Abstract
Shear stress is known to stimulate an intracellular free calcium concentration ([Ca2+]i) response in vascular endothelial cells (ECs). [Ca2+]i is a key second messenger for signaling that leads to vasodilation and EC survival. Although it is accepted that the shear-induced [Ca2+]i response is, in part, due to Ca2+ release from the endoplasmic reticulum (ER), the role of mitochondria (second largest Ca2+ store) is unknown. We hypothesized that the mitochondria play a role in regulating [Ca2+]i in sheared ECs. Cultured ECs, loaded with a Ca2+-sensitive fluorophore, were exposed to physiological levels of shear stress. Shear stress elicited [Ca2+]i transients in a percentage of cells with a fraction of them displaying oscillations. Peak magnitudes, percentage of oscillating ECs, and oscillation frequencies depended on the shear level. [Ca2+]i transients/oscillations were present when experiments were conducted in Ca2+-free solution (plus lanthanum) but absent when ECs were treated with a phospholipase C inhibitor, suggesting that the ER inositol 1,4,5-trisphosphate receptor is responsible for the [Ca2+]i response. Either a mitochondrial uncoupler or an electron transport chain inhibitor, but not a mitochondrial ATP synthase inhibitor, prevented the occurrence of transients and especially inhibited the oscillations. Knockdown of the mitochondrial Ca2+ uniporter also inhibited the shear-induced [Ca2+]i transients/oscillations compared with controls. Hence, EC mitochondria, through Ca2+ uptake/release, regulate the temporal profile of shear-induced ER Ca2+ release. [Ca2+]i oscillation frequencies detected were within the range for activation of mechanoresponsive kinases and transcription factors, suggesting that dysfunctional EC mitochondria may contribute to cardiovascular disease by deregulating the shear-induced [Ca2+]i response.
Keywords: fluid shear stress, endothelial cell, mitochondria, intracellular calcium, calcium oscillations
the ubiquitous second messenger calcium (Ca2+) is involved in regulating a variety of cellular processes from cell differentiation and proliferation to apoptosis via Ca2+-sensitive signaling pathways, transcription factor activation, and gene expression (7). Both in vitro and in vivo experiments have shown that fluid mechanical shear stress acting on vascular endothelial cells (ECs) causes changes in their intracellular free Ca2+ levels ([Ca2+]i), which have been implicated in endothelial nitric oxide (NO) synthase (eNOS) activation and vasodilation (2, 18, 23, 44, 56, 66, 75). While it is accepted that shear stress elicits a [Ca2+]i response, there is controversy regarding the characteristics of the response (single transient vs. oscillations) and the relative contribution of intracellular vs. extracellular Ca2+ in shear-induced [Ca2+]i signaling (1, 40, 46, 49, 77). In general, shear stress is thought to cause release of endogenous ATP that activates EC surface purinergic receptors (P2Rs), recently identified as the Gq/G11 protein-coupled P2Y2 subtype (61, 75, 76). Gq/G11 proteins activate phospholipase C (PLC), which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol 1,4,5-trisphosphate (IP3) (20, 37). IP3 activates its receptor (IP3R) on the endoplasmic reticulum (ER) and promotes ER Ca2+ release (6). Store depletion activates store-operated Ca2+ channels (SOCC) on the plasma membrane allowing Ca2+ influx from the extracellular space (6). However, shear stress was also shown to directly activate membrane-bound Gq/G11 proteins without the need for P2Rs (via its effect on the glycocalyx and junctional proteins), as well as transient receptor potential vanilloid type 4 channels on the plasma membrane leading to Ca2+ entry (15, 28, 54).
Although the ER (and, in muscle, its specialized form the sarcoplasmic reticulum) is the major intracellular Ca2+ store, attention has also been focused on the second largest store, the mitochondria. It is well known that excess Ca2+ uptake by mitochondria via the mitochondrial Ca2+ uniporter (MCU) can result in mitochondrial Ca2+ ([Ca2+]mt) overload that triggers the opening of the mitochondrial permeability transition pore (mtPTP) leading to cell apoptosis or necrosis, depending on ATP levels (47, 67). However, by uptaking and releasing Ca2+, the mitochondria also play a role in regulating [Ca2+]i in different cell types, including ECs, following exposure to submaximal cytokine concentrations (16, 19, 39, 51, 52). Since Ca2+ regulates the IP3R channel in a biphasic manner (stimulatory at low [Ca2+]i and inhibitory at high [Ca2+]i), the spatial proximity between ER and mitochondria makes the latter an important player in the control of ER Ca2+ release (8, 12, 22, 38, 62, 63, 73). In regards to mechanical forces, application of pressurized flow on isolated cardiomyocytes was found to trigger a [Ca2+]i transient that was inhibited when mitochondrial function was disrupted (5). Earlier work from our group and others on the effects of shear stress on EC mitochondrial respiration, redox status, and biogenesis has established the EC mitochondria as mechanosensitive organelles and as regulators of shear-induced gene expression (29, 30, 41, 42, 48, 70). However, their role in shaping the [Ca2+]i signals in sheared ECs is unknown.
In this study, we hypothesized that the mitochondria, in collaboration with the ER, contribute to [Ca2+]i regulation in cultured ECs exposed to steady laminar shear stress. Confluent human umbilical vein EC (HUVEC) monolayers were incubated with a Ca2+-sensitive fluorophore and sheared at different levels corresponding to venous or arterial blood flow, and the characteristics of temporal [Ca2+]i changes were quantified. Some monolayers were exposed to a low arterial level of shear stress (10 dyn/cm2) either in the absence or presence of extracellular Ca2+, a PLC inhibitor, a protonophore (to abolish the driving force for Ca2+ uptake by mitochondria), a mitochondrial electron transport chain (ETC) complex III inhibitor, a mitochondrial ATP synthase inhibitor, and an inhibitor of the main mitochondrial Ca2+ efflux channel [mitochondrial Na+/Ca2+ exchanger (mNCX)]. Within the time period examined, shear stress was found to increase the percentage of ECs that responded by raising [Ca2+]i at least once (responding ECs), the peak magnitude, the percentage of ECs that responded with repetitive transients (oscillating ECs, a fraction of the responding cells), and the oscillation frequency compared with static and was also found to decrease the time interval between shear initiation and the first [Ca2+]i peak compared with static. All the response parameters, with the exception of the percentage of responding cells, changed dose dependently with shear stress level. Repeating the experiments in Ca2+-free solution supplemented with lanthanum (La3+), to inhibit the plasma membrane Ca2+ ATPase (PMCA) pump and prevent Ca2+ extrusion (65), caused no significant changes to the shear-induced [Ca2+]i response suggesting that it majorly depends on Ca2+ release from intracellular stores. Use of the pharmacological inhibitors showed that the shear-induced [Ca2+]i response is due to activation of the PLC/IP3/IP3R pathway and that mitochondrial function is important for all [Ca2+]i transients but is essential for the occurrence of oscillations. Shear experiments in ECs with MCU knockdown provided additional evidence on the role of mitochondrial Ca2+ uptake in regulating the shear-induced [Ca2+]i response. Collectively, our findings suggest that Ca2+ shuttling between the ER and mitochondria is a critical component of shear-induced EC [Ca2+]i transients and oscillations and, thus, highlight a new mechanism by which the endothelial mitochondria regulate mechanotransduction.
MATERIALS AND METHODS
EC culture and labeling with a [Ca2+]i fluorophore.
Pooled primary HUVECs (VEC Technologies) were grown in complete EC growth medium (Cell Applications) in a tissue culture incubator (37°C, 5% CO2/95% air). Plastic slides of the highest optical quality (μ-Slide VI0.4; ibidi) were coated with 1% gelatin solution for 20 min. ECs were seeded on gelatin-coated slides in growth medium and became confluent within 48 h. EC monolayers were incubated in HEPES (20 mM)-buffered HBSS with Ca2+ and magnesium (Mg2+), but without phenol red (Thermo Fisher) for 1 h to allow for EC equilibration with the media used during shear exposure. For live monitoring of [Ca2+]i, EC monolayers were incubated with the Ca2+-sensitive fluorophore fluo-4 AM (Thermo Fisher) at 3 μM in HEPES-HBSS for 20 min in a tissue culture incubator. They were then washed three times in HEPES-HBSS and allowed an additional 5-min period at 37°C (to complete the deesterification of intracellular dye). Only ECs of up to passage 8 were used in this study.
EC shear stress exposure and chemical treatments.
EC monolayers in parallel-plate flow chambers (with the ibidi slides) were attached to a syringe pump (Harvard Apparatus) using Tygon tubing and placed in a stage-top incubator (Tokai Hit; maintained at 37°C) of an epifluorescence microscope equipped with a CCD camera (Nikon; 494-nm excitation/506-nm emission; ×20 magnification). For the first 2 min, the cells were maintained under static conditions to establish a [Ca2+]i baseline. At the end of 2 min, they were exposed to either a (continued) 5-min static period or a 5-min perfusion of HEPES-HBSS at a flow rate corresponding to a wall shear stress of either 1, 4, or 10 dyn/cm2 (ranging from venous to low arterial).
