Abstract
Objective
We have recently shown that mesenchymal stem cells (MSCs) embedded in a hyaluronic acid (HA) hydrogel and exposed to chondrogenic factors (transforming growth factor–β3 [TGF-β3]) produce a cartilage-like tissue in vitro. The current objective was to determine if these same factors could be combined immediately prior to implantation to induce a superior healing response in vivo relative to the hydrogel alone.
Design
Trochlear chondral defects were created in Yucatan mini-pigs (6 months old). Treatment groups included an HA hydrogel alone and hydrogels containing allogeneic MSCs, TGF-β3, or both. Six weeks after surgery, micro-computed tomography was used to quantitatively assess defect fill and subchondral bone remodeling. The quality of cartilage repair was assessed using the ICRS-II histological scoring system and immunohistochemistry for type II collagen.
Results
Treatment with TGF-β3 led to a marked increase in positive staining for collagen type II within defects (P < 0.05), while delivery of MSCs did not (P > 0.05). Neither condition had an impact on other histological semiquantitative scores (P > 0.05), and inclusion of MSCs led to significantly less defect fill (P < 0.05). For all measurements, no synergistic interaction was found between TGF-β3 and MSC treatment when they were delivered together (P > 0.05).
Conclusions
At this early healing time point, treatment with TGF-β3 promoted the formation of collagen type II within the defect, while allogeneic MSCs had little benefit. Combination of TGF-β3 and MSCs at the time of surgery did not produce a synergistic effect. An in vitro precultured construct made of these components may be required to enhance in vivo repair in this model system.
Keywords: cartilage, repair, mesenchymal stem cells, TGF-β3, animal models
Introduction
Focal defects in the articular cartilage occur due to sporting activities, trauma, or other activities of daily living. Given that these defects can impair quality of life1 and predispose the adjacent cartilage to progressive degeneration,2,3 these localized lesions are often surgically treated in young and/or active patient populations.4,5 In addition to currently available treatment options, including autologous cartilage implantation and osteochondral allografting,4,6-10 tissue engineering (TE) and regenerative medicine approaches have been pursued, combining various cell sources, scaffolding materials, and biochemical and biomechanical factors, to engineer cartilage formation either in vitro or in vivo.
Over the past 2 decades, steady advances in in vitro culture methods have culminated in a variety of TE approaches that can produce engineered constructs with biomechanical and biochemical properties on the order of native cartilage.11-20 As an example, our group has utilized an approach involving the combination of mesenchymal stem cells (MSCs) embedded within hyaluronic acid (HA) hydrogels. When exposed to chondrogenic factors, including transforming growth factor–β3 (TGF-β3), these constructs achieve near native biomechanical and biochemical properties during in vitro culture.12,20 However, it remains an open question as to whether these components must first be cultured in vitro to form tissue engineered cartilage or if they can be combined immediately prior to implantation to successfully induce cartilage repair in vivo.
Given that preculture would dramatically increase costs associated with therapeutic intervention, this study focused on the latter scenario, using our established porcine model of cartilage injury and repair.21,22 Although many large animal studies have applied exogenous TGF and MSCs in vivo for cartilage repair,22-44 few studies have used a full factorial design to determine the relative impact of one component versus another or to identify synergistic effects. Even in the small number of studies with full factorial designs, the results are unclear, with some studies reporting improved histological appearance when MSCs were combined with TGF-β,40 and others showing little improvement relative to TGF-β alone.39 Numerous variables likely contribute to these contrary results, including animal species, injury model, scaffolding material, growth factor type, dosage, and delivery method, cell type and number, and so on.
These differences suggest that generalized statements about the effects of a growth factor or cell type may not be possible and that it is necessary to explore the components of each TE system for each specific animal and injury model. As such, the objective of the current study was to determine whether growth factor or cell delivery (i.e., TGF-β3 or MSCs) within an HA hydrogel could induce a superior healing response in vivo when each factor was delivered alone or in combination in a porcine model of full thickness cartilage repair. We hypothesized that the combined treatment of MSCs and TGF-β3 within the HA hydrogels would result in the most robust positive healing response.
Methods
All animal procedures were performed at the Philadelphia VA Medical Center with approval from the Institutional Animal Care and Use Committee and in accordance with policies set forth by the National Institutes of Health. Eight adolescent, male Yucatan mini-pigs (6 months old, ~25-35 kg) were utilized (Sinclair Bioresources). Experimental groups included (Fig. 1B): (1) treatment with an acellular HA hydrogel (HA), (2) treatment with an HA hydrogel seeded with MSCs (HA/MSCs), (3) treatment with an HA hydrogel containing alginate microspheres encapsulating TGF-β3 (HA/TGF),45 and (4) treatment with an HA hydrogel seeded with MSCs and containing microspheres encapsulating TGF-β3 (HA/MSCs/TGF). Normal cartilage served as a control for all groups. To examine the early term effects of the various treatments, 1 animal with defects treated with HA, HA/MSCs, HA/TGF, and HA/MSCs/TGF (n = 1 per group) was euthanized at 2 weeks postoperatively. The remaining 7 animals were evaluated at 6 weeks postoperatively. Not all treatment groups were performed in the same set of animals, and other groups not reported here were also evaluated, giving rise to the unequal sample sizes (HA, n = 7; HA/MSCs, n = 4; HA/TGF, n = 7; HA/MSCs/TGF, n = 8). To minimize the number of animals used, an untreated control was not included in the study design, as this was described in our previous publication using this identical model.21 In that study, delivery of HA gel alone caused no statistically significant changes in the outcome measures described.
Figure 1.
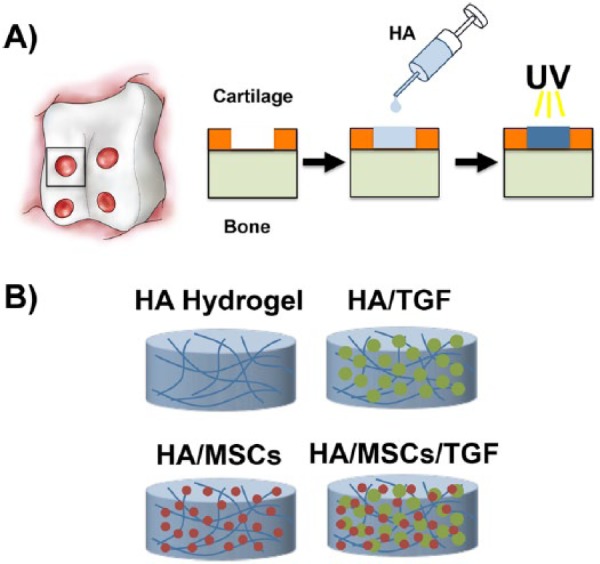
(A) Illustration of cartilage defects created in the trochlear groove and schematic of polymerization of the hyaluronic acid (HA) hydrogel within the defect via ultraviolet (UV) light. (B) Schematic of experimental groups. HA = hyaluronic acid; MSCs = mesenchymal stem cells; TGF = microspheres containing transforming growth factor-β3.
