A novel rat strain with a null mutation of the gene coding for the master antioxidant transcription factor nuclear factor (erythroid-derived 2)-like-2 (NRF2) has been developed. The paradoxical protective effect of low-dose angiotensin II infusion to ameliorate endothelial dysfunction and prevent microvascular rarefaction in salt-fed animals is absent in Nrf2−/− mutant rats.
Keywords: nuclear factor (erythroid-derived 2)-like-2, angiotensin II, endothelium, salt, nitric oxide, angiogenesis
Abstract
Nuclear factor (erythroid-derived 2)-like-2 (NRF2) is a master antioxidant and cell protective transcription factor that upregulates antioxidant defenses. In this study we developed a strain of Nrf2 null mutant rats to evaluate the role of reduced NRF2-regulated antioxidant defenses in contributing to endothelial dysfunction and impaired angiogenic responses during salt-induced ANG II suppression. Nrf2−/− mutant rats were developed using transcription activator-like effector nuclease technology in the Sprague-Dawley genetic background, and exhibited a 41-bp deletion that included the start codon for Nrf2 and an absence of immunohistochemically detectable NRF2 protein. Expression of mRNA for the NRF2-regulated indicator enzymes heme oxygenase-1, catalase, superoxide dismutase 1, superoxide dismutase 2, and glutathione reductase was significantly lower in livers of Nrf2−/− mutant rats fed high salt (HS; 4% NaCl) for 2 wk compared with wild-type controls. Endothelium-dependent dilation to acetylcholine was similar in isolated middle cerebral arteries (MCA) of Nrf2−/− mutant rats and wild-type littermates fed low-salt (0.4% NaCl) diet, and was eliminated by short-term (3 days) HS diet in both strains. Low-dose ANG II infusion (100 ng/kg sc) reversed salt-induced endothelial dysfunction in MCA and prevented microvessel rarefaction in wild-type rats fed HS diet, but not in Nrf2−/− mutant rats. The results of this study indicate that suppression of NRF2 antioxidant defenses plays an essential role in the development of salt-induced oxidant stress, endothelial dysfunction, and microvessel rarefaction in normotensive rats and emphasize the potential therapeutic benefits of directly upregulating NRF2-mediated antioxidant defenses to ameliorate vascular oxidant stress in humans.
NEW & NOTEWORTHY
A novel rat strain with a null mutation of the gene coding for the master antioxidant transcription factor nuclear factor (erythroid-derived 2)-like-2 (NRF2) has been developed. The paradoxical protective effect of low-dose angiotensin II infusion to ameliorate endothelial dysfunction and prevent microvascular rarefaction in salt-fed animals is absent in Nrf2−/− mutant rats.
a state of redox homeostasis is critical to maintaining normal vascular function. Elevated levels of reactive oxygen species (ROS) not only reduce nitric oxide availability, but also generate reactive nitrogen species such as peroxynitrite, which directly impairs signaling pathways mediating vascular relaxation (2, 3, 22, 26). In addition to its effects on the vasculature, increased oxidant stress has deleterious effects on multiple other organs and tissues, including lung (5, 35, 48, 49, 55), liver (44, 61), and brain (1, 53, 54, 63), emphasizing the importance of antioxidant defense systems throughout the body.
Nuclear factor (erythroid-derived 2)-like-2 (NRF2 or NFE2L2) is a redox-sensitive transcription factor that binds to the antioxidant response element (ARE) in the promoter region of hundreds of antioxidant and cytoprotective genes (21, 23). Together with its cytosolic inhibitor protein Kelch-like ECH-associated protein 1 (Keap1) (24), NRF2 senses oxidant/electrophilic stress and coordinates a transcriptional defense response. Recently, NRF2 has emerged as an attractive research target because dysregulation of NRF2 may provide a logical explanation for the association between oxidant stress and more than 200 human diseases (21).
Although Nrf2 knockout mice exist, the development of a Nrf2−/− mutant rat is significant and important because there is an extensive infrastructure of genetic and physiological data on the rat, the rat is better suited for physiological studies than the mouse because of its larger body size, and the rat is the preferred model within the pharmaceutical industry (25). An additional advantage of using transcription activator-like effector nuclease (TALEN) methodology or other gene-editing approaches to delete the Nrf2−/− gene in the rat is the availability of multiple specialized rat strains sensitized to model various human diseases, such as low-renin salt-sensitive hypertension (Dahl salt-sensitive rat), pulmonary hypertension (Fawn-hooded hypertensive rat), metabolic syndrome (obese Zucker rat and JCR rat), and nonobese type 2 diabetes mellitus [Goto-Kakizaki (GK) rat] that are not available in the mouse. The latter possibility could open new avenues of discovery regarding the role of NRF2 antioxidant defenses in various pathological conditions designed to mimic human disease.
High-salt (HS) diet suppresses renin release and reduces plasma angiotensin II (ANG II) levels. Congruent with studies in healthy human volunteers demonstrating endothelial dysfunction after short-term salt loading (58), elevated dietary salt intake in normotensive animals rapidly results in endothelial dysfunction (33, 46, 56), oxidant stress (31, 46, 65), suppressed antioxidant enzyme expression/activity (29), enhanced pro-oxidant enzyme activity (30), and impaired endothelial Ca2+ signaling (64).
In animal models, salt-induced vascular dysfunction can be prevented by acute scavenging of ROS (46) or, paradoxically, via intravenous infusion of a subpressor dose (3–5 ng·kg−1·min−1) of ANG II to restore physiological levels of ANG II in the blood (9, 10, 37, 38). Consistent with studies showing beneficial effects of low-dose ANG II infusion on collateral vessel development following coronary occlusion in Wistar-Kyoto (WKY) rats (50), low-dose ANG II infusion also prevents salt-induced microvascular rarefaction in HS-fed Sprague-Dawley (SD) rats (18) and impaired angiogenic responses to muscle stimulation in HS-fed SS.13BN consomic rats carrying a normally functioning renin gene in the Dahl salt-sensitive genetic background (8). A potential common denominator in all those effects of ANG II infusion is the recovery of vascular redox homeostasis. However, the relationships between HS diet, physiological ANG II levels, and the ARE-NRF2-KEAP1 axis have not been investigated.
In the present study, we developed a novel rat strain with a deletion of the Nrf2 gene to test the hypothesis that the NRF2 antioxidant defense system plays an important role in endothelial dysfunction and microvascular rarefaction associated with elevated dietary salt intake. An additional goal of these experiments was to use this novel rat strain to assess the potential role of NRF2 in mediating the protective effect of chronic low-dose ANG II infusion [to prevent salt-induced ANG II suppression (15, 64)] in ameliorating endothelial dysfunction and preventing microvascular rarefaction in rats fed a HS diet.
METHODS
Animals.
Initial experiments evaluating the expression of NRF2-regulated indicator enzymes in Nrf2−/− mutant rats and wild-type (WT) littermates used 8- to 10-wk-old male rats (see description below) that were maintained on low-salt (LS, 0.4% NaCl) diet or switched to a HS (4.0% NaCl) diet for 2 wk. To evaluate the role of NRF2 in mediating the protective effect of chronic low-dose ANG II infusion to restore endothelial function (33, 37, 38, 46, 60, 64, 65) and prevent microvascular rarefaction (16, 18), a second group of animals (short-term HS diet) was switched to HS diet for 3 days before receiving a subcutaneous osmotic minipump containing either isotonic saline or ANG II for an additional 3 days, as previously described (9, 46).
In preliminary experiments (not shown), we determined that maximal restoration of vasodilator responses to acetylcholine (ACh), and the stable prostacyclin analog iloprost and the G protein activator cholera toxin, and reduction of perfusate/superfusate Po2 to ∼40 torr in middle cerebral arteries (MCA) of HS-fed SD parental rats occurred during subcutaneous infusion of 100 ng·kg−1·min−1 ANG II, while infusion of lower doses (25 or 50 ng·kg−1·min−1) or higher doses (200 or 1,000 g·kg−1·min−1) of ANG II was less effective (or ineffective) in restoring vascular relaxation in response to these vasodilator stimuli, all of which were eliminated by HS diet. Based on those results, the 100 ng·kg−1·min−1 dose was used in the present experiments. That dose of ANG II also prevented salt-induced downregulation of Cu/Zn superoxide dismutase (SOD1) expression in cerebral arteries, mesenteric resistance arteries, and aortas of SD rats fed HS diet (not shown). All animal protocols were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Generation of Nrf2−/− mutant rats.
