Abstract
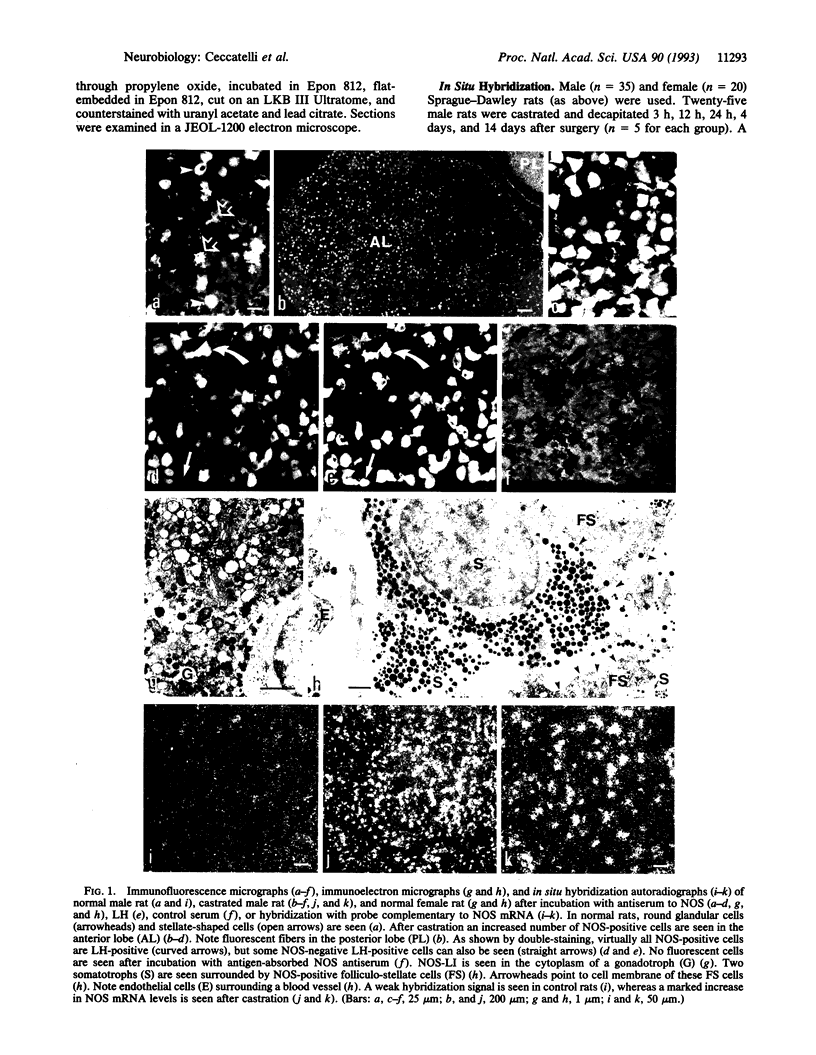
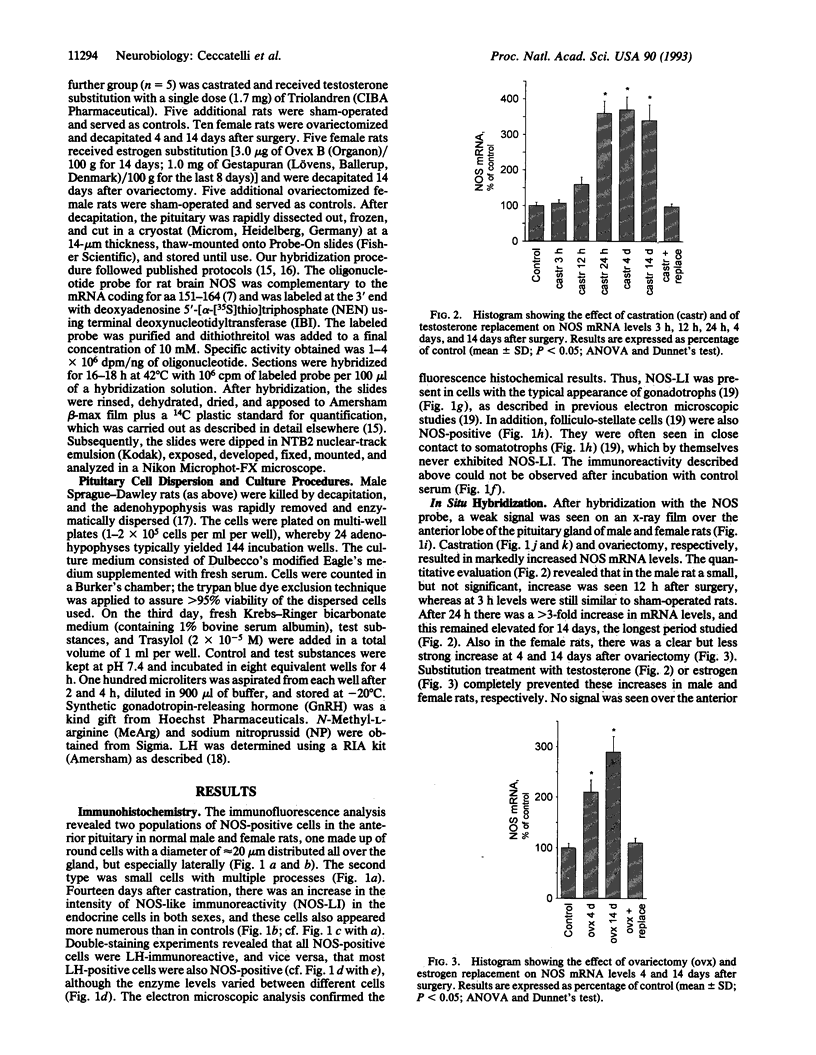
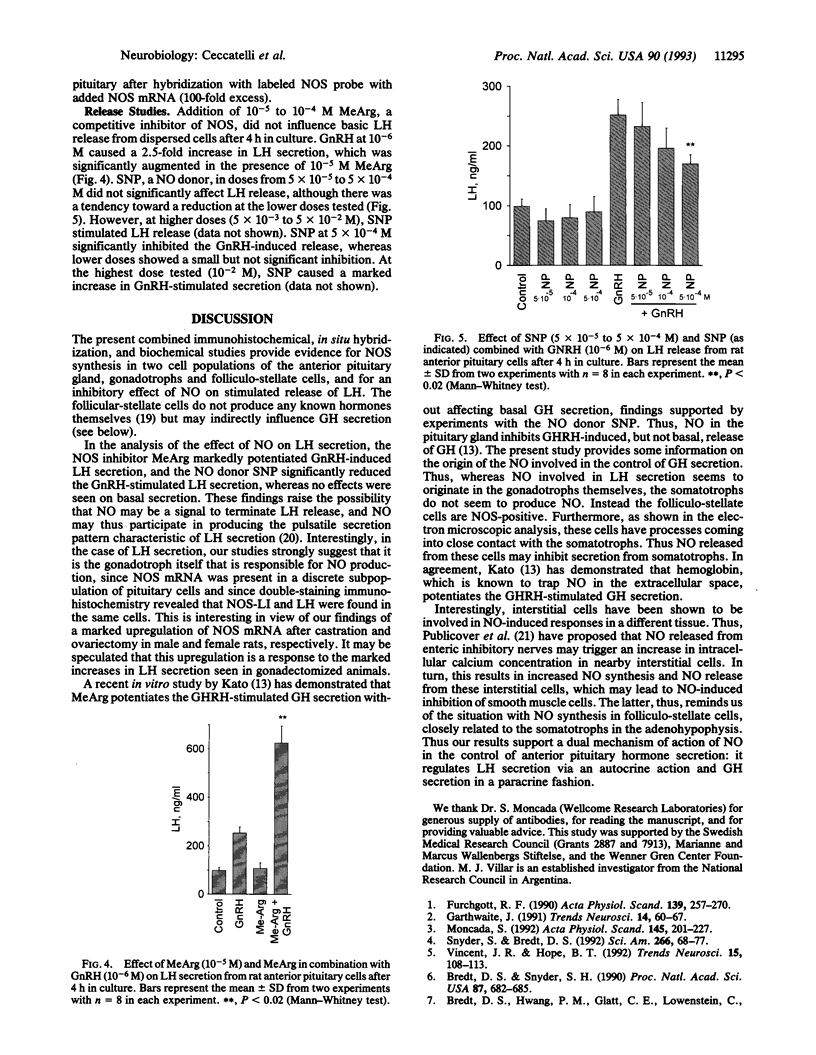
By using immunohistochemistry and in situ hybridization, we have demonstrated that the nitric oxide (NO)-synthesizing enzyme NO synthase is present in gonadotrophs and in folliculo-stellate cells of the anterior pituitary gland of male and female rats. A marked increase in levels of NO synthase protein and mRNA was observed after gonadectomy. In vitro studies on dispersed anterior pituitary cells suggest that NO inhibits gonadotropin-releasing-hormone-stimulated luteinizing hormone release. An inhibitory effect of NO has also been shown on growth-hormone-releasing-hormone-stimulated release of growth hormone [Kato, M. (1992) Endocrinology 131, 2133-2138]. Thus these findings support a dual mechanism for NO in the control of anterior pituitary hormone secretion, an autocrine mediation of luteinizing hormone release on gonadotrophs, and a paracrine effect on growth hormone secretion involving folliculo-stellate cells closely related to somatotrophs. We speculate that NO may participate in producing the pulsatile secretion patterns of these two pituitary hormones.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson K., Nilsen O. G., Toftgard R., Eneroth P., Gustafsson J. A., Battistini N., Agnati L. F. Increased amine turnover in several hypothalamic noradrenaline nerve terminal systems and changes in prolactin secretion in the male rat by exposure to various concentrations of toluene. Neurotoxicology. 1983 Winter;4(4):43–55. [PubMed] [Google Scholar]
- Bredt D. S., Glatt C. E., Hwang P. M., Fotuhi M., Dawson T. M., Snyder S. H. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991 Oct;7(4):615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Glatt C. E., Lowenstein C., Reed R. R., Snyder S. H. Cloned and expressed nitric oxide synthase structurally resembles cytochrome P-450 reductase. Nature. 