To evaluate the relative contribution of intracellular vs. extracellular Ca2+ in the shear-induced [Ca2+]i response, some EC monolayers were sheared in Ca2+-free HEPES-HBSS supplemented with 1 mM lanthanum (La3+; to block the PMCA) (65). To test for the role of the PLC/IP3 pathway in the [Ca2+]i response, some ECs were sheared in the presence of either the PLC inhibitor U73122 or its inactive analog U73343 (each at 0.5 μM). To investigate the role of [Ca2+]mt in the [Ca2+]i response, some ECs were preincubated (20 min) and sheared in the presence of the mitochondrial protonophore carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP; 0.5 or 2 μM). While FCCP at 0.5 and 2 μM is expected to partially and completely, respectively, dissipate the mitochondrial membrane potential (ΔΨm) and block [Ca2+]mt uptake, it also depolarizes the EC plasma membrane in a dose-dependent manner (14, 25, 59). To test for FCCP specificity, some monolayers were preincubated (20 min) and sheared in the presence of the mitochondrial ETC complex III inhibitor antimycin A (10 μM). To probe the importance of mitochondrial ATP in the [Ca2+]i response, some ECs were preincubated (20 min) and sheared in the presence of the mitochondrial ATP synthase inhibitor oligomycin (2 μM), both alone and in combination with FCCP or antimycin A. Last, to examine the role of [Ca2+]mt efflux, some monolayers were preincubated (20 min) and sheared in the presence of the mNCX inhibitor CGP37157 (2 or 10 μM). Chemical concentrations and, where applicable, preincubation times were chosen from previous Ca2+ studies in ECs, and, in some cases, from studies in other cell types (4, 5, 25, 39, 43, 51, 59). In particular for CGP37157, although it has been used as an mNCX inhibitor at concentrations between 1 and 20 μM (4, 5, 39, 50, 51), it is known to also partially inhibit the plasmalemmal NCX at ≥10 μM (13). All chemical inhibitors (U73122, U73343, FCCP, antimycin A, oligomycin, and CGP37157; from either Sigma or Thermo Fisher) were dissolved in DMSO for stock solutions. The final DMSO concentration in contact with ECs was <0.1%, and vehicle treatment was used as control. Due to visible loss of EC shape, no experiments were performed with monolayers in Ca2+-free HEPES-HBSS; EC monolayers in Ca2+-free solution supplemented with 1 mM La3+ did not appear any different compared with EC monolayers in HEPES-HBSS (58, 65).
Culture and shear exposure of a HUVEC line with stable MCU knockdown.
To probe the role of [Ca2+]mt uptake in the shear-induced [Ca2+]i response, while avoiding the ambiguity of chemical inhibitors, a HUVEC line with stable MCU knockdown was tested. Specifically, EA.hy926 cells (ATCC) were grown in DMEM (high glucose)/15% FBS, supplemented with 0.5% endothelial cell growth supplement, 100 U/ml penicillin, and 100 μg/ml streptomycin (Thermo Fisher) in a tissue culture incubator. EA.hy926 cells (5 × 105/well) grown in six-well plates were transduced with either MCU shRNA (TRCN0000133861; Broad Institute RNAi Consortium) lentivirus or nontargeting shRNA lentivirus and were selected with puromycin (2 μg/ml) 48 h posttransduction for 10 days, as previously described (33). MCU knockdown was confirmed both by quantitative (q)RT-PCR and by [Ca2+]mt dynamics measurements following cell exposure to histamine (33). For shear exposure, wild-type (WT) and MCU knockdown (MCU KD) cells were seeded on ibidi slides and cultured in DMEM/10% FBS for 48 h until confluency. WT and MCU KD cells were incubated in HEPES-HBSS for 1 h followed by a 20-min preincubation with fluo-4 AM (3 μM), 0.02% pluronic F-127 (Thermo Fisher), and 50 μM sulfinpyrazone (Sigma) in HEPES-HBSS, as previously described (33). They were then washed with HEPES-HBSS and exposed to shear stress, as described above for HUVECs.
Image acquisition and analysis.
Digital images of relative fluorescence changes were collected at a frequency of 1 Hz and stored in sequence using NIS-Elements Basic Research software (Nikon). ECs were outlined and separated from the background using the open source program CellProfiler (10). Outline images were superimposed onto the original images, where each region segmented by CellProfiler (corresponding to the surface area of a cell) was considered a region of interest (ROI). The average fluorescence intensity/ROI (F) was calculated in arbitrary units (AU) using the image processing toolbox in MATLAB (MathWorks). To account for variations in baseline F (AU) among ECs on the same slide (up to 2-fold) and the even greater variation in F among ECs in experiments performed on different days, F at each time point was normalized to its initial (for each experiment, this was the first digital frame acquired) fluorescence intensity (F0): (F − F0)/F0, and each ROI was analyzed independently. This method of image analysis was previously employed by the Segal group (69), who used fluo-4 to investigate temporal [Ca2+]i changes in ECs of freshly isolated EC tubes exposed to acetylcholine, and was found to enable the study of [Ca2+]i oscillations in individual ECs (contrary to spatial averaging of EC [Ca2+]i responses that masked the oscillations).
The normalized fluorescence response to shear stress was characterized by quantifying the following parameters: percentages of responding and oscillating cells, peak magnitude, time to peak, full duration at half-maximum (FDHM), and oscillation frequency. A peak was defined as a change in sign of the normalized fluorescence time derivative (slope changed from positive to negative) with an additional requirement being that the normalized fluorescence intensity should increase by at least 15%. Responding cells were cells that showed at least one peak during the 5-min shear exposure; oscillating cells were those that showed greater than or equal to two peaks during shear exposure. The percentage of responding cells per field of view was plotted both vs. time during 2-min static/5-min shear and as cumulative at the end of 5-min shear. The percentage of oscillating cells was reported as cumulative at the end of 5-min shear. Peak magnitude was defined as the normalized fluorescence magnitude of the largest peak (99.9% of the time, this was the first peak following shear initiation). Time to peak was defined as the time interval between shear initiation and the first peak in each responding cell. FDHM was the width of each peak at half of peak magnitude (in units of time). Oscillation frequency was the inverse of time period (the time interval between consecutive peaks) in each oscillating cell.
Statistical analysis.
Data of responding cells (%), peak magnitude, time to peak, FDHM, oscillating cells (%), and oscillation frequency (mHz) for each treatment are presented as means ± SE. Statistical differences for each of the above parameters among all shear levels (including static, 0 dyn/cm2) was done using a one-way ANOVA followed by Tukey's post hoc analysis. Statistical differences for each response parameter among chemical treatments and vehicle-treated shear (10 dyn/cm2) control were determined by one-way ANOVA followed by Dunnett's test. When responding cells (%) were plotted vs. time (min), time points during the 5-min shear period were compared with the average of the two time points in their corresponding (preshear) static period using one-way repeated-measures ANOVA followed by paired t-tests with the Bonferroni-Holm correction. To compare responding cells (%) and peak magnitude during the shear period, in the absence or presence of chemical treatments, to their corresponding (preshear) static values, two-way repeated-measures ANOVA followed by paired t-tests with the Bonferroni-Holm correction was used. To compare any response parameters between WT and MCU KD cells, Student's t-test was used. All analyses were performed on a PC using JMP statistical software (SAS Institute). P ≤ 0.05 was taken to indicate statistical significance. Last, the accuracy level of the shear-induced [Ca2+]i oscillation frequency measurements was determined based on a statistical formula that calculates the confidence interval for the mean of a Poisson distribution (central limit theorem; Ref. 60). For 20–30 cells in the microscope field of view (assuming that a peak in a cell does not affect the peaks in another cell, an assumption that can be challenged; Ref. 34) and a 5-min total image acquisition time at 1 Hz, the oscillation frequency measurement becomes more precise (precision is the half length of a 95% confidence interval) as the number of independent experiments increases. For example, at an oscillation frequency of 8 mHz (∼1 peak/2 min), three independent experiments allowed for 8% accuracy, whereas eight independent experiments put the estimate within 5% accuracy. However, at an oscillation frequency of 15 mHz (∼1 peak/min), three independent experiments allowed for 5% accuracy. Hence, in an effort to keep the accuracy of the measured oscillation frequencies at ≤5% precision, each mechanical or chemical treatment tested was repeated between four and eight times (independent experiments), depending on the oscillation frequency.
RESULTS
Shear stress induces a dose-dependent, transient and oscillatory [Ca2+]i response.
