Abstract
Purpose
Cardiac resynchronization therapy (CRT) improves outcomes in patients with heart failure, yet response rates are variable. We sought to determine whether physician-specified CRT programming was associated with improved outcomes.
Methods
Using data from the ALTITUDE remote follow-up cohort, we examined sensed atrioventricular (AV) and ventricular-to-ventricular (VV) programming and their associated outcomes in patients with de novo CRT from 2009–2010. Outcomes included arrhythmia burden, left ventricular (LV) pacing, and all-cause mortality at 4 years.
Results
We identified 5709 patients with de novo CRT devices; at the time of implant, 34 % (n=1959) had entirely nominal settings programmed, 40 % (n=2294) had only AV timing adjusted, 11 % (n=604) had only VV timing adjusted, and 15 % (n=852) had both AV and VV adjusted from nominal programming. Suboptimal LV pacing (<95 %) during follow-up was similar across groups; however, the proportion with atrial fibrillation (AF) burden >5%was lowest in the AV-only adjusted group (17.9 %) and highest in the nominal (27.7 %) and VV-only adjusted (28.3 %) groups. Adjusted all-cause mortality was significantly higher among patients with non-nominal AV delay >120 vs. <120 ms (adjusted heart rate (HR) 1.28, p=0.008) but similar when using the 180-ms cutoff (adjusted HR 1.13 for >180 vs. ≤180 ms, p=0.4).
Conclusions
Nominal settings for de novo CRT implants are frequently altered, most commonly the AV delay. There is wide variability in reprogramming. Patients with nominal or AV-only adjustments appear to have favorable pacing and arrhythmia outcomes. Sensed AV delays less than 120 ms are associated with improved survival.
Keywords: Cardiac resynchronization therapy, Optimization, Programing, Outcomes
1 Introduction
Cardiac resynchronization therapy (CRT) improves symptoms and survival in patients with refractory heart failure and conduction delay [1]. However, there is extensive variability in clinical response rates following CRT, with up to one third of patients deriving little or no clinical benefit [2]. Many studies have attempted to identify clinical predictors of response, including baseline electrocardiographic morphology, QRS duration, heart failure etiology, New York Heart Association class, and advanced echocardiographic parameters [3]. Additional studies have assessed the efficacy of electrocardiographic algorithms, echocardiography, or invasive assessments to determine ideal settings [4, 5]. Preliminary data suggested significant benefit to patient-specific optimization of atrioventricular (AV) timing and ventricular-to-ventricular (VV) timing [5, 6], yet subsequent clinical trials of an electrogram-based algorithm and echocardiography to determine optimal AV activation did not demonstrate clinical superiority for the endpoint of left ventricular end-systolic volume [7]. Therefore, providers are largely left to their own judgment as to the ideal programming for AV and VV delays in CRT patients.
While “nominal” values for AV timing and VV timing are preset by the manufacturer, operators may adjust or tailor these intervals at the time of implant to suit each specific patient. However, it is unknown how often this is done. Furthermore, it remains unclear if poor response can be partially explained by a “one-size-fits-all” approach to timing settings. Accordingly, we conducted a retrospective analysis of patients who underwent de novo CRT-D device and were enrolled in the ALTITUDE clinical science program. The objective of the study was to assess the use of physician-adjusted timing settings (versus nominal) in patients undergoing CRT, and their association with clinical outcomes. We hypothesized that physician-adjusted settings would be associated with improved biventricular pacing percentage and improved survival.
2 Methods
The design and methods of the ALTITUDE research program have been described previously [8]. Briefly, beginning in 2006, the ALTITUDE study has been updated with data from the LATITUDE US remote monitoring system (Boston Scientific, Natick, MA) for clinical research purposes. Uploaded LATITUDE data include device parameters, clinical diagnostics, and episodes. Participation in the ALTITUDE initiative is elective and governed by a data use agreement allowing for the use of such de-identified data for research purposes in accordance with Health Insurance Portability and Account-ability Act regulations. Less than 10 % of LATITUDE centers decline to contribute data to ALTITUDE [8].
The goal of the present analysis was to analyze non-nominal programming of CRT devices. Therefore, for the purpose of this study, the dataset was limited to de novo implants of a single model device (Boston Scientific Cognis N118 & N119), during a single implant period (2009–2010). Patients without functional biventricular pacing leads, those without programming settings available at implant, or without LATITUDE transmission within 6 months of implant were excluded. Finally, patients programmed to bradycardia modes other than DDD or DDDR were excluded.
We subsequently characterized CRT programming at implant (or within 10 days) and stratified patients by changes from nominal: (1) no change (all nominal settings), (2) sensed AV-only adjusted (hereafter “AVonly”), (3) VV-only adjusted, and (4) both sensed AV and VV adjusted. Since most CRT patients are atrially sensed, we utilized sensed AV delays (vs. paced AV delays) for the primary analyses. Notably, automatic algorithms such as Smart-AV are implemented at the level of the programmer, and therefore, only the resulting AV delays are included here. Baseline characteristics and other device programming parameters for these patients were compared. Distributions of programmed timing intervals were assessed relative to nominal values (180 ms for paced and 120 ms for sensed AV timing; 0 ms for VV timing). Additionally, analyses of sensed AV timing were conducted with binary stratification at (a) <120 vs. >120ms (excluding patients set to the nominal value, 120 ms) and (b) ≤180 vs. >180 ms.
As an assessment of baseline rhythm characteristics, device parameters and arrhythmias were measured within the first 6 months after implant (among patients with a LATITUDE transmission during that period). These included heart rate variability (HRV) parameters, patient activity, left ventricular (LV) pacing percentage, atrial fibrillation (AF) burden, and ventricular tachyarrhythmia therapies, each as measured by the device.
