Abstract
At normal body temperature, the two-pore potassium channels TREK-1 (K2P2.1/KCNK2), TREK-2 (K2P10.1/KCNK10), and TRAAK (K2P4.1/KCNK2) regulate cellular excitability by providing voltage-independent leak of potassium. Heat dramatically potentiates K2P channel activity and further affects excitation. This review focuses on the current understanding of the physiological role of heat-activated K2P current, and discusses the molecular mechanism of temperature gating in TREK-1, TREK-2, and TRAAK.
1. INTRODUCTION
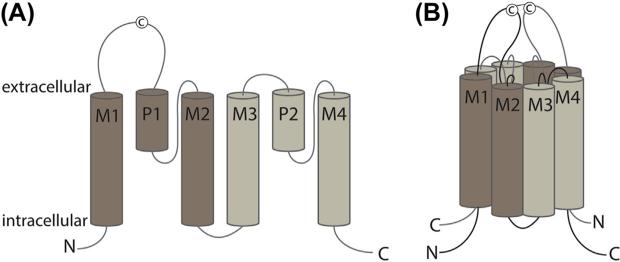
The two-pore potassium channels (K2P) contribute to the generation of an electric potential on the plasma membrane by providing voltage-independent “leak” of K+ ions (Enyedi & Czirjak, 2010). The K2Ps have a nonconventional topology: a mature channel is formed by two subunits, each containing two nonidentical pore-forming domains arranged in tandem (Figure 5.1). The K2P channels of the TREK/TRAAK group, which includes TREK-1 (K2P2.1, KCNK2), TREK-2 (K2P10.1, KCNK10), and TRAAK (K2P4.1, KCNK4), are expressed in various cell types, including neurons (Acosta et al., 2014; Fink et al., 1996; Kang & Kim, 2006; de la Pena et al., 2012; Talley, Solorzano, Lei, Kim, & Bayliss, 2001), cardiomyocytes (Xian Tao et al., 2006), and arterial myocytes (Bryan et al., 2006; Garry et al., 2007; Heyman et al., 2013). Temperature (Kang, Choe, & Kim, 2005; Maingret et al., 2000), mechanical force (Patel et al., 1998), general anesthetics (Patel et al., 1999), polyunsaturated fatty acids (Patel et al., 1998), and other compounds (Noel, Sandoz, & Lesage, 2011) potentiate the TREK/TRAAK-dependent background potassium efflux and suppress cellular excitability (Acosta et al., 2014; Dey et al., 2014).
Figure 5.1.
Membrane topology of K2P channels. (A) A topology diagram of a single K2P subunit with two pore-forming domains. (B) A mature channel is formed by two subunits covalently linked via the cysteines (C) in the first extracellular loop. M1–M4, trans-membrane segment 1–4; P1–2, pore helix 1–2. (See the color plate.)
TREK-1 is the most well-researched channel in the TREK/TRAAK group. Physiologically, TREK-1 contributes to the perception of temperature, pain, and mechanical force (Alloui et al., 2006; Noel et al., 2009; Plant, 2012); regulation of mood (Dominguez-Lopez, Howell, & Gobbi, 2012; Heurteaux et al., 2006; Kennard et al., 2005), anesthetic responses (Heurteaux et al., 2004); cardiac mechanoelectric feedback (Liu et al., 2008); and vasodilation (Bryan et al., 2006; Garry et al., 2007). Recent studies have suggested unexpected roles for TREK-1 in glutamate conductance (Hwang et al., 2014; Woo et al., 2012) and regulation of blood–brain barrier permeability (Bittner et al., 2013). Channel activity has been linked to several pathological conditions, such as cardiac hypertrophy (Wang et al., 2013), ischemia (Heurteaux et al., 2004; Laigle, Confort-Gouny, Le Fur, Cozzone, & Viola, 2012; Wu et al., 2013), and myocardial infarction (Zhao, Fu, Gao, Xie, & Cao, 2011).
Perhaps one of the most intriguing features of the TREK/TRAAK channels is the robust sensitivity to heat (Kang et al., 2005; Maingret et al., 2000). This feature is not found in other K2Ps, which are either insensitive to heat, such as the TASK channels (Bagriantsev, Peyronnet, Clark, Honore, & Minor, 2011), or poorly sensitive to heat, such as THIK-1 (K2P13.1, KCNK13) (Kang, Hogan, & Kim, 2013; Rajan et al., 2001). This review will focus on the physiological role and biophysical properties of the heat-evoked activity mediated by the TREK/TRAAK channels.
2. PHYSIOLOGICAL ROLE OF HEAT-ACTIVATED K2P CHANNELS
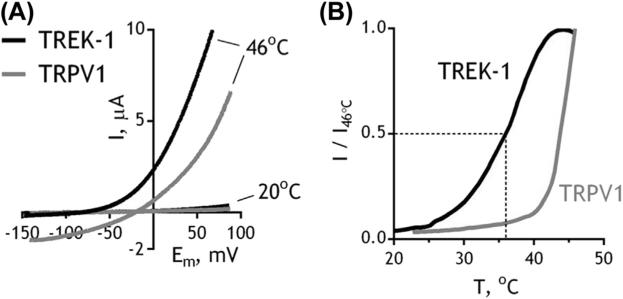
TREK-1, TREK-2, and TRAAK activate over a broad temperature range: the channels are silent at 14 °C and reach maximum activity above 40 °C (Kang & Kim, 2006; Maingret et al., 2000) (Figure 5.2(A) and (B)). The channels are expressed in the bodies of somatosensory neurons (Alloui et al., 2006; Maingret et al., 2000; Yamamoto, Hatakeyama, & Taniguchi, 2009) where they are thought to control excitation through regulation of temperature-dependent potassium “leak” (Dobler et al., 2007; Kang & Kim, 2006; Kang et al., 2013).
Figure 5.2.
A comparison of temperature activation profiles between TREK-1 and TRPV1. (A) Current–voltage plots showing the activity of TREK-1 and TRPV1 recorded by two-electrode voltage clamp in Xenopus oocytes at different temperatures. Currents were evoked in a “physiological” solution (2 mM KCl, 96 mM NaCl, 1.8 mM CaCl2, 2 mM MgCl2, 5 mM HEPES pH 7.4) by a 1-s-long voltage ramp from a holding potential of −80 mV. (B) Normalized activity of TREK-1 and TRPV1 at different temperatures, measured at 40 mV.
Decreased potassium efflux is expected to cause depolarization and potentiation of excitability. Accordingly, the deletion of KCNK2 and/or KCNK4 in mice stimulates firing rate of heat-sensitive somatosensory C-fibers (Alloui et al., 2006) and potentiates heat sensitivity in behavioral tests (Noel et al., 2009). The expression pattern of TREK/TRAAK significantly overlaps with that of TRPV1 (Yamamoto et al., 2009), a heat-activated nonselective cation channel (Caterina et al., 1997) (Figure 5.2(A) and (B)), which is essential for physiological sensitivity to noxious temperatures above 50 °C in behavioral tests (Caterina et al., 2000; Davis et al., 2000; Park et al., 2011; Pogorzala, Mishra, & Hoon, 2013). It was proposed that in the heat-sensing somatosensory neurons, the depolarizing effect of TRPV1 activation is counterbalanced by the hyperpolarizing activity of TREK/TRAAK. In KCNK2/4-knockout mice the balance is shifted toward TRPV1 activity, leading to increased heat sensitivity (Alloui et al., 2006; Noel et al., 2009).