The SD-Nfe212em1Mcwi mutant rat [hereafter called the Nrf2−/− mutant] developed for these studies was created on the SD genetic background using TALEN technology. In vitro-transcribed mRNAs encoding FLASH XTN TALENs targeting the sequence TAGTCCTGGCGGTGGCAATTCCAAGTCCATCATGCTGAGGGCGGACGCTGCGCTA within the first exon of the rat Nrf2 gene were obtained from Transposagen Biopharmaceuticals and injected (10 ng/μl) in SD (Crl:SD; Charles River Laboratories) embryos as previously described (12). Embryos were implanted in pseudopregnant females, and pups were genotyped using the following primers: 5′-TCCGCCTTTAAGTTCTTGTCCCGT-3′ (forward), 5′-AAGCCGGAGCTTCCTTCATACC-3′ (reverse). A candidate mutant founder was identified using the Surveyor Nuclease Assay (Transgenomic) (12) and backcrossed to the SD strain to establish a heterozygous mutant colony. Sanger sequencing confirmed germ line transmission of a 41-bp deletion (CTCAGCATGATGGACTTGGAATTGCCACCGCCAGGACTACA) within the first exon, eliminating the Nrf2 translation start codon (underlined and in bold). Experimental homozygous mutant and control sibling WT animals were generated for all studies by intercrossing heterozygotes and were genotyped using a simple sequence length polymorphism genotyping protocol on the Applied Biosystems 3730xl DNA Analyzer, where the forward primer above was tagged with an M13 tail (41).
Verification of Nrf2 mutation via polymerase chain reaction and immunohistochemistry.
To verify the mutation via PCR studies, total RNA was extracted from 25-mg kidney and liver samples from Nrf2 mutant and WT littermates using a commercially available kit (IBI Scientific). RNA was reversed transcribed to cDNA using an iScript cDNA synthesis kit (Bio-Rad). The cDNA was amplified via PCR with Platinum Taq Polymerase (Life Technologies) using the following primer sequences: 5′-CTATCTGCTGGTTCCCCACTGCT-3′ (forward) and 5′-GGCTGGCTGAATTGGGAGGA-3′ (reverse) to amplify the region targeted by the TALEN sequence. The thermal profile was 95οC denaturation for 5 min, 32 cycles of 95°C for 30 s, 61°C for 30 s, and 68°C for 40 s followed by 5 min of extension at 68°C. Amplification products were run on a precast 10% agarose gel (Bio-Rad), stained for 5–10 min with ethidium bromide, and imaged with ultraviolet light.
Successful knockout of NRF2 protein was obtained via immunohistochemistry in kidney slices from WT and Nrf2−/− mutant rats. For those studies, kidneys were obtained from Nrf2−/− mutant and WT rats maintained on LS diet. The kidneys were paraffin-embedded before transverse sectioning into 4-μm-thick slices. After the samples were deparaffinized, a standard immunohistochemistry protocol (40) was followed using dilutions of 1:50 anti-NRF2 primary antibody (Santa Cruz) and 1:100 horseradish peroxidase-conjugated anti-rabbit secondary antibody. Samples were viewed using the bright field filter on a Nikon Eclipse 80i microscope, and pictures were taken with a Nikon DS-Fi1 camera.
Evaluation of NRF2-regulated indicator enzymes via real-time PCR.
To evaluate the effect of the Nrf2 mutation on specific indicator enzymes known to be regulated by NRF2, we obtained 90- to 100-mg tissue samples from the liver of HS-fed Nrf2−/− mutant rats and WT littermates fed HS diet for 2 wk. RNA was isolated using a TRIzol procedure according to the manufacturer's instructions (Life Technologies). cDNA was synthesized using an iScript cDNA synthesis kit (Bio-Rad). Real-time quantitative PCR was carried out on an Applied Biosystems ABI Prism qPCR system using RT2 SYBR Green ROX qPCR Mastermix (Qiagen) with the following thermal profile: 50°C for 2 min, 95°C for 10 min, followed by 95°C for 15 s and 60°C for 60 s for 40 cycles. Expression of mRNA for the NRF2-regulated indicator enzymes heme oxygenase-1 (HO-1), SOD1, superoxide dismutase 2 (SOD2), catalase, and glutathione reductase (GSR) was evaluated using the following primer sequences: 1) HO-1: forward 5′-aagaggctaagaccgccttc-3′ and reverse 5′-cctctggcgaagaaactctg-3′; 2) SOD1: forward 5′-cgtcattcacttcgagcaga-3′ and reverse 5′-attgtccccatattgatgga-3′; 3) SOD2: forward 5′-gctggcttggcttcaataag-3′ and reverse 5′-acacatcaatccccagcagt-3′; 4) catalase: forward 5′-ttgacagagagcggattcct-3′ and reverse 5′-ctcaaacacctttgccttgg-3′; and 5) GSR: forward 5′-cgtgcactcggaattcatac-3′ and reverse 5′-gtaagcatcccgcttctcct-3′. Ribosomal subunit (r18S) primers (forward 5′-tgaggccatgattaagaggg-3′ and reverse 5′-agtcggcatcgtttatggtc-3′) were used as the internal control, and the ΔΔCT method was used to quantify the respective mRNA levels.
Response to vasodilators and microvessel density measurements.
MCA were isolated, mounted on glass micropipettes pressurized to 80 mmHg, and maintained at 37°C in bicarbonate-buffered physiological salt solution (PSS) equilibrated with a 21% O2-5% CO2-74% N2 gas mixture (11, 33, 56). To evaluate the potential role of Nrf2 in mediating the previously described protective effect of low-dose ANG II infusion to restore endothelium-dependent dilation, responses to increasing concentrations of the endothelium-dependent dilator ACh and the NO donor sodium nitroprusside (11, 37) were evaluated in MCA of the Nrf2−/− mutant rats and WT littermates fed either an LS or a short-term (3 days) HS diet. At the end of the experiment, active resting tone (T) was calculated as: T (%) = [(Dmax − Drest)/Dmax] × 100 where Dmax is the maximum diameter in Ca2+-free PSS and Drest is the diameter at the 80 mmHg equilibration pressure in normal PSS. Vessel responses to ACh were expressed as percent of the maximal dilator capacity of the artery [(diameter increase to ACh/maximum diameter increase in Ca2+-free PSS at 80 mmHg) × 100].
Microvessel density was assessed in the cremaster muscle of SD (parental strain) rats, Nrf2−/− rats, and WT littermates fed short-term HS diet and receiving an infusion of a low-dose ANG II or isotonic saline vehicle for 3 days. Vessels were visualized using rhodamine-labeled Griffonia simplicifolia-1 lectin as described previously (13, 17, 18), and the results were quantified by counting the intersections of vessels with a superimposed 19 × 14 grid generated using Metamorph Imaging software in 60–80 photographs taken in the cremaster muscles of each animal.
Cultured endothelial cell preparation.
Because translocation to the nucleus represents a critical step in NRF2 activation of antioxidant defense mechanisms, we determined whether ANG II affects the subcellular localization of NRF2 fluorescence in thoracic aortic endothelial cells cultured from male SD rats fed short-term HS diet. In those experiments, the animals were killed, and their thoracic aortas were removed and cleaned of adipose and connective tissues using sterile surgical tools. The aortas were cut into many smaller rings and incubated in microcentrifuge tubes containing 0.2% collagenase I (Sigma-Aldrich) solution in Hank's balanced salt solution for 45 min at room temperature. Tubes were centrifuged at 1,000 g for 10 min, and the supernatant was transferred to a cell culture flask containing growth medium for colony expansion and passaging. The growth medium used in these experiments was Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 1% antibiotic-antimycotic (containing 10,000 U/ml of penicillin, 10,000 μg/ml of streptomycin, and 25 μg/ml of Fungizone, an antifungal agent), and 400 μM l-alanyl-l-glutamine (GlutaMAX). All cell culture reagents were supplied by Life Technologies (Thermo Fisher Scientific), unless otherwise noted. On the 2nd or 3rd day of culture, nonadherent cells were removed, and the growth medium was replaced. The medium was changed every 3–4 days until flasks reached confluency, at which point the colonies were passaged. The experiments in this study used cells at passage number 1–2. Growth of an endothelial cell population was confirmed by visually inspecting the colonies weekly for the classic cobblestone morphology characteristic of endothelial cells and by confirming endothelial cell function using a tube-formation assay, as described by Hoffmann et al. (20).