Follow-up was censored at last LATITUDE transmission. Arrhythmia outcomes at follow-up were shock or anti-tachycardia pacing for ventricular tachyarrhythmias and changes in LATITUDE parameters. Changes in these parameters were calculated between the initial 6-month post-implant period and subsequent follow-up and included changes in patient activity, burden of AF, percent LV pacing, and HRV parameters (footprint, standard deviation of the average NN intervals [SDANN], and mean heart rate). Clinical outcome was measured as all-cause mortality and identified by cross-reference with the US Social Security Death Index (with surveillance out to 12 months after last follow-up).
2.1 Statistical methods
Pairwise comparisons of data across groups were tested using a t test for continuous variables, or a Wilcoxon rank-sum test for variables with extremely skewed distributions, or a chi-square test for categorical variables.
Landmark analyses were conducted, including all patients who survived at least 90 days post-implant. For these patients, day 0 was set at 90 days post-implant, and Kaplan-Meier curves of survival by group were plotted out to four subsequent years. For multivariable adjustment, a Cox proportional hazard model of time to death was used and adjusted for age, gender, defibrillation threshold (DFT) testing at implant, number of treatment zones, brady programming of DDD, LRL, VF detection rate, AF burden, LV pacing, and shock therapy.
2.2 Sensitivity analyses
To identify the durability of programming modifications at implant, we assessed the frequency of subsequent programming changes during follow-up. Additional, identical land-mark analyses for the mortality were also repeated only for patients with HRV data, with the additional variables SDANN, footprint, and mean heart rate adjusted for in the Cox model.
The study complies with the Declaration of Helsinki, and informed consent was waived under the common rule. All analyses were performed using SAS version 9.3.
3 Results
3.1 Baseline characteristics
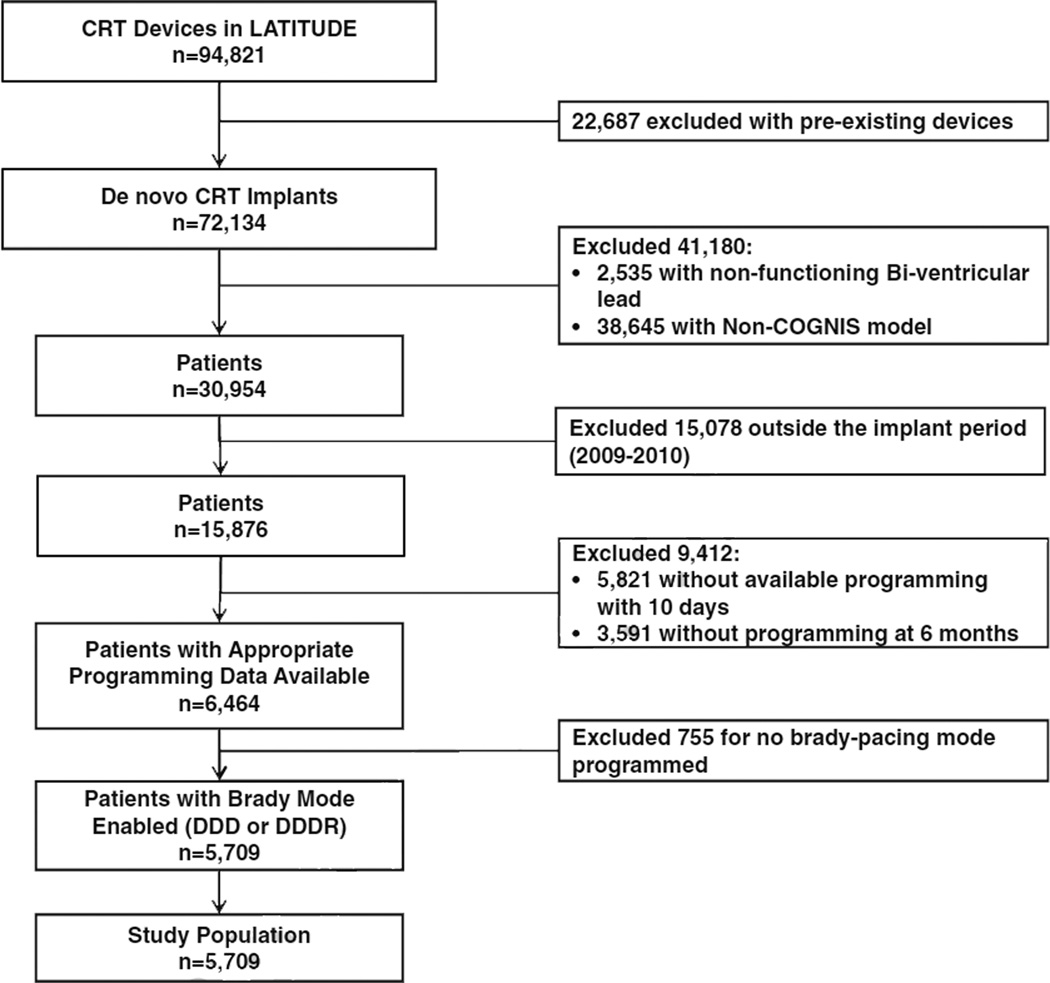
After exclusions (Fig. 1), the final analysis population included 5709 patients. Among these patients, 34 % (n=1959) had entirely nominal settings programmed, 40 % (n=2294) had only AV timing adjusted, 11 % (n=604) had only VV timing adjusted, and 15 % (n=852) had both AV and VV adjusted from nominal programming at the time of implant. Baseline characteristics of these groups are shown in Table 1. Age, gender, and defibrillation threshold testing were roughly similar across programming strategies. Patients with AV adjustment were less frequently programmed with rate-responsive bradycardia pacing modes (60 % DDD vs. 40 % DDDR), whereas pacing mode was more balanced among patients with nominal AV and VV timing, and in patients with any VV adjustment. Programming of ventricular tachyarrhythmia detection and therapy was similar across CRT programming groups.
Fig. 1.
Selection of analysis population, by exclusion criteria. AV atrioventricular timing, VV ventricular-to-ventricular timing
Table 1.
