Abstract
Nicotinic acetylcholine receptors (nAChRs) belong to the “Cys-loop” superfamily of ligand-gated ion channels that includes GABAA, glycine, and serotonin (5-HT3) receptors. There are 16 homologous mammalian nAChR subunits encoded by a multigene family. These subunits combine to form many different nAChR subtypes with various expression patterns, diverse functional properties, and differing pharmacological characteristics. Because cholinergic innervation is pervasive and nAChR expression is extremely broad, practically every area of the brain is impinged upon by nicotinic mechanisms. This review briefly examines the structural and functional properties of the receptor/channel complex itself. The review also summarizes activation and desensitization of nAChRs by the low nicotine concentrations obtained from tobacco. Knowledge of the three-dimensional structure and the structural characteristics of channel gating has reached an advanced stage. Likewise, the basic functional properties of the channel also are reasonably well understood. It is these receptor/channel properties that underlie the participation of nAChRs in nearly every anatomical region of the mammalian brain.
1. INTRODUCTION
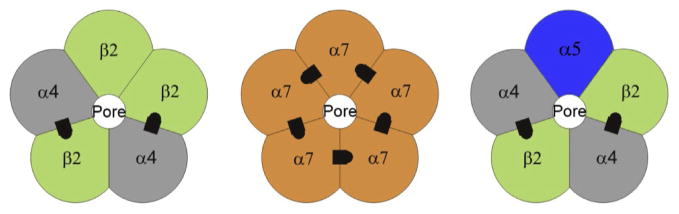
Mammalian nicotinic acetylcholine receptors (nAChRs) are composed on five subunits arranged around a water-filled pore (Fig. 1). The neuronal subunits are divided into the alpha (α2–α7, α9, and α10) and beta (β2–β4) classifications based on the presence of adjacent cysteine groups in the extracellular domain of only the α subunits (Albuquerque, Pereira, Alkondon, & Rogers, 2009; Dani & Balfour, 2011; Dani & Bertrand, 2007; Fasoli & Gotti, 2015; Lewis & Picciotto, 2013; McGehee & Role, 1995; McKay et al., 2007; Papke, 2014; Unwin, 2013; Zoli, Pistillo, & Gotti, 2014). The α8 subunit has been found in avian tissue but not in mammalian tissue. Much of the structural and functional diversity of neuronal nAChRs arises from the many possible subunit combinations. The two most commonly found nAChR subtypes in the mammalian brain are the α4β2 heteromeric and the α7 homomeric subunit combinations, which are didactically represented in Fig. 1 showing their agonist-binding sites.
Figure 1.
Didactic illustration of the nAChR subunits arranged as pentamers around the water-filled cation-permeable pore. The most common nAChRs in the brain are hetero-oligomeric α4β2 nAChRs and homo-oligomeric α7 nAChRs. The recognized ACh-binding sites are indicated by black asymmetric designs located between adjacent subunits. Adapted from Fig. 1B of McKay, Placzek, and Dani (2007).
The α4β2 is a subtype with high affinity for nicotine, and the α7 subtype is the main contributor to the α-bungarotoxin-binding sites of the brain. Because each subunit has sidedness and is not completely symmetrical, the placement of the many different subunits within the pentameric complex can produce thousands of different nAChR subtypes. For example, the α5 subunit may combine as an “accessory” subunit that does not contribute to the agonist-binding site (Fig. 1, right), but its presence modifies the functional properties of the receptor/channel complex. Another complicating feature is illustrated by considering the α4β2 heteromeric receptor that can exist as a 2(α4)–3(β2) receptor (represented in Fig. 1, left). It also can exist as a 3(α4)–2(β2) receptor, which can potentially have another agonist-binding site arising from the sidedness of the α subunit (Fasoli & Gotti, 2015). Therefore, each different pentameric complex can, in principle, produce a nAChR receptor/channel with different functional characteristics: e.g., opening, closing, and desensitizing kinetics; ionic conductance; cationic selectivity; and pharmacological properties. In practice, however, these subtypes commonly share many structural and functional properties, leading to the grouping of nAChRs into a few main neuronal nAChR subtype classifications. For example, those that contain the α7 subunit (α7*) as a homomeric or heteromeric receptor most commonly also have accompanying characteristics. They bind α-bungarotoxin, have relatively low affinity for nicotine and have relatively fast kinetics. Those that contain β2 (β2*) commonly have high affinity for nicotine, desensitize to low agonist concentrations, have relatively slow kinetics, and do not bind α-bungarotoxin. Because these broad nAChR categories include such a diverse collection of subtypes, not all the members perfectly follow these broad functional characteristics.
Other nAChR subtypes have a much more restricted distribution in the brain, but in some cases they can constitute the most abundant receptor subtype in a restricted brain area where they are expressed. For example, α3β4* nAChRs, which are commonly found in the peripheral nervous system, are expressed at high levels only in the medial habenula, interpeduncular nucleus, and locus coeruleus. α3β4* nAChRs have low affinity for nicotine and have much slower desensitization kinetics than α4β2 nAChRs (Fenster, Rains, Noerager, Quick, & Lester, 1997).
All the mammalian neuronal nAChR subtypes do share the general functional property of being permeable to small monovalent and divalent cations. The main conducting species under biological conditions are Na+, K+, and Ca2+. Agonists, such as endogenous acetylcholine (ACh) or exogenous nicotine (which can be obtained from smoking tobacco), stabilize the open conformation of the nAChR channel that transiently permeates small cations for several milliseconds before closing back to a resting state or closing to a desensitized state that is unresponsive to agonists. Brief exposure to high concentrations of the neurotransmitter, such as acetylcholine at a synaptic cleft, favors synchronous opening of the nAChRs’ pores. However, prolonged expose to low concentrations of nicotine, as obtained from tobacco use, produces some activation but also significant desensitization of nAChRs to the unresponsive closed state (Dani, Radcliffe, & Pidoplichko, 2000; Giniatullin, Nistri, & Yakel, 2005; Quick & Lester, 2002; Wooltorton, Pidoplichko, Broide, & Dani, 2003).
