Abstract
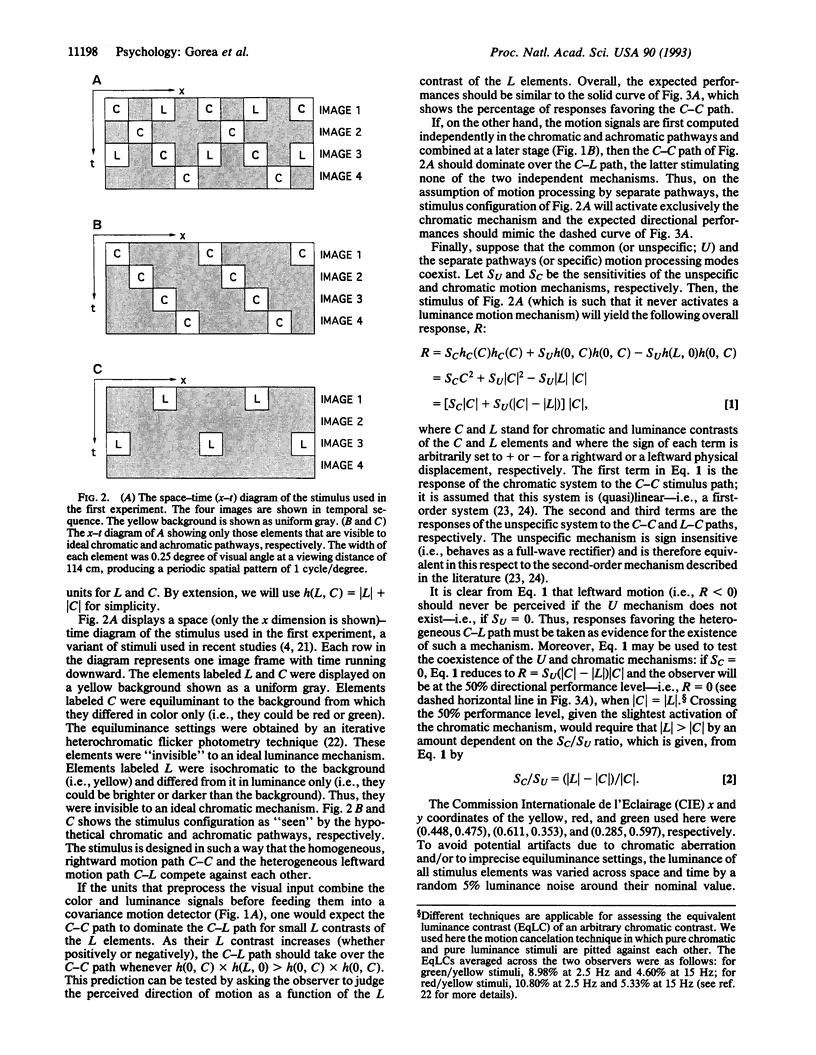
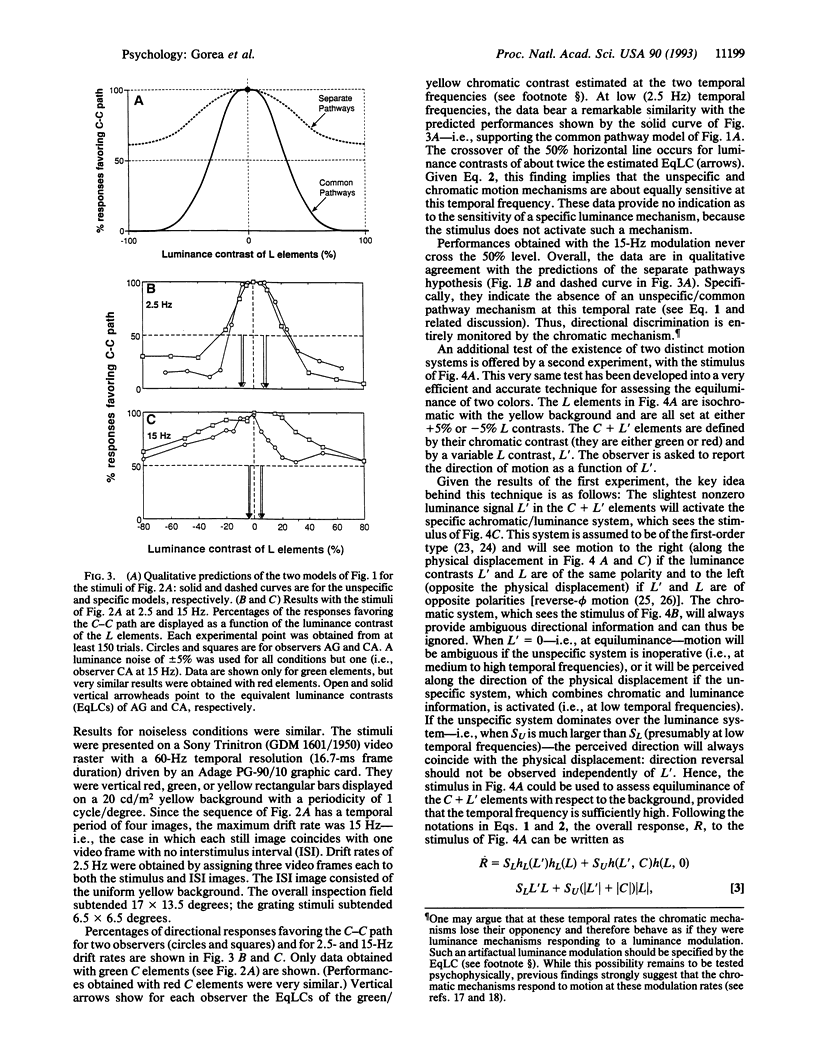
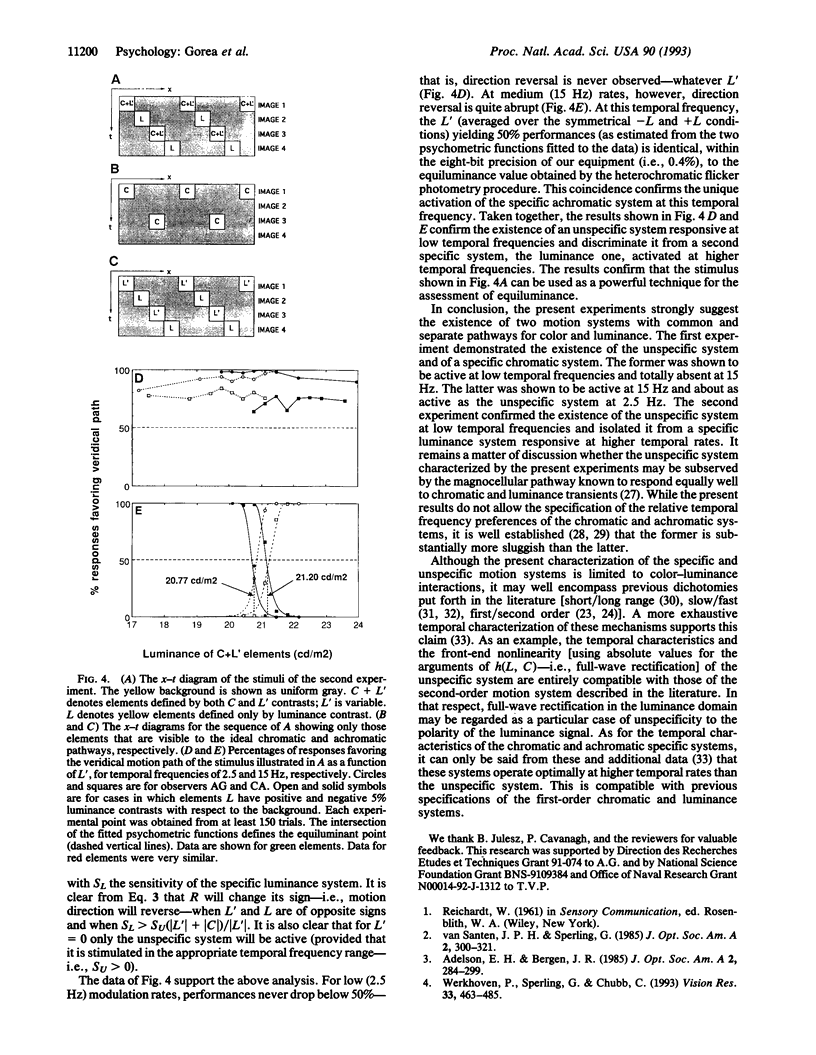
We present psychological experiments that reveal two motion systems, a specific and an unspecific one. The specific system prevails at medium to high temporal frequencies. It comprises at least two separate motion pathways that are selective for color and for luminance and that do not interact until after the motion signal is extracted separately in each. By contrast, the unspecific system prevails at low temporal frequencies and it combines color and luminance signals at an earlier stage, before motion extraction. The successful implementation of an efficient and accurate technique for assessing equiluminance corroborates further the main findings. These results offer a general framework for understanding the nature of interactions between color and luminance signals in motion perception and suggest that previously proposed dichotomies in motion processing may be encompassed by the specific/unspecific dichotomy proposed here.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelson E. H., Bergen J. R. Spatiotemporal energy models for the perception of motion. J Opt Soc Am A. 1985 Feb;2(2):284–299. doi: 10.1364/josaa.2.000284. [DOI] [PubMed] [Google Scholar]
- Agonie C., Gorea A. Equivalent luminance contrast of red-green drifting stimuli: dependency on luminance-color interactions and on the psychophysical task. J Opt Soc Am A. 1993 Jun;10(6):1341–1352. doi: 10.1364/josaa.10.001341. [DOI] [PubMed] [Google Scholar]
- Albrecht D. G., Hamilton D. B. Striate cortex of monkey and cat: contrast response function. J Neurophysiol. 1982 Jul;48(1):217–237. doi: 10.1152/jn.1982.48.1.217. [DOI] [PubMed] [Google Scholar]
- Almer A., Rudolph H., Hinnen A., Hörz W. Removal of positioned nucleosomes from the yeast PHO5 promoter upon PHO5 induction releases additional upstream activating DNA elements. EMBO J. 1986 Oct;5(10):2689–2696. doi: 10.1002/j.1460-2075.1986.tb04552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstis S. M. Phi movement as a subtraction process. Vision Res. 1970 Dec;10(12):1411–1430. doi: 10.1016/0042-6989(70)90092-1. [DOI] [PubMed] [Google Scholar]
- Anstis S. M., Rogers B. J. Illusory reversal of visual depth and movement during changes of contrast. Vision Res. 1975 Aug-Sep;15:957–961. doi: 10.1016/0042-6989(75)90236-9. [DOI] [PubMed] [Google Scholar]
