Abstract
Patients with functional pain disorders often complain of generalized sensory hypersensitivity, finding sounds, smells, or even everyday light aversive. The neural basis for this aversion is unknown, but cannot be attributed to a general increase in cortical sensory processing. Here we quantified the threshold for aversion to light in patients with fibromyalgia, a pain disorder thought to reflect dysregulation of brain pain-modulating systems. These individuals expressed discomfort at light levels substantially lower than healthy controls. Complementary studies in lightly anesthetized rat demonstrated that a subset of identified pain-modulating neurons in the rostral ventromedial medulla unexpectedly responds to light. Approximately half of the pain-facilitating “ON-cells” and pain-inhibiting “OFF-cells” sampled exhibited a change in firing with light exposure, shifting the system to a pro-nociceptive state with activation of ON-cells and suppression of OFF-cell firing. The change in neuronal firing did not require a trigeminal or posterior thalamic relay, but was blocked by inactivation of the olivary pretectal nucleus. Light exposure also resulted in a measurable but modest decrease in the threshold for heat-evoked paw withdrawal, as would be expected with engagement of this pain-modulating circuitry.
These data demonstrate integration of information about light intensity with somatic input at the level of single pain-modulating neurons in the brainstem of the rat under basal conditions. Taken together, our findings in rodents and humans provide a novel mechanism for abnormal photosensitivity, and suggest that light has the potential to engage pain-modulating systems such that normally innocuous inputs are perceived as aversive or even painful.
Keywords: pain-modulation, descending control, rostral ventromedial medulla, multisensory hypersensitivity, fibromyalgia
1. Introduction
Patients with functional pain disorders often complain of multisensory hypersensitivity, reporting that normal sounds, odors, and even everyday light are bothersome.20–21,58 This clinical observation is rarely quantified, and the underlying neural basis remains a puzzle. However, it is known that sensory acuity per se is not enhanced, nor is there amplified processing in primary sensory pathways.10,38–40,48 Here we focused on photosensitivity, and explored the possibility that intrinsic brain pain-modulating systems are engaged by light and thereby contribute to sensory hypersensitivity.
We first considered sensitivity to light in patients with fibromyalgia, a well-described but poorly understood pain disorder in which impaired pain-modulating systems are thought to play an important role.37,50,62 We determined that the threshold for light-evoked aversion and the ability to tolerate light in these patients was substantially reduced compared to healthy controls.
We next investigated a potential neural mechanism for abnormal generalized photosensitivity. The exacerbation of migraine headache pain by light and light-induced pain in the eye region in uveitis and other ophthalmic conditions are well known. These forms of photosensitivity are likely explained by a convergence of visual input with trigeminal nociceptive information at the level of the trigeminal nucleus in the caudal medulla or in the posterior thalamus.44–45 However, the well-documented direct interaction of visual input with pain sensory transmission pathways does not adequately explain a more general light intolerance such as that exhibited in fibromyalgia. We therefore considered an interaction of visual input with pain-modulating circuits in rats, and found that a subset of pain-modulating neurons in the rostral ventromedial medulla (RVM), “ON-cells” and “OFF-cells”,19 unexpectedly respond to light stimuli, even under basal conditions. These neurons are the output of an intrinsic pain-modulating system, and project to the dorsal horn where they modulate somatosensory processing. ON-cells exert a net facilitating influence on nociception, and OFF-cells a net inhibitory influence. A shift in the balance between these two populations can lead to enhanced or diminished pain. Further, experimental activation of ON-cells is aversive in rodents.12,26,30,33
Finally, in order to delineate the relevant circuitry through which photic input could reach the RVM, we investigated three possible relays: the olivary pretectal nucleus (OPt), the posterior thalamus (PO), and trigeminal sensory afferents (trigeminal ganglion, TG). The first is a known relay in the pupillary light reflex,53,63 while the latter two are sites where light-related information has been shown to converge with trigeminal nociceptive transmission pathways.44–45
Taken together, our findings provide a possible mechanism for abnormal photosensitivity in the absence of migraine or eye pathology. These data raise the possibility that light modulates pain processing such that normally innocuous inputs could be perceived as uncomfortable or aversive via activation of pain-facilitating ON-cells, and suppression of pain-inhibiting OFF-cells.
2. Methods
2.1 Human Study
2.1.1. Study Participants
Twenty-four women with fibromyalgia and 24 pain-free controls from the Pacific Northwest region were consented (IRB# 9036, Oregon Health & Science University). Inclusion criteria for fibromyalgia subjects were diagnosis based on 1990 ACR criteria,60 average overall pain scores of 6 or greater over the past month, and aged 21–70 years. Healthy controls were pain-free and matched within five years on fibromyalgia subjects’ age. Exclusion criteria for all included 1) significant eye pathology (glaucoma, blindness, macular degeneration), drugs known to alter pupillary dilation, centrally-acting drugs (opioids, serotonin-reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), antiepileptics); 2) diseases typically associated with altered light sensitivity or function of the melanopsin pathway (migraines, uveitis, iritis, dry eyes, glaucoma18), and 3) diagnosis of panic attacks, seizures, or post-concussive syndrome.
2.1.2. Procedure
On the testing day, current pain levels and associated symptoms were assessed using the Revised Fibromyalgia Impact Questionnaire (FIQR) or the Revised Symptom Impact Questionnaire (SIQR, a fibromyalgia-neutral revision developed to compare non-fibromyalgia subjects with fibromyalgia patients). These are validated instruments with good psychometric properties in both fibromyalgia and healthy controls.16,20
All subjects completed somatic pressure pain sensitivity testing using pressure algometry (Medoc Algomed, Ramat Yishai, Israel), measured in kg at mid-volar forearm of the subject’s dominant side.
Methods for measuring light aversion were adapted from studies of photophobia in migraine headache.14,41,54 Subjects were dark-adapted for 30 min in a dark room on a comfortable massage table with soothing music. Maintaining a dark environment, subjects were then led to the testing room and positioned with their heads on an adjustable chinrest, and diffuse light flashes were delivered in a Ganzfeld dome using an Espion E2 photostimulator. Stimuli were delivered for 2 s at 4 s intervals. The first stimulus measured 8 lux, and each subsequent stimulus was nominally twice as bright as the previous one. (Measured range: 8 to 67,360 lux. Measured, rather than nominal, brightness was used for all analyses.) On the first five trials (Discomfort), the researcher specified: “I will increase the brightness of the light; please let me know when you feel uncomfortable.” The stimulus train was discontinued when the subject indicated discomfort, and the mean intensity at which the subject indicated discomfort was determined on each of the five trials at two-minute intervals. Following five discomfort trials, a single trial on which the subject was asked to indicate intolerance was run, with the researcher specifying: “I will continue to raise the brightness of the light; please let me know when you can no longer tolerate it.” The stimulus protocol was stopped when the subject indicated intolerance. Thresholds for discomfort and intolerance to brightness were recorded.
2.1.3. Data analysis
Demographics (age, ethnicity), baseline clinical characteristics (current pain, associated symptoms), and overall impact of disease (FIQR, SIQR) were assessed and compared using independent sample t-tests (continuous variables) and chi-squared test (categorical variables). The primary outcome was difference in mean light discomfort thresholds between the fibromyalgia group and healthy controls.
Group means were compared to examine differences in light discomfort threshold and intolerance levels between the fibromyalgia group and controls. Light intensity was log2 transformed to normalize the data for analysis (two-tailed t-tests for independent groups) and presented as geometric mean with 95% confidence limits. In addition, a moderation analysis examined the relationship between light discomfort threshold (dependent variable) and pressure pain threshold (independent variable) in the two groups (independent variable) using the standard Baron-Kenny methodology.3 A similar moderation analysis examined the relationship between light discomfort threshold (dependent variable) and clinical pain (independent variable) in the two groups (independent variable).
2.2. Animal Study
All experimental procedures followed the guidelines of the National Institutes of Health and the Committee for Research and Ethical Issues of the International Association for the Study of Pain, and were approved by the Institutional Animal Care and Use Committee at the Oregon Health & Science University. Male Sprague-Dawley and Long-Evans rats (250–350 g) were obtained from Charles River.
Studies in lightly anesthetized animals included electrophysiological recordings, pharmacological inactivation, and behavioral withdrawal testing and were performed as described previously11,42 in a room under low (< 100 lux) ambient light conditions.