2. NICOTINIC RECEPTOR STRUCTURE
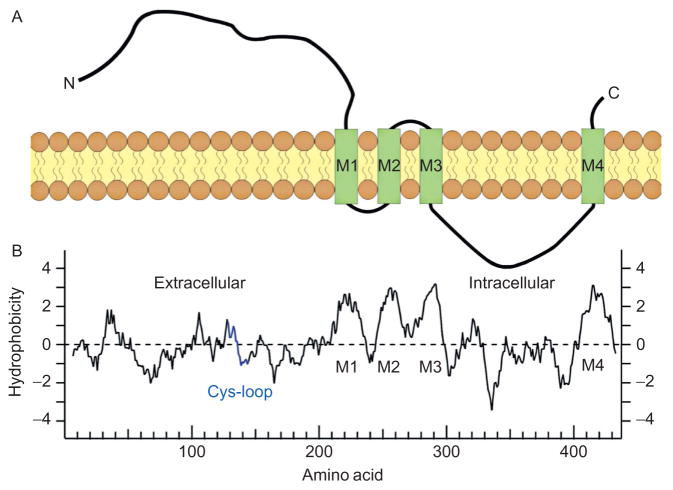
The neuronal nAChR subunits share a similar linear structure and transmembrane topology with the muscle α1 subunit (Fig. 2A) (Papke, 2014). The relatively long extracellular N-terminal domain contributes to ligand binding, followed by the three hydrophobic transmembrane regions (M1–M3), a large intracellular loop, a fourth transmembrane region (M4), and ultimately a short extracellular C-terminus (Fig. 2A). The general hydrophobicity plot for the alpha subunits suggests the basic structural domains, including the four transmembrane domains (Fig. 2B). The Cys-loop, which is shared by the whole gene superfamily, is created by a disulfide bond that links a 15 amino acid sequence contained within the large N-terminal extracellular domain. The M2 transmembrane segment in each subunit provides the main lining of the ionic pore with some contribution from the M1 segment where the pore widens (Bertrand, Galzi, Devillers-Thiery, Bertrand, & Changeux, 1993a; Dani, 1989; Karlin, 2002; Unwin & Fujiyoshi, 2012). The M1, M3, and M4 segments separate the pore-lining region from the hydrophobic membrane (Papke, 2014). The intracellular domains are quite variable among the different subunits. This variability has functional consequences for intracellular modifications, such as phosphorylation, and for linking to intracellular cytoskeletal elements that control cellular trafficking and influence surface distribution and clustering (Kracun, Harkness, Gibb, & Millar, 2008; Pollock, Pastoor, Katnik, Cuevas, & Wecker, 2009).
Figure 2.
Transmembrane topology of a nAChR subunit. (A) A didactic illustration of the linear structure of the nAChR subunit with four transmembrane domains (M1–M4) passing through the lipid bilayer member. (B) A plot of the hydrophobicity profile of a human α1 subunit. The profile is aligned with the linear representation of the subunit just above. Panel (A): Adapted from Fig. 1A of McKay et al. (2007). Panel (B): Adapted from Fig. 1A of Papke (2014).
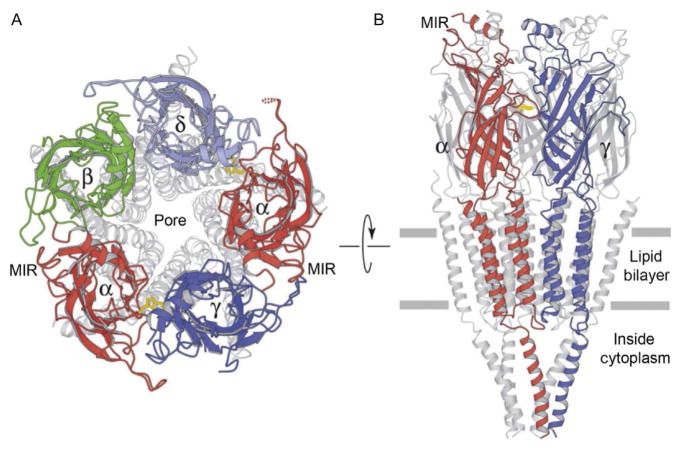
The basic structure of neuronal nicotinic receptors is homologous to the muscle nAChR (Karlin, 2002; Papke, 2014). Negative staining of the pseudo crystalline form of the muscle-type nAChRs isolated from the Torpedo Californica electric organ revealed the structure at 3.6 Å resolution (Unwin, 2005; Unwin, Miyazawa, Li, & Fujiyoshi, 2002). The muscle subunits that compose the receptor/channel are two α1 combined with β1, γ, δ, and the γ subunit is replaced by an ε subunit in the adult animal. The arrangement around the central pore is illustrated in a ribbon diagram in Fig. 3A. A lateral cross-section of the nAChR (Fig. 3B) displays an extracellular water-filled vestibule that is about 20 Å in diameter and extends 60 Å from the membrane surface into the synaptic cleft. The pore narrows at the level of the surface membrane, and permeant ions pass along this ionic pore for about 40 Å (Unwin, 2005).
Figure 3.
Illustrations of ribbon diagrams of the nAChR. (A) View from the top into the pore with only the upper most portion highlighted in colors (gray shades in the print version). (B) View from the side with only the front two subunits highlighted in colors (gray shades in the print version). The plane of the membrane is indicated by the two horizontal gray lines. Adapted from Fig. 3 of Unwin (2005).