To assess the effect of ANG II on the translocation of NRF2 to the nucleus, cultured aortic endothelial cells were incubated with either saline vehicle or 100 nM ANG II for 24 h. After the incubation period, the cells were fixed in a 1:1 acetone-methanol solution at −20°C for 20 min. Wells were rinsed with Dulbecco's phosphate-buffered saline (DPBS) and then blocked in blocking buffer (5% goat serum and 0.3% Triton X-100 in DPBS) for 1 h at room temperature. NRF2 was labeled by incubating the cover slips overnight at 4°C with a 1:200 dilution of primary antibody (anti-NRF2 antibody; Santa Cruz Biotechnology, Dallas, TX), rinsed with DPBS, and incubated in the dark for 4 h at room temperature with a fluorescent-labeled secondary antibody (1:1,000 Alexa 488 anti-rabbit) followed by a final rinse in DPBS. The cover slips were then adhered face down on a glass microscope slide using VECTASHIELD mounting medium with 4,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA), a fluorescent dye that emits a blue wavelength when bound to double-stranded DNA and is used in immunofluorescence studies as a nuclear marker (27).
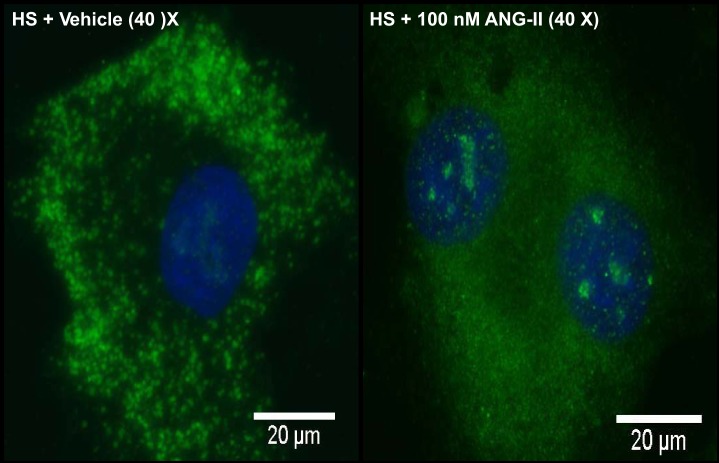
Slides were imaged on a Nikon Eclipse 80i microscope (Nikon, Tokyo, Japan) using MetaMorph imaging software (Molecular Devices, Sunnyvale, CA). Cells were located on the slide using the DAPI microscope filter; and green Nrf2 fluorescence was captured from five randomly selected regions. Later, the number of total nuclei imaged and the number of NRF2-positive nuclei [where DAPI and NRF2 fluorescence colocalized (see representative image in Fig. 4)] were manually counted in each image, and the results were quantified by expressing the number of NRF2-positive nuclei as a percentage of total nuclei visualized.
Fig. 4.
NRF2 staining (green) and nuclear 4,6-diamidino-2-phenylindole (DAPI) staining (blue) in aortic endothelial cells cultured from Sprague-Dawley (SD) rats fed HS (4% NaCl) diet incubated with vehicle (left) or 100 nM ANG II for 24 h (right).
Statistical methods.
Data are expressed as means ± SE. Differences between means were evaluated using Student's t-tests, one-way analysis of variance (ANOVA) with a Kruskal-Wallis test post hoc, or two-way ANOVAs with a Student-Newman-Keuls test post hoc, as appropriate. P < 0.05 was considered to be statistically significant.
RESULTS
Effect of Nrf2 mutation on baseline parameters.
The baseline parameters of body weight, mean arterial pressure (MAP), resting and maximum MCA diameter, and active tone were unaffected by the Nrf2 mutation, diet, or subcutaneous infusion of saline or ANG II (Table 1). These findings are consistent with previous reports in Nrf2−/− mice showing that elimination of Nrf2 does not affect growth or development (6) and also indicate that the Nrf2−/− mutation has no effect on active resting tone or structure of the MCA and does not increase arterial blood pressure under the conditions of our study.
Table 1.
Baseline parameters in wild-type and Nrf2−/− mutant rats
| Low-Salt Diets |
High-Salt Diets |
|||||
|---|---|---|---|---|---|---|
| Noninfused |
Saline-Infused |
ANG II-Infused |
||||
| Nrf2 WT | Nrf2 mutant | Nrf2 WT | Nrf2 mutant | Nrf2 WT | Nrf2 mutant | |
| Body wt, g | 290 ± 11.1 | 287 ± 26.7 | 281 ± 17.7 | 299 ± 22.2 | 291 ± 11.6 | 307 ± 11.5 |
| n | 4 | 4 | 8 | 4 | 6 | 6 |
| Blood pressure, mmHg | 92 ± 2.8 | 95 ± 2.6 | 95 ± 3.9 | 102 ± 3.0 | 95 ± 4.3 | 97 ± 2.9 |
| n | 4 | 4 | 4 | 3 | 3 | 5 |
| Maximum diameter, μm | 261 ± 5.3 | 241 ± 6.8 | 246 ± 4.8 | 248 ± 9.0 | 239 ± 2.7 | 243 ± 7.0 |
| n | 4 | 4 | 5 | 4 | 4 | 5 |
| Resting diameter, μm | 129 ± 9.6 | 111 ± 6.0 | 145 ± 12.5 | 154 ± 15.0 | 126 ± 12.1 | 136 ± 10.2 |
| n | 4 | 4 | 5 | 4 | 4 | 5 |
| Tone, % | 50.8 ± 3.39 | 53.9 ± 3.29 | 41.4 ± 4.47 | 37.4 ± 6.53 | 47.3 ± 4.48 | 43.9 ± 4.99 |
| n | 4 | 4 | 5 | 4 | 4 | 5 |
Values are means ± SE;
n, no. of rats. ANG II, angiotensin II; Nrf2, nuclear factor (erythroid-derived 2)-like-2; WT, wild type.
There were no significant differences between groups for any of the variables, P > 0.05 by 1-way ANOVA.
Verification of the Nrf2 mutation.