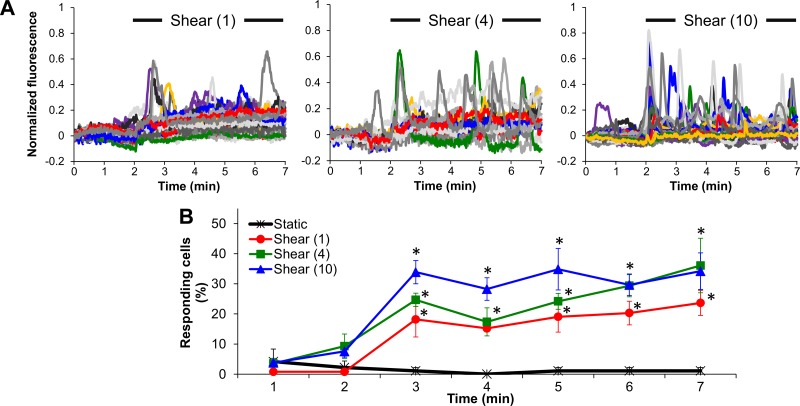
Fluo-4-loaded ECs were monitored for changes in fluorescence during a 2-min static period followed by either a 5-min exposure to shear stress (1, 4 or 10 dyn/cm2) or a continued 5-min static incubation. Characteristic normalized fluorescence traces for each cell in the microscope field of view (∼20–30 cells) demonstrated the occurrence of transient normalized fluorescence increases upon the onset and throughout shear exposure in a portion of cells, and the response appeared to intensify the higher the shear stress (Fig. 1A). There was a lack of transients throughout the static period relative to the shear period (Fig. 1A). When responding cells were counted every minute throughout the 7-min experiment and plotted at the end of that minute (as a percentage of cells in the field of view), it was found that shear stress at either 1, 4, or 10 dyn/cm2 significantly increased the number of responding cells within the first minute and maintained that increased response throughout the shear period compared with the corresponding (preshear) static period (Fig. 1B).
Fig. 1.
Shear stress causes endothelial cell (EC) intracellular free calcium concentration ([Ca2+]i) transient increases compared with static. A: characteristic normalized fluo-4 fluorescence signals vs. time during a 2-min static followed by a 5-min shear period at either 1, 4, or 10 dyn/cm2 (each colored line corresponds to a single cell in a microscope field of view; 10 colors are being repeated). B: responding cells (%) plotted every minute (at the end of each minute) during either a 7-min static period or a 2-min static period followed by a 5-min shear period (1, 4, or 10 dyn/cm2). Data are means ± SE (n = 4–8 independent experiments). *P < 0.05 vs. its corresponding (preshear) static period (average of the first 2-min data points).
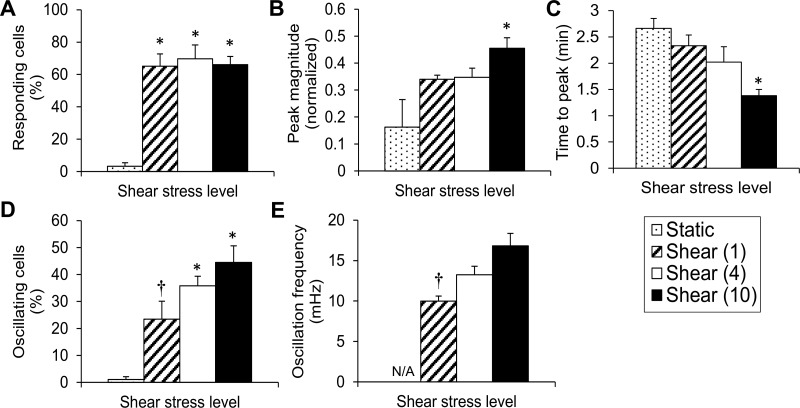
When the total number of responding cells throughout the shear period (as a percentage of cells in the field of view) was compared with the total number of responding cells during a 5-min static period, it was found that shear of either 1, 4, or 10 dyn/cm2 caused a significant increase in responding cells compared with static (notice that, even if the same cell responded more than once in the 5-min shear period, it was counted as just one responding cell), and there was no statistical difference among shear levels (Fig. 2A). Only the highest shear stress tested (10 dyn/cm2) caused a significant increase in mean peak magnitude (of responding cells) compared with static (Fig. 2B). The large SE of the mean peak magnitude at static was due to the limited number of peaks observed. The mean time to peak showed a decreasing trend with increasing shear, but only the highest shear stress significantly shortened the time between flow initiation and the first peak compared with static (Fig. 2C). FDHM values during a 5-min period at either 0 (static), 1, 4 or 10 dyn/cm2 were calculated at 18.5 ± 2.1, 29.3 ± 2.2, 23.2 ± 2.3, and 19.7 ± 1.4 s, respectively (means ± SE; n = 4–8 independent experiments). Although there was a trend of decreasing mean FDHM values with increasing shear between 1 and 10 dyn/cm2, no significant differences were found among different conditions. Either 4 or 10 dyn/cm2 resulted in a significant increase in the percentage of oscillating cells compared to static (Fig. 2D). Since it was extremely rare for a cell to oscillate under static conditions (Fig. 2D), oscillation frequencies were calculated only for cells that were sheared. Shear caused a graded response in mean oscillation frequencies in the range of ∼10–17 mHz with the oscillation frequency at 10 dyn/cm2 being significantly higher than that at 1 dyn/cm2 (Fig. 2E). Hence, [Ca2+]i transiently increases in sheared ECs, and every quantifiable measure of the shear-induced [Ca2+]i response, with the exception of responding cells (%) and FDHM, changes in proportion to the shear level.
Fig. 2.
Shear stress dose-dependently changes key quantifiable measures of the [Ca2+]i, response. A: responding cells (%) at the end of 5 min is plotted vs. shear stress level (0, 1, 4, or 10 dyn/cm2). B: magnitude of normalized fluorescence of the largest peak is averaged over all responding cells/experiment and independent experiments and plotted vs. shear stress level. C: time to first peak is processed and plotted as in B. D: oscillating cells (%) at the end of 5 min are plotted vs. shear stress level. E: oscillation frequency (mHz) of oscillating cells is plotted vs. shear stress level. Due to the shortage of oscillating cells under static, calculation of the oscillation frequency was labeled not applicable (N/A). Data are means ± SE (n = 4–8 independent experiments). *P < 0.05 vs. static. †P < 0.05 vs. the highest shear tested (10 dyn/cm2).
Shear-induced [Ca2+]i transients and oscillations depend on IP3-mediated ER Ca2+ release.
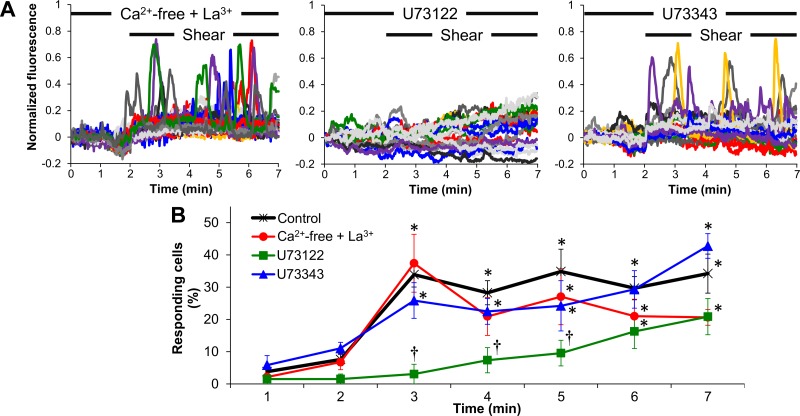
To examine the contribution of intracellular stores in the [Ca2+]i transients, shear experiments (10 dyn/cm2) were repeated in Ca2+-free solution supplemented with La3+ (thus in the absence of Ca2+ influx and concomitant prevention of Ca2+ extrusion). To assess the role of the PLC/IP3 pathway, ECs were sheared in regular solution in the presence of the PLC inhibitor U73122 or its inactive analog U73343. None of the treatments (Ca2+-free + La3+, U73122, or U73343) caused any significant changes in baseline fluorescence intensities (AU), where baseline was taken as either the fluorescence intensity of the first digital frame of the 2-min static or the average of the 2-min static before 5-min shear, compared with untreated (vehicle-treated) controls (not shown). When ECs were exposed to Ca2+-free solution supplemented with La3+ for 2-min static/5-min shear, shear caused a response that was indistinguishable from the response in regular solution (Fig. 3A). U73122 totally abolished all transients, whereas U73343 allowed for the shear-induced transients to occur. U73122 also resulted in a slow, gradual increase in normalized fluorescence in a portion of cells with time under shear (Fig. 3A). The percentage of responding cells vs. time during the 5-min shear period was significantly higher than that during the static period for the (shear) control, Ca2+-free + La3+, and U73343 conditions but not during the first 3-min shear in the presence of U73122 (Fig. 3B). Since the normalized fluorescence surpassed the 15% increase above baseline (our definition of a peak) beyond 3-min of shear in the presence of U73122, the last 2 min of shear data were categorized by the software as responses (and the cells as responding cells; Fig. 3B), despite the fact that no “real” peaks were observed in the digital images. In accordance to the above, only the first 3 min data points of responding cells (%) in the presence of U73122 were found to be significantly different from the corresponding time points in the control curve (Fig. 3B).
Fig. 3.
