Abstract
Background:
Attention-deficit hyperactivity disorder (ADHD) is the most common mental and behavioral disorder in school-aged children. This study evaluated the effect of osmotic-release oral system (OROS) methylphenidate (MPH) on cognitive function and academic performance of Chinese school-aged children with ADHD.
Methods:
This 12-week, prospective, multicenter, open-label, self-controlled study enrolled 153 Chinese school-aged children with ADHD and 41 non-ADHD children. Children with ADHD were treated with once-daily OROS-MPH (18 mg, 36 mg, or 54 mg). The primary endpoints were Inattention/Overactivity (I/O) with Aggression Conners Behavior Rating Scale (IOWA) and Digit Span Test at week 12 compared with baseline. Secondary endpoints included opposition/defiant (O/D) subscale of IOWA, Clinical Global Impression (CGI), Coding Test, Stroop Color-word Test, Wisconsin Card Sorting Test (WCST), academic performance on teacher-rated school examinations, and safety at week 12 compared with baseline. Both non-ADHD and ADHD children received the same frequency of cognitive operational test to avoid the possible bias caused by training.
Results:
A total of 128 patients were evaluated with cognitive assessments. The OROS-MPH treatment significantly improved IOWA Conners I/O subscale scores at week 12 (3.8 ± 2.3) versus baseline (10.0 ± 2.4; P < 0.0001). Digit Span Test scores improved significantly (P < 0.0001) with a high remission rate (81.1%) at week 12 versus baseline. A significant (P < 0.0001) improvement was observed in O/D subscale of IOWA, CGI, Coding Test, Stroop Color-word Test, WCST, and academic performance at week 12 versus baseline. Very few practice-related improvements were noticed in the non-ADHD group at week 12 compared with baseline. No serious adverse events and deaths were reported during the study.
Conclusions:
The OROS-MPH treatment effectively controlled symptoms of ADHD and significantly improved academic performance and cognitive function of Chinese school-aged children with ADHD. The treatment was found to be safe and generally well-tolerated over 12 weeks.
Trial Registration:
ClinicalTrials.gov, NCT01933880; http://clinicaltrials.gov/ct2/show/NCT01933880?term=CONCERTAATT4099&rank=1
Keywords: Academic Performance, Attention-deficit Hyperactivity Disorder, Cognitive Function, Osmotic-release Oral System-methylphenidate
INTRODUCTION
Attention-deficit hyperactive disorder (ADHD) is a common psychiatric disorder of childhood and adolescence with a worldwide prevalence of 5.3%,[1] and an estimated prevalence of 5-10% in school-aged children.[2] Primary school-aged children are affected approximately twice as frequently as adolescents; boys having twice the prevalence as girls.[3] It commonly manifests as cognitive dysfunction in working memory, verbal fluency, and executive processing speed[4,5] resulting in schooling difficulties such as disorders of learning and applying knowledge, along with limitations of family and social activities. The disorder may also continue into adolescence and adulthood, further impacting patients’ quality of life.[6,7,8]
Pharmacological therapy has been shown to reduce effectively the risk of decline in academic performance in children with ADHD.[8,9] Among the available therapies, methylphenidate (MPH) is one of the most widely investigated psychostimulants for the treatment of ADHD.[8,10,11,12] Once-daily osmotic-release oral system (OROS)-MPH is a long-acting formulation and has shown improved efficiency and higher remission rate compared to immediate-release methylphenidate (IR-MPH).[11] The OROS-MPH treatment improved executive functions of school-aged children with ADHD in areas such as working memory, sustaining attention, and impulsivity.[11]
In China, ADHD is the most common mental and behavioral disorder recognized in school-aged children.[13] A large number of studies separately focused on the cognitive improvement and academic performance in children with ADHD.[11,14,15,16,17] However, there are limited data available focusing on both the aspects in the Chinese population. In this study, we evaluated the effect of OROS-MPH on academic performance and cognitive function of Chinese school-aged children with ADHD.
METHODS
Study population
Boys and girls between 6 and 12 years of age with normal-intelligence (intelligence quotient ≥85), with a documented diagnosis of ADHD (Diagnostic Statistical Manual-Fourth Edition 314.00 and 314.01) weighing between 20 and 60 kg, with academic performance of the previous term, were enrolled in this study. Children having no history of taking psychotropic drugs in the last 6 months and currently taking effective IR-MPH (≤60 mg/d) were also included. The written informed consent form was obtained from the participants or their parents/guardians before enrolling into the study. Normal children, without ADHD between 7 and 13 years of age, weighing 20–60 kg, were also recruited as control to evaluate the practice effect. Both ADHD and normal children received the same frequency of cognitive operational test to avoid the possible bias caused by training.
Major exclusion criteria were evidence of any bipolar I or II affective disorder, anxiety disorder, general development disorder, schizophrenia, glaucoma, Tourette syndrome, hypertension and cardiovascular disease, any physical disease that can significantly reinforce the activity of the sympathetic nervous systems, serious gastrointestinal stenosis, dysphagia, and other serious somatic diseases. Patients were also excluded if they could not coordinate with the cognitive examination, highly sensitive to MPH, had taken or taking sympathomimetic agents such as β-adrenoreceptor blocking drugs, received or receiving monoamine oxidase inhibitor drugs such as clonidine, other α-2 adrenergic receptor agonist, tricyclic antidepressants, theophylline, or bishydroxycoumarin in the past 30 days, and history of drug or alcohol dependence.
The study was conducted from December 2009 to November 2010 at different centers in China, and the clinical protocol was approved by Independent Ethics Committees/Institutional Review Boards at each study center. The study was conducted in accordance with the ethical principles originating in the Declaration of Helsinki and in accordance with the ICH good clinical practice guidelines, applicable regulatory requirements and in compliance with the protocol. All participants provided written informed consent.
Study design
In this 12-week, prospective, open-label, multicenter, self-controlled clinical study (ClinicalTrials.gov, NCT01933880), patients received once-daily OROS-MPH 18 mg as the initial dose following a 4-week screening period. ADHD children, who were receiving other ADHD drugs except IR-MPH, were required to withdraw the drug for at least 3 days before baseline. For patients in the OROS-MPH treatment group, patients who received 5-mg IR-MPH 2 or 3 times a day were assigned to OROS-MPH 18 mg q.d. as recommended dosage, patients who received 10-mg IR-MPH 2 or 3 times a day were assigned to OROS-MPH 36 mg q.d. as recommended dosage, and patients who received 15-mg IR-MPH 2 or 3 times a day or a total daily dose of 60 mg were assigned to OROS-MPH 54 mg q.d. as recommended dosage. Dose was increased to 36 mg/d in week 1 (visit 2) which could be adjusted to 18 mg/d if the patient was intolerant and 54 mg/d in week 2 (visit 3) which could be adjusted to 36 or 18 mg if it was not well-tolerated. At week 3 (visit 4), doses were maintained at 18 mg/d, 36 mg/d, or 54 mg/d. In the remaining 9 weeks, patients were continuously treated with the optimized dose of OROS-MPH.
