Ad-RFP injected and Ad-GFP-Cre injected PGAT explants from 18-week-old obese Igf1 fl/fl mice. (n=4) (B) GFP expression in Ad-RFP treated (left side) and Ad-GFP-Cre treated (right side) and liver of 14-week-old lean Igf1fl/fl mice (n=3). (C) Igf1 gene expression in Ad-RFP treated (left side) and Ad-GFP-Cre treated (right side) PGAT of 14-week-old lean Igf1fl/fl mice (n=3) (D) Ad-RFP treated (left side) and Ad-GFP-Cre treated (right side) weight of 14-week-old lean Igf1fl/fl mice (n=3). All data are presented as mean + SD **p-value <0.01.
Deletion of Igf1 from adipocytes or macrophages does not alter adipose tissue in lean animals
To understand the contribution of adipocyte and macrophage derived IGF1 on adipose tissue development and function, we deleted Igf1 in either adipocytes or myeloid cells, including adipose tissue macrophages. C57BL/6 Igf1 fl/fl mice were intercrossed with mice carrying the Cre-recombinase driven by an Adiponectin promoter (ADIPO-IKO) or the Lysozyme 2 (Mac-IKO). Deletion occurred in each compartment as expected (Supplemental Figure 2).
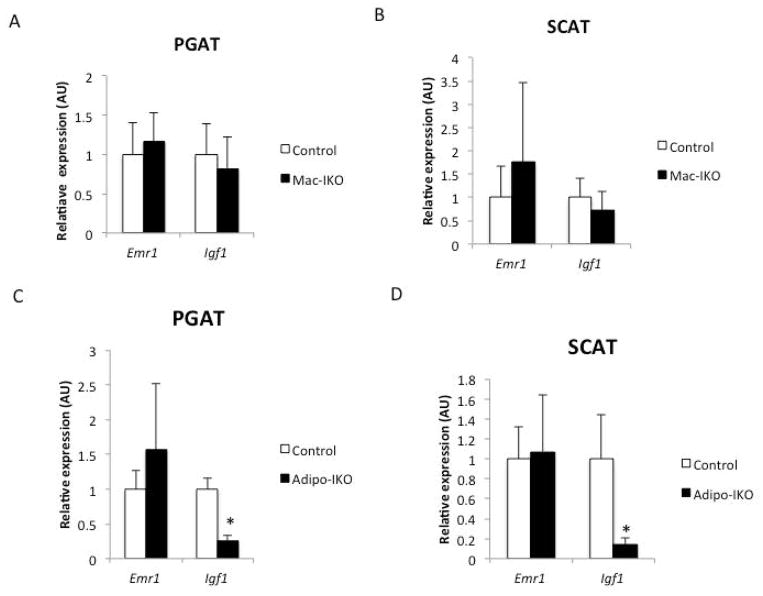
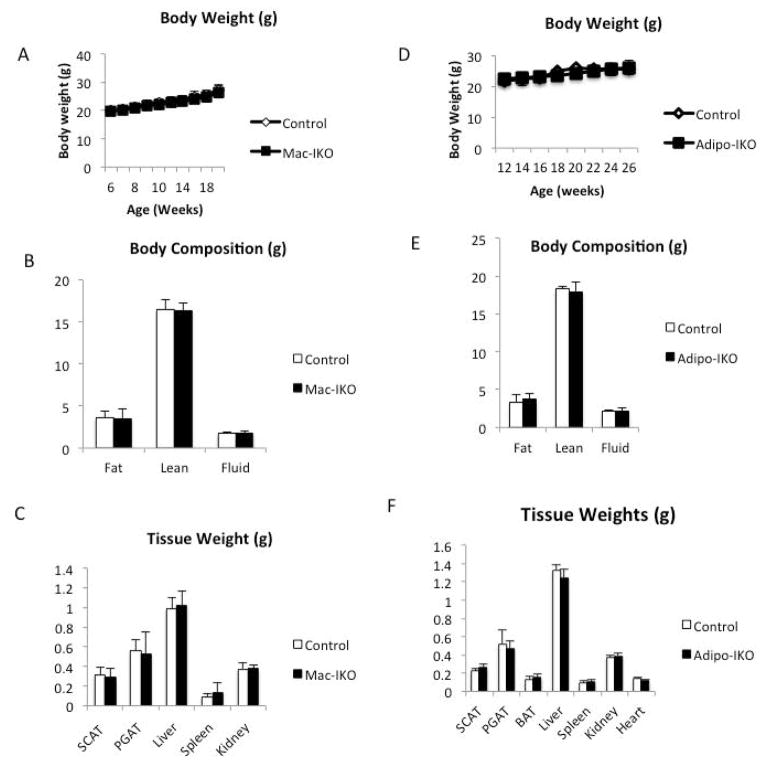
Consistent with expression data from purified cell populations, in lean animals deletion of adipocyte-derived Igf1 reduced overall Igf1 expression, whereas myeloid deletion had no consistent effect on fat Igf1 expression (Figure 3A and 3B). Despite a reduction in Igf1 in adipose tissue (Figure 3C and 3D), adipose tissue from Adipo-IKO mice had normal weight, morphology or histology (Figure 4D, 4E, 4F,5C and 5D). No functional defect in adipose tissue was apparent from systemic measures of lipid or glucose homeostasis in lean Adipo-IKO mice (Supplemental Table 2). As expected, given the small contribution of ATM-derived IGF1 in adipose tissue of lean animals there were also no consistent effects on adipose tissue or systemic measures of metabolism in the Mac-IKO mice (Figure 4A, 4B, 4C, 5A, 5B and Supplemental Table 1). Arguing that adipose tissue does not