When viewed from the side (Fig. 3B), the three main domains of the nAChR are observable. (1) The large extracellular domain that contains the agonist-binding sites and also creates the entrance vestibule to the pore. (2) The transmembrane domain that creates the water-filled, hydrophilic ionic pathway through the lipid bilayer membrane when the pore is open. (3) The intracellular domain that is the most highly variable among the subunits and contains sites for modifications and interaction with cytoplasmic elements. The main immunogenic region (MIR, Fig. 3) is a short amino acid sequence of the α1 subunit where many antibodies bind (Tzartos, Kokla, Walgrave, & Conti-Tronconi, 1988), including the autoantibodies to muscle nAChRs in human myasthenia gravis (Luo et al., 2009). This region is very diverse among the nAChR subunit family, but in some cases antibodies produced to the muscle α1 subunit’s MIR also bind to other neuronal nAChR subunits.
3. NICOTINIC RECEPTOR CHANNEL GATING
In most cases, there are two ACh-binding sites per muscle and heteromeric neuronal nAChR. Each binding site is formed by a pocket at the interface between adjacent subunits within the extracellular N-terminal domain (Albuquerque et al., 2009; Galzi et al., 1990; Karlin, 2002; Papke, 2014; Sine, 2002; Sine & Engel, 2006; Unwin, 2013). The situation is more complicated for the homomeric α7 nAChR subtype, where the sidedness of the interfaces between the alpha subunits provides five potential-binding sites (Fig. 1, middle). In the case of the muscle receptors, the two ACh molecules bind at the interface between the α and γ subunits (or α–ε in the adult form) and between the α and δ subunits (Fig. 4, where only the α and γ-binding site is indicated). The blue (light gray in the print version) shading in Fig. 4 indicates the protein structure pulling closer together when ACh binds. The red (dark gray in the print version) sphere indicates a bound ACh molecule in the binding pocket at the α–γ interface. The yellow (light gray in the print version) arrows indicate the general structural movement of the protein, including the C-loop (or loop C) closing over the ACh in the binding site. In the α4β2 receptor (Fig. 1, left), the ACh molecules bind between the α4 and β2 subunits as indicated. Therefore, both α and β subunits contribute to the pharmacology of the heteromeric-binding site.
Figure 4.
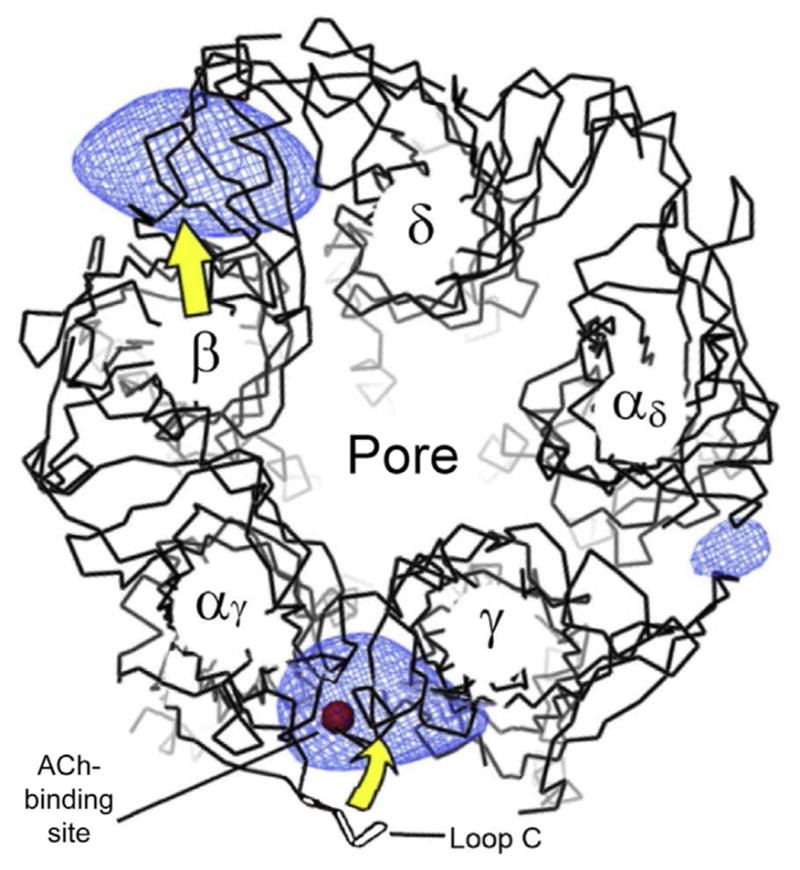
Illustration of the structural changes induced by ACh (red (dark gray in the print version) sphere) binding into the pocket formed by the closing of loop C, as viewed from the top into the pore. The blue (light gray in the print version) shaded regions represent the most significant increases in density of the open channel relative to the closed channel. The yellow (light gray in the print version) arrows indicate the general structural displacement caused by opening. Adapted from Fig. 2 of Unwin and Fujiyoshi (2012).
The extracellular ligand-binding domain consists of six loops: three on the principal side of the α subunit and three on the adjacent subunit (Williams, Stokes, Horenstein, & Papke, 2011). Two important loops in the N-terminal extracellular domain are the Cys-loop (Fig. 2B) and the C-loop (Fig. 4). In the 3D crystal structure of the α1 subunit, the Cys-loop is a 13 amino acid sequence linked by a cysteine disulfide bond located at the bottom of a beta-barrel that lies in close proximity to the extracellular M2–M3 loop (Fig. 2A). When an agonist, such as nicotine or ACh enters the binding site, the C-loop moves and covers the ligand (Celie et al., 2004) (Fig. 4). The ligand-binding process also requires participation of a series of aromatic residues whose structural arrangement is shared by all members of the Cys-loop family of channels (Taly, Corringer, Guedin, Lestage, & Changeux, 2009).