Similarly, TREK/TRAAK expression significantly overlaps with TRPM8 (Yamamoto et al., 2009), a nonselective cold-activated ion channel (McKemy, Neuhausser, & Julius, 2002; Peier et al., 2002) responsible for the detection of mild (nonnoxious) cold (Bautista et al., 2007; Colburn et al., 2007; Dhaka et al., 2007; Knowlton et al., 2013). While the deletion of KCNK2 alone does not significantly affect cold responses in the sensory periphery (Alloui et al., 2006), the deletion of KCNK4 or a combined deletion of KCNK2 and KCNK4 potentiates cold sensitivity of somatosensory neurons, and facilitates cold avoidance in behavioral tests (Descoeur et al., 2011; Noel et al., 2009). Thus, TREK-1 and TRAAK appear to modify both cold and warm perception, apparently via their effects on excitability of somatosensory neurons. To actively affect temperature sensation, TREK-1 and TRAAK should be expressed in the nerve terminals in the skin. In support of this, it was reported that the channels traffic along peripheral axons (Bearzatto, Lesage, Reyes, Lazdunski, & Laduron, 2000). However, a direct evidence for colocalization of TREK/TRAAK and TRPV1 or TRPM8 in afferent endings is, to our knowledge, missing.
Though explored in the sensory periphery, the molecular basis of thermosensitivity in other types of neurons is unclear. The wide neuronal distribution of TREK/TRAAK suggests that their heat-evoked activity may play a role in various regions of central and peripheral nervous systems (Maingret et al., 2000; Talley et al., 2001). The K2Ps were suggested to contribute to temperature-dependent neuronal excitability in the hippocampus (de la Pena et al., 2012), Grueneberg ganglion (Stebe, Schellig, Lesage, Breer, & Fleischer, 2013), and preoptic thermoregulatory area of the hypothalamus (Wechselberger, Wright, Bishop, & Boulant, 2006), a region that determines the set point for body temperature (Kobayashi, Hori, Matsumura, & Hosokawa, 2006; Zhao & Boulant, 2005).
It should be noted, however, that apart from their effects on thermosensitivity, the deletion of KCNK2 and/or KCNK4 produces a number of other striking phenotypes, including altered anesthetic (Heurteaux et al., 2004; Vallee, Rostain, & Risso, 2009) and mechanical sensitivity (Noel et al., 2009), increased susceptibility to epilepsy (Heurteaux et al., 2004) and decompression sickness (Vallee, Meckler, Risso, & Blatteau, 2012), and resistance to depression (Heurteaux et al., 2006). Unexpectedly, even though TREK-1 and TRAAK share overall topology, functional properties, and expression pattern (Medhurst et al., 2001; Talley et al., 2001), the deletion of KCNK2 potentiates ischemia (Heurteaux et al., 2004), while the deletion of KCNK4 protects against it (Laigle et al., 2012). Thus, the plethora of KCNK2−/− and KCNK4−/− phenotypes strongly suggests that TREK-1 and TRAAK are integral to a number of physiological processes. Therefore, cell-type-specific deletions of the KCNK genes will be essential to clarify the exact contribution of these channels in heat sensitivity.
3. MOLECULAR MECHANISM OF TEMPERATURE GATING OF TREK-1, TREK-2, AND TRAAK
3.1 Characteristics of temperature-activated K2P current
Despite their importance for physiology, TREK-1, TREK-2, and TRAAK remain pharmacological orphans (Bagriantsev et al., 2013), which complicates their analysis in native cells. Most of our knowledge about temperature properties of these channels comes from heterologous systems, such as HEK293 and COS7 cells, and Xenopus oocytes (Bagriantsev, Clark, & Minor, 2012; Kang et al., 2005; Maingret et al., 2000). At room temperature, TREK-1 exhibits only background potassium leak, which increases with temperature, reaching maximum at ~42 °C (Figure 5.2). Interestingly, TREK-1 has its half-maximal temperature activation point (T1/2) at ~37 °C, implying that the midpoint of the channel's dynamic range is centered on the homeostatic thermal set point for most mammals. If this property is maintained in native cells, then TREK-1 activity is set to be maximally sensitive to minute variations in physiological temperature.
The heat activation profile of TREK-1 is notably different from those exhibited by the members of the transient receptor potential family, such as TRPV1 (Caterina et al., 1997; Gracheva et al., 2011). The temperature–activity relationship for TRPV1 has a clearly identifiable inflection point at ~42 °C, after which the rate of change in current per degree Celsius dramatically increases. This point, often referred to as temperature activation threshold, is difficult to identify with regard to TREK-1 (Figure 5.2(B)), because the equilibrium between closed and open channels shifts over a much broader temperature range. An alternative way to define a temperature activation threshold is to define a point at which channel activity begins to significantly exceed background noise. This approach, however, is problematic with regard to TREK-1, because of the “leaky” nature of its current, which increases linearly with the number of channels on the surface. Another difference is in the Q10 value, which reports fold change in current amplitude over 10 °C. For TRPV1, Q10 estimates vary, depending on the expression system, but in most cases they are around 20 (Caterina et al., 1997; Gracheva et al., 2011), whereas the K2Ps exhibit a more modest change of about 10 (Bagriantsev et al., 2012; Kang et al., 2005; Maingret et al., 2000). In summary, even though TREK-1 is heat sensitive, it has a rather modest Q10 and does not have a clearly identifiable temperature activation threshold, at least when measured in heterologous expression systems.
3.2 Contribution of the extracellular C-type gate
Like most other potassium channels, K2Ps have the canonical Thr-X-Gly-Phe/Tyr-Gly ion selectivity sequence in the structure of the outer pore (Brohawn, del Marmol, & MacKinnon, 2012, 2013; Miller & Long, 2012). In K2Ps, the selectivity filter region is a key part of an extracellular gate, which is often referred to as “C-type”-like, because it functions in a way similar to the C-type inactivation gate of voltage-gated potassium channels (Zilberberg, Ilan, & Goldstein, 2001).
During activation, the C-type gate of TREK-1 undergoes structural rearrangements, becoming more potassium selective. Saturation of the selectivity filter with high concentration of extracellular potassium (150 mM) stabilizes the gate in an open conformation and renders it insensitive to gating by extracellular protons (Cohen, Ben-Abu, Hen, & Zilberberg, 2008). Using sensitivity to potassium as readout, it was established that the C-type gate mediates temperature sensitivity of TREK-1 and TREK-2, suggesting that the gating mechanism is conserved among heat-sensitive K2Ps (Bagriantsev et al., 2011).