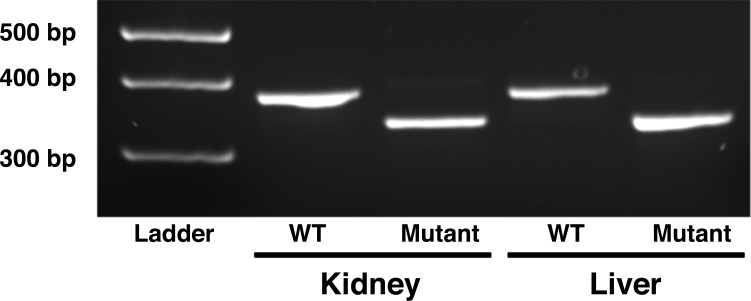
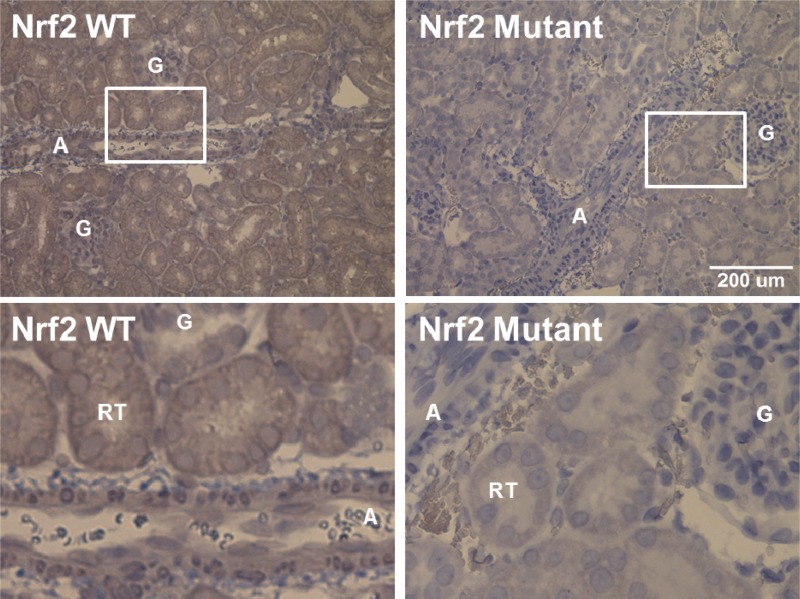
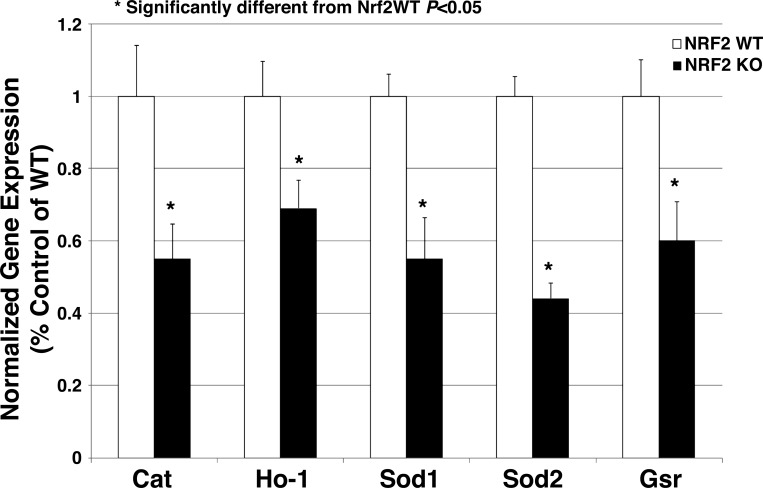
PCR amplification of the region containing the DNA deletion showed the expected full PCR products of 389 bases for the WT Nrf2 transcript samples, whereas Nrf2−/− mutant samples showed smaller bands at 348 bases, indicative of the 41-base deletion mutation (Fig. 1). In the immunohistochemical studies (Fig. 2), kidneys from WT rats showed widespread dark brown staining, indicative of ubiquitous NRF2 protein localization (6, 23), that was absent in kidneys from rats homozygous for the Nrf2 mutation. The absence of NRF2 protein in those structures provided further verification that we had successfully generated an Nrf2 null rat. Expression of mRNA for the NRF2-regulated indicator enzymes catalase, HO-1, SOD1, SOD2, and GSR was also significantly lower in livers of Nrf2−/− rats fed HS diet for 2 wk compared with those from HS-fed WT littermates (Fig. 3).
Fig. 1.
Effect of nuclear factor (erythroid-derived 2)-like-2 (Nrf2) gene mutation on cDNA from liver and kidney. An expected product of 389 bp is represented in wild-type (WT) tissues. Transcription activator-like effector nuclease (TALEN)-mediated mutagenesis resulted in a 41-base deletion, represented by the smaller 348-bp band in homozygous mutant tissues. Organs were from high-salt (HS)-fed, saline-infused rats.
Fig. 2.
Effect of Nrf2 gene mutation on immunohistological detection of NRF2 in the whole kidney. NRF2 staining (brown) is markedly absent from renal structures [artery (A), glomerulus (G), and renal tubule (RT)] in the kidneys of low-salt (LS)-fed Nrf2−/− mutant rats (right) compared with LS-fed WT animals (left). Images on top were photographed with a ×20 objective, and images on bottom are enlargements of the areas indicated by the white boxes in the images on top. Scale bar on top = 200 μm.
Fig. 3.
Expression of mRNA for the NRF2-regulated indicator enzymes catalase (Cat), heme oxygenase-1 (Ho-1), superoxide dismutase 1 (Sod1), superoxide dismutase 2 (Sod2), and glutathione reductase (Gsr) in livers of Nrf2−/− mutant rats fed HS diet for 2 wk compared with those from HS-fed WT controls. Data are expressed as mean percent of WT control ± SE for 3 animals. *P < 0.05, Student's t-test.
ANG II-induced translocation of NRF2 to the nucleus of cultured endothelial cells.
Cultured aortic endothelial cells from SD rats fed HS diet showed diffuse fluorescence of NRF2 in the cytoplasm, consistent with binding of NRF2 by KEAP1 anchored to the cytoskeleton. Incubation of the cells with 100 nM ANG II for 24 h caused translocation of NRF2 to the cell nucleus, as indicated by a shift in NRF2 fluorescence away from the cytosol and into distinct regions of the nucleus (Fig. 4), and a significant increase in NRF2-positive nuclei [from 36 ± 9% NRF2-positive cells with vehicle treatment (n = 6) to 72 ± 15% NRF2-positive cells after 24 h incubation with ANG II (n = 6); P < 0.05, Student's t-test].
Effect of low-dose ANG II infusion on responses of MCA to ACh in Nrf2−/− mutant rats fed HS diet.
Because low-dose ANG II infusion reduces vascular oxidant stress and restores endothelium-dependent vascular relaxation in HS-fed rats (33, 37, 38, 60, 64, 65) and hamsters (46), we performed experiments to evaluate endothelial function in isolated MCA from WT and NRF2−/− mutant rats. In those experiments, MCA of Nrf2−/− mutant rats and WT littermates maintained on LS diet dilated to a similar extent in response to ACh while HS diet eliminated ACh-induced dilation [as previously reported in other experimental models (33, 37, 60)] in MCA from both WT and Nrf2−/− mutant rats (Fig. 5) (34). Chronic infusion of ANG II restored ACh-induced dilation in MCA of WT controls fed short-term HS diet, but this protective effect of ANG II infusion to restore endothelium-dependent vasodilation to ACh was completely absent in MCA of HS-fed Nrf2−/− mutant rats (Fig. 6). MCA of Nrf2−/− mutant rats and WT littermates fed either LS or HS diet exhibited similar dilations in response to the NO donor sodium nitroprusside (not shown), indicating that neither HS diet nor the Nrf2 mutation affect vascular smooth muscle NO sensitivity in this experimental model.
Fig. 5.
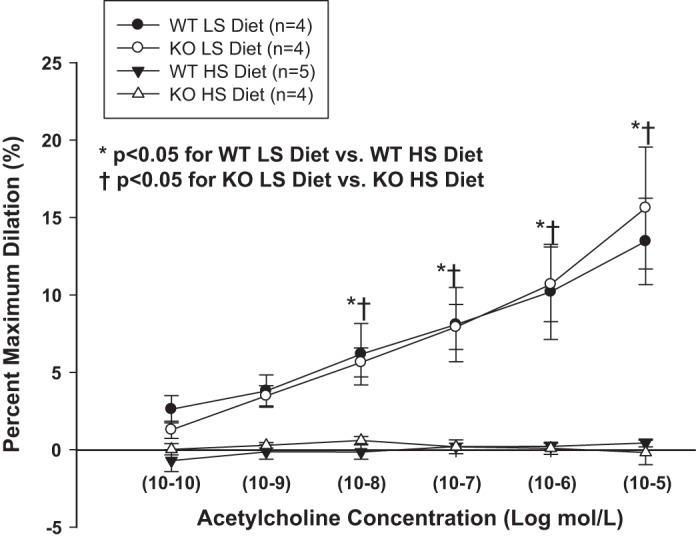
Responses to the endothelium-dependent vasodilator acetylcholine (ACh) in middle cerebral arteries (MCA) of Nrf2−/− mutant rats [knockout (KO)] and WT littermates maintained on an LS (0.4% NaCl) diet or short-term (3 days) HS (4% NaCl) diet. Data are summarized as mean percent of maximum dilation in Ca2+-free physiological salt solution (PSS) (± SE) for 4–5 animals/group. *P < 0.05. WT LS diet vs. WT HS diet and †P < 0.05, KO LS diet vs. KO HS diet by 2-way ANOVA with Student Newman-Keuls test post hoc.
Fig. 6.