- Archer T. K., Cordingley M. G., Wolford R. G., Hager G. L. Transcription factor access is mediated by accurately positioned nucleosomes on the mouse mammary tumor virus promoter. Mol Cell Biol. 1991 Feb;11(2):688–698. doi: 10.1128/mcb.11.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer T. K., Lefebvre P., Wolford R. G., Hager G. L. Transcription factor loading on the MMTV promoter: a bimodal mechanism for promoter activation. Science. 1992 Mar 20;255(5051):1573–1576. doi: 10.1126/science.1347958. [DOI] [PubMed] [Google Scholar]
- Archer T. K., Tam S. P., Deugau K. V., Deeley R. G. Apolipoprotein C-II mRNA levels in primate liver. Induction by estrogen in the human hepatocarcinoma cell line, HepG2. J Biol Chem. 1985 Feb 10;260(3):1676–1681. [PubMed] [Google Scholar]
- Bresnick E. H., Bustin M., Marsaud V., Richard-Foy H., Hager G. L. The transcriptionally-active MMTV promoter is depleted of histone H1. Nucleic Acids Res. 1992 Jan 25;20(2):273–278. doi: 10.1093/nar/20.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr K. D., Richard-Foy H. Glucocorticoids locally disrupt an array of positioned nucleosomes on the rat tyrosine aminotransferase promoter in hepatoma cells. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9300–9304. doi: 10.1073/pnas.87.23.9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato A. C., Henderson D., Ponta H. The hormone response element of the mouse mammary tumour virus DNA mediates the progestin and androgen induction of transcription in the proviral long terminal repeat region. EMBO J. 1987 Feb;6(2):363–368. doi: 10.1002/j.1460-2075.1987.tb04763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cato A. C., Miksicek R., Schütz G., Arnemann J., Beato M. The hormone regulatory element of mouse mammary tumour virus mediates progesterone induction. EMBO J. 1986 Sep;5(9):2237–2240. doi: 10.1002/j.1460-2075.1986.tb04490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh P., Anstis S. The contribution of color to motion in normal and color-deficient observers. Vision Res. 1991;31(12):2109–2148. doi: 10.1016/0042-6989(91)90169-6. [DOI] [PubMed] [Google Scholar]
- Cavanagh P., Arguin M., von Grünau M. Interattribute apparent motion. Vision Res. 1989;29(9):1197–1204. doi: 10.1016/0042-6989(89)90065-5. [DOI] [PubMed] [Google Scholar]
- Cavanagh P., Boeglin J., Favreau O. E. Perception of motion in equiluminous kinematograms. Perception. 1985;14(2):151–162. doi: 10.1068/p140151. [DOI] [PubMed] [Google Scholar]
- Cavanagh P., Mather G. Motion: the long and short of it. Spat Vis. 1989;4(2-3):103–129. doi: 10.1163/156856889x00077. [DOI] [PubMed] [Google Scholar]
- Cavanagh P., Tyler C. W., Favreau O. E. Perceived velocity of moving chromatic gratings. J Opt Soc Am A. 1984 Aug;1(8):893–899. doi: 10.1364/josaa.1.000893. [DOI] [PubMed] [Google Scholar]