Another important element of the outer gate is the pore helix, which plays a key role in maintaining proper conformation of the selectivity filter in various ion channel classes (Alagem, Yesylevskyy, & Reuveny, 2003; Cordero-Morales et al., 2006). Crystal structures of TWIK-1 and TRAAK revealed that the pore helix of the K2Ps is located in a typical orientation relative to the selectivity filter (Brohawn et al., 2012, Brohawn, Campbell, & Mackinnon, 2013; Miller & Long, 2012). Mutations in the pore helix dramatically affect gating of various K2Ps, including TWIK-1 (Chatelain et al., 2012), TREK-1, TASK-1, and TASK-3 (Bagriantsev et al., 2012). A G137I mutation in the pore helix 1 of TREK-1 (Figure 5.3) stabilizes the channel in an open, potassium-selective conformation and abrogates gating by temperature. The same effect can be achieved by saturating the selectivity filter with high concentrations of the permeant ion. Thus, temperature responses are significantly attenuated under the conditions of a rigid outer gate, regardless of whether this conformation is achieved by high concentration of extracellular potassium or via a mutation (Bagriantsev et al., 2011, 2012). These data strongly suggest that temperature affects TREK-1 and TREK-2 activity through opening or closing the C-type gate.
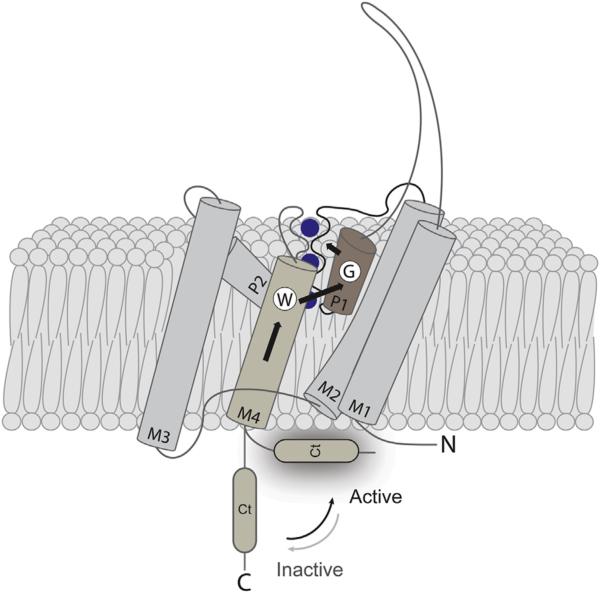
Figure 5.3.
A hypothetical cartoon model of how Ct affects TREK-1 activity. A cartoon model of a single TREK-1 subunit showing a hypothetical mechanism of channel activation by temperature. It was proposed that increasing temperature facilitates the transition of Ct from inactive to active conformation, leading to stabilization of an open conformation of the selectivity filter via interaction between Trp275 (W) and Gly137 (G) from a single TREK-1 subunit (Bagriantsev et al., 2012). M1–M4, transmembrane segment 1–4; P1–2, pore helix 1–2; Ct, C-terminal domain. Blue spheres depict potassium ions. (See the color plate.)
The importance of the C-type gate for temperature activation is in accord with the general role of this region in mediating TREK-1 responses to a broad spectrum of gating commands. In addition to temperature, the C-type gate mediates TREK-1 gating by intracellular pH (Piechotta et al., 2011), extracellular pH (Cohen et al., 2008; Ma, Yu et al., 2011; Sandoz, Douguet, Chatelain, Lazdunski, & Lesage, 2009), a small molecule activator ML67-33 (Bagriantsev et al., 2013), phosphorylation (Bagriantsev et al., 2012), and, possibly, membrane stretch (Bagriantsev et al., 2011; Piechotta et al., 2011). Therefore, in TREK-1, the C-type gate is the most crucial, and possibly the sole structural element that controls channel opening in response to different gating modalities, including temperature. In this regard, TREK-1 is similar to cyclic-nucleotide-gated channels, which rely almost exclusively on the extracellular C-type gate for function (Contreras, Srikumar, & Holmgren, 2008; Furini & Domene, 2011).
3.3 Contribution of the intracellular bundle crossing region
Activity of many potassium channels depends on the movement of the lower activation gate, known as the bundle crossing. The gate is formed by the C-terminal regions of the “inner” transmembrane helixes, topologically corresponding to the M2 and M4 segments of the K2Ps. The bundle crossing region may play a role in the gating of TASK-2 (Niemeyer, Cid, Pena-Munzenmayer, & Sepulveda, 2010) and the fly K2P KCNK0 (Ben-Abu, Zhou, Zilberberg, & Yifrach, 2009), but its role in the gating of TREK-1, TREK-2, or TRAAK by temperature or other modalities remains unconfirmed.
Several studies have inquired into the role of bundle crossing in heat-sensitive K2P channel function. Random mutagenesis of TREK-1 identified gain-of-function mutations in various channel regions, except in the C-terminal parts of the M2 and M4 transmembrane helices, suggesting that the potential lower gate exists in a predominantly open conformation (Bagriantsev et al., 2011). An elegant study established that the TREK-1 blocker tetrahexylammonium has unobstructed access to its binding site immediately below the selectivity filter even when the channel is closed (Piechotta et al., 2011). This work demonstrated the absence of a physical barrier between the cytosol and the outer gate, which further supports the idea that the potential bundle crossing region is locked in an open conformation and does not regulate ion flow.
Finally, a crystal structure of TRAAK revealed an opening between the inner helixes measuring ~10 Å (Brohawn et al., 2012), which is a wider opening than in open-state voltage-gated potassium channel structure (Long, Tao, Campbell, & MacKinnon, 2007). Thus, the existence of a functional bundle crossing region in heat-activated K2Ps has not yet found experimental support. Though it remains possible that the inner helices of the K2Ps may form a gate under certain conditions, the selectivity-filter-based extracellular C-type gate remains the only confirmed physical element that mediates temperature sensitivity of heat-activated K2P channels.
3.4 Contribution of the intracellular C-terminal domain
The C-terminal domain (Ct) is a major intracellular region of TREK-1 that mediates the reception of a number of regulatory commands (Noel et al., 2011). Deletion of Ct produces a striking effect on TREK-1, leading to suppression of basal activity (Patel et al., 1998) and decreased sensitivity to intra-cellular protons, mechanical force (Maingret, Patel, Lesage, Lazdunski, & Honore, 1999), and temperature (Maingret et al., 2000). These studies have established a key role for Ct in regulation of various modulatory responses, including sensitivity to heat.
However, the plethora of observed effects suggests that a complete deletion of Ct may produce a global impact on the TREK-1 molecule. A more subtle way to probe the importance of Ct for function is to decouple it from the pore-forming domain by introducing a flexible linker at the junction between M4 and Ct. This strategy has been successfully used to decouple cross-talk between functional domains of various classes of ion channels (Findeisen & Minor, 2009; Su, Anishkin, Kung, & Saimi, 2011). With regard to TREK-1, a triple-glycine (3G) or a triple-alanine (3A) linker introduced between M4 and Ct renders the channel insensitive to metabolic stimuli that converge on Ct, suggesting that the mutations obliterate functional communication between Ct and the gating apparatus. Importantly, the C-type gate remains functional in these mutants, as determined by measuring sensitivity to extracellular pH. At the same time, TREK-1 3G and 3A mutants become insensitive to heat (Bagriantsev et al., 2012), suggesting that functional coupling between Ct and the C-type gate is essential for heat sensitivity. These experiments have two major implications. First, they show that Ct is critical for normal heat sensitivity of TREK-1. Second, they clarify the role of the C-type gate: even though the gate mediates channel responses to heat, it lacks robust intrinsic temperature sensitivity that transforms into function.
