Abstract
Skeletal muscle is the major constituent of lean body mass and essential for the body's locomotor function. Women have less muscle mass (and more body fat) than men and are therefore not able to exert the same absolute maximal force as men. The difference in body composition between the sexes is evident from infancy but becomes most marked after puberty (when boys experience an accelerated growth spurt) and persists into old age. During early adulthood until approximately the fourth decade of life, muscle mass is relatively stable, both in men and women, but then begins to decline, and the rate of loss is slower in women than in men. In this review we discuss the underlying mechanisms responsible for the age-associated sexual dimorphism in muscle mass (as far as they have been elucidated to date) and highlight areas that require more research to advance our understanding of the control of muscle mass throughout life.
Keywords: aging, hypertrophy, muscle protein, protein turnover
skeletal muscle is the major constituent (>50%) of lean body mass; it is essential for the body's locomotor function, posture, and thermoregulation, and is the major site of insulin-stimulated glucose uptake. In individual myocytes, force development is directly proportional to the physiological cross-sectional area. Accordingly, changes in muscle mass are accompanied by almost perfectly proportional increments in strength in healthy and well-conditioned muscle (39, 109). In the general population, however, there is often a dissociation between muscle mass and muscle strength because of neuromuscular adaptations to habitual activities and factors that influence muscle quality (e.g., muscle architecture, innervation, deposition of noncontractile material such as fat and connective tissue) (72, 84, 111).
REGULATION OF MUSCLE MASS
Although skeletal muscle mass is highly heritable—reported heritability estimates range from ≥85% in children, adolescents, and young adults (66, 108) to 50-85% in middle-aged and older adults (4, 85, 96)—it is a highly dynamic organ and can rapidly increase or decrease in size. In healthy people at rest, muscle protein turns over (is renewed/remodeled) at a rate of approximately 1–2% per day. When muscle mass is constant, the rate of muscle protein synthesis (MPS) over the course of a day matches the rate of muscle protein breakdown (MPB). An imbalance between MPS and MPB results in either a net gain (MPS > MPB) or net loss (MPB > MPS) of muscle mass. In healthy people, contractile/physical activity and food intake are the major regulators of MPS and MPB (92) as outlined in Fig. 1 (top). During basal, postabsorptive conditions (overnight fast), the rate of MPB exceeds the rate of MPS, resulting in net loss of muscle protein. Meal intake compensates for these losses because hyperaminoacidemia/protein ingestion and insulin stimulate MPS and inhibit MPB, resulting in net muscle protein accretion. The net muscle protein anabolic response to a meal is largely determined by the amount of protein ingested because amino acids stimulate MPS in a dose-dependent manner up to ∼20 g of protein (“ceiling”), whereas the concentration of insulin necessary to achieve maximal suppression of MPB (∼15–30 μU/ml) already occurs after consuming a small amount of carbohydrate or protein (11, 43, 82). Exercise (both resistance and endurance) stimulates both MPS and, to a lesser extent, also MPB; consequently, net muscle protein balance after a bout of exercise in the postabsorptive state remains negative, whereas the net protein balance becomes positive after exercise in the postprandial state and exceeds the net balance after meal intake alone (83). Conversely, muscle disuse suppresses MPS (117) and initially (within the first few days) upregulates but then downregulates markers of proteolysis (25, 107). Accordingly, resistance exercise training can rapidly increase muscle size [by approximately 5–10% within 3–6 mo (2, 70)] whereas muscle disuse (as a result of casting or joint immobilization or even just reduced ambulation) rapidly results in loss of muscle mass [approximately 2–5% per wk (117)]. Inadequate protein intake [i.e., less than the recommended daily allowance (RDA) of 0.8 g·kg−1·day−1] results in loss of lean body and muscle mass [approximately 0.2–0.5% per week during energy balance (13, 21, 22) and up to ∼1% per day during starvation (56)], whereas adding protein to a diet that already contains the RDA of protein has no effect on lean body and muscle mass (9, 13, 115). Probably as important as total daily protein intake is the distribution of dietary protein intake over the course of the day (6, 68), owing both to the refractory “muscle-full” phenomenon (12) and the “ceiling” effect of the amino acid/protein-MPS dose response relationship (11, 82).
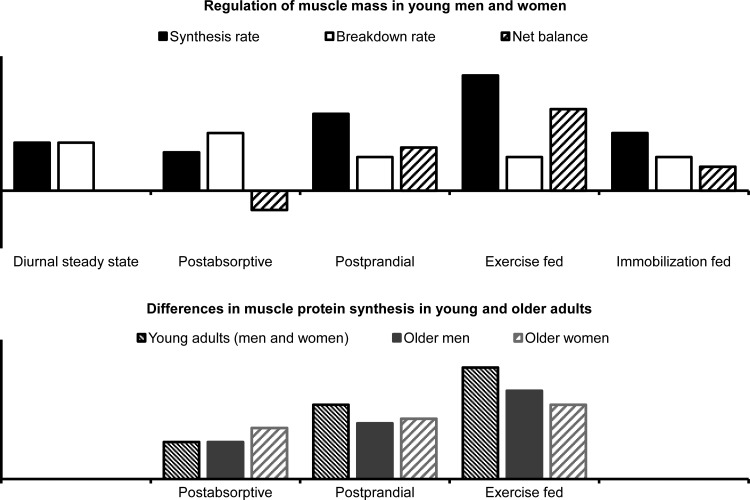
Fig. 1.
Schematic presentation of the regulation of muscle mass and age-associated changes in muscle protein synthesis. Top: in healthy young and middle-aged adults, when muscle mass is constant, the rate of muscle protein synthesis (MPS) over the course of a day matches the rate of muscle protein breakdown (MPB). An imbalance between MPS and MPB results in either a net gain (MPS > MPB) or net loss (MPB > MPS) of muscle mass. During basal, postabsorptive conditions (overnight fast), the rate of MPB exceeds the rate of MPS, resulting in net loss of muscle protein. Meal intake compensates for these losses because protein/amino acids and insulin stimulate MPS and inhibit MPB, resulting in net muscle protein accretion. Exercise (both resistance and endurance) stimulates MPS, and the net protein balance after exercise in the postprandial state exceeds the net balance after meal intake alone. Conversely, muscle disuse suppresses MPS and initially (within the first few days) upregulates but then downregulates markers of proteolysis and blunts the anabolic response to hyperaminoacidemia/protein ingestion. There are no established sex differences in the regulation of muscle protein turnover in young and middle-aged adults. Bottom: aging is associated with an increase in the basal rate of MPS in women (but not in men) and both men and women have a blunted MPS response to amino acids/protein and exercise but the reduction is greater in women than in men. Potential differences in MPB between older men and women have yet to be evaluated.
