Abstract
Objective
To study if a culture of chondrocytes can be obtained from pathologic hyaline cartilage (PHC) fragments.
Design
Twenty-five men and 9 women with osteochondritis dissecans (OCD) in 11 cases, arthrosis in 13 patients, and trauma in the remaining 10 cases were included. The PHC fragments and a small sample of the next healthy cartilage were extracted by arthroscopy. According to the appearance, the PHC samples were divided into fixed (3 cases), flapped (6 patients), or loose bodies (25 cases), depending on the attachment degree of the cartilage to the subchondral bone. Approximately half of each pathologic sample and the whole healthy one were digested to isolate the cells trying to establish the cell culture.
Results
We were able to establish a cell culture in 7 out of 34 (20.6%) PHC samples (positive samples), whereas in the remaining 27 (79.4%) no cell growth was observed (negative samples). Most of the negative samples were loose bodies (P = 0.005) taken from patients with OCD or arthrosis (P = 0.001) with an evolution time of more than 1 year (P < 0.001). The best binary logistic regression model (P < 0.001) showed that the only factor affecting the establishment of cell culture was the evolution time (P = 0.044).
Conclusion
It is possible to culture chondrocytes from osteochondral fragments if they are traumatic, within a year of injury and not from fragments due to arthrosis or OCD.
Keywords: chondrocytes, cells, articular cartilage, tissue, knee, joint involved
Introduction
Osteochondritis dissecans (OCD) is defined as an orthopedic enigma. Despite the introduction of arthroscopy in the 1970s, which has allowed us to make a more definitive assessment of the size and condition of the lesion, and magnetic resonance imaging shortly after, in the early 1980s, which has offered us an early diagnosis, accurate location and stability, doubts, and controversies continue.
We do not exactly know the etiology, prognosis, and natural history of OCD. Many of the treatments that have been proposed do not make sense, especially when today we have serious doubts about the viability and survival of loose bodies, which is the end stage of the process in many cases. We have studied the viability in more than 60 cases by culturing the cartilage that surrounds the source of nearby osteochondritis and healthy cartilage, and it was found that cells do not grow in loose bodies but they do in healthy tissue (personal observations).
It is known that the mature hyaline articular cartilage is avascular, aneural, and alymphatic and had no capacity for regeneration.1 Today, both articular cartilage nutrition routes, synovial route or vascular route, are accepted. The first route—synovial—is defended by many authors.2-6 Yonetani et al.7 found viable hyaline cartilage in OCD cartilage fragments. Shapiro et al.8 also observed, as in idiopathic necrosis of the femoral head, the hyaline articular cartilage was still alive.
The main result for many authors is that the synovial pathway is shown as an undisputed route: the fragment is viable, can be reinserted into the bed, and even if it is enlarged, it can be cropped beforehand and adapted for its insertion in the lesion. The second pathway or vascular route was defended by many authors9-12; however, some authors13,14 found that in the adult knee the only area with limited vascularity was located in the anterior medial femoral condyle and not in the back or the middle and that such a fact was associated with more OCD.
We have operated OCD in knees for several years, and depending on size, age, location of the lesion, and stability, we performed different surgical procedures: extraction of the fragment, abrasion, microfracture or drilling of the bed, in other cases with a semidetached fragment, we reinserted it after abrasing the bed with screws and/or biodegradable needles. Finally in the detached fragments, loose bodies, we reinserted them onto the abrased bed, if they were not very old and their shape and size allowed it, fixing them with screws and/or biodegradable needles. However, these techniques were successful only in few cases, and although the positive outcome of the process has allowed these patients to continue leading a normal life for several years after, in most cases the techniques failed and the implanted fragment became loose, while showing a very thin appearance and with the screws having damaged the cartilage of the tibial plateau.
The aim of the present work was to study whether a culture of chondrocytes can be obtained from these pathologic hyaline cartilage (PHC) fragments. For this purpose, we have tried to culture the OCD anchored/adhered fragments, loose bodies, and flapped fragments, as per the following scheme:
Anchored/adhered articular hyaline cartilage fragments covering the OCD and the nearby healthy cartilage
Flapped cartilage fragments and the nearby healthy cartilage
Loose body fragments of cartilage and the nearby healthy cartilage
Materials and Methods
Patients
PHC fragments were extracted from the knee of 34 patients (25 men and 9 women) of age 34.4 ± 11.7 years (mean ± SD). All the patients signed an informed consent, and the study was approved by the hospital’s ethics committee.