Children were evaluated by the Inattention/Overactivity (I/O) with Aggression, Inattention/Overactivity (I/O) with Aggression (IOWA) Conners Behavior Rating Scale at baseline and at weeks 1, 2, 3, 7, and 12. Clinical Global Impression-Improvement (CGI-I) scale was used at baseline and at week 12. Psychological, cognitive, and academic assessments were performed at baseline and at week 12. The patient disposition in the study is briefly summarized in Figure 1.
Figure 1.
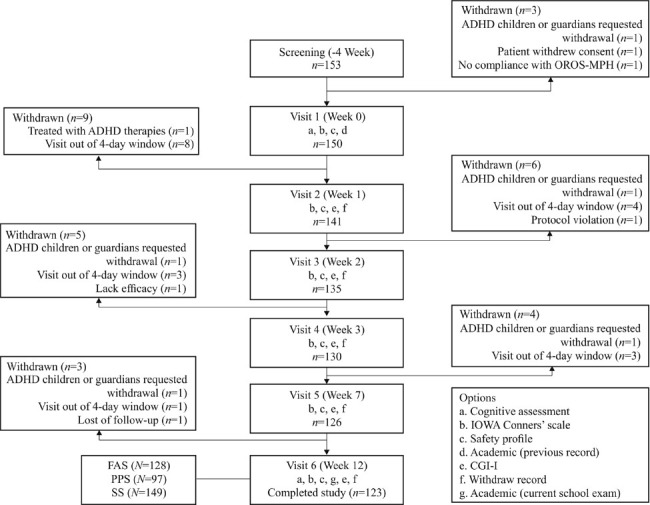
Patient disposition: Cognitive assessments include Digit Span Test, Stroop Color-word Test, Wisconsin Card Sorting Test, Coding Test. CGI: Clinical Global Impression; FAS: Full analysis set; IOWA Conners: Inattention/Overactivity with Aggression Conners Behavior Rating Scale; OROS-MPH: Osmotic-Release Oral System Methylphenidate; PPS: Per protocol set; SS: Safety set.
Efficacy assessment
The primary efficacy outcomes were I/O subscale of parent-rated IOWA Conners scale and Digit Span Test. The IOWA Conners scale, used to assess the therapeutic effect, consisted of 10 items, which were separated into two subscales, I/O, and oppositional/defiance (O/D). All items were evaluated by a 4-point scale (0 = not at all, 1 = occasionally, 2 = frequently, and 3 = always)[18] at baseline, each visit and the end of the study. Digit Span Test, a subtest of China-Wechsler Intelligence Scale for children,[19] was used to access the verbal working memory consisting of both Digit Span Forward (order Digit Span) and Digit Span Backward (reverse Digit Span) tests. Children were requested to read numbers of increasing length, repeating them either forward or backward, and tested with different number sets[20] at each visit and the end of the study versus baseline. The parents rated the symptoms (items in the subscale) of the children at home and at each visit. In this test, one-point improvement is considered clinically significant, since even one-point improvement means recalling one more figure.[4]
The other efficacy outcomes included: (1) O/D subscale and sum score of parent-rated IOWA Conners scale; (2) CGI-I rated on a 7-point scale (1 = very much improved to 7 = very much worse), based on a review of parents’ ratings and interviews;[16] (3) Remission rate, defined as the rate of children with total score ≤5 based on IOWA Conners Behavior Rating Scale evaluated by parents; (4) Stroop Color-word Test (four tasks: Word reading, color reading, word reading of color-words and color naming of color-words)[5] to record completion times and the number of errors made during each task/test; (5) Coding Test (Digit Symbol Substitution Test; consisted of digit-symbol pairs): Children read the list of digits and drew corresponding symbols, and the number of correct symbols within 150 s time-limit was assessed; (6) Wisconsin Card Sorting Test (WCST), consisted of 13-item and measured the ability to display flexibility during schedule changes with a number of stimulus cards on which the shapes of different color, quantity and designs were read by the children. Children were then requested to match those cards with additional cards under changing rules. The number of “Right” and “Wrong” matches and the time children took to learn new rules, as well as the mistakes, were measured;[21] (7) Academic performance was assessed by comparing the Chinese language and mathematical achievements based on teacher-rated records of school examination scores at baseline and at week 12; and (8) Number of participants compliant (calculated by the percentage of dose [actual dose × 100/theoretical dose]) to the treatment.
Safety assessment
Children had a general physical and blood examination (if required) before initiating OROS-MPH. Blood pressure, pulse rate, concomitant medications, adverse events (AEs), and treatment review were conducted at baseline and at each visit.
Statistical analysis
The data were analyzed using SAS 9.1.3 (SAS Institute Inc., Cary, NC, USA). The primary analysis was based on the full analysis set that comprised children who received at least one dose of OROS-MPH and had at least one efficacy assessment. Perprotocol set was defined as a set of all children who reasonably followed the protocol procedures and had good compliance (80–120% adherence) to the treatment drug, and had Conners scale score at all visits. Safety analysis was performed in the safety set population that comprised children who received at least one dose of OROS-MPH.
Demographic and baseline characteristics were summarized and evaluated for distributional analysis. Paired t-test was used for the normally distributed data, and the Wilcoxon signed-rank test was used for the nonparametric analysis. The last-observation-carried-forward approach was used to impute the missing data for primary efficacy outcomes. No multiplicity adjustment was made. The mean values were displayed as mean ± standard deviation (SD) and the mean difference was exhibited with the 95% confidence interval (CI). All hypothesis tests are double tailed tests, with 0.05 as the significance level.
RESULTS
Participants
Overall, 153 children with ADHD were enrolled [Figure 1], of which 123 completed the study, while the analysis was based on the full analysis set (n = 128) [Table 1]. Of the 41 non-ADHD children recruited, 40 completed the study with one participant withdrawing the consent.
Table 1.
Demographic data at baseline in both ADHD and non-ADHD groups
| Characteristics | ADHD group (n = 128) | Non-ADHD group (n = 40) |
|---|---|---|
| Gender, n (%) | ||
| Boys | 108 (84.4) | 23 (57.5) |
| Girls | 20 (15.6) | 17 (42.5) |
| Ethnicity, n (%) | ||
| Han | 124 (96.9) | 39 (97.5) |
| Others | 4 (3.1) | 1 (2.5) |
| Body weight (kg), mean ± SD | 33.0 ± 9.3 | 37.5 ± 8.3 |
| Age (years), median (mean ± SD) | 9 (9.2 ± 1.5) | 10 (9.7 ± 1.7) |
| Age distribution, n (%) | ||
| ≤7 years | 14 (10.9) | 7 (17.5) |
| 8 years | 34 (26.6) | 3 (7.5) |
| 9 years | 32 (25.0) | 7 (17.5) |
| 10 years | 23 (18.0) | 9 (22.5) |
| 11 years | 13 (10.2) | 8 (20.0) |
| ≥12 years | 12 (9.4) | 6 (15.0) |
ADHD: Attention-deficit hyperactivity disorder; SD: Standard deviation.