- Chubb C., Sperling G. Drift-balanced random stimuli: a general basis for studying non-Fourier motion perception. J Opt Soc Am A. 1988 Nov;5(11):1986–2007. doi: 10.1364/josaa.5.001986. [DOI] [PubMed] [Google Scholar]
- Cordingley M. G., Hager G. L. Binding of multiple factors to the MMTV promoter in crude and fractionated nuclear extracts. Nucleic Acids Res. 1988 Jan 25;16(2):609–628. doi: 10.1093/nar/16.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordingley M. G., Riegel A. T., Hager G. L. Steroid-dependent interaction of transcription factors with the inducible promoter of mouse mammary tumor virus in vivo. Cell. 1987 Jan 30;48(2):261–270. doi: 10.1016/0092-8674(87)90429-6. [DOI] [PubMed] [Google Scholar]
- DeYoe E. A., Van Essen D. C. Concurrent processing streams in monkey visual cortex. Trends Neurosci. 1988 May;11(5):219–226. doi: 10.1016/0166-2236(88)90130-0. [DOI] [PubMed] [Google Scholar]
- Fiering S., Northrop J. P., Nolan G. P., Mattila P. S., Crabtree G. R., Herzenberg L. A. Single cell assay of a transcription factor reveals a threshold in transcription activated by signals emanating from the T-cell antigen receptor. Genes Dev. 1990 Oct;4(10):1823–1834. doi: 10.1101/gad.4.10.1823. [DOI] [PubMed] [Google Scholar]
- Gorea A., Papathomas T. V., Kovacs I. Motion perception with spatiotemporally matched chromatic and achromatic information reveals a "slow" and a "fast" motion system. Vision Res. 1993 Dec;33(17):2515–2534. doi: 10.1016/0042-6989(93)90132-g. [DOI] [PubMed] [Google Scholar]
- Gorea A., Papathomas T. V. Motion processing by chromatic and achromatic visual pathways. J Opt Soc Am A. 1989 Apr;6(4):590–602. doi: 10.1364/josaa.6.000590. [DOI] [PubMed] [Google Scholar]
- Gowland P. L., Buetti E. Mutations in the hormone regulatory element of mouse mammary tumor virus differentially affect the response to progestins, androgens, and glucocorticoids. Mol Cell Biol. 1989 Sep;9(9):3999–4008. doi: 10.1128/mcb.9.9.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiochon-Mantel A., Loosfelt H., Lescop P., Sar S., Atger M., Perrot-Applanat M., Milgrom E. Mechanisms of nuclear localization of the progesterone receptor: evidence for interaction between monomers. Cell. 1989 Jun 30;57(7):1147–1154. doi: 10.1016/0092-8674(89)90052-4. [DOI] [PubMed] [Google Scholar]
- Karttunen J., Shastri N. Measurement of ligand-induced activation in single viable T cells using the lacZ reporter gene. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3972–3976. doi: 10.1073/pnas.88.9.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D. H. Spatio-temporal frequency chracteristics of color-vision mechanisms. J Opt Soc Am. 1974 Jul;64(7):983–990. doi: 10.1364/josa.64.000983. [DOI] [PubMed] [Google Scholar]
