Abstract
Purpose:
To customize clinical practice guidelines (CPGs) for cataract management in the Iranian population.
Methods:
First, four CPGs (American Academy of Ophthalmology 2006 and 2011, Royal College of Ophthalmologists 2010, and Canadian Ophthalmological Society 2008) were selected from a number of available CPGs in the literature for cataract management. All recommendations of these guidelines, together with their references, were studied. Each recommendation was summarized in 4 tables. The first table showed the recommendation itself in clinical question components format along with its level of evidence. The second table contained structured abstracts of supporting articles related to the clinical question with their levels of evidence. The third table included the customized recommendation of the internal group respecting its clinical advantage, cost, and complications. In the fourth table, the internal group their recommendations from 1 to 9 based on the customizing capability of the recommendation (applicability, acceptability, external validity). Finally, customized recommendations were sent one month prior to a consensus session to faculty members of all universities across the country asking for their comments on recommendations.
Results:
The agreed recommendations were accepted as conclusive while those with no agreement were discussed at the consensus session. Finally, all customized recommendations were codified as 80 recommendations along with their sources and levels of evidence for the Iranian population.
Conclusion:
Customization of CPGs for management of adult cataract for the Iranian population seems to be useful for standardization of referral, diagnosis and treatment of patients.
Keywords: Adult, Cataract, Iran, Practice Guideline
INTRODUCTION
Cataract is the progressive deterioration of optical qualities of the crystalline lens.[1,2] The most common form is age-related cataract which affects a high percentage of people over the age of 50 years.[2] Cataract has been reported to be the cause of 47.8% of all cases of blindness worldwide and are a serious health issue particularly in developing countries.[3] In Iran, cataract is are highly prevalent, and based on two studies performed on Tehran citizens over 40 years, a prevalence of 12% to 19.1% has been reported.[4,5] Another study from Varamin district has reported cataract as the cause of blindness in 31.7% of cases and severe visual acuity impairment in 47.5% of cases.[6] Currently, the only option for visual rehabilitation in human subjects with cataract is surgical removal of the lens,[7] which is one of the most successful surgical treatments.[2] This surgery has vastly changed during the past 20 years,[8] and with advances in microscopic surgery and intraocular lens (IOL) manufacturing, the quality of vision after surgery has dramatically improved, leading to more cases of surgical treatment being performed.[9]
National clinical practice guidelines (CPGs) include clinical recommendations based on systematically verified evidence. These guidelines are developed to evaluate the effectiveness, safety, and efficiency of inventions considering domestic factors and justly distributed health care. These guidelines have an impact on decision-making by both clinicians and health care authorities.[10,11]
The present guideline for management of cataract in adult patients has been customized in order to comply with guidelines of the Fifth National Development Program of the Islamic Republic of Iran, particularly section D of the 32nd amendment calling for customizing national CPGs for the healthcare system and providing fair access to continuous and qualified health care as one of the main goals of the health care system. We also stressed adhering to the 75th strategic goal of the Iranian Ministry of Health and Medical Education regarding use of national medical guidelines and establishment of evidence-based practices. This guideline was customized in the Ophthalmic Knowledge Management Unit (KMU) at Shahid Beheshti University of Medical Sciences in 2011 to fulfill requirements by the Iranian Ministry of Health and Medical Education.[12,13]
Iranian cataract management guidelines contain 80 clinical recommendations with the aim of standardizing and customizing different aspects of cataract management in adults including etiology, progression, indication for intervention, diagnosis, treatment, and pre-and post-surgical care.
METHODS
A group including general ophthalmologists as well as cornea, vitreoretinal and strabismus subspecialists, masters of optometry and biostatistics, expert searcher, head of the Office for healthcare standards, Deputy of Curative Affairs, Iran Ministry of Health and Medical Education representative participated in this effort.
Search for Related Guidelines and Screen Extracted Guidelines
First, medical data resources including National Guideline Clearinghouse, Guidelines International Network, National Institute for Clinical Excellence, Scottish Intercollegiate Guidelines Network, National Health and Medical Research Council, and New Zealand Guidelines Group were searched to find national guidelines related to our subject. All guidelines were evaluated based on their quality characteristics such as structural soundness, availability of the full guideline, and being up-to-date. In the next step, guidelines which fulfilled all of the above-mentioned requirements were scored by using AGREE instrument. This evaluation instrument included 23 questions covering different aspects of the guideline, and the guidelines were given a score of 0 to 100. Finally, based on their scores, the guidelines from the American Academy of Ophthalmology (2006 and 2011),[2,14] the Royal College of Ophthalmologists,[9] and the Canadian Ophthalmological Society[1] with total scores of 85, 81, 80, and 75, respectively, were selected as reference guidelines.
The Process of Evaluating the Selected Clinical Guidelines
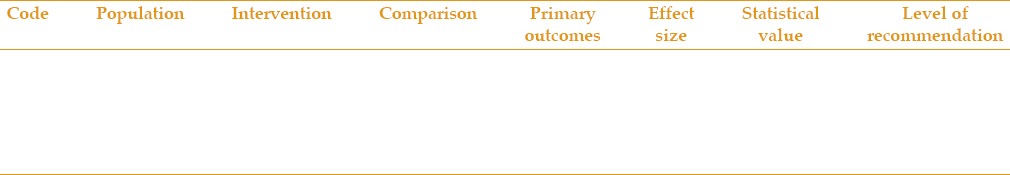
We first designed the questions based on the selected CPGs, then categorized them according to their precedence in the treatment process of cataract [Table 1].
Table 1.
Algorithm of the clinical practice guidelines

Evaluating the content of referenced guidelines
The recommendations of the selected guidelines which have addressed each question were included in Table 2. Those recommendations which had similar populations or interventions were placed in the same table. To fill this table, we first recorded four parts of questions which included the patient population or the disease (P), intervention or exposure (I), comparison (C), and finally, outcome (O), and then entered the main related recommendation. We also entered technical breakthroughs if suggested by reliable scientific organizations or countries as new recommendations.
Table 2.
Analysis of recommendation

Organizing the recommendations in tables helped us recognize different aspects of the final guideline. This method also optimized the search for related evidence.
Analysis of the evidence supporting each recommendation
Abstracts of all supporting evidence for each recommendation were extracted. We also searched the Coherence Library, Trip Database (Evidence-based synopses), Bandolier, and PubMed (Clinical queries section) databases from 2007 to 2012 for the clinical questions related to each suggested recommendation. These databases are known as reliable sources for information with highest levels of evidence based on systematic review, meta-analysis, and clinical trial studies. All these abstracts were criticised by our team, and their related details were entered in Table 3. If there was any ambiguity in the abstract, the full text was studied.
Table 3.
Analysis of evidences
The level of evidences have been determined based on Tables 4 and 5. If there was more than one recommendation for a clinical question and those recommendations did not have the same level of evidence, the recommendation with a higher level of evidence was selected.
Table 4.
Level of evidence
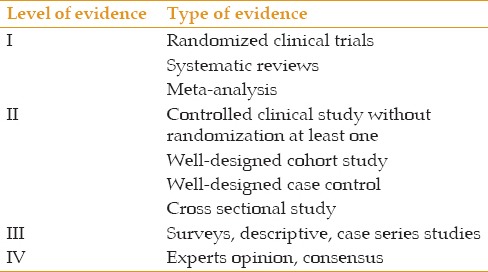
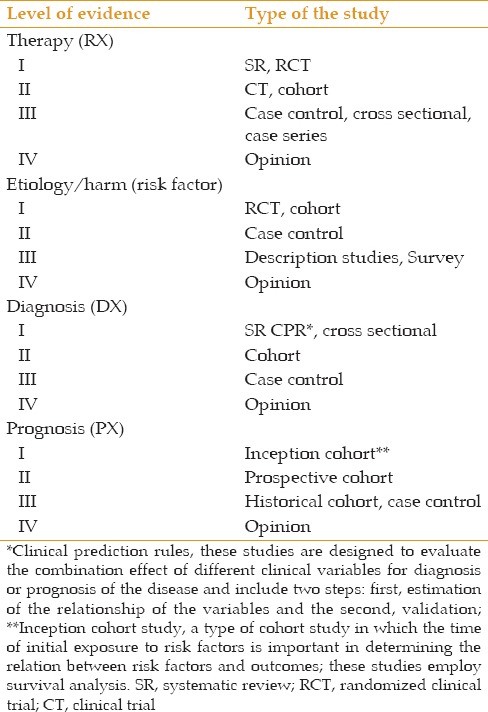
Table 5.
Level of evidence according to the type of study
Analysis of the clinical advantage of recommendations
After completing the supporting evidence and choosing the recommendation with the highest level of evidence, the related recommendation was entered in Table 6. Next, the internal group evaluated the recommendation in terms of its benefits, side effects, and treatment cost.
Table 6.
Clinical advantages of recommendations

The benefit of an intervention is different from its outcome. It includes the ability to manage the treatment at lower levels of the health care system, at specialty and subspecialty levels, decrease admission rate and duration, decreased the need for surgical treatment, increase patient satisfaction, allow faster recovery, reduce the need for post-treatment visits, and minimize patient pain and suffering.
We also categorized side effects based on their nature and severity as well as the effectiveness based on the supporting evidences.
Finally, our team, as an internal reviewer, gave low, moderate, or high scores to the clinical advantages of each recommendation.
Customizing the recommendations
Customization of each recommendation was evaluated in Table 7. In this table, the applicability, external validity, and acceptability of each recommendation was determined, and each recommendation was given a score of low,[1,2,3] moderate,[4,5,6] or high.[7,8,9]
Table 7.
Adaptability of the recommendation

Consensus Process
First consensus (External peer review)
All customized recommendations and related data [Tables 2, 3, 6 and 7] were sent to expert in the field of cataract surgery at different public hospitals supervised by medical universities (Tabriz University of Medical Sciences, Shahid Beheshti University of Medical Sciences, Iran University of Medical Sciences, Tehran University of Medical Sciences, Ahvaz Jundishapur University of Medical Sciences, Zahedan University of Medical Sciences, and Mashhad University of Medical Sciences) after removing the internal scores.
Participants were asked to:
Evaluate the recommendations and determine the clinical advantage score, customized potential score, and total score for each recommendation
Add any evidence related to recommendations that were not included in our search, which could change the final decision, if possible.
Evaluation of total scores
After collecting the external peer review scores, agreement among the external peer review was assessed by our team, and the agreed recommendations were considered as final customized recommendations.
Second consensus
The external and internal reviewers gathered again and discussed non-agreed recommendations, some of which were modified and rescored to achieve agreement from both groups.
Final Assessment of Recommendations
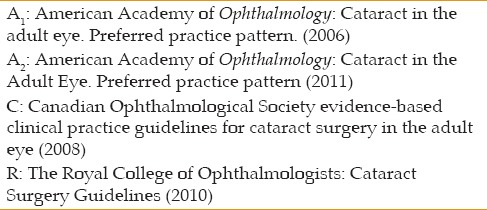
All recommendation were re-evaluated by our team, and the final recommendations were recorded in a file with special codes in abbreviations, which included the name of the adopted CPG [Table 8], its level of evidence, and the number for its reference. For example (C4), (I), indicates that the recommendation has stemmed from recommendation number 4 from the Canadian Ophthalmological Society clinical practice guidelines for adult cataract surgery, a Clinical Practice Guideline with level of evidence of I.
Table 8.
Abbreviations of the reference guidelines
RESULTS
The result of customizing 4 prior guidelines for the Iranian population may be summarized into the 80 recommendations classified below.