The patients’ diagnosis was OCD in 11 cases, arthrosis in 13 patients, and trauma in the remaining 10 cases. Once diagnosed, the PHC fragment and a small sample of the nearby healthy cartilage were extracted by arthroscopy and transported to the lab in 25 mL of Dulbecco’s Modified Eagle’s Medium (DMEM) (Lonza Group Ltd., Basel, Switzerland).
Based on the aspect of the PHC fragment, we classified the samples into 3 types: (a) fixed, when the PHC fragment was attached to the subchondral bone but beneath the fragment the cartilage and the subchondral bone were not joined; (b) flapped fragment when it was attached to the subchondral bone by one or more points and the fragment was almost loose; or (c) loose body when the PHC fragment was completely detached from the subchondral bone. In 3 cases (8.8%) the PHC fragment was fixed, in 6 patients (17.7%) the fragment was flapped, and in the remaining 25 (73.5%) the fragment was a loose body.
Cell Cultures
Approximately half of each PHC fragment and the whole healthy cartilage were digested with collagenase to isolate the cells and then cultured with DMEM supplemented with 10% fetal bovine serum. Each sample was placed in a petri dish and cut into small fragments using a sterile razor blade. The fragments were carefully placed in a sterile 50 mL tube and isolation of cells was performed by enzymatic digestion with 1 mg/mL collagenase A (Roche Diagnostics GmbH, Mannheim, Germany) at 37°C overnight. The digested samples were filtered through a 70 µm gauge nylon cell strainer (BD Falcon, Franklin Lakes, NJ) connected to a clean 50 mL tube. To recover the maximum number of cells, the cell strainer was washed with 10 mL of DMEM. The sample was centrifuged for 5 minutes at 1,800 rpm and room temperature, the supernatant was discarded, and additional 20 mL of DMEM was added to the tube. The tube was centrifuged for 5 minutes at 1,500 rpm and room temperature, the supernatant was discarded, and the pellet was resuspended in DMEM supplemented with 10% of fetal bovine serum (Lonza), L-glutamine, and penicillin-streptomycin. The number of viable cells was estimated using the trypan-blue exclusion method in a Neubauer’s chamber.
Gene Expression Analysis
RNA was precipitated from the other half of the pathologic tissue with isopropanol after a sequential treatment with TRIzol (Invitrogen) and chloroform. RNA was reverse-transcribed using random hexanucleotides with the First Strand cDNA Synthesis Kit for RT-PCR (AMV) (Roche Diagnostics GmbH) following the manufacturer’s instructions.
The determination of the relative levels of expression of aggrecan, type I collagen (Col-I), and type II collagen (Col-II) was carried out by RT-PCR in a LightCycler 1.5 as previously reported15 using the expression of the housekeeping glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene as reference. Each sample was analyzed by duplicate and all genes were studied in the same PCR run.
Histology and Immunohistochemistry
In 9 cases we had enough pathologic tissue to perform a histological study. The remaining tissue was fixed in 10% buffered neutral formalin and paraffin embedded following standard procedure. Routine sections were stained with hematoxylin and eosin and Masson’s Trichrome and Safranin O. Selected sections were immunostained for Type II Collagen (clone 2B1.5, Master Diagnostica, Granada, Spain) and for S100 Protein (Polyclonal, DAKO, Barcelona, Spain) using a FLEX-Ready-to-Use immunohistochemical system (Envision, Glostrup, Denmark) and an automated immunostainer (DAKO Autostainer Plus, Dako). Appropriate controls were included.
Morphometric studies were performed to quantify cellularity in all histologic samples. The number of chondrocytes per surface unit was quantified in cartilage histologic sections stained with hematoxylin-eosin, using the image analysis software Leica QWin. For each sample a 1 mm2 section was analyzed, from a representative cartilage area, using sequential images taken with a digital camera DC300 paired to an optical microscope Leica DM5000B at 20× magnification.
Statistical Analysis
Statistical analysis was performed using the IBM SPSS Statistics version 22 for Windows software. Continuous variables were expressed as the mean ± standard deviation, and the normality was checked with the Kolmogorov-Smirnov test. The continuous variables studied were “Age,” “Body Mass Index,” “Aggrecan expression,” “Col-I expression,” and “Col-II expression” and were compared using Student’s t test or the Wilcoxon signed-rank test. Categorical variables were expressed as counts and/or percentage and were compared with the χ2 test. The categorical variables included in the study were “Gender” (0 = man, 1 = woman), “Dominance” (0 = left-handed, 1 = right-handed, 2 = ambidextrous), “Diagnostic” (0 = osteochondritis dissecans, 1 = arthrosis, 2 = traumatic), “Appearance” (0 = fixed, 1 = flapped, 2 = loose body), “Knee” (0 = right, 1 = left), “Place” (0 = lateral femoral condyle, 1 = medial femoral condyle, 2 = patella, 3 = trochlea, 4 = lateral femoral condyle, patella, and trochlea, 5 = patella and trochlea, 6 = lateral femoral condyle, medial femoral condyle, patella, and trochlea), “Time of evolution” (0 = less than 1 month, 1 = from 1 month to 1 year, 2 = more than 1 year), and “Cell growth” (0 = No, 1 = Yes).