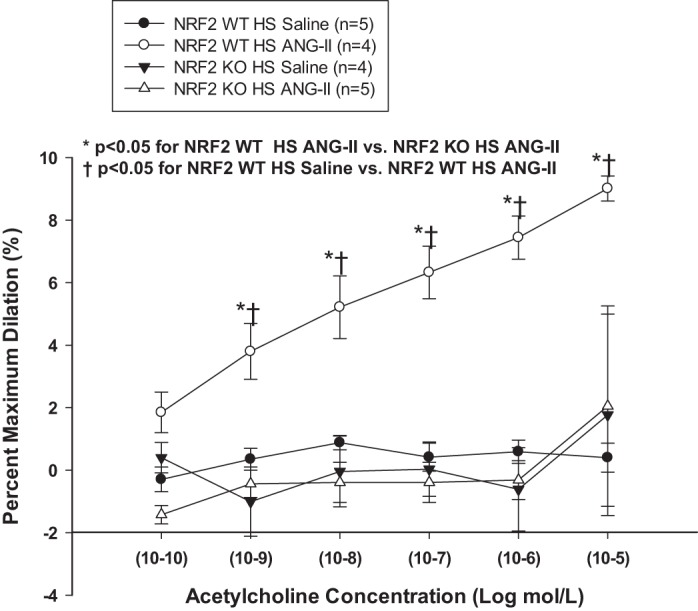
Responses to endothelium-dependent vasodilator ACh in MCA of Nrf2−/− mutant (KO) rats and WT littermates fed HS (4% NaCl) diet and infused with either saline vehicle or low-dose (100 ng·kg−1·min−1) ANG II via sc osmotic minipump for 3 days. Data are summarized as mean percent of maximum dilation in Ca2+-free PSS (± SE) for 4–5 animals/treatment group. *P < 0.05. WT HS diet + ANG II vs. KO HS diet + ANG II and †P < 0.05. WT HS diet + ANG II vs. WT HS diet + saline by 2-way ANOVA with Student Newman-Keuls test post hoc.
Effect of low-dose ANG II infusion on salt-induced microvascular rarefaction in Nrf2−/− rats.
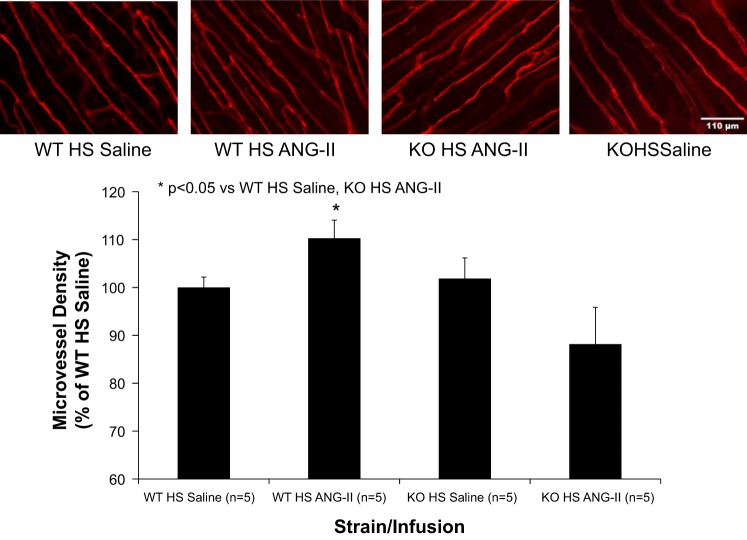
Elevated dietary salt intake is also associated with a reduced density of microvessels (microvascular rarefaction) in SD rats (18). Consistent with that report (18), microvessel density was significantly lower in the cremaster muscle of SD parental rats fed HS diet for 3 days compared with LS controls, and microvascular rarefaction in SD rats fed short-term HS diet was prevented by subcutaneous infusion of a low dose of ANG II (100 ng·kg−1·min−1) (Fig. 7). ANG II infusion also increased microvessel density in the cremaster muscle of WT rats fed short-term HS diet, but not in cremaster muscles of HS-fed Nrf2−/− mutant rats (Fig. 8).
Fig. 7.
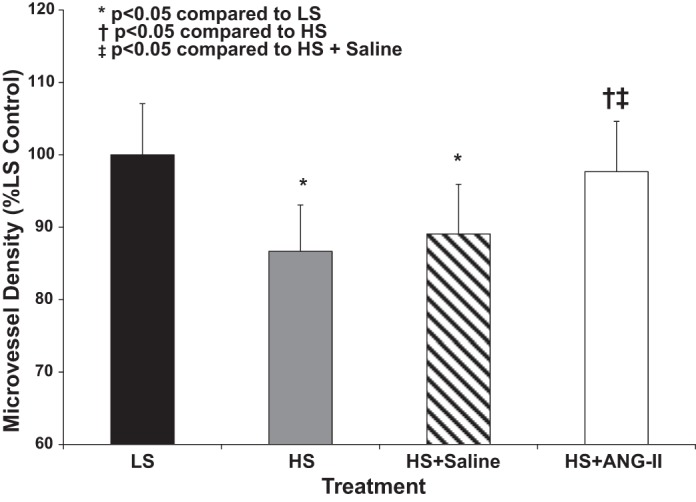
Effect of HS diet on microvessel density in cremaster muscles of SD parental rats fed LS (0.4% NaCl) diet and HS (4% NaCl) diet for 3 days (± 100 ng·kg−1·min−1 sc ANG II infusion). Data are expressed as mean percent of LS control ± SE for 5–6 animals. *P < 0.05 vs. LS control, †P < 0.05 vs. HS, and ‡P < 0.05 vs. HS + isotonic saline vehicle by 1-way ANOVA with Kruskal-Wallis test post hoc.
Fig. 8.
Effect of low-dose ANG II infusion (100 ng·kg−1·min−1 sc) on microvessel density in the cremaster muscle of WT and Nrf2−/− mutant (KO) rats fed HS (4% NaCl) diet for 3 days. Scale bar = 110 μm. Data are expressed as mean percent of WT saline-infused control. *P < 0.05 vs. HS-fed controls infused with isotonic saline vehicle by 1-way ANOVA with Kruskal-Wallis test post hoc.
DISCUSSION
In the present study, we developed a novel rat strain (SD-Nfe212em1Mcwi) in which a deletion mutation of the Nrf2 gene was created in the SD genetic background. We used this novel genetic rat strain to test the hypothesis that suppression of the NRF2 antioxidant defense system contributes to endothelial dysfunction arising from salt-induced ANG II suppression and to evaluate the potential role of NRF2 in contributing to the protective effect of low-dose ANG II infusion to ameliorate endothelial dysfunction and prevent microvascular rarefaction in HS-fed rats.
Although Nrf2 knockout mice exist, there is a substantially greater body of background knowledge regarding the physiology of the rat vs. the mouse, and the small size of the mouse presents substantial challenges for in vivo physiological studies because it precludes many of the experimental procedures that are extremely successful in the rat, e.g., blood pressure monitoring combined with serial blood sampling in conscious animals (15, 19, 34). In this regard, the availability of a Nrf2−/− mutant rat strain provides significant advantages for detailed physiological studies. Of particular importance in regard to the present experiments is that the renin-angiotensin system in mice is very different from that in rats (4, 7), which more closely resembles that of the human (7). For example, ANG II infusion leads to a substantial increase in MAP in rats but not mice (4, 7), and aortic contractions in response to ANG II are substantially greater in rats vs. mice (4, 7). Angiotensin-converting enzyme inhibition and losartan produce a 50–90% greater fall in MAP in conscious mice vs. rats (7). ANG II causes medial hypertrophy in rat arteries but adventitial expansion in mouse arteries (4), and ANG II infusion reduces ANG II receptor density by 78% in mouse kidney and 29% in mouse spleen, with no effect in rats (4). In light of those findings, Cassis et al. (4) concluded that considerably higher doses of ANG II are necessary to elicit physiological effects in mice vs. rats. In light of existing reports that plasma ANG II levels in conscious mice and rats are not significantly different (7), the above findings strongly suggest that caution is necessary when using mouse models to study the effects of physiological ANG II levels on the cardiovascular system.