contribute substantially to circulating IGF1, none of the mutant mice had plasma [IGF1] that differed from that control mice (Data not shown). These data demonstrate that adipocyte and macrophage-derived IGF1 are dispensable for normal adipose tissue development and function in lean animals without metabolic stress.
Figure 3. In lean animals adipose tissue IGF1 is primarily adipocyte-derived.
F4/80 (Emr1) and Igf1 expression in (A) perigonadal and (B) subcutaneous adipose tissue of 24-week-old lean mice and those in which IGF1 had been genetically deleted from myeloid cells (Mac-IKO) (n=9–16). F4/80 (Emr1) and Igf1 expression in (C) perigonadal or (C) subcutaneous adipose tissue of 26-week-old lean control mice and those in which IGF1 had been genetically deleted from adipocytes (Adipo-IKO) (n=5). All data are presented as mean + SD *p-value <0.05.
Figure 4. Neither myeloid nor adipocyte IGF1 is required for normal development in lean animals.
(A) Body weights of 6- to 24-week-old lean control and Mac-IKO male mice (n=9–16). (B) Body compositions (fat, lean, and fluid mass) of 24-week-old lean control and Mac-IKO male mice as measured by MRI (n=9–16). (C) Organ weights of 24-week-old lean control and Mac-IKO male mice (n=9–16). All data are presented as mean + SD. (D) Body weights of 12- to 26-week old lean control and Adipo-IKO male mice. n=5/genotype. (E) Body compositions of 26-week-old lean control and Adipo-IKO male mice. n=5/genotype. (F) Organ weights of 26-week-old lean control and Adipo-IKO mice. n=5/genotype. All data are presented as mean + SD.
Figure 5. Adipose tissue develops normally in the absence of adipocyte or myeloid IGF1 in lean animals.
(A) PGAT histology (hemotoxylin and eosin stained) and (B) adipocyte size (area um2) distribution of 24-week-old lean control and Mac-IKO male mice (n=9–16). (C) PGAT histology (hemotoxylin and eosin stained) and (D) adipocyte size (area um2) distribution of 26-week-old lean control and Adipo-IKO male mice. All data are presented as mean + SD.