Binary logistic regression (BLR) analysis was performed in order to explore the influence of the above-described variables on the response (cell culture set-up). Thus, dependent variable was defined as “Cell growth” (0 = No, 1 = Yes). Overall significance was assessed by the log of likelihood ratio with the χ2 test, and goodness-of-fit was studied by the Hosmer-Lemeshow test. Statistical significance of the coefficients in the regression equation was contrasted with the Wald test. Odds ratios (ORs) and their respective 95% confidence intervals (CIs) were also estimated. To study the ability of the definitive model in the discrimination between the 2 values of the “Cell growth” variable, receiver operating characteristic (ROC) curve was constructed using the predicted probability values estimated with this model as the test variable and “Cell growth yes = 1” as the value of the state variable.16 Cutoff probability value to discriminate between “Cell growth: yes” and “Cell growth: no” was estimated by examining the ROC curve coordinates.
In all comparisons and parameter estimation a value of P < 0.05 (2-sided) were considered statistically significant.
Results
Throughout the article, we will name “positive samples” those cartilage fragments in which the cell culture was successful. On the contrary, the cartilage fragments in which the cell culture could not be established will be named “negative samples.” In this study, among the PHC samples we had 7 (20.6%) positive samples out of the 34 studied, whereas the remaining 27 (79.4%) cases were negative samples. All the healthy cartilage samples taken at the same time as the pathologic samples were positive. The cell growth was normal in all the cases and no special event was observed during the cell culture. In Table 1, the influence of several factors on the establishment of the cell cultures is depicted. Age, gender, body mass index, dominance, and the knee from which the pathological cartilage fragments were collected were similar between positive and negative samples. Only the appearance, the distribution of the diagnosis, and the time of evolution were statistically different between the groups (P = 0.005, P = 0.001, and P < 0.001, respectively; χ2 tests) (Table 1). Thus, most of the positive samples were taken from patients with trauma and an evolution time less than 1 year. On the contrary, in most of the pathologic samples, completely detached from the subchondral bone (loose bodies) or taken from patients with OCD or arthrosis with a time of evolution of more than 1 year, the cell culture could not be set up. No statistically significant differences were observed between positive and negative samples regarding the anatomical place of the knee from where the pathologic cartilage fragment was taken (Table 2).
Table 1.
Influence of Several Factors in the Establishment of Cell Culture From the Pathologic Cartilage Fragments (Univariate Analysis).
| Cell Culture |
|||
|---|---|---|---|
| Factor | Yes | No | P Value |
| Age (years)a | 32.29 ± 11.81 | 35.00 ± 11.86 | 0.593 |
| Body mass indexa | 22.74 ± 1.71 | 25.72 ± 4.47 | 0.097 |
| Gender | 0.888 | ||
| Male | 5 | 20 | |
| Female | 2 | 7 | |
| Dominance | 0.653 | ||
| Left-handed | 0 | 2 | |
| Right-handed | 7 | 24 | |
| Ambidextrous | 0 | 1 | |
| Knee | |||
| Right | 5 | 12 | 0.203 |
| Left | 2 | 15 | |
| Aspect | 0.005 | ||
| Fixed | 1 | 2 | |
| Flapped | 4 | 2 | |
| Loose body | 2 | 23 | |
| Diagnosis | 0.001 | ||
| OD | 0 | 11 | |
| Arthrosis | 1 | 12 | |
| Trauma | 6 | 4 | |
| Time of evolution | <0.001 | ||
| Less than 1 month | 3 | 1 | |
| Between 1 month and 1 year | 3 | 2 | |
| More than 1 year | 1 | 24 | |
OD = osteochondritis dissecans.
Expressed as the mean ± standard deviation. Statistical significance is represented in boldface.
Table 2.