The M2 segment lines the ion channel along the axis of symmetry so that it also provides the amino acids for the explicit gate that closes the pore. The closure gate is located near the middle of the membrane-spanning portion of the channel (near Z=0 in Fig. 5), where hydrophobic residues approach each other to narrow the closed structure of the pore (Fig. 5, black dashed contour). The hydrophobic environment is energetically unfavorable for ion permeation. Thus, this gate, which is composed of three rings of hydrophobic residues, prevents passage of permeant ions when the channel is in the closed conformation (Hilf & Dutzler, 2008; Unwin & Fujiyoshi, 2012). This area of the M2 region is allosterically coupled with the agonist-binding region (Taly et al., 2009). The analysis of bacterial proteins homologue to nAChRs has suggested that channel opening is produced by the concerted tilting of the M2 helices, the M2–M3 loop, and the M3 segment (Popot, Demel, Sobel, Van Deenen, & Changeux, 1978). A series of interacting residues participate to transmit the agonist-binding conformational changes to the channel gate (Lee & Sine, 2005; Sine & Engel, 2006). Unwin and Fujiyoshi (2012) and Unwin (2013) have presented evidence indicating that the M2 transmembrane domain also converts from a bent conformation to a more straightened conformation by flexing in a way that moves the hydrophobic gate residues in a radial direction away from the axis of the pore (Fig. 5, red (dark gray in the print version) contour).
Figure 5.
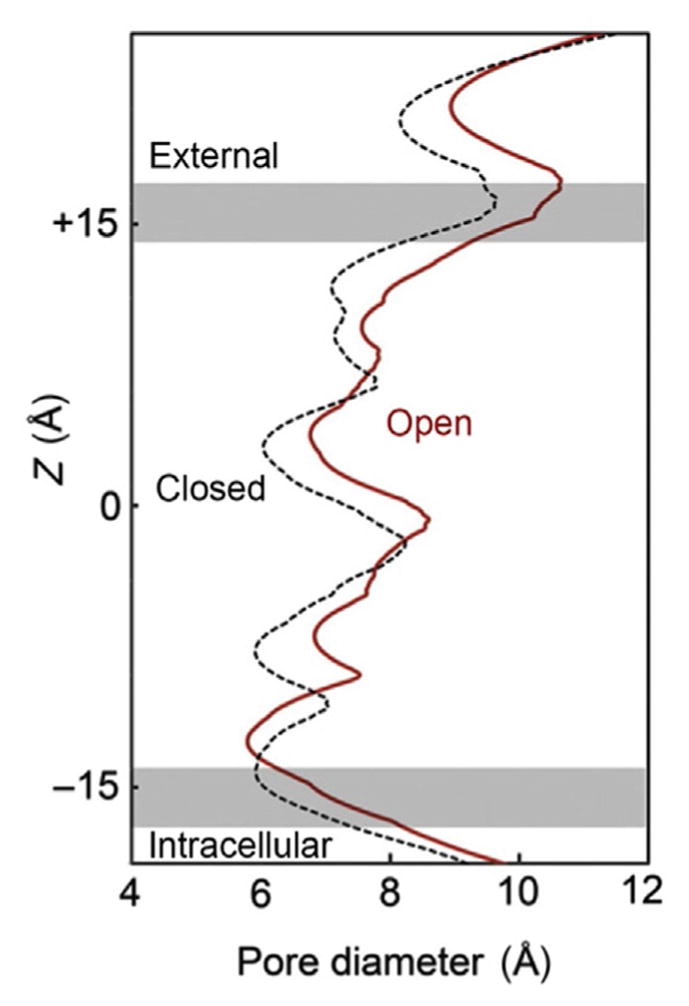
Representation of the structural change of the nAChR pore when transitioning from closed (dashed black) to open (red (dark gray in the print version)). The pore is represented to run vertically with the gray horizontal lines delimiting the position of the lipid bilayer membrane. Upon opening, the diameter of the pore increases in the constricting hydrophobic region near the middle of the membrane (at Z =0), and the narrowest region shifts near to the intracellular membrane surface where the pore is lined by polar residues (near Z =−14). Adapted from Fig. 10 of Unwin and Fujiyoshi (2012).
Structural models in conjunction with single-channel current measurements of the muscle-like nAChR revealed invariant charged amino acids that electrostatically couple α subunit-binding domains, ultimately linking them to the channel-forming α-helix. Movement of these structures underlies nAChR channel gating. During channel opening, the narrowest region of the pore moves from near the middle of channel to near the intracellular membrane surface where the pore is lined by polar residues (Fig. 5, red (dark gray in the print version) contour). The width of the narrow region moves from a hydrophobic to a hydrophilic lining region of the pore. The pore’s narrowest region does not become much wider, but it does become amenable to the permeation of cations by providing a polar, hydrophilic pathway. Permeation studies and structural data indicate that the narrowest cross-section near the inner surface of the membrane is short (3–6 Å) and about 6–7 Å in diameter (Dani, 1989; Karlin, 2002; Unwin, 2005).