During early adulthood until approximately the fourth decade of life, muscle mass is relatively stable but then begins to decline at a rate of approximately 1–2% per decade in healthy people and more during times of illness and reduced mobility and in people with chronic diseases (17). The age-associated decline in muscle strength usually proceeds at a faster rate (i.e., approximately 2–3% decline in strength per year) than the decline in muscle mass because of additional age-associated changes to muscle architecture, innervation, and deposition of noncontractile material (fat and connective tissue) (72, 111). The age-associated decline in muscle mass affects everyone, even master-athletes (33, 88), and is largely due to anabolic resistance [i.e., a blunted increase of MPS in response to exercise and nutrients (61, 80) and a blunted suppression of MPB by insulin (123)]. Some investigators also report a reduced basal rate of MPS in older adults, whereas others report no difference in basal rates of muscle protein turnover between young and older adults (60).
SEXUAL DIMORPHISM IN MUSCLE MASS
At any given total body weight, young and middle-aged women have less muscle mass and more body fat than age-matched men (65, 78) and are therefore not able to exert the same maximal force as men (38, 40); however, strength relative to muscle cross-sectional area or muscle mass is nearly the same in men and women (67, 73). The difference in body composition between the sexes is evident from infancy but becomes most marked after puberty (when boys experience an accelerated growth spurt) and persists into old age (63, 120) (Fig. 2). The aging-associated loss of muscle mass in women coincides with the onset of menopause, is accelerated during the transition into menopause (3) and then proceeds at a slower rate (∼0.25% per year) than in men (∼0.40% per year) (37, 62). The hypertrophic response to exercise training and the atrophic response to muscle disuse are similar in young men and women (54, 129), but both are reduced in older women compared with older men (10, 15, 20).
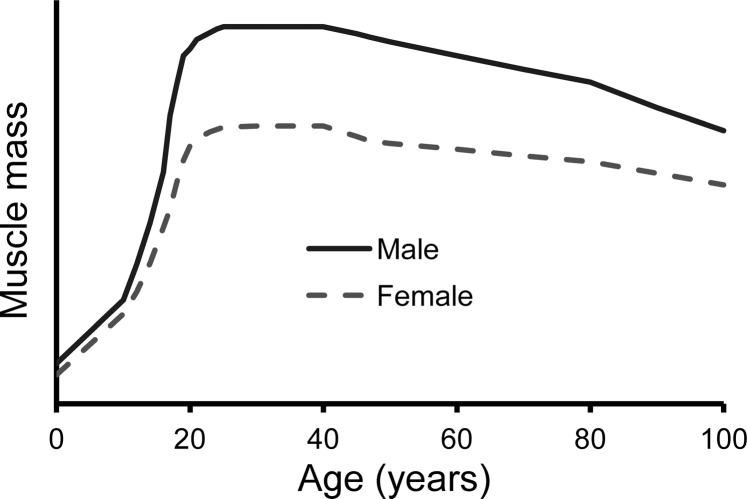
Fig. 2.
Muscle mass throughout life in men and women. At any given total body weight, girls and women have less muscle mass than boys and men. The difference is evident from infancy but becomes most marked after puberty (when boys experience an accelerated growth spurt) and persists into old age. The aging-associated loss of muscle mass, which starts in midlife, coincides with the onset of menopause in women, is accelerated during the transition into menopause, and then proceeds at a slower rate in women than in men. Advanced age (∼80 yr and older), periods of inactivity, and disease accelerate the catabolic process.
SEXUAL DIMORPHISM IN MUSCLE PROTEIN TURNOVER
Young and Middle-Aged Adults
Several groups of investigators, including our own, have compared the rates of muscle protein (mixed or myofibrillar fraction) turnover in young and middle-aged (18–45 yr old) men and women by using the traditional primed, constant amino acid infusion technique to calculate the fractional turnover rate of muscle protein (i.e., by evaluating the rate of incorporation of the tracer amino acid into muscle protein relative to the enrichment of a validated precursor amino acid pool for protein synthesis with the tracer amino acid); none found differences between the sexes in the rates of MPS and anabolic signaling in muscle during basal postabsorptive conditions (27, 41, 57, 69, 86, 99, 101, 121) or the acute response to nutritional stimuli (99, 101, 121) or resistance-type exercise (27, 74, 121). Measurement of MPB in vivo in people is technically challenging and only two studies to date have compared the rate of MPB in men and women (41, 57). Both found that the basal rate of MPB is not different in young men and women. Two studies compared surrogate markers of proteolytic activity in muscle (e.g., the expression of genes encoding ubiquitin-mediated proteolysis pathway components) and under basal conditions at rest also found no difference between the sexes (121, 122). Taken together, these data are consistent with a stable muscle mass during young and middle-age adulthood (i.e., a state in which MPS and MPB reflect remodeling of muscle but do not result in net growth or loss) and similar hypertrophic responses to resistance exercise training in men and women (1, 54, 93, 118). To our knowledge, the MPS and MPB responses to atrophy-inducing stimuli (reduced activity or protein intake) have not been compared in men and women. It is possible, but unlikely that muscle protein turnover responses during atrophy are different in men and women, because the net atrophic response to muscle disuse is not different in young men and women (129).
In a unique study, Scalzo et al. (95) compared the 4-wk-long cumulative MPS rate during sprint interval training in men and women by using a novel, 2H2O-based method. Mixed, cytosolic, and mitochondrial protein fraction synthesis rates were measured over the entire 4-wk period by 1) using gas chromatography/mass spectrometry (GC/MS) analysis of alanine in proteins and 2) using liquid chromatography/mass spectrometry (LC/MS) analysis of dozens of tryptic peptides of positively identified muscle proteins. They reported that the MPS rate in the mixed, cytosolic, and mitochondrial fractions (based on the GC/MS analysis) was almost 50% greater in men than in women and the synthesis rates of individual proteins (analyzed by LC/MS) was ∼10% greater in men than women. These results raise the possibility that it requires a substantial amount of time to capture potentially subtle but important differences in muscle protein turnover between men and women that are missed when using the traditional, short-term (several hours) primed, constant amino acid infusion technique to calculate the fractional turnover rate of muscle protein. However, the 2H2O method used by Scalzo et al. (95) has some limitations that need to be considered when interpreting the results. First, the method used by Scalzo et al. (95) relies on body water enrichment as the precursor enrichment for MPS, whereas the true precursor enrichment for MPS is that of the tRNA-bound amino acids in muscle. Accordingly, the reported synthesis rates reflect the fraction of protein synthesized de novo (by incorporating hydrogen from body water into the precursor amino acid alanine, which is then incorporated into muscle protein), not the total rate of protein synthesis (which includes protein synthesis from newly synthesized amino acids as well as “recycled” amino acids). Moreover, there is sexual dimorphism in alanine metabolism (41, 52, 94) that could affect the contribution of de novo synthesized alanine to the total plasma alanine pool or the plasma-to-tRNA enrichment ratio and hence the enrichment of alanine in the direct precursor pool for protein synthesis in muscle relative to the body water enrichment. It is therefore possible that the differences in protein turnover reported by Scalzo et al. (95) are attributable to differences in the alanine and body water precursor enrichment equilibrium (due to differences in alanine metabolism between men and women) rather than to actual differences in muscle protein synthesis (i.e., the rate of incorporation of alanine into muscle protein) between men and women. Lastly, Scalzo et al. (95) do not provide baseline data, at rest or data from nonexercising control groups; therefore, it is unclear whether the reported differences represent a differential response to exercise training or reflect baseline differences in muscle protein turnover between men and women in their cohort.