To form the HA hydrogel, methacrylated HA was synthesized by reacting methacrylic anhydride (Sigma) and 74 kDa HA (Lifecore) as previously described.17,18,20 Two days before surgery, the HA macromer was sterilized by exposure to an ultraviolet lamp for 15 minutes. Afterward, a solution of 1.5% HA (mass/volume) with 0.05% Irgacure-2959 photoinitiator (Ciba-Geigy) was produced in phosphate buffered saline.
Alginate microspheres containing TGF-β3 were prepared as previously described using an emulsion/gelation technique.45 Alginic acid sodium salt (Sigma) was dissolved in deionized water (2.3% w/v) with bovine serum albumin (1% w/v) combined with TGF-β3 (R&D Systems) in solution to achieve a final alginate concentration of 2% (w/v) and a TGF-β3 concentration of 12.5 μg/mL. The alginate/TGF-β3 mixture was added dropwise to an excess of olive oil under stirring conditions. Tween 80 (1% (v/v)) was used as a surfactant. The emulsion was allowed to mix for 3 minutes. Afterward, a calcium chloride solution (200 mM) was added dropwise, and the alginate was allowed to crosslink for 15 minutes. The solution was then centrifuged at 1500g for 5 minutes to isolate the microspheres. After removal of the supernatant, particles were resuspended in 2-propanol to remove the residue oil and centrifuged again. This washing process was repeated a total of 3 times, followed by 4 washes with sterile deionized water to remove the 2-propanol. To enable controlled release of the TGF-β3, microspheres were coated with nanofilm layers of poly(allylamine hydrochloride) (molecular weight [MW] 15 kDa) and poly(sodium 4-styrenesulfonate) (MW 1 MDa) (Sigma), as previously described.45,46 This approach results in a roughly linear release of TGF-β3 for up to 5 to 7 days in vitro. For in vivo implantation, microspheres were mixed with HA solution to obtain a final concentration of 2 μg/mL of TGF-β3 and 1% (w/v) of HA solution.
MSCs were obtained from 2 mini-pigs (6-month-old males) in a parallel study immediately after euthanasia. A Jamshidi needle was used to obtain a bone marrow aspirate from the iliac crest. Marrow was diluted in Dulbecco’s modified Eagle’s medium (DMEM) containing heparin. Following centrifugation, marrow was plated onto tissue culture plates in basal medium (DMEM with 10% fetal bovine serum and 1% penicillin/streptomycin/fungizone as previously described.47 MSC colonies formed within 2 weeks. Cells were frozen in liquid nitrogen until further use. Before surgery, cells were plated at 20,000 cells/cm2 and expanded to passage 2 in basal media. Cells from the 2 donors were then pooled. For the 2-week time point, MSCs were tagged with a fluorescent dye (Vybrant DiD, Molecular Probes) to enable tracking in vivo. Immediately prior to implantation, cells were resuspended in HA solution to achieve a concentration of 60 million cells/mL and 1% HA.
For surgery, animals were sedated with ketamine and xylazine, and anesthesia was maintained throughout with isoflurane. A lateral parapatellar arthrotomy through a medial-based knee incision was made to the stifle joint, and the patella was retracted medially to expose the trochlear groove of the femur. Full-thickness chondral defects (4 mm diameter) were created bilaterally in the trochlear groove (4 defects per joint) using a biopsy punch as previously described (Fig. 1A).21 The cartilage was completely removed (~2 mm thickness) without macroscopic removal of the subchondral bone. No microfracture was performed. For all groups, HA hydrogels were polymerized in situ via exposure to ultraviolet light (365 nm, 1 mW/cm2, Omnicure S2000, Lumen Dynamics Group) for 10 minutes. Defect volume was ~0.025 mL, allowing for delivery of 50 ng of TGF-β3 and 1.5 million MSCs per defect. Previous studies have indicated that no other means of fixation are required to keep the hydrogels within the defects.21,22
After defect creation and repair, layered closure was performed using absorbable sutures. Animals were provided bupivacaine and carprofen for postoperative pain control. Animals were allowed free movement and weight bearing as tolerated, with no specific rehabilitation protocols prescribed. At 2 or 6 weeks postoperatively, animals were euthanized with an overdose of pentobarbital. Afterward, hind limbs were disarticulated at the hip and the trochlear groove of each joint was carefully exposed. After gross inspection and imaging, individual cartilage defects with underlying bone as well as normal osteochondral samples were isolated and fixed in 4% paraformaldehyde.
For the samples at 2 weeks, cell nuclei in whole mount samples were stained with Hoechst stain. Using an Olympus Fluoview FV1000 confocal microscope (Olympus America Inc., Center Valley, PA), confocal stacks were acquired from the edge of the defect to a depth of 100 µm from the cartilage surface. Samples were then dehydrated, paraffin embedded, sectioned to 6 µm, and stained to assess cell morphology (hematoxylin and eosin).
For the 6-week time point, micro-computed tomography (μCT) was performed to assess the 3D morphometry of the healing cartilage and bone (Viva CT75, Scanco).48-50 Specimens were first μCT scanned to image the bone (70 kVp, 110 µA). Samples were then placed in an iodine-based contrast solution (Lugol’s solution, Sigma) for 48 hours and rescanned using the same parameters to visualize the cartilage. Bone volume per total volume (BV/TV) was calculated for the first 2 mm and for a region 3 to 5 mm beneath the original defect for each specimen.21 Degree of defect fill was determined as a percentage of the total defect volume from the contrast-enhanced micro-computed tomography (µCT) images.
Following µCT, samples were decalcified (Formical 2000, Decal Chemical Corporation) for 1 week and prepared for histology as above to assess cell morphology (hematoxylin & eosin) and matrix content (proteoglycan and collagen/fibrous matrix via Safranin O and fast green, respectively). Slides were scored using a modified ICRS-II system51 by 5 blinded reviewers, with scores averaged across reviewers.