Baseline characteristics, stratified by programming at implant
| Nominal (n=1559) |
AV adjusted (n=2294) |
p value (vs. nominal) |
VV adjusted (n=604) |
p value (vs. nominal) |
AV and VV adjusted (n=852) |
p value (vs. nominal) |
|
|---|---|---|---|---|---|---|---|
| Age | 69.8 (11.3) | 68.8 (11.1) | 0.007 | 69.8 (11.8) | 0.997 | 68.8 (11.5) | 0.030 |
| Male | 73.7 % | 69.9 % | 0.006 | 73.8 % | 0.964 | 71.0 % | 0.133 |
| DFT at implant | 77.6 % | 83.8 % | <0.0001 | 80.3 % | 0.166 | 81.9 % | 0.011 |
| Last shock energy (J) | 19.97 (6.31) | 19.43 (6.35) | 0.011 | 19.97 (5.98) | 0.956 | 19.45 (5.55) | 0.069 |
| Bradycardia programming | |||||||
| DDD | 52.5 % | 59.8 % | <0.0001 | 48.2 % | 0.065 | 59.0 % | 0.001 |
| DDDR | 47.5 % | 40.2 % | <0.0001 | 51.8 % | 0.065 | 41.0 % | 0.001 |
| LRL | 61.5 (7.3) | 59.9 (7.5) | <0.0001 | 60.2 (9.4) | 0.000 | 58.7 (8.9) | <0.0001 |
| Tachyarrhythmia programming | |||||||
| 1 therapy zone | 16.5 % | 15.9 % | 0.580 | 8.9 % | <0.0001 | 11.4 % | 0.0004 |
| 2 therapy zones | 69.7 % | 71.2 % | 0.269 | 75.3 % | 0.007 | 75.7 % | 0.001 |
| 3 therapy zones | 13.8 % | 12.9 % | 0.377 | 15.7 % | 0.232 | 12.9 % | 0.534 |
| VT detection rate | 175 (15) | 176 (14) | 0.224 | 176 (14) | 0.564 | 175 (14) | 0.290 |
| VF detection rate | 207 (16) | 207 (16) | 0.379 | 209 (15) | 0.009 | 207 (16) | 0.736 |
| ATP therapy “on” in VT zone/s | 94.3 % | 95.2 % | 0.227 | 95.9 % | 0.134 | 96.1 % | 0.051 |
Numbers are % or mean (standard deviation)
AV atrioventricular timing, VV ventricular-to-ventricular timing, DFT defibrillation threshold testing, LRL lower rate limit
3.2 Distribution of AV and VV intervals
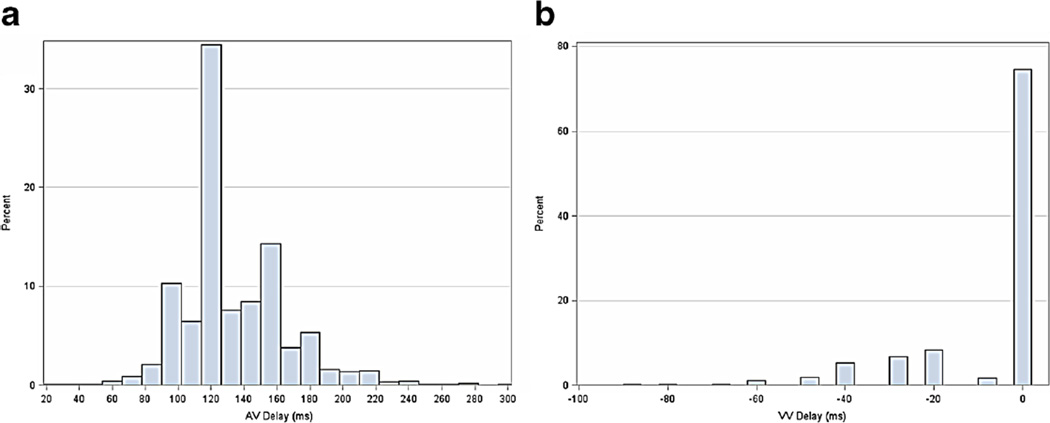
Distributions of AV and VV times, over the entire cohort, are shown in Fig. 2a, b. Nearly 80 % of patients had VV programmed to the nominal value (0 ms), whereas the nominal sensed AV delay was used in 30%. There appeared to be more heterogeneity in programming of AV timing versus VV timing, including use of long AV delays. Programming changes during follow-up occurred in approximately one third of patients in each baseline group (nominal, AV-only, VV-only, and both adjusted; Supplemental Material).
Fig. 2.
a, b Distribution of programming parameters within 10 days of implant, across the entire analysis population. AV atrioventricular timing, VV ventricular-to-ventricular timing
3.3 Heart rhythm and pacing outcomes within 6-months of implant
Baseline rhythm characteristics within 6 months of implant are shown in Table 2. Overall, patient activity was low (6–7 %), although it was higher in the AV adjusted group. HRV was similar across all four groups as demonstrated by multiple indices. During this initial period, AF burden >5 % was 11–12 % in patients with any AV adjustment, and 22–23 % in patients with all nominal or VV-only adjusted settings. Patients were atrially paced a minority of the time, across groups. Suboptimal LV pacing (<95 %) was highest in the nominal (17.6 %) and AV-only adjusted (17.7 %) groups. Overall, 6–7 % experienced a shock within 6 months of implant.
Table 2.