While temperature almost certainly produces conformational changes throughout the channel, in some regions these changes may have more profound functional implications. The absence of temperature responses in TREK-1 3G and 3A mutants strongly suggest that heat exerts only minimal functional impact on the gate. Instead, current data are most consistent with the idea that temperature affects the intracellular Ct domain, which then potentiates opening of the heat-insensitive C-type gate via an allosteric mechanism (Bagriantsev et al., 2012). This mechanism is in accord with the general gating paradigm established for TREK-1, whereby different sensory elements, such as the extracellular proton sensor His126 (Cohen et al., 2008) or the polymodal intracellular sensor Ct, affect channel function by converging on a common C-type-like extracellular gate (Bagriantsev et al., 2011).
How Ct senses temperature remains unclear. It was proposed (Chemin et al., 2005) that regulatory factors that converge on Ct, such as phospholipids (Lopes et al., 2005), polyunsaturated fatty acids (Patel et al., 1998), intracellular pH (Honore, Maingret, Lazdunski, & Patel, 2002), and phosphorylation (Murbartian, Lei, Sando, & Bayliss, 2005), affect TREK-1 via modulating the affinity of Ct to the phospholipids in the inner leaflet of the plasma membrane. Optical probing of GFP-tagged Ct by total internal reflection fluorescence microscopy provided strong support for this hypothesis by showing that increased association of Ct with the plasma membrane correlates with TREK-1 activation, while dissociation leads to channel inhibition (Sandoz, Bell, & Isacoff, 2011). The dynamic interaction between Ct and the plasma membrane appears to mediate channel responses to a broad range of stimuli, including protons, various metabolites, and the antidepressant drug fluoxetine (Prozac) (Kennard et al., 2005; Sandoz et al., 2011). It is therefore possible, but not confirmed, that heat affects the channel via a similar mechanism, whereby increased temperature facilitates the association between Ct and plasma membrane, leading to channel opening via an allosteric effect on the extracellular C-type gate (Figure 5.3).
3.5 The mechanism connecting the heat-sensing and gating domains of TREK-1
The topological localization of the Ct and the C-type gate of TREK-1 on the opposite sides of the plasma membrane necessitates the existence of a mechanism connecting the domains. Random site-directed mutagenesis identified the N-terminal (near-extracellular) region of M4 as a key element controlling the C-type gate (Bagriantsev et al., 2011). Within this region, the site corresponding to Trp275 of TREK-1 is critical for gating various K2P channels, including TWIK-1 (Chatelain et al., 2012), TREK-2, TASK-1, TASK-2, and TASK-3 (Bagriantsev et al., 2011). Mutagenic analysis showed that substitution of Trp275 with serine, or another amino acid with smaller side chain, stabilizes the selectivity filter of TREK-1 in an open, potassium-selective conformation, causing significant attenuation of temperature responses.
The determination of TRAAK crystal structure showed that the N-terminal portion of M4 is tightly packed against the pore helix 1, and that the side chain of Trp262 (Trp275 in TREK-1) extends to Gly124, a position equivalent to Gly137 in TREK-1 (Brohawn et al., 2012). The close opposition of the two key regulators of the C-type gate provides a mechanistic explanation for how the N-terminal segment of M4 could transmit structural deformations of Ct to the gate. In another high-resolution TRAAK structure, the M4 segment has moved, leading to a rotamer switch of the side chain of Trp262 away from Gly124 and the disappearance of a tight interaction between the N-terminal segment of M4 and pore helix 1 (Brohawn et al., 2013). Thus, functional and structural data suggest that the C-type gate and Ct can be coupled via M4. In this model, a shift in the dynamic equilibrium between membrane-bound and dissociated conformations of Ct can displace M4, causing significant changes around the selectivity filter and thus affecting channel function (Figure 5.3).
A functional TREK-1 molecule contains two Cts. While each Ct is connected to the pore via the M4, it remained unclear whether each Ct affects the gate via the cis- or trans-M4 segment (or both). This question was resolved using TREK-1 concatamers bearing activating (E306A) and decoupling (3G) mutations in the same or neighboring Ct. The E306A mutation stimulates the association of Ct with the plasma membrane (Chemin et al., 2005; Sandoz et al., 2011), and leads to channel activation via stabilization of the C-type gate (Bagriantsev et al., 2012; Piechotta et al., 2011). When present in only one Ct of a TREK-1 concatamer, E306A causes half-maximal stabilization of the gate. This effect can be eliminated by decoupling the mutated, but not the neighboring wild-type, Ct with a 3G mutation (Bagriantsev et al., 2012). These data showed that Cts act independently, and that the mechanism coupling each Ct and the C-type gate involves M4 segments of the same subunit, i.e., in cis configuration.
While selective activation of only one Ct in a TREK-1 by temperature is experimentally challenging, it is possible to compare temperature responses of TREK-1 concatamers with one Ct decoupled from the pore. Experiments showed that decoupling of any one Ct significantly attenuates temperature response, but does not eliminate it. Thus, while both C termini are required to achieve full temperature activation, a partial effect can be achieved with only one Ct (Bagriantsev et al., 2012). The similarity between the effects of E306A and temperature suggest that TREK-1 activation through Ct proceeds through a similar mechanism, regardless of the nature of the activating stimulus.
4. HEAT- AND MECHANOSENSITIVITY OF K2PS: DIFFERENT FACETS OF THE SAME PROCESS?
Heat-sensitive K2Ps are truly polymodal ion channels. In addition to temperature, intra- and extracellular protons, fatty acids, phospholipids, and various small molecule compounds, TREK-1, TREK-1, and TRAAK are potently activated by membrane stretch (Bang, Kim, & Kim, 2000; Honore, Patel, Chemin, Suchyna, & Sachs, 2006; Lesage, Terrenoire, Romey, & Lazdunski, 2000; Maingret, Fosset, Lesage, Lazdunski, & Honore, 1999, Maingret, Patel et al., 1999; Patel et al., 1998). Mutations that affect Ct function (Honore et al., 2002; Maingret, Patel et al., 1999; Patel et al., 1998) or stabilize the C-type gate (Bagriantsev et al., 2011) attenuate TREK-1 mechanosensitivity, highlighting a general importance of these regions for TREK-1 gating. Even though the exact role of these domains in mechanosensitivity requires clarification, it is tempting to speculate that temperature and mechanical force activate TREK-1 via similar mechanisms, whereby Ct serves as a mechanosensor and affects channel function via the C-type gate.