Comparison of the Anatomical Place of the Knee from Which the Pathologic Cartilage Fragment Was Taken in Positive and Negative Samples (χ2 Test).
| Cell Culture |
|||
|---|---|---|---|
| Place of the Knee | Yes (Positive Samples) | No (Negative Samples) | P Value |
| Medial femoral condyle (MFC) | 4 | 14 | 0.446 |
| Lateral femoral condyle (LFC) | 0 | 4 | |
| Patella | 2 | 6 | |
| Trochlea | 1 | 0 | |
| Patella + Trochlea | 0 | 1 | |
| Patella + Trochlea + MFC | 0 | 1 | |
| Patella + Trochlea + MFC + LFC | 0 | 1 | |
A part of the PHC fragment was processed to study the relative expression of aggrecan, Col-II, and Col-I in positive and negative samples (Fig. 1). The mean relative expression of aggrecan and Col-II was significantly higher in positive than in negative samples (P = 0.005 and P < 0.001, respectively; Student’s t test), suggesting that viable chondrocytes are present in these samples. By contrast, the mean relative expression of Col-I was statistically higher in negative than in positive samples (P < 0.001; Student’s t test), indicating that these PHC fragments had no viable hyaline cartilage.
Figure 1.
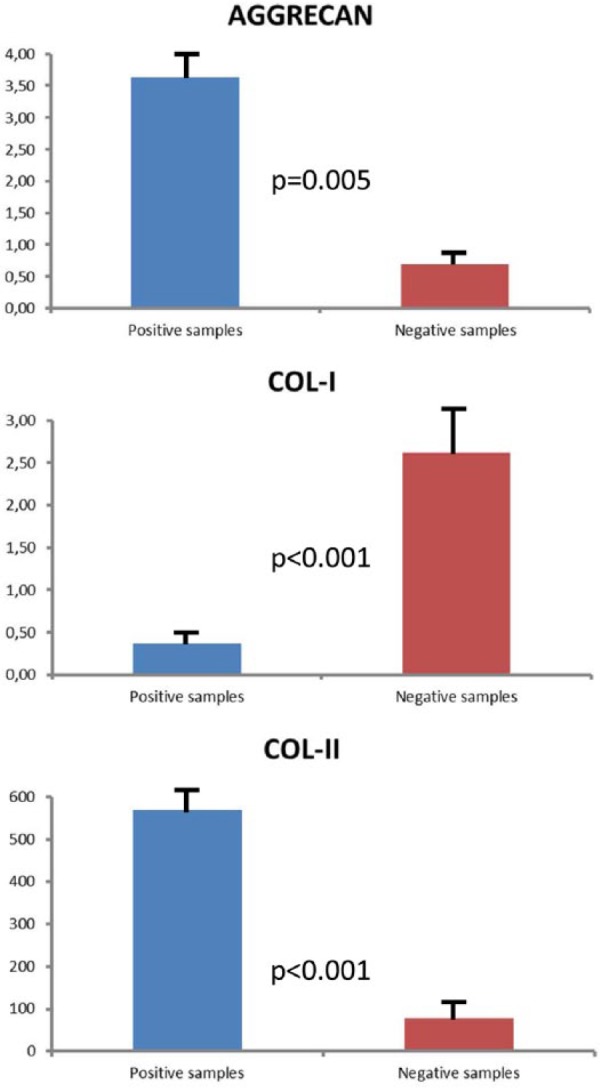
Mean expression of aggrecan, Col-I, and Col-II in positive and negative samples.
A histological study was performed in 9 of the extracted pathologic cartilage fragments (4 positive and 5 negative samples). All the samples showing positive cell cultures had well-differentiated hyaline cartilage (Fig. 2). Three of them were flapped fragments (the first with a time of evolution of less than 1 month, the second between 1 month and 1 year, and the third with a time of evolution of more than 1 year). The remaining positive sample belonged to a loose body with an evolution time less than 1 month. PHC fragments from the negative samples showed well-differentiated hyaline cartilage in 4 (3 loose bodies and 1 flapped fragment) of the 5 cases, with the remaining one being of fibroblastic nature (corresponding to a flapped cartilage fragment) (Fig. 3). All of them were cartilage fragments with a time of evolution of more than 1 year. In both positive and negative samples, trabecular bone tissue could be found (Fig. 4). Protein S100 expression was found in all pathologic fragments with hyaline cartilage (8 out of 9) but not in the sample with fibroblastic cells (Fig. 5). Type II collagen was only expressed in the samples showing hyaline cartilage (Fig. 6).
Figure 2.