Shear-induced [Ca2+]i increases are due to inositol 1,4,5-trisphosphate (IP3)-dependent endoplasmic reticulum (ER) Ca2+ release. A: characteristic normalized fluorescence signals vs. time for endothelial cells (ECs) during a 2-min static incubation followed by shear (10 dyn/cm2) in either Ca2+-free solution supplemented with La3+ or in regular solution in the presence of either U73122 or U73343 (each colored line corresponds to a single cell in a microscope field of view). B: responding cells (%) vs. time (every minute) for control (shear at 10 dyn/cm2), Ca2+-free + La3+, U73122, or U73343. Data are means ± SE (n = 4–8 independent experiments). *P < 0.05 vs. its corresponding (preshear) static period. †P < 0.05 vs. control (at the corresponding time point).
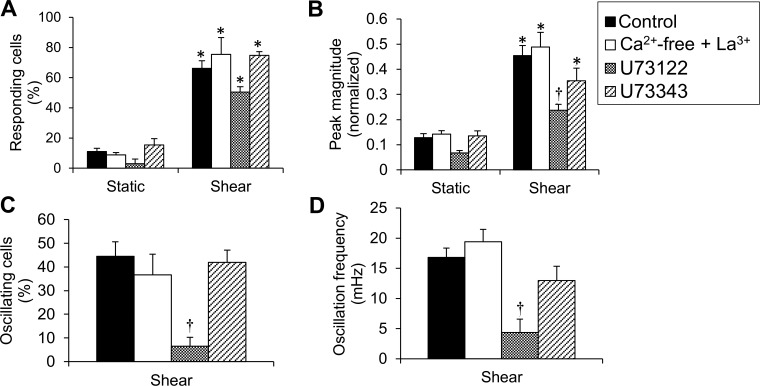
Responding cells (%) throughout the shear period for either Ca2+-free + La3+, U73122, or U73343 were significantly higher compared with responding cells (%) during the corresponding static period (Fig. 4A). Although this comparison was made between a 2-min static period and a 5-min shear period, it should be mentioned that the percentage of responding cells during a 2-min static period is not statistically significantly different from that of responding cells during a 5-min static period under any condition tested (not shown, except in the case of static control; compare Figs. 4A with 2A). No significant difference was found among different treatments during shear (Fig. 4A). Peak magnitudes were significantly higher under shear vs. static for Ca2+-free + La3+ and U73343, and only the peak magnitude under shear in the presence of U73122 was significantly lower than the peak magnitude of control (Fig. 4 B). Time to peak was not significantly affected by either Ca2+-free + La3+ or U73343 and was significantly increased by U73122 (due to the slow rise in background fluorescence) (not shown). There was no FDHM to measure in the case of U73122, and FDHM was not significantly affected by any chemical treatment compared with control (not shown). U73122, but not U73343, significantly inhibited both the percentage of oscillating cells and the oscillation frequency compared with control (Fig. 4, C and D). The data collectively suggest that the ER, via activation of the PLC/IP3 pathway, regulates the shear-induced [Ca2+]i response, in terms of both [Ca2+]i transients and oscillations.
Fig. 4.
ER Ca2+ release is required for shear-induced [Ca2+]i transients and oscillations. A: responding cells (%) throughout the (preshear) static period vs. the shear period for control, Ca2+-free + La3+, U73122, and U73343. B: peak magnitude of normalized fluorescence during static vs. shear for the same treatments as in A. C: oscillating cells (%) for the same treatments (since there were no oscillating cells during static, data were plotted only for ECs under shear). D: oscillation frequency (mHz) for the same treatments. Data are means ± SE (n = 4–8 independent experiments). *P < 0.05 vs. static under the same treatment. †P < 0.05 vs. control.
Shear-induced [Ca2+]i transients and oscillations depend on EC mitochondria.
To assess the role of mitochondrial function in the shear-induced [Ca2+]i response, ECs were both preincubated and sheared in the presence of either FCCP (0.5 or 2 μM), antimycin A (10 μM), oligomycin (2 μM) with/without FCCP (2 μM) or antimycin A (10 μM), or CGP37157 (2 or 10 μM). None of the treatments caused any significant changes in baseline fluorescence intensities (AU) compared with untreated controls (not shown). In separate experiments, fluorescence was monitored during the 20-min (static) preincubation period. In the case of FCCP and antimycin A, normalized fluorescence increased following addition of the chemical and then it declined back to the original baseline value within ∼3 min, whereas, in the case of oligomycin and CGP37157, it remained unchanged throughout the preincubation period (not shown), further confirming that all EC monolayers started the 2-min static/5-min shear exposure at comparable baseline fluorescence values independently of treatment.
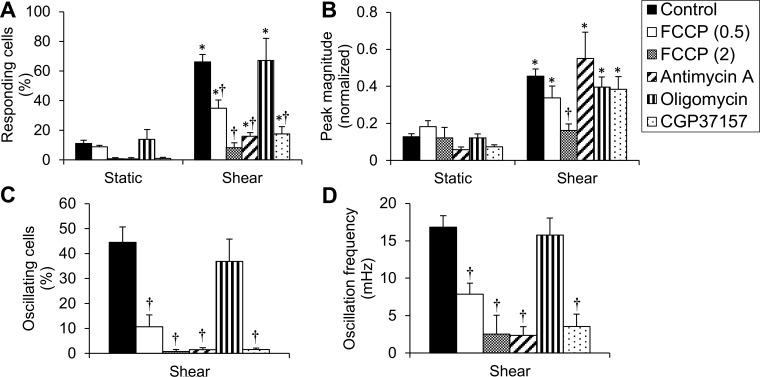
FCCP inhibited the shear-induced increases in normalized fluorescence in a dose-dependent manner (Fig. 5, A and B); at 2 μM, it totally abolished the response (Fig. 5B). To confirm that the FCCP effect on the [Ca2+]i response was due to its action on ΔΨm/[Ca2+]mt, shear experiments were repeated using ECs treated with antimycin A (10 μM), an inhibitor of the mitochondrial ETC that contributes to the generation of ΔΨm. Antimycin A allowed for a single transient of normalized fluorescence in a percentage of cells but inhibited the oscillations (Fig. 5, A and B). To examine whether mitochondrial ATP is needed for the shear-induced response, oligomycin was tested; no significant difference was found compared with control (Fig. 5B). Since, in the presence of either the high FCCP concentration or antimycin A, there is no driving force for the mitochondrial ATP synthase and ATP may be consumed as the ATP synthase operates in reverse mode, experiments were repeated with combined FCCP or antimycin A plus oligomycin; the effect was the same as in either compound alone (not shown). These data suggested that the effects of FCCP and antimycin A on the shear-induced [Ca2+]i response are not due to mitochondrial ATP depletion but rather due to inhibition of [Ca2+]mt transport. To probe the role of [Ca2+]mt efflux in the [Ca2+]i response, ECs were both preincubated and sheared in the presence of the mNCX inhibitor CGP37157 (2 or 10 μM). At 2 μM, CGP37157 did not have any significant effect on the shear-induced [Ca2+]i response (not shown). However, at 10 μM (a concentration with potential nonspecific effects), it showed an inhibitory effect similar to that by antimycin A; it allowed for a single transient in a percentage of cells and blocked the oscillations (only CGP37157 at 10 μM is shown in Figs. 5 and 6). The responding cells (%) vs. time plot showed that FCCP (0.5 μM), antimycin A, and CGP37157 at the first minute of shear and oligomycin throughout the 5-min shear were significantly higher than the (preshear) static period (Fig. 5B). In addition, FCCP (2 μM) throughout the shear period and FCCP (0.5), antimycin A, and CGP37157 for the last 4 min of shear were significantly lower than the (shear) control at corresponding times (Fig. 5B).
Fig. 5.
Shear-induced [Ca2+]i transients and oscillations require Ca2+ buffering by mitochondria. A: characteristic normalized fluorescence signals vs. time for ECs during a 2-min static incubation followed by shear (10 dyn/cm2) in the presence of either FCCP (0.5), antimycin A, or CGP37157. B: responding cells (%) vs. time (every min) for control, FCCP (0.5 or 2 μM), antimycin A, oligomycin. or CGP37157. Data are means ± SE (n = 4–8 independent experiments). *P < 0.05 vs. its corresponding (preshear) static period. †P < 0.05 vs. control (at the corresponding time point).
Fig. 6.
EC mitochondria regulate the shear-induced [Ca2+]i transients and oscillations. A: responding cells (%) throughout the (preshear) static period vs. the shear period for control, FCCP (0.5 or 2 μM), antimycin A, oligomycin, and CGP37157. B: peak magnitude of normalized fluorescence during static vs. shear for the same treatments as in A. C: oscillating cells (%) for the same treatments. D: oscillation frequency (mHz) for the same treatments. Data are means ± SE (n = 4–8 independent experiments). *P < 0.05 vs. static under the same treatment. †P < 0.05 vs. control.