Medications
At baseline, 12 (9.4%) children with ADHD were taking IR-MPH. The mean course of OROS-MPH treatment was 80.1 days and total compliance was 93.6%, in which the total compliance of 17 participants was <80% (13.4%), 110 cases between 80% and 120% (86.6%) and no cases >120%. During the optimized treatment phase (weeks 3–12), 73.5% children received OROS-MPH 18 mg once-daily and 26.5% received 36 mg once-daily (weeks 7–12).
Primary efficacy outcomes
The OROS-MPH treatment showed continuous increase in the parent IOWA Conners I/O scores at weeks 1, 2, 3, 7, and 12 versus baseline. At week 12, the mean I/O scores of the short version of the parents questionnaire of IOWA Conners score of OROS-MPH treatment were significantly higher than the baseline [P < 0.0001; Table 2 and Figure 2]. The OROS-MPH treatment showed therapeutic response (efficacy rate; 89.8%) in 114 children with ADHD. At week 12, the mean total scores and reverse recitation of Digit Span Test score was significantly higher than the baseline [P < 0.0001; Table 3].
Table 2.
IOWA Conners scale, remission rate, and CGI-I in Chinese patients with ADHD (full analysis set)
| Items | Baseline | Week 1 | Week 2 | Week 3 | Week 7 | Week 12 |
|---|---|---|---|---|---|---|
| Parent-rated IOWA Conners scale (mean ± SD), LOCF | ||||||
| N (missing) | 127 (1) | 127 (1) | 127 (1) | 127 (1) | 127 (1) | 127 (1) |
| I/O subscale | 10.0 ± 2.4 | 7.3 ± 2.6* | 6.0 ± 2.4* | 5.0 ± 2.3* | 4.3 ± 2.2* | 3.8 ± 2.3* |
| 95% CI | 9.6–10.4 | 6.9–7.8 | 5.5–6.4 | 4.6–5.4 | 3.9–4.6 | 3.4–4.2 |
| O/D subscale | 8.7 ± 3.2 | 6.7 ± 3.2 | 5.5 ± 3.0 | 4.4 ± 2.6 | 3.7 ± 2.1 | 3.3 ± 2.4* |
| 95% CI | 8.1–9.3 | 6.1–7.2 | 4.9–6 | 3.9–4.8 | 3.3–4.1 | 2.8–3.7 |
| Sum | 18.7 ± 5.1 | 14.0 ± 5.4 | 11.4 ± 5.0 | 9.3 ± 4.5 | 7.9 ± 3.7 | 6.9 ± 4.3* |
| 95% CI | 17.8–19.6 | 13.1–15.0 | 10.5–12.4 | 8.4–10.1 | 7.2–8.5 | 6.1–7.7 |
| Remission rate (the score of IOWA Conners I/O subscale is <5 points) (n = 127), n (%), LOCF | ||||||
| Patients | – | 33 (26.0) | 66 (52.0) | 85 (66.9) | 100 (78.7) | 103 (81.1) |
| 95% CI | – | 18.5–34.3 | 42.6–60.5 | 57.5–74.5 | 70.0–84.9 | 72.5–86.9 |
| CGI-I (n = 128), n (%) | ||||||
| N (missing) | – | 127 (1) | 121 (7) | 117 (11) | 114 (14) | 111(17) |
| 1 = Very much improved | – | 20 (15.7) | 28 (23.1) | 29 (24.8) | 35 (30.7) | 36 (32.4) |
| 2 = Much improved | – | 59 (46.5) | 53 (43.8) | 44 (37.6) | 41 (36.0) | 46 (41.4) |
| 3 = Minimally improved | – | 33 (26.0) | 28 (23.1) | 33 (28.2) | 28 (24.6) | 25 (22.5) |
| 4 = No change | – | 15 (11.8) | 10 (8.3) | 11 (9.4) | 7 (6.1) | 3 (2.7) |
| 5 = Minimally worse | – | 0 | 2 (1.6) | 0 | 3 (2.6) | 1 (0.9) |
| 6 = Much worse | – | 0 | 0 | 0 | 0 | 0 |
| 7 = Very much worse | – | 0 | 0 | 0 | 0 | 0 |
*P<0.001 versus baseline within the subgroup (self-controlled); two-sided P value for paired t-test or Wilcoxon signed-rank test on change. –: No data/not analyzed; ADHD: Attention-deficit hyperactivity disorder; CGI-I: Clinical Global Impression-Improvement; CI: Confidence interval; I/O: Attention/overactivity; IOWA Conners’: Inattention/Overactivity with Aggression Conners Behavior Rating Scale; LOCF: Last-observation-carried-forward; O/D: Opposition/defiant; SD: Standard deviation.
Figure 2.

Remission rate and parent-rated IOWA Conners scale of Chinese children. (a) Remission rate and I/O subscale of IOWA Conners; (b) O/D subscale of IOWA Conners. Remission rate and I/O subscale of IOWA Conners recorded in 6 visits (weeks 0, 1, 2, 3, 7, and 12). Mean scores of week 0–12 are significantly reduced (*P < 0.0001), compared with that of baseline. I/O: Inattention/Overactivity; O/D: Opposition/defiant; IOWA Conners: Inattention/Overactivity with Aggression Conners Behavior Rating Scale.
Table 3.