1991 Jun 27;351(6329):714–718. doi: 10.1038/351714a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagerlind A., Friberg K., Bean A. J., Hökfelt T. Sensitive mRNA detection using unfixed tissue: combined radioactive and non-radioactive in situ hybridization histochemistry. Histochemistry. 1992 Aug;98(1):39–49. doi: 10.1007/BF00716936. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. The 1989 Ulf von Euler lecture. Studies on endothelium-dependent vasodilation and the endothelium-derived relaxing factor. Acta Physiol Scand. 1990 Jun;139(2):257–270. doi: 10.1111/j.1748-1716.1990.tb08923.x. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991 Feb;14(2):60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Horvath E., Kovacs K. Fine structural cytology of the adenohypophysis in rat and man. J Electron Microsc Tech. 1988 Apr;8(4):401–432. doi: 10.1002/jemt.1060080410. [DOI] [PubMed] [Google Scholar]
- Kato M. Involvement of nitric oxide in growth hormone (GH)-releasing hormone-induced GH secretion in rat pituitary cells. Endocrinology. 1992 Nov;131(5):2133–2138. doi: 10.1210/endo.131.5.1330492. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S. The 1991 Ulf von Euler Lecture. The L-arginine: nitric oxide pathway. Acta Physiol Scand. 1992 Jul;145(3):201–227. doi: 10.1111/j.1748-1716.1992.tb09359.x. [DOI] [PubMed] [Google Scholar]
- Nakano H., Fawcett C. P., McCann S. M. Enzymatic dissociation and short-term culture of isolated rat anterior pituitary cells for studies on the control of hormone secretion. Endocrinology. 1976 Feb;98(2):278–288. doi: 10.1210/endo-98-2-278. [DOI] [PubMed] [Google Scholar]
- Publicover N. G., Hammond E. M., Sanders K. M. Amplification of nitric oxide signaling by interstitial cells isolated from canine colon. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2087–2091. doi: 10.1073/pnas.90.5.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riveros-Moreno V., Beddell C., Moncada S. Nitric oxide synthase. Structural studies using anti-peptide antibodies. Eur J Biochem. 