Responding cells (%) throughout shear for either FCCP (0.5), antimycin A, oligomycin or CGP37157 were significantly higher compared with responding cells (%) under static, whereas either dose of FCCP, antimycin A, or CGP37157 resulted in a significant decrease in responding cells (%) compared with control (Fig. 6A). Peak magnitudes were significantly higher under shear vs. static for FCCP (0.5 μM), antimycin A, oligomycin, and CGP37157, and only the peak magnitude under FCCP (2 μM) was significantly lower than the peak magnitude of control (Fig. 6B). CGP37157 (and antimycin A almost) significantly decreased the mean time to peak compared with control [not shown; it should be mentioned that time to peak is not meaningful in the case of FCCP (2), due to the limited number of peaks]. FDHM was not significantly affected by any chemical treatment compared with control (not shown). FCCP at either concentration and antimycin A and CGP37157 significantly inhibited both the oscillating cells (%) and the oscillation frequency compared with control (Fig. 6, C and D). Either FCCP (2 μM) or antimycin A combined with oligomycin gave similar results regarding responding cells (%), peak magnitude, time to peak, FDHM, oscillating cells (%), and oscillation frequency as either did alone (not shown). The data collectively suggest that healthy mitochondria are necessary for both shear-induced [Ca2+]i single transients and oscillations, with the oscillations being especially dependent on [Ca2+]mt handling.
Shear-induced [Ca2+]i transients and oscillations depend on [Ca2+]mt uptake.
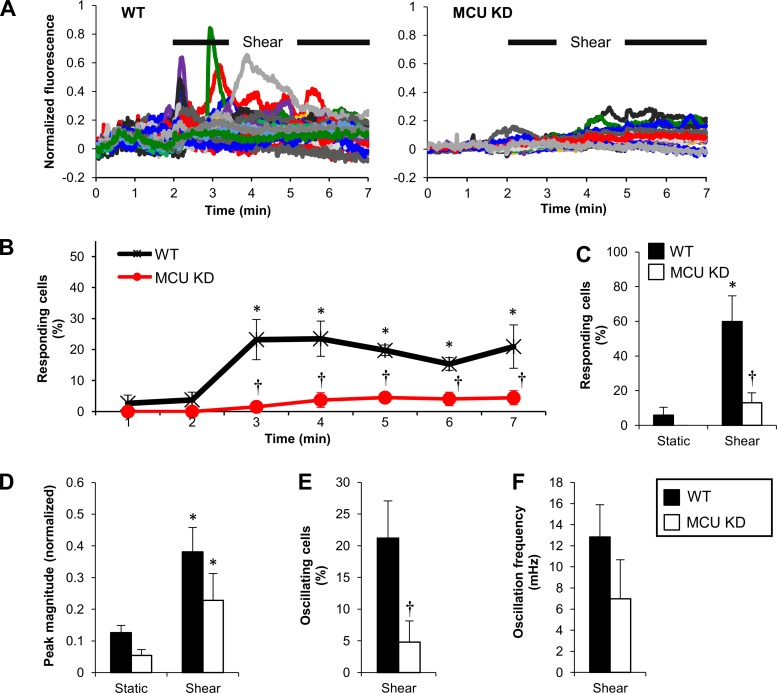
Baseline fluo-4 fluorescence (AU) in WT cells was not significantly different from that in MCU KD cells (not shown). When these cells were sheared, WT responded similar to HUVEC controls, whereas MCU KD showed a diminished response (Fig. 7A). The responding cells (%) vs. time plot verified that a significant portion of WT cells increased their fluorescence at each minute during the 5-min shear compared with the 2-min static (Fig. 7B). The percentage of responding cells in MCU KD was significantly lower than in WT at each minute during shear (Fig. 7B); this was also true throughout shear exposure (Fig. 7C). The mean peak magnitude in either WT or MCU KD was significantly higher compared with static but not significantly different from each other, although MCU KD had a lower mean (Fig. 7D). Mean time to peak was significantly increased by MCU KD compared with WT (not shown). The percentage of oscillating cells was significantly inhibited in MCU KD compared with WT (Fig. 7E). The oscillation frequency in MCU KD was lower but did not reach significant difference, compared with WT (Fig. 7F). The MCU KD data support the earlier findings using mitochondrial chemical inhibitors by pointing to the importance of [Ca2+]mt uptake in shaping the shear-induced [Ca2+]i transients and oscillations.
Fig. 7.
EC mitochondrial Ca2+ ([Ca2+]mt) uptake is required for shear-induced [Ca2+]i transients and oscillations. A: characteristic normalized fluorescence signals vs. time for wild-type (WT) and mitochondrial Ca2+ uniporter (MCU) knockdown (KD) ECs during a 2-min static incubation followed by shear (10 dyn/cm2). B: responding cells (%) vs. time for WT and MCU KD. Data are means ± SE (n = 4–8 independent experiments). *P < 0.05 vs. corresponding static. †P < 0.05 vs. WT (at each time point). C: responding cells (%) throughout static and shear for wild-type (WT) and MCU knockdown (KD). D: peak magnitude during static and shear for WT and MCU KD. E and F: oscillating cells (%) and oscillation frequency, respectively, during shear of WT and MCU KD. For C–F: *P < 0.05 vs. corresponding static. †P < 0.05 vs. WT.
DISCUSSION
In the present study, we demonstrated that the EC [Ca2+]i response to fluid shear stress is, at least in part, in the form of oscillations, and quantitative characteristics of the oscillatory behavior, such as peak magnitude, oscillating cells (%), and oscillation frequency, vary in proportion to the shear stress level. Both the initiation and temporal propagation of [Ca2+]i oscillations are driven by the release of Ca2+ from the ER via the PLC/IP3/IP3R pathway, but they also require healthy mitochondria that are able to uptake and release Ca2+ in collaboration with the ER IP3R.
Shear-induced [Ca2+]i oscillations.
Before this work, others documented asynchronous [Ca2+]i transients in sheared ECs in the absence of exogenous ATP, both in vitro and in vivo, as well as the dependence of [Ca2+]i peak magnitude on the level of shear stress (18, 40, 66). Instead of spatially averaging fluorescence signals across the microscope field of view (that would show only an initial [Ca2+]i peak at the onset of shear followed by a slow decline to baseline values), we chose to monitor the characteristics of [Ca2+]i signaling vs. time for each individual cell, as previously (69). This analysis, when applied to shear experiments at arterial shear stress (10 dyn/cm2), showed that, within a 5-min time window, the majority of ECs respond to shear stress with repetitive transients (oscillations) (∼60% of total cells responded, but ∼70% of the responding cells oscillated; Fig. 2, A and D). Due to the uneven surface of the monolayer and various cell orientations, ECs that either respond minimally or not at all may be the ones that encounter shearing forces of lower values or at certain angles (compared with the cell's axis; Ref. 74). It is also possible that cells that responded with single transients are those that just exhibited their first oscillation and, if flow was continued beyond 5 min, they might have presented subsequent peaks.
Ca2+ shuttling between ER and mitochondria in sheared ECs.
Repeating the shear experiments in Ca2+-free solution supplemented with La3+, as well as in the presence of U73122 or its inactive analog in regular solution, suggested that both single transients and oscillations depend on Ca2+ release via the ER IP3R (Figs. 3 and 4). Shear stress is known to cause a rapid rise in IP3 levels, and IP3 was found to remain elevated for >5 min after the flow onset (57). Although U73122 abolished the response, it also caused a gradual [Ca2+]i increase in ∼50% of total cells during shear (Figs. 3A and 4A), which could be due to slow Ca2+ efflux from the ER (incomplete PLC inhibition) and/or Ca2+ influx across the plasma membrane.
To probe the role of mitochondria in the shear-induced [Ca2+]i response, we preincubated ECs with mitochondrial bioenergetics modulators before shear exposure, as in earlier studies where they first made mitochondria dysfunctional and then subjected the cells to either chemical (16, 39, 51) or mechanical stimulation (4, 5, 52). Use of FCCP, antimycin A, oligomycin, and CGP37157 (the latter, with ambiguity regarding its specificity) in regular solution pointed to the role of mitochondrial function (beyond ATP production) in shaping the shear-induced [Ca2+]i response (Figs. 5 and 6). Healthy mitochondria were especially important for [Ca2+]i oscillations, based on the fact that FCCP (0.5) and antimycin A decreased the number of responding cells by ∼50 and ∼75%, respectively, but inhibited the number of oscillating cells by ∼80 and ∼95%, respectively, compared with control (Fig. 6, A and C). Shear experiments using an EC line with MCU knockdown confirmed that [Ca2+]mt transport (uptake) is the mitochondrial property necessary for [Ca2+]i transients and oscillations (Fig. 7). MCU KD decreased both the responding and the oscillating cells by ∼75% compared with WT (Fig. 7, C and E), despite the fact that fewer cells oscillated in EA.hy926 WT compared with HUVECs (Figs. 2D and 7E). None of the inhibitors (with the exception of FCCP at 2 μM) nor MCU KD had a significant effect on peak magnitude compared with respective controls (Figs. 6B and 7D), probably because the magnitude of the first peak (the largest peak in 99.9% of experiments) is primarily regulated by IP3 levels and IP3-mediated ER Ca2+ release.