Assessments of cognitive function and academic performance in Chinese patients with ADHD and non-ADHD participants (full analysis set)
| Items | ADHD group (n = 128) | Non-ADHD (n = 40) | ||
|---|---|---|---|---|
| Baseline | Week 12 | Baseline | Week 12 | |
| Digit Span Test, LOCF | ||||
| N (missing) | 128 (0) | 128 (0) | 40 (0) | 40 (0) |
| Forward | 7.7 ± 1.2 | 8.0 ± 1.1* | 8.2 ± 0.9† | 8.3 ± 0.9 |
| 95% CI | 7.5–7.9 | 7.9–8.2 | 7.9–8.5 | 8.0–8.6 |
| Backward | 4.0 ± 1.5 | 4.6 ± 1.4* | 5.5 ± 1.8† | 5.9 ± 1.6*,‡ |
| 95% CI | 3.7–4.2 | 4.4–4.8 | 4.9–6.1 | 5.4–6.4 |
| Total | 11.7 ± 2.2 | 12.7 ± 2.1* | 13.7 ± 2.4† | 14.2 ± 2.3*,‡ |
| 95% CI | 11.3–12.1 | 12.3–13.0 | 12.9–14.5 | 13.4–14.9 |
| Stroop Color-word Test | ||||
| N (missing) | 128 (0) | 111 (17) | 40 (0) | 40 (0) |
| Stroop 1 | ||||
| CT (s) | 20.6 ± 17.5 | 19.0 ± 15.4* | 6.2 ± 2.3† | 5.7 ± 1.8‡ |
| 95% CI | 17.5–23.6 | 16.1–21.9 | 5.5–6.9 | 5.1–6.3 |
| NE | 1.4 ± 2.4 | 0.9 ± 1.4* | 0 ± 0.2† | 0 ± 0.2‡ |
| 95% CI | 1.0–1.8 | 0.6–1.1 | 0–0.1 | 0–0.1 |
| Stroop 2 | ||||
| CT (s) | 19.3 ± 14.8 | 18.3 ± 13.7* | 7.2 ± 2.6† | 6.7 ± 2.3‡ |
| 95% CI | 16.7–21.9 | 15.7–20.9 | 6.3–8.0 | 5.9–7.4 |
| NE | 1.1 ± 1.8 | 0.7 ± 1.2* | 0.1 ± 0.3† | 0 ± 0.2‡ |
| 95% CI | 0.8–1.4 | 0.5–0.9 | 0–0.2 | 0–0.1 |
| Stroop 3 | ||||
| CT (s) | 18.7 ± 14.5 | 16.9 ± 12.2* | 6.9 ± 2.6† | 6.5 ± 2.4‡ |
| 95% CI | 16.1–21.2 | 14.6–19.2 | 6.0–7.7 | 5.7–7.3 |
| NE | 0.9 ± 1.5 | 0.6 ± 0.9* | 0.1 ± 0.4† | 0.1 ± 0.3‡ |
| 95% CI | 0.7–1.2 | 0.4–0.8 | 0–0.2 | 0–0.2 |
| Stroop 4 | ||||
| CT (s) | 22.9 ± 11.3 | 19.4 ± 9.9* | 11.8 ± 4.4† | 10.5 ± 4.2*,‡ |
| 95% CI | 20.9–24.8 | 17.5–21.2 | 20.8–24.8 | 9.1–11.8 |
| NE | 1.5 ± 1.9 | 0.7 ± 1.3* | 0.2 ± 0.5† | 0.4 ± 0.9 |
| 95% CI | 1.1–1.8 | 0.4–0.9 | 0.1–0.4 | 0.1–0.6 |
| WCST | ||||
| N (missing) | 128 (0) | 111 (17) | 40 (0) | 40 (0) |
| Trials administered | 125.1 ± 8.7 | 116.4 ± 17.7* | 120.8 ± 15.5 | 117.1 ± 17.4 |
| 95% CI | 123.6–126.7 | 113.1–119.7 | 115.9–125.8 | 111.5–122.6 |
| Total categories completed | 3.2 ± 1.6 | 4.1 ± 1.5* | 3.3 ± 1.7 | 4.0 ± 1.3 |
| 95% CI | 2.9–3.5 | 3.8–4.4 | 2.8–3.8 | 3.6–4.4 |
| Total correct response | 72.9 ± 15.6 | 80.3 ± 12.2* | 77.9 ± 12.3 | 84.7 ± 12.3* |
| 95% CI | 70.1–75.6 | 78–82.6 | 74–81.8 | 80.8–88.7 |
| Total response errors | 52.3 ± 18.6 | 36.1 ± 16.9* | 42.9 ± 17.8 | 32.3 ± 15.1* |
| 95% CI | 49.0–55.5 | 33.0–39.3 | 37.2–48.6 | 27.5–37.2 |
| Total correct response (%) | 58.7 ± 13.8 | 70.1 ± 11.6* | 65.5 ± 12.6 | 73.3 ± 10.5* |
| 95% CI | 56.3–61.1 | 67.9–72.3 | 61.5–69.6 | 70.0–76.7 |
| Trials to complete first categories | 22.6 ± 22.3 | 23.4 ± 19.0 | 25.2 ± 24.4 | 26.5 ± 22.8 |
| 95% CI | 18.7–26.5 | 19.8–26.9 | 17.4–33.0 | 19.2–33.8 |
| Conceptual level responses (%) | 46.4 ± 18.6 | 61.1 ± 17.2* | 54.7 ± 18.5† | 65.0 ± 16.0* |
| 95% CI | 43.1–49.6 | 57.8–64.3 | 48.8–60.6 | 59.9–70.1 |
| Perseverative response | 33.7 ± 20.5 | 22.0 ± 16.2* | 26.5 ± 14.1† | 18.6 ± 13.0* |
| 95% CI | 30.1–37.3 | 19.0–25.0 | 22–31 | 14.5–22.7 |
| Perseverative errors | 28.7 ± 15.2 | 19.4 ± 12.5* | 23.0 ± 11.0† | 16.4 ± 9.8* |
| 95% CI | 26.0–31.4 | 17.0–21.7 | 19.4–26.5 | 13.3–19.6 |
| Perseverative errors (%) | 22.7 ± 11.7 | 16.1 ± 9.2* | 18.5 ± 8.1† | 13.6 ± 7.2* |
| 95% CI | 20.6–24.7 | 14.3–17.8 | 15.9–21.0 | 11.3–15.9 |
| Nonperseverative errors | 23.5 ± 11.6 | 16.8 ± 9.2* | 20.0 ± 8.9 | 15.8 ± 7.7 |
| 95% CI | 21.5–25.6 | 15.0–18.5 | 17.1–22.8 | 13.3–18.3 |
| Failure to maintain set | 1.7 ± 1.6 | 2.0 ± 1.8 | 2.3 ± 1.6 | 2.5 ± 1.7 |
| 95% CI | 1.5–2.0 | 1.7–2.4 | 1.7–2.8 | 2.0–3.1 |
| Learning to learn | −2.5 ± 6.4 | −1.9 ± 3.8 | −2.4 ± 6.3 | −1.3 ± 4.4 |
| 95% CI | −3.6–−1.4 | −2.6–−1.1 | −4.4–−0.3 | −2.7–0.1 |
| Coding Test | 63.4 ± 40.1 | 73.2 ± 41.4* | 77.4 ± 44.8 | 83.1 ± 44.2 |
| 95% CI | 56.3–70.4 | 65.4–81.0 | 63.0–91.7 | 68.9–97.2 |
| Academic performance | ||||
| Chinese achievement | 77.4 ± 13.8 | 83.9 ± 10.7* | 94.2 ± 4.3† | 94.6 ± 4.4‡ |
| 95% CI | 74.9–79.8 | 81.9–86.0 | 92.8–95.6 | 93.2–96.0 |
| Mathematical achievement | 78.9 ± 15.4 | 86.0 ± 11.6* | 96.2 ± 3.6† | 96.1 ± 3.6‡ |
| 95% CI | 76.2–81.5 | 83.9–88.2 | 95.1–97.4 | 95.0–97.3 |
Data are expressed as mean ± SD. *P<0.001 versus baseline in the same subgroup (self-controlled; using paired t-test or Wilcoxon signed-rank test); †P<0.05 versus OROS-MPH treated group at the baseline; ‡P<0.001 versus OROS-MPH treated group at week 12 (using t-test or Wilcoxon rank sum test). ADHD: Attention-deficit hyperactivity disorder; CI: Confidence interval; CT: Completion time; LOCF: Last-observation-carried-forward; NE: Number of errors; s: Seconds; SD: Standard deviation; OROS-MPH: Osmotic-Release Oral System Methylphenidate; WCST: Wisconsin Card Sorting Test.