Older Adults
Few studies have evaluated potential differences in muscle protein turnover between older men and older women, and all of them so far have focused solely on MPS; potential differences in MPB between older men and women have been evaluated only by assessing surrogate markers of proteolytic activity (e.g., muscle MAFbx, MuRF1, and FOXO3A mRNA expression), which do not reliably reflect actual rates of MPB (43).
In a series of studies, we have demonstrated that aging affects MPS differently in men and women. In carefully matched young and older men and women, we found that the basal rate of MPS is greater in older women than older men, and older women, compared with older men, have a blunted anabolic response to mixed meal ingestion, combined intravenous infusion of amino acids and insulin, and exercise training (100–102). In addition, we found no difference in the basal rate of MPS between healthy young and older men but a greater rate of MPS in older compared with young women (101, 103). Older men and women both exhibited a blunted anabolic response to nutritional stimuli compared with young men and women (101). These findings are consistent with a slower rate of muscle loss in older women compared with older men (37, 62) [because the greater basal rate of MPS compensates for the greater anabolic deficit in women than in men (100, 101)] and the blunted hypertrophic response to exercise training in older women compared with older men (10, 15). The greater basal rate of MPS in older women in our study was associated with a greater capacity for protein synthesis [i.e., more ribosomal RNA (77)] and a more active translational process at the elongation stage of protein synthesis (40% reduced phosphorylation of muscle eEF2Thr56) (100). In addition, we and others found an age-related upregulation of stimulatory muscle growth regulatory genes in women (91, 103) but not in men (29, 59). The reduced anabolic response to feeding in older women, on the other hand, was associated with reduced stimulation of translation initiation (phosphorylation of muscle eIF4ESer209 and eIF4E-BP1Thr37/46) (100). In addition, it has been reported that older women, but not older men, have an impaired hyperemic response to exercise (64, 87), which could limit amino acid supply to muscle and thereby blunt the anabolic response to exercise in older women.
Bukhari and colleagues (18) studied only older women and, consistent with the results from our studies, reported basal myofibrillar protein synthesis rates that are at least 50% higher (∼0.07%/h) than those reported by the same group of investigators and others for older men (0.025–0.043%/h) (24, 61, 80, 126, 127) and young men and women (0.026–0.041%/h) (5, 7, 24, 42, 125). Moreover, the anabolic response to ingestion of 20 g of whey protein (∼25%) in their study was much less than that reported for older men (∼65%) in studies that used the same experimental protocol (19, 127). Hansen et al. (49), also studied older women only, and they too reported a much higher basal myofibrillar protein synthesis (∼0.08% per h) than typically observed in older men and no increase in response to exercise in older women.
Henderson and colleagues (53) evaluated basal rates of MPS in young and older men and women and found a greater rate of MPS in women than in men but no sex × age interaction. However, that study included young men and women who were healthy, but only older men with hypogonadism and older women with low serum dehydroepiandrosterone concentration, which may have confounded the results. Hypoandrogenemia is associated with a reduced lean body mass (58), and treatment with testosterone increases the muscle protein synthesis rate (14, 45, 103, 113).
Markofski and colleagues (69) performed a post hoc analysis of historic basal MPS rate measurements in young and older men and women made over a 10-yr period and found no difference between young and older subjects and men and women. However, there are reasons to believe that this study design made it impossible to pick up the ∼30% greater MPS rate in older women (0.058%/h) compared with older men and young men and women (all ∼0.045%/h) we (99–103) and others (18, 49) have reported. First, the average MPS values for individual years ranged from ∼0.055%/h in 2009 to ∼0.075%/h in 2005, and the variance of the MPS rate measurements during the early years was nearly double that in the later years. This suggests that either there were considerable differences among the various cohorts (possibly due to differences in their sex and age distribution) or technical reasons that resulted in less precise measurements early on. Second, the use of oral sex steroid preparations, which can affect MPS (see sex hormone effects on muscle protein turnover), was not an exclusion criterion.
In addition, several investigators have compared the basal rates of MPS between young and older men only and reported no difference or a lower basal MPS rate in older compared with young men (e.g., 24, 28, 61, 116, 119); only one other study, to our knowledge, has compared MPS rates in young and older women only and found no difference (23).
In summary, the composite of the results obtained in older men and women suggest that aging is associated with an increase in the basal rate of MPS in women (but not in men), and that both older men and older women (compared with young men and women) have a blunted MPS response to amino acids/protein and exercise but the reduction is greater in women than in men (Fig. 1, bottom).
SEX HORMONE EFFECTS ON MUSCLE PROTEIN TURNOVER
Considering the well-known anabolic effect of testosterone, which is mediated by its stimulatory effect on MPS (14, 45, 103, 113) and possibly also an inhibitory effect on MPB (34), it may seem surprising that there are no differences in muscle protein turnover between young men and women (see sexual dimorphism in muscle protein turnover, Young and Middle-Aged Adults). This is most likely due to the fact that during young and middle-age adulthood when muscle mass is constant, MPS and MPB simply reflect turning over of a stable muscle mass (i.e., remodeling of muscle). In fact, the rapid growth of lean body and muscle mass during adolescence and early adulthood in boys is most certainly mediated by the surge in testosterone secretion (36, 55), whereas growth of lean body and muscle mass in girls simply proceeds in parallel with overall skeletal growth (i.e., constant relative to height); the surge in ovarian hormone production during puberty in girls stimulates growth of fat mass but does not suppress lean tissue and muscle growth (120). Together, the continued growth in lean body and muscle mass in adolescent boys and the increase in fat mass in adolescent girls result in the well-established sexual dimorphism in body composition in adulthood. On the other hand, it is intriguing that differences in muscle protein turnover between men and women become apparent in older age (see sexual dimorphism in muscle protein turnover, Older Adults) because the loss of testosterone with aging in men, although significant, is very small (51, 116) compared with the change in female sex steroid availability at the onset of menopause. This would suggest that the female sex steroids, estrogens and progesterone, are important regulators of muscle protein turnover. Indeed, studies conducted in rodents suggest that female sex steroids suppress MPS (110). Interpretation of these results, however, is complicated by the ovariectomy-induced changes in physical activity and body weight in rats (35, 44). The results from studies that evaluated the effect of female sex steroids on muscle protein turnover in people are conflicting. Several studies found that the basal rate of MPS is greater in older, postmenopausal compared with young, premenopausal women (101, 103); lower in women who received estrogen replacement therapy after surgically induced menopause than age-matched postmenopausal women (49); and lower in women who use certain types of oral, hormonal contraceptives (i.e., Lindynette) compared with those who do not (47). These results are consistent with a suppressive effect of female sex steroids on MPS. On the other hand, numerous other studies found no difference in the rate of MPS between women who use certain other oral steroidal contraceptives (i.e., Cilest) and those who do not (47), and no difference between women who were studied during the follicular and luteal phases of their menstrual cycles (74), which would suggest that female sex steroids are not important regulators of muscle protein turnover. It is possible that menstrual cycle and oral contraceptive use-induced changes in female sex steroid availability during young and middle-age adulthood are often too subtle compared with the prevailing hormone milieu, which makes it difficult to evaluate their effect until cessation of ovarian function with menopause. Interpretation of the results from these studies is also difficult because of their cross-sectional design, the difference in progestin potency in various oral contraceptive formulations, and potential confounding influences [e.g., differences in free testosterone, IGF-1, and insulin concentrations between hormone-treated and untreated women (47, 49)], which may have been induced by the oral hormone treatments (97, 104) that were used in those studies. To avoid these confounding influences, we recently evaluated the effects of systemically delivered estradiol and micronized progesterone and found that transdermally delivered estradiol has no effect on MPS or the expression of several genes in muscle that encode proteins involved in the regulation of muscle mass, whereas vaginally delivered micronized progesterone has potent stimulatory effects on MPS and MYOD1 mRNA expression (103). Congruent with the results from our study, other investigators found that estradiol has no effect on basal muscle growth stimulatory factor expression in ovariectomized female and orchidectomized male mice and rats (30, 31, 106, 112, 114), whereas combined (estrogen plus progestin) hormone replacement stimulates growth regulatory gene expression in muscle of postmenopausal women (26, 90). In addition, the results from studies conducted in rodents demonstrate that 1) both testosterone and estrogen can counteract the disuse-induced loss of muscle atrophy (32, 105, 124, 128), 2) estrogen is necessary for the recovery of muscle loss in ovariectomized rats (16, 71, 98) and augments muscle myoD expression during recovery (114), and 3) hormone replacement therapy stimulates myogenic gene expression after muscle-damaging eccentric exercise in postmenopausal women (26). Testosterone, on the other hand, does not affect recovery from disuse (50). In summary, these results suggest that during adulthood testosterone and progesterone are probably important for muscle maintenance, whereas estradiol could be a critical mediator for muscle growth following/during periods of muscle atrophy (Table 1); this helps explain the blunted anabolic response to exercise training in older women compared with younger women and old men.