Local IGF1 maintains PGAT mass in high fat fed and cold-challenged animals
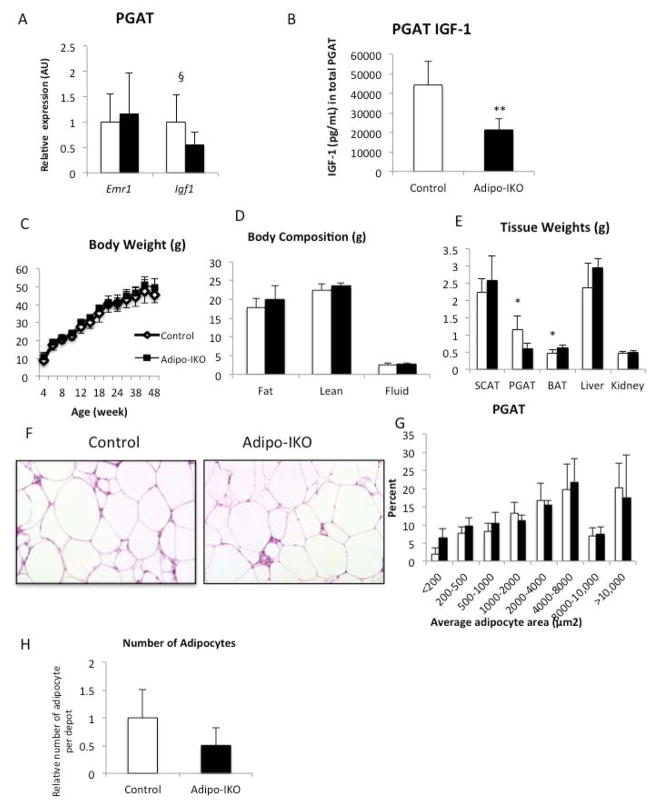
In vitro IGF1 regulates differentiation, promotes survival and suppresses lipolysis of adipocytes (7, 9). Obesity induces adipocyte stress and increases lipolysis, (28) and hence, we hypothesized that local IGF1 may play a critical role in expanse and metabolic function of adipose tissue in obese individuals. To test this, we fed Adipo-IKO mice a high fat diet for 16 weeks. In vivo, deletion of adipocyte-derived Igf1 had a depot specific effect on adipose tissue mass: subcutaneous adipose tissue mass, morphology and histology were not affected, but the mass of PGAT was reduced by ~25% (Figure 6E). Histological analysis revealed no reduction in adipocyte size but to account for a reduction in mass there was a reduction in adipocyte number, implying a defect in obesity-induced adipocyte hyperplasia or survival (Figure 6F, 6G and 6H).
Figure 6. Adipocyte-derived IGF-1 is required to maintain perigonadal adipose tissue mass during chronic high fat feeding.
(A) F4/80 (Emr1) and Igf1 expression in PGAT of 48-week-old control (white bars) and Adipo-IKO (black bars) obese male mice (n=5–7). (B) IGF1 protein concentration in PGAT of 48-week-old control and Adipo-IKO obese male mice. (n=5–7) (C) Body weights of 4- to 48-week-old control (empty circles) and Adipo-IKO (filled circles) high fat fed male mice. (D)Body compositions of 48-week-old male mice (n=5–7). (E) SCAT, PGAT, BAT, liver, and kidney weights of 45-week-old control (white bars) and Adipo-IKO (black bars) high fat fed mice (n=5–7). (F)PGAT histology (H&E stained) of 48-week-old male obese mice. (G) Quantification of perigonadal adipocyte size from figure 8F. (H) Relative adipocyte number in PGAT of 45-week-old control (white bars) and Adipo-IKO (black bars) high fat fed mice (n=5–7) p-value § =0.08, * <0.05 **<0.01. All data are presented as mean + SD.