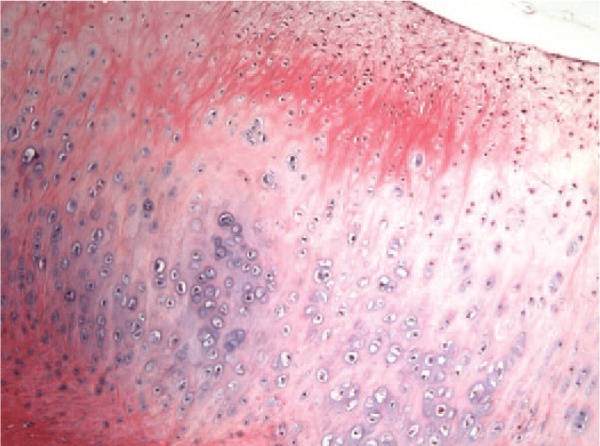
Pathologic cartilage fragment from the surface of a sample in which the culture could be established (positive samples) showing well-differentiated hyaline cartilage (magnification: 100×).
Figure 3.
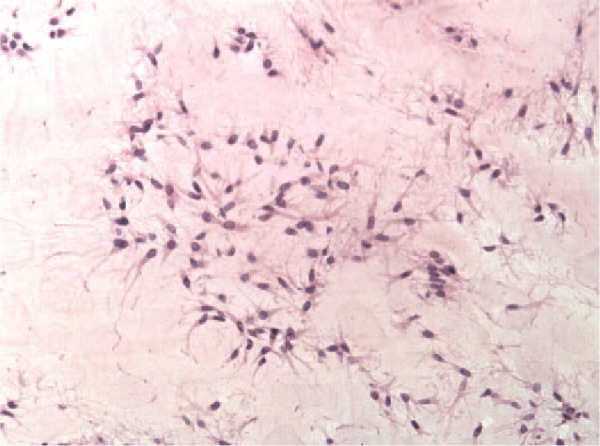
Pathologic cartilage fragment corresponding to a flapped cartilage fragment showing tissue of a fibroblastic nature (magnification: 200×).
Figure 4.
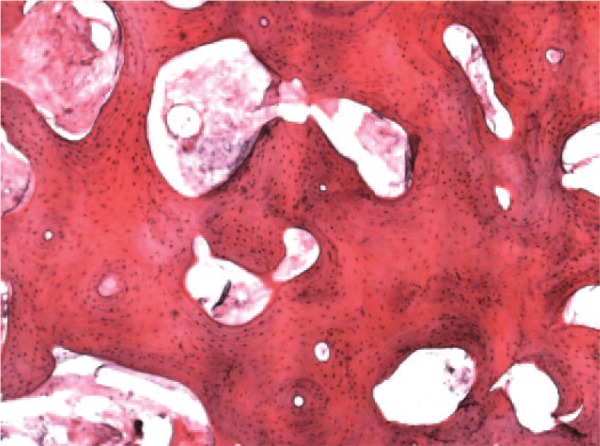
Trabecular bone tissue taken from a positive sample (magnification: 40×).
Figure 5.
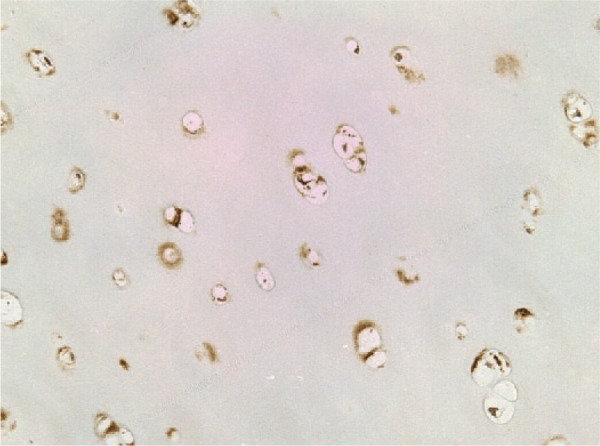
Immunostaining study showing expression of S100 protein in a pathologic sample with hyaline cartilage (magnification: 400×).
Figure 6.