Baseline rhythm characteristics (within 6 months of implant), stratified by programming at implant
| Nominal (n=1559) |
AV adjusted (n=2294) |
p value (vs. nominal) |
VV adjusted (n=604) |
p value (vs. nominal) |
AV and VV adjusted (n=852) |
p value (vs. nominal) |
|
|---|---|---|---|---|---|---|---|
| Patient activity (%) | 6.7 (4.0) | 7.3 (4.3) | <0.0001 | 6.5 (4.0) | 0.2 | 6.9 (4.0) | 0.3 |
| HRV SDANN | 70.1 (20.4) | 70.6 (18.9) | 0.6 | 70.1 (20.3) | 1 | 70.7 (20.3) | 0.6 |
| HRV footprint | 28.2 (9) | 28.3 (8.7) | 0.9 | 27.5 (9.5) | 0.3 | 28.3 (9.2) | 0.9 |
| HRV mean HR | 77.2 (8.2) | 77.2 (8.3) | 0.9 | 77.4 (9.3) | 0.7 | 76.6 (8.8) | 0.2 |
| AF burden >5 % | 21.7 % | 11.7 % | <0.0001 | 22.0 % | 0.9 | 11.0 % | <0.0001 |
| Atrial pacing (%) | 32 (36) | 27 (33) | 0.01 | 27 (35) | 0.0001 | 27 (35) | 0.005 |
| LV pacing <95 % | 17.6 % | 17.7 % | 0.9 | 15.9 % | 0.3 | 14.9 % | 0.08 |
| Any shock within 6 months of implant | 6.1 % | 6.9 % | 0.3 | 7.6 % | 0.2 | 7.4 % | 0.2 |
| ATP therapy | 12.9 % | 16.7 % | 0.0005 | 16.2 % | 0.04 | 18.2 % | 0.0002 |
Numbers are % or mean (standard deviation)
AV atrioventricular timing, VV ventricular-to-ventricular timing, ATP anti-tachycardia pacing, AF atrial fibrillation, LV left ventricular, HRV heart rate variability, SDANN standard deviation of the average NN intervals, HR heart rate
3.4 LATITUDE parameters during follow-up
Overall, mean follow-up was 2.5±1.1 years across CRT programming strata. Patients in the AV-only adjusted group had increased activity (7.5 vs. 6.9–7.3 % for other groups) and increased measures of HRV compared with other programming groups (Table 3). Burden of AF remained lowest in patients with AV-only adjusted, but the increase in AF burden was similar across groups. Proportion of patients with <95 % LV pacing was lowest in the nominal and AV-only adjusted groups (approximately 17%), compared with VV-only adjusted (19.2 %) and both adjusted (18.5 %). For both anti-tachycardia pacing and shocks, patients with both AV and VV adjusted had the highest rates (32.7 % with ATP, 16.7 % with shocks).
Table 3.
Unadjusted outcomes through last follow-up
| Nominal (n=1559) |
AV adjusted (n=2294) |
p value (vs. nominal) |
VV adjusted (n=604) |
p value (vs. nominal) |
AV and VV adjusted (n=852) |
p value (vs. nominal) |
|
|---|---|---|---|---|---|---|---|
| Follow-up, days | 956 (328) | 983 (322) | 0.009 | 944 (344) | 0.5 | 952 (330) | 0.8 |
| PCT activity | 7 (4.2) | 7.5 (4.6) | 0.0005 | 6.9 (4.3) | 0.8 | 7.3 (4.2) | 0.2 |
| Change from baseline | 0.00 (1.4) | −0.03 (1.6) | 0.5 | −0.09 (1.5) | 0.2 | 0.03 (1.5) | 0.8 |