The limited reduction in PGAT mass and adipocyte number did not measurable decrease overall body weight (Figure 6C and 6D). Although reductions in “metabolically good” subcutaneous are associated with lipodystrophic phenotypes, reduction in intra-abdominal fat specifically is unusual and would not be expected to impair systemic metabolism. Consistent with these observations there were no effects on systemic glucose or lipid homeostasis in the high fat fed obese Adipo-IKO mice (Supplemental Table 3 and Supplemental Figure 3). Similarly, there was no evidence of hepatic ectopic lipid deposition beyond that seen in control littermates on a high fat diet (Data not shown).
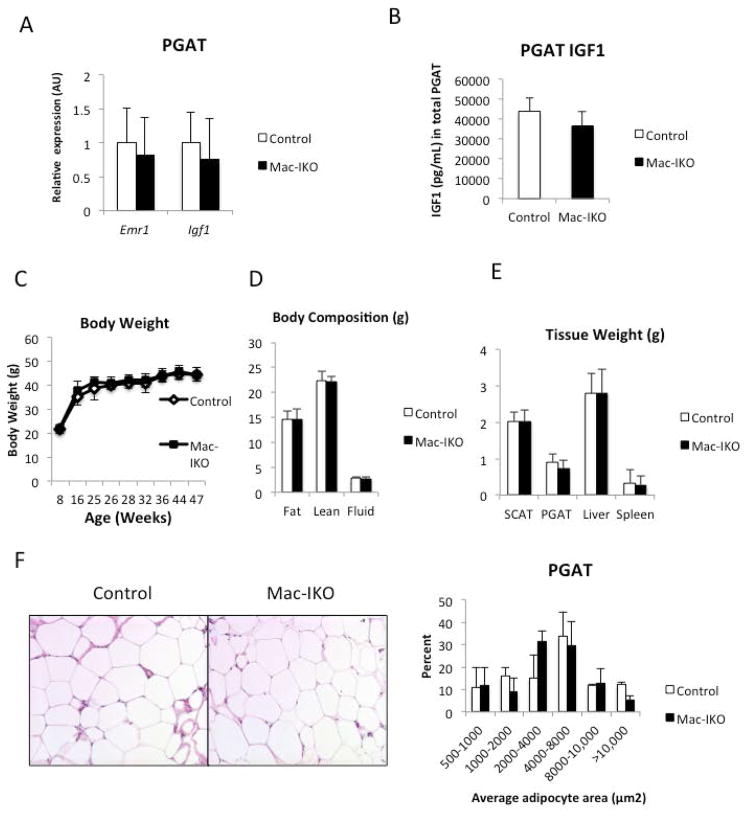
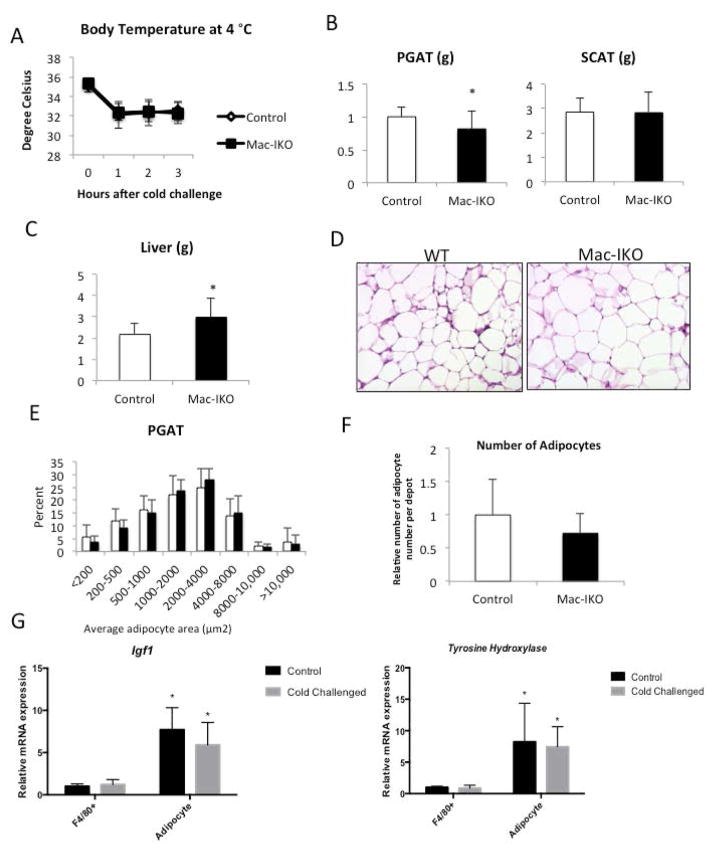
In contrast to our findings in Adipo-IKO mice, we did not find any consistent alterations in adipose tissue mass, adipocyte number or systemic metabolism in Mac-IKO (Figure 7E and 7F; Supplemental Table 4). We have previously shown that in response to lipolysis macrophages accumulate in adipose tissue depots (23); this is especially pronounced in obese animals. To test whether ATM-derived Igf1 might play a role in local regulation in response to the natural lipolytic stimulus of a cold challenged, we placed Mac-IKO and control littermate mice at 4 °C. During a cold challenge mice mobilize TG as substrate for uncoupled oxidation and increase food intake to maintain fat depot mass. Igf1 expression is maintained in whole adipose tissue despite a trend for a reduction in adipocyte Igf1 expression (Supplemental Figure 5; Figure 8G). Recent reports have found a recruitment of macrophages to subcutaneous and BAT fat depots during a cold challenge (29), that may in part maintain local IGF1. However, in Mac-IKO mice, a cold challenge reduced PGAT mass compared to PGAT from control animals (Figure 8C). Previous studies have revealed CCR2-dependent recruitment of macrophages to adipose tissue depots during a cold challenge. Some of these macrophages, the M2-polarized