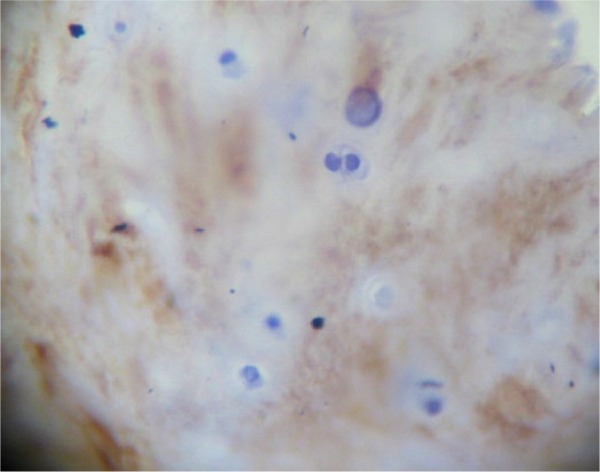
Expression of collagen type II was only found in the pathologic samples with hyaline cartilage (magnification: 400×).
The number of cells was quantified but showed no significant differences between positive and negative samples (390.17 cells/mm2 in the negative group vs. 389.00 cells/mm2 in the positive group).
A global BLR analysis model was constructed to study the effect of all the variables included in the present study with “Cell growth” as dependent variable (0 = no, 1 = yes). After the exclusion of the nonsignificant variables (one by one), the best fitting model obtained (the model that includes significant variables and those variables that make the model to fit well to data) is shown in Table 3. Log of likelihood ratio contrasted by the χ2 test demonstrated that the model was highly significant (χ2 = 21.02; degrees of freedom [df] = 4; P < 0.001). The Hosmer-Lemeshow test that evaluates the differences between the probabilities predicted by the model, and those observed, showed that the goodness of fit of the model was acceptable (χ2 = 4.5; df = 8; P = 0.808). As shown in Table 3, the time of evolution was statistically significant, adjusting by the appearance of the fragment (fixed, flapped, or loose body) and the body mass index values. The ORs estimated for the variables included in the model are shown in Table 3. The goodness of fit of the model was not acceptable if the variable body mass index values were excluded from the model (χ2 = 5.17; df = 4; P = 0.270).
Table 3.
Factors Associated with the Establishment of Cell Cultures from Loose Bodies in the Logistic Regression Analysis.
| Factor | Coefficient (B) | P Value | OR | 95% CI of OR |
|---|---|---|---|---|
| Time of evolutiona | 0.044 | |||
| Time of evolution (1) | 4.452 | 0.029 | 85.81 | 1.60 – 4614.91 |
| Time of evolution (2) | 5.222 | 0.028 | 185.28 | 1.74 – 19699.16 |
| Appearance | −1.644 | 0.143 | ||
| BMI | −0.401 | 0.273 | ||
| Constant | 9.767 | 0.228 |
OR = odds ratio; CI = confidence interval; BMI = body mass index.
Categorical variable (1 = between 1 month and 1 year; 2 = more than 1 year).
The probability values estimated with the above-described model were used to construct an ROC curve taking “Cell growth=yes” as the state variable (supplement file). Area under the ROC curve (AUC) was 0.963 (95% CI = 0.896-1.000; P < 0.001), indicating that the probabilities predicted by the model were very similar to those observed.
Discussion
One of the controversial facts in traumatology is the origin and the treatment of OCD, with some authors even claiming for the use of these pathologic cartilage fragments as a source of chondrocytes for a future autologous chondrocyte implantation. In this work, we have studied the viability of the cells isolated from these pathologic fragments. In our study, a cell culture could be set up in only 7 out of the 34 pathologic fragments included. This result showed that not in all cases the pathologic fragments derived from an OCD can be used as a cell source for an autologous chondrocyte implantation, and it explains why the fixation of the pathological cartilage fragment is not always maintained and it does not lead to the healing of the damaged cartilage. The reason of this result is unclear. Some authors have found that the cells from detached cartilage could lose their viability17 or might even have a reduced synthesis of extracellular matrix.18 Maybe, the disruption between the cartilage and the subchondral bone is responsible for this finding, and in these cases the signaling pathways between both compartments are cut, avoiding the communication between the articular cartilage and the subchondral bone. Our results support this idea since we have found that in the loose body fragments the probability to establish the cell culture was much lower than in those flapped and fixed fragments.