Non-surgical Treatments/Risk Factors for Cataract Progression
It is recommended that ophthalmologists suggest that patients use sunglasses and hats with visors to reduce sun ray (UV-B) damage and progression of cortical cataracts.[15,16,17] (I), (A1,2)
Based on a high level of evidence, supplements such as antioxidants, Vit C, Vit E, Vit A and beta-carotene do not prevent or reduce the rate of progression of cataracts.[18,19,20] (I), (A1,2)
Considering the proven effect of quitting cigarette smoking in limiting the progression of cataracts, it is recommended that ophthalmologists ask all patients with lens opacity to quit smoking.[21,22,23,24,25] (I), (A1,2 R3-3)
It is recommended to inform patients about the increased risk of cataracts after long-term use of oral or inhaled steroids, and alternative medications should be used, if possible.[26,27] (II), (A1)>
The use of steroids, amitriptyline, statins, anti-diabetes pills, insulin, and potassium-sparing diuretics increase the risk of cataracts and cause faster progression of the condition, but aspirin and thiazides do not have a role in this respect. Discontinuation of cataract-causing drugs may be recommended by physicians, if possible.[28,29] (I), (A1)
It is recommended that the increased risk of cataract be discussed with diabetic patients[30,31,32] (II), (A1)
It is recommended that the increased risk of accidents and bone fractures be discussed with patients who have cataracts but do not wish to be operated on.[33,34,35,36,37] (I), (C4).
Surgical Treatments/Preoperative/Indication of Cataract Surgery Based on Visual Acuity
-
8.
If cataract is the main and primary cause of visual loss, surgery is indicated. (IV), (consensus C19)
-
9.
Cataract surgery is recommended when vision loss is not corrected with non-surgical methods meet patients' needs.[38,39] (IV), (A22, C2)
-
10.
It is recommended that in evaluating cataracts and determining the time of surgery, with the exception of visual acuity, other factors such as need for far sight, near sight, and sight under different lighting conditions be considered.[40,41,42,43,44,45] (I), (A1)
-
11.
In patients who have less than standard visual acuity according to their occupation (like drivers, soldiers, and pilots) and wish to continue their occupation, cataract surgery is indicated even when there are not many functional deficits under normal life circumstances. (IV), (consensus C3).
Surgical Treatments/Preoperative/Indication for Fellow Eye Cataract Surgery
-
12.
Indication for surgery on the second eye is the same as the first eye, but in some cases of anisometropia, earlier operation is recommended. The time between surgeries should be long enough for detecting possible complications of the first procedure such as endophthalmitis or residual refractive errors. (IV), (consensus, C11)
-
13.
In bilateral cataract cases, it is recommended to perform surgery in separate sessions in order to achieve better binocular vision.[46,47,48,49,50] (I), (R5.2)
-
14.
Because of the chance of endophthalmitis and bilateral toxic anterior segment syndrome (TASS), simultaneous surgery on both eyes is not recommended.[51,52,53] (III), (C12)
-
15.
In one-eyed cataract patients, surgery is recommended if the advantages of performing the surgery are higher than its side effects.[54,55] (III), (C10)
-
16.
Based on scientific evidence, performing cataract surgery might reduce intraocular pressure in patients who also have closed angle glaucoma.[56,57,58,59,60,61,62,63] (II), (A2 6)
-
17.
If there is a chance of blindness due to increased intraocular pressure after cataract surgery and glaucoma surgery, simultaneous surgery is recommended. (IV), (consensus, C18)
-
18.
Cataract surgery is indicated in patients with phacomorphic glaucoma, lens-induced uveitis, and posterior segment diseases, if lens opacity limits fundus examination or treatment. (IV), (consensus, C5).
Surgical Treatments/Preoperative/Assessment/Eye Assessment
-
19.
It is recommended for all cataract surgeons to answer the following questions prior to surgery in order to prevent or reduce some risk factors:[64]
Is the cataract the main cause of vision loss?
Is there any other eye disease like glaucoma that might worsen because of cataract surgery?
Is there any eye disease that might complicate the surgical process? (I), (C7)
-
20.
The surgeon should refer the patients to facilities with more expertise if the surgery seems to be very complicated, has a high risk of complications, or the surgeon does not have enough experience.[65] (II), (C8)
-
21.
α-blockers (tamsulosin) should be discontinued before surgery because of the high risk of floppy iris syndrome (FIS) and other serious complications.[66,67,68,69,70,71] (I), (A2, 5)
-
22.
Due to the absence of serious vision related side effects, discontinuation of anti-coagulant medications (except for Warfarin and Clopidogrel) is not recommended.[72,73,74,75] (I), (C26, R7-5-1)
-
23.
If a surgeon encounters a higher incidence of endophthalmitis compared to what has been reported in the literature, it is recommended to search for the source of this complication by taking serial microbial cultures from personnel, surgery room, and devices as well as controlling the sterilization process. Also preps of eyelashes with 10% betadine and preps of the cul-de-sac and conjunctiva with 5% betadine are recommended. (IV), (consensus, C57 and external consensus)
-
24.
It is recommended that a form, including the eye undergoing surgery, IOL power, medications used by the patients, previous diseases, etc., be prepared at all eye surgical centers and filled out immediately before surgery by the surgeon or the operating room staff. It is also recommended that patient education brochures be given to all patients or their accompanying person after surgery (including information about post-surgical pain, introduction of drugs, time for post-operative visits, etc.).[76,77,78,79,80,81] (II), (A2 11).
Surgical Treatments/Preoperative/Assessment/Lab Assessment
Surgical Treatments/Preoperative/Assessment/IOL Assessment
-
26.
It is recommended to repeat A-scan biometry in the following circumstances:[84,85]
- Axial length more than 26mm or less than 21mm
- Keratometry more than 47 diopters or less than 41 diopters
- Astigmatism more than 2.5D
- Axial length difference more than 0.7 between the two eyes
- Keratometry difference of more than 0.9 between the two eyes (III), (R 8-7-4)
-
27.
It is recommended that optical (laser interferometry) biometry or utrasonography with immersion technique be used to find the exact axial length. Both methods are superior to contact method.[86,87] (I), (C31)
-
28.
In eyes with abnormal size, the surgeon should use Holladay 2 or Haigis formulas to achieve the best post-operative refractive outcomes. (IV) (consensus, C33)
-
29.
To achieve the best postoperative refractive results, it is recommended to calculate the lens power using the new generation formulas (Hoffer Q, SRK/T, Holladay).[88] (III), (C32)
-
30.
Surgeons should optimize their IOL A-constant based on their postoperative refractive results and consider it in IOL power calculation. (IV), (consensus, C34)
-
31.
The effect of A-constant optimization on IOL power prediction is more pronounced when using the modern IOL power calculation formulas. In some cases, the percentage of eyes with acceptable refractive range after surgery (Target refraction ± 1D) can be increased by 20% when A-constant optimization is applied. (IV), (consensus, R 8-7-5)
-
32.
The A-constant provided by the manufacturer which is calculated by contact sonography is different from optical optimized A-constant. To improve the A-constant of newer intraocular lenses in optical biometry, ULIB online website should be utilized. (IV) (consensus, R8-7-1)
-
33.
Patients with a history of refractive surgery should be informed about the possibility of errors in IOL power calculation and postoperative refraction even when utilizing adjustments. (IV), (consensus, C38)
-
34.
In patients who have a history of keratorefractive surgery, surgeons should be aware of the possibility of unusual postoperative refractive results and should calculate the real corneal refractive power using adjusted formulas, utilizing patient preoperative findings, or direct calculation of the central corneal power using SimKs or Scheimpflug methods. It is ideal to apply several methods for calculating the IOL power. (IV), (consensus, C36)
-
35.
It is recommended that the past medical and ocular history, including keratometry and biometry results, of all patients undergoing keratorefractive surgery be kept by the treatment center.[89] (II), C37)
-
36.
It is recommended to use IOLs with sharp angles to lessen the chance of posterior capsule opacity.[90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109] (I), (R10, C62)
-
37.
The use of anterior aspheric IOLs is recommended to achieve better contrast sensitivity and visual performance particularly during the night. It should be noted that in conditions like zonular rupture and astigmatism, which increase the chance of IOL decentration, or after hyperopic corneal refractive surgery, spherical IOLs should be implanted.[110,111,112] (I), (C42, R 9-7-4)
-
38.
In the process of using multifocal or accommodative IOLs which correct different degrees of presbyopia, careful patient selection and consultation, informing the patient about the results, and utilizing necessary preoperative examinations are vital. (IV), (consensus, C44)
-
39.
It is recommended that multifocal and adjustable lenses be used based on patient needs and desires, and after giving the patient complete information about the advantages and disadvantages of such lenses, including glare/haloes, posterior capsular opacity and reduction of contrast sensitivity.[113,114,115] (I), R 9-7-1)
-
40.
To use toric IOLs in patients with regular preoperative corneal astigmatism, accurate preoperative calculations, correct marking of the steep axis, and implanting the IOL in correct axis should be considered to eliminate undesirable surgical induced astigmatism. (IV), (consensus, C43)
-
41.
Visual outcomes after cataract surgery and implantation of UV filter IOLs and blue filter IOLs are comparable.[116,117] (I), (New) - (IV), (Internal and external consensus)
-
42.
When the PCIOL is placed in the ciliary sulcus, decreasing the power by 0 to 1.5 diopters should be considered. (IV), (consensus, C35).
Surgical Treatments/Preoperative/Risk Factors of Cataract Surgery
-
43.
It is preferable that the waiting time for cataract surgery be less than 2 to 3 months to avoid the chance of visual deterioration and possible accidents.[118,119] (I), (R 4)
-
44.
Patients with cataracts and at danger of the consequences of reduced vision, namely falls, fractures or accidents, should undergo surgical correction as soon as possible.[120,121,122,123,124,125] (I), (C6)
-
45.
It is recommended that all patients with cataracts be informed about the chance of PCO before surgery.[90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109] (III), (R10)
-
46.
It is recommended that the chance of aggravation of diabetic retinopathy be discussed with patients with diabetes and lens opacity who are undergoing cataract surgery.[126,127] (III), (C21)
-
47.
If the surgeon finds a serious condition in the pre-surgical examinations like tractional retinal detachment, it is recommended that consultation for performing combined vitrectomy and cataract surgery be considered.[128] (III), (C20)
-
48.
Treatment of proliferative diabetic retinopathy (PDR) and clinically significant macular edema (CSME) should be performed before cataract surgery, if possible. (IV), (consensus, C20)
-
49.
It is recommended that the risk of retinal detachment be discussed with high-risk patients (patients with posterior capsule tear and consequent anterior vitrectomy, age less than 60 years, male sex, and high myopia). They should also be informed of the warning signs and symptoms of retinal detachment to facilitate early diagnosis and treatment.[129,130,131] (I), (C64)
-
50.
In patients with Fuchs' dystrophy and cataract, if the chance of corneal decompensation is considerable, combined cataract and corneal transplantation could be considered. (IV), (consensus, C17)
-
51.
Anterior chamber depth less than 2.5mm in patients with pseudoexfoliation (PEX) increases the chance of complications during the cataract surgery.
Accompanying recommendation: It is recommended that cataract surgery in these patients be performed by a more experienced surgeon in order to decrease the risk of complications.[132] (II), (C23)
-
52.
Cataract surgery after trabeculectomy (TX) increases the chances of TX failure. A shorter time interval between the two surgeries increases the risk of this failure[133] (I), (New) - (IV), (Internal and external consensus)
-
53.
It is recommended that patients with age-related macular degeneration (ARMD) undergo cataract surgery only if there is a chance for improved vision. Informing the patient about the chance of worsening of macular degeneration following cataract surgery is necessary.[134,135,136,137] (I) (C 15).
Surgical Treatments/Intraoperative
-
54.
Treatment of blepharitis before surgery and also prep of the eyelashes with 10% betadine and prep of the cul-de-sac and conjunctiva with 5% betadine immediately before surgery is recommended to decrease the chance of endophthalmitis.[138,139,140,141,142,143] (II), (C55)
-
55.