Secondary efficacy outcomes
Opposition/defiant subscale of Inattention/Overactivity with Aggression score and remission rate
At week 12, OROS-MPH significantly decreased the mean (SD) O/D scores (5.5 ± 3.6; P < 0.0001) and the total scores (12.0 ± 6.2; P < 0.0001) of IOWA Conners scale in children with ADHD compared with baseline [Table 2]. A continuous increase was observed in the remission rate at all visits compared with baseline [Table 2 and Figure 2]. In the OROS-MPH treatment group, remission rate was 26% (33 children) and 81.1% (103 children) at weeks 1 and 12, respectively. The reciprocal relationship between remission rate and I/O subscale of parent-rated IOWA Conners scale are provided in Figure 2.
Clinical Global Impression-Improvement
The OROS-MPH treatment showed benefits in the global assessment of efficacy at each visit [Table 2]. At week 12, the CGI-I scale showed improvements in 96.4% of children receiving OROS-MPH with ratings of “minimally improved” (25 [22.5%] patients), “much improved” (46 [41.4%] patients), and “very much improved (36 [32.4%] patients).
Coding Test (Digit Symbol Substitution Test)
At week 12, OROS-MPH significantly increased the mean ± SD Coding Test score [6.8 ± 8.9; P < 0.0001; Table 3] in children with ADHD, but not in the non-ADHD children (5.7 ± 21.7; P > 0.05) compared with baseline.
Stroop Color-word Test
A significant decrease in the completion times in the Stroop Color-word Test 1, Test 2, Test 3 (all P < 0.0001), and Test 4 (P = 0.0005) was observed in children with ADHD at week 12 compared with baseline. Significant higher completion time for Stroop Color-word Test 4 was observed at baseline, for children with ADHD as compared with non-ADHD children, but was not significant at week 12. Further, no significant difference for other tests (Tests 1, 2, and 3) was observed between the groups at baseline. In children with ADHD, a significant decrease in the number of errors in the Stroop Color-word Test 1 (mean ± SD: 0.6 ± 1.8; P < 0.0005), Test 2 (0.5 ± 1.5; P = 0.0009), Test 3 (0.4 ± 1.2; P = 0.0009), and Test 4 (0.8 ± 1.8; P < 0.0001) was observed at week 12 compared with baseline, which was not observed in non-ADHD children (P > 0.05) [Table 3].
Wisconsin Card Sorting Test
Compared with baseline, OROS-MPH treatment showed a significant difference (P < 0.0001) in 10 of 13 parameters in WCST [Table 3]. At week 12, OROS-MPH treatment significantly (P < 0.0001) decreased the administered responses (Ra; mean ± SD: 8.5 ± 17.2), persistent responses (Rp; 11.7 ± 20.8), persistent error responses (Rpe; 9.4 ± 15.8), and non-persistent error responses (nRpe; 7.2 ± 12.6) in WCST compared with baseline. At week 12, OROS-MPH treatment significantly decreased (P < 0.0001) error responses (Re; 16.5 ± 20.9), completed categories (Cc; 1.0 ± 1.8), correct responses (Rc; 8.0 ± 17.6), percentage of correct responses (Rc%; 11.8 ± 15.0), and the percentage of conceptual level responses (Rf%; 15.2 ± 22.2) of WCST compared with baseline. The Re of non-ADHD Children was significantly decreased (10.6 ± 14.6) compared with baseline (P = 0.0001). No significant increase (0.3 ± 2.2) in the “Failure to Maintain Set” (Fm) was observed in the children with ADHD compared with baseline (P > 0.05). The OROS-MPH treatment did not cause any significant change (P > 0.05) in the “Learning to Learn” at Week 12 versus baseline (0.4 ± 7.5). A significant increase in Cc (0.7 ± 1.5; P = 0.0051) and Rc (6.8 ± 13.7; P = 0.0032) was observed in non-ADHD children at week 12 compared with baseline. No significant increase was observed in first response (Rf) in WCST compared with baseline. The OROS-MPH treatment significantly decreased (7.2 ± 12.6) nRpe at week 12 compared with baseline (P < 0.0001). No significant change in Ra and Rf was observed in the non-ADHD Children at week 12 compared with baseline (P > 0.05). Significant differences were observed between the two groups in WCST scores at baseline (all P < 0.05); however, no significant differences were observed at week 12.
Academic performance
At week 12, OROS-MPH treatment significantly increased the Chinese achievement score (5.5 ± 10.2; P < 0.0001) and mathematical achievement score (7.3 ± 10.3; P < 0.0001) compared with baseline, which was not seen in the non-ADHD children (P > 0.05) [Table 3]. Both children, with ADHD and non-ADHD, received the same frequency of cognitive operational tests (such as Digit Span, Stroop Color-word Test, Coding Test, and WSCT).
Safety
A total of 149 patients were included in the safety analysis set. No serious AEs and deaths were reported during the study. A total of 40 AEs were reported in 24 children, which were considered to be related to OROS-MPH treatment. Most commonly reported AEs were loss of appetite (12.7%), poor sleep quality (2.0%), and insomnia [2.0%; Table 4 and Figure 3]. No obvious change in blood pressure and pulse rate was recorded.
Table 4.
Safety evaluations in Chinese patients with ADHD (safety set)
| Adverse events | Week 0 | Week 1 | Week 2 | Week 3 | Week 7 | Week 12 |
|---|---|---|---|---|---|---|
| Poor sleep | 6 (4.0) | 13 (9.3) | 11 (8.1) | 6 (4.6) | 4 (3.2) | 2 (1.6) |
| Tic | 0 | 0 | 1 (0.7) | 2 (1.5) | 1 (0.8) | 2 (1.6) |
| Poor appetite | 8 (5.4) | 46 (32.9) | 30 (22.2) | 21 (16.1) | 17 (13.5) | 15 (12.2) |
Data are expressed as n (%). ADHD: Attention-deficit hyperactivity disorder.
Figure 3.