2.2.1. Electrophysiological Recording
RVM neurons were isolated and characterized as ON-cells, OFF-cells, or NEUTRAL-cells. This mutually exclusive and exhaustive classification is based on firing patterns relative to nocifensor withdrawals.19 At the withdrawal, “ON-cells” become active (if not already active), “OFF-cells” cease firing (if active), and NEUTRAL-cells do not change firing rate.
2.2.2. Behavioral Testing
For thermal nociceptive testing, a Peltier device (Yale Instrumentation, New Haven, CT) was lightly applied to the plantar surface of the left hindpaw, and heated at a constant rate of 1.2 °C/s from 35 °C to a maximum of 53 °C. To avoid damage to the paw, the heat stimulus was removed when the animal initiated movement of the stimulated paw (as indicated in EMG).
To characterize RVM responses to light and determine the effect of light-exposure on nociceptive withdrawal, diffused light stimuli (30 s, 18×103 lux, comparable to that employed by Okamoto et al.45 and less than the brightest stimulus intensity employed by Noseda et al.44 or in the human studies described above) were delivered using a fiber-optic source (Dolan-Jenner Fiber-Lite, Dolan-Jenner Industries, Buxborough, MA) placed 5 cm from the right eye. For the Long-Evans strain, pupils were dilated to eliminate differences in the amount of light reaching the retina due to any variations in the pupillary light reflex between animals or from trial to trial. For neurons with background activity, the neuron was considered “photosensitive” if firing rate during the 30-s stimulus changed by at least 50% relative to background activity, and “light-insensitive” if any change was less than 50%. In the case of ON-cells without spontaneous activity, the cell was considered responsive if at least 10 spikes occurred during the stimulus. These criteria were chosen based on the known changes in firing during heat-evoked withdrawal. In the present experiments, OFF-cell firing changed 75 ± 3% (mean ± SEM) at the time of the paw withdrawal, and ON-cell firing changed 229 ± 15% relative to pre-stimulus baseline, and withdrawal-related and light-evoked changes in firing of light-sensitive ON- and OFF-cells were comparable (see Results). The 50% criterion is also conservative relative to the 25% change in threshold used by others.44 Our data may therefore underestimate the incidence of photoresponsiveness in this region.
2.2.3. Experimental Protocols
RVM extracellular single-unit recording
Sprague-Dawley (albino, n = 75) and Long-Evans (black-hooded, n = 17) rats were used in this set of experiments. There was no difference between the strains in incidence of photoresponsiveness (Fisher’s Exact Test, p = 0.58), so data were combined for all analyses. To assess the responsiveness of identified RVM ON-, OFF-, and NEUTRAL-cells to light, stimuli were applied at 5-min intervals, alternating noxious heat and light, with two or three trials for each stimulus. Interleaving noxious heat and light trials at predefined intervals provides a consistent pattern of noxious stimulation and confirms anesthetic stability. This approach also avoids confounds that arise when the delivery of the stimulus is determined by the experimenter who is monitoring the activity of the cell. In this set of experiments, more than one cell could be characterized in an individual animal as the electrode was moved through the RVM, and 176 cells (90 ON-cells, 73 OFF-cells, and 13 NEUTRAL-cells) were recorded in the 92 rats.
Inactivation of potential relays of light information to the RVM
An inactivation strategy was used to determine whether light stimuli influence the RVM via trigeminal afferents, the posterior thalamus, or the olivary pretectal nucleus. These experiments were performed in a subset of the animals described above. After isolating a photosensitive ON-cell or OFF-cell, baseline data were collected (interleaved responses to heat and light stimuli at 5 min intervals, 2x each stimulus as described above). Lidocaine (4%) was microinjected into the right trigeminal ganglion (TG, 1 μl over 3 min), left posterior thalamus (PO, 500 nl over 3 min), or left olivary pretectal nucleus (OPt, 200 nl over 2 min) using a 1-μl Hamilton syringe connected to a glass micropipette (OD 75–80 μm, PO and OPt) or a metal cannula (33 ga, TG) with PE50 tubing. Although lidocaine blocks fibers of passage as well as local cell bodies, it has the advantage of a short duration of action, which allowed us to verify that the RVM response recovered after the block. Evoked responses were monitored for an additional 30 minutes. Coordinates for the different targets were as follows. TG: 3.0 mm caudal and 2.6 mm lateral to bregma, 10.1 to 10.5 mm from brain surface. PO: 5.6 mm anterior to interaural line, 2.5 mm lateral to midline, 5.0 mm below brain surface. These coordinates targeted injection sites near the dorsal border of the PO, since the majority of cells responding to both dural and light stimulation are found near or above the dorsal border of the PO.44 OPt: 4.2 mm anterior to interaural line and 1.4 mm lateral to midline, 4.5 mm below brain surface.
Effect of light stimulus on nociceptive threshold
To determine whether the threshold for heat-evoked paw withdrawal was altered during exposure to light, we alternated heat trials at 5-min intervals with and without concomitant light stimulation, for a total of six trials (3 Heat alone, 3 Light+Heat). In trials with light stimulation, the light stimulus was turned on for 30 s, and the heat stimulus triggered approximately 10 s into the light stimulus. Heat was terminated when the animal withdrew from the heat stimulus. (Sprague-Dawley strain, n = 10).
2.2.4. Histology
Microinjection sites were marked by fluorescent beads (FluoSpheres, Invitrogen, Eugene, OR) included in the lidocaine solution. Recording sites were marked with an electrolytic lesion at the conclusion of the experiment. Recording and injection sites were mapped using the atlas of Paxinos and Watson.47 Data from sites outside of the boundaries of the target structures were excluded from analysis.
2.2.5. Data Analysis
Data are presented as mean + SEM. Spontaneous firing is determined from 30-s sampling intervals immediately preceding each stimulus trial. The 30-s pre-stimulus interval was adopted based on the original description of periodicity of these cell classes,2,25 and captures the current state of the neuron (active or inactive). Because the spontaneous firing of these neurons is not stationary, use of a longer baseline is precluded.
We also determined the duration of the longest single silent period beginning during the light stimulus or associated with the heat-evoked withdrawal (OFF-cells) or the total spike count in active periods beginning during the light stimulus (ON-cells) or associated with the heat-evoked withdrawal. To facilitate comparisons between stimulus modalities and groups, reflex- and stimulus-related changes in activity for both ON- and OFF-cells were also expressed as a percent of the background firing by comparing firing rates at the time of the withdrawal (3 s interval beginning 0.5 s prior to the withdrawal) or during the light stimulus to that in the 30 s before stimulus onset. A ceiling of 500% of baseline was imposed as a conservative approach to limit impact of neurons with low spontaneous activity. Peak firing was defined for ON-cells as the firing rate in the single highest 0.5 s bin associated with paw withdrawal (3 s window beginning 0.5 s before the withdrawal) or during the light stimulus (30 s duration).
Cell parameters in photosensitive and light-insensitive populations were compared using two-tailed nonparametric tests (Wilcoxon’s signed ranks, Mann-Whitney U), with p values of less than 0.05 considered statistically significant. Correlations were calculated using Spearman’s rho.
3. Results
3.1. Patients with fibromyalgia demonstrate profound photosensitivity
Female patients with fibromyalgia and age-matched healthy controls were enrolled (n = 24/group). Symptom severity was assessed using validated questionnaire instruments (FIQR/SIQR).20 Patients had significantly higher mean scores on the FIQR/SIQR than healthy controls, indicating moderate to severe fibromyalgia impact (Table 1). Patients self-reported greater sensory sensitivity (auditory, visual, and olfactory stimuli) using this instrument.
Table 1. Baseline demographics and clinical characteristics.
