Abstract
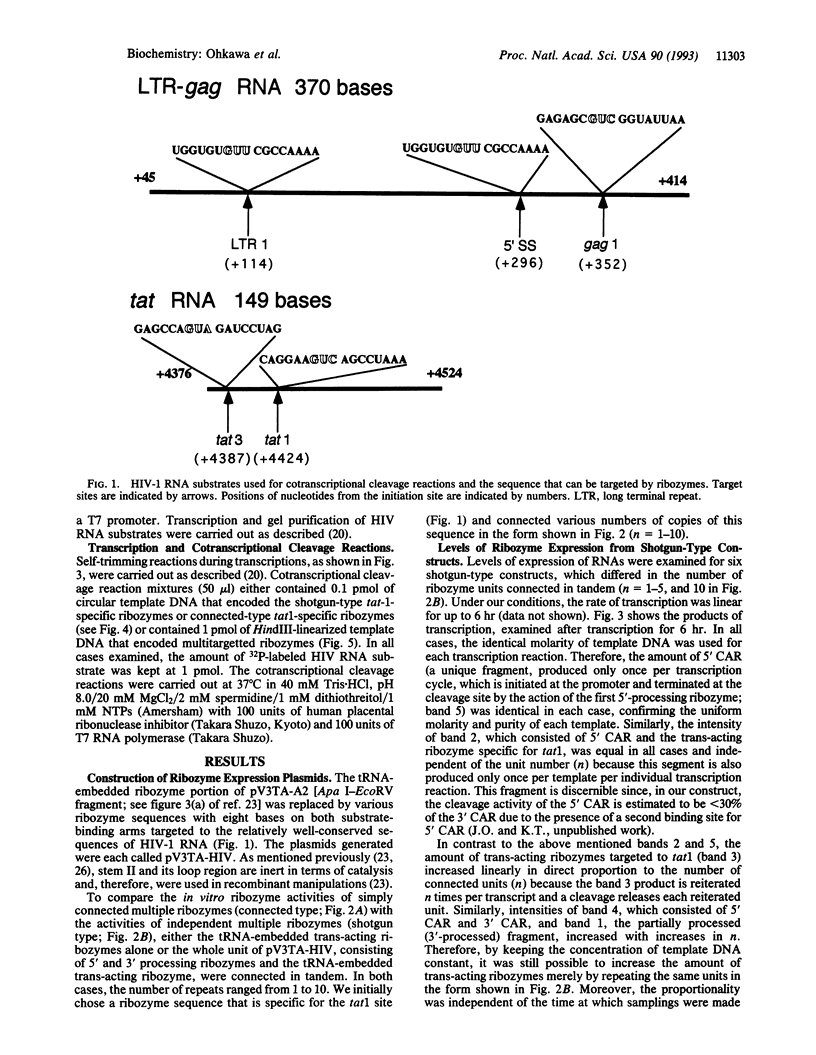
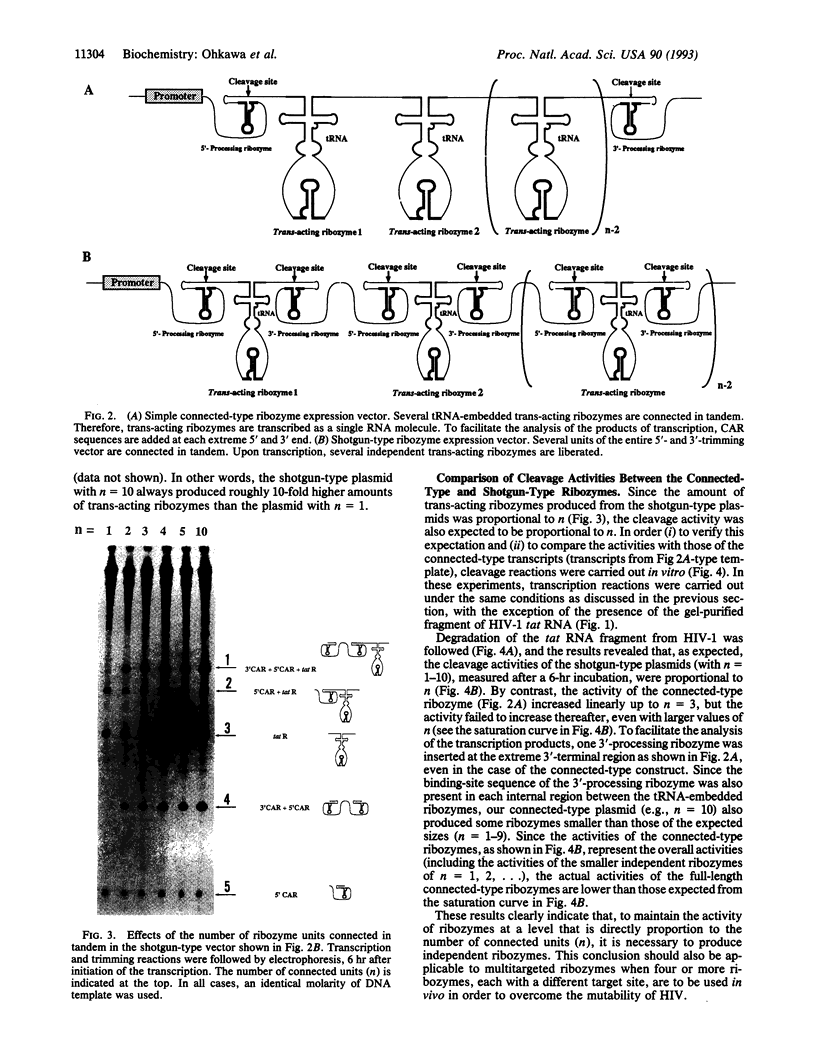
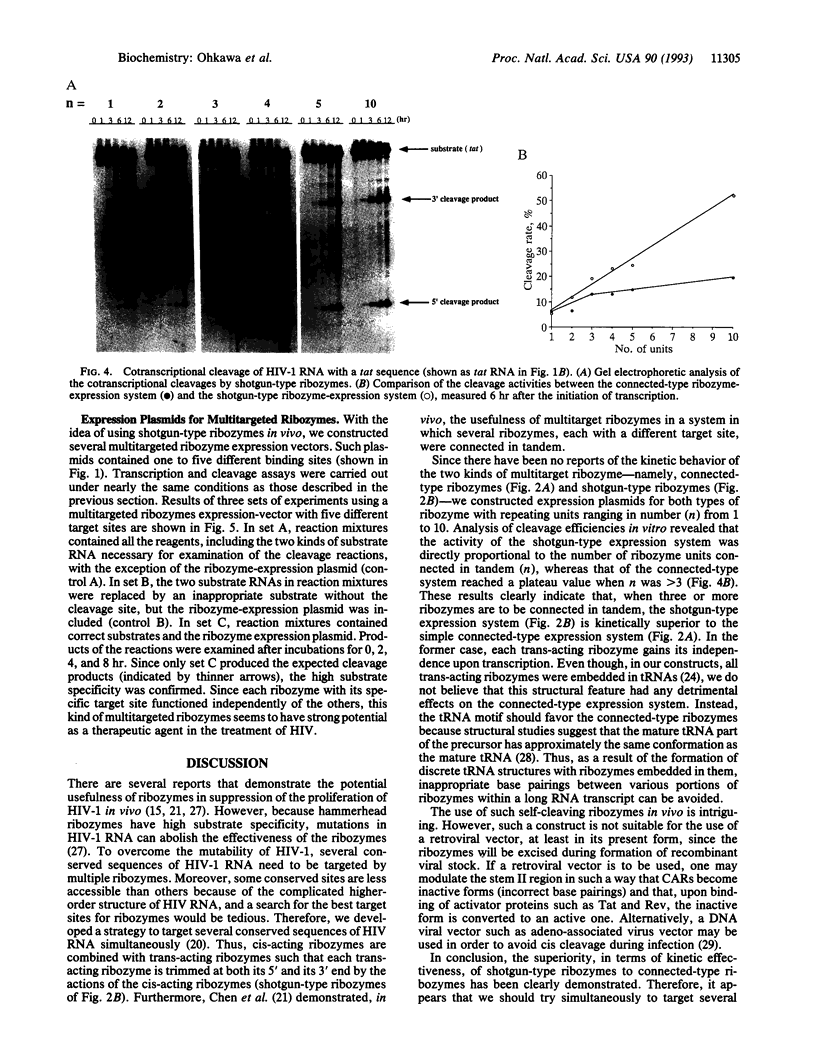
The kinetic behavior of ribozymes derived from two types of multiple-ribozyme expression vector were examined. In some cases, multiple ribozymes were expressed as a single RNA molecule and all the ribozymes were simply connected in tandem (connected type). In other cases, multiple ribozymes were flanked by cis-acting ribozymes at both their 5' and 3' ends so that, upon transcription, multiple ribozymes were trimmed at both their 5' and 3' ends, with resultant liberation of multiple independent ribozymes (shotgun type). When levels of ribozyme expression were examined for the shotgun-type vector, the level of the ribozyme transcript was found to be proportional to the number of units (n) connected in tandem. Accordingly, the activities of the shotgun-type ribozymes, in terms of the cleavage of HIV-1 RNA in vitro, were also found to be proportional to the number of units connected in tandem (n). By contrast, the activities of the connected-type ribozymes reached plateau values at around n = 3. These results indicate that, when the shotgun-type expression system is used, it is theoretically possible to generate various independent ribozymes, each specific for a different target site, without sacrificing the activity of any individual ribozyme.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S. Ribonuclease P: an enzyme with a catalytic RNA subunit. Adv Enzymol Relat Areas Mol Biol. 1989;62:1–36. doi: 10.1002/9780470123089.ch1. [DOI] [PubMed] [Google Scholar]
- Buzayan J. M., Gerlach W. L., Bruening G. Satellite tobacco ringspot virus RNA: A subset of the RNA sequence is sufficient for autolytic processing. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8859–8862. doi: 10.1073/pnas.83.23.8859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. J., Banerjea A. C., Harmison G. G., Haglund K., Schubert M. Multitarget-ribozyme directed to cleave at up to nine highly conserved HIV-1 env RNA regions inhibits HIV-1 replication--potential effectiveness against most presently sequenced HIV-1 isolates. Nucleic Acids Res. 1992 Sep 11;20(17):4581–4589. doi: 10.1093/nar/20.17.