In the present study, the deletion mutation in the first exon of the Nrf2 gene of the SD-Nfe212em1Mcwi rats included the start codon for NRF2 translation, and was transmitted to mRNA, as verified in cDNA by the presence of a truncated PCR product (Fig. 1) and the absence of NRF2 protein evaluated by immunohistochemistry in whole kidney sections (Fig. 2). Verification of this deletion mutation in the Nrf2−/− rat is important since it supports the hypothesis that any changes in vascular reactivity or microvessel density in the Nrf2−/− mutant rats (or in the ability of low-dose ANG II infusion to modify those parameters) compared with responses in WT littermates are due to loss of the antioxidant and cell protective effects of the NRF2 protein in the mutant rats. Supporting the hypothesis that loss of NRF2 has an adverse effect on antioxidant mechanisms in mutant rats fed HS diet is the finding that the expression of mRNA for the NRF2-regulated indicator genes Ho-1, catalase, Sod1, Sod2, and Gsr was significantly lower in livers of HS-fed Nrf2−/− mutant rats compared with those of WT controls (Fig. 3).
Classically, high levels of ANG II exert pro-oxidant effects by stimulating the vascular NADPH oxidase isoforms and increasing the production of superoxide anions (14, 28, 39, 57). However, new evidence suggests that there is a paradoxical antioxidant role for plasma ANG II levels within the physiological range. For example, in salt-insensitive (for blood pressure) animal models, suppression of plasma ANG II by an HS diet leads to changes in vascular function similar to those occurring with high-ANG II models, including oxidant stress (65, 66) and endothelial dysfunction (33), without changes in mean arterial blood pressure (10, 32, 38, 60). Infusion of a low (subpressor) dose of ANG II to prevent salt-induced ANG II suppression (15, 64) not only restores endothelium-dependent vascular relaxation but also reduces vascular oxidant stress in arteries of HS-fed rats (65) and hamsters (46). In the context of the present study, this is important because it demonstrates that exposure to chronically low levels of circulating ANG II (with HS diet or likely in low renin forms of hypertension) leads to vascular oxidant stress independent of the complicating effects of elevated blood pressure and reinforces the importance of NRF2 in mediating the tonic effects of physiological ANG II levels in maintaining vascular antioxidant defenses under normal physiological conditions.
Surprisingly, the antioxidant effects of low-dose ANG II infusion are mediated via the ANG II type 1 (AT1) receptor for ANG II (46). The protective effect of low-dose ANG II infusion to restore endothelium-dependent vasodilation in salt-fed animals depends on transactivation of the epidermal growth factor receptor in response to ANG II binding to its AT1 receptor, with subsequent activation of ERK1/2 signaling and, ultimately, new protein synthesis (37). Consistent with this interpretation, studies in other laboratories have shown that ERK1/2 signaling promotes the translocation of NRF2 to the cell nucleus in other experimental models (45, 67, 68). Taken together, these findings suggest that the protective effect of ANG II infusion to reduce vascular oxidant stress and restore endothelium-dependent dilation in salt-fed animals is mediated via activation of the NRF2 antioxidant defense system in response to AT1 receptor activation and subsequent activation of the ERK1/2 signaling pathway (37). In the present study, the observation that ANG II promoted translocation of NRF2 to the nucleus in cultured aortic endothelial cells from SD parental rats (Fig. 4) is also consistent with the hypothesis that the NRF2 antioxidant defense system plays an important role in the protective effects of low-dose ANG II infusion to restore endothelial function (33, 37, 46) (Fig. 6) and prevent microvascular rarefaction (18) (Fig. 8) in salt-fed animals.
Previous studies have shown that HS diet leads to striking structural changes in arterioles and capillaries (16) and to a significant reduction in microvessel density (microvascular rarefaction) (18). Microvascular rarefaction in salt-fed animals is due to salt-induced ANG II suppression and is prevented by restoring normal plasma ANG II levels via chronic infusion of a low dose of ANG II (18). In the present study, the failure of low-dose ANG II infusion to restore microvessel density in the HS-fed Nrf2−/− mutant rats indicates that activation of NRF2-regulated antioxidant defenses plays a crucial role in mediating the protective effect of ANG II infusion to prevent microvascular rarefaction in HS-fed animals, and also suggests that tonic activation of the NRF2 antioxidant defense system may contribute to the ability of physiological levels of ANG II to maintain normal microvessel density.
Yun and coworkers (62) postulated that angiogenic responses and collateral vessel formation are likely to depend upon an optimal level of ROS within a relatively narrow “redox window.” As such, the failure of low-dose ANG II infusion to increase microvessel density in the cremaster muscle of Nrf2−/− rats could be due to elevated levels of ROS in the tissue as a result of reduced expression and activity of NRF2-regulated antioxidant enzymes in the tissue of the mutant rats. Supporting the latter hypothesis are the findings of Reed et al. (50), who reported that low-dose ANG II infusion improved collateral flow following repeated coronary occlusion in WKY rats but failed to improve collateral vessel formation in the JCR rat model of syndrome X characterized by elevated levels of tissue oxidant stress. By contrast, high-dose ANG II infusion, expected to generate higher ROS levels in the tissue, abrogated coronary collateral formation in the WKY rats, and AT1 receptor blockade with candesartan improved coronary collateral flow in the JCR rats but failed to improve coronary flow in the WKY rats (50). In a similar fashion, low-dose ANG II infusion in the presence of elevated levels of tissue oxidant stress would likely fail to restore increased microvessel density in the HS-fed Nrf2−/− mutant rats.
In the vascular reactivity studies, endothelium-dependent dilation to ACh was identical in MCA from Nrf2−/− mutant rats and WT littermates fed LS diet (Fig. 5), demonstrating that loss of NRF2 does not impair endothelial function in the absence of salt-induced ANG II suppression and oxidant stress. Consistent with previous reports in salt-fed SD rats (32, 33, 37, 60) and hamsters (46), HS diet eliminated ACh-induced dilation of MCA from both the WT and the Nrf2−/− mutant rats. The most important and novel finding of the present study was that the protective effect of ANG II infusion to restore endothelium-dependent dilation to ACh that was present in MCA of HS-fed WT controls was completely eliminated in the Nrf2−/− mutant rats (Fig. 6).
Overall, the results of the current study support the hypothesis that the protective effect of low-dose ANG II infusion to restore vascular relaxation and prevent microvascular rarefaction in salt-fed rats is mediated via activation of the NRF2 antioxidant defense mechanism and suggest a mechanism by which physiological ANG II levels play a protective role in the vasculature through the maintenance of redox homeostasis. In this paradigm, binding of ANG II to the AT1 receptor transactivates the epidermal growth factor receptor (37), leading to ERK1/2-mediated phosphorylation of component(s) of the Nrf2/Keap1 complex and translocation of NRF2 to the cell nucleus where it binds to the ARE consensus sequence in the promoter region of antioxidant and cell protective genes. NRF2 binding to the ARE would, in turn, ameliorate the oxidant stress occurring in response to HS diet (65) and restore redox homeostasis necessary for the preservation of normal endothelial cell function, notably endothelial intracellular Ca2+ concentration signaling (64) and NO release (31, 56, 64–66), and also maintain tissue ROS levels within the narrow redox window (62) necessary to preserve angiogenic responses, as postulated by Reed et al. (50) and Yun et al. (62).
One question that remains unanswered is the potential effects of ANG II-induced NRF2 activation on ROS production itself. Previous studies (65) have shown that inhibition of nitric oxide synthase, cyclooxygenase, and cytochrome P-450 enzymes all lead to a reduction in vascular oxidant stress in small mesenteric resistance arteries of HS-fed SD rats, suggesting that antioxidant defenses in HS-fed rats are reduced and therefore unable to buffer ROS production by any enzyme that produces superoxide as part of its normal catalytic activity. However, elucidation of the effect of the NRF2 system in directly regulating ROS formation itself in resistance arteries (especially during salt-induced ANG II suppression) clearly appears to be an important area for future investigation.
The NRF2 oxidant stress response plays an important role in a variety of pathophysiological states, including diseases of the lung (5, 48, 49) and liver (44, 61), neurodegeneration (59), and cancer (43, 47, 51, 52). In humans, Nrf2 mRNA and protein are downregulated in alveolar macrophages collected from patients with chronic obstructive pulmonary disease and long-time smokers (35, 55). Recently Marczak and coworkers (36) reported that the −653G polymorphism within the NRF2 promoter region is associated with reduced forearm blood flow and increased vascular resistance in healthy African-American volunteers and also significantly reduces NRF2 transcription in vitro (36).