| HRV SDANN | 73.6 (20.7) | 72.9 (18.8) | 0.5 | 73 (21.4) | 0.7 | 73.5 (22.4) | 0.9 |
| Change from baseline | 0.83 (9.8) | 0.92 (10.2) | 0.9 | 0.21 (9.7) | 0.5 | 0.71 (10.2) | 0.9 |
| HRV footprint | 29 (8.9) | 28.8 (9) | 0.8 | 29.1 (10.5) | 0.9 | 28.9 (9.6) | 0.9 |
| Change from baseline | 0.24 (3.8) | 0.14 (3.9) | 0.7 | 0.63 (0.9) | 0.3 | −0.13 (4.5) | 0.2 |
| HRV mean HR | 77 (8) | 77.1 (8.2) | 0.8 | 77.2 (10.2) | 0.8 | 76.5 (9.5) | 0.4 |
| Change from baseline | −0.45 (3.4) | −0.47 (3.7) | 0.9 | −0.28 (4.4) | 0.6 | −0.08 (4.9) | 0.2 |
| AF burden >5 % | 27.7 % | 17.9 % | <0.0001 | 28.3 % | 0.8 | 18.9 % | <0.0001 |
| Change from baseline | 4.00 % (21.0 %) | 4.00 % (18.0 %) | 0.8 | 3.00 % (19.0 %) | 0.9 | 4.00 % (17.0 %) | 0.6 |
| Atrial pacing (%) | 33 (37) | 31 (35) | 0.6 | 29 (36) | 0.001 | 32 (37) | 0.8 |
| Change from baseline | 1.00 (32) | 4.00 (29) | 0.03 | 2.00 (32) | 0.9 | 5.00 (32) | 0.06 |
| LV pacing <95 % | 17.3 % | 17.8 % | 0.6 | 19.2 % | 0.3 | 18.5 % | 0.4 |
| Change from baseline | 0.00 % (13.0 %) | −1.00 % (14.0 %) | 0.8 | −1.00 % (12.0 %) | 0.046 | −2.00 % (15.0 %) | 0.09 |
| ATP therapy | 27.6 % | 29.5 % | 0.2 | 31.0 % | 0.1 | 32.7 % | 0.006 |
| Shock during follow-up | 13.1 % | 13.4 % | 0.8 | 12.9 % | 0.9 | 16.7 % | 0.01 |
| Death | 18.6 % | 19.1 % | 0.7 | 20.7 % | 0.3 | 19.2 % | 0.7 |
Numbers are % or mean (standard deviation)
AV atrioventricular timing, VV ventricular-to-ventricular timing, ATP anti-tachycardia pacing, AF atrial fibrillation, LV left ventricular, HRV heart rate variability, SDANN standard deviation of the average NN intervals, HR heart rate
3.5 Mortality
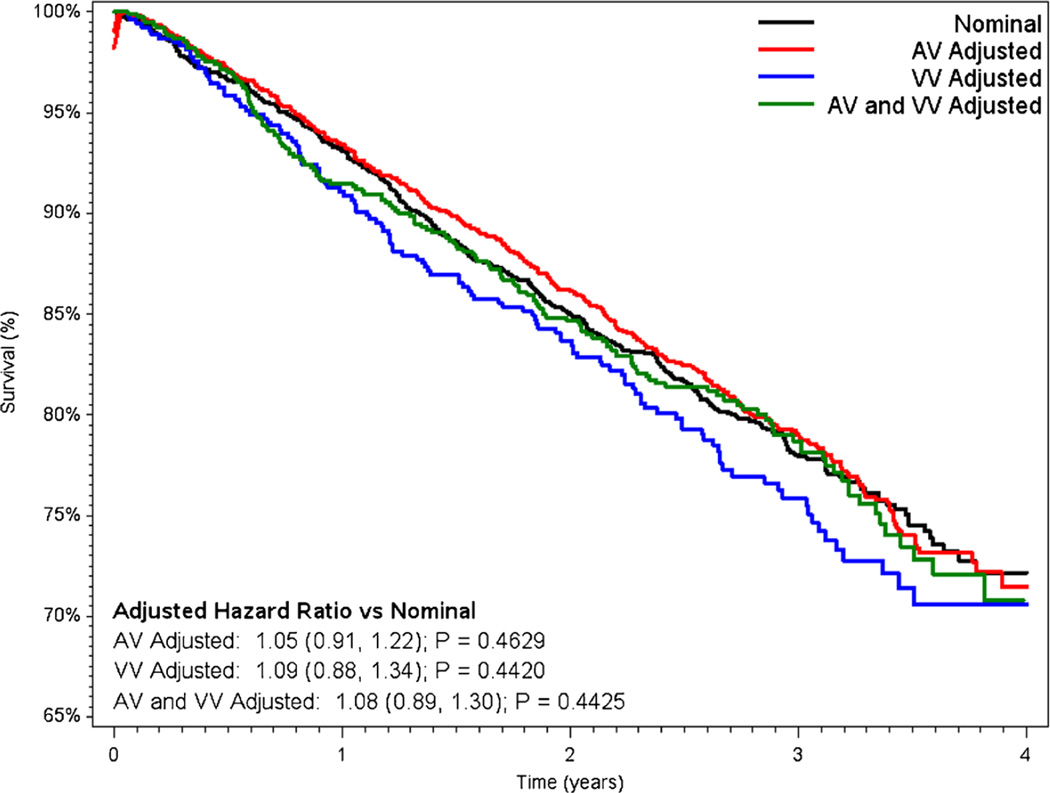
Unadjusted mortality was 18.6 % for patients with nominal settings at baseline, 19.1 % among those with AV-only adjustment, 20.7 % in patients with VV-only adjustment had the worst survival, and 19.2 % in patients with both AV and VV adjusted (P=NS for each pairwise comparison vs. nominal). Results of the Cox proportional hazards adjustment for all-cause mortality are shown in Fig. 3. After adjustment, risk of mortality was similar between patients programmed nominally and those with AV-only adjustment (adjusted heart rate (HR) 1.05 for AV-only vs. nominal; 95 % confidence interval (CI) 0.91–1.22). There was slightly higher mortality in patients with VV-only adjustment (adjusted HR 1.09 vs. nominal, 95 % CI 0.88–1.34) and in those with both AV and VV adjustment (adjusted HR 1.08 vs. nominal, 95% CI 0.89–1.30); however, none of the differences were statistically significant. Results of multivariable models for all-cause mortality adjusted for HRV yielded results similar to the primary analysis (Supplemental Material).