Comparisons between the two groups were accomplished using t-test for continuous variables and χ2 test for categorical variables.
| Characteristic | FM Group n = 24 |
Control Group n = 24 |
p |
|---|---|---|---|
| Age (mean (SD) in years) | 50 (14) | 47 (14) | 0.48 |
| Ethnicity (% Caucasian) | 92 | 92 | 1.00 |
| FIQR/SIQR total score (mean (SD)) | 53.1 (17.9) | 2.9 (2.8) | < 0.001 |
| FIQR/SIQR symptoms (mean (SD)) | 56.8 (14.8) | 5.1 (3.6) | <0.001 |
| FIQR/SIQR level of pain (mean (SD)) | 5.6 (2.9) | 0.6 (1.6) | < 0.001 |
| FIQR/SIQR level of sensitivity to loud noises, bright lights, odors, or cold (mean (SD)) | 6.6 (2.6) | 0.8 (1.2) | 0.001 |
Pressure and light sensitivity analyses
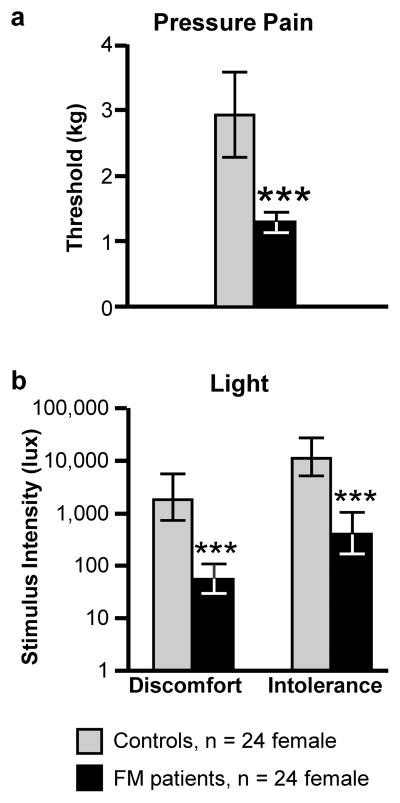
Pressure pain thresholds for fibromyalgia patients were significantly lower than those for healthy controls, consistent with the presentation of this disorder (Fig. 1a).
Fig. 1. Photosensitivity in fibromyalgia patients.
Thresholds for (a) pressure-pain and (b) light-evoked discomfort and intolerance were determined in fibromyalgia patients and age-matched healthy controls. As would be expected based on the clinical presentation of this disorder, the fibromyalgia group exhibited a significantly lower threshold for pressure-evoked pain. They also reported light-evoked “discomfort” and “intolerance” at substantially lower levels than controls. (t-test compared to control, ***p < 0.001). Data presented as mean with 95% confidence limits.
To determine photosensitivity in patients with FM, we adopted procedures previously used in patients with migraine to measure thresholds for light-evoked discomfort and intolerance.14,41,54 Both discomfort and intolerance thresholds were significantly lower in the fibromyalgia group than controls (Fig. 1b). Light discomfort and pressure pain thresholds were correlated (Pearson’s r = 0.55, p = 0.0001). However, when fibromyalgia status was examined as a moderator to the relationship between light discomfort and pressure pain,3 status was a statistically significant predictor of light discomfort (Table 2); pressure pain was no longer significantly correlated with light threshold when status was included in the model (p = 0.238). This indicates that fibromyalgia status explains more of the variance in light threshold than pressure pain threshold (Adj R2 for light threshold and status = 0.47, F2,45 = 21.46, p < 0.001).
Table 2. Moderation analysis.
Relationship between light discomfort threshold (dependent variable) and pressure pain threshold (independent variable) in normal and fibromyalgia groups (independent variable). Pressure pain rating was not significantly correlated with light discomfort threshold when group was included in the model, indicating that fibromyalgia status (group) explained more of the variance than pressure pain threshold.
| Model | Unstandardized Coefficients | Standardized Coefficients | t | Signif | |
|---|---|---|---|---|---|
|
| |||||
| B | Std. Error | Beta | |||
|
| |||||
| Constant | 8.679 | 1.459 | 5.948 | < 0.001 | |
|
|
|||||
| Pressure Threshold | 0.556 | 0.466 | 0.170 | 1.195 | 0.238 |
|
|
|||||
| Status (FM vs. control) | −4.476 | 1.107 | −0.575 | −4.045 | < 0.001 |
Light discomfort thresholds were also correlated with clinical pain ratings (r = −0.66, p < 0.0001). However, a moderator analysis again indicated that fibromyalgia status moderated the relationship, since light threshold was not significantly correlated with clinical pain rating when fibromyalgia was included in the model (p = 0.19). This indicates that fibromyalgia status explains more of the variance than clinical pain ratings as such (Adj R2 for light threshold and status = 0.47, F2,45 = 21.80, p < 0.001).
3.2. Identified brainstem pain-modulating neurons respond to visual light in the rat
Pain-modulating neurons were recorded in the RVM of normal, lightly anesthetized rats using standard in vivo extracellular single-cell recording techniques. A total of 176 RVM neurons was recorded in 92 animals (1 to 5 cells per animal), with 73 classified as OFF-cells, 90 as ON-cells, and 13 as NEUTRAL-cells. NEUTRAL-cells do not respond during noxious-evoked behaviors, and whether these neurons have a role in pain-modulation remains an open question.26
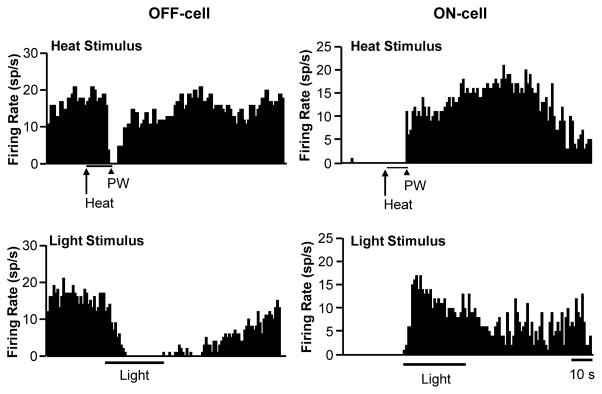
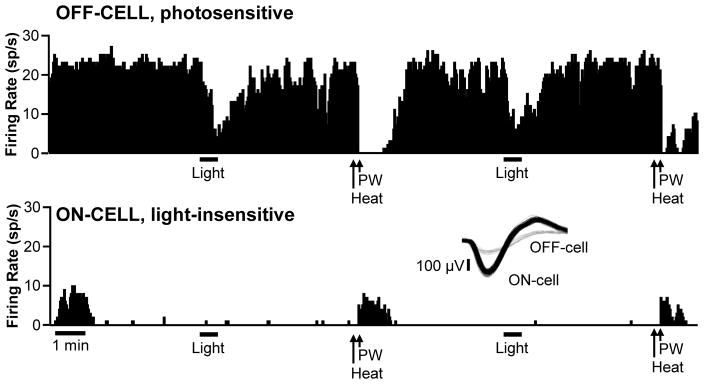
RVM ON- and OFF-cells are defined by changes in activity associated with behavioral responses to noxious stimuli.19 Remarkably, a substantial subset of these neurons exhibited a robust change in firing during exposure to bright light (approx. 18,000 lux shone in one eye, an intensity comparable to that used in prior studies of photosensitive neurons in the trigeminal system and posterior thalamus).44–45 Examples of an OFF-cell and ON-cell that exhibited light-related changes in firing are shown in Fig. 2. The OFF-cell showed complete inhibition of firing associated with the heat-evoked paw withdrawal, pausing for 5.87 s. This cell also showed a pause in firing during photostimulation, with complete inhibition lasting over 26 seconds, most of the stimulus application. The ON-cell, recorded in a different animal, showed a vigorous heat-evoked reflex-related response, with peak firing at approximately 18 spikes/s and an average of 351 evoked spikes per reflex-related burst of activity. The response to light was comparable in this neuron, with peak firing again 18 sp/s, and an average of 483 total evoked spikes.
Fig. 2. Representative OFF-cell (left) and ON-cell (right) responding during light stimulus.
Ratemeter records (1 s bins) with heat onset (Heat) and paw withdrawal (PW), as well as the duration of light stimulus shown below each trace. The OFF-cell demonstrated a clear pause in firing at the time of the paw withdrawal and during the light stimulus. The ON-cell also responded during both stimuli.