4. CATIONIC PERMEABILITY OF THE NICOTINIC RECEPTOR PORE
Mammalian nAChRs are cation selective, being permeable to small monovalent and divalent cations that can fit through the narrowest hydrophilic region of the open pore (Albuquerque et al., 2009; Dani, 1989; Dani & Bertrand, 2007; Dani & Eisenman, 1987). When the linear sequences of homologous cationic nAChR and anionic channel domains are aligned, a proline residue in the anionic channel is found to be missing from the short intracellular segment between M1 and M2 of the nAChRs (as illustrated in Fig. 2A). Also near the inner mouth of the nAChR pore a negatively charged glutamate residue of the nAChR channel is missing from anionic channels, and a valine in M2 is replaced by a threonine in the channel lining of the nAChR (Galzi et al., 1992). When the amino acids of the anionic channel are inserted such that the absent proline is provided, the negatively charged glutamate is removed, and the polar threonine is replaced by valine, then the homomeric α7 nAChR is converted from cationic to anionic selectivity (Galzi et al., 1992).
Although sodium and potassium carry most of the nAChR current, calcium makes a significant contribution (Albuquerque et al., 2009; Dani, 2001; Dani & Mayer, 1995; Fucile, 2004; Vernino, Amador, Luetje, Patrick, & Dani, 1992; Vernino, Rogers, Radcliffe, & Dani, 1994). While nAChR activity causes depolarization, the divalent cation permeability plays an important physiological role by supplying ionic signals, including calcium (Bertrand, Galzi, Devillers-Thiery, Bertrand, & Changeux, 1993b; Dani & Bertrand, 2007; Decker & Dani, 1990; Gray, Rajan, Radcliffe, Yakehiro, & Dani, 1996; McGehee, Heath, Gelber, Devay, & Role, 1995; Vernino et al., 1992). The relative permeability of calcium to sodium estimated from permeability ratios is ~0.1 for muscle, ~2.0 for heteromeric neuronal, and ≥10 for homomeric α7 or the heteromeric α9/α10 nAChRs, which are expressed in cochlear hair cells (Bertrand et al., 1993b; Castro & Albuquerque, 1995; Fayuk & Yakel, 2005; Haghighi & Cooper, 2000; Lipovsek et al., 2014; Seguela, Wadiche, Dineley-Miller, Dani, & Patrick, 1993; Vernino et al., 1992). The higher calcium permeability of the α7 nAChRs arises from the arrangement of charged residues at the inner mouth of the ionic pore and polar residues in the outer part of the channel. These entrance vestibules form the transition from bulk solution to the narrow selectivity filter of the channel (Dani, 1986). For the nAChR, these vestibules have an overall net negative charge that enhances the cationic selectivity and contributes to the relatively high conductance of the nAChR channel. Substitution of the negatively charged glutamate residue found at the inner mouth of the α7 nAChRs by the neutral alanine residue suppresses calcium permeability (Bertrand et al., 1993b). Similarly, replacement of the α7 leucine at the extracellular entrance to the pore by threonine dramatically reduces the calcium permeability. However, substitution of the leucine by threonine at another polar ring of amino acids within the pore (position 247) did not alter divalent ionic selectivity, but altered agonist/antagonist relationships and aspects of desensitization (Bertrand et al., 1993a; Revah et al., 1991). These data illustrate the importance of particular conserved amino acids and the complex relationship between the structure of the pore and the resulting function.
The most basic conformational states of the nAChR are the closed state at rest, the open state, and the desensitized state. The kinetic rate at which the nicotinic receptor proceeds through the various conformational states and the selectivity with which it conducts cations in the open state depend on many factors, including the subunit composition. Therefore, the extensive nAChR diversity has the potential to produce many different responses to endogenous or exogenous agonists. The intensity of the membrane depolarization, the kinetics of gating activation, the rates of desensitization and recovery from desensitization, the size of the ionic signal, the pharmacology, and the regulatory controls of the ACh response all depend on the subunit composition of the nAChRs. In addition, the local environmental and regulatory factors influence the function of nAChRs. These influences include peptide transmitters, various protein kinases, the cytoskeleton, and calcium. Although calcium modulation can act intracellularly, nAChRs also are allosterically modulated by extracellular calcium, leading to dramatic changes in the channel opening probability (Amador & Dani, 1995; Mulle, Lena, & Changeux, 1992; Vernino et al., 1992). This modulation occurs over the physiological concentration range of external calcium. Therefore, high levels of neuronal activity that can diminish extracellular calcium (Wiest, Eagleman, King, & Montague, 2000) could cause a negative feedback that lowers the opening probability of nAChRs.
To add further complexity, the three basic conformational states (rest, open, and desensitized) do not account for the actual kinetic properties of nicotinic receptors. Rather, there are multiple conformations involved in the gating (Auerbach, 2014). Desensitization, in particular, encompasses many time constants (Steinbach & Sine, 1987). Thus, there may be short-and long-lived states of desensitization. Long exposures to low concentrations of agonist will favor deeper levels of desensitization, and this situation is often the case for smokers who maintain low concentrations of nicotine throughout the day (Dani & Heinemann, 1996; De Biasi & Dani, 2011).
5. NICOTINIC RECEPTOR RESPONSE TO NICOTINE FROM TOBACCO
Tobacco smoking activates and desensitizes nAChRs as 20–100 nM nicotine (Brody et al., 2006; Rose, Behm, Westman, & Coleman, 1999) reaches throughout the brain (Dani, Kosten, & Benowitz, 2014). Although many areas of the brain participate, nicotinic receptors of the midbrain dopamine (DA) area are particularly important during the initiation of the addiction process (Dani et al., 2014; De Biasi & Dani, 2011). On the midbrain DA and GABA neurons’ cell bodies and postsynaptically, many of the nAChRs contain α4β2 subunits that have a high affinity for nicotine. When nicotine first arrives in the midbrain DA area, it excites nAChRs, particularly the high-affinity α4β2* nAChRs and related nAChR subtypes and, to a lesser degree, the lower-affinity α7* nAChRs. Activation of the presynaptic nAChRs (commonly but not exclusively α7* nAChRs) enhances the release of glutamate (Dani et al., 2000; Mansvelder & McGehee, 2000, 2002). Simultaneously, activity of postsynaptic (and somatic) α4β2* nAChRs depolarizes DA neurons leading to enhanced action potential firing (Zhang et al., 2009). This depolarization and firing of the DA neurons helps to relieve the divalent cation block of NMDA receptors and, thus, enables the NMDA receptors to participate in long-term synaptic potentiation of glutamatergic afferents onto midbrain dopamine neurons.