Fig. 3.
Unadjusted Kaplan-Meier survival curves, stratified by baseline programming. Hazard ratios adjusted for age, gender, DFT at implant, number of treatment zones, brady programming of DDD, LRL, VF detection rate, AF burden, LV pacing, and shock therapy. AV atrioventricular timing, VV ventricular-to-ventricular timing, DFT defibrillation threshold, LRL lower rate limit, VF ventricular fibrillation, AF atrial fibrillation, LV left ventricular
3.6 Short versus long AV delays
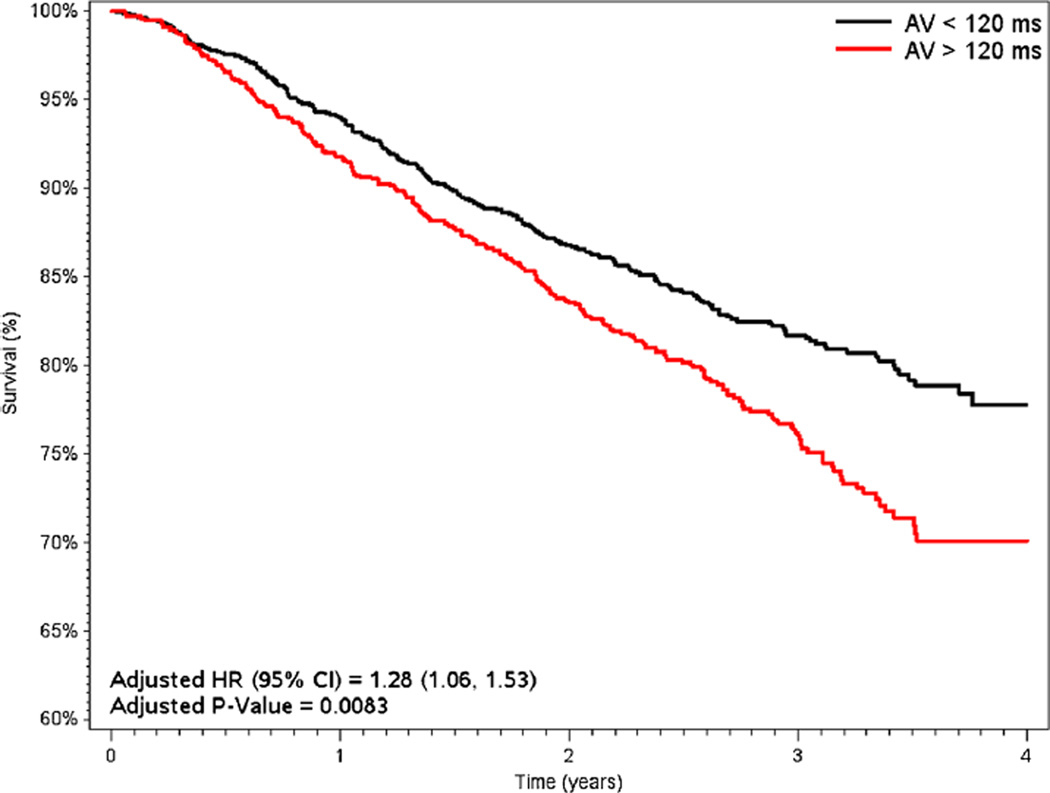
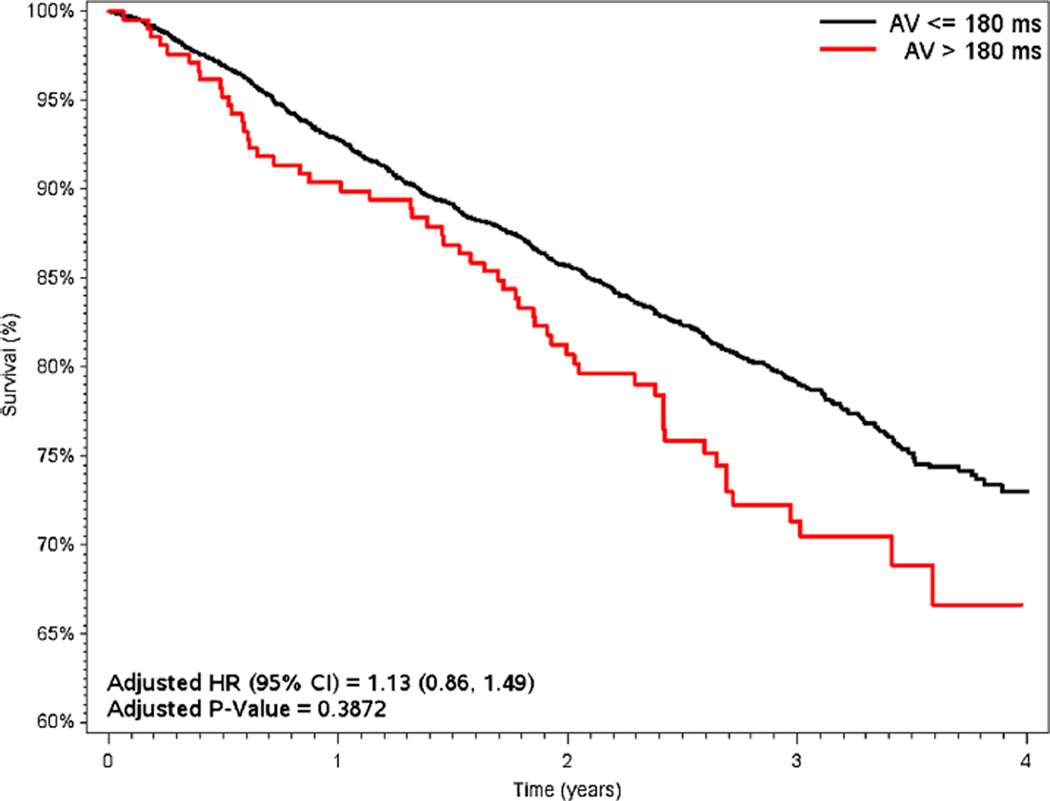
Survival results according to short and long AV delay programming are shown in Figs. 4 and 5. When stratifying by AV adjustment (<120 ms [1046/3146, 33 %] vs. >120 ms [2100/3146, 67 %]; ≤180 ms [4674/4953, 94 %] vs. >180 ms [279/4953, 6 %]), unadjusted mortality consistently favored patients with short AV timing (15.6 % for <120 ms vs. 21 % for >120 ms, p=0.0003; 16.2 % for ≤180 ms vs. 21.8 % for >180 ms, p<0.0001). In adjusted analyses, mortality remained significantly lower for patients with AV delay <120 ms (adjusted HR 1.28 vs. >120 ms, 95 % CI 1.06–1.53, p= 0.0083) but was similar for patients with AV delay ≤180 ms (adjusted HR 1.13 vs. >180 ms, 95 % CI 0.86–1.49, p=0.4).
Fig. 4.
Unadjusted Kaplan-Meier survival curves, dichotomized by baseline sensed AV programming (nominal=120 ms). Hazard ratios adjusted for age, gender, DFT at implant, number of treatment zones, brady programming of DDD, LRL, VF detection rate, AF burden, LV pacing, and shock therapy. AV atrioventricular timing, VV ventricular-to-ventricular timing, DFT defibrillation threshold, LRL lower rate limit, VF ventricular fibrillation, AF atrial fibrillation, LV left ventricular
Fig. 5.
Unadjusted Kaplan-Meier survival curves, dichotomized by baseline sensed AV programming at 180 ms (nominal=120 ms). Hazard ratios adjusted for age, gender, DFT at implant, number of treatment zones, brady programming of DDD, LRL, VF detection rate, AF burden, LV pacing, and shock therapy. AV atrioventricular timing, VV ventricular-to-ventricular timing, DFT defibrillation threshold, LRL lower rate limit, VF ventricular fibrillation, AF atrial fibrillation, LV left ventricular
3.7 Sensitivity analysis
In sensitivity analyses, findings were consistent when using paced AV delay settings, instead of sensed AV delay (see Supplemental Material). However, among patients with non-nominal paced AV delay (180ms), there did not appear to be a survival advantage of short, paced AV delays (≤120 or <180 ms).