Although oxidant stress is a hallmark of multiple cardiovascular diseases, clinical trials involving direct administration of antioxidants have been surprisingly disappointing (42). As a result, there is growing interest in direct upregulation of endogenous antioxidant systems, including NRF2, as potential therapeutic approaches to pathological conditions characterized by increased oxidant stress. For example, in whole animal models, inducers of NRF2 expression attenuate ischemic damage from stroke and mitochondrial stress (1, 53, 54). Sulforaphane-induced NRF2 activation also improves many aspects of blood-brain barrier function following brain injury produced by controlled cortical impact, including maintenance of endothelial cell markers, maintenance of tight junction proteins in the microvasculature, and decreased edema, indicative of a less permeable blood-brain barrier in vivo (63).
By employing a novel Nrf2 mutant rat strain, we have shown that NRF2 is necessary for the protective effect of low-dose ANG II infusion to ameliorate endothelial dysfunction and prevent microvascular rarefaction in the face of an elevated dietary salt intake. However, the potential importance of the Nrf2−/− mutant rat strain extends far beyond insight into the novel protective role of physiological levels of ANG II in maintaining normal endothelial function because of the global nature of the NRF2 system. For example, Hybertson et al. (21) noted that dysregulation of NRF2-regulated genes provides a logical explanation for the direct and indirect connections between oxidant stress and more than 200 human diseases and that NRF2 can modulate the expression of hundreds of different genes that affect multiple processes ranging from immune and inflammatory responses, tissue remodeling and fibrosis, carcinogenesis and metastasis, and even cognitive dysfunction. As such, direct upregulation of the NRF2 antioxidant defense system could prove to be a highly beneficial therapeutic and preventative strategy for pathophysiological conditions associated with increased oxidant stress, especially in light of the disappointing results of clinical trials involving direct administration of antioxidants (42). In this regard, crucial insights into the role of NRF2 in these processes and the mechanisms of NRF2 action could be obtained from studies of the SD-Nfe212em1Mcwi mutant rat strain, or other Nrf2−/− mutant rat strains developed in disease-sensitized genetic backgrounds.
GRANTS
This work was supported by NIH Grants R01-HL-65289-12, R56-HL-65289-13A1, and R21-OD-018309-01.
DISCLOSURES
MCW may one day receive royalties on the sale of genetically modified rats.
AUTHOR CONTRIBUTIONS
Author contributions: J.R.P., B.T.E., A.M.G., and J.H.L. conception and design of research; J.R.P., K.E.K., M.C.C., and A.M.G. performed experiments; J.R.P., K.E.K., M.C.C., A.M.G., and J.H.L. analyzed data; J.R.P., K.E.K., M.C.C., B.T.E., A.M.G., and J.H.L. interpreted results of experiments; J.R.P., K.E.K., M.C.C., and A.M.G. prepared figures; J.R.P. and J.H.L. drafted manuscript; J.R.P., K.E.K., M.C.C., B.T.E., A.M.G., and J.H.L. edited and revised manuscript; J.R.P., K.E.K., M.C.C., B.T.E., A.M.G., and J.H.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Brian Weinberg and Lynn Dondlinger for outstanding technical contributions and Glenn Slocum for advice and assistance in the immunohistochemistry studies and cultured cell studies.
REFERENCES
- 1.Alfieri A, Srivastava S, Siow RC, Modo M, Fraser PA, Mann GE. Targeting the Nrf2-Keap1 antioxidant defence pathway for neurovascular protection in stroke. J Physiol 589: 4125–4136, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez B, Rubbo H, Kirk M, Barnes S, Freeman BA, Radi R. Peroxynitrite-dependent tryptophan nitration. Chem Res Toxicol 9: 390–396, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Brzezinska AK, Gebremedhin D, Chilian WM, Kalyanaraman B, Elliott SJ. Peroxynitrite reversibly inhibits Ca2+-activated K+ channels in rat cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol 278: H1883–H1890, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Cassis LA, Huang J, Gong MC, Daugherty A. Role of metabolism and receptor responsiveness in the attenuated responses to angiotensin II in mice compared to rats. Regul Pept 117: 107–116, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Chan K, Kan YW. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc Natl Acad Sci USA 96: 12731–12736, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan K, Lu R, Chang JC, Kan YW. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc Natl Acad Sci USA 93: 13943–13948, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cholewa BC, Meister CJ, Mattson DL. Importance of the renin-angiotensin system in the regulation of arterial blood pressure in conscious mice and rats. Acta Physiol Scand 183: 309–320, 2005. [DOI] [PubMed] [Google Scholar]
- 8.de Resende MM, Greene AS. Effect of ANG II on endothelial cell apoptosis and survival and its impact on skeletal muscle angiogenesis after electrical stimulation. Am J Physiol Heart Circ Physiol 294: H2814–H2821, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durand MJ, Lombard JH. Low-dose angiotensin II infusion restores vascular function in cerebral arteries of high salt-fed rats by increasing copper/zinc superoxide dimutase expression. Am J Hypertens 26: 739–747, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Durand MJ, Raffai G, Weinberg BD, Lombard JH. Angiotensin-(1–7) and low-dose angiotensin II infusion reverse salt-induced endothelial dysfunction via different mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol 299: H1024–H1033, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fredricks KT, Liu Y, Rusch NJ, Lombard JH. Role of endothelium and arterial K+ channels in mediating hypoxic dilation of middle cerebral arteries. Am J Physiol Heart Circ Physiol 267: H580–H586, 1994. [DOI] [PubMed] [Google Scholar]
- 12.Geurts AM, Cost GJ, Remy S, Cui X, Tesson L, Usal C, Menoret S, Jacob HJ, Anegon I, Buelow R. Generation of gene-specific mutated rats using zinc-finger nucleases. Methods Mol Biol 597: 211–225, 2010. [DOI] [PubMed] [Google Scholar]
- 13.Greene AS, Lombard JH, Cowley AW Jr, Hansen-Smith FM. Microvessel changes in hypertension measured by Griffonia simplicifolia I lectin. Hypertension 15: 779–783, 1990. [DOI] [PubMed] [Google Scholar]
- 14.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 74: 1141–1148, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Gross V, Kurth TM, Skelton MM, Mattson DL, Cowley AW Jr. Effects of daily sodium intake and ANG II on cortical and medullary renal blood flow in conscious rats. Am J Physiol Regul Integr Comp Physiol 274: R1317–R1323, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Hansen-Smith FM, Morris LW, Greene AS, Lombard JH. Rapid microvessel rarefaction with elevated salt intake and reduced renal mass hypertension in rats. Circ Res 79: 324–330, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Hansen-Smith FM, Watson L, Lu DY, Goldstein I. Griffonia simplicifolia I: fluorescent tracer for microcirculatory vessels in nonperfused thin muscles and sectioned muscle. Microvasc Res 36: 199–215, 1988. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez I, Cowley AW Jr, Lombard JH, Greene AS. Salt intake and angiotensin II alter microvessel density in the cremaster muscle of normal rats. Am J Physiol Heart Circ Physiol 263: H664–H667, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Hinojosa-Laborde C, Greene AS, Cowley AW. Autoregulation of the systemic circulation in conscious rats. Hypertension 11: 685–691, 1988. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann BR, Wagner JR, Prisco AR, Janiak A, Greene AS. Vascular endothelial growth factor-A signaling in bone marrow-derived endothelial progenitor cells exposed to hypoxic stress. Physiol Genomics 45: 1021–1034, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med 32: 234–246, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Ischiropoulos H, Zhu L, Chen J, Tsai M, Martin JC, Smith CD, Beckman JS. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys 298: 431–437, 1992. [DOI] [PubMed] [Google Scholar]
- 23.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236: 313–322, 1997. [DOI] [PubMed] [Google Scholar]