The choice of local anesthesia for cataract surgery is made based on the patient and surgeon's preference.[144,145,146,147,148,149] (I) (R 7-2-2)
-
56.
Phacoemulsification using a small incision is the preferred method of cataract surgery compared to extracapsular cataract extraction since it causes less astigmatism, achieves fast and better visual outcomes, and has fewer complications.[150,151,152] (I) (C45)
-
57.
Smaller incisions (3.2 mm) during cataract surgery are recommended due to less astigmatism and less short-term changes in the cornea.[153,154] (I), (C46)
-
58.
Implantation of foldable IOLs is preferable to rigid IOLs since they require smaller incisions, cause less astigmatism, provide better visual acuity, and cause less postoperative inflammation.[155] (I) (C40)
-
59.
Staining of the anterior capsule is recommended in mature cataracts, white cataracts, complicated cataracts and in children.[156] (I) (C50)
-
60.
Smaller capsulorhexis (4.5mm to 5mm) which can completely cover the margin of the IOL optic are preferable to larger ones since they decrease the chance of PCO.[157] (I), (C47)
-
61.
It is recommended that hydrodissection and hydrodelineation be performed routinely to reduce tension on the zonulae, facilitate cortical removal, and reduce the chance of PCO.[158] (I) (C48)
-
62.
The surgeon should ask patients about the history of using alpha adrenergic blocker medications (e.g. Tamsulosin), which cause intraoperative floppy iris syndrome. If there is a positive history, atropine drops and intraocular epinephrine should be used both before and during surgery. The surgeon should also use less fluid circulation and more viscoelastic materials as well as iris retractors and pupil rings to reduce the chance of floppy iris syndrome. Due to chance of bleeding, iris sphincterotomy should be avoided.[159,160,161,162,163,164] (I), (A25), (IV), (consensus C53)
-
63.
To reduce surgical complications, a small pupil should be enlarged appropriately. To achieve this goal, different methods can be used based on surgeon's experience and methods with less trauma. Usual methods include stretching hooks, rings, and pupil retractors, and using ophthalmic visco-surgical devices (OVDs).[165] (IV) (consensus, C52)
-
64.
Performing phacoemulsification with a torsional probe is preferred to conventional longitudinal probes since there is less chance of corneal trauma.[166,167,168,169,170,171] (I), (New) - (IV), (Internal and external consensus)
-
65.
In patients with local or general zonular weakness, applying capsular tension rings is recommended to achieve better control during surgery, reduce complications, and provide better IOL centration.[172] (I), (51)
-
66.
Considering the reduced incidence of side effects of using injectors (as compared to forceps) for implanting IOLs such as less wound damage, less IOLs damage, and a lower chance of endophthalmitis, it is recommended to implant the IOL using its special injector.[173] (III), (C41)
-
67.
In the absence of inadequate capsular bag support, the surgeon should determine a suitable IOL (considering lens power and material) to be implanted in the ciliary sulcus.[174,175,176,177,178] (III), (A2, 10)
-
68.
In case of posterior capsular rupture and inadequate capsular support for in- the-bag IOL implantation, ACIOLs, scleral fixation PCIOLs, or iris fixation IOLs may be used.[179] (III), (C39)
69- 1- Intracameral antibiotic injections are not recommended to reduce the chance of endophthalmitis due to the toxic effects and likelihood of reduction in corneal endothelial cells.[180,181,182,183,184,185,186,187] (III), (A28) - (IV), (Internal and external consensus)
69-2- Subconjunctival antibiotic injection may be recommended to reduce the chance of postoperative endophthalmitis with weak level of evidence.[180,181,182,183,184,185,186,187] (III), (A28).
Surgical Treatments/Postoperative/Complications
-
70.
Use of high frequency postoperative antibiotics is recommended to reduce the chance of endophthalmitis.[180,181,182,183,184,185,186,187] (III), (A2 8) - (IV), (Internal and external consensus)
-
71.
To treat postoperative cystoid macular edema (CME), topical non-steroidal anti-inflammatory drugs (NSAIDs) such as ketoralac 0.5 percent are recommended as the first step of therapy.[188,189] (I), (R4) - (IV), (Internal and external consensus)
-
72.
Before performing Nd: YAG laser capsulotomy for treatment of PCO, patients at high risk of retinal detachment (RD) such as posterior capsule rupture in the other eye, axial length more than 23 mm, and male sex, should be informed of the possibility of RD following laser capsulotomy.[190] (II), (C63)
-
73.
Postoperative intraocular inflammation is considered as a risk factor for corneal, trabecular and retinal complications, therefore the use of steroids or NSAIDs immediately after surgery to reduce inflammation is recommended. (IV), (consensus, C54)
-
74.
Because of the common use of antibiotics, resistance to several antibacterial agents such as penicillin and fluoroquinolones for treatment of postoperative staphylococcal endophthalmitis is observed. Ophthalmologists should be aware of this problem and consider it when prescribing post-operative antibiotics (resistance to ciprofloxacin has also been reported in several reports).[191,192,193,194,195,196,197] (III), (A27)
-
75.
Although toxic anterior segment syndrome (TASS) is rare, ophthalmologists should be aware of its predisposing factors such as posterior capsule rupture, vitreous loss, and potential complications.[198,199] (III), (A29)
-
76.
It is recommended to consider complications like dysphotopsia and lens capsule opacity when choosing the IOLs.[200] (I), (C65).
Surgical Treatments/Postoperative/Follow-up
-
77.
All patients undergoing phacoemulsification surgery should be aware of the postoperative examination schedule and possible side effects of the surgery. It is recommended to perform the first postoperative examination up to 24 hours after the surgery. The exact timing of other postoperative visits depends on the circumstances of the operation and the method used, complications, and surgeon and patient preference. (IV) (consensus, C59-60) - (IV), (Internal and external consensus)
-
78.
In high risk conditions like monocular patients, glaucomatous eyes or glaucoma suspects and surgical complications, the first visit should be performed by 24 hours after surgery and more frequent follow-up examinations are needed. Patients should be instructed to inform their ophthalmologists as soon as possible in case of severe reduction of vision, increased pain, redness, or inflammation around the eye as these might be signs of endophthalmitis. (IV) (consensus, C61)
-
79.
Final evaluation of refractive power should be performed 2 weeks postoperatively in patients with small corneal incisions (under 3.5mm) and after 6 weeks in patients with larger incisions or extracapsular operations. More postoperative examinations during this period, or even after it, are recommended based on special circumstances of the surgery, postoperative complications, the need for removing corneal sutures, and also evaluating intraocular pressure (IV), (consensus, C61).
-
80.
To evaluate patient satisfaction, visual assessment using a high contrast Snellen chart is not enough. Standard questionnaires like the VF14 will provide greater insight into patient satisfaction. (IV), (consensus, R12-6) - (IV), (Internal and external consensus).
DISCUSSION
The current CPG for treatment of cataracts in adult Iranian patients was customized based on the standard method suggested by the Iranian Ministry of Health and Medical Education at the knowledge management unit (KMU) of Shahid Beheshti University of Medical Sciences. This guideline includes 80 recommendations covering preoperative, intraoperative, and postoperative aspects related to adult cataract management. In the process of preparing the guidelines, preliminary consensus was not achieved in 15 out of 80 recommendations. These 15 recommendations were further discussed in internal panels, and 6 primary recommendations were reapproved, 8 recommendations were modified, and one recommendation was omitted. The changes were applied to 8 recommendations as follows: Recommendation number 5, which initially recommended that “the use of steroid drugs, amitriptyline, statins, and potassium-sparing diuretics increase the risk of cataract or its progression, and the use of aspirin and thiazides do not increase this chance”, was modified to “The use of steroids, amitriptyline, statins, anti-diabetes pills, insulin, and potassium-sparing diuretics increase the risk of cataracts and cause faster progression of the condition, but aspirin and thiazides do not have a role in this respect. Discontinuation of cataract-causing drugs may be recommended by physicians, if possible.”
Recommendation number 23, which stated, “If a surgeon encounters a higher incidence of endophthalmitis compared to what has been reported in the literature or in instances that the risk of postoperative endophthalmitis is higher due to complicated surgery, the postoperative injection of intracameral or subconjunctival antibiotics should be considered.” was changed to “If a surgeon encounters a higher incidence of endophthalmitis compared to what has been reported in the literature, it is recommended to search for the source of this complication by taking serial microbial cultures from personnel, surgery room, and its devices as well as controlling the sterilization process. Also, preparation of eyelashes with 10% betadine and preparation of the cul-de-sac and conjunctiva with 5% Betadine is recommended.”
Recommendation number 25 initially stated, “Considering the available data, preoperative examination does not increase the safety of cataract surgery. If the patient does not suffer from any special condition or diseases, performing routine preoperative exams are not recommended.” Considering the condition among Iranian patients and the fact that they might not have any previous systemic evaluation, after panel discussion this was changed to: “Performing general medical preoperative examinations, even when using local anesthesia, is recommended.”
Recommendation number 39 which stated that “The use of multifocal or adjustable lenses should be based on the necessity and the desire of the patient and after giving the patient a good understanding of advantages and disadvantages (glare/haloes, PCO)”, was changed to “It is recommended that multifocal and adjustable lenses be used based on patient needs and desires, and after giving the patient complete information about the advantages and disadvantages of such lenses, including glare/haloes, posterior capsular opacity and reduction of contrast sensitivity).”
The first version of recommendation number 50 stated: “In cataract patients with Fuchs' dystrophy, if the surgeon gives a chance of corneal decompression, the cataract and corneal surgeries are better to be combined.” After panel discussion, the term “is better” was changed to term “could”, and the final recommendation was:” In patients with Fuchs' dystrophy and cataract, if the chance of corneal decompensation is considerable, combined cataract and corneal transplantation can be considered.”
The initial statement for recommendation number 64 was “Performing phacoemulsification using a torsional probe is recommended compared to the use of conventional longitudinal probes since there is less chance of corneal trauma.” This recommendation was finalized in the discussion panel with a minor change as follows: “Performing phacoemulsification with a torsional probe is preferred to conventional longitudinal probes since there is less chance of corneal trauma.”
Two recommendations which were about antibiotic usage during surgery and postoperatively were discussed in the panel and the primary recommendation of “inject proper antibiotic in the anterior chamber to reduce the chance of endophthalmitis” was rejected by panel members, and intracameral prophylaxis antibiotic was not recommended as mentioned in recommendation 69-1.
Finally, before the panel discussion, recommendation number 71 suggested the use of NSAIDS for treatment of postoperative acute cystoid macular edema was not recommended, but it was changed to, “To treat postoperative cystoid macular edema (CME), topical non-steroidal anti-inflammatory drugs (NSAIDs) such as ketoralac 0.5 percent are recommended as the first step of therapy.”
At the end of the discussion panel, all final recommendations were edited and finalized as final recommendations.
This guideline, as compared to the cited CPGs which were used, has more detailed recommendations considering different aspects of cataract surgery, including important pre-, intra-, and postoperative aspects of the surgery. For example, the Canadian Ophthalmological Society evidence-based clinical practice guidelines for cataract surgery in the adult eye (2008) contains 65 recommendations, but the present guideline consists of 80 recommendations. On the other hand, this customized guideline has the benefit of considering clinical advantages and practicality among Iranian patients, so its use among the Iranian population should be more effective.
In conclusion, the current clinical practice guideline for adult cataract management was customized for an Iranian adult population at KMU at the Ophthalmic Research Center, Shahid Beheshti University of Medical Sciences as suggested by the Standardization and CPG Development Office, Deputy of Curative Affairs, and the Iranian Ministry of Health and Medical Education. Therefore, its recommendations can be used to standardize different aspects in the management of adult patients with cataracts.