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten M., Birnstiel M. L. Ribozyme mediated destruction of RNA in vivo. EMBO J. 1989 Dec 1;8(12):3861–3866. doi: 10.1002/j.1460-2075.1989.tb08564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dropulić B., Lin N. H., Martin M. A., Jeang K. T. Functional characterization of a U5 ribozyme: intracellular suppression of human immunodeficiency virus type 1 expression. J Virol. 1992 Mar;66(3):1432–1441. doi: 10.1128/jvi.66.3.1432-1441.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of virusoid RNA is performed by the proposed 55-nucleotide active site. Cell. 1987 Jul 3;50(1):9–16. doi: 10.1016/0092-8674(87)90657-x. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Hutchins C. J., Rathjen P. D., Forster A. C., Symons R. H. Self-cleavage of plus and minus RNA transcripts of avocado sunblotch viroid. Nucleic Acids Res. 1986 May 12;14(9):3627–3640. doi: 10.1093/nar/14.9.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisziewicz J., Sun D., Klotman M., Agrawal S., Zamecnik P., Gallo R. Specific inhibition of human immunodeficiency virus type 1 replication by antisense oligonucleotides: an in vitro model for treatment. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11209–11213. doi: 10.1073/pnas.89.23.11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller H. F., Hoffarth V., Zimniak L. Unusual resistance of peptidyl transferase to protein extraction procedures. Science. 1992 Jun 5;256(5062):1416–1419. doi: 10.1126/science.1604315. [DOI] [PubMed] [Google Scholar]
- Peliska J. A., Benkovic S. J. Mechanism of DNA strand transfer reactions catalyzed by HIV-1 reverse transcriptase. Science. 1992 Nov 13;258(5085):1112–1118. doi: 10.1126/science.1279806. [DOI] [PubMed] [Google Scholar]
- Piccirilli J. A., McConnell T. S., Zaug A. J., Noller H. F., Cech T. R. Aminoacyl esterase activity of the Tetrahymena ribozyme. Science. 1992 Jun 5;256(5062):1420–1424. doi: 10.1126/science.1604316. [DOI] [PubMed] [Google Scholar]