- King W. J., Greene G. L. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984 Feb 23;307(5953):745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- Ko M. S., Nakauchi H., Takahashi N. The dose dependence of glucocorticoid-inducible gene expression results from changes in the number of transcriptionally active templates. EMBO J. 1990 Sep;9(9):2835–2842. doi: 10.1002/j.1460-2075.1990.tb07472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovaćs I., Julesz B. Depth, motion, and static-flow perception at metaisoluminant color contrast. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10390–10394. doi: 10.1073/pnas.89.21.10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laybourn P. J., Kadonaga J. T. Threshold phenomena and long-distance activation of transcription by RNA polymerase II. Science. 1992 Sep 18;257(5077):1682–1685. doi: 10.1126/science.1388287. [DOI] [PubMed] [Google Scholar]
- Lefebvre P., Berard D. S., Cordingley M. G., Hager G. L. Two regions of the mouse mammary tumor virus long terminal repeat regulate the activity of its promoter in mammary cell lines. Mol Cell Biol. 1991 May;11(5):2529–2537. doi: 10.1128/mcb.11.5.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtler A., Hager G. L. Induction of multiple replacement mutations by oligonucleotide-directed mutagenesis with extended mismatch primers. Gene Anal Tech. 1987 Nov-Dec;4(6):111–118. doi: 10.1016/0735-0651(87)90009-4. [DOI] [PubMed] [Google Scholar]
- Logothetis N. K., Schiller P. H., Charles E. R., Hurlbert A. C. Perceptual deficits and the activity of the color-opponent and broad-band pathways at isoluminance. Science. 1990 Jan 12;247(4939):214–217. doi: 10.1126/science.2294602. [DOI] [PubMed] [Google Scholar]
- Murray I., MacCana F., Kulikowski J. J. Contribution of two movement detecting mechanisms to central and peripheral vision. Vision Res. 1983;23(2):151–159. doi: 10.1016/0042-6989(83)90138-4. [DOI] [PubMed] [Google Scholar]
- Nishida S., Takeuchi T. The effects of luminance on affinity of apparent motion. Vision Res. 1990;30(5):709–721. doi: 10.1016/0042-6989(90)90097-5. [DOI] [PubMed] [Google Scholar]
- Papathomas T. V., Gorea A., Julesz B. Two carriers for motion perception: color and luminance. Vision Res. 1991;31(11):1883–1892. doi: 10.1016/0042-6989(91)90183-6. [DOI] [PubMed] [Google Scholar]