Acknowledgments
We are immensely grateful to the external faculty members of all universities for scoring the recommendations of the clinical practice guidelines and their valuable comments.
We would like to show our gratitude to Ms. Bahar Kheiri for checking the evidences in terms of statistic criteria and all her efforts. We also would like to thanks Ms. Shirin Mohebikho for assistant with the literature review and coordination. Moreover, we really appreciate Ms. Soheila Khoshneshin for their kind attempts for typing the CPGs and tables.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Canadian Ophthalmological Society Cataract Surgery Clinical Practice Guideline Expert Committee. Canadian Ophthalmological Society evidence-based clinical practice guidelines for cataract surgery in the adult eye. Can J Ophthalmol. 2008;43(Suppl 1):S7–57. doi: 10.1139/i08-133. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Ophthalmology. Cataract in the adult eye (2011) [Last assessed on 2013 Sep 19]. Available at: http://one.aaoorg/preferred-practice-pattern/cataract-in-adult-eye-ppp-october-2011 .
- 3.Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 4.Hashemi H, Hatef E, Fotouhi A, Feizzadeh A, Mohammad K. The prevalence of lens opacities in Tehran: The Tehran Eye Study. Ophthalmic Epidemiol. 2009;16:187–192. doi: 10.1080/09286580902863031. [DOI] [PubMed] [Google Scholar]
- 5.Javadi MA, Rezaie A, Karimian F, Amini H, Pakravan M, Nouri Mahdavi K, et al. Prevalence of cataract in subjects over 40 years old in Tehran city. Bina J Ophthalmol. 2004;9:309–317. [Google Scholar]
- 6.Rajavi Z, Katibeh M, Ziaei H, Fardesmaeilpour N, Sehat M, Ahmadieh H, et al. Rapid assessment of avoidable blindness in Iran. Ophthalmology. 2011;118:1812–1818. doi: 10.1016/j.ophtha.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 7.Huang W, Huang G, Wang D, Yin Q, Foster PJ, He M. Outcomes of cataract surgery in urban southern China: The Liwan Eye Study. Invest Ophthalmol Vis Sci. 2011;52:16–20. doi: 10.1167/iovs.10-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schein OD, Katz J, Bass EB, Tielsch JM, Lubomski LH, Feldman MA, et al. The value of routine preoperative medical testing before cataract surgery. Study of Medical Testing for Cataract Surgery. N Engl J Med. 2000;342:168–175. doi: 10.1056/NEJM200001203420304. [DOI] [PubMed] [Google Scholar]
- 9.The Royal College of Ophthalmologists. Cataract surgery guidelines. 2010. [Last assessed on 2013 Sep 3]. Available at: http://www.rcophth.ac.uk/core/core_picker/download.asp?lid=544 .
- 10.National Guideline Clearinghouse. Inclusion of Clinical Practice Guidelines in NGC. [Last assessed on 2013 Sep 5]. Available at: http://www.guideline.gov/about/inclusion-criteria.aspx .
- 11.Jacobson PD. Transforming clinical practice guidelines into legislative mandates: Proceed with abundant caution. JAMA. 2008;299:208–210. doi: 10.1001/jama.2007.12. [DOI] [PubMed] [Google Scholar]
- 12. [Last assessed on 2013 Sep 18]. http://www.hbi.ir/NSite/FullStory/News/?Id=1200 .
- 13.Fifth Five Year Development Plan of the Islamic Republic of Iran (1390-1394) [Last assessed on 2013 Sep 07]. Farsi. Available at: http://rc.majlis.ir/fa/law/show/790196 .
- 14.National Guideline Clearinghouse. Cataract in the adult eye. 2006. [Last assessed on 2013 Sep 14]. Available at: http://www.guideline.gov/content.aspx?id=36090 .
- 15.McCarty CA, Mukesh BN, Fu CL, Taylor HR. The epidemiology of cataract in Australia. Am J Ophthalmol. 1999;128:446–465. doi: 10.1016/s0002-9394(99)00218-4. [DOI] [PubMed] [Google Scholar]
- 16.McCarty CA, Nanjan MB, Taylor HR. Attributable risk estimates for cataract to prioritize medical and public health action. Invest Ophthalmol Vis Sci. 2000;41:3720–3725. [PubMed] [Google Scholar]
- 17.McCarty CA, Taylor HR. A review of the epidemiologic evidence linking ultraviolet radiation and cataracts. Dev Ophthalmol. 2002;35:21–31. doi: 10.1159/000060807. [DOI] [PubMed] [Google Scholar]
- 18.Mathew MC, Ervin AM, Tao J, Davis RM. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst Rev. 2012;6:CD004567. doi: 10.1002/14651858.CD004567.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi R, Hayashi S, Arai K, Chikuda M, Obara Y. Effects of antioxidant supplementation on mRNA expression of glucose-6-phosphate dehydrogenase, β-actin and 18S rRNA in the anterior capsule of the lens in cataract patients. Exp Eye Res. 2012;96:48–54. doi: 10.1016/j.exer.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Huang HY, Caballero B, Chang S, Alberg A, Semba R, Schneyer C, et al. Multivitamin/mineral supplements and prevention of chronic disease. Evid Rep Technol Assess (Full Rep) 2006;139:1–117. [PMC free article] [PubMed] [Google Scholar]
- 21.Christen WG, Glynn RJ, Ajani UA, Schaumberg DA, Buring JE, Hennekens CH, et al. Smoking cessation and risk of age-related cataract in men. JAMA. 2000;284:713–716. doi: 10.1001/jama.284.6.713. [DOI] [PubMed] [Google Scholar]
- 22.Christen WG, Manson JE, Seddon JM, Glynn RJ, Buring JE, Rosner B, et al. A prospective study of cigarette smoking and risk of cataract in men. JAMA. 1992;268:989–993. [PubMed] [Google Scholar]
- 23.West S, Munoz B, Emmett EA, Taylor HR. Cigarette smoking and risk of nuclear cataracts. Arch Ophthalmol. 1989;107:1166–1169. doi: 10.1001/archopht.1989.01070020232031. [DOI] [PubMed] [Google Scholar]
- 24.Pederson LL, Baskerville JC, Wanklin JM. Multivariate statistical models for predicting change in smoking behavior following physician advice to quit smoking. Prev Med. 1982;11:536–549. doi: 10.1016/0091-7435(82)90067-6. [DOI] [PubMed] [Google Scholar]
- 25.Ockene JK. Smoking intervention: the expanding role of the physician. Am J Public Health. 1987;77:782–783. doi: 10.2105/ajph.77.7.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smeeth L, Boulis M, Hubbard R, Fletcher AE. A population based case-control study of cataract and inhaled corticosteroids. Br J Ophthalmol. 2003;87:1247–1251. doi: 10.1136/bjo.87.10.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ernst P, Baltzan M, Deschênes J, Suissa S. Low-dose inhaled and nasal corticosteroid use and the risk of cataracts. Eur Respir J. 2006;27:1168–1174. doi: 10.1183/09031936.06.00043005. [DOI] [PubMed] [Google Scholar]
- 28.Klein BE, Klein R, Lee KE, Danforth LG. Drug use and five-year incidence of age-related cataracts: The Beaver Dam Eye Study. Ophthalmology. 2001;108:1670–1674. doi: 10.1016/s0161-6420(01)00656-x. [DOI] [PubMed] [Google Scholar]
- 29.Foody J. Statin use associated with increased risk of cataract, myopathy, liver dysfunction and acute renal failure with varying numbers needed to harm. Evid Based Med. 2010;15:187–188. doi: 10.1136/ebm1103. [DOI] [PubMed] [Google Scholar]
- 30.Klein BE, Klein R, Lee KE. Diabetes, cardiovascular disease, selected cardiovascular disease risk factors, and the 5-year incidence of age-related cataract and progression of lens opacities: The Beaver Dam Eye Study. Am J Ophthalmol. 1998;126:782–790. doi: 10.1016/s0002-9394(98)00280-3. [DOI] [PubMed] [Google Scholar]
- 31.Leske MC, Wu SY, Hennis A, Connell AM, Hyman L, Schachat A. Diabetes, hypertension, and central obesity as cataract risk factors in a black population. The Barbados Eye Study. Ophthalmology. 1999;106:35–41. doi: 10.1016/s0161-6420(99)90003-9. [DOI] [PubMed] [Google Scholar]
- 32.Hennis A, Wu SY, Nemesure B, Leske MC Barbados Eye Studies Group. Risk factors for incident cortical and posterior subcapsular lens opacities in the Barbados Eye Studies. Arch Ophthalmol. 2004;122:525–530. doi: 10.1001/archopht.122.4.525. [DOI] [PubMed] [Google Scholar]
- 33.Subzwari S, Desapriya E, Scime G, Babul S, Jivani K, Pike I. Effectiveness of cataract surgery in reducing driving-related difficulties: A systematic review and meta-analysis. Inj Prev. 2008;14:324–328. doi: 10.1136/ip.2007.017830. [DOI] [PubMed] [Google Scholar]
- 34.Sach TH, Foss AJ, Gregson RM, Zaman A, Osborn F, Masud T, et al. Falls and health status in elderly women following first eye cataract surgery: An economic evaluation conducted alongside a randomised controlled trial. Br J Ophthalmol. 2007;91:1675–1679. doi: 10.1136/bjo.2007.118687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owsley C, McGwin G, Jr, Sloane M, Wells J, Stalvey BT, Gauthreaux S. Impact of cataract surgery on motor vehicle crash involvement by older adults. JAMA. 2002;288:841–849. doi: 10.1001/jama.288.7.841. [DOI] [PubMed] [Google Scholar]
- 36.Gillespie LD, Robertson MC, Gillespie WJ, Lamb SE, Gates S, Cumming RG, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;2:146. doi: 10.1002/14651858.CD007146.pub2. [DOI] [PubMed] [Google Scholar]
- 37.Harwood RH, Foss AJ, Osborn F, Gregson RM, Zaman A, Masud T. Falls and health status in elderly women following first eye cataract surgery: a randomised controlled trial. Br J Ophthalmol. 2005;89:53–59. doi: 10.1136/bjo.2004.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quintana JM, Escobar A, Aróstegui I. Development of appropriateness explicit criteria for cataract extraction by phacoemulsification. BMC Health Serv Res. 2006;6:23. doi: 10.1186/1472-6963-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cutler DM, McClellan M. Is technological change in medicine worth it? Health Aff (Millwood) 2001;20:11–29. doi: 10.1377/hlthaff.20.5.11. [DOI] [PubMed] [Google Scholar]
- 40.Schein OD, Steinberg EP, Cassard SD, Tielsch JM, Javitt JC, Sommer A. Predictors of outcome in patients who underwent cataract surgery. Ophthalmology. 1995;102:817–23. doi: 10.1016/s0161-6420(95)30952-9. [DOI] [PubMed] [Google Scholar]
- 41.Brenner MH, Curbow B, Javitt JC, Legro MW, Sommer A. Vision change and quality of life in the elderly. Response to cataract surgery and treatment of other chronic ocular conditions. Arch Ophthalmol. 1993;111:680–685. doi: 10.1001/archopht.1993.01090050114040. [DOI] [PubMed] [Google Scholar]