After the initial exposure to nicotine and potentiation of glutamatergic afferents onto the DA neurons, there is significant, but incomplete, desensitization of particularly the high-affinity α4β2* nAChR subtypes. Thus, α4β2 nAChRs that are predominantly expressed on GABA neurons are significantly desensitized, decreasing normal afferent cholinergic drives onto the local GABAergic circuitry. Consequently, there is decreased GABAergic inhibition onto the DA neurons. The DA neurons from the posterior ventral tegmental area that provide the main projection to the nucleus accumbens commonly express α6 and β3 subunits with the α4 and β2 subunits (Leslie, Mojica, & Reynaga, 2013; Zhao-Shea et al., 2011). At the low concentrations of nicotine achieved by smokers, the presence of the α6 subunit, particularly in α6α4β2* nAChRs, slows the rate and degree of desensitization seen with the higher affinity α4β2 nAChRs (Liu, Zhao-Shea, McIntosh, Gardner, & Tapper, 2012). Therefore, those α6-containing receptors are important to maintain the more prolonged activation of DA neurons caused by nicotine from tobacco (Leslie et al., 2013; Pidoplichko et al., 2004).
The desensitization of nAChRs arising from the relatively long-lived nicotine from tobacco has other immediate effects. The sensitive nAChR subtypes at cholinergic synapses are desensitized by prolonged nicotine. Thus, smoking will turn down the gain for activity arriving via nicotinic cholinergic synapses because fewer nAChRs will be able to respond to the synaptic ACh release. In summary, nicotine not only sends inappropriate information through the mesocorticolimbic DA system but also decreases the amplitude for normal nicotinic cholinergic information processing (Dani et al., 2014).
6. CONCLUSION
Nicotinic receptors of the brain share a basic fundamental property: they mediate a cationic conductance upon binding agonist. The tremendous diversity of nAChR subtypes provides the structural and functional flexibility necessary for them to play multiple, varied roles (Zoli et al., 2014). Broad, sparse cholinergic projects throughout the brain ensure that nicotinic mechanisms modulate the neuronal excitability of relatively wide circuits (Albuquerque et al., 2009; Dani & Bertrand, 2007). Presynaptic and preterminal nicotinic receptors regulate the release of many neurotransmitters. Postsynaptic nAChRs contribute a neuroanatomically varied, but usually small, component of fast excitatory transmission. Nonsynaptic nAChRs modulate many neurotransmitter systems by influencing input impedance, neuronal set point, and neuronal excitability. While this review focused on nAChRs of the mammalian brain, nAChRs also are widely distributed and play even more diverse roles in the peripheral nervous system and in non-neuronal tissue. Thus, from a rather simple, basic underlying function (i.e., cationic permeability in response to agonist), nAChRs serve an extraordinary array of roles.
Acknowledgments
Research in the laboratory and effort for this review was supported by the following NIH grants: NIDA DA09411 and NINDS NS21229.
References
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiological Reviews. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador M, Dani JA. Mechanism for modulation of nicotinic acetylcholine receptors that can influence synaptic transmission. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1995;15:4525–4532. doi: 10.1523/JNEUROSCI.15-06-04525.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A. Agonist activation of a nicotinic acetylcholine receptor. Neuropharmacology. 2014;96:150–156. doi: 10.1016/j.neuropharm.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Galzi JL, Devillers-Thiery A, Bertrand S, Changeux JP. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal alpha 7 nicotinic receptor. Proceedings of the National Academy of Sciences of the United States of America. 1993b;90:6971–6975. doi: 10.1073/pnas.90.15.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D, Galzi JL, Devillers-Thiery A, Bertrand S, Changeux JP. Stratification of the channel domain in neurotransmitter receptors. Current Opinion in Cell Biology. 1993a;5:688–693. doi: 10.1016/0955-0674(93)90141-c. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D, et al. Cigarette smoking saturates brain alpha 4 beta 2 nicotinic acetylcholine receptors. Archives of General Psychiatry. 2006;63:907–915. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro NG, Albuquerque EX. Alpha-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophysical Journal. 1995;68:516–524. doi: 10.1016/S0006-3495(95)80213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celie PH, van Rossum-Fikkert SE, van Dijk WJ, Brejc K, Smit AB, Sixma TK. Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron. 2004;41:907–914. doi: 10.1016/s0896-6273(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Dani JA. Ion-channel entrances influence permeation. Net charge, size, shape, and binding considerations. Biophysical Journal. 1986;49:607–618. doi: 10.1016/S0006-3495(86)83688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA. Open channel structure and ion binding sites of the nicotinic acetylcholine receptor channel. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1989;9:884–892. doi: 10.1523/JNEUROSCI.09-03-00884.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA. Overview of nicotinic receptors and their roles in the central nervous system. Biological Psychiatry. 2001;49:166–174. doi: 10.1016/s0006-3223(00)01011-8. [DOI] [PubMed] [Google Scholar]