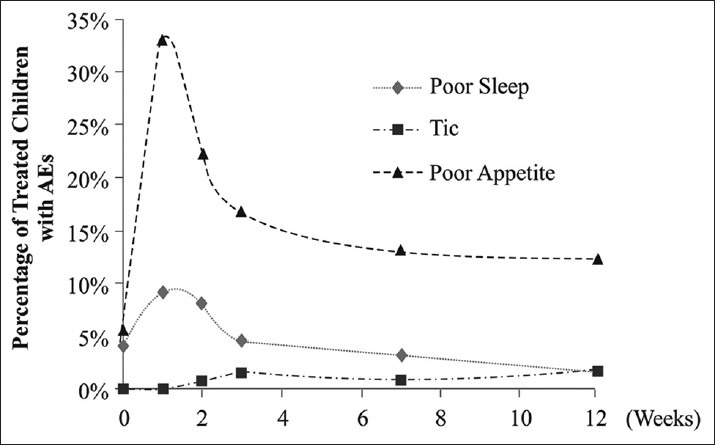
Incidence of adverse events in Chinese children with attention-deficit hyperactivity disorder. The percentage of treated children who reported AEs was plotted. The trend of three common AEs (poor sleep, tic, and poor appetite) at each visit (week 0, week 1, week 2, week 3, week 7, and week 12) is depicted.
DISCUSSION
Long-term studies in patients with ADHD have shown the impact of treatment on emotional behavior, academic achievement, and social functioning,[22,23] which suggest that the goal of pharmacotherapy is not only to improve the symptoms, but also to restore optimal functionality in the emotional, behavioral, academic, and social functioning. To the best of our knowledge, this is the first study in Chinese school-aged children that assessed both cognitive and academic improvements with OROS-MPH treatment. Improvements were seen in psychological, cognitive, and academic performance over 12 weeks. The majority of the children were 8–9 years old (42.9%); the prevalence rate in this age group is comparable to another Chinese study, in which the prevalence rate of ADHD in children aged 7–9 years was higher (>6%) than in the other age groups.[24] Similarly, more boys compared to girls (>5:1) had ADHD in this study, which is in line with the existing literature.[11,24,25,26,27]
A continuous increase in the remission rate and a decrease in the IOWA Conners I/O scores were observed during the treatment period. The changes were rapid in the initial 2–3 weeks indicating that most children with ADHD may improve faster during the first 3 weeks (50% improvement versus baseline) of treatment; however, the improvement continued until week 12 (62% improvement versus baseline). These improvements are consistent with the previously published studies, both in Chinese[25] and non-Chinese children.[9,26] A study in Chinese population in Taiwan of China showed 44.2% of symptomatic remission at week 4, 58.8% at week 6, and 59.6% at week 10 (average dose: 36.7 mg/d).[26] In another study,[28] the remission rate (Swanson, Nolan and Pelham, version IV scale, Chinese version [SNAP-IV-C]) was 30.7% after 48-weeks treatment with 30.16 mg/d dose. In comparison, lower average dose (22.8 mg/d) was observed in the current study that resulted in a higher and faster remission. The difference in the remission rate can be attributed to several factors such as the difference in baseline data, prevalence of comorbidities (e.g., oppositional defiant disorder, somatization disorder), family history of psychiatric disorders, and proportion of children with ADHD who had received previous IR-MPH (9.6%; rest were drug naïve). Further, a high compliance rate (93.6%) and enrollment of patients with mild ADHD and the difference in remission definition and evaluation tools may have contributed to the difference in the remission rates in the current study. We defined remission rate as the rate of children with average total score ≤5 based on IOWA Conners I/O subscale, whereas the other studies[9,26,28] used SNAP-IV-C scale where <1 (“not at all” or “just a little” in ADHD symptom) score on each of the 18 ADHD items was considered remission rate.
Significant improvements were noted in both forward and backward performances in the Digit Span Test. These results are consistent with the previous findings in other studies with MPH[29] and OROS-MPH.[30] Moreover, there was no significant difference in positive recitation between the ADHD and non-ADHD children at week 12, which shows a short term memory improvement in the children with ADHD to such an extent that they may appear normal. Though, a similar pattern was not seen for the negative recitation test as it necessitates special processes above and beyond moving backward along a time continuum.[31,32] However, the improvement could not reach the clinical relevance of one-point improvement compared with baseline.
Stroop Color-word Test is associated with cognitive flexibility and resistance to interference with outside stimuli. Significant improvements in all the Stroop Color-word Tests were observed. Cognitive assessment tests such as Digit Span backward, Digit Span sum, and Stroop Color-word Test 4 and 7 parameters in WCST may possess testing practice effect, where the total effect can be due to “practice” and/or “treatment drug.” Significant improvements were observed in cognitive assessments in this study. Practice-related improvements were not observed in the non-ADHD children. Hence, it can be concluded that the observed improvements are more likely to be due to the OROS-MPH treatment itself rather than the effect of testing practice.
At baseline, a significant difference was observed between ADHD children and non-ADHD children for Re, Rc%, Rf%, and perspective response tests in WCST and Stroop Test 4. However, at week 12, the difference between the groups was nonsignificant, suggesting that the OROS-MPH treatment substantially improved the behavior of children with ADHD and they appeared to be no different from the normal children.
In the present study, overall, children with ADHD showed significant improvements in verbal working memory, mental flexibility, and learning ability. These results are in line with the existing literature where MPH has showed significant improvements in cognition and executive functions.[11,30]
Coding Test or Digit Symbol Substitution Test is a useful indicator of change in sensory processing performance and is a sensitive measure of frontal lobe executive functions.[33,34] This test has been proven highly effective owing to its simplicity and sensitivity to various psychiatric and neurological disorders.[35,36,37,38,39] In this study, significant improvements were noted in ADHD children in psychomotor and processing speeds, high-speed visuomotor/visuospatial tracking, and attention as well as working memory. However, this was not observed in non-ADHD children. In another study analyzing the intellectual skill difficulties and attention problems,[40] hydrocephalus and ADHD children showed poor scores versus normal children in the Coding Test. Thus, indicating that ADHD and hydrocephalus children had problems in focusing/executing attention and sustaining attention.
An open-label, nonrandomized study design is a major limitation for this study. Although normal children without ADHD were recruited into the study, they were not used for the final analysis as the groups were not comparable. Also, the methods used for the academic performance evaluation in the current study are not standard methods. The two most commonly used methods, absolute score in standard test[41] and converted percentile of this score in individual tests,[12] were not used as these tests are not available at different centers in China. Further, there is no standard academic test that has been designed for Chinese children. Demographic and social factors may have an impact on school performance. The percentile comparison would not be suitable as our participants came from seven different centers or hospitals located in different provinces.