4 Discussion
We examined de novo CRT programming in more than 5700 patients enrolled in remote monitoring. Based upon these analyses, there are three primary findings. First, AV and VV intervals are often adjusted, and these changes are highly variable. Second, nominal programming and AV adjustment only are associated with improved biventricular pacing percentage and lower atrial arrhythmia burden. Despite differences in pacing and arrhythmia outcomes, survival was not significantly different according to AV and VV adjustment. Finally, and perhaps most importantly, there may be a survival advantage to short, sensed AV delay programming (<120 ms), and this strategy deserves a prospective, randomized trial.
The optimal programing of AV and VV timing for patients with CRT is not known. Optimization using either advanced imaging or invasive hemodynamics has improved response rates and outcomes in some studies [5, 6]. However, complex electrocardiographic algorithms to set the AV delay, such as Smart-AV and QuickOpt, have failed to “optimize” response rates in randomized controlled trials [7, 9]. The conflicting data have led many to conclude that a highly individualized approach is warranted, and there are data to support improved outcomes for patients managed in an “optimization” clinic [10]. Yet such clinics are not universally available nor are they uniformly implemented. Therefore, most implanting physicians must attempt to identify the ideal settings on their own, often at the time of implant. However, additional, device-based algorithms with continuous optimization, such as AdaptiveCRT, may help still prove helpful in reducing non-responder rates [11, 12]. In the absence of additional, randomized data, shorter AV delays may be preferable in empiric CRT programming.
Overall, more than 70 % of the CRT population had some alteration from nominal values at implant, and one third of the remaining patients had modifications during follow-up. Modification of the nominal sensed AV delay was most common, with or without VV adjustment (VV-only modification accounted for a minority of patients). The use of long AV delays was common in these patients with new CRT, and patients in whom only the AV delay was modified from base-line appeared to have favorable arrhythmia outcomes.
When not selecting the nominal value (120 ms), providers used a variety of settings above and below 120 ms. Recent data from the MADIT-CRT trial suggest that short AV delays (<120 ms) are associated with improved clinical outcomes, including reductions in heart failure and death [13]. In ALTITUDE, AV adjustment was associated with improved arrhythmia outcomes including lower AF burden and greater biventricular pacing percentage. Furthermore, among patients who were programmed non-nominal, a short AV delay (<120 ms) was associated with significantly lower all-cause mortality. In contrast to the closely controlled environment of the MADIT-CRT randomized clinical trial, these data are from a nationwide, observational cohort and yield consistent findings. While some have suggested that the association between short AV delays and improved survival is due to the selection of healthier patients with shorter AV delays [14], the consistency of this finding across different clinical settings and different populations lends further support to the hypothesis that shorter AV delays may lead to improved CRT treatment effects. However, additional study of the impact of AV programming on clinical outcomes in prospective clinical trials is warranted prior to broadly implementing this strategy in clinical practice.
In programming of VV timing, the predominant use of 0 ms LV offset contradicts studies demonstrating −40 ms may improve outcomes in many patients [1, 15]. However, a minority of patients, either initially or at follow-up, had VV timing adjustment and it appears that patients who did tended to have worse clinical outcomes. While this may represent more aggressive care for patients with worse underlying disease or suboptimal lead position, it may also reflect deleterious consequences of poor VV timing and another opportunity to improve response rates.
Lastly, the use of programming strategies to suppress AF remains of great interest. Our data demonstrate higher rates of significant AF in patients programmed nominally or with VV-only adjustment vs. those with any AV adjustment. This difference persisted during follow-up. Since we excluded patients programmed to VVI, all of the patients in our cohort had the opportunity for AV timing adjustment, and this may contribute to lower rates of AF. The recent results of the MINERVA study demonstrated the potential for device programming interventions to alter AF progression [16], and such interventions could be of great benefit to the high-risk population of patients with heart failure and AF being treated with CRT. However, we cannot exclude the possibility that this effect is due to baseline arrhythmias (e.g., high AF burden) influencing subsequent programming.
5 Limitations
This study utilized a national database of patients enrolled in remote follow-up from centers consenting to participate in voluntary de-identified data-sharing scientific program. Thus, it is limited in the data elements captured and may represent a selected cohort that is not generalizable to CRT patients followed without remote monitoring. Furthermore, arrhythmia episodes are device-identified, without physician adjudication. Given the observational nature of our study, in order to account for potential confounding, we utilized multivariable adjustment. The adjustment methods included the use of heart rate variability, a factor strongly associated with all-cause survival; however, it is possible that residual confounding exists.
6 Conclusions
Nominal device programming settings are frequently modified in patients undergoing de novo implant of CRT devices. Though programmed timing is highly variable, AV adjustment is the most common and appears to be associated with improved survival.
Supplementary Material
Acknowledgments
Funding sources This analysis was conducted with support from Boston Scientific. Dr. Steinberg was funded by NIH T-32 training grant no. 5T32 HL 7101–38.
Conflict of interest The following relationships exist related to this presentation: BS reports modest educational support from Medtronic; SW, PJ, and KS are employees of Boston Scientific; KJ reports research grant support from Medtronic; DH served in an advisory capacity of St. Jude Medical, Boston Scientific, Medtronic; speaks at educational venues of St. Jude Medical, Boston Scientific, Medtronic, Biotronik, and Sorin Medical; and is a steering committee member of St. Jude Medical, Medtronic; JD does not report any relevant disclosures; CF-M serves as a consultant to Boston Scientific; BP has received consulting fees from Boston Scientific; NV received research grants and consultancies from Biotronik, Boston Scientific, Medtronic, St. Jude Medical; JP reports receiving grants for clinical research from ARCA biopharma, Boston Scientific, GE Healthcare, Johnson & Johnson, and ResMed as well as consultancies to Janssen Scientific Affairs and Spectranetics.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10840-015-0058-5) contains supplementary material, which is available to authorized users.