- Peterson C. L., Herskowitz I. Characterization of the yeast SWI1, SWI2, and SWI3 genes, which encode a global activator of transcription. Cell. 1992 Feb 7;68(3):573–583. doi: 10.1016/0092-8674(92)90192-f. [DOI] [PubMed] [Google Scholar]
- Picard D., Kumar V., Chambon P., Yamamoto K. R. Signal transduction by steroid hormones: nuclear localization is differentially regulated in estrogen and glucocorticoid receptors. Cell Regul. 1990 Feb;1(3):291–299. doi: 10.1091/mbc.1.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piña B., Brüggemeier U., Beato M. Nucleosome positioning modulates accessibility of regulatory proteins to the mouse mammary tumor virus promoter. Cell. 1990 Mar 9;60(5):719–731. doi: 10.1016/0092-8674(90)90087-u. [DOI] [PubMed] [Google Scholar]
- Ramachandran V. S., Gregory R. L. Does colour provide an input to human motion perception? Nature. 1978 Sep 7;275(5675):55–56. doi: 10.1038/275055a0. [DOI] [PubMed] [Google Scholar]
- Reese J. C., Katzenellenbogen B. S. Examination of the DNA-binding ability of estrogen receptor in whole cells: implications for hormone-independent transactivation and the actions of antiestrogens. Mol Cell Biol. 1992 Oct;12(10):4531–4538. doi: 10.1128/mcb.12.10.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik A., Schütz G., Stewart A. F. Glucocorticoids are required for establishment and maintenance of an alteration in chromatin structure: induction leads to a reversible disruption of nucleosomes over an enhancer. EMBO J. 1991 Sep;10(9):2569–2576. doi: 10.1002/j.1460-2075.1991.tb07797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard-Foy H., Hager G. L. Sequence-specific positioning of nucleosomes over the steroid-inducible MMTV promoter. EMBO J. 1987 Aug;6(8):2321–2328. doi: 10.1002/j.1460-2075.1987.tb02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud G., Roux J., Pictet R., Grange T. In vivo footprinting of rat TAT gene: dynamic interplay between the glucocorticoid receptor and a liver-specific factor. Cell. 1991 Nov 29;67(5):977–986. doi: 10.1016/0092-8674(91)90370-e. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Colby C. L. The responses of single cells in the lateral geniculate nucleus of the rhesus monkey to color and luminance contrast. Vision Res. 1983;23(12):1631–1641. doi: 10.1016/0042-6989(83)90177-3. [DOI] [PubMed] [Google Scholar]