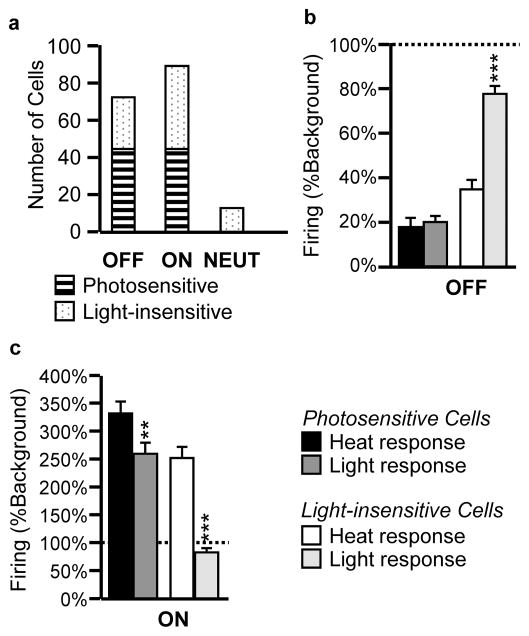
Using a 50% change in firing relative to background activity to define a neuron as photosensitive, we determined that 61% of the OFF-cells and 50% of the ON-cells examined in these experiments responded to light (Fig. 3a). None of the NEUTRAL-cells studied showed a change in firing associated with the light stimulus (Fig. 3a).
Fig. 3. Distribution of photosensitivity across RVM ON-cell, OFF-cell and NEUTRAL-cell populations, and comparison of heat- and light-related responses.
a. A change in firing of at least 50% relative to ongoing firing prior to stimulus onset was considered a “response” to the visual stimulus. Approximately half of the OFF- and ON-cell populations demonstrated a response to photic input, whereas none of the NEUTRAL –cells examined were light-sensitive. b and c. Firing of photosensitive and light-insensitive OFF-cells (b) and ON-cells (c) at the time of heat-evoked paw withdrawal or during the light stimulus as a percent of firing prior to stimulus onset. **p < 0.01, ***p < 0.001 compared to heat-evoked, reflex-related changes using Wilcoxon’s signed ranks test. Data presented as mean + SEM percent of background firing prior to stimulus onset. No change would be 100% of baseline (dotted line).
We next attempted to determine how light-evoked changes in firing compared to those evoked by noxious somatic stimuli. The photosensitive OFF-cells exhibited comparable slowing at the time of the heat-evoked paw-withdrawal and during light exposure (Fig. 3b, heat and light responses for “Photosensitive Cells”), and the decreases in firing rate during noxious heat and light were not significantly different. As would be expected given the definition of photosensitivity, the light-evoked change in firing of the OFF-cells considered light-insensitive was significantly less than the change in firing associated with the heat-evoked paw withdrawal of the same neurons (Fig. 3b, heat and light responses for “Light-insensitive Cells”).
Photoresponsive ON-cells showed increases in firing during light exposure as well as at the time of paw withdrawal (Fig. 3c, heat and light responses for “Photosensitive Cells”), although the increase associated with the heat-evoked paw withdrawal was significantly greater for these neurons than during light exposure. Peak firing rate associated with the heat-evoked reflex was also significantly greater than that during light exposure (heat: 32.5 ± 3.1 sp/s, light: 22.6 ± 2.6 sp/s, p < 0.0001 Wilcoxon’s signed ranks test). As would be expected, the light-evoked change in firing of the ON-cells considered light-insensitive was significantly less than that associated with heat-evoked paw withdrawal of the same neurons (Fig. 3c, heat and light responses for “Light-insensitive cells”).
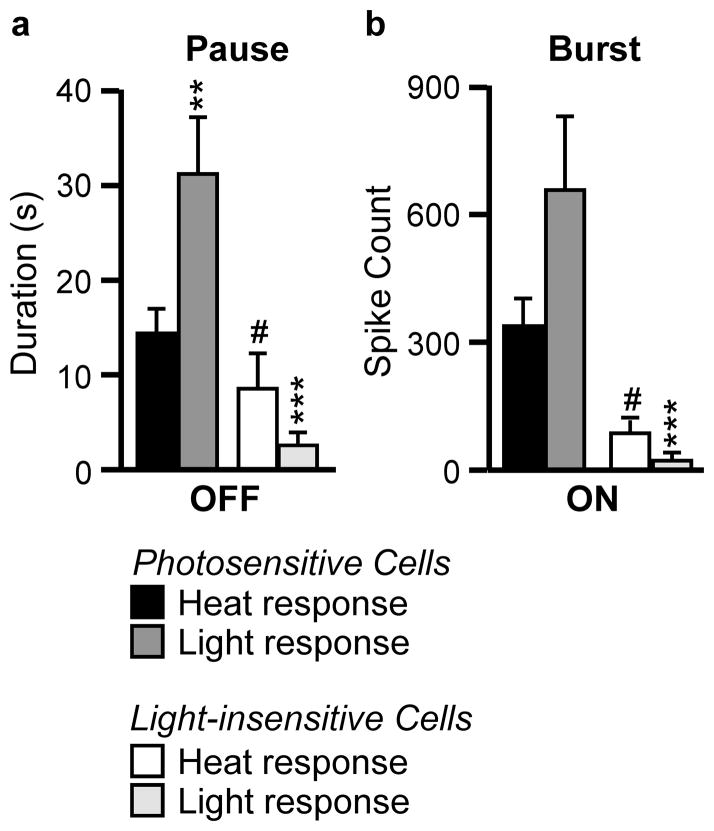
Analysis of the OFF-cell “pause” associated with heat and during light, and the ON-cell “burst” associated with heat and during light confirms that both classes respond to light. Fig. 4a plots the pause duration (in seconds) and Fig. 4b the burst activity (total evoked spikes) for photosensitive and light-insensitive OFF- and ON-cells. In both cases, the light-evoked response is at least as large as the heat-evoked, reflex-related response in the photosensitive subgroups.
Fig. 4. OFF-cell pause (a, duration in s) and ON-cell burst (b, total evoked spikes) associated with heat-evoked withdrawal or the light stimulus.
Neurons more responsive during nociceptive withdrawal were also more responsive to the light stimulus. **p < 0.01, ***p < 0.001 compared to heat-evoked changes using Wilcoxon’s signed ranks test. #p < 0.05 compared to light-responsive neurons of that class using Mann-Whitney U. Data presented as mean + SEM.
Since not all ON- and OFF-cells responded to light exposure, we also considered the possibility that light-insensitive cells might be less responsive to sensory inputs more generally. Consistent with this notion, heat-evoked, reflex-related changes in firing were different for light-responsive versus non-responsive cells, a difference seen in both the OFF-cell and ON-cell samples (Fig. 4a, b). Indeed, noxious heat-related changes and light-evoked changes were significantly correlated (OFF-cells: r = 0.62, p < 0.0001, ON-cells: r = 0.34, p = 0.01). When considering photosensitive neurons only, the light-related pause exhibited by the OFF-cells was significantly longer than that associated with heat-evoked withdrawal (Fig. 4a). However, this difference presumably reflects the fact that the light stimulus was applied for a full 30 s, whereas the heat stimulus was terminated when the animal withdrew the paw. A similar trend was seen for the ON-cell burst, although this was not statistically significant (Fig. 4b).
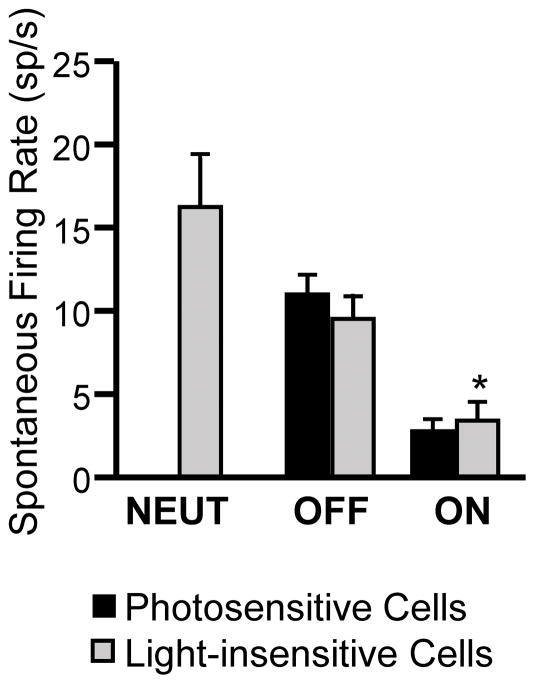
We next considered whether photosensitivity was related to spontaneous firing rate of the neuron under study, as a measure of overall excitability. As shown in Fig. 5, spontaneous firing rates of photosensitive and light-insensitive OFF-cells were comparable, at approximately 10 spikes/s. Light-insensitive ON-cells exhibited a slightly higher spontaneous firing rate (Mann-Whitney U, p = 0.045). Thus, photosensitivity was not related to a higher firing rate in the ON-cell population or a lower spontaneous firing rate in the OFF-cell population, suggesting that photosensitivity does not simply reflect overall excitability of the neuron, at least as reflected in spontaneous activity.