Contributor Information
Benjamin A. Steinberg, Email: benjamin.steinberg@duke.edu.
Scott Wehrenberg, Email: Scott.Wehrenberg@bsci.com.
David L. Hayes, Email: dhayes@mayo.edu.
Niraj Varma, Email: varman@ccf.org.
Brian D. Powell, Email: powell.brian17@gmail.com.
John D. Day, Email: johndaymd@gmail.com.
Kenneth M. Stein, Email: kenneth.stein@bsci.com.
Paul W. Jones, Email: Paul.Jones@bsci.com.
References
- 1.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. The New England Journal of Medicine. 2005;352(15):1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 2.Dickstein K, Vardas PE, Auricchio A, Daubert JC, Linde C, McMurray J, et al. 2010 Focused update of ESC guide-lines on device therapy in heart failure: an update of the 2008 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC guidelines for cardiac and resynchronization therapy. Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Europace. 2010;12(11):1526–1536. doi: 10.1093/europace/euq392. [DOI] [PubMed] [Google Scholar]
- 3.Hsu JC, Solomon SD, Bourgoun M, McNitt S, Goldenberg I, Klein H, et al. Predictors of super-response to cardiac resynchronization therapy and associated improvement in clinical outcome: the MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy) study. Journal of the American College of Cardiology. 2012;59(25):2366–2373. doi: 10.1016/j.jacc.2012.01.065. [DOI] [PubMed] [Google Scholar]
- 4.Ritter P, Padeletti L, Gillio-Meina L, Gaggini G. Determination of the optimal atrioventricular delay in DDD pacing. Comparison between echo and peak endocardial acceleration measurements. Europace. 1999;1(2):126–130. doi: 10.1053/eupc.1998.0032. [DOI] [PubMed] [Google Scholar]
- 5.Gold MR, Niazi I, Giudici M, Leman RB, Sturdivant JL, Kim MH, et al. A prospective comparison of AV delay programming methods for hemodynamic optimization during cardiac resynchronization therapy. Journal of Cardiovascular Electrophysiology. 2007;18(5):490–496. doi: 10.1111/j.1540-8167.2007.00770.x. [DOI] [PubMed] [Google Scholar]
- 6.Burri H, Sunthorn H, Somsen A, Zaza S, Fleury E, Shah D, et al. Optimizing sequential biventricular pacing using radionuclide ventriculography. Heart Rhythm. 2005;2(9):960–965. doi: 10.1016/j.hrthm.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 7.Ellenbogen KA, Gold MR, Meyer TE, Fernndez Lozano I, Mittal S, Waggoner AD, et al. Primary results from the SmartDelay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation. 2010;122(25):2660–2668. doi: 10.1161/CIRCULATIONAHA.110.992552. [DOI] [PubMed] [Google Scholar]
- 8.Saxon LA, Hayes DL, Gilliam FR, Heidenreich PA, Day J, Seth M, et al. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation. 2010;122(23):2359–2367. doi: 10.1161/CIRCULATIONAHA.110.960633. [DOI] [PubMed] [Google Scholar]
- 9.Kamdar R, Frain E, Warburton F, Richmond L, Mullan V, Berriman T, et al. A prospective comparison of echocardiography and device algorithms for atrioventricular and interventricular interval optimization in cardiac resynchronization therapy. Europace. 2010;12(1):84–91. doi: 10.1093/europace/eup337. [DOI] [PubMed] [Google Scholar]
- 10.Mullens W, Grimm RA, Verga T, Dresing T, Starling RC, Wilkoff BL, et al. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. Journal of the American College of Cardiology. 2009;53(9):765–773. doi: 10.1016/j.jacc.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 11.Birnie D, Lemke B, Aonuma K, Krum H, Lee KL, Gasparini M, et al. Clinical outcomes with synchronized left ventricular pacing: analysis of the adaptive CRT trial. Heart Rhythm. 2013;10(9):1368–1374. doi: 10.1016/j.hrthm.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Martin DO, Lemke B, Birnie D, Krum H, Lee KL, Aonuma K, et al. Investigation of a novel algorithm for synchronized left-ventricular pacing and ambulatory optimization of cardiac resynchronization therapy: results of the adaptive CRT trial. Heart Rhythm. 2012;9(11):1807–1814. doi: 10.1016/j.hrthm.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Brenyo A, Kutyifa V, Moss AJ, Mathias A, Barsheshet A, Pouleur AC, et al. Atrioventricular delay programming and the benefit of cardiac resynchronization therapy in MADIT-CRT. Heart Rhythm. 2013;10(8):1136–1143. doi: 10.1016/j.hrthm.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Verdino RJ. Goldilocks and the importance of AV intervals in cardiac resynchronization—how to best find the AV interval that is not too long, not too short, but just right for patients. Heart Rhythm. 2013;10(8):1144–1145. doi: 10.1016/j.hrthm.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Bogaard MD, Meine M, Tuinenburg AE, Maskara B, Loh P, Doevendans PA. Cardiac resynchronization therapy beyond nominal settings: who needs individual programming of the atrioventricular and interventricular delay? Europace. 2012;14(12):1746–1753. doi: 10.1093/europace/eus170. [DOI] [PubMed] [Google Scholar]
- 16.Funck RC, Boriani G, Manolis AS, Puererfellner H, Mont L, Tukkie R, et al. The MINERVA study design and rationale: a controlled randomized trial to assess the clinical benefit of minimizing ventricular pacing in pacemaker patients with atrial tachyarrhythmias. American Heart Journal. 2008;156(3):445–451. doi: 10.1016/j.ahj.2008.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.