- Preston B. D., Poiesz B. J., Loeb L. A. Fidelity of HIV-1 reverse transcriptase. Science. 1988 Nov 25;242(4882):1168–1171. doi: 10.1126/science.2460924. [DOI] [PubMed] [Google Scholar]
- Prody G. A., Bakos J. T., Buzayan J. M., Schneider I. R., Bruening G. Autolytic processing of dimeric plant virus satellite RNA. Science. 1986 Mar 28;231(4745):1577–1580. doi: 10.1126/science.231.4745.1577. [DOI] [PubMed] [Google Scholar]
- Roberts J. D., Bebenek K., Kunkel T. A. The accuracy of reverse transcriptase from HIV-1. Science. 1988 Nov 25;242(4882):1171–1173. doi: 10.1126/science.2460925. [DOI] [PubMed] [Google Scholar]
- Sarver N., Cantin E. M., Chang P. S., Zaia J. A., Ladne P. A., Stephens D. A., Rossi J. J. Ribozymes as potential anti-HIV-1 therapeutic agents. Science. 1990 Mar 9;247(4947):1222–1225. doi: 10.1126/science.2107573. [DOI] [PubMed] [Google Scholar]
- Sarver N., Rossi J. Gene therapy: a bold direction for HIV-1 treatment. AIDS Res Hum Retroviruses. 1993 May;9(5):483–487. doi: 10.1089/aid.1993.9.483. [DOI] [PubMed] [Google Scholar]
- Shimayama T., Nishikawa F., Nishikawa S., Taira K. Nuclease-resistant chimeric ribozymes containing deoxyribonucleotides and phosphorothioate linkages. Nucleic Acids Res. 1993 Jun 11;21(11):2605–2611. doi: 10.1093/nar/21.11.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira K., Nakagawa K., Nishikawa S., Furukawa K. Construction of a novel RNA-transcript-trimming plasmid which can be used both in vitro in place of run-off and (G)-free transcriptions and in vivo as multi-sequences transcription vectors. Nucleic Acids Res. 1991 Oct 11;19(19):5125–5130. doi: 10.1093/nar/19.19.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuyama N., Ohkawa J., Inokuchi Y., Shirai M., Sato A., Nishikawa S., Taira K. Construction of a tRNA-embedded-ribozyme trimming plasmid. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1271–1279. doi: 10.1016/s0006-291x(05)81543-8. [DOI] [PubMed] [Google Scholar]
- von Weizsäcker F., Blum H. E., Wands J. R. Cleavage of hepatitis B virus RNA by three ribozymes transcribed from a single DNA template. Biochem Biophys Res Commun. 1992 Dec 15;189(2):743–748. doi: 10.1016/0006-291x(92)92264-x. [DOI] [PubMed] [Google Scholar]