To assess the deposition of type II collagen via immunohistochemistry, sections were deparaffinized, rehydrated, and subjected to proteinase K antigen retrieval. Sections were incubated with a type II collagen antibody (5 µg/mL; Developmental Studies Hybridoma Bank, University of Iowa) for 1 hour. After washing, the antibody was detected using the Millipore Immunoperoxidase Secondary Detection System (EMD Millipore Corporation). After brightfield imaging, images were converted to grayscale, and the area of the defect was outlined using ImageJ (National Institutes of Health). The images were thresholded to match the positive staining in the original image. The percent positive staining was computed as the number of black (positive) pixels divided by the total number of pixels in the defect.
Statistical analyses were performed using SPSS (version 21, IBM). Normality of each dataset was verified using the Kolmogorov-Smirnoff test. Given that all data for the experimental groups was normally distributed, a 2-way analysis of variance was used for most outcome measures, using treatment with TGF-β3 or MSCs as independent factors. For µCT analysis, the zone within the subchondral bone was an additional independent factor. After performing the analysis of variance, Bonferroni or Games-Howell post hoc tests were performed, depending on whether or not the variances were equal between groups. Significance was set at P < 0.05. All experimental groups were also compared with the normal control via either t tests or Mann-Whitney U tests, depending on the normality of the control data. To control for type I error, a Bonferroni correction was used, and significance was set at P < 0.013 for these comparisons.
Results
At the time of surgery, a small amount of bleeding occurred during defect creation. Bleeding was minimized prior to hydrogel implantation, and all hydrogels readily gelled in the defects within 10 minutes and maintained complete defect fill prior to closure. All animals were mobile/standing on the day of or the day following surgery. No animals had noticeable gait deficits after 1 week.
Two weeks after surgery, defects within one animal were examined to assess the patency of the hydrogel within the defect as well as the presence of delivered MSCs. Confocal imaging revealed the presence of both implanted MSCs and endogenous cells at the central defect surface for the HA/MSCs and HA/MSCs/TGF groups (Fig. 2A). At the defect edge, the implanted MSCs remained within the hydrogel and were not present in the adjacent cartilage. Endogenous cells were also present within the defect for the HA and HA/TGF groups (Fig. 2A). Histological staining of the cells and matrix via hematoxylin and eosin showed the presence of the hydrogel within the defect site for all groups (Fig. 2B). Partial incorporation of the construct was observed, and a hypercellular, fibrous matrix surrounded the hydrogel within the defect site for all groups.
Figure 2.
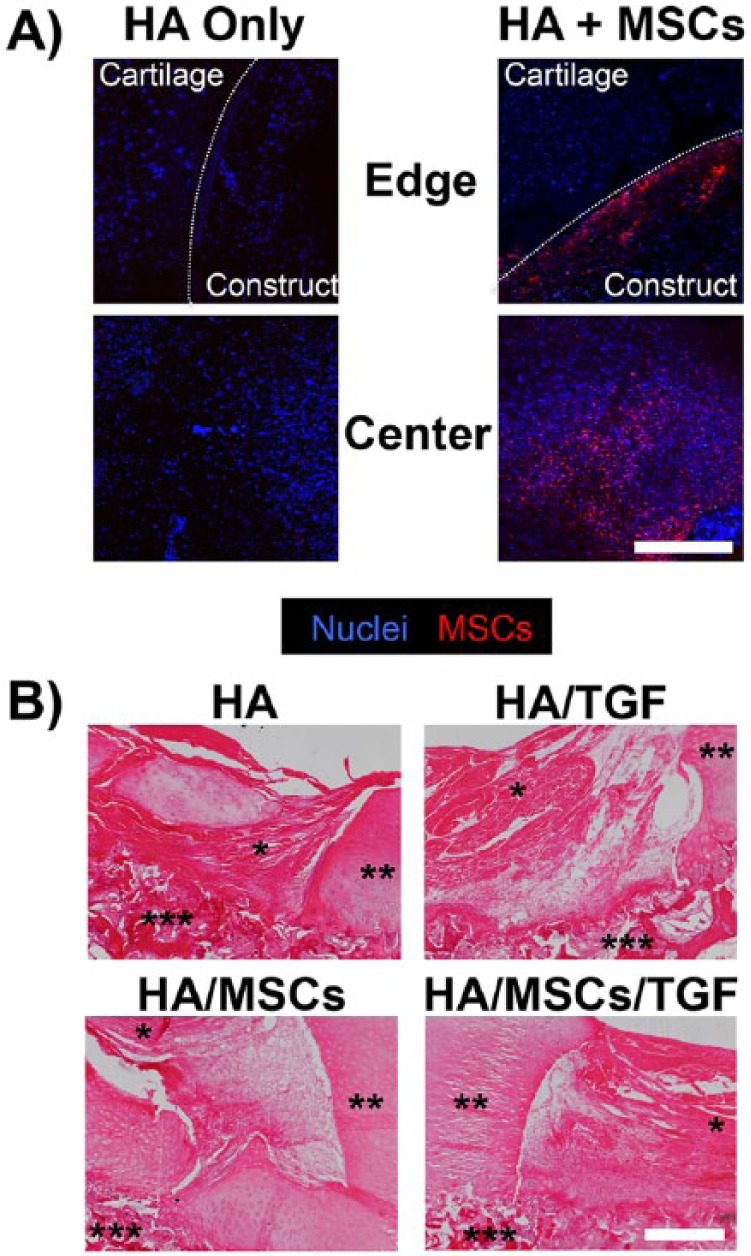
Short-term integration of hyaluronic acid hydrogels and persistence of delivered stem cells. (A) Confocal images of defect site after 2 weeks in vivo following treatment with hyaluronic acid hydrogels only or with mesenchymal stem cells (scale bar = 500 µm; HA = hyaluronic acid; MSCs = mesenchymal stem cells). All cell nuclei labeled blue. Implanted cells also marked with cell tracker (red). Host cell nuclei show as blue. (B) Hematoxylin and eosin staining of cell nuclei and matrix showing incorporation of the HA hydrogels for all groups at 2 weeks postimplantation (*HA hydrogel, **adjacent cartilage, ***underlying bone, scale bar = 500 µm; TGF = microspheres containing transforming growth factor–β3).
Six weeks after surgery, a white/red fibrous tissue was present within the defects with incomplete filling (Fig. 3A). Little to no damage was found in the adjacent cartilage, and no marked differences were noted between experimental groups. From μCT measurements, mean values for defect fill ranged from 70% for the HA/MSCs/TGF group to 87% for the HA group (Fig. 3B). No statistically significant effect was observed with the inclusion of TGF-β3 (P > 0.05). Interestingly, groups containing MSCs had defect fill values 12% to 18% lower than defects without MSCs (P < 0.05). The interaction term between treatment with TGF-β3 and MSCs was not statistically significant (P > 0.05).