- Dani JA, Balfour DJ. Historical and current perspective on tobacco use and nicotine addiction. Trends in Neurosciences. 2011;34:383–392. doi: 10.1016/j.tins.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annual Review of Pharmacology and Toxicology. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Dani JA, Eisenman G. Monovalent and divalent cation permeation in acetylcholine receptor channels. Ion transport related to structure. The Journal of General Physiology. 1987;89:959–983. doi: 10.1085/jgp.89.6.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16:905–908. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Dani JA, Kosten TR, Benowitz NL. The pharmacology of nicotine and tobacco. In: Ries RK, Fiellin DA, Miller SC, Saitz R, editors. The ASAM principles of addiction medicine. 5. chapter 12 Philadelphia, PA: Wolters Kluwer; 2014. pp. 201–216. [Google Scholar]
- Dani JA, Mayer ML. Structure and function of glutamate and nicotinic acetylcholine receptors. Current Opinion in Neurobiology. 1995;5:310–317. doi: 10.1016/0959-4388(95)80043-3. [DOI] [PubMed] [Google Scholar]
- Dani JA, Radcliffe KA, Pidoplichko VI. Variations in desensitization of nicotinic acetylcholine receptors from hippocampus and midbrain dopamine areas. European Journal of Pharmacology. 2000;393:31–38. doi: 10.1016/s0014-2999(00)00003-0. [DOI] [PubMed] [Google Scholar]
- De Biasi M, Dani JA. Reward, addiction, withdrawal to nicotine. Annual Review of Neuroscience. 2011;34:105–130. doi: 10.1146/annurev-neuro-061010-113734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker ER, Dani JA. Calcium permeability of the nicotinic acetylcholine receptor: The single-channel calcium influx is significant. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1990;10:3413–3420. doi: 10.1523/JNEUROSCI.10-10-03413.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasoli F, Gotti C. Structure of neuronal nicotinic receptors. Current Topics in Behavioral Neurosciences. 2015;23:1–17. doi: 10.1007/978-3-319-13665-3_1. [DOI] [PubMed] [Google Scholar]
- Fayuk D, Yakel JL. Ca2+ permeability of nicotinic acetylcholine receptors in rat hippocampal CA1 interneurones. The Journal of Physiology. 2005;566:759–768. doi: 10.1113/jphysiol.2005.089789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster CP, Rains MF, Noerager B, Quick MW, Lester RA. Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1997;17:5747–5759. doi: 10.1523/JNEUROSCI.17-15-05747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucile S. Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium. 2004;35:1–8. doi: 10.1016/j.ceca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Galzi JL, Devillers-Thiery A, Hussy N, Bertrand S, Changeux JP, Bertrand D. Mutations in the channel domain of a neuronal nicotinic receptor convert ion selectivity from cationic to anionic. Nature. 1992;359:500–505. doi: 10.1038/359500a0. [DOI] [PubMed] [Google Scholar]
- Galzi JL, Revah F, Black D, Goeldner M, Hirth C, Changeux JP. Identification of a novel amino acid alpha-tyrosine 93 within the cholinergic ligands-binding sites of the acetylcholine receptor by photoaffinity labeling. Additional evidence for a three-loop model of the cholinergic ligands-binding sites. The Journal of Biological Chemistry. 1990;265:10430–10437. [PubMed] [Google Scholar]
- Giniatullin R, Nistri A, Yakel JL. Desensitization of nicotinic ACh receptors: Shaping cholinergic signaling. Trends in Neurosciences. 2005;28:371–378. doi: 10.1016/j.tins.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Haghighi AP, Cooper E. A molecular link between inward rectification and calcium permeability of neuronal nicotinic acetylcholine alpha3beta4 and alpha4beta2 receptors. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2000;20:529–541. doi: 10.1523/JNEUROSCI.20-02-00529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilf RJ, Dutzler R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature. 2008;452:375–379. doi: 10.1038/nature06717. [DOI] [PubMed] [Google Scholar]
- Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nature Reviews Neuroscience. 2002;3:102–114. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- Kracun S, Harkness PC, Gibb AJ, Millar NS. Influence of the M3–M4 intracellular domain upon nicotinic acetylcholine receptor assembly, targeting and function. British Journal of Pharmacology. 2008;153:1474–1484. doi: 10.1038/sj.bjp.0707676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WY, Sine SM. Principal pathway coupling agonist binding to channel gating in nicotinic receptors. Nature. 2005;438:243–247. doi: 10.1038/nature04156. [DOI] [PubMed] [Google Scholar]
- Leslie FM, Mojica CY, Reynaga DD. Nicotinic receptors in addiction pathways. Molecular Pharmacology. 2013;83:753–758. doi: 10.1124/mol.112.083659. [DOI] [PubMed] [Google Scholar]
- Lewis AS, Picciotto MR. High-affinity nicotinic acetylcholine receptor expression and trafficking abnormalities in psychiatric illness. Psychopharmacology. 2013;229:477–485. doi: 10.1007/s00213-013-3126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipovsek M, Fierro A, Perez EG, Boffi JC, Millar NS, Fuchs PA, et al. Tracking the molecular evolution of calcium permeability in a nicotinic acetylcholine receptor. Molecular Biology and Evolution. 2014;31:3250–3265. doi: 10.1093/molbev/msu258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhao-Shea R, McIntosh JM, Gardner PD, Tapper AR. Nicotine persistently activates ventral tegmental area dopaminergic neurons via nicotinic acetylcholine receptors containing alpha4 and alpha6 subunits. Molecular Pharmacology. 2012;81:541–548. doi: 10.1124/mol.111.076661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Taylor P, Losen M, de Baets MH, Shelton GD, Lindstrom J. Main immunogenic region structure promotes binding of conformation-dependent myasthenia gravis autoantibodies, nicotinic acetylcholine receptor conformation maturation, and agonist sensitivity. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29:13898–13908. doi: 10.1523/JNEUROSCI.2833-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. Journal of Neurobiology. 2002;53:606–617. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Role LW. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annual Review of Physiology. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- McKay BE, Placzek AN, Dani JA. Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochemical Pharmacology. 2007;74:1120–1133. doi: 10.1016/j.bcp.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle C, Lena C, Changeux JP. Potentiation of nicotinic receptor response by external calcium in rat central neurons. Neuron. 1992;8:937–945. doi: 10.1016/0896-6273(92)90208-u. [DOI] [PubMed] [Google Scholar]