The opposite result has been found by other authors.19-21 These authors have found that the detached cartilage retains its properties and some cells that can be used for chondrocyte implantation can be obtained from them. One explanation of this discrepancy may be that the viability of the cells could be influenced by the time elapsed between the detachment of the cartilage and the moment when the pathological cartilage fragment is excised by a surgical procedure and the cells are isolated from the fragment. In fact, in our work we have found that the establishment of cell culture was affected by the diagnosis, the moment when the detached fragment was produced (the fragment became detached) and the appearance of the pathologic cartilage fragment: negative samples were more frequent in patients with OCD or arthrosis, when the fragment is a loose body and when the time of evolution was higher than 1 year (univariate analysis). In a multivariate analysis, the evolution time was the only statistically significant factor affecting the isolation of viable cells to start a cell culture, but in the model, the appearance of the fragment and the body mass index should be included. The inclusion of the appearance of the fragment in the statistical model, although without significance, supports the results found in the univariate analysis. We do not have a biological explanation for the inclusion of the body mass index in the model; it was only included for mathematical reasons. Touten et al.22 have reported that although the anchoring of old fragments could be considered, the reduction of osteochondral bodies should be done as soon as possible because extracellular matrix and type II collagen expression could deteriorate after increasing the time period. Giannini et al.21 found that the detached osteochondral fragments harvested from the ankle joint were a good source of cells for autologous chondrocyte implantation. Their results confirm our findings since they included 20 patients with osteochondral lesion in the talus of traumatic origin and with a mean time elapsing from the traumatic event to the fragment collection of 10 months.
We have also studied the expression pattern of types I and II collagens as well as the aggrecan expression of the pathological fragment. We have found that the expression of type I collagen was statistically higher in the negative samples whereas in the positive ones a statistically higher expression of aggrecan and type II collagen was found, indicating that at least in these fragments in which we were able to establish a cell culture, the phenotype of these cells were of chondral nature. We have studied the histology of 9 samples (5 negative and 4 positive). All the positive samples had well-differentiated hyaline cartilage, with expression of type II collagen. Taking these results together with those obtained from real-time PCR shows that these pathologic cartilage fragments had viable chondrocytes, which were able to grow after isolation from the corresponding PHC and which were able to synthesize the appropriate extracellular matrix too. These results are in agreement with those published by other authors concerning the use of these detached fragments as a source of chondrocytes.19-21 However, in the negative samples, 2 histological patterns were found: most samples (4 out of 5) had well-differentiated hyaline cartilage, whereas the remaining sample was composed of fibroblastic cells. As we have not studied the expression of other collagens or procollagens, for example, type IIA procollagen, characteristic of prechondrocytes, type X collagen, synthesized mainly by hyperthrophic chondrocytes or type III collagen, characteristic of dedifferentiated chondrocytes, we cannot establish the maturation status of the chondrocytes from the pathological cartilage samples in which a cell culture cannot be obtained. We have no clear explanation for this fact; maybe the time of evolution is so long that these cells lose their viability, or as we have explained before, they are loose body fragments in which signaling between cells and subchondral bone has been impaired, avoiding the establishment of cell cultures in these cases. However, future studies taking into account the evolution time and the expression pattern of high collagen and procollagen types will be carried out in order to elucidate this issue.
In conclusion, our study demonstrates that not all the pathologic cartilage fragments detached from the knee articular cartilage are a good source of cells for a cell culture for a future autologous implantation. Our results suggest that time elapsed between the loose body formation and the excision of the fragment is the main factor affecting the cell culture setup. Another factor that we have to take into account is the degree of detachment between the cartilage fragment and the subchondral bone. All these factors may affect not only the usefulness of the pathologic cartilage fragments as a source of chondrocytes for a future implantation but also the success of the anchoring of these fragments to the subchondral bone for the treatment of cartilage lesions. Since we consider that the knowledge of the future behavior of these fragments is very important for choosing one or other treatment, further studies will be planned to completely clarify the role of each factor. Although it is possible to culture chondrocytes from osteochondral fragments if they are traumatic, within a year of injury, the quality of cells from such fragments regarding cartilage formation capacity and suitability for clinical use needs further evaluation.
Supplementary Material
Footnotes
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work has been financed by the Fundacion Pedro Guillen.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The study was approved by the hospital’s ethics committee.