Fig. 5. Spontaneous firing for each cell class comparing photosensitive and light-insensitive neurons.
There is no difference between photosensitive and light-insensitive neurons within the OFF-cell population, while spontaneous firing of the light-insensitive ON-cells was slightly greater. *p < 0.05 compared to photosensitive neurons (Wilcoxon’s signed ranks test). Data presented as mean + SEM.
Finally, we considered the possibility that photosensitive neurons were found only in a subset of animals. The example in Fig. 6 shows an OFF- and ON-cell recorded simultaneously on a single electrode, with the OFF-cell light-responsive but the ON-cell unresponsive. We determined the percentage of cells that were light responsive from all experiments in which more than one cell was recorded per animal. This was found to range from 0 to 100%, averaging 48.3 ± 4.8%, which was comparable to the proportion seen in the overall sample of 176 neurons. Therefore, photosensitivity was not specific to certain animals, but was a property of individual neurons, and photosensitive and insensitive neurons were intermingled.
Fig. 6. Simultaneous recording from a photosensitive OFF-cell and light-insensitive ON-cell demonstrates that light-sensitive and -insensitive cells are intermingled.
Ratemeter records (1 s bins) with heat onset and paw withdrawal as well as the duration of light stimulus shown below each trace. The OFF-cell firing slowed or ceased at the time of the paw withdrawal and during the light stimulus. By contrast, the ON-cell responded at the time of paw withdrawal, but not during light exposure. Inset shows the waveforms for the two units, with the ON-cell in black, OFF-cell in gray.
In sum, RVM pain-modulating neurons display a range of responsiveness to light, with only a subset demonstrating a substantial change (> 50%), and photosensitivity is to some extent related to the responsiveness of that same neuron during noxious thermal stimulation, although not to spontaneous firing rate.
3.3. Light exposure lowers the threshold for noxious heat-evoked withdrawal
We next considered the functional implications of light-evoked responses in RVM neurons. Given that ON-cells exert a net pro-nociceptive effect, and that OFF-cells have a net anti-nociceptive effect,26,30 one would predict that light exposure should produce a fairly small, but measurable behavioral hyperalgesia. To test this, we compared the thresholds for heat-evoked withdrawal with and without concomitant light exposure in alternate trials in a separate set of experiments. The threshold was lowered, modestly but significantly, in trials where heat was applied during light exposure compared to ambient light (47.6 ± 0.17 during light exposure vs. 47.9 ± 0.20 °C in ambient light (approx. 100 lux), t9 = 3.1, p = 0.01, n = 10). This change in threshold is small, consistent with the previously reported modulation of nociceptive threshold by spontaneous fluctuations in ON- and OFF-cell firing,25,27,42 and with the fact that only a subset of RVM neurons was activated by light in these animals.
3.4. Photic input is relayed to pain-modulating systems via the olivary pretectal nucleus
Using an inactivation strategy, we next tested three candidate relays through which information about light could gain access to the RVM: trigeminal primary sensory neurons, which are required for light-evoked activity in the spinal trigeminal nucleus,45 the posterior thalamus, which is known to receive convergent input from trigeminal (dural) afferents and retina,44 and the olivary pretectal nucleus, a reasonable candidate because of its known role in irradiance detection.53,63
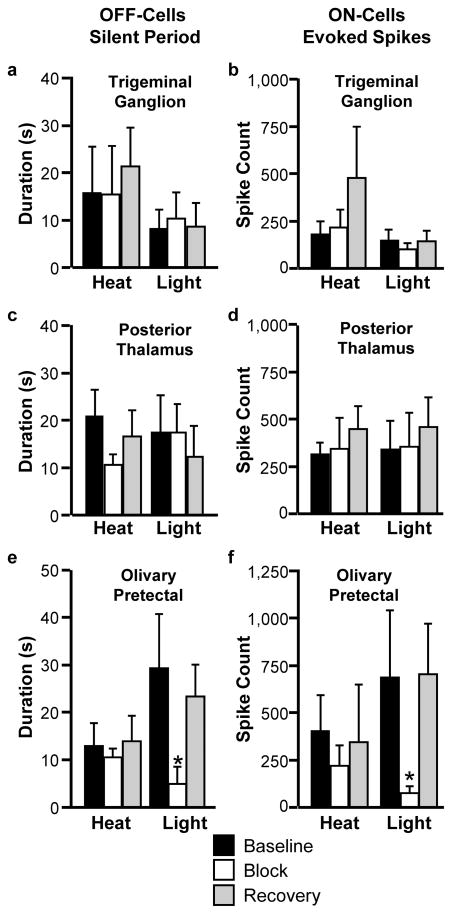
Following a baseline period during which ON- and OFF-cell responses associated with heat-evoked withdrawal reflexes and light stimulation were determined, lidocaine was microinjected into the trigeminal ganglion (TG) ipsilateral to the light stimulus, the contralateral posterior thalamus (PO, since the projection is mostly contralateral44) or contralateral olivary pretectal nucleus (OPt, again, the projection being almost entirely contralateral64). Block of TG or PO had no effect on the responses of OFF- and ON-cells to either heat or light (Fig. 7a–d). By contrast, inactivation of OPt attenuated or blocked the light-related responses of these neurons, without affecting responses during heat-evoked withdrawal (Fig. 7e, f). Fig. 8 shows an example of an almost complete block of a light-evoked response of an RVM ON-cell following inactivation of OPt, with subsequent recovery. Placements of the injectors in the PO and OPt regions are shown in Fig. 9.
Fig. 7. Inactivation of olivary pretectal nucleus, but not trigeminal ganglion or posterior thalamus, interferes with the light-evoked changes in firing of OFF-cells (a,c,e) and ON-cells (b,d,f).
For OFF-cells, parameter is duration of reflex-related pause (heat-evoked withdrawal) or longest silent period beginning during the light stimulus. For ON-cells, parameter is total evoked spikes associated with heat-evoked withdrawal or longest continuous active period beginning during the light stimulus. *p < 0.05 compared to pre-block baseline, Wilcoxon’s signed ranks test. OFF-cells: n = 6 (TG), 7 (PO), and 8 (OPt). ON-cells: n = 10 (TG), 7 (PO), and 6 (OPt). Data presented as mean + SEM.
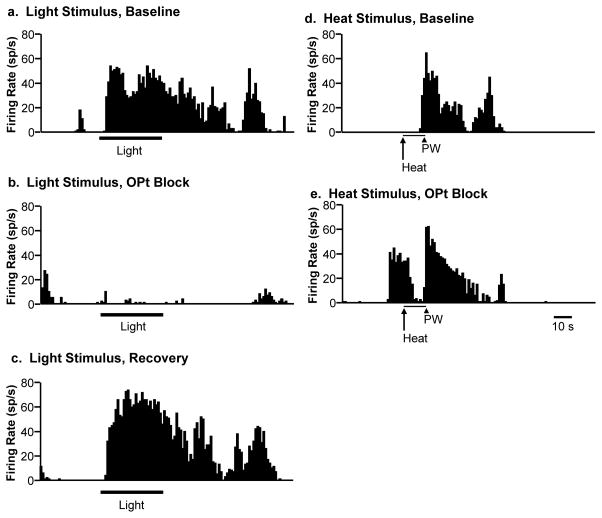
Fig 8. Representative example showing profound suppression of light-evoked responding of an RVM ON-cell by inactivation of the OPt.
Light-evoked activity in baseline (a), during lidocaine block of OPt (b), and during subsequent recovery (c). Reflex-related burst of activity that defines this neuron as an ON-cell, with activation linked to heat-evoked paw withdrawal (PW) in baseline (d) and during OPt block (e). Light-evoked activation of this neuron was almost eliminated by OPt block, whilst heat-evoked reflex-related activation was unaffected. Ratemeter records with 1 s bins. The duration of the light stimulus is indicated by the bar below the trace.