- 42.Desai P, Reidy A, Minassian DC, Vafidis G, Bolger J. Gains from cataract surgery: Visual function and quality of life. Br J Ophthalmol. 1996;80:868–873. doi: 10.1136/bjo.80.10.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bilbao A, Quintana JM, Escobar A, García S, Andradas E, Baré M, et al. IRYSS-Cataract Group. Responsiveness and clinically important differences for the VF-14 index, SF-36, and visual acuity in patients undergoing cataract surgery. Ophthalmology. 2009;116:418–424. doi: 10.1016/j.ophtha.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 44.Datta S, Foss AJ, Grainge MJ, Gregson RM, Zaman A, Masud T, et al. The importance of acuity, stereopsis, and contrast sensitivity for health-related quality of life in elderly women with cataracts. Invest Ophthalmol Vis Sci. 2008;49:1–6. doi: 10.1167/iovs.06-1073. [DOI] [PubMed] [Google Scholar]
- 45.Desai P, Reidy A, Minassian DC. Profile of patients presenting for cataract surgery in the UK: National data collection. Br J Ophthalmol. 1999;83:893–896. doi: 10.1136/bjo.83.8.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laidlaw A, Harrad R. Can second eye cataract extraction be justified? Eye (Lond) 1993;7(Pt 5):680–686. doi: 10.1038/eye.1993.155. [DOI] [PubMed] [Google Scholar]
- 47.Henderson BA, Schneider J. Same-day cataract surgery should not be the standard of care for patients with bilateral visually significant cataract. Surv Ophthalmol. 2012;57:580–583. doi: 10.1016/j.survophthal.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 48.Talbot EM, Perkins A. The benefit of second eye cataract surgery. Eye (Lond) 1998;12(Pt 6):983–989. doi: 10.1038/eye.1998.254. [DOI] [PubMed] [Google Scholar]
- 49.Javitt JC, Steinberg EP, Sharkey P, Schein OD, Tielsch JM, Diener M, et al. Cataract surgery in one eye or both. A billion dollar per year issue. Ophthalmology. 1995;102:1583–1592. doi: 10.1016/s0161-6420(95)30824-x. [DOI] [PubMed] [Google Scholar]
- 50.Laidlaw DA, Harrad RA, Hopper CD, Whitaker A, Donovan JL, Brookes ST, et al. Randomised trial of effectiveness of second eye cataract surgery. Lancet. 1998;352:925–929. doi: 10.1016/s0140-6736(97)12536-3. [DOI] [PubMed] [Google Scholar]
- 51.Ozdek SC, Onaran Z, Gürelik G, Konuk O, Tekinşen A, Hasanreisoğlu B. Bilateral endophthalmitis after simultaneous bilateral cataract surgery. J Cataract Refract Surg. 2005;31:1261–1262. doi: 10.1016/j.jcrs.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Kashkouli MB, Salimi S, Aghaee H, Naseripour M. Bilateral Pseudomonas aeruginosa endophthalmitis following bilateral simultaneous cataract surgery. Indian J Ophthalmol. 2007;55:374–375. doi: 10.4103/0301-4738.33825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arshinoff SA, Strube YN, Yagev R. Simultaneous bilateral cataract surgery. J Cataract Refract Surg. 2003;29:1281–1291. doi: 10.1016/s0886-3350(03)00052-x. [DOI] [PubMed] [Google Scholar]
- 54.Pomberg ML, Miller KM. Functional visual outcomes of cataract extraction in monocular versus binocular patients. Am J Ophthalmol. 2004;138:125–132. doi: 10.1016/j.ajo.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 55.Bergwerk KL, Miller KM. Outcomes of cataract surgery in monocular patients. J Cataract Refract Surg. 2000;26:1631–1637. doi: 10.1016/s0886-3350(00)00440-5. [DOI] [PubMed] [Google Scholar]
- 56.Shingleton BJ, Gamell LS, O'Donoghue MW, Baylus SL, King RJ. Long-term changes in intraocular pressure after clear corneal phacoemulsification: Normal patients versus glaucoma suspect and glaucoma patients. J Cataract Refract Surg. 1999;25:885–890. doi: 10.1016/s0886-3350(99)00107-8. [DOI] [PubMed] [Google Scholar]
- 57.Tennen DG, Masket S. Short-and long-term effect of clear corneal incisions on intraocular pressure. J Cataract Refract Surg. 1996;22:568–570. doi: 10.1016/s0886-3350(96)80010-1. [DOI] [PubMed] [Google Scholar]
- 58.Tong JT, Miller KM. Intraocular pressure change after sutureless phacoemulsification and foldable posterior chamber lens implantation. J Cataract Refract Surg. 1998;24:256–262. doi: 10.1016/s0886-3350(98)80208-3. [DOI] [PubMed] [Google Scholar]
- 59.Poley BJ, Lindstrom RL, Samuelson TW, Schulze R., Jr Intraocular pressure reduction after phacoemulsification with intraocular lens implantation in glaucomatous and no glaucomatous eyes: Evaluation of a causal relationship between the natural lens and open-angle glaucoma. J Cataract Refract Surg. 2009;35:1946–1955. doi: 10.1016/j.jcrs.2009.05.061. [DOI] [PubMed] [Google Scholar]
- 60.Vizzeri G, Weinreb RN. Cataract surgery and glaucoma. Curr Opin Ophthalmol. 2010;21:20–24. doi: 10.1097/ICU.0b013e328332f562. [DOI] [PubMed] [Google Scholar]
- 61.Shrivastava A, Singh K. The effect of cataract extraction on intraocular pressure. Curr Opin Ophthalmol. 2010;21:118–122. doi: 10.1097/ICU.0b013e3283360ac3. [DOI] [PubMed] [Google Scholar]
- 62.Hayashi K, Hayashi H, Nakao F, Hayashi F. Effect of cataract surgery on intraocular pressure control in glaucoma patients. J Cataract Refract Surg. 2001;27:1779–1786. doi: 10.1016/s0886-3350(01)01036-7. [DOI] [PubMed] [Google Scholar]
- 63.Friedman DS, Jampel HD, Lubomski LH, Kempen JH, Quigley H, Congdon N, et al. Surgical strategies for coexisting glaucoma and cataract: An evidence-based update. Ophthalmology. 2002;109:1902–1913. doi: 10.1016/s0161-6420(02)01267-8. [DOI] [PubMed] [Google Scholar]
- 64.Muhtaseb M, Kalhoro A, Ionides A. A system for preoperative stratification of cataract patients according to risk of intraoperative complications: A prospective analysis of 1441 cases. Br J Ophthalmol. 2004;88:1242–1246. doi: 10.1136/bjo.2004.046003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Osborne SA, Severn P, Bunce CV, Fraser SG. The use of a pre-operative scoring system for the prediction of phacoemulsification case difficulty and the selection of appropriate cases to be performed by trainees. BMC Ophthalmol. 2006;6:38. doi: 10.1186/1471-2415-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang DF, Campbell JR. Intraoperative floppy iris syndrome associated with tamsulosin. J Cataract Refract Surg. 2005;31:664–673. doi: 10.1016/j.jcrs.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 67.Chang DF, Braga-Mele R, Mamalis N, Masket S, Miller KM, Nichamin LD, et al. ASCRS Cataract Clinical Committee. ASCRS White Paper: Clinical review of intraoperative floppy-iris syndrome. J Cataract Refract Surg. 2008;34:2153–2162. doi: 10.1016/j.jcrs.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 68.Chang DF, Osher RH, Wang L, Koch DD. Prospective multicenter evaluation of cataract surgery in patients taking tamsulosin (Flomax) Ophthalmology. 2007;114:957–964. doi: 10.1016/j.ophtha.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 69.Chang DF, Braga-Mele R, Mamalis N, Masket S, Miller KM, Nichamin LD, et al. ASCRS Cataract Clinical Committee. Clinical experience with intraoperative floppy-iris syndrome. Results of the 2008 ASCRS member survey. J Cataract Refract Surg. 2008;34:1201–1209. doi: 10.1016/j.jcrs.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 70.Bell CM, Hatch WV, Fischer HD, Cernat G, Paterson JM, Gruneir A, et al. Association between tamsulosin and serious ophthalmic adverse events in older men following cataract surgery. JAMA. 2009;301:1991–1996. doi: 10.1001/jama.2009.683. [DOI] [PubMed] [Google Scholar]
- 71.Chatziralli IP, Sergentanis TN. Risk factors for intraoperative floppy iris syndrome: A meta-analysis. Ophthalmology. 2011;118:730–735. doi: 10.1016/j.ophtha.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 72.Katz J, Feldman MA, Bass EB, Lubomski LH, Tielsch JM, Petty BG, et al. Study of medical testing for cataract surgery team. Risks and benefits of anticoagulant and antiplatelet medication use before cataract surgery. Ophthalmology. 2003;110:1784–1788. doi: 10.1016/S0161-6420(03)00785-1. [DOI] [PubMed] [Google Scholar]
- 73.Benzimra JD, Johnston RL, Jaycock P, Galloway PH, Lambert G, Chung AK, et al. The cataract national dataset electronic multicentre audit of 55,567 operations: Antiplatelet and anticoagulant medications. Eye (Lond) 2009;23:10–16. doi: 10.1038/sj.eye.6703069. [DOI] [PubMed] [Google Scholar]
- 74.Jamula E, Anderson J, Douketis JD. Safety of continuing warfarin therapy during cataract surgery: A systematic review and meta-analysis. Thromb Res. 2009;124:292–299. doi: 10.1016/j.thromres.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 75.Dunn AS, Turpie AG. Perioperative management of patients receiving oral anticoagulants: A systematic review. Arch Intern Med. 2003;163:901–908. doi: 10.1001/archinte.163.8.901. [DOI] [PubMed] [Google Scholar]
- 76.Recommendations of American Academy of Ophthalmology Wrong-Site Task Force. 2008. Available at: http://one.aao.org/CE/Practice Guidelines/Patient.aspx .
- 77.de Vries EN, Prins HA, Crolla RM, den Outer AJ, van Andel G, van Helden SH, et al. Effect of a comprehensive surgical safety system on patient outcomes. N Engl J Med. 2010;363:1928–1937. doi: 10.1056/NEJMsa0911535. [DOI] [PubMed] [Google Scholar]
- 78.Kelly SP, Jalil A. Wrong intraocular lens implant; learning from reported patient safety incidents. Eye (Lond) 2011;25:730–734. doi: 10.1038/eye.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stahel PF, Sabel AL, Victoroff MS, Varnell J, Lembitz A, Boyle DJ, et al. Wrong-site and wrong-patient procedures in the universal protocol era: Analysis of a prospective database of physician self-reported occurrences. Arch Surg. 2010;145:978–984. doi: 10.1001/archsurg.2010.185. [DOI] [PubMed] [Google Scholar]
- 80.Pennsylvania Patient Safety Authority. The evidence base for the principles for reliable performance of the Universal Protocol. 2010. [Last assessed on 2013 July 20]. Available at: www.patientsafetyauthority.org/EducationalTools/PatientSafetyTools/PWSS/Documents/u_principles.pdf .
- 81.Pennsylvania Patient Safety Authority. Quarterly update: the evidence base for best practices for preventing wrong-site surgery. Pa Patient SafAdvis [online] 2010. Dec, [Last assessed on 2011 July 20]. Available at: www.patientsafetyauthority.org/ADVISORIES/AdvisoryLibrary/2010/Dec7%284%29/Pages/151.aspx .