ones, produce catecholamines (29). However, cold challenge of mice did not induce expression of Tyrosine hydroxylase, the gene that encodes the committed step in catecholamine synthesis, in ATMs from these mice (Figure 8G). These data argue that myeloid-derived IGF1 maintains adipose tissue mass during an acute metabolic challenge and does not effect catecholamine expression.
Figure 7. ATM-derived IGF-1 is not required for adipose tissue development during the onset of obesity.
(A) F4/80(Emr1) and Igf1 mRNA gene expression of PGAT in 45-week-old control and Mac-IKO obese male mice (n=5–6/genotype) (B) Total IGF1 protein level (as measured by ELISA) of PGAT in 45-week-old control and Mac-IKO obese male mice (n=5–6). (C) Body weights of 4- to 45-week-old control and Mac-IKO obese male mice. Mice were fed high-fat diet from 5-week of age. (n=8–15) (D) Body compositions of 45-week-old control and Mac-IKO obese male mice. n=5–6 per genotype. (E) Tissue weights of 45-week-old control and Mac-IKO obese male mice (n=5–6) (F) PGAT histology (H&E stained) of 45-week-old control and Mac-IKO obese male mice (n=5–6). All data are presented as mean ± SD.
Figure 8. ATM-derived IGF1 is required to maintain perigonadal adipose tissue during a cold challenge in obese animals.
(A) Body temperature of 46-week-old control (white diamonds) and Mac-IKO mice (filled circles) during a cold challenge at 4 °C (n= 8–15). (B) PGAT, and SCAT weights of cold challenged 46-week-old male obese mice. (C) Liver weight of cold challenged 46-week-old obese mice (D) PGAT histology, (E) adipocyte size distribution and (F) adipocyte number of PGAT from 46-week-old male obese mice following a cold challenged for 3 hours at 4 ° C. (G) 9-week old lean male mice were placed at either 25°C or 4°C for 72 hours and F4/80+ macrophage and adipocytes were purified. Expression of Igf1 and Tyrosine Hydroxylase (Th) were measured qPCR (n=10 per genotype), *p-value<0.05 relative to F4/80+ cells. All data are presented as means + SD. *p-value <0.05.