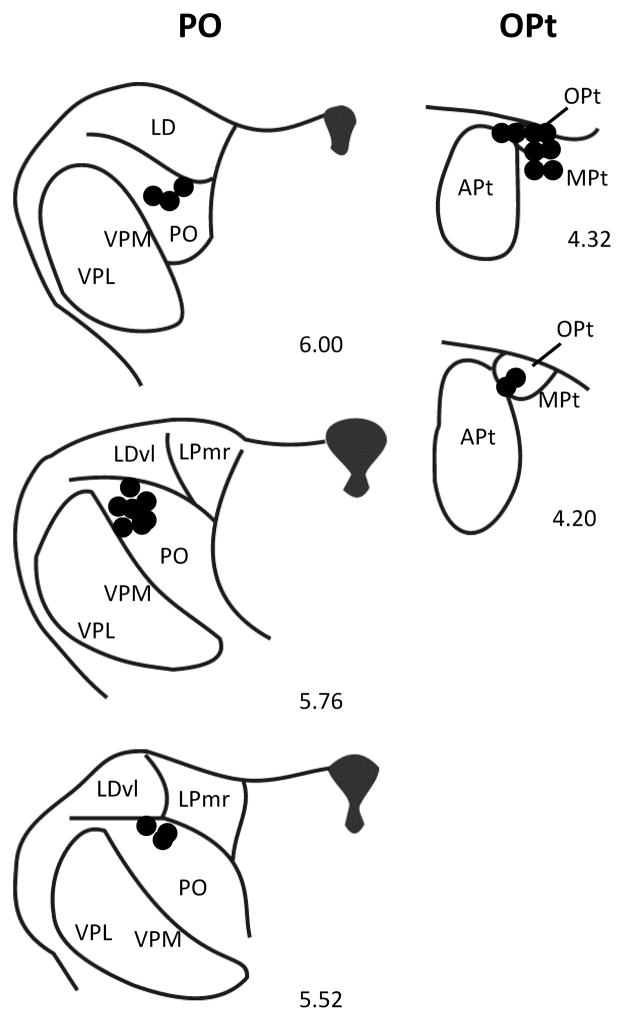
Fig. 9.
Histologically verified locations of injection sites in PO (left) and OPt. Apt: anterior pretectal nucleus; LD: lateral dorsal thalamic nucleus; LDvl: lateral dorsal thalamic nucleus, ventrolateral part; LPmr: lateral posterior thalamic nucleus, mediorostral part; MPt: medial pretectal nucleus; OPt: olivary pretectal nucleus; PO: posterior thalamic nucleus; VPL: ventroposterolateral thalamic nucleus; VPM: ventromedial thalamic nucleus. Distance rostral to interaural line is indicated for each section.
4. Discussion
Photosensitivity has not previously been quantified in individuals with fibromyalgia, although it is widely recognized clinically.20 We determined that women with fibromyalgia experience discomfort at light levels substantially lower than those required to elicit this sensation in age-matched healthy controls, and that they are unable to tolerate light levels well within the tolerance of the controls. Further, fibromyalgia status predicted abnormal photosensitivity even when controlling for pressure-pain threshold.
Despite complaints of multi-modal hypersensitivity in these patients, sensory acuity per se is not enhanced, nor is there amplified processing in primary sensory pathways.10,38–40,48 Consequently, the abnormal photosensitivity observed here in fibromyalgia patients is unlikely to represent a simple hypersensitivity of visual sensory systems. Our finding in rodents, that light can modulate the firing of brainstem pain-modulating neurons, even under basal conditions, therefore provides a plausible mechanism by which light could increase sensitivity to somatic inputs, causing normal somatosensory afferent traffic to be perceived as aversive or even painful. Additional studies, in both humans and rodents, are clearly needed to examine this hypothesis. Investigation of photoresponsiveness of RVM neurons in rodent chronic pain models will be particularly crucial.
Dysfunction of brainstem pain-modulating systems is thought to play an important role in the pathophysiology of fibromyalgia and other “functional pain disorders” where an obvious peripheral trigger cannot easily be identified.37,50,62 We therefore considered the possibility that photic input could activate identified pain-modulating neurons in the RVM, a primary output node of brainstem pain-modulating circuitry. Light activated a subset of RVM ON-cells and suppressed the firing of a subset of OFF-cells. Since the net effect of ON-cell activation is pro-nociceptive and aversive, while the net effect of OFF-cell activation is anti-nociceptive,28,30,33 light exposure tipped the balance in this system to a more pro-nociceptive state.
Consistent with the idea that light could induce a pro-nociceptive state, light exposure led to a behaviorally measurable decrease in nociceptive withdrawal threshold in the rodent study. Under the conditions of this experiment, light-associated behavioral hyperalgesia was modest, consistent with the known effect of suppressing a net inhibitory tone mediated by OFF-cells,25,27,42 and with the fact that only a subset of the ON- and OFF-cell populations responded during the light stimulus under the conditions of our experiments. It will be important to define stimulus-response curves for light-related engagement of this system in future studies, but the magnitudes of the light- and noxious heat-evoked changes in firing of the photosensitive neurons were correlated. This raises the possibility that additional ON- and OFF-cells could be recruited into the photoresponsive population under conditions and chronic pain models where these neurons are known to be sensitized to somatic input, such as persistent inflammation, nerve injury, or models of migraine headache.9,11,17 In migraine for example, sensitization of RVM ON- and OFF-cells to light as well as somatosensory input would be consistent with the mutual potentiation of visual and somatic discomfort reported by migraineurs.8,15,36,54 It will therefore be important to determine whether the light-evoked responses of RVM neurons are enhanced in migraine models and other hyperalgesic states. Conversely, it would be of interest to determine whether clinical pain is influenced by light in individuals with fibromyalgia or other pain disorders attributed to dysregulated pain-modulating systems. Sex differences may also be important. The human subjects were female, while the rodents were all male, and future studies should examine both sexes within each species. However, fibromyalgia is now recognized as occurring in males as well as females, and the clinical picture is much the same in the two sexes, apart from women having more tender points and depression.5,24,59 In any case, the goal of the present studies was not to directly compare photosensitivity in human and rodent, but to characterize and quantify abnormal photosensitivity in a chronic pain population (specifically fibromyalgia), and define a possible underlying neural mechanism.
We did not investigate the light-evoked output from the RVM. However, this region is known to project to the dorsal horn. Light-induced activation of RVM ON-cells could therefore lower pain thresholds through this pathway, modulating dorsal horn processing of somatic sensory input. In effect, the visual input is converted to a general somatic discomfort. (Light-evoked changes in RVM activity could also modulate light-evoked responses in somatosensory pathways, e.g., trigeminal complex or thalamus.) Alternatively or in addition, the extensive ascending projections of the RVM to limbic forebrain32,56–57 could play some role in producing light-induced aversion. Although the function of the ascending RVM projections has not been investigated, it is not unreasonable to think that they could contribute to the aversion resulting from ON-cell activation.33 Ascertaining whether the descending and/or ascending projections from RVM contribute to abnormal photosensitivity would therefore be of significant interest.
Finally, we considered the relays through which information about light levels is conveyed to the RVM. The RVM is not known to receive direct retinal input, but since RVM ON- and OFF-cells respond to noxious stimulation delivered over the entire body surface, we tested the possibility that these neurons derive their sensitivity to light via nociceptive transmission neurons receiving convergent irradiance input or other light-induced input from the eye, such as the trigeminal system or posterior thalamus13,44–45 Although we cannot state with certainty that the entire posterior thalamus or trigeminal system was silenced with our microinjection, even with the large volumes employed here, these relays are unlikely to have a major role, since RVM responses to light were unchanged following lidocaine microinjection in either structure. This finding also implies that light-evoked responses in RVM do not represent a noxious ocular event, which would presumably be mediated by the trigeminal system. By contrast, inactivation of the OPt effectively eliminated the RVM response to light, while the responses associated with heat-evoked withdrawal were unaffected. OPt has classically been recognized as a relay in the pupillary light reflex.53,63 It is also an important target for intrinsically photosensitive retinal ganglion cells,22–23 which are thought to mediate light aversion in mice.52 Although the OPt itself does not appear to project to the RVM directly, it does project to the Edinger-Westphal nucleus and lateral parabrachial complex, either of which could influence the RVM through direct projections.4,6–7,34–35,56
In conclusion, the present experiments document substantial light intolerance in patients with fibromyalgia, and raise the possibility that this abnormal photosensitivity could be explained by abnormal engagement of pain-facilitating systems by light. Pain-modulating systems are widely thought to be dysfunctional in fibromyalgia and other functional pain disorders,50,62 and our experiments in normal rodents demonstrate that endogenous pain-modulating systems receive input related to light levels. We also provide evidence that this input is relayed via the OPt, and not via trigeminal sensory pathways, and that the influence of light on pain-modulating neurons has functional consequences. These findings add to the known inputs to the RVM that have the potential to modulate nociceptive responsiveness, including sensory inputs and top-down influences from higher centers such as periaqueductal gray, amygdala, and hypothalamus.1,29,31–32,42–43,46,55–57
Our findings may extend beyond fibromyalgia to other pain disorders that are considered to reflect altered pain-modulation circuitry.49,51,61,65 Dysfunction of descending control is difficult to pinpoint in individual patients, and typically involves the use of noxious stimuli. Although direct comparisons between nocturnal rodents and diurnal humans must be approached with caution, our finding that some brainstem pain-modulating neurons respond to light, even under basal conditions, suggests that abnormal photosensitivity should be investigated as a potential index of dysregulated descending control in patients with a range of pain disorders.