Our findings agree with studies on cells treated with submaximal concentrations of IP3-generating cytokines: agonist-induced [Ca2+]i oscillations were abolished in cell lines expressing a mutant IP3R with a reduced sensitivity to Ca2+, suggesting that IP3R and [Ca2+]i form a positive feedback loop that confers the regenerative properties on ER Ca2+ release and is crucial for the generation of oscillatory [Ca2+]i patterns (55). In a highly cited article, Ishii et al. (39) measured Ca2+ dynamics within intracellular stores (ER and mitochondria) simultaneously with [Ca2+]i in HeLa cells. Analysis of Ca2+ signals in the absence or presence of mitochondrial bioenergetics modulators revealed that the first oscillation is generated by Ca2+ release from the ER with a fraction of Ca2+ being taken up by mitochondria, whereas subsequent oscillations are initiated by Ca2+ release from mitochondria, which triggers regenerative Ca2+ release from the ER (39). Lately, the role of the MCU in regenerative ER Ca2+ release was demonstrated in activated mast cells, where either increasing the FCCP concentration or knocking down the MCU inhibited the [Ca2+]i oscillations (65). Last, Malli et al. (51) showed that, in ECs treated with histamine, the [Ca2+]i increase is mostly due to Ca2+ release from the ER and mitochondria and that the mitochondria uptake some of the Ca2+ released from the ER and also release some of their Ca2+ back to the ER. All the above together with the fact that the majority of the EC mitochondria are located in the perinuclear region and 50–55% of them colocalize with the ER (25, 27) support our major finding that the EC mitochondria are active players in the shear-induced [Ca2+]i signaling.
[Ca2+]i homeostasis in sheared ECs.
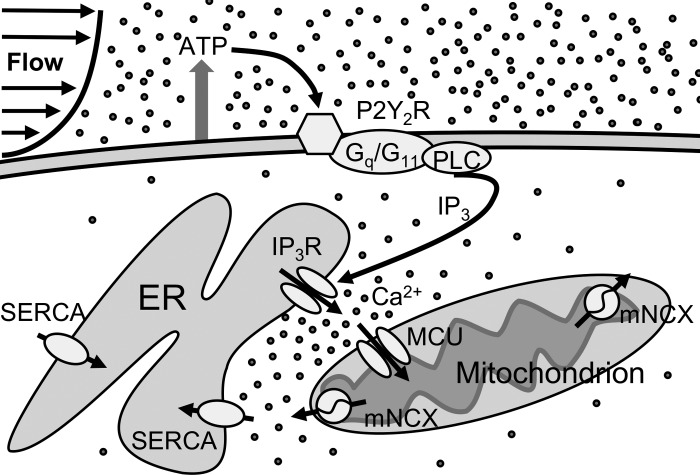
Figure 8 shows a schematic illustration of proposed Ca2+ signaling in ECs exposed to shear stress that includes the shuttling of Ca2+ between ER and mitochondria, based on the present and previous studies. Briefly, shear forces acting on ECs are expected to cause endogenous ATP release and subsequent P2R activation of plasma membrane-bound Gq/G11 proteins (61, 75, 76). Following the PLC/IP3/IP3R-mediated Ca2+ release from the ER, there is high [Ca2+]i locally near IP3Rs, which, based on the literature, may result in Ca2+-dependent IP3R inactivation (1st [Ca2+]i peak) (8, 38). Some of that Ca2+ is rapidly transported into the mitochondria via the MCU, and afterwards extruded from the organelle via the mNCX (22, 62, 63). The notion of Ca2+ exchange between ER and mitochondria is supported by the presence of ER-mitochondrial junctions called “mitochondria-associated membranes” (MAMs) (12, 62). In the subcellular region between ER and mitochondria, Ca2+ uptake by mitochondria is thought to suppress the Ca2+ inhibitory effect on IP3R allowing for continued ER Ca2+ release (thus facilitating the occurrence of the 1st transient and of subsequent oscillations, based on our findings in MCU KD ECs) (11, 65, 72). [Ca2+]mt release is known to assist in refilling the ER Ca2+ store and, by increasing [Ca2+]i levels locally in that region, it may also provide feedback control of ER Ca2+ release (with some studies reporting activation and others claiming inhibition of ER Ca2+ release) (3, 32, 39, 51). Due to the CGP37157 lack of specificity at the concentration used in this study, the specific role of [Ca2+]mt release could not be clarified. Evidence in different cell types suggests that sustaining the oscillations for longer times requires Ca2+ entry through SOCC (58).
Fig. 8.
Schematic diagram of proposed Ca2+ signaling in ECs exposed to shear stress. Shear stress, mainly via ATP binding to P2Y2R, is thought to activate the G protein/PLC/IP3 pathway. IP3 activates the IP3R and causes Ca2+ release from the ER, which at high concentrations may deactivate the IP3R in the subcellular region between ER and mitochondria. Both [Ca2+]mt uptake via the MCU and [Ca2+]mt release via the mNCX may differentially regulate the activation of IP3R locally and, thus, play a role in shaping the first [Ca2+]i transient and, in particular, the subsequent oscillations. [Ca2+]mt release is thought to also help refill the ER store via the sarco-endoplasmic reticulum Ca2+-ATPase (SERCA).
EC decoding of the shear-induced [Ca2+]i oscillations.
[Ca2+]i oscillations occur in all cells in response to low doses of agonists and, in nonexcitable cells, mostly arise because both IP3 and [Ca2+]i regulate the IP3R with [Ca2+]i exerting a biphasic effect (38, 58, 71, 73). It is thought that cells prefer an oscillatory [Ca2+]i response over a single, sustained one to avoid Ca2+ overload and desensitization of Ca2+-dependent processes. Oscillations allow low levels of stimulation to activate responses by imparting information to Ca2+-sensitive proteins, which decode it based on the oscillation characteristics: amplitude, frequency, FDHM, duty cycle [the product of frequency (Hz) and FDHM (s)], or cumulative duration [the product of duty cycle and total time of exposure to a stimulus (s)] (58, 68). The multiple decoding mechanisms allow for a lot more diverse information to be stored in oscillations than in a sustained response. For most cells and stimuli, it is the changes in frequency (and duty cycle) that determine the extent of activation of decoder proteins and, ultimately, the cell response (64, 68). Our observed frequency (∼17 mHz) and duty cycle (∼0.017 s−1 × 20 s = 0.34) at arterial shear stress fall within, or close to, the range reported for agonist-induced nuclear factor (NF)-κB activation and stimulation of vascular cell adhesion molecule (VCAM)-1 expression in ECs (68), an expected result, since laminar shear stress is known to transiently activate NF-κB and upregulate VCAM-1 (36, 79). Our values also fall within the range for Ca2+ clamping-induced phosphorylation of extracellular signal-regulated kinases (ERK) 1/2 in HeLa cells (45), which agrees with the fact that shear stress activates ERK1/2 in ECs (78).
Another important decoder in ECs is the Ca2+/calmodulin-dependent protein kinase II (CaMKII). Our measured frequency for arterial flow is an order of magnitude lower than (but the duty cycle is within) the range for CaMKII activation in neurons (21); our frequency is closer to that shown to activate CaMKII in fertilized eggs (53). CaMKII has four isoforms encoded by different genes giving rise to multiple splice variants (9); this may affect the requirements for its activation by [Ca2+]i oscillations in different cell types. Furthermore, blood plasma contains a low concentration of ATP (≤200 nM), and the combination of exogenous ATP with shear stress may increase the oscillation frequency bringing it to the level required for EC CaMKII activation (26, 66). Since eNOS is activated by CaMKII upon the onset of shear stress (44), inhibition of [Ca2+]i oscillations is expected to block the early rise in shear-induced NO production. Sustained, shear-induced NO release is known to require eNOS phosphorylation by Akt, a process thought to be Ca2+ independent (17, 44). However, it was recently shown that shear-induced ATP release activates P2Y2R and Gq/G11 proteins, which phosphorylate platelet/endothelial cell adhesion molecule (PECAM)-1 and vascular endothelial growth factor receptor 2 (VEGFR2) leading to Akt phosphorylation and eNOS activation/NO release, and either P2Y2R or Gq/G11 deficiency blocks both the [Ca2+]i increase and the PECAM-1/VEGFR2/Akt/eNOS activation (75). P2Y2R-mediated [Ca2+]i increase is also important for NO production in static ECs exposed to ATP (61). Based on all the above, we can speculate that inhibition of [Ca2+]mt transport, via its effect on the occurrence and the frequency of [Ca2+]i oscillations (Figs. 6, 7), may have a negative impact on sustained NO production by sheared ECs.
In conclusion, our major finding that sheared-induced [Ca2+]i oscillations in ECs require Ca2+ buffering by mitochondria highlights the important role of these organelles in mechanotranduction. Although it is known that steady and pulsatile flows increase EC [Ca2+]i and NO production, whereas oscillatory flow (encountered in atheroprone regions of human arteries) does not (24, 31, 35), the effects of these flows on [Ca2+]mt buffering have not been delineated. It is possible that cardiovascular diseases associated with altered hemodynamics and endothelial dysfunction arise due to faulty regulation of [Ca2+]mt buffering and shear-induced [Ca2+]i oscillations.