The exact structural changes that accompany temperature-evoked gating of the K2Ps and other ion channels, are unclear (Clapham & Miller, 2011; Chowdhury et al., 2014). Significant advances have been made with regard to understanding how ion channels are gated by mechanical force, but this largely pertains to prokaryotic channels, such as MscS and MscL. In these channels, membrane stretch invokes drastic structural changes, leading to stabilization of an open conformation with the pore large enough to allow passage of not only ions, but also bulky metabolites (Sukharev & Sachs, 2012). Mammalian mechanogated ion channels, such as the K2Ps, Piezo1 and 2 (Coste et al., 2010, Coste et al., 2012), and others (Arnadottir & Chalfie, 2010; Delmas, Hao, & Rodat-Despoix, 2011), exhibit significant ion (or at least charge) preference and presumably require relatively subtle structural perturbations that do not profoundly impact selectivity. This assumption is exemplified in the case of TREK-1 and TRAAK, which remain potassium selective under both low and high membrane tension (Brohawn, Su, & Mackinnon, 2014). The actual gating mechanism(s) that transform membrane tension into channel opening is unclear, but it may, similar to heat-evoked gating, involve stabilization of the C-type gate. It is important to note that such mechanism would not require any additional components other than the lipid membrane, as purified K2Ps exhibit robust mechanosensitivity in artificial membranes (Berrier et al., 2013; Brohawn et al., 2014). The emerging similarity in the mechanism of heat- and mechanoactivation of K2Ps suggests that these studies may eventually converge to provide a unifying explanation of how the apparently different physical cues affect channel function.
5. FUTURE STUDIES OF K2P CHANNEL THERMAL SENSITIVITY
5.1 Are K2Ps intrinsically heat sensitive?
Among key unsolved questions is the requirement of intracellular factors for heat sensitivity of TREK-1 and other K2Ps. If such a component (or components) exists, it should be ubiquitous and evolutionarily conserved, as TREK-1 is robustly temperature sensitive in amphibian and mammalian cells (Bagriantsev et al., 2011, 2012; Kang et al., 2005; Maingret et al., 2000). Patch excision was reported to obliterate heat sensitivity of the K2Ps in COS7 cells (Kang et al., 2005; Maingret et al., 2000), demonstrating that cell integrity is crucial for temperature activation. Possibly, patch excision leads to dissociation of a cytosolic component (Noel et al., 2011) which interacts with TREK-1 and which is mandatory for heat sensitivity, while the channel itself lacks intrinsic thermal properties. To demonstrate this convincingly would require investigation of thermal properties of purified ion channels in artificial membranes. Recently, this method was used to demonstrate that the nonselective heat-activated ion channel TRPV1 does not require additional proteinaceous components for temperature response (Cao, Cordero-Morales, Liu, Qin, & Julius, 2013).
5.2 What are the physiological roles of the heat-activated K2P current?
In addition to the proposed role in somatosensory heat responses, temperature-activated K2P channels were suggested to contribute to temperature-dependent excitability of the neurons in the hippocampus (de la Pena et al., 2012), Grueneberg ganglion (Stebe et al., 2013), and pre-optic area (POA) of the hypothalamus (Wechselberger et al., 2006). The thermoregulatory region of POA contains numerous thermosensitive neurons which are thought to act as sensors for internal temperature. Temperature increases firing rate of a subpopulation of POA neurons (Zhao & Boulant, 2005) through an unknown mechanism. Interestingly, the half-maximal temperature activation point (T1/2) of TREK-1 expressed in Xenopus oocytes is very close to normal body temperature. While temperature properties of K2P channels in native and heterologous systems may differ, it is worth noting that since temperature changes produce the most significant effect precisely at T1/2, the heat-activated K2Ps are uniquely positioned to sense minute variations of body temperature. In this regard, it is interesting to consider the contribution of heat-evoked potassium current to the physiology of cells and tissues where TREK-1 or other K2Ps have been shown to play functional roles, such as in lung epithelial cells (Davis & Cowley, 2006; Roan, Waters, Teng, Ghosh, & Schwingshackl, 2014; Schwingshackl, Teng, Ghosh, & Waters, 2013), myometrium (Heyman et al., 2013; Wu, Singer, & Buxton, 2012), and endothelium of blood vessels (Bittner et al., 2013; Garry et al., 2007; Namiranian et al., 2010).
While it is well established that K2Ps mediate potassium efflux, several reports showed that potassium selectivity of TREK-1 (Bagriantsev et al., 2011; Cohen et al., 2008; Thomas, Plant, Wilkens, McCrossan, & Goldstein, 2008) and other K2Ps (Chatelain et al., 2012; Ma, Zhang, & Chen, 2011) can be significantly compromised, leading to increased permeability to sodium. Thus, available evidence suggests that K2Ps may lose selectivity to potassium under physiologically relevant conditions. Hypothetically, a near-complete loss of cation selectivity may turn TREK-1/-2/TRAAK into a heat-activated excitatory ion channel similar to TRPV1, i.e., its own functional antipode.
Finally, we note that the striking dependence of TREK-1/-2/TRAAK activity on temperature indicates that this factor must be taken into account in any study investigating K2P channel function in physiological context. As discussed above, multiple modalities that regulate K2P channel function act via the extracellular C-type gate. Therefore, studying the activity of heat-sensitive K2Ps at room temperature, i.e., under conditions when the gate does not receive the tonic activating stimulus it receives at 37 °C, will complicate the analysis of the physiological role of these channels.
ACKNOWLEDGMENTS
We thank Willem Laursen for comments on the manuscript. This work was supported by a grant from the American Heart Association (14SDG17880015) to S.N.B., and by fellowships from the Beckman Foundation and Alfred P. Sloan Foundation to E.O.G.