- 82.Schein OD, Katz J, Bass EB, Tielsch JM, Lubomski LH, Feldman MA, et al. The value of routine preoperative medical testing before cataract surgery. Study of Medical Testing for Cataract Surgery. N Engl J Med. 2000;342:168–175. doi: 10.1056/NEJM200001203420304. [DOI] [PubMed] [Google Scholar]
- 83.Keay L, Lindsley K, Tielsch J, Katz J, Schein O. Routine preoperative medical testing for cataract surgery. Cochrane Database Syst Rev. 2009:CD007293. doi: 10.1002/14651858.CD007293.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Knox Cartwright NE, Johnston RL, Jaycock PD, Tole DM, Sparrow JM. The Cataract National Dataset electronic multicentre audit of 55,567 operations: When should IOLMaster biometric measurements be rechecked? Eye (Lond) 2010;24:894–900. doi: 10.1038/eye.2009.196. [DOI] [PubMed] [Google Scholar]
- 85.Hashemi H, Mohammadi SF, Majdi M, KhabazKhoob M, Zare Mehrjerdi H, Mazori A, et al. Residual refractive errors following cataract surgery and its determinants. Bina J Ophthalmol. 2010;15:263–273. [Google Scholar]
- 86.Schelenz J, Kammann J. Comparison of contact and immersion techniques for axial length measurement and implant power calculation. J Cataract Refract Surg. 1989;15:425–428. doi: 10.1016/s0886-3350(89)80062-8. [DOI] [PubMed] [Google Scholar]
- 87.Packer M, Fine IH, Hoffman RS, Coffman PG, Brown LK. Immersion A-scan compared with partial coherence interferometry: Outcomes analysis. J Cataract Refract Surg. 2002;28:239–242. doi: 10.1016/s0886-3350(01)01259-7. [DOI] [PubMed] [Google Scholar]
- 88.Narváez J, Zimmerman G, Stulting RD, Chang DH. Accuracy of intraocular lens power prediction using the Hoffer Q, Holladay 1, Holladay 2, and SRK/T formulas. J Cataract Refract Surg. 2006;32:2050–2053. doi: 10.1016/j.jcrs.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 89.Aramberri J. Intraocular lens power calculation after corneal refractive surgery: Double-K method. J Cataract Refract Surg. 2003;29:2063–2068. doi: 10.1016/s0886-3350(03)00957-x. [DOI] [PubMed] [Google Scholar]
- 90.Nishi O, Nishi K, Wickström K. Preventing lens epithelial cell migration using intraocular lenses with sharp rectangular edges. J Cataract Refract Surg. 2000;26:1543–1549. doi: 10.1016/s0886-3350(00)00426-0. [DOI] [PubMed] [Google Scholar]
- 91.Meacock WR, Spalton DJ, Boyce JF, Jose RM. Effect of optic size on posterior capsule opacification: 5.5 mm versus 6.0 mm AcrySof intraocular lenses. J Cataract Refract Surg. 2001;27:1194–1198. doi: 10.1016/s0886-3350(01)00855-0. [DOI] [PubMed] [Google Scholar]
- 92.Meacock WR, Spalton DJ. Effect of intraocular lens haptic compressibility on the posterior lens capsule after cataract surgery. J Cataract Refract Surg. 2001;27:1366–1371. doi: 10.1016/s0886-3350(01)01024-0. [DOI] [PubMed] [Google Scholar]
- 93.Hollick EJ, Spalton DJ, Meacock WR. The effect of capsulorhexis size on posterior capsular opacification: One-year results of a randomized prospective trial. Am J Ophthalmol. 1999;128:271–279. doi: 10.1016/s0002-9394(99)00157-9. [DOI] [PubMed] [Google Scholar]
- 94.Boyce JF, Bhermi GS, Spalton DJ, El-Osta AR. Mathematical modeling of the forces between an intraocular lens and the capsule. J Cataract Refract Surg. 2002;28:1853–1859. doi: 10.1016/s0886-3350(02)01490-6. [DOI] [PubMed] [Google Scholar]
- 95.Apple DJ, Peng Q, Visessook N, Werner L, Pandey SK, Escobar-Gomez M, et al. Eradication of posterior capsule opacification: documentation of a marked decrease in Nd:YAG laser posterior capsulotomy rates noted in an analysis of 5416 pseudophakic human eyes obtained postmortem. Ophthalmology. 2001;108:505–518. doi: 10.1016/s0161-6420(00)00589-3. [DOI] [PubMed] [Google Scholar]
- 96.Meacock WR, Spalton DJ, Boyce J, Marshall J. The effect of posterior capsule opacification on visual function. Invest Ophthalmol Vis Sci. 2003;44:4665–4669. doi: 10.1167/iovs.02-0634. [DOI] [PubMed] [Google Scholar]
- 97.Channell MM, Beckman H. Intraocular pressure changes after neodymium-YAG laser posterior capsulotomy. Arch Ophthalmol. 1984;102:1024–1026. doi: 10.1001/archopht.1984.01040030826025. [DOI] [PubMed] [Google Scholar]
- 98.Steinert RF, Puliafito CA, Kumar SR, Dudak SD, Patel S. Cystoid macular edema, retinal detachment, and glaucoma after Nd:YAG laser posterior capsulotomy. Am J Ophthalmol. 1991;112:373–380. doi: 10.1016/s0002-9394(14)76242-7. [DOI] [PubMed] [Google Scholar]
- 99.Tetz MR, Apple DJ, Price FW, Jr, Piest KL, Kincaid MC, Bath PE. A newly described complication of neodymium-YAG laser capsulotomy: Exacerbation of an intraocular infection. Case report. Arch Ophthalmol. 1987;105:1324–1325. doi: 10.1001/archopht.1987.01060100026011. [DOI] [PubMed] [Google Scholar]
- 100.Olsen GM, Olson RJ. Prospective study of cataract surgery, capsulotomy, and retinal detachment. J Cataract Refract Surg. 1995;21:136–139. doi: 10.1016/s0886-3350(13)80500-7. [DOI] [PubMed] [Google Scholar]
- 101.Koch DD, Liu JF, Gill EP, Parke DW., 2nd Axial myopia increases the risk of retinal complications after neodymium-YAG laser posterior capsulotomy. Arch Ophthalmol. 1989;107:986–990. doi: 10.1001/archopht.1989.01070020048027. [DOI] [PubMed] [Google Scholar]
- 102.Mayer E, Cadman D, Ewings P, Twomey JM, Gray RH, Claridge KG, et al. A 10 year retrospective survey of cataract surgery and endophthalmitis in a single eye unit: injectable lenses lower the incidence of endophthalmitis. Br J Ophthalmol. 2003;87:867–869. doi: 10.1136/bjo.87.7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vasavada AR, Dholakia SA, Raj SM, Singh R. Effect of cortical cleaving hydrodissection on posterior capsule opacification in age-related nuclear cataract. J Cataract Refract Surg. 2006;32:1196–1200. doi: 10.1016/j.jcrs.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 104.Cheng JW, Wei RL, Cai JP, Xi GL, Zhu H, Li Y, et al. Efficacy of different intraocular lens materials and optic edge designs in preventing posterior capsular opacification: A meta-analysis. Am J Ophthalmol. 2007;143:428–436. doi: 10.1016/j.ajo.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 105.Wren SM, Spalton DJ, Jose R, Boyce J, Heatley CJ. Factors that influence the development of posterior capsule opacification with a polyacrylic intraocular lens. Am J Ophthalmol. 2005;139:691–695. doi: 10.1016/j.ajo.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 106.Findl O, Buehl W, Menapace R, Sacu S, Georgopoulos M, Rainer G. Long-term effect of sharp optic edges of a polymethyl methacrylate intraocular lens on posterior capsule opacification: A randomized trial. Ophthalmology. 2005;112:2004–2008. doi: 10.1016/j.ophtha.2005.06.021. Epub 2005 Sep 15. [DOI] [PubMed] [Google Scholar]
- 107.Heatley CJ, Spalton DJ, Kumar A, Jose R, Boyce J, Bender LE. Comparison of posterior capsule opacification rates between hydrophilic and hydrophobic single-piece acrylic intraocular lenses. J Cataract Refract Surg. 2005;31:718–724. doi: 10.1016/j.jcrs.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 108.Sacu S, Menapace R, Findl O, Kiss B, Buehl W, Georgopoulos M. Long-term efficacy of adding a sharp posterior optic edge to a three-piece silicone intraocular lens on capsule opacification: Five-year results of a randomized study. Am J Ophthalmol. 2005;139:696–703. doi: 10.1016/j.ajo.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 109.Findl O, Buehl W, Bauer P, Sycha T. Interventions for preventing posterior capsule opacification. Cochrane Database Syst Rev. 2010:CD003738. doi: 10.1002/14651858.CD003738.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Packer M, Fine IH, Hoffman RS, Piers PA. Improved functional vision with a modified prolate intraocular lens. J Cataract Refract Surg. 2004;30:986–992. doi: 10.1016/j.jcrs.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 111.Cuthbertson FM, Dhingra S, Benjamin L. Objective and subjective outcomes in comparing three different aspheric intraocular lens implants with their spherical counterparts. Eye (Lond) 2009;23:877–883. doi: 10.1038/eye.2008.122. [DOI] [PubMed] [Google Scholar]
- 112.Kamlesh, Dadeya S, Kaushik S. Contrast sensitivity and depth of focus with aspheric multifocal versus conventional monofocal intraocular lens. Can J Ophthalmol. 2001;36:197–201. doi: 10.1016/s0008-4182(01)80040-5. [DOI] [PubMed] [Google Scholar]
- 113.Takakura A, Iyer P, Adams JR, Pepin SM. Functional assessment of accommodating intraocular lenses versus monofocal intraocular lenses in cataract surgery: Metaanalysis. J Cataract Refract Surg. 2010;36:380–388. doi: 10.1016/j.jcrs.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 114.Leyland M, Pringle E. Multifocal versus monofocal intraocular lenses after cataract extraction. Cochrane Database Syst Rev. 2006:CD003169. doi: 10.1002/14651858.CD003169.pub2. [DOI] [PubMed] [Google Scholar]
- 115.Interventional procedure overview of the implantation of accommodating intraocular lenses during cataract surgery. [Last assessed on 2013 Sep 5]. Available at: http://www.nice.org.uk/nicemedia/live/11284/31693/31693.pdf .
- 116.Zhu XF, Zou HD, Yu YF, Sun Q, Zhao NQ. Comparison of blue light-filtering IOLs and UV light-filtering IOLs for cataract surgery: A meta-analysis. PLoS One. 2012;7:e33013. doi: 10.1371/journal.pone.0033013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schmack I, Schimpf M, Stolzenberg A, Conrad-Hengerer I, Hengerer FH, Dick HB. Visual quality assessment in patients with orange-tinted blue light-filtering and clear ultraviolet light-filtering intraocular lenses. J Cataract Refract Surg. 2012;38:823–832. doi: 10.1016/j.jcrs.2011.12.028. [DOI] [PubMed] [Google Scholar]
- 118.Desai P, Reidy A, Minassian DC. Profile of patients presenting for cataract surgery in the UK: National data collection. Br J Ophthalmol. 1999;83:893–896. doi: 10.1136/bjo.83.8.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Waiting for cataracts. [Last assessed on 2013 Sep 10]. Available at: www.medicine.ox.ac.uk/bandolier/journal.2007 .