Table 1.
Effects of sex hormones on regulation of skeletal soft tissue mass
| Testosterone | Estradiol | Progesterone | |
|---|---|---|---|
| Mixed muscle/myofibrillar protein synthesis rate | |||
| Rest | ↑ | - | ↑ |
| Loss during disuse | ↓ | ↓ | ? |
| Recovery from disuse | - | ↑ | ? |
| Muscle collagen synthesis | |||
| Rest | ? | - ↓ | ? |
| Response to exercise | ? | - ↑ | ? |
| Tendon collagen synthesis rate | |||
| Rest | ? | - ↓ | ? |
| Response to exercise | ? | - ↓ | ? |
↑ indicates stimulatory effect; ↓ indicates inhibitory effects; - indicates no effect; ? indicates effect not known; multiple symbols indicate equivocal findings.
NONCONTRACTILE PROTEINS
Little is known about the turnover of noncontractile proteins that provide structural support and are critical for force transfer from the muscle to the skeleton and joint stabilization (i.e, collagenous proteins of the extracellular matrix and tendons and ligaments). Overall, the turnover rate of these proteins is similar to that of contractile proteins (∼0.05%/h) and they are very responsive to changes in muscle loading (they increase with exercise and decrease with unloading) (25, 76, 81). However, unlike contractile proteins, they are not responsive to nutritional stimuli (8, 79). Muscle collagen turnover is not different in men and women at rest, and increases to the same extent in response to exercise in young men and women; however, the effect of exercise is blunted in postmenopausal women and may be restored with estrogen replacement therapy (49, 74, 89). Tendon collagen turnover, on the other hand, is ∼50% slower in women than in men, and women (unlike men) do not increase tendon collagen synthesis in response to exercise, regardless of age (46, 48, 75, 89). This could have detrimental consequences because it might adversely affect the balance between force-producing (contractile proteins) and force-transmitting proteins (tendons) and could help explain the greater risk of soft tissue injury during physical activity in women than men. The effect of sex hormones on tendon collagen turnover has been assessed by only two studies and the results are equivocal (46, 89).
SUMMARY, CONCLUSION, AND FUTURE PERSPECTIVES
Women have less muscle mass than men, and the greater muscle mass in men is most likely due to a testosterone-driven growth spurt in adolescence. During young and middle-age adulthood, when muscle mass is stable, there are no differences in muscle protein turnover between men and women, either at rest or after exercise. However, the age-associated decline in muscle mass, which starts around the fourth decade of life, is slower in women than in men and older women appear to be less responsive to the hypertrophic effect of exercise training. The specific mechanisms responsible for this phenomenon are not entirely clear but the relative preservation of muscle mass in older women compared with older men appears to be driven by an age-associated increase in the expression of growth regulatory genes and MPS, whereas the blunted hypertrophic response to exercise training in older women may, at least in part, be due to an impaired hyperemic response and limited substrate supply for MPS. How much of the differences between men and women can be attributed to differences in the sex hormone milieu is unclear because the results from studies evaluating the effect of sex steroids are equivocal and often difficult to interpret because orally delivered synthetic sex steroids can cause changes in circulating anabolic and catabolic hormone concentrations. In addition, women of all ages fail to adapt tendon collagen synthesis to increased loading, which might adversely affect the balance between force-producing (contractile proteins) and force-transmitting (tendons) proteins. Carefully designed and adequately powered prospective studies that focus on the combined study of MPS and MPB in older men and women are needed to fully appreciate the age-associated differences in muscle protein turnover while mechanistic studies (including, for example, exercise and nutritional dose-response studies and sex hormone interventions) will help delineate the sex-specific pathophysiology of sarcopenia.
GRANTS
The authors received salary support from NIH grants DK-94483, DK-56341 (Washington University School of Medicine Nutrition and Obesity Research Center), and UL1 TR-000448 (Washington University School of Medicine Clinical Translational Science Award), including KL2 subaward TR-000450.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.M. conception and design of research; G.I.S. and B.M. interpreted results of experiments; B.M. drafted manuscript; G.I.S. and B.M. edited and revised manuscript; G.I.S. and B.M. approved final version of manuscript.