- Papke RL. Merging old and new perspectives on nicotinic acetylcholine receptors. Biochemical Pharmacology. 2014;89:1–11. doi: 10.1016/j.bcp.2014.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoplichko VI, Noguchi J, Areola OO, Liang Y, Peterson J, Zhang T, et al. Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learning & Memory. 2004;11:60–69. doi: 10.1101/lm.70004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock VV, Pastoor T, Katnik C, Cuevas J, Wecker L. Cyclic AMP-dependent protein kinase A and protein kinase C phosphorylate alpha4beta2 nicotinic receptor subunits at distinct stages of receptor formation and maturation. Neuroscience. 2009;158:1311–1325. doi: 10.1016/j.neuroscience.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popot JL, Demel RA, Sobel A, Van Deenen LL, Changeux JP. Interaction of the acetylcholine (nicotinic) receptor protein from Torpedo marmorata electric organ with monolayers of pure lipids. European Journal of Biochemistry. 1978;85:27–42. doi: 10.1111/j.1432-1033.1978.tb12209.x. [DOI] [PubMed] [Google Scholar]
- Quick MW, Lester RA. Desensitization of neuronal nicotinic receptors. Journal of Neurobiology. 2002;53:457–478. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- Revah F, Bertrand D, Galzi JL, Devillers-Thiery A, Mulle C, Hussy N, et al. Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature. 1991;353:846–849. doi: 10.1038/353846a0. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Coleman RE. Arterial nicotine kinetics during cigarette smoking and intravenous nicotine administration: Implications for addiction. Drug and Alcohol Dependence. 1999;56:99–107. doi: 10.1016/s0376-8716(99)00025-3. [DOI] [PubMed] [Google Scholar]
- Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: A nicotinic cation channel highly permeable to calcium. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sine SM. The nicotinic receptor ligand binding domain. Journal of Neurobiology. 2002;53:431–446. doi: 10.1002/neu.10139. [DOI] [PubMed] [Google Scholar]
- Sine SM, Engel AG. Recent advances in Cys-loop receptor structure and function. Nature. 2006;440:448–455. doi: 10.1038/nature04708. [DOI] [PubMed] [Google Scholar]
- Steinbach JH, Sine SM. Function of nicotinic acetylcholine receptors. Society of General Physiologists Series. 1987;41:19–42. [PubMed] [Google Scholar]
- Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP. Nicotinic receptors: Allosteric transitions and therapeutic targets in the nervous system. Nature Reviews Drug Discovery. 2009;8:733–750. doi: 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- Tzartos SJ, Kokla A, Walgrave SL, Conti-Tronconi BM. Localization of the main immunogenic region of human muscle acetylcholine receptor to residues 67–76 of the alpha subunit. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:2899–2903. doi: 10.1073/pnas.85.9.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. Journal of Molecular Biology. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Unwin N. Nicotinic acetylcholine receptor and the structural basis of neuromuscular transmission: Insights from Torpedo postsynaptic membranes. Quarterly Reviews of Biophysics. 2013;46:283–322. doi: 10.1017/S0033583513000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N, Fujiyoshi Y. Gating movement of acetylcholine receptor caught by plunge-freezing. Journal of Molecular Biology. 2012;422:617–634. doi: 10.1016/j.jmb.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin N, Miyazawa A, Li J, Fujiyoshi Y. Activation of the nicotinic acetylcholine receptor involves a switch in conformation of the alpha subunits. Journal of Molecular Biology. 2002;319:1165–1176. doi: 10.1016/S0022-2836(02)00381-9. [DOI] [PubMed] [Google Scholar]
- Vernino S, Amador M, Luetje CW, Patrick J, Dani JA. Calcium modulation and high calcium permeability of neuronal nicotinic acetylcholine receptors. Neuron. 1992;8:127–134. doi: 10.1016/0896-6273(92)90114-s. [DOI] [PubMed] [Google Scholar]
- Vernino S, Rogers M, Radcliffe KA, Dani JA. Quantitative measurement of calcium flux through muscle and neuronal nicotinic acetylcholine receptors. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1994;14:5514–5524. doi: 10.1523/JNEUROSCI.14-09-05514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest MC, Eagleman DM, King RD, Montague PR. Dendritic spikes and their influence on extracellular calcium signaling. Journal of Neurophysiology. 2000;83:1329–1337. doi: 10.1152/jn.2000.83.3.1329. [DOI] [PubMed] [Google Scholar]
- Williams DK, Stokes C, Horenstein NA, Papke RL. The effective opening of nicotinic acetylcholine receptors with single agonist binding sites. The Journal of General Physiology. 2011;137:369–384. doi: 10.1085/jgp.201010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooltorton JR, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Zhang L, Liang Y, Siapas AG, Zhou FM, Dani JA. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29:4035–4043. doi: 10.1523/JNEUROSCI.0261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao-Shea R, Liu L, Soll LG, Improgo MR, Meyers EE, McIntosh JM, et al. Nicotine-mediated activation of dopaminergic neurons in distinct regions of the ventral tegmental area. Neuropsychopharmacology. 2011;36:1021–1032. doi: 10.1038/npp.2010.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoli M, Pistillo F, Gotti C. Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology. 2014;96:302–311. doi: 10.1016/j.neuropharm.2014.11.003. [DOI] [PubMed] [Google Scholar]