Discussion
The high expression of IGF1 by adipose tissue (6), classic in vitro IGF1 studies of adipocyte differentiation and metabolism (7), and the adipose tissue phenotypes of mice harboring deletion of the IGF1 receptors in adipocytes (10) all argued that local IGF1 has important development and metabolic functions in fat. Previous receptor studies had implicated the adipocyte as a source of Igf1 (30). Indeed, we found that adipocytes express Igf1 at high levels, contributing the majority of its expression in adipose tissue from lean animals. However, in addition to adipocytes, we found that ATMs are an important source of IGF1 in fat. This observation echoes findings from muscle and tumor microenvironment in which macrophage IGF1 contributes substantially to local production (21, 31). In these systems, Igf1 plays homeostatic roles, responding to stress. In the case of muscle, macrophage-derived Igf1 is critical for the response to injury (21), while in certain neoplasm tumor associated macrophage-derived trophic factors such as IGF1 have been implicated in the response to tumor induced hypoxia (18, 32). Our data argue that in adipose tissue, macrophage-derived IGF1 plays a role in response to an acute metabolic stress.
During a cold challenge when non-shivering thermogenesis is activated, adipose tissue lipolysis is increased but adipose tissue mass is not reduced. Instead, animals increase caloric intake and maintain fat mass. While circulating and local catecholamines concentrations rise in adipose tissue during a cold challenge and are known to be catabolic, inducing lipolysis in adipose tissue (29), the identity of any anabolic signals that help maintain adipose tissue mass during a cold challenge have remained obscure. Our data now identify ATM-derived IGF1 as critical for maintaining adipose tissue mass during a cold challenge, at least in depots that have little thermogenic potential, e.g. perigonadal fat. Deletion of IGF1 from ATMs did not have a consistent effect on other measured phenotype, although in some cohorts the serum concentration of non-esterified fatty acids was higher (Data not shown).
In contrast to the effects of ATM-derived IGF1 on an acute metabolic stress, adipocyte-derived IGF1 is required to maintain fat mass but in response to a chronic metabolic stress – high fat feeding. With high fat feeding C57BL/6 mice gain weight with a marked increase in adiposity. Overall, Adipo-IKO mice had similar adiposity to littermate controls but a specific reduction in PGAT mass suggests that local IGF1 from adipocytes is important for maintaining PGAT mass during the development of obesity. The specificity of the phenotype is intriguing. During the development of obesity, PGAT undergoes extensive remodeling more so than other depots (33). While little is known about the signals regulating the remodeling process our findings suggest that IGF1 may play a role.
Our studies found that the expression of Igf1 is dynamically regulated and maintained even during the development of obesity when changes in the expression by adipocytes appear to be balanced by larger numbers of ATMs. We also were surprised to find that our in vivo attempt to acutely lower Igf1 expression via target deletion was not successful, despite our ability to do so ex vivo. These findings suggest that a dynamic system exists to maintain IGF1 expression. Given that obesity is not associated with consistent changes in circulating GH concentrations our findings argue that another regulatory mechanism is at work. Indeed, previous studies have found that the macrophage regulate Igf1 expression via a non-canonical promoter site (19). Our findings suggest a model in which adipocytes reduce their expression of Igf1 as they undergo hypertrophy and that this reduction is sensed leading to recruitment of macrophages that in part maintain IGF1 in fat. The system by which ATMs maintain Igf1 expression, however, can be overwhelmed in the absence of all adipocyte-derived IGF1. Similarly, following an acute loss of IGF1 or an acute stress, adipocytes cannot adapt and increase Igf1 expression.