REFERENCES
- 1.Abe T, DeHoyos DV, Pollock ML, Garzarella L. Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur J Appl Physiol 81: 174–180, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Ahtiainen JP, Pakarinen A, Alen M, Kraemer WJ, Hakkinen K. Short vs long rest period between the sets in hypertrophic resistance training: influence on muscle strength, size, and hormonal adaptations in trained men. J Strength Cond Res 19: 572–582, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Aloia JF, McGowan DM, Vaswani AN, Ross P, Cohn SH. Relationship of menopause to skeletal and muscle mass. Am J Clin Nutr 53: 1378–1383, 1991. [DOI] [PubMed] [Google Scholar]
- 4.Arden NK, Spector TD. Genetic influences on muscle strength, lean body mass, and bone mineral density: a twin study. J Bone Miner Res 12: 2076–2081, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Areta JL, Burke LM, Camera DM, West DW, Crawshay S, Moore DR, Stellingwerff T, Phillips SM, Hawley JA, Coffey VG. Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short-term energy deficit. Am J Physiol Endocrinol Metab 306: E989–E997, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Areta JL, Burke LM, Ross ML, Camera DM, West DW, Broad EM, Jeacocke NA, Moore DR, Stellingwerff T, Phillips SM, Hawley JA, Coffey VG. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J Physiol 591: 2319–2331, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 92: 1080–1088, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Babraj JA, Cuthbertson DJ, Smith K, Langberg H, Miller B, Krogsgaard MR, Kjaer M, Rennie MJ. Collagen synthesis in human musculoskeletal tissues and skin. Am J Physiol Endocrinol Metab 289: E864–E869, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Baer DJ, Stote KS, Paul DR, Harris GK, Rumpler WV, Clevidence BA. Whey protein but not soy protein supplementation alters body weight and composition in free-living overweight and obese adults. J Nutr 141: 1489–1494, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bamman MM, Hill VJ, Adams GR, Haddad F, Wetzstein CJ, Gower BA, Ahmed A, Hunter GR. Gender differences in resistance-training-induced myofiber hypertrophy among older adults. J Gerontol A Biol Sci Med Sci 58: 108–116, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol 552: 315–324, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohe J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol 532: 575–579, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bray GA, Smith SR, de Jonge L, Xie H, Rood J, Martin CK, Most M, Brock C, Mancuso S, Redman LM. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA 307: 47–55, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brodsky IG, Balagopal P, Nair KS. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men–a clinical research center study. J Clin Endocrinol Metab 81: 3469–3475, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Brose A, Parise G, Tarnopolsky MA. Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. J Gerontol A Biol Sci Med Sci 58: 11–19, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Brown M, Foley A, Ferreria JA. Ovariectomy, hindlimb unweighting, and recovery effects on skeletal muscle in adult rats. Aviat Space Environ Med 76: 1012–1018, 2005. [PubMed] [Google Scholar]
- 17.Buford TW, Anton SD, Judge AR, Marzetti E, Wohlgemuth SE, Carter CS, Leeuwenburgh C, Pahor M, Manini TM. Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res Rev 9: 369–383, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bukhari SS, Phillips BE, Wilkinson DJ, Limb MC, Rankin D, Mitchell WK, Kobayashi H, Greenhaff PL, Smith K, Atherton PJ. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. Am J Physiol Endocrinol Metab 308: E1056–E1065, 2015. [DOI] [PubMed] [Google Scholar]
- 19.Burd NA, Yang Y, Moore DR, Tang JE, Tarnopolsky MA, Phillips SM. Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. Br J Nutr 108: 958–962, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Callahan DM, Tourville TW, Miller MS, Hackett SB, Sharma H, Cruickshank NC, Slauterbeck JR, Savage PD, Ades PA, Maughan DW, Beynnon BD, Toth MJ. Chronic disuse and skeletal muscle structure in older adults: sex-specific differences and relationships to contractile function. Am J Physiol Cell Physiol 308: C932–C943, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castaneda C, Charnley JM, Evans WJ, Crim MC. Elderly women accommodate to a low-protein diet with losses of body cell mass, muscle function, and immune response. Am J Clin Nutr 62: 30–39, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Castaneda C, Gordon PL, Fielding RA, Evans WJ, Crim MC. Marginal protein intake results in reduced plasma IGF-I levels and skeletal muscle fiber atrophy in elderly women. J Nutr Health Aging 4: 85–90, 2000. [PubMed] [Google Scholar]
- 23.Chevalier S, Goulet ED, Burgos SA, Wykes LJ, Morais JA. Protein anabolic responses to a fed steady state in healthy aging. J Gerontol A Biol Sci Med Sci 66: 681–688, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19: 422–424, 2005. [DOI] [PubMed] [Google Scholar]
- 25.de Boer MD, Selby A, Atherton P, Smith K, Seynnes OR, Maganaris CN, Maffulli N, Movin T, Narici MV, Rennie MJ. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol 585: 241–251, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dieli-Conwright CM, Spektor TM, Rice JC, Sattler FR, Schroeder ET. Influence of hormone replacement therapy on eccentric exercise induced myogenic gene expression in postmenopausal women. J Appl Physiol 107: 1381–1388, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dreyer HC, Fujita S, Glynn EL, Drummond MJ, Volpi E, Rasmussen BB. Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex. Acta Physiol (Oxf) 199: 71–81, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol 104: 1452–1461, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drummond MJ, Miyazaki M, Dreyer HC, Pennings B, Dhanani S, Volpi E, Esser KA, Rasmussen BB. Expression of growth-related genes in young and older human skeletal muscle following an acute stimulation of protein synthesis. J Appl Physiol 106: 1403–1411, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enns DL, Iqbal S, Tiidus PM. Oestrogen receptors mediate oestrogen-induced increases in post-exercise rat skeletal muscle satellite cells. Acta Physiol (Oxf) 194: 81–93, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Enns DL, Tiidus PM. Estrogen influences satellite cell activation and proliferation following downhill running in rats. J Appl Physiol 104: 347–353, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Evans WJ, Ivy JL. Effects of testosterone propionate on hindlimb-immobilized rats. J Appl Physiol Respir Environ Exerc Physiol 52: 1643–1647, 1982. [DOI] [PubMed] [Google Scholar]