Acknowledgments
Supported by grants from NIH (NS082020, MMH), the Medical Research Foundation of Oregon (OH), and the Fibromyalgia Information Foundation. KT was supported by T32 AG023477.
Footnotes
The authors declare no conflict of interest.
References
- 1.Baez MA, Brink TS, Mason P. Roles for pain modulatory cells during micturition and continence. J Neurosci. 2005;25:384–394. doi: 10.1523/JNEUROSCI.3536-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbaro NM, Heinricher MM, Fields HL. Putative nociceptive modulatory neurons in the rostral ventromedial medulla of the rat display highly correlated firing patterns. Somatosens Mot Res. 1989;6:413–425. doi: 10.3109/08990228909144684. [DOI] [PubMed] [Google Scholar]
- 3.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic and statistical consderations. J Pers Soc Psych. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 4.Beitz AJ. The nuclei of origin of brain stem enkephalin and substance P projections to the rodent nucleus raphe magnus. Neuroscience. 1982;7:2753–2768. doi: 10.1016/0306-4522(82)90098-7. [DOI] [PubMed] [Google Scholar]
- 5.Bennett RM, Friend R, Marcus D, Bernstein C, Han BK, Yachoui R, Deodhar A, Kaell A, Bonafede P, Chino A, Jones KD. Criteria for the diagnosis of fibromyalgia: Validation of the modified 2010 preliminary American College of Rheumatology criteria and the development of alternative criteria. Arthritis Care Res. 2014;66:1364–1373. doi: 10.1002/acr.22301. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi R, Corsetti G, Rodella L, Tredici G, Gioia M. Supraspinal connections and termination patterns of the parabrachial complex determined by the biocytin anterograde tract-tracing technique in the rat. J Anat. 1998;193(Pt 3):417–430. doi: 10.1046/j.1469-7580.1998.19330417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: Evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 1999;415:285–312. [PubMed] [Google Scholar]
- 8.Boulloche N, Denuelle M, Payoux P, Fabre N, Trotter Y, Geraud G. Photophobia in migraine: An interictal PET study of cortical hyperexcitability and its modulation by pain. J Neurol Neurosurg Psychiatry. 2010;81:978–984. doi: 10.1136/jnnp.2009.190223. [DOI] [PubMed] [Google Scholar]
- 9.Carlson JD, Maire JJ, Martenson ME, Heinricher MM. Sensitization of pain-modulating neurons in the rostral ventromedial medulla after peripheral nerve injury. J Neurosci. 2007;27:13222–13231. doi: 10.1523/JNEUROSCI.3715-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrillo-de-la-Pena MT, Vallet M, Perez MI, Gomez-Perretta C. Intensity dependence of auditory-evoked cortical potentials in fibromyalgia patients: A test of the generalized hypervigilance hypothesis. J Pain. 2006;7:480–487. doi: 10.1016/j.jpain.2006.01.452. [DOI] [PubMed] [Google Scholar]
- 11.Cleary DR, Heinricher MM. Adaptations in responsiveness of brainstem pain-modulating neurons in acute compared with chronic inflammation. Pain. 2013;154:845–855. doi: 10.1016/j.pain.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Felice M, Eyde N, Dodick D, Dussor GO, Ossipov MH, Fields HL, Porreca F. Capturing the aversive state of cephalic pain preclinically. Ann Neurol. 2013;74:257–265. doi: 10.1002/ana.23922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolgonos S, Ayyala H, Evinger C. Light-induced trigeminal sensitization without central visual pathways: Another mechanism for photophobia. Invest Ophthalmol Vis Sci. 2011;52:7852–7858. doi: 10.1167/iovs.11-7604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drummond PD. A quantitative assessment of photophobia in migraine and tension headache. Headache. 1986;26:465–469. doi: 10.1111/j.1526-4610.1986.hed2609465.x. [DOI] [PubMed] [Google Scholar]
- 15.Drummond PD, Woodhouse A. Painful stimulation of the forehead increases photophobia in migraine sufferers. Cephalalgia. 1993;13:321–324. doi: 10.1046/j.1468-2982.1993.1305321.x. [DOI] [PubMed] [Google Scholar]
- 16.Dunkl PR, Taylor AG, McConnell GG, Alfano AP, Conaway MR. Responsiveness of fibromyalgia clinical trial outcome measures. J Rheumatol. 2000;27:2683–2691. [PubMed] [Google Scholar]
- 17.Edelmayer RM, Vanderah TW, Majuta L, Zhang ET, Fioravanti B, De Felice M, Chichorro JG, Ossipov MH, King T, Lai J, Kori SH, Nelsen AC, Cannon KE, Heinricher MM, Porreca F. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol. 2009;65:184–193. doi: 10.1002/ana.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feigl B, Mattes D, Thomas R, Zele AJ. Intrinsically photosensitive (melanopsin) retinal ganglion cell function in glaucoma. Invest Ophthalmol Vis Sci. 2011;52:4362–4367. doi: 10.1167/iovs.10-7069. [DOI] [PubMed] [Google Scholar]
- 19.Fields HL, Heinricher MM. Anatomy and physiology of a nociceptive modulatory system. Philos Trans of the R Soc Lond B Biol Sci. 1985;308:361–374. doi: 10.1098/rstb.1985.0037. [DOI] [PubMed] [Google Scholar]
- 20.Friend R, Bennett RM. Distinguishing fibromyalgia from rheumatoid arthritis and systemic lupus in clinical questionnaires: An analysis of the revised fibromyalgia impact questionnaire (FIQR) and its variant, the symptom impact questionnaire (SIQR), along with pain locations. Arthritis Res Ther. 2011;13:R58. doi: 10.1186/ar3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geisser ME, Glass JM, Rajcevska LD, Clauw DJ, Williams DA, Kileny PR, Gracely RH. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J Pain. 2008;9:417–422. doi: 10.1016/j.jpain.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 22.Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23:7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hauser W, Kuhn-Becker H, von Wilmoswky H, Settan M, Brahler E, Petzke F. Demographic and clinical features of patients with fibromyalgia syndrome of different settings: A gender comparison. Gend Med. 2011;8:116–125. doi: 10.1016/j.genm.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Heinricher MM, Barbaro NM, Fields HL. Putative nociceptive modulating neurons in the rostral ventromedial medulla of the rat: Firing of on- and off-cells is related to nociceptive responsiveness. Somatosens Mot Res. 1989;6:427–439. doi: 10.3109/08990228909144685. [DOI] [PubMed] [Google Scholar]
- 26.Heinricher MM, Fields HL. Central nervous system mechanisms of pain modulation. In: McMahon S, Koltzenburg M, Tracey I, Turk DC, editors. Wall and Melzack’s Textbook of Pain. 6. London: Elsevier; 2013. pp. 129–142. [Google Scholar]
- 27.Heinricher MM, Kaplan HJ. GABA-mediated inhibition in rostral ventromedial medulla: Role in nociceptive modulation in the lightly anesthetized rat. Pain. 1991;47:105–113. doi: 10.1016/0304-3959(91)90017-R. [DOI] [PubMed] [Google Scholar]
- 28.Heinricher MM, Maire JJ, Lee D, Nalwalk JW, Hough LB. Physiological basis for inhibition of morphine and improgan antinociception by CC12, a P450 epoxygenase inhibitor. J Neurophysiol. 2010;104:3222–3230. doi: 10.1152/jn.00681.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinricher MM, Neubert MJ, Martenson ME, Gonçalves L. Prostaglandin E2 in the medial preoptic area produces hyperalgesia and activates pain-modulating circuitry in the rostral ventromedial medulla. Neuroscience. 2004;128:389–398. doi: 10.1016/j.neuroscience.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 30.Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hentall ID, Budhrani VM. The interpeduncular nucleus excites the on-cells and inhibits the off-cells of the nucleus raphe magnus. Brain Res. 1990;522:322–324. doi: 10.1016/0006-8993(90)91476-w. [DOI] [PubMed] [Google Scholar]