GRANTS
This work was supported by National Institutes of Health Grants R01-GM-109882, R01-HL-086699, R01-HL-119306, and 1S10-RR-027327 (to M. Madesh), R15-HL-121778 (to N. M. Tsoukias), and R21-HL-106392; a scientific cooperation grant between São Paulo Research Foundation, Brazil and Ohio State University; and an American Heart Association Grant-in-Aid (to B. R. Alevriadou).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.G.S., M.M., N.M.T., and B.R.A. conception and design of research; C.G.S., J.A.J., and S.S. performed experiments; C.G.S., J.A.J., N.M.T., and B.R.A. analyzed data; C.G.S. and B.R.A. interpreted results of experiments; C.G.S. prepared figures; C.G.S., S.S., and B.R.A. edited and revised manuscript; B.R.A. drafted manuscript; B.R.A. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. H. N. Nagaraja, Ohio State University Biostatistics, and N. Dolman, Thermo Fisher (Molecular Probes), for expert advice on statistical analysis and fluorescence data analysis, respectively. We thank Dr. R. J. Giedt for preliminary experiments.
REFERENCES
- 1.Ando J, Yamamoto K. Flow detection and calcium signaling in vascular endothelial cells. Cardiovasc Res 99: 260–268, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Andrews AM, Jaron D, Buerk DG, Barbee KA. Shear stress-induced NO production is dependent on ATP autocrine signaling and capacitative calcium entry. Cell Mol Bioeng 7: 510–520, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnaudeau S, Kelley WL, Walsh JV Jr, Demaurex N. Mitochondria recycle Ca2+ to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J Biol Chem 276: 29430–29439, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Belmonte S, Morad M. “Pressure-flow”-triggered intracellular Ca2+ transients in rat cardiac myocytes: possible mechanisms and role of mitochondria. J Physiol 586: 1379–1397, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belmonte S, Morad M. Shear fluid-induced Ca2+ release and the role of mitochondria in rat cardiac myocytes. Ann NY Acad Sci 1123: 58–63, 2008. [DOI] [PubMed] [Google Scholar]
- 6.Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta 1793: 933–940, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351: 751–754, 1991. [DOI] [PubMed] [Google Scholar]
- 9.Cai H, Liu D, Garcia JG. CaM Kinase II-dependent pathophysiological signalling in endothelial cells. Cardiovasc Res 77: 30–34, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, Golland P, Sabatini DM. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol 7: R100, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins TJ, Lipp P, Berridge MJ, Li W, Bootman MD. Inositol 1,4,5-trisphosphate-induced Ca2+ release is inhibited by mitochondrial depolarization. Biochem J 347: 593–600, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol 174: 915–921, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czyz A, Kiedrowski L. Inhibition of plasmalemmal Na+/Ca2+ exchange by mitochondrial Na+/Ca2+ exchange inhibitor 7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one (CGP-37157) in cerebellar granule cells. Biochem Pharmacol 66: 2409–2411, 2003. [DOI] [PubMed] [Google Scholar]
- 14.de la Fuente S, Fonteriz RI, de la Cruz PJ, Montero M, Alvarez J. Mitochondrial free [Ca2+] dynamics measured with a novel low-Ca2+ affinity aequorin probe. Biochem J 445: 371–376, 2012. [DOI] [PubMed] [Google Scholar]
- 15.dela Paz NG, Melchior B, Shayo FY, Frangos JA. Heparan sulfates mediate the interaction between platelet endothelial cell adhesion molecule-1 (PECAM-1) and the Galphaq/11 subunits of heterotrimeric G proteins. J Biol Chem 289: 7413–7424, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delmotte P, Yang B, Thompson MA, Pabelick CM, Prakash YS, Sieck GC. Inflammation alters regional mitochondrial Ca2+ in human airway smooth muscle cells. Am J Physiol Cell Physiol 303: C244–C256, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999. [DOI] [PubMed] [Google Scholar]
- 18.Duza T, Sarelius IH. Localized transient increases in endothelial cell Ca2+ in arterioles in situ: implications for coordination of vascular function. Am J Physiol Heart Circ Physiol 286: H2322–H2331, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Eisner V, Csordas G, Hajnoczky G. Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle–pivotal roles in Ca(2+) and reactive oxygen species signaling. J Cell Sci 126: 2965–2978, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erb L, Liao Z, Seye CI, Weisman GA. P2 receptors: intracellular signaling. Pflügers Arch 452: 552–562, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Eshete F, Fields RD. Spike frequency decoding and autonomous activation of Ca2+-calmodulin-dependent protein kinase II in dorsal root ganglion neurons. J Neurosci 21: 6694–6705, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filippin L, Magalhaes PJ, Di Benedetto G, Colella M, Pozzan T. Stable interactions between mitochondria and endoplasmic reticulum allow rapid accumulation of calcium in a subpopulation of mitochondria. J Biol Chem 278: 39224–39234, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Fisslthaler B, Dimmeler S, Hermann C, Busse R, Fleming I. Phosphorylation and activation of the endothelial nitric oxide synthase by fluid shear stress. Acta Physiol Scand 168: 81–88, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Gambillara V, Chambaz C, Montorzi G, Roy S, Stergiopulos N, Silacci P. Plaque-prone hemodynamics impair endothelial function in pig carotid arteries. Am J Physiol Heart Circ Physiol 290: H2320–H2328, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Giedt RJ, Pfeiffer DR, Matzavinos A, Kao CY, Alevriadou BR. Mitochondrial dynamics and motility inside living vascular endothelial cells: Role of bioenergetics. Ann Biomed Eng 52: 348–356, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorman MW, Feigl EO, Buffington CW. Human plasma ATP concentration. Clin Chem 53: 318–325, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Graier WF, Frieden M, Malli R. Mitochondria and Ca2+ signaling: old guests, new functions. Pflügers Arch 455: 375–396, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gudi S, Nolan JP, Frangos JA. Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc Natl Acad Sci USA 95: 2515–2519, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han Z, Chen YR, Jones CI 3rd Meenakshisundaram G, Zweier JL, Alevriadou BR. Shear-induced reactive nitrogen species inhibit mitochondrial respiratory complex activities in cultured vascular endothelial cells. Am J Physiol Cell Physiol 292: C1103–C1112, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Han Z, Varadharaj S, Giedt RJ, Zweier JL, Szeto HH, Alevriadou BR. Mitochondria-derived reactive oxygen species mediate heme oxygenase-1 expression in sheared endothelial cells. J Pharmacol Exp Ther 329: 94–101, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Helmlinger G, Berk BC, Nerem RM. Calcium responses of endothelial cell monolayers subjected to pulsatile and steady laminar flow differ. Am J Physiol Cell Physiol 269: C367–C375, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Hernandez-SanMiguel E, Vay L, Santo-Domingo J, Lobaton CD, Moreno A, Montero M, Alvarez J. The mitochondrial Na+/Ca2+ exchanger plays a key role in the control of cytosolic Ca2+ oscillations. Cell Calcium 40: 53–61, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman NE, Chandramoorthy HC, Shanmughapriya S, Zhang X, Rajan S, Mallilankaraman K, Gandhirajan RK, Vagnozzi RJ, Ferrer LM, Sreekrishnanilayam K, Natarajaseenivasan K, Vallem S, Force T, Choi ET, Cheung JY, Madesh M. MICU1 motifs define mitochondrial calcium uniporter binding and activity. Cell Rep 5: 1576–1588, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong D, Jaron D, Buerk DG, Barbee KA. Heterogeneous response of microvascular endothelial cells to shear stress. Am J Physiol Heart Circ Physiol 290: H2498–H2508, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Hsiai TK, Hwang J, Barr ML, Correa A, Hamilton R, Alavi M, Rouhanizadeh M, Cadenas E, Hazen SL. Hemodynamics influences vascular peroxynitrite formation: Implication for low-density lipoprotein apo-B-100 nitration. Free Radic Biol Med 42: 519–529, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu Q, Deshpande S, Irani K, Ziegelstein RC. [Ca2+]i oscillation frequency regulates agonist-stimulated NF-kappaB transcriptional activity. J Biol Chem 274: 33995–33998, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Hubbard KB, Hepler JR. Cell signalling diversity of the Gqalpha family of heterotrimeric G proteins. Cell Signal 18: 135–150, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Iino M, Endo M. Calcium-dependent immediate feedback control of inositol 1,4,5-triphosphate-induced Ca2+ release. Nature 360: 76–78, 1992. [DOI] [PubMed] [Google Scholar]