Figure 3.
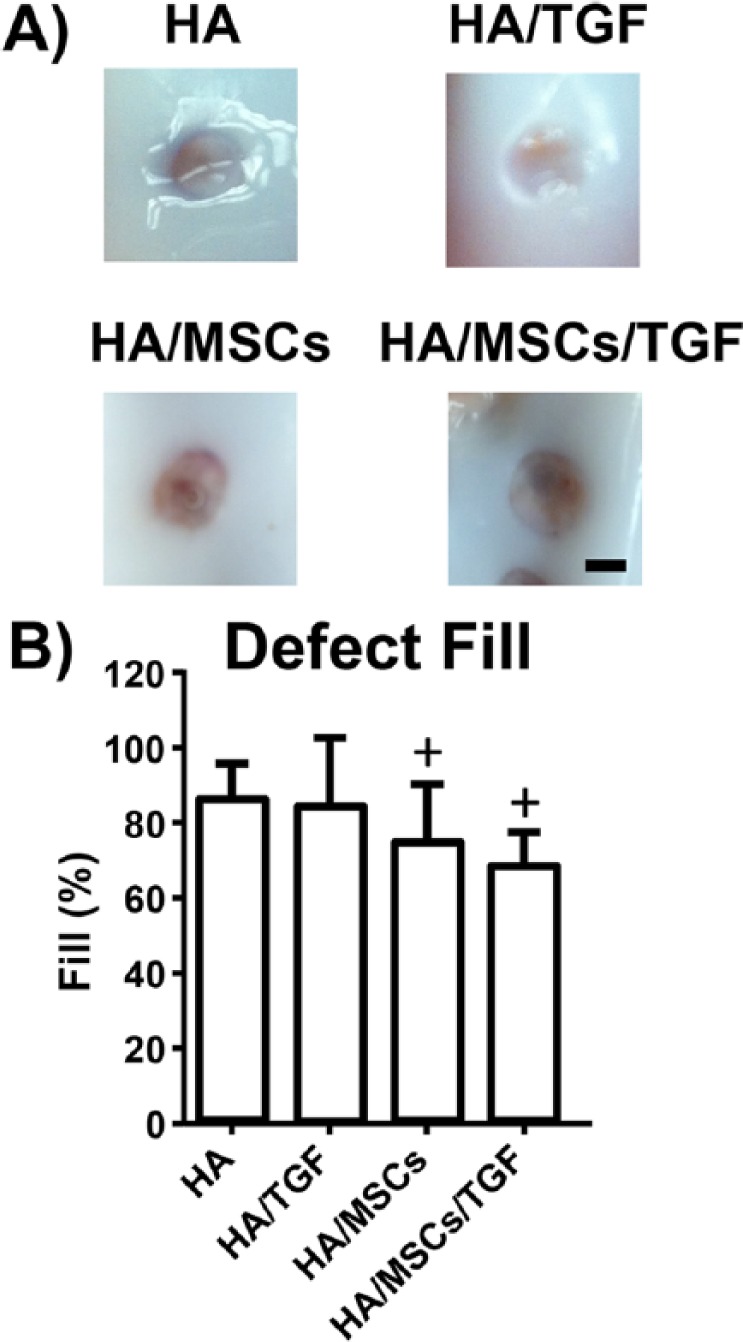
(A) Gross images of cartilage defects after 6 weeks of healing (scale bar = 2 mm; HA = hyaluronic acid; MSCs = mesenchymal stem cells; TGF = microspheres containing transforming growth factor-β3). (B) Quantification of defect fill via micro-computed tomography reconstruction (+P < 0.05 vs. non-MSC groups).
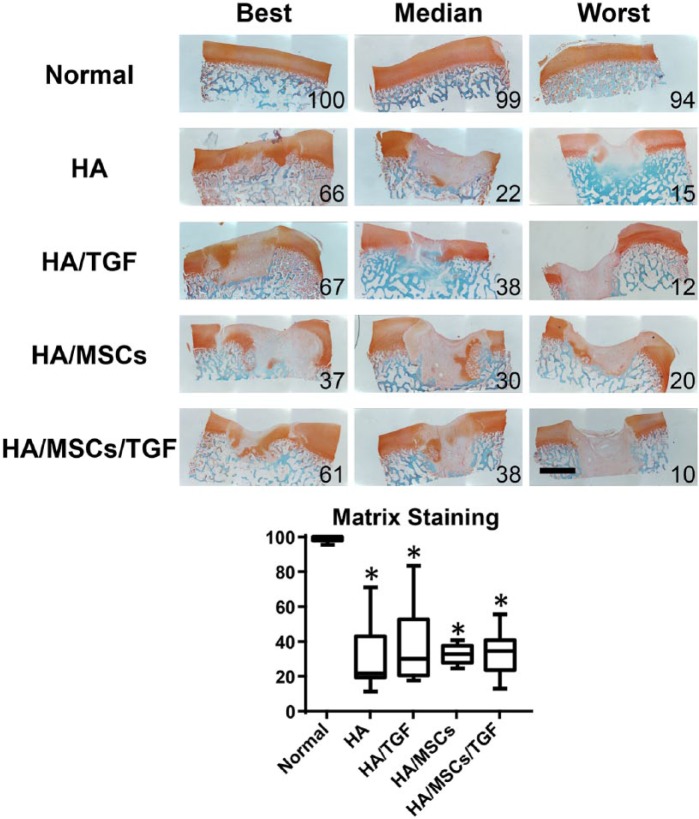
Histological evaluation of proteoglycans and fibrous tissue deposition at the repair site was assessed via Safranin O/Fast Green staining (Fig. 4). Most specimens had at least some positive staining for proteoglycans, although the amount of staining was highly variable between the best and worst samples. The best samples featured robust staining for proteoglycans, while the worst samples were filled with almost entirely fibrous tissue. Semiquantitative ICRS-II scoring (Fig. 4) of matrix staining revealed similar mean values ranging from 21 for the HA group to 34 for the HA/MSC/TGF group. No effect was observed due to treatment with either TGF-β3 or MSCs (P > 0.05). However, all experimental groups had lower matrix staining scores compared with normal controls (P < 0.05).
Figure 4.