- 33.Faulkner JA, Larkin LM, Claflin DR, Brooks SV. Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol 34: 1091–1096, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Ferrando AA, Sheffield-Moore M, Paddon-Jones D, Wolfe RR, Urban RJ. Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab 88: 358–362, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Fonseca H, Powers SK, Goncalves D, Santos A, Mota MP, Duarte JA. Physical inactivity is a major contributor to ovariectomy-induced sarcopenia. Int J Sports Med 33: 268–278, 2012. [DOI] [PubMed] [Google Scholar]
- 36.Forbes GB. The effect of anabolic steroids on lean body mass: the dose response curve. Metabolism 34: 571–573, 1985. [DOI] [PubMed] [Google Scholar]
- 37.Forbes GB, Reina JC. Adult lean body mass declines with age: some longitudinal observations. Metabolism 19: 653–663, 1970. [DOI] [PubMed] [Google Scholar]
- 38.Ford LE, Detterline AJ, Ho KK, Cao W. Gender- and height-related limits of muscle strength in world weightlifting champions. J Appl Physiol 89: 1061–1064, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol 88: 1321–1326, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol 71: 644–650, 1991. [DOI] [PubMed] [Google Scholar]
- 41.Fujita S, Rasmussen BB, Bell JA, Cadenas JG, Volpi E. Basal muscle intracellular amino acid kinetics in women and men. Am J Physiol Endocrinol Metab 292: E77–E83, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol 586: 6049–6061, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, Rennie MJ. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab 295: E595–E604, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greising SM, Carey RS, Blackford JE, Dalton LE, Kosir AM, Lowe DA. Estradiol treatment, physical activity, and muscle function in ovarian-senescent mice. Exp Gerontol 46: 685–693, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griggs RC, Kingston W, Jozefowicz RF, Herr BE, Forbes G, Halliday D. Effect of testosterone on muscle mass and muscle protein synthesis. J Appl Physiol 66: 498–503, 1989. [DOI] [PubMed] [Google Scholar]
- 46.Hansen M, Kongsgaard M, Holm L, Skovgaard D, Magnusson SP, Qvortrup K, Larsen JO, Aagaard P, Dahl M, Serup A, Frystyk J, Flyvbjerg A, Langberg H, Kjaer M. Effect of estrogen on tendon collagen synthesis, tendon structural characteristics, and biomechanical properties in postmenopausal women. J Appl Physiol 106: 1385–1393, 2009. [DOI] [PubMed] [Google Scholar]
- 47.Hansen M, Langberg H, Holm L, Miller BF, Petersen SG, Doessing S, Skovgaard D, Trappe T, Kjaer M. Effect of administration of oral contraceptives on the synthesis and breakdown of myofibrillar proteins in young women. Scand J Med Sci Sports 21: 62–72, 2011. [DOI] [PubMed] [Google Scholar]
- 48.Hansen M, Miller BF, Holm L, Doessing S, Petersen SG, Skovgaard D, Frystyk J, Flyvbjerg A, Koskinen S, Pingel J, Kjaer M, Langberg H. Effect of administration of oral contraceptives in vivo on collagen synthesis in tendon and muscle connective tissue in young women. J Appl Physiol 106: 1435–1443, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Hansen M, Skovgaard D, Reitelseder S, Holm L, Langbjerg H, Kjaer M. Effects of estrogen replacement and lower androgen status on skeletal muscle collagen and myofibrillar protein synthesis in postmenopausal women. J Gerontol A Biol Sci Med Sci 67: 1005–1013, 2012. [DOI] [PubMed] [Google Scholar]
- 50.Harjola VP, Jankala H, Harkonen M. The effect of androgen status on skeletal muscle myosin heavy chain mRNA and protein levels in rats recovering from immobilization. Eur J Appl Physiol 83: 427–433, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 86: 724–731, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Haymond MW, Karl IE, Clarke WL, Pagliara AS, Santiago JV. Differences in circulating gluconeogenic substrates during short-term fasting in men, women, and children. Metabolism 31: 33–42, 1982. [PubMed] [Google Scholar]
- 53.Henderson GC, Dhatariya K, Ford GC, Klaus KA, Basu R, Rizza RA, Jensen MD, Khosla S, O'Brien P, Nair KS. Higher muscle protein synthesis in women than men across the lifespan, and failure of androgen administration to amend age-related decrements. FASEB J 23: 631–641, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hubal MJ, Gordish-Dressman H, Thompson PD, Price TB, Hoffman EP, Angelopoulos TJ, Gordon PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Seip RL, Clarkson PM. Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc 37: 964–972, 2005. [PubMed] [Google Scholar]
- 55.Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, Lenzi A, Fabbri A. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf) 63: 280–293, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Jackson JM, Blaine D, Powell-Tuck J, Korbonits M, Carey A, Elia M. Macro- and micronutrient losses and nutritional status resulting from 44 days of total fasting in a non-obese man. Nutrition 22: 889–897, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Jahn LA, Barrett EJ, Genco ML, Wei L, Spraggins TA, Fryburg DA. Tissue composition affects measures of postabsorptive human skeletal muscle metabolism: comparison across genders. J Clin Endocrinol Metab 84: 1007–1010, 1999. [DOI] [PubMed] [Google Scholar]
- 58.Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab 81: 4358–4365, 1996. [DOI] [PubMed] [Google Scholar]
- 59.Kim JS, Kosek DJ, Petrella JK, Cross JM, Bamman MM. Resting and load-induced levels of myogenic gene transcripts differ between older adults with demonstrable sarcopenia and young men and women. J Appl Physiol 99: 2149–2158, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Koopman R, van Loon LJ. Aging, exercise, and muscle protein metabolism. J Appl Physiol 106: 2040–2048, 2009. [DOI] [PubMed] [Google Scholar]
- 61.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol 587: 211–217, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kyle UG, Genton L, Hans D, Karsegard L, Slosman DO, Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr 55: 663–672, 2001. [DOI] [PubMed] [Google Scholar]
- 63.Kyle UG, Genton L, Slosman DO, Pichard C. Fat-free and fat mass percentiles in 5225 healthy subjects aged 15 to 98 years. Nutrition 17: 534–541, 2001. [DOI] [PubMed] [Google Scholar]
- 64.La Favor JD, Kraus RM, Carrithers JA, Roseno SL, Gavin TP, Hickner RC. Sex differences with aging in nutritive skeletal muscle blood flow: impact of exercise training, nitric oxide, and alpha-adrenergic-mediated mechanisms. Am J Physiol Heart Circ Physiol 307: H524–H532, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee SJ, Janssen I, Heymsfield SB, Ross R. Relation between whole-body and regional measures of human skeletal muscle. Am J Clin Nutr 80: 1215–1221, 2004. [DOI] [PubMed] [Google Scholar]
- 66.Loos R, Thomis M, Maes HH, Beunen G, Claessens AL, Derom C, Legius E, Derom R, Vlietinck R. Gender-specific regional changes in genetic structure of muscularity in early adolescence. J Appl Physiol 82: 1802–1810, 1997. [DOI] [PubMed] [Google Scholar]
- 67.Lynch NA, Metter EJ, Lindle RS, Fozard JL, Tobin JD, Roy TA, Fleg JL, Hurley BF. Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol 86: 188–194, 1999. [DOI] [PubMed] [Google Scholar]