REFERENCES
- Acosta C, Djouhri L, Watkins R, Berry C, Bromage K, Lawson SN. TREK2 expressed selectively in IB4-binding C-fiber nociceptors hyperpolarizes their membrane potentials and limits spontaneous pain. Journal of Neuroscience. 2014;34:1494–1509. doi: 10.1523/JNEUROSCI.4528-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagem N, Yesylevskyy S, Reuveny E. The pore helix is involved in stabilizing the open state of inwardly rectifying K+ channels. Biophysical Journal. 2003;85:300–312. doi: 10.1016/S0006-3495(03)74475-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloui A, Zimmermann K, Mamet J, Duprat F, Noel J, Chemin J, et al. TREK-1, a K+ channel involved in polymodal pain perception. EMBO Journal. 2006;25:2368–2376. doi: 10.1038/sj.emboj.7601116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnadottir J, Chalfie M. Eukaryotic mechanosensitive channels. Annual Review of Biophysics. 2010;39:111–137. doi: 10.1146/annurev.biophys.37.032807.125836. [DOI] [PubMed] [Google Scholar]
- Bagriantsev SN, Ang KH, Gallardo-Godoy A, Clark KA, Arkin MR, Renslo AR, et al. A high-throughput functional screen identifies small molecule regulators of temperature- and mechano-sensitive K2P channels. ACS Chemical Biology. 2013;8:1841–1851. doi: 10.1021/cb400289x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagriantsev SN, Clark KA, Minor DL., Jr. Metabolic and thermal stimuli control K(2P)2.1 (TREK-1) through modular sensory and gating domains. EMBO Journal. 2012;31:3297–3308. doi: 10.1038/emboj.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagriantsev SN, Peyronnet R, Clark KA, Honore E, Minor DL., Jr. Multiple modalities converge on a common gate to control K(2P) channel function. EMBO Journal. 2011;30:3594–3606. doi: 10.1038/emboj.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang H, Kim Y, Kim D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. Journal of Biological Chemistry. 2000;275:17412–17419. doi: 10.1074/jbc.M000445200. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, et al. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Bearzatto B, Lesage F, Reyes R, Lazdunski M, Laduron PM. Axonal transport of TREK and TRAAK potassium channels in rat sciatic nerves. Neuroreport. 2000;11:927–930. doi: 10.1097/00001756-200004070-00006. [DOI] [PubMed] [Google Scholar]
- Ben-Abu Y, Zhou Y, Zilberberg N, Yifrach O. Inverse coupling in leak and voltage-activated K+ channel gates underlies distinct roles in electrical signaling. Nature Structural & Molecular Biology. 2009;16:71–79. doi: 10.1038/nsmb.1525. [DOI] [PubMed] [Google Scholar]
- Berrier C, Pozza A, de Lacroix de Lavalette A, Chardonnet S, Mesneau A, Jaxel C, et al. The purified mechanosensitive channel TREK-1 is directly sensitive to membrane tension. Journal of Biological Chemistry. 2013;288:27307–27314. doi: 10.1074/jbc.M113.478321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner S, Ruck T, Schuhmann MK, Herrmann AM, Maati HM, Bobak N, et al. Endothelial TWIK-related potassium channel-1 (TREK1) regulates immune-cell trafficking into the CNS. Nature Medicine. 2013;19:1161–1165. doi: 10.1038/nm.3303. [DOI] [PubMed] [Google Scholar]
- Brohawn SG, Campbell EB, MacKinnon R. Domain-swapped chain connectivity and gated membrane access in a Fab-mediated crystal of the human TRAAK K+ channel. Proc Natl Acad Sci U S A. 2013;110:2129–2134. doi: 10.1073/pnas.1218950110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn SG, del Marmol J, MacKinnon R. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science. 2012;335:436–441. doi: 10.1126/science.1213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brohawn SG, Su Z, MacKinnon R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc Natl Acad Sci U S A. 2014;111:3614–3619. doi: 10.1073/pnas.1320768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan RM, Jr., You J, Phillips SC, Andresen JJ, Lloyd EE, Rogers PA, et al. Evidence for two-pore domain potassium channels in rat cerebral arteries. American Journal of Physiology - Heart and Circulatory Physiology. 2006;291:H770–H780. doi: 10.1152/ajpheart.01377.2005. [DOI] [PubMed] [Google Scholar]
- Cao E, Cordero-Morales JF, Liu B, Qin F, Julius D. TRPV1 channels are intrinsically heat sensitive and negatively regulated by phosphoinositide lipids. Neuron. 2013;77:667–679. doi: 10.1016/j.neuron.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chatelain FC, Bichet D, Douguet D, Feliciangeli S, Bendahhou S, Reichold M, et al. TWIK1, a unique background channel with variable ion selectivity. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5499–5504. doi: 10.1073/pnas.1201132109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemin J, Patel AJ, Duprat F, Lauritzen I, Lazdunski M, Honore E. A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO Journal. 2005;24:44–53. doi: 10.1038/sj.emboj.7600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Jarecki BW, Chanda B. A molecular framework for temperature-dependent gating of ion channels. Cell. 2014;158:1148–1158. doi: 10.1016/j.cell.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE, Miller C. A thermodynamic framework for understanding temperature sensing by transient receptor potential (TRP) channels. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19492–19497. doi: 10.1073/pnas.1117485108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Ben-Abu Y, Hen S, Zilberberg N. A novel mechanism for human K2P2.1 channel gating. Facilitation of C-type gating by protonation of extracellular histidine residues. Journal of Biological Chemistry. 2008;283:19448–19455. doi: 10.1074/jbc.M801273200. [DOI] [PubMed] [Google Scholar]
- Colburn RW, Lubin ML, Stone DJ, Jr., Wang Y, Lawrence D, D'Andrea MR, et al. Attenuated cold sensitivity in TRPM8 null mice. Neuron. 2007;54:379–386. doi: 10.1016/j.neuron.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Contreras JE, Srikumar D, Holmgren M. Gating at the selectivity filter in cyclic nucleotide-gated channels. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3310–3314. doi: 10.1073/pnas.0709809105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-Morales JF, Cuello LG, Zhao Y, Jogini V, Cortes DM, Roux B, et al. Molecular determinants of gating at the potassium-channel selectivity filter. Nature Structural and Molecular Biology. 2006;13:311–318. doi: 10.1038/nsmb1069. [DOI] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483:176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KA, Cowley EA. Two-pore-domain potassium channels support anion secretion from human airway Calu-3 epithelial cells. Pflugers Archiv. 2006;451:631–641. doi: 10.1007/s00424-005-1505-4. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- de la Pena E, Malkia A, Vara H, Caires R, Ballesta JJ, Belmonte C, et al. The influence of cold temperature on cellular excitability of hippocampal networks. PLoS One. 2012;7:e52475. doi: 10.1371/journal.pone.0052475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P, Hao J, Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nature Reviews Neuroscience. 2011;12:139–153. doi: 10.1038/nrn2993. [DOI] [PubMed] [Google Scholar]