Histological staining (Safranin O/fast green) for proteoglycans (red) and collagens (green) of full thickness cartilage defects treated with hyaluronic acid (HA) hydrogels, microspheres containing transforming growth factor–β (TGF), and mesenchymal stem cells (MSCs) showing entire defect and adjacent normal tissue. Numbers represent overall histological score for that specimen (*P < 0.05 vs. normal; scale bar = 2 mm).
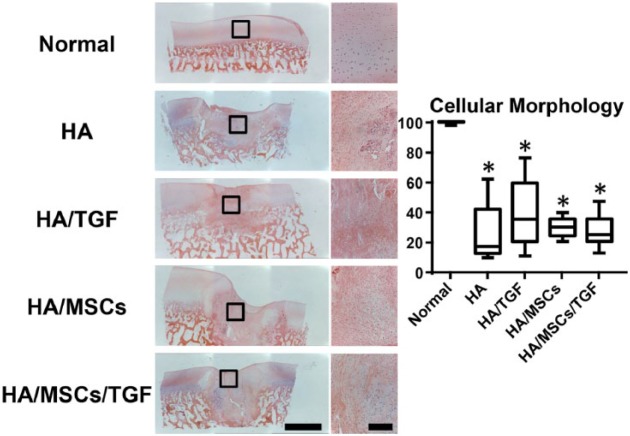
Via hematoxylin and eosin staining (Fig. 5), the hydrogel was not readily observed at the 6-week time point. In all experimental groups, cell morphology ranged from rounded cells to more elongated ones, which generally corresponded to regions of proteoglycan staining and fibrous tissue formation, respectively (Fig. 4). Semiquantification of cell morphology revealed similar trends as for matrix staining. Scores for the experimental groups were similar, with median values ranging from 17 to 35. Again, no effect was observed due to treatment with either TGF-β3 or MSCs (P > 0.05), and all treatment groups had scores 59% to 72% lower than normal controls (P < 0.05). Other scoring categories, such as cell clustering at the surface, surface architecture, basal integration, vascularization, and tidemark formation, revealed similar trends (Suppl. Fig. S1).
Figure 5.
Histological staining (hematoxylin and eosin) for cells and matrix of full thickness cartilage defects treated with hyaluronic acid (HA) hydrogels, microspheres containing transforming growth factor–β (TGF), and mesenchymal stem cells (MSCs) showing entire defect and adjacent normal tissue (*P < 0.05 vs. normal; scale bar = 2 mm).
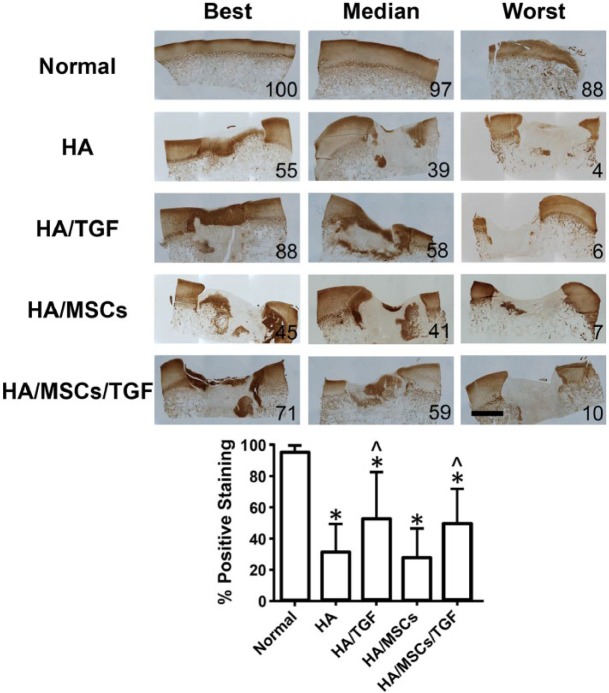
Deposition of type II collagen was assessed via immunohistochemistry (Fig. 6). All groups showed some level of positive staining, although the amount of staining had considerable variability between the best and worst specimens. Quantification of type II collagen staining showed that treatment with TGF-β3 led to increased staining intensity for the HA/TGF and HA/MSCs/TGF groups (53% ± 29% and 50% ± 22%, respectively) relative to the HA and HA/MSCs groups (32% ± 17% and 29% ± 18%, respectively, P < 0.05). The presence of MSCs had little effect, as comparisons between the HA and HA/MSCs groups and between the HA/TGF and HA/MSCs/TGF groups were not statistically significant (P > 0.05). The interaction term between treatment with TGF-β3 and MSCs was also not statistically significant (P > 0.05). All groups had significantly lower positive staining compared with the normal tissue (96% ± 3%, P < 0.05).
Figure 6.
Immunostaining for collagen type II showing entire defect following treatment with hyaluronic acid (HA) hydrogels, microspheres containing transforming growth factor–β (TGF), and mesenchymal stem cells (MSCs) (*P < 0.05 vs. normal, ^P < 0.05 vs. non-TGF groups; scale bar = 2 mm).
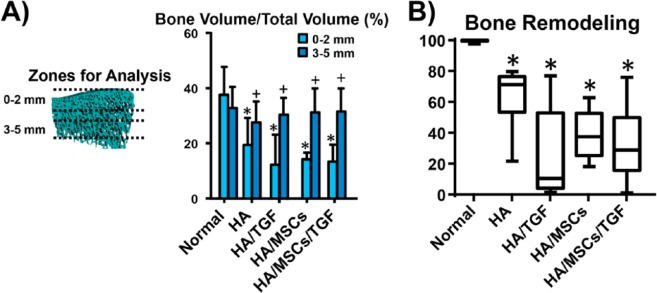
Finally, the amount of subchondral bone remodeling underneath the defects was quantified via μCT and histological scoring (Fig. 7). No effect was observed due to treatment with either TGF-β3 or MSCs (P > 0.05), but a significant effect was found for zone of analysis (P < 0.05). The interaction term between these variables was not statistically significant (P > 0.05). All experimental groups had lower BV/TV values near the original cartilage/bone interface (within 2 mm) relative to a region further from the original cartilage/bone interface (3-5 mm) (P < 0.05). Near the interface, these groups were also 48% to 68% lower than normal (P < 0.05), while no differences were found relative to normal controls in the region further removed from the interface (P > 0.05). Histological scoring for bone remodeling, which considers the entirety of the bone remodeling response, revealed a wide range of scores for the experimental groups. No statistically significant differences were found due to treatment with TGF-β3 or MSCs (P > 0.05), and scores for the experimental groups ranged from 36% to 71% lower than normal controls (P < 0.05).