Defining more clearly the local anabolic and catabolic signals that regulate adipose tissue has the potential to identify pathways that are dysregulated in metabolic disease and therapeutic targets to improve the development and maintenance of healthy adipose tissue.
Supplementary Material
Known Previously.
IGF1 regulates adipocyte differentiation and development in vitro
Paradoxically, deletion of IGF1 Receptors in adipose tissue inCreases adipose tissue mass through a poorly-defined feedback mechanism
Local, in vivo role of IGF1 in adipose tissue development and function is not known
Novel Findings.
Through coordinated regulation adipocytes and macrophages express IGF1 in adipose tissue
In unstressed adipose tissue IGF1 is not required for development or metabolism
In metabolically stressed states - obesity and cold challenge - adipocyte and macrophage IGF1 maintain tissue mass and homeostasis
Acknowledgments
Grant Support: This work was supported by research and center grants from the NIH (DK066525, DK101942, DK063608, DK026687) and predoctoral training support for HRC (DK007647).
We thank Deroik LeRoith and Shoshona Yakar for mice carrying the Igf1-floxed allele. These experiments benefited greatly from discussions with other members of the Ferrante Laboratory and the Berrie Diabetes Center and Drs. Henry Ginsberg, Domenico Accili and Ira Goldberg. This research was supported by grants from NIH, including R01DK066525, R01DK101942 and support from the Columbia University Diabetes Research Center (P30DK063608), New York Obesity Nutrition Research Center (P30DK026687) and Institute for Human Nutrition (T32DK007647).
Footnotes
Author Contributions: HRC, HJK and AWF conceived and designed the experiments. HRC, HJK and XX performed all studies and collected all data. HRC, HJK and AWF reviewed, analyzed and interpreted the data. The manuscript was written by HRC & AWF.
Conflict of Interest: None of the authors have any conflicts of interest.
References
- 1.Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocrine reviews. 2001;22(1):53–74. doi: 10.1210/edrv.22.1.0419. [DOI] [PubMed] [Google Scholar]
- 2.Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75(1):73–82. [PubMed] [Google Scholar]
- 3.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75(1):59–72. [PubMed] [Google Scholar]
- 4.Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, et al. IGF-I is required for normal embryonic growth in mice. Genes & development. 1993;7(12B):2609–17. doi: 10.1101/gad.7.12b.2609. [DOI] [PubMed] [Google Scholar]
- 5.Sjogren K, Liu JL, Blad K, Skrtic S, Vidal O, Wallenius V, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(12):7088–92. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yakar S, Liu JL, Stannard B, Butler A, Accili D, Sauer B, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(13):7324–9. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scavo LM, Karas M, Murray M, Leroith D. Insulin-like growth factor-I stimulates both cell growth and lipogenesis during differentiation of human mesenchymal stem cells into adipocytes. The Journal of clinical endocrinology and metabolism. 2004;89(7):3543–53. doi: 10.1210/jc.2003-031682. [DOI] [PubMed] [Google Scholar]
- 8.Entingh-Pearsall A, Kahn CR. Differential roles of the insulin and insulin-like growth factor-I (IGF-I) receptors in response to insulin and IGF-I. The Journal of biological chemistry. 2004;279(36):38016–24. doi: 10.1074/jbc.M313201200. [DOI] [PubMed] [Google Scholar]
- 9.Smith PJ, Wise LS, Berkowitz R, Wan C, Rubin CS. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. The Journal of biological chemistry. 1988;263(19):9402–8. [PubMed] [Google Scholar]
- 10.Kloting N, Koch L, Wunderlich T, Kern M, Ruschke K, Krone W, et al. Autocrine IGF-1 action in adipocytes controls systemic IGF-1 concentrations and growth. Diabetes. 2008;57(8):2074–82. doi: 10.2337/db07-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boucher J, Tseng YH, Kahn CR. Insulin and insulin-like growth factor-1 receptors act as ligand-specific amplitude modulators of a common pathway regulating gene transcription. The Journal of biological chemistry. 2010;285(22):17235–45. doi: 10.1074/jbc.M110.118620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LeRoith D, Yakar S. Mechanisms of disease: metabolic effects of growth hormone and insulin-like growth factor 1. Nature clinical practice Endocrinology & metabolism. 2007;3(3):302–10. doi: 10.1038/ncpendmet0427. [DOI] [PubMed] [Google Scholar]