- 24.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13: 76–86, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacob HJ, Lazar J, Dwinell MR, Moreno C, Geurts AM. Gene targeting in the rat: advances and opportunities. Trends Genet 26: 510–518, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kagota S, Tamashiro A, Yamaguchi Y, Sugiura R, Kuno T, Nakamura K, Kunitomo M. Downregulation of vascular soluble guanylate cyclase induced by high salt intake in spontaneously hypertensive rats. Br J Pharmacol 134: 737–744, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapuscinski J. DAPI: a DNA-specific fluorescent probe. Biotech Histochem 70: 220–233, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells: Nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res 88: 888–894, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Lenda DM, Boegehold MA. Effect of a high salt diet on microvascular antioxidant enzymes. J Vasc Res 39: 41–50, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Lenda DM, Boegehold MA. Effect of a high-salt diet on oxidant enzyme activity in skeletal muscle microcirculation. Am J Physiol Heart Circ Physiol 282: H395–H402, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Lenda DM, Sauls BA, Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am J Physiol Heart Circ Physiol 279: H7–H14, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Rusch NJ, Lombard JH. Loss of endothelium and receptor-mediated dilation in pial arterioles of rats fed a short-term high salt diet. Hypertension 33: 686–688, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Lombard JH, Sylvester FA, Phillips SA, Frisbee JC. High-salt diet impairs vascular relaxation mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol 284: H1124–H1133, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Makino A, Skelton MM, Zou AP, Roman RJ, Cowley AW Jr. Increased renal medullary oxidative stress produces hypertension. Hypertension 39: 667–672, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, Singh A, Chen L, Zhuang X, Hogg J, Pare P, Tuder RM, Biswal S. Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med 178: 592–604, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Marczak ED, Marzec J, Zeldin DC, Kleeberger SR, Brown NJ, Pretorius M, Lee CR. Polymorphisms in the transcription factor NRF2 and forearm vasodilator responses in humans. Pharmacogenet Genomics 22: 620–628, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McEwen ST, Balus SF, Durand MJ, Lombard JH. Angiotensin II maintains cerebral vascular relaxation via EGF receptor transactivation and ERK1/2. Am J Physiol Heart Circ Physiol 297: H1296–H1303, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McEwen ST, Schmidt JR, Somberg L, de la Cruz L, Lombard JH. Time-course and mechanisms of restored vascular relaxation by reduced salt intake and angiotensin II infusion in rats fed a high-salt diet. Microcirculation 16: 220–234, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res 90: E58–E65, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Moreno C, Hoffman M, Stodola TJ, Didier DN, Lazar J, Geurts AM, North PE, Jacob HJ, Greene AS. Creation and characterization of a renin knockout rat. Hypertension 57: 614–619, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moreno C, Kennedy K, Andrae JW, Jacob HJ. Genome-wide scanning with SSLPs in the rat. Methods Mol Med 108: 131–138, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Myung SK, Ju W, Cho B, Oh SW, Park SM, Koo BK, Park BJ, Group KMAS . Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta-analysis of randomised controlled trials. Br Med J 346: f10, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohta T, Iijima K, Miyamoto M, Nakahara I, Tanaka H, Ohtsuji M, Suzuki T, Kobayashi A, Yokota J, Sakiyama T, Shibata T, Yamamoto M, Hirohashi S. Loss of Keap1 function activates Nrf2 and provides advantages for lung cancer cell growth. Cancer Res 68: 1303–1309, 2008. [DOI] [PubMed] [Google Scholar]
- 44.Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun 339: 79–88, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Papaiahgari S, Kleeberger SR, Cho HY, Kalvakolanu DV, Reddy SP. NADPH oxidase and ERK signaling regulates hyperoxia-induced Nrf2-ARE transcriptional response in pulmonary epithelial cells. J Biol Chem 279: 42302–42312, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Priestley JR, Buelow MW, McEwen ST, Weinberg BD, Delaney M, Balus SF, Hoeppner C, Dondlinger L, Lombard JH. Reduced angiotensin II levels cause generalized vascular dysfunction via oxidant stress in hamster cheek pouch arterioles. Microvasc Res 89: 134–145, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, Kensler TW. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci USA 98: 3410–3415, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J Clin Invest 114: 1248–1259, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rangasamy T, Guo J, Mitzner WA, Roman J, Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, Biswal S. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med 202: 47–59, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reed R, Kolz C, Potter B, Rocic P. The mechanistic basis for the disparate effects of angiotensin II on coronary collateral growth. Arterioscler Thromb Vasc Biol 28: 61–67, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Shibata T, Ohta T, Tong KI, Kokubu A, Odogawa R, Tsuta K, Asamura H, Yamamoto M, Hirohashi S. Cancer related mutations in NRF2 impair its recognition by Keap1-Cul3 E3 ligase and promote malignancy. Proc Natl Acad Sci USA 105: 13568–13573, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shibata T, Saito S, Kokubu A, Suzuki T, Yamamoto M, Hirohashi S. Global downstream pathway analysis reveals a dependence of oncogenic NF-E2-related factor 2 mutation on the mTOR growth signaling pathway. Cancer Res 70: 9095–9105, 2010. [DOI] [PubMed] [Google Scholar]
- 53.Shih AY, Imbeault S, Barakauskas V, Erb H, Jiang L, Li P, Murphy TH. Induction of the Nrf2-driven antioxidant response confers neuroprotection during mitochondrial stress in vivo. J Biol Chem 280: 22925–22936, 2005. [DOI] [PubMed] [Google Scholar]
- 54.Shih AY, Li P, Murphy TH. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J Neurosci 25: 10321–10335, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suzuki M, Betsuyaku T, Ito Y, Nagai K, Nasuhara Y, Kaga K, Kondo S, Nishimura M. Down-regulated NF-E2-related factor 2 in pulmonary macrophages of aged smokers and patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 39: 673–682, 2008. [DOI] [PubMed] [Google Scholar]
- 56.Sylvester FA, Stepp DW, Frisbee JC, Lombard JH. High-salt diet depresses acetylcholine reactivity proximal to NOS activation in cerebral arteries. Am J Physiol Heart Circ Physiol 283: H353–H363, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res 90: 1205–1213, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Tzemos N, Lim PO, Wong S, Struthers AD, MacDonald TM. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension 51: 1525–1530, 2008. [DOI] [PubMed] [Google Scholar]
- 59.Vargas MR, Johnson DA, Sirkis DW, Messing A, Johnson JA. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci 28: 13574–13581, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weber DS, Lombard JH. Elevated salt intake impairs dilation of skeletal muscle resistance arteries via angiotensin II suppression. Am J Physiol Heart Circ Physiol 278: H500–H506, 2000. [DOI] [PubMed] [Google Scholar]
- 61.Xu W, Shao L, Zhou C, Wang H, Guo J. Upregulation of Nrf2 expression in non-alcoholic fatty liver and steatohepatitis. Hepatogastroenterology 58: 2077–2080, 2011. [DOI] [PubMed] [Google Scholar]
- 62.Yun J, Rocic P, Pung YF, Belmadani S, Carrao AC, Ohanyan V, Chilian WM. Redox-dependent mechanisms in coronary collateral growth: the “redox window” hypothesis. Antioxid Redox Signal 11: 1961–1974, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci 27: 10240–10248, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu J, Drenjancevic-Peric I, McEwen S, Friesema J, Schulta D, Yu M, Roman RJ, Lombard JH. Role of superoxide and angiotensin II suppression in salt-induced changes in endothelial Ca2+ signaling and NO production in rat aorta. Am J Physiol Heart Circ Physiol 291: H929–H938, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries J. Vasc Res 44: 382–390, 2007. [DOI] [PubMed] [Google Scholar]
- 66.Zhu J, Mori T, Huang T, Lombard JH. Effect of high-salt diet on NO release and superoxide production in rat aorta. Am J Physiol Heart Circ Physiol 286: H575–H583, 2004. [DOI] [PubMed] [Google Scholar]
- 67.Zipper LM, Mulcahy RT. Erk activation is required for Nrf2 nuclear localization during pyrrolidine dithiocarbamate induction of glutamate cysteine ligase modulatory gene expression in HepG2 cells. Toxicol Sci 73: 124–134, 2003. [DOI] [PubMed] [Google Scholar]
- 68.Zipper LM, Mulcahy RT. Inhibition of ERK and p38 MAP kinases inhibits binding of Nrf2 and induction of GCS genes. Biochem Biophys Res Commun 278: 484–492, 2000. [DOI] [PubMed] [Google Scholar]