Figure 7.
Effects of treatment on subchondral bone remodeling. (A) Quantification of bone volume per total volume via micro-computed tomography at regions 0 to 2 mm and 3 to 5 mm under the original injury site (*P < 0.05 vs. normal, +P < 0.05 vs. 0- to 2-mm zone for same group). (B) Histological scoring of bone remodeling via the ICRS-II scoring system (*p<0.05 vs. normal).
Discussion
In this study, we evaluated the individual and synergistic effects of growth factor (TGF-β3) and cell (MSCs) delivery within an HA hydrogel on cartilage repair and subchondral bony remodeling in a porcine model of full thickness cartilage repair. Most interestingly, TGF-β3 led to a marked increase in positive staining for collagen type II within the defects, while delivery of MSCs did not. Neither TGF-β3 nor MSCs had an impact on other histological semiquantitative scoring, and in fact, delivery of MSCs led to significantly less defect fill as assessed by µCT. For all these outcome measurements, no synergy was found between TGF-β3 and MSC treatment when delivered together, contrary to our hypothesis that the combined treatment with MSCs and TGF-β3 in the HA hydrogels would result in the most robust positive healing response.
By 6 weeks, TGF-β3 elicited a significant improvement in the formation of type II collagen, but had little impact on defect fill or histological measures, including proteoglycan staining. Many studies have examined the use of TGF-β3 for cartilage repair in vivo,22-27 but direct comparisons are difficult due to differences in the delivery mechanism of the growth factor, the dose, or the isoform used. In our group, a recent study in mini-pigs showed that delivery of TGF-β3 from fibrous HA scaffolds with a similar release profile combined with microfracture also increased collagen type II content by 12 weeks.22 In addition, delivery of TGF-β3 improved overall semiquantitative histological scoring of the repair tissue in those defects. From the current study, it is unclear whether later time points would reveal similar improvements in histological measurements due to TGF-β3 treatment. The dose and timing of TGF-β3 delivery used in this study was based on in vitro results by our group showing that delivery of 50 to 100 ng/mL of TGF-β3 over a 1-week period was sufficient to induce and maintain chondrogenesis of MSCs52 over 12 weeks, with this transient high-dose delivery resulting in superior outcomes compared to lower doses delivered over the same 12 week time period. The alginate microspheres used in this study have been shown to mediate controlled release of TGF-β3 for up to one week in vitro.45 We did not directly assess the impact of the in vivo growth factor release profile, but there is some evidence in the pig model that controlled release of growth factors provides better long-term repair tissue at 12 months relative to a bolus delivery.23-25 Additional controlled studies are warranted. The isoform of TGF could also explain differences between studies; however, Ng et al.13 have shown similar results in a direct comparison between TGF-β3 and TGF-β1 on in vitro chondrogenesis.
Our study showed little positive impact of allogeneic MSCs on the repair response despite their presence in significant numbers in the repair tissue, confirmed using cell labeling. In fact, we found significantly less defect fill, and although not statistically significant, the groups containing MSCs had the poorest median scores in terms of subchondral bone remodeling. There are a host of studies in the literature on the effects of MSCs on cartilage healing, with some showing positive effects and others showing little impact, or even a negative one.28-38 Again, a variety of differences exist between studies making comparisons difficult, including species and age of the animals, delivery method (injection vs. within a scaffold), the source of the MSCs within the body, the in vitro preculture methods used, and the use of autologous cells, allogeneic cells, or xenogeneic cells. In our study, we isolated MSCs from the bone marrow of a donor pig of similar age, expanded in serum containing media, and implanted those cells within an HA hydrogel into a different animal. It is unclear whether or not autologous cells would achieve better results in this model, and future studies on this topic are warranted.
Other large animal studies have precultured MSCs in vitro within a chemically defined media known to induce chondrogenesis prior to implantation.28-34 In the ovine model, Marquass et al.34 showed that such preculture of MSCs could result in better in vivo outcomes relative to non-precultured MSCs or articular chondrocytes. However, Chang et al.33 found dissimilar findings in the pig model, with undifferentiated MSCs providing superior repair versus MSCs precultured in the presence of TGF-β3. Further complicating the issue, Miot et al.28 found that 2 weeks of preculture within a scaffold material was best for articular chondrocytes in terms of potential for in vivo repair in the goat model, relative to both shorter (2 days) and longer (6 weeks) periods of preculture. Our recent work using HA hydrogels is consistent with this finding in that the best results in an in vitro “integration” assay were found for an intermediate culture period.20 These findings were dependent on the maturation trajectory of the construct properties with time, not the maturation state at the time of “implantation.” However, additional studies are still needed to confirm this hypothesis in vivo,20 and the mechanism by which in vitro preculture might enhance in vivo results has not yet been elucidated. One potential mechanism is that a construct with specific mechanical function and extracellular matrix content may allow more appropriate load transfer to the cells, which in turn, may elicit even more matrix production and more appropriate mechanical function. In addition, culture within highly controlled in vitro conditions can ensure consistent chondrogenesis of the MSCs prior to implantation. At the right dosage, such short-term delivery of chondrogenic factors might allow the MSCs to maintain a chondrogenic phenotype even under less ideal conditions within the joint.
This study focused on the effects of MSCs and TGF-β delivered within hydrogels on cartilage repair, and was not designed to compare the potential contributions of the subchondral bone and synovium on the repair response. However, it is likely that both may play a role. In the current and previous work, we have noted a “covering” over the implanted hydrogels that forms at the articular surface and likely involves some cells from the synovium.21 At the same time, upon creation of the defects, a small amount of bleeding occurred from the subchondral bone, and remodeling of the bone was noted as in our previous studies,21 which also likely plays a role in the development of the repair tissue.
The combination of MSCs and TGF did not produce positive synergistic results for any of the outcome measures in this study, similar to some other studies.43,44 Yet, others have found the addition of TGF delivery with MSCs enhanced results relative to MSCs alone.41,42 Many of these studies lack full factorial designs, however, preventing the determination of individual and synergistic effects of the TGF and MSCs. Even in the small number of studies with full factorial designs, the results are unclear. For example, Mrugala et al.40 used a partial-thickness defect model in sheep and found that the addition of MSCs with TGF-β3 within a chitosan powder vehicle appeared to have a small additional benefit relative to the application of TGF-β3 and chitosan alone. However, Im and Lee39 reported that combined delivery of adipose derived stem cells with TGF-β2 and BMP-7 for the treatment osteochondral defects in rabbits did not improve the histological appearance of the repair relative to growth factor treatment alone. Neither study revealed a positive effect due to the cells alone. As above, comparisons between studies are difficult due to a variety of experimental differences.