- 120.Hodge W, Horsley T, Albiani D, Baryla J, Belliveau M, Buhrmann R, et al. The consequences of waiting for cataract surgery: A systematic review. CMAJ. 2007;176:1285–1290. doi: 10.1503/cmaj.060962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Conner-Spady B, Sanmartin C, Sanmugasunderam S, De Coster C, Lorenzetti D, McLaren L, et al. A systematic literature review of the evidence on benchmarks for cataract surgery waiting time. Can J Ophthalmol. 2007;42:543–551. [PubMed] [Google Scholar]
- 122.Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9:CD007146. doi: 10.1002/14651858.CD007146.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Church J, Goodall S, Norman R, Haas M. An economic evaluation of community and residential aged care falls prevention strategies in NSW. N S W Public Health Bull. 2011;22:60–68. doi: 10.1071/NB10051. [DOI] [PubMed] [Google Scholar]
- 124.Hodge W, Horsley T, Albiani D, Baryla J, Belliveau M, Buhrmann R, et al. The consequences of waiting for cataract surgery: a systematic review. CMAJ. 2007;176:1285–1290. doi: 10.1503/cmaj.060962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Desapriya E, Subzwari S, Scime-Beltrano G, Samayawardhena LA, Pike I. Vision improvement and reduction in falls after expedited cataract surgery Systematic review and metaanalysis. J Cataract Refract Surg. 2010;36:13–19. doi: 10.1016/j.jcrs.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 126.Mittra RA, Borrillo JL, Dev S, Mieler WF, Koenig SB. Retinopathy progression and visual outcomes after phacoemulsification in patients with diabetes mellitus. Arch Ophthalmol. 2000;118:912–917. [PubMed] [Google Scholar]
- 127.Antcliff RJ, Poulson A, Flanagan DW. Phacoemulsification in diabetics. Eye (Lond) 1996;10:737–741. doi: 10.1038/eye.1996.171. [DOI] [PubMed] [Google Scholar]
- 128.Lahey JM, Francis RR, Kearney JJ. Combining phacoemulsification with pars plana vitrectomy in patients with proliferative diabetic retinopathy: A series of 223 cases. Ophthalmology. 2003;110:1335–1339. doi: 10.1016/S0161-6420(03)00454-8. [DOI] [PubMed] [Google Scholar]
- 129.Powe NR, Schein OD, Gieser SC, Tielsch JM, Luthra R, Javitt J, et al. Synthesis of the literature on visual acuity and complications following cataract extraction with intraocular lens implantation. Cataract Patient Outcome Research Team. Arch Ophthalmol. 1994;112:239–252. doi: 10.1001/archopht.1994.01090140115033. [DOI] [PubMed] [Google Scholar]
- 130.Clark A, Morlet N, Ng JQ, Preen DB, Semmens JB. Risk for retinal detachment after phacoemulsification: A whole-population study of cataract surgery outcomes. Arch Ophthalmol. 2012;130:882–888. doi: 10.1001/archophthalmol.2012.164. [DOI] [PubMed] [Google Scholar]
- 131.Haug SJ, Bhisitkul RB. Risk factors for retinal detachment following cataract surgery. Curr Opin Ophthalmol. 2012;23:7–11. doi: 10.1097/ICU.0b013e32834cd653. [DOI] [PubMed] [Google Scholar]
- 132.Küchle M, Viestenz A, Martus P, Händel A, Jünemann A, Naumann GO. Anterior chamber depth and complications during cataract surgery in eyes with pseudoexfoliation syndrome. Am J Ophthalmol. 2000;129:281–285. doi: 10.1016/s0002-9394(99)00365-7. [DOI] [PubMed] [Google Scholar]
- 133.Husain R, Liang S, Foster PJ, Gazzard G, Bunce C, Chew PT, et al. Cataract surgery after trabeculectomy: The effect on trabeculectomy function. Arch Ophthalmol. 2012;130:165–170. doi: 10.1001/archophthalmol.2011.329. [DOI] [PubMed] [Google Scholar]
- 134.Wang JJ, Klein R, Smith W, Klein BE, Tomany S, Mitchell P. Cataract surgery and the 5-year incidence of late-stage age-related maculopathy: pooled findings from the Beaver Dam and Blue Mountains eye studies. Ophthalmology. 2003;110:1960–1967. doi: 10.1016/s0161-6420(03)00816-9. [DOI] [PubMed] [Google Scholar]
- 135.Casparis H, Lindsley K, Kuo IC, Sikder S, Bressler NB. Surgery for cataracts in people with age-related macular degeneration. Database Syst Rev. 2012 J;6:CD006757. doi: 10.1002/14651858.CD006757.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Klein BE, Howard KP, Lee KE, Iyengar SK, Sivakumaran TA, Klein R. The Relationship of Cataract and Cataract Extraction to Age-related Macular Degeneration: The Beaver Dam Eye Study. Ophthalmology. 2012;119:1628–1633. doi: 10.1016/j.ophtha.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ho L, Boekhoorn SS, Liana, van Duijn CM, Uitterlinden AG, Hofman A, et al. Cataract surgery and the risk of aging macula disorder: The rotterdam study. Invest Ophthalmol Vis Sci. 2008;49:4795–4800. doi: 10.1167/iovs.08-2066. [DOI] [PubMed] [Google Scholar]
- 138.Speaker MG, Milch FA, Shah MK, Eisner W, Kreiswirth BN. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology. 1991;98:639–649. doi: 10.1016/s0161-6420(91)32239-5. [DOI] [PubMed] [Google Scholar]
- 139.Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98:1769–1775. doi: 10.1016/s0161-6420(91)32052-9. [DOI] [PubMed] [Google Scholar]
- 140.Ciulla TA, Starr MB, Masket S. Bacterial endophthalmitis prophylaxis for cataract surgery: An evidence-based update. Ophthalmology. 2002;109:13–24. doi: 10.1016/s0161-6420(01)00899-5. [DOI] [PubMed] [Google Scholar]
- 141.Wu PC, Li M, Chang SJ, Teng MC, Yow SG, Shin SJ, et al. Risk of endophthalmitis after cataract surgery using different protocols for povidone- iodine preoperative disinfection. J Ocul Pharmacol Ther. 2006;22:54–61. doi: 10.1089/jop.2006.22.54. [DOI] [PubMed] [Google Scholar]
- 142.Kelly SP, Mathews D, Mathews J, Vail A. Reflective consideration of postoperative endophthalmitis as a quality marker. Eye (Lond) 2007;21:1419–1426. doi: 10.1038/sj.eye.6701996. [DOI] [PubMed] [Google Scholar]
- 143.Kamalarajah S, Silvestri G, Sharma N, Khan A, Foot B, Ling RC, et al. Surveillance of endophthalmitis following cataract surgery in the UK. Eye (Lond) 2004;18:580–587. doi: 10.1038/sj.eye.6700645. [DOI] [PubMed] [Google Scholar]
- 144.Davison M, Padroni S, Bunce C, Rüschen H. Sub-Tenon's anaesthesia versus topical anaesthesia for cataract surgery. Cochrane Database Syst Rev. 2007;3:CD006291. doi: 10.1002/14651858.CD006291.pub2. [DOI] [PubMed] [Google Scholar]
- 145.Ezra DG, Allan BD. Topical anaesthesia alone versus topical anaesthesia with intracameral lidocaine for phacoemulsification. Cochrane Database Syst Rev. 2007;3:CD005276. doi: 10.1002/14651858.CD005276.pub2. [DOI] [PubMed] [Google Scholar]
- 146.Alhassan MB, Kyari F, Ejere HO. Peribulbar versus retrobulbar anaesthesia for cataract surgery. Cochrane Database Syst Rev. 2008;3:CD004083. doi: 10.1002/14651858.CD004083.pub2. [DOI] [PubMed] [Google Scholar]
- 147.OD Schein, DS Friedman, LA Fleisher, LH Lubomski, J Magaziner, M Sprintz, et al. Anesthesia Management During Cataract Surgery: Summary. [Last Assessed on 2013 Sep 13]. Available at: http://www.ncbi.nlm.nih.gov/books/NBK11949 .
- 148.Eke T, Thompson JR. Serious complications of local anaesthesia for cataract surgery: A 1 year national survey in the United Kingdom. Br J Ophthalmol. 2007;91:470–475. doi: 10.1136/bjo.2006.106005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhao LQ, Zhu H, Zhao PQ, Wu QR, Hu YQ. Topical anesthesia versus regional anesthesia for cataract surgery: A meta-analysis of randomized controlled trials. Ophthalmology. 2012;119:659–667. doi: 10.1016/j.ophtha.2011.09.056. [DOI] [PubMed] [Google Scholar]
- 150.Minassian DC, Rosen P, Dart JK, Reidy A, Desai P, Sidhu M, et al. Extracapsular cataract extraction compared with small incision surgery by phacoemulsification: A randomised trial. Br J Ophthalmol. 2001;85:822–829. doi: 10.1136/bjo.85.7.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yasmin Riaz, Aeesha NJ Malik, Jennifer R. Evans Phacoemulsification with posterior chamber intraocular lens versus manual small incision cataract surgery (MSICS) with posterior chamber intraocular lens for age-related. Cochrane Database Syst Rev. 2012;4:CD008811. doi: 10.1002/14651858.CD008811.pub2. [DOI] [PubMed] [Google Scholar]
- 152.Yasmin Riaz, Jod S Mehta, Richard Wormald, Jennifer R Evans, Allen Foster, Thulasiraj Ravilla, et al. Surgical interventions for age-related cataract. Cochrane Database Syst Rev. 2006;4:CD001323. doi: 10.1002/14651858.CD001323.pub2. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hayashi K, Hayashi H, Nakao F, Hayashi F. The correlation between incision size and corneal shape changes in sutureless cataract surgery. Ophthalmology. 1995;102:550–556. doi: 10.1016/s0161-6420(95)30983-9. [DOI] [PubMed] [Google Scholar]
- 154.Ang M, Evans JR, Mehta JS. Manual small incision cataract surgery (MSICS) with posterior chamber intraocular lens versus extracapsular cataract extraction (ECCE) with posterior chamber intraocular lens for age-related cataract. Cochrane Database Syst Rev. 2012;4:CD008811. doi: 10.1002/14651858.CD008811.pub2. [DOI] [PubMed] [Google Scholar]
- 155.Oshika T, Nagahara K, Yaguchi S, Emi K, Takenaka H, Tsuboi S, et al. Three year prospective, randomized evaluation of intraocular lens implantation through 3.2 and 5.5 mm incisions. J Cataract Refract Surg. 1998;24:509–514. doi: 10.1016/s0886-3350(98)80293-9. [DOI] [PubMed] [Google Scholar]
- 156.Jacobs DS, Cox TA, Wagoner MD, Ariyasu RG, Karp CL. Capsule staining as an adjunct to cataract surgery: A report from the American Academy of Ophthalmology. Ophthalmology. 2006;113:707–713. doi: 10.1016/j.ophtha.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 157.Hollick EJ, Spalton DJ, Meacock WR. The effect of capsulorhexis size on posterior capsular opacification: one-year results of a randomized prospective trial. Am J Ophthalmol. 1999;128:271–279. doi: 10.1016/s0002-9394(99)00157-9. [DOI] [PubMed] [Google Scholar]
- 158.Vasavada AR, Dholakia SA, Raj SM, Singh R. Effect of cortical cleaving hydrodissection on posterior capsule opacification in age-related nuclear cataract. J Cataract Refract Surg. 2006;32:1196–2000. doi: 10.1016/j.jcrs.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 159.Lorente R, de Rojas V, Vázquez de Parga P, Moreno C, Varela J, Landaluce ML, et al. Intracameral Phenylephrine 1.5% for Prophylaxis against Intraoperative Floppy Iris Syndrome: Prospective, Randomized Fellow Eye Study. Ophthalmology. 2012;119:2053–2058. doi: 10.1016/j.ophtha.2012.04.028. doi: 10.1016/j.ophtha.2012.04.028. Epub 2012 Jun 17. [DOI] [PubMed] [Google Scholar]
- 160.Gupta S, Sharma A, Bansal N. Intra-operative floppy iris syndrome. J Indian Med Assoc. 2011;109:740–741. [PubMed] [Google Scholar]