- 68.Mamerow MM, Mettler JA, English KL, Casperson SL, Arentson-Lantz E, Sheffield-Moore M, Layman DK, Paddon-Jones D. Dietary protein distribution positively influences 24-h muscle protein synthesis in healthy adults. J Nutr 144: 876–880, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Markofski MM, Dickinson JM, Drummond MJ, Fry CS, Fujita S, Gundermann DM, Glynn EL, Jennings K, Paddon-Jones D, Reidy PT, Sheffield-Moore M, Timmerman KL, Rasmussen BB, Volpi E. Effect of age on basal muscle protein synthesis and mTORC1 signaling in a large cohort of young and older men and women. Exp Gerontol 65: 1–7, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McCall GE, Byrnes WC, Dickinson A, Pattany PM, Fleck SJ. Muscle fiber hypertrophy, hyperplasia, and capillary density in college men after resistance training. J Appl Physiol 81: 2004–2012, 1996. [DOI] [PubMed] [Google Scholar]
- 71.McClung JM, Davis JM, Wilson MA, Goldsmith EC, Carson JA. Estrogen status and skeletal muscle recovery from disuse atrophy. J Appl Physiol 100: 2012–2023, 2006. [DOI] [PubMed] [Google Scholar]
- 72.McGregor RA, Cameron-Smith D, Poppitt SD. It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Healthspan 3: 9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miller AE, MacDougall JD, Tarnopolsky MA, Sale DG. Gender differences in strength and muscle fiber characteristics. Eur J Appl Physiol Occup Physiol 66: 254–262, 1993. [DOI] [PubMed] [Google Scholar]
- 74.Miller BF, Hansen M, Olesen JL, Flyvbjerg A, Schwarz P, Babraj JA, Smith K, Rennie MJ, Kjaer M. No effect of menstrual cycle on myofibrillar and connective tissue protein synthesis in contracting skeletal muscle. Am J Physiol Endocrinol Metab 290: E163–E168, 2006. [DOI] [PubMed] [Google Scholar]
- 75.Miller BF, Hansen M, Olesen JL, Schwarz P, Babraj JA, Smith K, Rennie MJ, Kjaer M. Tendon collagen synthesis at rest and after exercise in women. J Appl Physiol 102: 541–546, 2007. [DOI] [PubMed] [Google Scholar]
- 76.Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol 567: 1021–1033, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Millward DJ, Garlick PJ, James WP, Nnanyelugo DO, Ryatt JS. Relationship between protein synthesis and RNA content in skeletal muscle. Nature 241: 204–205, 1973. [DOI] [PubMed] [Google Scholar]
- 78.Mingrone G, Marino S, DeGaetano A, Capristo E, Heymsfield SB, Gasbarrini G, Greco AV. Different limit to the body's ability of increasing fat-free mass. Metabolism 50: 1004–1007, 2001. [DOI] [PubMed] [Google Scholar]
- 79.Mittendorfer B, Andersen JL, Plomgaard P, Saltin B, Babraj JA, Smith K, Rennie MJ. Protein synthesis rates in human muscles: neither anatomical location nor fibre-type composition are major determinants. J Physiol 563: 203–211, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, Phillips SM. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci 70: 57–62, 2015. [DOI] [PubMed] [Google Scholar]
- 81.Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab 288: E1153–E1159, 2005. [DOI] [PubMed] [Google Scholar]
- 82.Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 89: 161–168, 2009. [DOI] [PubMed] [Google Scholar]
- 83.Morton RW, McGlory C, Phillips SM. Nutritional interventions to augment resistance training-induced skeletal muscle hypertrophy. Front Physiol 6: 245, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Narici MV, Roi GS, Landoni L, Minetti AE, Cerretelli P. Changes in force, cross-sectional area and neural activation during strength training and detraining of the human quadriceps. Eur J Appl Physiol Occup Physiol 59: 310–319, 1989. [DOI] [PubMed] [Google Scholar]
- 85.Nguyen TV, Howard GM, Kelly PJ, Eisman JA. Bone mass, lean mass, and fat mass: same genes or same environments? Am J Epidemiol 147: 3–16, 1998. [DOI] [PubMed] [Google Scholar]
- 86.Parise G, Mihic S, MacLennan D, Yarasheski KE, Tarnopolsky MA. Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed-muscle protein synthesis. J Appl Physiol 91: 1041–1047, 2001. [DOI] [PubMed] [Google Scholar]
- 87.Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Proctor DN. Sex-specific influence of aging on exercising leg blood flow. J Appl Physiol 104: 655–664, 2008. [DOI] [PubMed] [Google Scholar]
- 88.Pearson SJ, Young A, Macaluso A, Devito G, Nimmo MA, Cobbold M, Harridge SD. Muscle function in elite master weightlifters. Med Sci Sports Exerc 34: 1199–1206, 2002. [DOI] [PubMed] [Google Scholar]
- 89.Pingel J, Langberg H, Skovgard D, Koskinen S, Flyvbjerg A, Frystyk J, Kjaer M, Hansen M. Effects of transdermal estrogen on collagen turnover at rest and in response to exercise in postmenopausal women. J Appl Physiol 113: 1040–1047, 2012. [DOI] [PubMed] [Google Scholar]
- 90.Pollanen E, Ronkainen PH, Suominen H, Takala T, Koskinen S, Puolakka J, Sipila S, Kovanen V. Muscular transcriptome in postmenopausal women with or without hormone replacement. Rejuvenation Res 10: 485–500, 2007. [DOI] [PubMed] [Google Scholar]
- 91.Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Myogenic gene expression at rest and after a bout of resistance exercise in young (18–30 yr) and old (80–89 yr) women. J Appl Physiol 101: 53–59, 2006. [DOI] [PubMed] [Google Scholar]
- 92.Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW. Control of the size of the human muscle mass. Annu Rev Physiol 66: 799–828, 2004. [DOI] [PubMed] [Google Scholar]
- 93.Roth SM, Ivey FM, Martel GF, Lemmer JT, Hurlbut DE, Siegel EL, Metter EJ, Fleg JL, Fozard JL, Kostek MC, Wernick DM, Hurley BF. Muscle size responses to strength training in young and older men and women. J Am Geriatr Soc 49: 1428–1433, 2001. [DOI] [PubMed] [Google Scholar]
- 94.Sarwar G, Botting HG, Collins M. A comparison of fasting serum amino acid profiles of young and elderly subjects. J Am Coll Nutr 10: 668–674, 1991. [DOI] [PubMed] [Google Scholar]
- 95.Scalzo RL, Peltonen GL, Binns SE, Shankaran M, Giordano GR, Hartley DA, Klochak AL, Lonac MC, Paris HL, Szallar SE, Wood LM, Peelor FF 3rd, Holmes WE, Hellerstein MK, Bell C, Hamilton KL, Miller BF. Greater muscle protein synthesis and mitochondrial biogenesis in males compared with females during sprint interval training. FASEB J 28: 2705–2714, 2014. [DOI] [PubMed] [Google Scholar]
- 96.Seeman E, Hopper JL, Young NR, Formica C, Goss P, Tsalamandris C. Do genetic factors explain associations between muscle strength, lean mass, and bone density? A twin study. Am J Physiol Endocrinol Metab 270: E320–E327, 1996. [DOI] [PubMed] [Google Scholar]
- 97.Shifren JL, Desindes S, McIlwain M, Doros G, Mazer NA. A randomized, open-label, crossover study comparing the effects of oral versus transdermal estrogen therapy on serum androgens, thyroid hormones, and adrenal hormones in naturally menopausal women. Menopause 14: 985–994, 2007. [DOI] [PubMed] [Google Scholar]
- 98.Sitnick M, Foley AM, Brown M, Spangenburg EE. Ovariectomy prevents the recovery of atrophied gastrocnemius skeletal muscle mass. J Appl Physiol 100: 286–293, 2006. [DOI] [PubMed] [Google Scholar]