- 13.Nakae J, Kido Y, Accili D. Distinct and overlapping functions of insulin and IGF-I receptors. Endocrine reviews. 2001;22(6):818–35. doi: 10.1210/edrv.22.6.0452. [DOI] [PubMed] [Google Scholar]
- 14.Boulet SL, Alexander GR, Salihu HM, Pass M. Macrosomic births in the united states: determinants, outcomes, and proposed grades of risk. American journal of obstetrics and gynecology. 2003;188(5):1372–8. doi: 10.1067/mob.2003.302. [DOI] [PubMed] [Google Scholar]
- 15.Marks V, Teale JD. Tumours producing hypoglycaemia. Diabetes/metabolism reviews. 1991;7(2):79–91. doi: 10.1002/dmr.5610070202. [DOI] [PubMed] [Google Scholar]
- 16.Musaro A, McCullagh KJ, Naya FJ, Olson EN, Rosenthal N. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature. 1999;400(6744):581–5. doi: 10.1038/23060. [DOI] [PubMed] [Google Scholar]
- 17.Xian L, Wu X, Pang L, Lou M, Rosen CJ, Qiu T, et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nature medicine. 2012;18(7):1095–101. doi: 10.1038/nm.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollard JW. Trophic macrophages in development and disease. Nature reviews Immunology. 2009;9(4):259–70. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gow DJ, Sester DP, Hume DA. CSF-1, IGF-1, and the control of postnatal growth and development. Journal of leukocyte biology. 2010;88(3):475–81. doi: 10.1189/jlb.0310158. [DOI] [PubMed] [Google Scholar]
- 20.Rom WN, Basset P, Fells GA, Nukiwa T, Trapnell BC, Crysal RG. Alveolar macrophages release an insulin-like growth factor I-type molecule. The Journal of clinical investigation. 1988;82(5):1685–93. doi: 10.1172/JCI113781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu H, Huang D, Saederup N, Charo IF, Ransohoff RM, Zhou L. Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25(1):358–69. doi: 10.1096/fj.10-171579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(10):2596–605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. The Journal of clinical investigation. 2010;120(10):3466–79. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AW., Jr Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell metabolism. 2013;18(6):816–30. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bourgeois F, Alexiu A, Lemonnier D. Dietary-induced obesity: effect of dietary fats on adipose tissue cellularity in mice. The British journal of nutrition. 1983;49(1):17–26. doi: 10.1079/bjn19830006. [DOI] [PubMed] [Google Scholar]
- 26.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56(12):2910–8. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 27.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation. 2003;112(12):1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. The Journal of clinical investigation. 2011;121(6):2094–101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480(7375):104–8. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boucher J, Mori MA, Lee KY, Smyth G, Liew CW, Macotela Y, et al. Impaired thermogenesis and adipose tissue development in mice with fat-specific disruption of insulin and IGF-1 signalling. Nature communications. 2012;3:902. doi: 10.1038/ncomms1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denduluri SK, Idowu O, Wang Z, Liao Z, Yan Z, Mohammed MK, et al. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes & Diseases. 2015;2(1):13–25. doi: 10.1016/j.gendis.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fritz JM, Tennis MA, Orlicky DJ, Lin H, Ju C, Redente EF, et al. Depletion of tumor-associated macrophages slows the growth of chemically induced mouse lung adenocarcinomas. Frontiers in immunology. 2014;5:587. doi: 10.3389/fimmu.2014.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nature medicine. 2013;19(10):1338–44. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.