As for academic performance, both the Chinese language and mathematical achievement scores were improved in the children; improvement in mathematical score is more prominent than that of the Chinese score, consistent with the existing literature.[42] The majority of AEs were observed within the 1 week and the incidence of AEs declined consistently from Week 2 until Week 12 [Figure 3], indicating that OROS-MPH treatment is well-tolerated after the first couple of weeks of treatment. Safety results are consistent with previously published studies with OROS-MPH.[8,25]
In conclusion, this open-label study suggests that the OROS MPH improves academic and cognitive performance in Chinese school-aged children with ADHD. The treatment was safe and generally well-tolerated over the period of 12 weeks.
Financial support and sponsorship
The study presented in this report was supported by Xi’an Janssen Pharmaceutical Ltd.
Conflicts of interest
Ms. Jian-Min Zhuo and Dr. Sheng-Nan Xie are full-time salaried employees of Xi’an-Janssen Pharmaceutical Ltd. Drs. Yi Zheng, Hong-Yun Gao, Zhi-Wei Yang, Fu-Jun Jia, Fang Fang, and Rong Li have served on advisory boards of Xi’an Janssen Pharmaceutical Ltd., and Eli Lilly and Company.
Acknowledgments
We acknowledge Mr. Shreekant Sharma (SIRO Clinpharm Pvt., Ltd.) for providing writing assistance and Dr. Jia-Zhi Qu (Xi’an Janssen Pharmaceutical Ltd.) for additional editorial support for the development of this manuscript. The authors also thank the study participants of the study, without whom the study would never have been accomplished, and the investigators for their participation in the study.
Footnotes
Edited by: Yuan-Yuan Ji
REFERENCES
- 1.Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–8. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 2.Scahill L, Schwab-Stone M. Epidemiology of ADHD in school-age children. Child Adolesc Psychiatr Clin N Am. 2000;9:541–55. vii. [PubMed] [Google Scholar]
- 3.Effective Health Care Review. Comparative Effectiveness Review Number 44. Attention Deficit Hyperactivity Disorder: Effectiveness of Treatment in At-Risk Preschoolers; Long-Term Effectiveness in All Ages; and Variability in Prevalence, Diagnosis and Treatment. U.S. Department of Health and Human Services. 2011. [Last cited on 2015 Jan 12]. Available from: http://www.effectivehealthcare.ahrq.gov/ehc/products/191/818/CER44-ADHD_20111021.pdf .
- 4.Li SC, Lewandowsky S. Forward and backward recall: Different retrieval processes. J Exp Psychol Learn Mem Cogn. 1995;21:837–47. [Google Scholar]
- 5.Hong HJ, Lee JB, Kim JS, Seo WS, Koo BH, Bai DS, et al. Impairment of concept formation ability in children with ADHD: Comparisons between lower grades and higher grades. Psychiatry Investig. 2010;7:177–88. doi: 10.4306/pi.2010.7.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loe IM, Feldman HM. Academic and educational outcomes of children with ADHD. J Pediatr Psychol. 2007;32:643–54. doi: 10.1093/jpepsy/jsl054. [DOI] [PubMed] [Google Scholar]
- 7.Das D, Cherbuin N, Butterworth P, Anstey KJ, Easteal S. A population-based study of attention deficit/hyperactivity disorder symptoms and associated impairment in middle-aged adults. PLoS One. 2012;7:e31500. doi: 10.1371/journal.pone.0031500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mattos P, Louzã MR, Palmini AL, de Oliveira IR, Rocha FL. A multicenter, open-label trial to evaluate the quality of life in adults with ADHD treated with long-acting methylphenidate (OROS MPH): Concerta Quality of Life (CONQoL) study. J Atten Disord. 2013;17:444–8. doi: 10.1177/1087054711434772. [DOI] [PubMed] [Google Scholar]
- 9.Steele M, Weiss M, Swanson J, Wang J, Prinzo RS, Binder CE. A randomized, controlled effectiveness trial of OROS-methylphenidate compared to usual care with immediate-release methylphenidate in attention deficit-hyperactivity disorder. Can J Clin Pharmacol. 2006;13:e50–62. [PubMed] [Google Scholar]
- 10.Venkatraman VK, Aizenstein HJ, Newman AB, Yaffe K, Harris T, Kritchevsky S, et al. Lower digit symbol substitution score in the oldest old is related to magnetization transfer and diffusion tensor imaging of the white matter. Front Aging Neurosci. 2011;3:11. doi: 10.3389/fnagi.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wigal SB, Wigal T, Schuck S, Brams M, Williamson D, Armstrong RB, et al. Academic, behavioral, and cognitive effects of OROS® methylphenidate on older children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2011;21:121–31. doi: 10.1089/cap.2010.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zoëga H, Rothman KJ, Huybrechts KF, Ólafsson Ö, Baldursson G, Almarsdóttir AB, et al. A population-based study of stimulant drug treatment of ADHD and academic progress in children. Pediatrics. 2012;130:e53–62. doi: 10.1542/peds.2011-3493. [DOI] [PubMed] [Google Scholar]
- 13.Lv XZ, Shu Z, Zhang YW, Wu SS, Zhan SY. Effectiveness and safety of methylphenidate and atomoxetine for attention deficit hyperactivity disorder: A systematic review (in Chinese) Chin J Contemp Pediatr. 2011;13:365–9. [PubMed] [Google Scholar]
- 14.Wietecha L, Williams D, Shaywitz S, Shaywitz B, Hooper SR, Wigal SB, et al. Atomoxetine improved attention in children and adolescents with attention-deficit/hyperactivity disorder and dyslexia in a 16 week, acute, randomized, double-blind trial. J Child Adolesc Psychopharmacol. 2013;23:605–13. doi: 10.1089/cap.2013.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rapport MD, Orban SA, Kofler MJ, Friedman LM. Do programs designed to train working memory, other executive functions, and attention benefit children with ADHD? A meta-analytic review of cognitive, academic, and behavioral outcomes. Clin Psychol Rev. 2013;33:1237–52. doi: 10.1016/j.cpr.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Yang P, Chung LC, Chen CS, Chen CC. Rapid improvement in academic grades following methylphenidate treatment in attention-deficit hyperactivity disorder. Psychiatry Clin Neurosci. 2004;58:37–41. doi: 10.1111/j.1440-1819.2004.01190.x. [DOI] [PubMed] [Google Scholar]
- 17.Balthazor MJ, Wagner RK, Pelham WE. The specificity of the effects of stimulant medication on classroom learning-related measures of cognitive processing for attention deficit disorder children. J Abnorm Child Psychol. 1991;19:35–52. doi: 10.1007/BF00910563. [DOI] [PubMed] [Google Scholar]