- 99.Smith GI, Atherton P, Reeds DN, Mohammed BS, Jaffery H, Rankin D, Rennie MJ, Mittendorfer B. No major sex differences in muscle protein synthesis rates in the postabsorptive state and during hyperinsulinemia-hyperaminoacidemia in middle-aged adults. J Appl Physiol 107: 1308–1315, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith GI, Atherton P, Villareal DT, Frimel TN, Rankin D, Rennie MJ, Mittendorfer B. Differences in muscle protein synthesis and anabolic signaling in the postabsorptive state and in response to food in 65–80 year old men and women. PLoS One 3: e1875, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smith GI, Reeds DN, Hall AM, Chambers KT, Finck BN, Mittendorfer B. Sexually dimorphic effect of aging on skeletal muscle protein synthesis. Biol Sex Differ 3: 11, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smith GI, Villareal DT, Sinacore DR, Shah K, Mittendorfer B. Muscle protein synthesis response to exercise training in obese, older men and women. Med Sci Sports Exerc 44: 1259–1266, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Smith GI, Yoshino J, Reeds DN, Bradley D, Burrows RE, Heisey HD, Moseley AC, Mittendorfer B. Testosterone and progesterone, but not estradiol, stimulate muscle protein synthesis in postmenopausal women. J Clin Endocrinol Metab 99: 256–265, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sonnet E, Lacut K, Roudaut N, Mottier D, Kerlan V, Oger E. Effects of the route of oestrogen administration on IGF-1 and IGFBP-3 in healthy postmenopausal women: results from a randomized placebo-controlled study. Clin Endocrinol (Oxf) 66: 626–631, 2007. [DOI] [PubMed] [Google Scholar]
- 105.Sugiura T, Ito N, Goto K, Naito H, Yoshioka T, Powers SK. Estrogen administration attenuates immobilization-induced skeletal muscle atrophy in male rats. J Physiol Sci 56: 393–399, 2006. [DOI] [PubMed] [Google Scholar]
- 106.Svensson J, Movérare-Skrtic S, Windahl S, Swanson C, Sjögren K. Stimulation of both estrogen and androgen receptors maintains skeletal muscle mass in gonadectomized male mice but mainly via different pathways. J Mol Endocrinol 45: 45–57, 2010. [DOI] [PubMed] [Google Scholar]
- 107.Tesch PA, von Walden F, Gustafsson T, Linnehan RM, Trappe TA. Skeletal muscle proteolysis in response to short-term unloading in humans. J Appl Physiol 105: 902–906, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thomis MA, Van Leemputte M, Maes HH, Blimkie CJ, Claessens AL, Marchal G, Willems E, Vlietinck RF, Beunen GP. Multivariate genetic analysis of maximal isometric muscle force at different elbow angles. J Appl Physiol 82: 959–967, 1997. [DOI] [PubMed] [Google Scholar]
- 109.Tonson A, Ratel S, Le Fur Y, Cozzone P, Bendahan D. Effect of maturation on the relationship between muscle size and force production. Med Sci Sports Exerc 40: 918–925, 2008. [DOI] [PubMed] [Google Scholar]
- 110.Toth MJ, Poehlman ET, Matthews DE, Tchernof A, MacCoss MJ. Effects of estradiol and progesterone on body composition, protein synthesis, and lipoprotein lipase in rats. Am J Physiol Endocrinol Metab 280: E496–E501, 2001. [DOI] [PubMed] [Google Scholar]
- 111.Trappe T. Influence of aging and long-term unloading on the structure and function of human skeletal muscle. Appl Physiol Nutr Metab 34: 459–464, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tsai WJ, McCormick KM, Brazeau DA, Brazeau GA. Estrogen effects on skeletal muscle insulin-like growth factor 1 and myostatin in ovariectomized rats. Exp Biol Med (Maywood) 232: 1314–1325, 2007. [DOI] [PubMed] [Google Scholar]
- 113.Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, Ferrando A. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol Endocrinol Metab 269: E820–E826, 1995. [DOI] [PubMed] [Google Scholar]
- 114.Velders M, Schleipen B, Fritzemeier KH, Zierau O, Diel P. Selective estrogen receptor-beta activation stimulates skeletal muscle growth and regeneration. FASEB J 26: 1909–1920, 2012. [DOI] [PubMed] [Google Scholar]
- 115.Verdijk LB, Jonkers RA, Gleeson BG, Beelen M, Meijer K, Savelberg HH, Wodzig WK, Dendale P, van Loon LJ. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr 89: 608–616, 2009. [DOI] [PubMed] [Google Scholar]
- 116.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA 286: 1206–1212, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wall BT, Dirks ML, van Loon LJ. Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Rev 12: 898–906, 2013. [DOI] [PubMed] [Google Scholar]
- 118.Walts CT, Hanson ED, Delmonico MJ, Yao L, Wang MQ, Hurley BF. Do sex or race differences influence strength training effects on muscle or fat? Med Sci Sports Exerc 40: 669–676, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Welle S, Thornton C, Jozefowicz R, Statt M. Myofibrillar protein synthesis in young and old men. Am J Physiol Endocrinol Metab 264: E693–E698, 1993. [DOI] [PubMed] [Google Scholar]
- 120.Wells JC. Sexual dimorphism of body composition. Best Pract Res Clin Endocrinol Metab 21: 415–430, 2007. [DOI] [PubMed] [Google Scholar]
- 121.West DW, Burd NA, Churchward-Venne TA, Camera DM, Mitchell CJ, Baker SK, Hawley JA, Coffey VG, Phillips SM. Sex-based comparisons of myofibrillar protein synthesis after resistance exercise in the fed state. J Appl Physiol 112: 1805–1813, 2012. [DOI] [PubMed] [Google Scholar]
- 122.Whitman SA, Wacker MJ, Richmond SR, Godard MP. Contributions of the ubiquitin-proteasome pathway and apoptosis to human skeletal muscle wasting with age. Pflugers Arch 450: 437–446, 2005. [DOI] [PubMed] [Google Scholar]
- 123.Wilkes EA, Selby AL, Atherton PJ, Patel R, Rankin D, Smith K, Rennie MJ. Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age-related sarcopenia. Am J Clin Nutr 90: 1343–1350, 2009. [DOI] [PubMed] [Google Scholar]
- 124.Wimalawansa SM, Chapa MT, Wei JN, Westlund KN, Quast MJ, Wimalawansa SJ. Reversal of weightlessness-induced musculoskeletal losses with androgens: quantification by MRI. J Appl Physiol 86: 1841–1846, 1999. [DOI] [PubMed] [Google Scholar]
- 125.Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr 99: 86–95, 2014. [DOI] [PubMed] [Google Scholar]
- 126.Yang Y, Breen L, Burd NA, Hector AJ, Churchward-Venne TA, Josse AR, Tarnopolsky MA, Phillips SM. Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br J Nutr 108: 1780–1788, 2012. [DOI] [PubMed] [Google Scholar]
- 127.Yang Y, Churchward-Venne TA, Burd NA, Breen L, Tarnopolsky MA, Phillips SM. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr Metab (Lond) 9: 57, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yarrow JF, Conover CF, Beggs LA, Beck DT, Otzel DM, Balaez A, Combs SM, Miller JR, Ye F, Aguirre JI, Neuville KG, Williams AA, Conrad BP, Gregory CM, Wronski TJ, Bose PK, Borst SE. Testosterone dose dependently prevents bone and muscle loss in rodents after spinal cord injury. J Neurotrauma 31: 834–845, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yasuda N, Glover EI, Phillips SM, Isfort RJ, Tarnopolsky MA. Sex-based differences in skeletal muscle function and morphology with short-term limb immobilization. J Appl Physiol 99: 1085–1092, 2005. [DOI] [PubMed] [Google Scholar]