- Descoeur J, Pereira V, Pizzoccaro A, Francois A, Ling B, Maffre V, et al. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Molecular Medicine. 2011;3:266–278. doi: 10.1002/emmm.201100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey D, Eckle VS, Vitko I, Sullivan KA, Lasiecka ZM, Winckler B, et al. A potassium leak channel silences hyperactive neurons and ameliorates status epilepticus. Epilepsia. 2014;55:203–213. doi: 10.1111/epi.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Dobler T, Springauf A, Tovornik S, Weber M, Schmitt A, Sedlmeier R, et al. TRESK two-pore-domain K+ channels constitute a significant component of background potassium currents in murine dorsal root ganglion neurons. Journal of Physiology. 2007;585:867–879. doi: 10.1113/jphysiol.2007.145649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Lopez S, Howell R, Gobbi G. Characterization of serotonin neurotransmission in knockout mice: implications for major depression. Reviews in the Neurosciences. 2012;23:429–443. doi: 10.1515/revneuro-2012-0044. [DOI] [PubMed] [Google Scholar]
- Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiological Reviews. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- Findeisen F, Minor DL., Jr. Disruption of the IS6-AID linker affects voltage-gated calcium channel inactivation and facilitation. Journal of General Physiology. 2009;133:327–343. doi: 10.1085/jgp.200810143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, et al. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO Journal. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- Furini S, Domene C. Gating at the selectivity filter of ion channels that conduct Na+ and K+ ions. Biophysical Journal. 2011;101:1623–1631. doi: 10.1016/j.bpj.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry A, Fromy B, Blondeau N, Henrion D, Brau F, Gounon P, et al. Altered acetylcholine, bradykinin and cutaneous pressure-induced vasodilation in mice lacking the TREK1 potassium channel: the endothelial link. EMBO Reports. 2007;8:354–359. doi: 10.1038/sj.embor.7400916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracheva EO, Cordero-Morales JF, Gonzalez-Carcacia JA, Ingolia NT, Manno C, Aranguren CI, et al. Ganglion-specific splicing of TRPV1 underlies infrared sensation in vampire bats. Nature. 2011;476:88–91. doi: 10.1038/nature10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurteaux C, Guy N, Laigle C, Blondeau N, Duprat F, Mazzuca M, et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO Journal. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurteaux C, Lucas G, Guy N, El Yacoubi M, Thummler S, Peng XD, et al. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nature Neuroscience. 2006;9:1134–1141. doi: 10.1038/nn1749. [DOI] [PubMed] [Google Scholar]
- Heyman NS, Cowles CL, Barnett SD, Wu YY, Cullison C, Singer CA, Leblanc N, Buxton IL. TREK-1 currents in smooth muscle cells from pregnant human myometrium. Am J Physiol Cell Physiol. 2013;305:C632–642. doi: 10.1152/ajpcell.00324.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore E, Maingret F, Lazdunski M, Patel AJ. An intracellular proton sensor commands lipid- and mechano-gating of the K(+) channel TREK-1. EMBO Journal. 2002;21:2968–2976. doi: 10.1093/emboj/cdf288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore E, Patel AJ, Chemin J, Suchyna T, Sachs F. Desensitization of mechano-gated K2P channels. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:6859–6864. doi: 10.1073/pnas.0600463103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang EM, Kim E, Yarishkin O, Woo DH, Han KS, Park N, et al. A disulphide-linked heterodimer of TWIK-1 and TREK-1 mediates passive conductance in astrocytes. Nature Communications. 2014;5:3227. doi: 10.1038/ncomms4227. [DOI] [PubMed] [Google Scholar]
- Kang D, Choe C, Kim D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. Journal of Physiology. 2005;564:103–116. doi: 10.1113/jphysiol.2004.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Hogan JO, Kim D. THIK-1 (K2P13.1) is a small-conductance background K(+) channel in rat trigeminal ganglion neurons. Pflugers Arch. 2014;466:1289–1300. doi: 10.1007/s00424-013-1358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Kim D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. American Journal of Physiology - Cell Physiology. 2006;291:C138–C146. doi: 10.1152/ajpcell.00629.2005. [DOI] [PubMed] [Google Scholar]
- Kennard LE, Chumbley JR, Ranatunga KM, Armstrong SJ, Veale EL, Mathie A. Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. British Journal of Pharmacology. 2005;144:821–829. doi: 10.1038/sj.bjp.0706068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, et al. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. Journal of Neuroscience. 2013;33:2837–2848. doi: 10.1523/JNEUROSCI.1943-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Hori A, Matsumura K, Hosokawa H. Point: heat-induced membrane depolarization of hypothalamic neurons: a putative mechanism of central thermosensitivity. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2006;290:R1479–R1480. doi: 10.1152/ajpregu.00655.2005. discussion R1484. [DOI] [PubMed] [Google Scholar]
- Laigle C, Confort-Gouny S, Le Fur Y, Cozzone PJ, Viola A. Deletion of TRAAK potassium channel affects brain metabolism and protects against ischemia. PLoS One. 2012;7:e53266. doi: 10.1371/journal.pone.0053266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage F, Terrenoire C, Romey G, Lazdunski M. Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi, and Gq protein-coupled receptors. Journal of Biological Chemistry. 2000;275:28398–28405. doi: 10.1074/jbc.M002822200. [DOI] [PubMed] [Google Scholar]
- Liu X, Huang H, Wang W, Wang J, Sachs F, Niu W. Stretch-activated potassium channels in hypotonically induced blebs of atrial myocytes. Journal of Membrane Biology. 2008;226:17–25. doi: 10.1007/s00232-008-9135-3. [DOI] [PubMed] [Google Scholar]
- Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- Lopes CM, Rohacs T, Czirjak G, Balla T, Enyedi P, Logothetis DE. PIP2 hydrolysis underlies agonist-induced inhibition and regulates voltage gating of two-pore domain K+ channels. Journal of Physiology. 2005;564:117–129. doi: 10.1113/jphysiol.2004.081935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Fosset M, Lesage F, Lazdunski M, Honore E. TRAAK is a mammalian neuronal mechano-gated K+ channel. Journal of Biological Chemistry. 1999;274:1381–1387. doi: 10.1074/jbc.274.3.1381. [DOI] [PubMed] [Google Scholar]
- Maingret F, Lauritzen I, Patel AJ, Heurteaux C, Reyes R, Lesage F, et al. TREK-1 is a heat-activated background K(+) channel. EMBO Journal. 2000;19:2483–2491. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. Journal of Biological Chemistry. 1999;274:26691–-26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- Ma XY, Yu JM, Zhang SZ, Liu XY, Wu BH, Wei XL, et al. External Ba2+ block of the two-pore domain potassium channel TREK-1 de fines conformational transition in its selectivity filter. Journal of Biological Chemistry. 2011;286:39813–39822. doi: 10.1074/jbc.M111.264788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Zhang X, Chen H. TWIK-1 two-pore domain potassium channels change ion selectivity and conduct inward leak sodium currents in hypokalemia. Science Signaling. 2011;4:ra37. doi: 10.1126/scisignal.2001726. [DOI] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Medhurst AD, Rennie G, Chapman CG, Meadows H, Duckworth MD, Kelsell RE, et al. Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Brain Research Molecular Brain Research. 2001;86:101–114. doi: 10.1016/s0169-328x(00)00263-1. [DOI] [PubMed] [Google Scholar]
- Miller AN, Long SB. Crystal structure of the human two-pore domain potassium channel K2P1. Science. 2012;335:432–436. doi: 10.1126/science.1213274. [DOI] [PubMed] [Google Scholar]
- Murbartian J, Lei Q, Sando JJ, Bayliss DA. Sequential phosphorylation mediates receptor- and kinase-induced inhibition of TREK-1 background potassium channels. Journal of Biological Chemistry. 2005;280:30175–30184. doi: 10.1074/jbc.M503862200. [DOI] [PubMed] [Google Scholar]