References
- 1. Iwamoto M, Ohta Y, Larmour C, Enomoto-Iwamoto M. Toward regeneration of articular cartilage. Birth Defects Res C Embryo Today. 2013;99:192-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Giera M, Ioan-Facsinay A, Toes R, Gao F, Dalli J, Deelder AM, et al. Lipid and lipid mediator profiling of human synovial fluid in rheumatoid arthritis patients by means of LC-MS/MS. Biochim Biophys Acta. 2012;1821:1415-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kokebie R, Aggarwal R, Lidder S, Hakimiyan AA, Rueger DC, Block JA, et al. The role of synovial fluid markers of catabolism and anabolism in osteoarthritis, rheumatoid arthritis and asymptomatic organ donors. Arthritis Res Ther. 2011;13:R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hui AY, McCarty WJ, Masuda K, Firestein GS, Sah RL. A systems biology approach to synovial joint lubrication in health, injury, and disease. Wiley Interdiscip Rev Syst Biol Med. 2012;4:15-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Y, Wei L, Zeng L, He D, Wei X. Nutrition and degeneration of articular cartilage. Knee Surg Sports Traumatol Arthrosc. 2013;21:1751-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fam H, Bryant JT, Kontopoulou M. Rheological properties of synovial fluids. Biorheology. 2007;44:59-74. [PubMed] [Google Scholar]
- 7. Yonetani Y, Matsuo T, Nakamura N, Natsuume T, Tanaka Y, Shiozaki Y, et al. Fixation of detached osteochondritis dissecans lesions with bioabsorbable pins: clinical and histologic evaluation. Arthroscopy. 2010;26:782-9. [DOI] [PubMed] [Google Scholar]
- 8. Shapiro F, Connolly S, Zurakowski D, Menezes N, Olear E, Jimenez M, et al. Femoral head deformation and repair following induction of ischemic necrosis: a histologic and magnetic resonance imaging study in the piglet. J Bone Joint Surg Am. 2009;91:2903-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mobasheri A. The future of osteoarthritis therapeutics: emerging biological therapy. Curr Rheumatol Rep. 2013;15:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leyh M, Seitz A, Dürselen L, Schaumburger J, Ignatius A, Grifka J, et al. Subchondral bone influences chondrogenic differentiation and collagen production of human bone marrow-derived mesenchymal stem cells and articular chondrocytes. Arthritis Res Ther. 2014;16:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakasa T, Adachi N, Kato T, Ochi M. Correlation between subchondral bone plate thickness and cartilage degeneration in osteoarthritis of the ankle. Foot Ankle Int. 2014;35:1341-9. [DOI] [PubMed] [Google Scholar]
- 12. Imhof H, Breitenseher M, Kainberger F, Rand T, Trattnig S. Importance of subchondral bone to articular cartilage in health and disease. Top Magn Reson Imaging. 1999;10:180-92. [DOI] [PubMed] [Google Scholar]
- 13. Malinin T, Ouellette EA. Articular cartilage nutrition is mediated by subchondral bone: a long-term in baboons. Osteoarthritis Cartilage. 2000;8:483-91. [DOI] [PubMed] [Google Scholar]
- 14. Reddy AS, Frederick RW. Evaluation of the intraosseous and extraosseous blood supply to the distal femoral condyles. Am J Sports Med. 1998;26:415-9. [DOI] [PubMed] [Google Scholar]
- 15. Guillén-García P, Rodríguez-Iñigo E, Guillén-Vicente I, Caballero-Santos R, Guillén-Vicente M, Abelow S, et al. Increasing the dose of autologous chondrocytes improves articular cartilage repair: histological and molecular study in the sheep animal model. Cartilage. 2014;5:114-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561-77. [PubMed] [Google Scholar]
- 17. Kuroki K, Cook JL, Tomlinson JL, Kreeger JM. In vitro characterization of chondrocytes isolated from naturally occurring osteochondrosis lesions of the humeral hip of dogs. Am J Vet Res. 2002;63:186-93. [DOI] [PubMed] [Google Scholar]
- 18. Tomlinson JL, Cook JL, Kuroki K, Kreeger JM, Anderson MA. Biochemical characterization of cartilage affected by osteochondritis dissecans in the humeral hip of dogs. Am J Vet Res. 2001;62:876-81. [DOI] [PubMed] [Google Scholar]
- 19. Aurich M, Hoffmann GO, Mückley T, Mollenhauer J, Rolauffs B. In vitro phenotypic modulation of chondrocyte from knees of patients with osteochondritis dissecans. J Bone Joint Surg Br. 2012;94:62-7. [DOI] [PubMed] [Google Scholar]
- 20. Pascual-Garrido C, Tanoira I, Musculo DL, Ayerza MA, Makino A. Viability of loose body fragments in osteochondritis dissecans of knee. A series of cases. Int Orthop. 2010;34:827-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Giannini S, Buda R, Grigolo B, Vannini F, De Franceschi L, Facchini A. The detached osteochondral fragment as a source of cells for autologous chondrocyte implantation (ACI) in the ankle joint. Osteoarthritis Cartilage. 2005;13:601-7. [DOI] [PubMed] [Google Scholar]
- 22. Touten Y, Adachi N, Deie M, Tanaka N, Ochi M. Histologic evaluation of osteochondral loose bodies and repaired tissues after fixation. Arthroscopy. 2007;23:188-96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