- Strair R. K., Towle M., Smith B. R. Retroviral mediated gene transfer into bone marrow progenitor cells: use of beta-galactosidase as a selectable marker. Nucleic Acids Res. 1990 Aug 25;18(16):4759–4762. doi: 10.1093/nar/18.16.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straka C., Hörz W. A functional role for nucleosomes in the repression of a yeast promoter. EMBO J. 1991 Feb;10(2):361–368. doi: 10.1002/j.1460-2075.1991.tb07957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor I. C., Workman J. L., Schuetz T. J., Kingston R. E. Facilitated binding of GAL4 and heat shock factor to nucleosomal templates: differential function of DNA-binding domains. Genes Dev. 1991 Jul;5(7):1285–1298. doi: 10.1101/gad.5.7.1285. [DOI] [PubMed] [Google Scholar]
- Troscianko T. Perception of random-dot symmetry and apparent movement at and near isoluminance. Vision Res. 1987;27(4):547–554. doi: 10.1016/0042-6989(87)90041-1. [DOI] [PubMed] [Google Scholar]
- Tzukerman M., Zhang X. K., Hermann T., Wills K. N., Graupner G., Pfahl M. The human estrogen receptor has transcriptional activator and repressor functions in the absence of ligand. New Biol. 1990 Jul;2(7):613–620. [PubMed] [Google Scholar]
- Werkhoven P., Sperling G., Chubb C. The dimensionality of texture-defined motion: a single channel theory. Vision Res. 1993 Mar;33(4):463–485. doi: 10.1016/0042-6989(93)90253-s. [DOI] [PubMed] [Google Scholar]
- Workman J. L., Taylor I. C., Kingston R. E. Activation domains of stably bound GAL4 derivatives alleviate repression of promoters by nucleosomes. Cell. 1991 Feb 8;64(3):533–544. doi: 10.1016/0092-8674(91)90237-s. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Nolan G. P., Pollock R., Prockop S., Li S. C., Herzenberg L. A., Alt F. W. A novel fluorescence-based system for assaying and separating live cells according to VDJ recombinase activity. Mol Cell Biol. 1990 Apr;10(4):1697–1704. doi: 10.1128/mcb.10.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga S. K., Peterson C. L., Herskowitz I., Yamamoto K. R. Roles of SWI1, SWI2, and SWI3 proteins for transcriptional enhancement by steroid receptors. Science. 1992 Dec 4;258(5088):1598–1604. doi: 10.1126/science.1360703. [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Yamamoto K. R. Reversible and persistent changes in chromatin structure accompany activation of a glucocorticoid-dependent enhancer element. Cell. 1984 Aug;38(1):29–38. doi: 10.1016/0092-8674(84)90523-3. [DOI] [PubMed] [Google Scholar]
- van Santen J. P., Sperling G. Elaborated Reichardt detectors. J Opt Soc Am A. 1985 Feb;2(2):300–321. doi: 10.1364/josaa.2.000300. [DOI] [PubMed] [Google Scholar]
- von der Ahe D., Janich S., Scheidereit C., Renkawitz R., Schütz G., Beato M. Glucocorticoid and progesterone receptors bind to the same sites in two hormonally regulated promoters. Nature. 1985 Feb 21;313(6004):706–709. doi: 10.1038/313706a0. [DOI] [PubMed] [Google Scholar]