1993 Aug 1;215(3):801–808. doi: 10.1111/j.1432-1033.1993.tb18095.x. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Bredt D. S. Biological roles of nitric oxide. Sci Am. 1992 May;266(5):68-71, 74-7. doi: 10.1038/scientificamerican0592-68. [DOI] [PubMed] [Google Scholar]
- Southern C., Schulster D., Green I. C. Inhibition of insulin secretion by interleukin-1 beta and tumour necrosis factor-alpha via an L-arginine-dependent nitric oxide generating mechanism. FEBS Lett. 1990 Dec 10;276(1-2):42–44. doi: 10.1016/0014-5793(90)80502-a. [DOI] [PubMed] [Google Scholar]
- Springall D. R., Riveros-Moreno V., Buttery L., Suburo A., Bishop A. E., Merrett M., Moncada S., Polak J. M. Immunological detection of nitric oxide synthase(s) in human tissues using heterologous antibodies suggesting different isoforms. Histochemistry. 1992 Nov;98(4):259–266. doi: 10.1007/BF00271040. [DOI] [PubMed] [Google Scholar]
- Terenghi G., Riveros-Moreno V., Hudson L. D., Ibrahim N. B., Polak J. M. Immunohistochemistry of nitric oxide synthase demonstrates immunoreactive neurons in spinal cord and dorsal root ganglia of man and rat. J Neurol Sci. 1993 Aug;118(1):34–37. doi: 10.1016/0022-510x(93)90242-q. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Hope B. T. Neurons that say NO. Trends Neurosci. 1992 Mar;15(3):108–113. doi: 10.1016/0166-2236(92)90021-y. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Kimura H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience. 1992;46(4):755–784. doi: 10.1016/0306-4522(92)90184-4. [DOI] [PubMed] [Google Scholar]
- Welsh N., Eizirik D. L., Bendtzen K., Sandler S. Interleukin-1 beta-induced nitric oxide production in isolated rat pancreatic islets requires gene transcription and may lead to inhibition of the Krebs cycle enzyme aconitase. Endocrinology. 1991 Dec;129(6):3167–3173. doi: 10.1210/endo-129-6-3167. [DOI] [PubMed] [Google Scholar]