- 39.Ishii K, Hirose K, Iino M. Ca2+ shuttling between endoplasmic reticulum and mitochondria underlying Ca2+ oscillations. EMBO Rep 7: 390–396, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.James NL, Harrison DG, Nerem RM. Effects of shear on endothelial cell calcium in the presence and absence of ATP. FASEB J 9: 968–973, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Jones CI 3rd, Han Z, Presley T, Varadharaj S, Zweier JL, Ilangovan G, Alevriadou BR. Endothelial cell respiration is affected by the oxygen tension during shear exposure: role of mitochondrial peroxynitrite. Am J Physiol Cell Physiol 295: C180–C191, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim B, Lee H, Kawata K, Park JY. Exercise-mediated wall shear stress increases mitochondrial biogenesis in vascular endothelium. PLoS One 9: e111409, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kokoska ER, Wolff AB, Smith GS, Miller TA. Epidermal growth factor-induced cytoprotection in human intestinal cells involves intracellular calcium signaling. J Surg Res 88: 97–103, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Kuchan MJ, Frangos JA. Role of calcium and calmodulin in flow-induced nitric oxide production in endothelial cells. Am J Physiol Cell Physiol 266: C628–C636, 1994. [DOI] [PubMed] [Google Scholar]
- 45.Kupzig S, Walker SA, Cullen PJ. The frequencies of calcium oscillations are optimized for efficient calcium-mediated activation of Ras and the ERK/MAPK cascade. Proc Natl Acad Sci USA 102: 7577–7582, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwan HY, Leung PC, Huang Y, Yao X. Depletion of intracellular Ca2+ stores sensitizes the flow-induced Ca2+ influx in rat endothelial cells. Circ Res 92: 286–292, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Lemasters JJ, Qian T, He L, Kim JS, Elmore SP, Cascio WE, Brenner DA. Role of mitochondrial inner membrane permeabilization in necrotic cell death, apoptosis, and autophagy. Antioxid Redox Signal 4: 769–781, 2002. [DOI] [PubMed] [Google Scholar]
- 48.Li R, Beebe T, Cui J, Rouhanizadeh M, Ai L, Wang P, Gundersen M, Takabe W, Hsiai TK. Pulsatile shear stress increased mitochondrial membrane potential: implication of Mn-SOD. Biochem Biophys Res Commun 388: 406–412, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu B, Lu S, Zheng S, Jiang Z, Wang Y. Two distinct phases of calcium signalling under flow. Cardiovasc Res 91: 124–133, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Malli R, Frieden M, Osibow K, Zoratti C, Mayer M, Demaurex N, Graier WF. Sustained Ca2+ transfer across mitochondria is essential for mitochondrial Ca2+ buffering, store-operated Ca2+ entry, and Ca2+ store refilling. J Biol Chem 278: 44769–44779, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Malli R, Frieden M, Trenker M, Graier WF. The role of mitochondria for Ca2+ refilling of the endoplasmic reticulum. J Biol Chem 280: 12114–12122, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Mann ZF, Duchen MR, Gale JE. Mitochondria modulate the spatio-temporal properties of intra- and intercellular Ca2+ signals in cochlear supporting cells. Cell Calcium 46: 136–146, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Markoulaki S, Matson S, Ducibella T. Fertilization stimulates long-lasting oscillations of CaMKII activity in mouse eggs. Dev Biol 272: 15–25, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol 298: H466–H776, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyakawa T, Mizushima A, Hirose K, Yamazawa T, Bezprozvanny I, Kurosaki T, Iino M. Ca2+-sensor region of IP3 receptor controls intracellular Ca2+ signaling. EMBO J 20: 1674–1680, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mo M, Eskin SG, Schilling WP. Flow-induced changes in Ca2+ signaling of vascular endothelial cells: effect of shear stress and ATP. Am J Physiol Heart Circ Physiol 260: H1698–H1707, 1991. [DOI] [PubMed] [Google Scholar]
- 57.Nollert MU, Eskin SG, McIntire LV. Shear stress increases inositol trisphosphate levels in human endothelial cells. Biochem Biophys Res Commun 170: 281–287, 1990. [DOI] [PubMed] [Google Scholar]
- 58.Parekh AB. Decoding cytosolic Ca2+ oscillations. Trends Biochem Sci 36: 78–87, 2011. [DOI] [PubMed] [Google Scholar]
- 59.Park KS, Jo I, Pak K, Bae SW, Rhim H, Suh SH, Park J, Zhu H, So I, Kim KW. FCCP depolarizes plasma membrane potential by activating proton and Na+ currents in bovine aortic endothelial cells. Pflügers Arch 443: 344–352, 2002. [DOI] [PubMed] [Google Scholar]
- 60.Patil VV, Kulkarni HV. Comparison of confidence intervals for the Poisson mean: some new aspects. REVSTAT Stat J 10: 211–227, 2012. [Google Scholar]
- 61.Raqeeb A, Sheng J, Ao N, Braun AP. Purinergic P2Y2 receptors mediate rapid Ca(2+) mobilization, membrane hyperpolarization and nitric oxide production in human vascular endothelial cells. Cell Calcium 49: 240–248, 2011. [DOI] [PubMed] [Google Scholar]
- 62.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13: 566–578, 2012. [DOI] [PubMed] [Google Scholar]
- 63.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280: 1763–1766, 1998. [DOI] [PubMed] [Google Scholar]
- 64.Salazar C, Politi AZ, Hofer T. Decoding of calcium oscillations by phosphorylation cycles: analytic results. Biophys J 94: 1203–1215, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Samanta K, Douglas S, Parekh AB. Mitochondrial calcium uniporter MCU supports cytoplasmic Ca2+ oscillations, store-operated Ca2+ entry and Ca2+-dependent gene expression in response to receptor stimulation. PLoS One 9: e101188, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen J, Luscinskas FW, Connolly A, Dewey CF, Gimbrone MA. Fluid shear stress modulates cytosolic free calcium in vascular endothelial cells. Am J Physiol Cell Physiol 262: C384–C390, 1992. [DOI] [PubMed] [Google Scholar]
- 67.Smaili SS, Hsu YT, Youle RJ, Russell JT. Mitochondria in Ca2+ signaling and apoptosis. J Bioenerg Biomembr 32: 35–46, 2000. [DOI] [PubMed] [Google Scholar]
- 68.Smedler E, Uhlen P. Frequency decoding of calcium oscillations. Biochim Biophys Acta 1840: 964–969, 2014. [DOI] [PubMed] [Google Scholar]
- 69.Socha MJ, Domeier TL, Behringer EJ, Segal SS. Coordination of intercellular Ca2+ signaling in endothelial cell tubes of mouse resistance arteries. Microcirculation 19: 757–770, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takabe W, Jen N, Ai L, Hamilton R, Wang S, Holmes K, Dharbandi F, Khalsa B, Bressler S, Barr ML, Li R, Hsiai TK. Oscillatory shear stress induces mitochondrial superoxide production: implication of NADPH oxidase and c-Jun NH2-terminal kinase signaling. Antioxid Redox Signal 15: 1379–1388, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uhlen P, Fritz N. Biochemistry of calcium oscillations. Biochem Biophys Res Commun 396: 28–32, 2010. [DOI] [PubMed] [Google Scholar]
- 72.Vay L, Hernandez-Sanmiguel E, Santo-Domingo J, Lobaton CD, Moreno A, Montero M, Alvarez J. Modulation of Ca2+ release and Ca2+ oscillations in HeLa cells and fibroblasts by mitochondrial Ca2+ uniporter stimulation. J Physiol 580: 39–49, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walsh C, Barrow S, Voronina S, Chvanov M, Petersen OH, Tepikin A. Modulation of calcium signalling by mitochondria. Biochim Biophys Acta 1787: 1374–1382, 2009. [DOI] [PubMed] [Google Scholar]
- 74.Wang C, Baker BM, Chen CS, Schwartz MA. Endothelial cell sensing of flow direction. Arterioscler Thromb Vasc Biol 33: 2130–216, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang S, Iring A, Strilic B, Albarran Juarez J, Kaur H, Troidl K, Tonack S, Burbiel JC, Muller CE, Fleming I, Lundberg JO, Wettschureck N, Offermanns S. P2Y(2) and Gq/G(11) control blood pressure by mediating endothelial mechanotransduction. J Clin Invest 125: 3077–3086, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yamamoto K, Furuya K, Nakamura M, Kobatake E, Sokabe M, Ando J. Visualization of flow-induced ATP release and triggering of Ca2+ waves at caveolae in vascular endothelial cells. J Cell Sci 124: 3477–3483, 2011. [DOI] [PubMed] [Google Scholar]
- 77.Yamamoto K, Korenaga R, Kamiya A, Ando J. Fluid shear stress activates Ca2+ influx into human endothelial cells via P2X4 purinoceptors. Circ Res 87: 385–391, 2000. [DOI] [PubMed] [Google Scholar]
- 78.Yeh LH, Park YJ, Hansalia RJ, Ahmed IS, Deshpande SS, Goldschmidt-Clermont PJ, Irani K, Alevriadou BR. Shear-induced tyrosine phosphorylation in endothelial cells requires Rac1-dependent production of ROS. Am J Physiol Cell Physiol 276: C838–C847, 1999. [DOI] [PubMed] [Google Scholar]
- 79.Zhu L, Luo Y, Chen T, Chen F, Wang T, Hu Q. Ca2+ oscillation frequency regulates agonist-stimulated gene expression in vascular endothelial cells. J Cell Sci 121: 2511–2518, 2008. [DOI] [PubMed] [Google Scholar]