- Namiranian K, Lloyd EE, Crossland RF, Marrelli SP, Taffet GE, Reddy AK, et al. Cerebrovascular responses in mice deficient in the potassium channel, TREK-1. American Journal of Physiology - Regulatory Integrative and Comparative Physiology. 2010;299:R461–R469. doi: 10.1152/ajpregu.00057.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemeyer MI, Cid LP, Pena-Munzenmayer G, Sepulveda FV. Separate gating mechanisms mediate the regulation of K2P potassium channel TASK-2 by intraand extracellular pH. Journal of Biological Chemistry. 2010;285:16467–16475. doi: 10.1074/jbc.M110.107060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel J, Sandoz G, Lesage F. Molecular regulations governing TREK and TRAAK channel functions. Channels (Austin) 2011;5:402–409. doi: 10.4161/chan.5.5.16469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, et al. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO Journal. 2009;28:1308–1318. doi: 10.1038/emboj.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park U, Vastani N, Guan Y, Raja SN, Koltzenburg M, Caterina MJ. TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. Journal of Neuroscience. 2011;31:11425–11436. doi: 10.1523/JNEUROSCI.1384-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nature Neuroscience. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Maingret F, Lesage F, Fink M, Duprat F, et al. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO Journal. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Piechotta PL, Rapedius M, Stansfeld PJ, Bollepalli MK, Ehrlich G, Andres-Enguix I, et al. The pore structure and gating mechanism of K2P channels. EMBO Journal. 2011;30:3607–3619. doi: 10.1038/emboj.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant LD. A role for K2P channels in the operation of somatosensory nociceptors. Frontiers in Molecular Neuroscience. 2012;5:21. doi: 10.3389/fnmol.2012.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogorzala LA, Mishra SK, Hoon MA. The cellular code for Mammalian thermosensation. Journal of Neuroscience. 2013;33:5533–5541. doi: 10.1523/JNEUROSCI.5788-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S, Wischmeyer E, Karschin C, Preisig-Muller R, Grzeschik KH, Daut J, et al. THIK-1 and THIK-2, a novel subfamily of tandem pore domain K+ channels. Journal of Biological Chemistry. 2001;276:7302–7311. doi: 10.1074/jbc.M008985200. [DOI] [PubMed] [Google Scholar]
- Roan E, Waters CM, Teng B, Ghosh M, Schwingshackl A. The 2-Pore domain potassium channel TREK-1 regulates stretch-induced Detachment of alveolar epithelial cells. PLoS One. 2014;9:e89429. doi: 10.1371/journal.pone.0089429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz G, Bell SC, Isacoff EY. Optical probing of a dynamic membrane interaction that regulates the TREK1 channel. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:2605–2610. doi: 10.1073/pnas.1015788108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz G, Douguet D, Chatelain F, Lazdunski M, Lesage F. Extracellular acidification exerts opposite actions on TREK1 and TREK2 potassium channels via a single conserved histidine residue. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14628–14633. doi: 10.1073/pnas.0906267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwingshackl A, Teng B, Ghosh M, Waters CM. Regulation of monocyte chemotactic protein-1 secretion by the two-pore-domain potassium (K2P) channel TREK-1 in human alveolar epithelial cells. American Journal of Translational Research. 2013;5:530–542. [PMC free article] [PubMed] [Google Scholar]
- Stebe S, Schellig K, Lesage F, Breer H, Fleischer J. The thermosensitive potassium channel TREK-1 contributes to coolness-evoked responses of Grueneberg ganglion neurons. Cellular and molecular neurobiology. 2014;34:113–122. doi: 10.1007/s10571-013-9992-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Anishkin A, Kung C, Saimi Y. The core domain as the force sensor of the yeast mechanosensitive TRP channel. Journal of General Physiology. 2011;138:627–640. doi: 10.1085/jgp.201110693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukharev S, Sachs F. Molecular force transduction by ion channels: diversity and unifying principles. Journal of Cell Science. 2012;125:3075–3083. doi: 10.1242/jcs.092353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. Journal of Neuro-science. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Plant LD, Wilkens CM, McCrossan ZA, Goldstein SA. Alternative translation initiation in rat brain yields K2P2.1 potassium channels permeable to sodium. Neuron. 2008;58:859–870. doi: 10.1016/j.neuron.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee N, Meckler C, Risso JJ, Blatteau JE. Neuroprotective role of the TREK-1 channel in decompression sickness. Journal of Applied Physiology. 2012;112:1191–1196. doi: 10.1152/japplphysiol.01100.2011. [DOI] [PubMed] [Google Scholar]
- Vallee N, Rostain JC, Risso JJ. Low susceptibility to inert gases and pressure symptoms in TREK-1-deficient mice. Neuroreport. 2009;20:343–347. doi: 10.1097/wnr.0b013e328322ca4d. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang M, Li P, Yuan H, Feng N, Peng Y, et al. An increased TREK-1-like potassium current in ventricular myocytes during rat cardiac hypertrophy. Journal of Cardiovascular Pharmacology. 2013;61:302–310. doi: 10.1097/FJC.0b013e318280c5a9. [DOI] [PubMed] [Google Scholar]
- Wechselberger M, Wright CL, Bishop GA, Boulant JA. Ionic channels and conductance-based models for hypothalamic neuronal thermosensitivity. American Journal of Physiology - Regulatory Integrative and Comparative Physiology. 2006;291:R518–R529. doi: 10.1152/ajpregu.00039.2006. [DOI] [PubMed] [Google Scholar]
- Woo DH, Han KS, Shim JW, Yoon BE, Kim E, Bae JY, et al. TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell. 2012;151:25–40. doi: 10.1016/j.cell.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Wu X, Liu Y, Chen X, Sun Q, Tang R, Wang W, et al. Involvement of TREK-1 activity in astrocyte function and neuroprotection under simulated ischemia conditions. Journal of Molecular Neuroscience. 2013;49:499–506. doi: 10.1007/s12031-012-9875-5. [DOI] [PubMed] [Google Scholar]
- Wu YY, Singer CA, Buxton IL. Variants of stretch-activated two-pore potassium channel TREK-1 associated with preterm labor in humans. Biology of Reproduction. 2012;87:96. doi: 10.1095/biolreprod.112.099499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian Tao L, Dyachenko V, Zuzarte M, Putzke C, Preisig-Muller R, Isenberg G, et al. The stretch-activated potassium channel TREK-1 in rat cardiac ventricular muscle. Cardiovascular Research. 2006;69:86–97. doi: 10.1016/j.cardiores.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Hatakeyama T, Taniguchi K. Immunohistochemical colocalization of TREK-1, TREK-2 and TRAAK with TRP channels in the trigeminal ganglion cells. Neuroscience Letters. 2009;454:129–133. doi: 10.1016/j.neulet.2009.02.069. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Boulant JA. Temperature effects on neuronal membrane potentials and inward currents in rat hypothalamic tissue slices. Journal of Physiology. 2005;564:245–257. doi: 10.1113/jphysiol.2004.075473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao LN, Fu L, Gao QP, Xie RS, Cao JX. Regional differential expression of TREK-1 at left ventricle in myocardial infarction. Canadian Journal of Cardiology. 2011;27:826–833. doi: 10.1016/j.cjca.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Zilberberg N, Ilan N, Goldstein SA. KCNKO: opening and closing the 2-P-domain potassium leak channel entails “C-type” gating of the outer pore. Neuron. 2001;32:635–648. doi: 10.1016/s0896-6273(01)00503-7. [DOI] [PubMed] [Google Scholar]