- 18.Waschbusch DA, Willoughby MT. Parent and teacher ratings on the IOWA conners rating scale. J Psychopathol Behav Assess. 2008;30:180–92. [Google Scholar]
- 19.Wechsler D. Administration and Scoring Manual. 4th ed. San Antonio, TX: Harcourt Assessment, Inc; 2003. Wechsler intelligence scale for children. [Google Scholar]
- 20.Shelton JT, Elliott EM, Hill BD, Calamia MR, Gouvier WD. A comparison of laboratory and clinical working memory tests and their prediction of fluid intelligence. Intelligence. 2009;37:283. doi: 10.1016/j.intell.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tchanturia K, Davies H, Roberts M, Harrison A, Nakazato M, Schmidt U, et al. Poor cognitive flexibility in eating disorders: Examining the evidence using the Wisconsin Card Sorting Task. PLoS One. 2012;7:e28331. doi: 10.1371/journal.pone.0028331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss G, Hechtman L, Milroy T, Perlman T. Psychiatric status of hyperactives as adults: A controlled prospective 15-year follow-up of 63 hyperactive children. J Am Acad Child Psychiatry. 1985;24:211–20. doi: 10.1016/s0002-7138(09)60450-7. [DOI] [PubMed] [Google Scholar]
- 23.Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective follow-up study. J Am Acad Child Adolesc Psychiatry. 1990;29:546–57. doi: 10.1097/00004583-199007000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Zhou KY, Gao MH, Yang CH, Zhang JN, Chen YZ, Song JZ, et al. An epidemiological survey of attention deficit hyperactivity disorder in school-age children in Shenzhen (in Chinese) Chin J Contemp Pediatr. 2012;14:689–92. [PubMed] [Google Scholar]
- 25.Zheng Y, Wang YF, Qin J, Wang LW, Zou LP, Jin XM, et al. Prospective, naturalistic study of open-label OROS methylphenidate treatment in Chinese school-aged children with attention-deficit/hyperactivity disorder. Chin Med J. 2011;124:3269–74. [PubMed] [Google Scholar]
- 26.Chou WJ, Chen SJ, Chen YS, Liang HY, Lin CC, Tang CS, et al. Remission in children and adolescents diagnosed with attention-deficit/hyperactivity disorder via an effective and tolerable titration scheme for osmotic release oral system methylphenidate. J Child Adolesc Psychopharmacol. 2012;22:215–25. doi: 10.1089/cap.2011.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brault MC, Lacourse É. Prevalence of prescribed attention-deficit hyperactivity disorder medications and diagnosis among Canadian preschoolers and school-age children: 1994-2007. Can J Psychiatry. 2012;57:93–101. doi: 10.1177/070674371205700206. [DOI] [PubMed] [Google Scholar]
- 28.Tzang RF, Wang YC, Yeh CB, Hsu CD, Liang HY, Yang PC, et al. Naturalistic exploration of the effect of osmotic release oral system-methylphenidate on remission rate and functional improvement in Taiwanese children with attention-deficit-hyperactivity disorder. Psychiatry Clin Neurosci. 2012;66:53–63. doi: 10.1111/j.1440-1819.2011.02289.x. [DOI] [PubMed] [Google Scholar]
- 29.Tsai CS, Huang YS, Wu CL, Hwang FM, Young KB, Tsai MH, et al. Long-term effects of stimulants on neurocognitive performance of Taiwanese children with attention-deficit/hyperactivity disorder. BMC Psychiatry. 2013;13:330. doi: 10.1186/1471-244X-13-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang L, Cao Q, Shuai L, Li H, Chan RC, Wang Y. Comparative study of OROS-MPH and atomoxetine on executive function improvement in ADHD: A randomized controlled trial. Int J Neuropsychopharmacol. 2012;15:15–26. doi: 10.1017/S1461145711001490. [DOI] [PubMed] [Google Scholar]
- 31.Murdock BB. Developing TODAM: Three models for serial-order information. Mem Cognit. 1995;23:631–45. doi: 10.3758/bf03197264. [DOI] [PubMed] [Google Scholar]
- 32.Thomas JG, Milner HR, Haberlandt KF. Forward and backward recall: Different response time patterns, same retrieval order. Psychol Sci. 2003;14:169–74. doi: 10.1111/1467-9280.01437. [DOI] [PubMed] [Google Scholar]
- 33.Vilkki J, Holst P. Mental programming after frontal lobe lesions: Results on digit symbol performance with self-selected goals. Cortex. 1991;27:203–11. doi: 10.1016/s0010-9452(13)80124-4. [DOI] [PubMed] [Google Scholar]
- 34.Parkin AJ, Java RI. Deterioration of frontal lobe function in normal aging: Influences of fluid intelligence versus perceptual speed. Neuropsychology. 1999;13:539–45. doi: 10.1037//0894-4105.13.4.539. [DOI] [PubMed] [Google Scholar]
- 35.Yamanouchi N, Okada S, Kodama K, Sakamoto T, Sekine H, Hirai S, et al. Effects of MRI abnormalities on WAIS-R performance in solvent abusers. Acta Neurol Scand. 1997;96:34–9. doi: 10.1111/j.1600-0404.1997.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 36.Demakis GJ, Sawyer TP, Fritz D, Sweet JJ. Incidental recall on WAIS-R digit symbol discriminates Alzheimer's and Parkinson's diseases. J Clin Psychol. 2001;57:387–94. doi: 10.1002/jclp.1020. [DOI] [PubMed] [Google Scholar]
- 37.Ratti MT, Bo P, Giardini A, Soragna D. Chronic alcoholism and the frontal lobe: Which executive functions are imparied? Acta Neurol Scand. 2002;105:276–81. doi: 10.1034/j.1600-0404.2002.0o315.x. [DOI] [PubMed] [Google Scholar]
- 38.Blekher TM, Yee RD, Kirkwood SC, Hake AM, Stout JC, Weaver MR, et al. Oculomotor control in asymptomatic and recently diagnosed individuals with the genetic marker for Huntington's disease. Vision Res. 2004;44:2729–36. doi: 10.1016/j.visres.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Nakahachi T, Iwase M, Takahashi H, Honaga E, Sekiyama R, Ukai S, et al. Discrepancy of performance among working memory-related tasks in autism spectrum disorders was caused by task characteristics, apart from working memory, which could interfere with task execution. Psychiatry Clin Neurosci. 2006;60:312–8. doi: 10.1111/j.1440-1819.2006.01507.x. [DOI] [PubMed] [Google Scholar]
- 40.Bakar EE, Bakar B, Taner YI, Akalan N. Evaluation of the intellectual skill problems of hydrocephalic children: A clinical study. Turk Neurosurg. 2009;19:29–35. [PubMed] [Google Scholar]
- 41.Burlison JD, Dwyer WO. Risk screening for ADHD in a college population: Is there a relationship with academic performance? J Atten Disord. 2013;17:58–63. doi: 10.1177/1087054711423628. [DOI] [PubMed] [Google Scholar]
- 42.Williamson D, Murray DW, Damaraju CV, Ascher S, Starr HL. Methylphenidate in children with ADHD with or without learning disability. J Atten Disord. 2014;18:95–104. doi: 10.1177/1087054712443411. [DOI] [PubMed] [Google Scholar]