- 32.Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Forebrain projections of the rostral nucleus raphe magnus shown by iontophoretic application of choleratoxin b in rats. Neurosci Lett. 1996;216:151–154. doi: 10.1016/0304-3940(96)13013-5. [DOI] [PubMed] [Google Scholar]
- 33.Hirakawa N, Tershner SA, Fields HL, Manning BH. Bi-directional changes in affective state elicited by manipulation of medullary pain-modulatory circuitry. Neuroscience. 2000;100:861–871. doi: 10.1016/s0306-4522(00)00329-8. [DOI] [PubMed] [Google Scholar]
- 34.Klooster J, Beckers HJ, Vrensen GF, van der Want JJ. The peripheral and central projections of the Edinger-Westphal nucleus in the rat. A light and electron microscopic tracing study. Brain Res. 1993;632:260–273. doi: 10.1016/0006-8993(93)91161-k. [DOI] [PubMed] [Google Scholar]
- 35.Klooster J, Vrensen GF, Muller LJ, van der Want JJ. Efferent projections of the olivary pretectal nucleus in the albino rat subserving the pupillary light reflex and related reflexes. A light microscopic tracing study. Brain Res. 1995;688:34–46. doi: 10.1016/0006-8993(95)00497-e. [DOI] [PubMed] [Google Scholar]
- 36.Kowacs PA, Piovesan EJ, Werneck LC, Tatsui CE, Lange MC, Ribas LC, da Silva HP. Influence of intense light stimulation on trigeminal and cervical pain perception thresholds. Cephalalgia. 2001;21:184–188. doi: 10.1046/j.1468-2982.2001.00178.x. [DOI] [PubMed] [Google Scholar]
- 37.Lautenbacher S, Rollman GB. Possible deficiencies of pain modulation in fibromyalgia. Clin J Pain. 1997;13:189–196. doi: 10.1097/00002508-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Sola M, Pujol J, Wager TD, Garcia-Fontanals A, Blanco-Hinojo L, Garcia-Blanco S, Poca-Dias V, Harrison BJ, Contreras-Rodriguez O, Monfort J, Garcia-Fructuoso F, Deus J. Altered functional magnetic resonance imaging responses to nonpainful sensory stimulation in fibromyalgia patients. Arthritis Rheumatol. 2014;66:3200–3209. doi: 10.1002/art.38781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorenz J. Hyperalgesia or hypervigilance? An evoked potential approach to the study of fibromyalgia syndrome. Z Rheumatol. 1998;57(Suppl 2):19–22. doi: 10.1007/s003930050228. [DOI] [PubMed] [Google Scholar]
- 40.Lotsch J, Kraetsch HG, Wendler J, Hummel T. Self-ratings of higher olfactory acuity contrast with reduced olfactory test results of fibromyalgia patients. Int J Psychophysiol. 2012;86:182–186. doi: 10.1016/j.ijpsycho.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Main A, Dowson A, Gross M. Photophobia and phonophobia in migraineurs between attacks. Headache. 1997;37:492–495. doi: 10.1046/j.1526-4610.1997.3708492.x. [DOI] [PubMed] [Google Scholar]
- 42.Martenson ME, Cetas JS, Heinricher MM. A possible neural basis for stress-induced hyperalgesia. Pain. 2009;142:236–244. doi: 10.1016/j.pain.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGaraughty S, Heinricher MM. Microinjection of morphine into various amygdaloid nuclei differentially affects nociceptive responsiveness and RVM neuronal activity. Pain. 2002;96:153–162. doi: 10.1016/s0304-3959(01)00440-7. [DOI] [PubMed] [Google Scholar]
- 44.Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, Burstein R. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13:239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okamoto K, Tashiro A, Chang Z, Bereiter DA. Bright light activates a trigeminal nociceptive pathway. Pain. 2010;149:235–242. doi: 10.1016/j.pain.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oliveras JL, Vos B, Martin G, Montagne J. Electrophysiological properties of ventromedial medulla neurons in response to noxious and non-noxious stimuli in the awake, freely moving rat: A single-unit study. Brain Res. 1989;486:1–14. doi: 10.1016/0006-8993(89)91271-7. [DOI] [PubMed] [Google Scholar]
- 47.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, compact. 6. Amsterdam: Academic Press; 2009. [Google Scholar]
- 48.Peters ML, Vlaeyen JW, van Drunen C. Do fibromyalgia patients display hypervigilance for innocuous somatosensory stimuli? Application of a body scanning reaction time paradigm. Pain. 2000;86:283–292. doi: 10.1016/S0304-3959(00)00259-1. [DOI] [PubMed] [Google Scholar]
- 49.Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states--maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141–154. doi: 10.1016/j.berh.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Staud R. Abnormal endogenous pain modulation is a shared characteristic of many chronic pain conditions. Expert Rev Neurother. 2012;12:577–585. doi: 10.1586/ern.12.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Staud R. The important role of CNS facilitation and inhibition for chronic pain. Int J Clin Rheumatol. 2013;8:639–646. doi: 10.2217/ijr.13.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson S, Recober A, Vogel TW, Kuburas A, Owens JA, Sheffield VC, Russo AF, Stone EM. Light aversion in mice depends on nonimage-forming irradiance detection. Behav Neurosci. 2010;124:821–827. doi: 10.1037/a0021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trejo LJ, Cicerone CM. Cells in the pretectal olivary nucleus are in the pathway for the direct light reflex of the pupil in the rat. Brain Res. 1984;300:49–62. doi: 10.1016/0006-8993(84)91340-4. [DOI] [PubMed] [Google Scholar]
- 54.Vanagaite J, Pareja JA, Storen O, White LR, Sand T, Stovner LJ. Light-induced discomfort and pain in migraine. Cephalalgia. 1997;17:733–741. doi: 10.1046/j.1468-2982.1997.1707733.x. [DOI] [PubMed] [Google Scholar]
- 55.Vanegas H, Barbaro NM, Fields HL. Midbrain stimulation inhibits tail-flick only at currents sufficient to excite rostral medullary neurons. Brain Res. 1984;321:127–133. doi: 10.1016/0006-8993(84)90688-7. [DOI] [PubMed] [Google Scholar]
- 56.Verner TA, Pilowsky PM, Goodchild AK. Retrograde projections to a discrete apneic site in the midline medulla oblongata of the rat. Brain Res. 2008;1208:128–136. doi: 10.1016/j.brainres.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 57.Vertes RP. Brainstem afferents to the basal forebrain in the rat. Neuroscience. 1988;24:907–935. doi: 10.1016/0306-4522(88)90077-2. [DOI] [PubMed] [Google Scholar]
- 58.Wilbarger JL, Cook DB. Multisensory hypersensitivity in women with fibromyalgia: Implications for well being and intervention. Arch Phys Med Rehabil. 2011;92:653–656. doi: 10.1016/j.apmr.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR preliminary diagnostic criteria for fibromyalgia. J Rheumatol. 2011;38:1113–1122. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 60.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 61.Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): Its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010;23:611–615. doi: 10.1097/ACO.0b013e32833c348b. [DOI] [PubMed] [Google Scholar]
- 63.Young MJ, Lund RD. The anatomical substrates subserving the pupillary light reflex in rats: Origin of the consensual pupillary response. Neuroscience. 1994;62:481–496. doi: 10.1016/0306-4522(94)90381-6. [DOI] [PubMed] [Google Scholar]
- 64.Young MJ, Lund RD. The retinal ganglion cells that drive the pupilloconstrictor response in rats. Brain Res. 1998;787:191–202. doi: 10.1016/s0006-8993(97)01473-x. [DOI] [PubMed] [Google Scholar]
- 65.Yunus MB. Fibromyalgia and overlapping disorders: The unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36:339–356. doi: 10.1016/j.semarthrit.2006.12.009. [DOI] [PubMed] [Google Scholar]