- 161.Theodossiadis PG, Achtsidis V, Theodoropoulou S, Tentolouris N, Komninos C, Fountas KN. The effect of alpha antagonists on pupil dynamics: Implications for the diagnosis of intraoperative floppy iris syndrome. Am J Ophthalmol. 2012;153:620–626. doi: 10.1016/j.ajo.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 162.Santaella RM, Destafeno JJ, Stinnett SS, Proia AD, Chang DF, Kim T. The effect of alpha1-adrenergic receptor antagonist tamsulosin (Flomax) on iris dilator smooth muscle anatomy. Ophthalmology. 2010;117:1743–1749. doi: 10.1016/j.ophtha.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 163.Ford RL, Sallam A, Towler HM. Intraoperative floppy iris syndrome associated with risperidone intake. Eur J Ophthalmol. 2011;21:210–211. doi: 10.5301/ejo.2010.4698. [DOI] [PubMed] [Google Scholar]
- 164.Chen AA, Kelly JP, Bhandari A, Wu MC. Pharmacologic prophylaxis and risk factors for intraoperative floppy-iris syndrome in phacoemulsification performed by resident physicians. J Cataract Refract Surg. 2010;36:898–905. doi: 10.1016/j.jcrs.2009.12.039. [DOI] [PubMed] [Google Scholar]
- 165.Akman A, Yilmaz G, Oto S, Akova YA. Comparison of various pupil dilatation methods for phacoemulsification in eyes with a small pupil secondary to pseudoexfoliation. Ophthalmology. 2004;111:1693–1698. doi: 10.1016/j.ophtha.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 166.Park YG, Chung SH, Joo CK. Comparison of microcoaxial with standard clear corneal incisions in torsional handpiece cataract surgery. Ophthalmologica. 2012;227:55–59. doi: 10.1159/000329599. [DOI] [PubMed] [Google Scholar]
- 167.Vasavada AR, Vasavada V, Vasavada VA, Praveen MR, Johar SR, Gajjar D, et al. Comparison of the effect of torsional and microburst longitudinal ultrasound on clear corneal incisions during phacoemulsification. J Cataract Refract Surg. 2012;38:833–839. doi: 10.1016/j.jcrs.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 168.Vasavada AR, Raj SM, Patel U, Vasavada V, Vasavada V. Comparison of torsional and microburst longitudinal phacoemulsification: A prospective, randomized, masked clinical trial. Ophthalmic Surg Lasers Imaging. 2010;41:109–114. doi: 10.3928/15428877-20091230-20. [DOI] [PubMed] [Google Scholar]
- 169.Rekas M, Montés-Micó R, Krix-Jachym K, Kluś A, Stankiewicz A, Ferrer-Blasco T. Comparison of torsional and longitudinal modes using phacoemulsification parameters. J Cataract Refract Surg. 2009;35:1719–1724. doi: 10.1016/j.jcrs.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 170.Fakhry MA, El Shazly MI. Torsional ultrasound mode versus combined torsional and conventional ultrasound mode phacoemulsification for eyes with hard cataract. Clin Ophthalmol. 2011;5:973–978. doi: 10.2147/OPTH.S22879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Liu Y, Zeng M, Liu X, Luo L, Yuan Z, Xia Y, et al. Torsional mode versus conventional ultrasound mode phacoemulsification: randomized comparative clinical study. J Cataract Refract Surg. 2007;33:287–292. doi: 10.1016/j.jcrs.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 172.Bayraktar S, Altan T, Küçüksümer Y, Yilmaz OF. Capsular tension ring implantation after capsulorhexis in phacoemulsification of cataracts associated with pseudoexfoliation syndrome. Intraoperative complications and early postoperative findings. J Cataract Refract Surg. 2001;27:1620–1628. doi: 10.1016/s0886-3350(01)00965-8. [DOI] [PubMed] [Google Scholar]
- 173.Mayer E, Cadman D, Ewings P, Twomey JM, Gray RH, Claridge KG, et al. A 10 year retrospective survey of cataract surgery and endophthalmitis in a single eye unit: Injectable lenses lower the incidence of endophthalmitis. Br J Ophthalmol. 2003;87:867–869. doi: 10.1136/bjo.87.7.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chang DF, Masket S, Miller KM, Braga-Mele R, Little BC, Mamalis N, et al. ASCRS Cataract Clinical Committee. Complications of sulcus placement of single-piece acrylic intraocular lenses: Recommendations for backup IOL implantation following posterior capsule rupture. J Cataract Refract Surg. 2009;35:1445–1458. doi: 10.1016/j.jcrs.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 175.Bayramlar H, Hepsen IF, Yilmaz H. Myopic shift from the predicted refraction after sulcus fixation of PMMA posterior chamber intraocular lenses. Can J Ophthalmol. 2006;41:78–82. doi: 10.1016/S0008-4182(06)80072-4. [DOI] [PubMed] [Google Scholar]
- 176.Gimbel HV, DeBroff BM. Intraocular lens optic capture. J Cataract Refract Surg. 2004;30:200–206. doi: 10.1016/j.jcrs.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 177.Altmann GE, Nichamin LD, Lane SS, Pepose JS. Optical performance of 3 intraocular lens designs in the presence of decentration. J Cataract Refract Surg. 2005;31:574–585. doi: 10.1016/j.jcrs.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 178.Wang L, Koch DD. Effect of decentration of wavefront-corrected intraocular lenses on the higher-order aberrations of the eye. Arch Ophthalmol. 2005;123:1226–1230. doi: 10.1001/archopht.123.9.1226. [DOI] [PubMed] [Google Scholar]
- 179.Wagoner MD, Cox TA, Ariyasu RG, Jacobs DS, Karp CL. American Academy of Ophthalmology Intraocular lens implantation in the absence of capsular support: A report by the American Academy of Ophthalmology. Ophthalmology. 2003;110:840–859. doi: 10.1016/s0161-6420(02)02000-6. [DOI] [PubMed] [Google Scholar]
- 180.Vazirani J, Basu S. Role of topical, subconjunctival, intracameral, and irrigative antibiotics in cataract surgery. Curr Opin Ophthalmol. 2013;24:60–65. doi: 10.1097/ICU.0b013e32835a93be. [DOI] [PubMed] [Google Scholar]
- 181.Barry P, Seal DV, Gettinby G, Lees F, Peterson M, Revie CW ESCRS Endophthalmitis Study Group. ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery: Preliminary report of principal results from a European multicenter study. J Cataract Refract Surg. 2006;32:709. doi: 10.1016/j.jcrs.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 182.Jensen MK, Fiscella RG, Crandall AS, Moshirfar M, Mooney B, Wallin T, et al. A retrospective study of endophtalmitis rates comparing quinolone antibiotics. Am J Ophthalmol. 2005;139:141–148. doi: 10.1016/j.ajo.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 183.Jensen MK, Fiscella RG, Moshirfar M, Mooney B. Third-and fourth-generation fluoroquinolones: Retrospective comparison of endophthalmitis after cataract surgery performed over 10 years. J Cataract Refract Surg. 2008;34:1460–1467. doi: 10.1016/j.jcrs.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 184.Ng JQ, Morlet N, Bulsara MK, Semmens JB. Reducing the risk for endophthalmitis after cataract surgery: Population-based nested case-control study: Endophthalmitis population study of Western Australia sixth report. J Cataract Refract Surg. 2007;33:269–280. doi: 10.1016/j.jcrs.2006.10.067. [DOI] [PubMed] [Google Scholar]
- 185.Romero-Aroca P, Méndez-Marin I, Salvat-Serra M, Fernández-Ballart J, Almena-Garcia M, Reyes-Torres J. Results at seven years after the use of intracamerular cefazolin as an endophthalmitis prophylaxis in cataract surgery. BMC Ophthalmology. 2012;12:2. doi: 10.1186/1471-2415-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shorstein NH, Winthrop KL, Herrinton LJ. Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a Northern California eye department. J Cataract Refract Surg. 2013;39:8–14. doi: 10.1016/j.jcrs.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 187.Yu-Wai-Man P, Morgan SJ, Hildreth AJ, Steel DH, Allen D. Efficacy of intracameral and subconjunctival cefuroxime in preventing endophthalmitis after cataract surgery. J Cataract Refract Surg. 2008;34:447–451. doi: 10.1016/j.jcrs.2007.10.041. [DOI] [PubMed] [Google Scholar]
- 188.Sivaprasad S, Bunce C, Crosby-Nwaobi R. Non-steroidal anti-inflammatory agents for treating cystoid macular oedema following cataract surgery. Cochrane Database Syst Rev. 2012;2:CD004239. doi: 10.1002/14651858.CD004239.pub3. Review. [DOI] [PubMed] [Google Scholar]
- 189.Abeysiri P, Wormald R, Bunce C. Prophylactic non-steroidal anti-inflammatory agents for the prevention of cystoid macular oedema after cataract surgery. Cochrane Database Syst Rev. 2011 CD006683pub2. [Google Scholar]
- 190.Tuft SJ, Minassian D, Sullivan P. Risk factors for retinal detachment after cataract surgery: A case-control study. Ophthalmology. 2006;113:650–656. doi: 10.1016/j.ophtha.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 191.Recchia FM, Busbee BG, Pearlman RB, Carvalho-Recchia CA, Ho AC. Changing trends in the microbiologic aspects of postcataract endophthalmitis. Arch Ophthalmol. 2005;123:341–346. doi: 10.1001/archopht.123.3.341. [DOI] [PubMed] [Google Scholar]
- 192.Mollan SP, Gao A, Lockwood A, Durrani OM, Butler L. Postcataract endophthalmitis: Incidence and microbial isolates in a United Kingdom region from 1996 through 2004. J Cataract Refract Surg. 2007;33:265–268. doi: 10.1016/j.jcrs.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 193.Deramo VA, Lai JC, Winokur J, Luchs J, Udell IJ. Visual outcome and bacterial sensitivity after methicillin-resistant Staphylococcus aureus-associated acute endophthalmitis. Am J Ophthalmol. 2008;145:413–417. doi: 10.1016/j.ajo.2007.10.020. Epub 2008 Jan 11. [DOI] [PubMed] [Google Scholar]
- 194.Deramo VA, Lai JC, Fastenberg DM, Udell IJ. Acute endophthalmitis in eyes treated prophylactically with gatifloxacin and moxifloxacin. Am J Ophthalmol. 2006;142:721–725. doi: 10.1016/j.ajo.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 195.Recchia FM, Busbee BG, Pearlman RB, Carvalho-Recchia CA, Ho AC. Changing trends in the microbiologic aspects of postcataract endophthalmitis. Arch Ophthalmol. 2005;123:341–346. doi: 10.1001/archopht.123.3.341. [DOI] [PubMed] [Google Scholar]
- 196.Altan T, Acar N, Kapran Z, Unver YB, Yurttaser S, Küçüksümer Y, et al. Acute-onset endophthalmitis after cataract surgery: Success of initial therapy, visual outcomes, and related factors. Retina. 2009;29:606–612. doi: 10.1097/IAE.0b013e3181953a31. [DOI] [PubMed] [Google Scholar]
- 197.Olson R, Donnenfeld E, Bucci FA, Jr, Price FW, Jr, Raizman M, Solomon K, et al. Methicillin resistance of Staphylococcus species among health care and nonhealth care workers undergoing cataract surgery. Clin Ophthalmol. 2010;4:1505–1514. doi: 10.2147/OPTH.S14333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cutler Peck CM, Brubaker J, Clouser S, Danford C, Edelhauser HE, Mamalis N. Toxic anterior segment syndrome: common causes. J Cataract Refract Surg. 2010;36:1073–1080. doi: 10.1016/j.jcrs.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 199.Sengupta S, Chang DF, Gandhi R, Kenia H, Venkatesh R. Incidence and long-term outcomes of toxic anterior segment syndrome at Aravind Eye Hospital. J Cataract Refract Surg. 2011;37:1673–1678. doi: 10.1016/j.jcrs.2011.03.053. [DOI] [PubMed] [Google Scholar]
- 200.Wallin TR, Hinckley M, Nilson C, Olson RJ. A clinical comparison of single-piece and three-piece truncated hydrophobic acrylic intraocular lenses. Am J Ophthalmol. 2003;136:614–619. doi: 10.1016/s0002-9394(03)00418-5. [DOI] [PubMed] [Google Scholar]