One interesting finding in this work was the fact that the HA hydrogel degraded rapidly in vivo, with partial incorporation at 2 weeks and complete incorporation by 6 weeks. Importantly, little apparent inflammation response was observed due to the degradation of the HA at either time point. In our previous in vivo studies using the mini-pig model,21,22 no impact was observed for HA scaffolds alone relative to untreated control defects for any of the outcome measures presented here. This was true for both HA hydrogels and electrospun HA fibers. As such, no untreated control group was included in this study.
Collectively, these data suggest that in the early-term healing response, TGF-β3 can have a positive impact on the formation of collagen type II within the defect, while allogeneic MSCs added little benefit toward the promotion of the healing response. Combination of TGF-β3 and MSCs has no synergistic effect in this model. Studies are warranted to determine whether combination of these factors can provide a superior repair long-term. Furthermore, the role of age in the repair response should be further explored, given the decrement in endogenous progenitor cells with aging. In conclusion, these data suggest that combination of MSCs with growth factors and scaffold materials followed by a period of preculture using a traditional in vitro tissue engineering approach may provide a better outcome compared with direct implantation of cells and chondrogenic factors.
Supplementary Material
Footnotes
Supplementary material for this article is available on the Cartilage website at http://cart.sagepub.com/supplemental.
Authors’ Note: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs. No funding source had a role in the study design, collection, analysis and interpretation of data, writing of the manuscript, or the decision to submit the manuscript for publication.
Ethical Approval: Ethical approval was not sought for the present study because human subjects or samples were not involved in this study.
Animal Welfare: The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation.
Acknowledgments and Funding: The authors thank Liming Bian, Megan Farrell, Tristan Driscoll, Sylvia Qu, and Marc Bostrom for their technical assistance. This work was supported by the National Institutes of Health (R01 EB008722 and F32 AR062971), the Department of Veterans Affairs (I01 RX000700), the AO Foundation, and the Orthopaedic Research and Education Foundation (Resident Clinician Scientist Training Grant).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1. Heir S, Nerhus TK, Rotterud JH, Loken S, Ekeland A, Engebretsen L, et al. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;38:231-7. [DOI] [PubMed] [Google Scholar]
- 2. Guettler JH, Demetropoulos CK, Yang KH, Jurist KA. Osteochondral defects in the human knee: Influence of defect size on cartilage rim stress and load redistribution to surrounding cartilage. Am J Sports Med. 2004;32:1451-8. [DOI] [PubMed] [Google Scholar]
- 3. Wang Y, Ding C, Wluka AE, Davis S, Ebeling PR, Jones G, et al. Factors affecting progression of knee cartilage defects in normal subjects over 2 years. Rheumatology (Oxford). 2006;45:79-84. [DOI] [PubMed] [Google Scholar]
- 4. Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am J Sports Med. 2010;38:1259-71. [DOI] [PubMed] [Google Scholar]
- 5. Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33:295-306. [DOI] [PubMed] [Google Scholar]
- 6. Steadman JR, Rodkey WG, Briggs KK. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg. 2002;15:170-6. [PubMed] [Google Scholar]
- 7. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;(391 Suppl):S362-9. [DOI] [PubMed] [Google Scholar]
- 8. Hangody L, Kish G, Karpati Z, Szerb I, Udvarhelyi I. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. A preliminary report. Knee Surg Sports Traumatol Arthrosc. 1997;5:262-7. [DOI] [PubMed] [Google Scholar]
- 9. Baumbach K, Petersen JP, Ueblacker P, Schroder J, Gopfert C, Stork A, et al. The fate of osteochondral grafts after autologous osteochondral transplantation: a one-year follow-up study in a minipig model. Arch Orthop Trauma Surg. 2008;128:1255-63. [DOI] [PubMed] [Google Scholar]
- 10. Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640-5. [DOI] [PubMed] [Google Scholar]
- 11. Obradovic B, Martin I, Padera RF, Treppo S, Freed LE, Vunjak-Novakovic G. Integration of engineered cartilage. J Orthop Res. 2001;19:1089-97. [DOI] [PubMed] [Google Scholar]
- 12. Erickson IE, Kestle SR, Zellars KH, Farrell MJ, Kim M, Burdick JA, et al. High mesenchymal stem cell seeding densities in hyaluronic acid hydrogels produce engineered cartilage with native tissue properties. Acta Biomater. 2012;8:3027-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ng KW, O’Conor CJ, Kugler LE, Cook JL, Ateshian GA, Hung CT. Transient supplementation of anabolic growth factors rapidly stimulates matrix synthesis in engineered cartilage. Ann Biomed Eng. 2011;39:2491-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheng NC, Estes BT, Awad HA, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells by a porous scaffold derived from native articular cartilage extracellular matrix. Tissue Eng Part A. 2009;15:231-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vinardell T, Sheehy EJ, Buckley CT, Kelly DJ. A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Eng Part A. 2012;18:1161-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, et al. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-β3. Osteoarthritis Cartilage. 2007;15:1025-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Erickson IE, Kestle SR, Zellars KH, Dodge GR, Burdick JA, Mauck RL. Improved cartilage repair via in vitro pre-maturation of msc-seeded hyaluronic acid hydrogels. Biomed Mater. 2012;7:024110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burdick JA, Chung C, Jia XQ, Randolph MA, Langer R. Controlled degradation and mechanical behavior of photopolymerized hyaluronic acid networks. Biomacromolecules. 2005;6:386-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Erickson IE, Huang AH, Chung C, Li RT, Burdick JA, Mauck RL. Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Eng Part A. 2009;15:1041-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher MB, Henning EA, Soegaard NB, Dodge GR, Steinberg DR, Mauck RL. Maximizing cartilage formation and integration via a trajectory-based tissue engineering approach. Biomaterials. 2014;35:2140-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fisher MB, Belkin NS, Milby AH, Henning EA, Bostrom M, Kim M, et al. Cartilage repair and subchondral bone remodeling in response to focal lesions in a mini-pig model: implications for tissue engineering. Tissue Eng Part A. 2015;21:850-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim IL, Pfeifer CP, Fisher MB, Saxena V, Meloni GR, Kwon MY, et al. Fibrous scaffolds with varied fiber chemistry and growth factor delivery promote repair in a porcine cartilage defect model. Tissue Eng Part A. 2015;21:2680-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gotterbarm T, Richter W, Jung M, Berardi Vilei S, Mainil-Varlet P, Yamashita T, et al. An in vivo study of a growth-factor enhanced, cell free, two-layered collagen-tricalcium phosphate in deep osteochondral defects. Biomaterials. 2006;27:3387-95. [DOI] [PubMed] [Google Scholar]
- 24. Hunziker EB. Growth-factor-induced healing of partial-thickness defects in adult articular cartilage. Osteoarthritis Cartilage. 2001;9:22-32. [DOI] [PubMed] [Google Scholar]
- 25. Hunziker EB, Driesang IM, Morris EA. Chondrogenesis in cartilage repair is induced by members of the transforming growth factor-beta superfamily. Clin Orthop Relat Res. 2001;(391 Suppl):S171-81. [DOI] [PubMed] [Google Scholar]
- 26. Lee CH, Cook JL, Mendelson A, Moioli EK, Yao H, Mao JJ. Regeneration of the articular surface of the rabbit synovial joint by cell homing: a proof of concept study. Lancet. 2010;376:440-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469:2706-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miot S, Brehm W, Dickinson S, Sims T, Wixmerten A, Longinotti C, et al. Influence of in vitro maturation of engineered cartilage on the outcome of osteochondral repair in a goat model. Eur Cell Mater. 2012;23:222-36. [DOI] [PubMed] [Google Scholar]
- 29. Liu K, Zhou GD, Liu W, Zhang WJ, Cui L, Liu X, et al. The dependence of in vivo stable ectopic chondrogenesis by human mesenchymal stem cells on chondrogenic differentiation in vitro. Biomaterials. 2008;29:2183-92. [DOI] [PubMed] [Google Scholar]
- 30. Dashtdar H, Rothan HA, Tay T, Ahmad RE, Ali R, Tay LX, et al. A preliminary study comparing the use of allogenic chondrogenic pre-differentiated and undifferentiated mesenchymal stem cells for the repair of full thickness articular cartilage defects in rabbits. J Orthop Res. 2011;29:1336-42. [DOI] [PubMed] [Google Scholar]
- 31. Pei M, He F, Li J, Tidwell JE, Jones AC, McDonough EB. Repair of large animal partial-thickness cartilage defects through intraarticular injection of matrix-rejuvenated synovium-derived stem cells. Tissue Eng Part A. 2013;19:1144-54. [DOI] [PubMed] [Google Scholar]
- 32. Moretti M, Wendt D, Dickinson SC, Sims TJ, Hollander AP, Kelly DJ, et al. Effects of in vitro preculture on in vivo development of human engineered cartilage in an ectopic model. Tissue Eng. 2005;11:1421-8. [DOI] [PubMed] [Google Scholar]
- 33. Chang CH, Kuo TF, Lin CC, Chou CH, Chen KH, Lin FH, et al. Tissue engineering-based cartilage repair with allogenous chondrocytes and gelatin-chondroitin-hyaluronan tri-copolymer scaffold: a porcine model assessed at 18, 24, and 36 weeks. Biomaterials. 2006;27:1876-88. [DOI] [PubMed] [Google Scholar]
- 34. Marquass B, Schulz R, Hepp P, Zscharnack M, Aigner T, Schmidt S, et al. Matrix-associated implantation of predifferentiated mesenchymal stem cells versus articular chondrocytes: in vivo results of cartilage repair after 1 year. Am J Sports Med. 2011;39:1401-12. [DOI] [PubMed] [Google Scholar]
- 35. Li WJ, Chiang H, Kuo TF, Lee HS, Jiang CC, Tuan RS. Evaluation of articular cartilage repair using biodegradable nanofibrous scaffolds in a swine model: a pilot study. J Tissue Eng Regen Med. 2009;3:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saw KY, Anz A, Merican S, Tay YG, Ragavanaidu K, Jee CS, et al. Articular cartilage regeneration with autologous peripheral blood progenitor cells and hyaluronic acid after arthroscopic subchondral drilling: a report of 5 cases with histology. Arthroscopy. 2011;27:493-506. [DOI] [PubMed] [Google Scholar]
- 37. Lee JC, Min HJ, Lee S, Seong SC, Lee MC. Effect of chondroitinase abc on adhesion and behavior of synovial membrane-derived mesenchymal stem cells in rabbit partial-thickness chondral defects. J Orthop Res. 2013;31:1293-301. [DOI] [PubMed] [Google Scholar]
- 38. Zhou G, Liu W, Cui L, Wang X, Liu T, Cao Y. Repair of porcine articular osteochondral defects in non-weightbearing areas with autologous bone marrow stromal cells. Tissue Eng. 2006;12:3209-21. [DOI] [PubMed] [Google Scholar]
- 39. Im GI, Lee JH. Repair of osteochondral defects with adipose stem cells and a dual growth factor-releasing scaffold in rabbits. J Biomed Mater Res B Appl Biomater. 2010;92:552-60. [DOI] [PubMed] [Google Scholar]
- 40. Mrugala D, Bony C, Neves N, Caillot L, Fabre S, Moukoko D, et al. Phenotypic and functional characterisation of ovine mesenchymal stem cells: application to a cartilage defect model. Ann Rheum Dis. 2008;67:288-95. [DOI] [PubMed] [Google Scholar]
- 41. Wang W, Li B, Yang J, Xin L, Li Y, Yin H, et al. The restoration of full-thickness cartilage defects with BMSCS and TGF-β1 loaded PLGA/fibrin gel constructs. Biomaterials. 2010;31:8964-73. [DOI] [PubMed] [Google Scholar]
- 42. Fan H, Tao H, Wu Y, Hu Y, Yan Y, Luo Z. TGF-β3 immobilized PLGA-gelatin/chondroitin sulfate/hyaluronic acid hybrid scaffold for cartilage regeneration. J Biomed Mater Res A. 2010;95:982-92. [DOI] [PubMed] [Google Scholar]
- 43. Guo X, Park H, Young S, Kretlow JD, van den Beucken JJ, Baggett LS, et al. Repair of osteochondral defects with biodegradable hydrogel composites encapsulating marrow mesenchymal stem cells in a rabbit model. Acta Biomater. 2010;6:39-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Miller RE, Grodzinsky AJ, Vanderploeg EJ, Lee C, Ferris DJ, Barrett MF, et al. Effect of self-assembling peptide, chondrogenic factors, and bone marrow–derived stromal cells on osteochondral repair. Osteoarthritis Cartilage. 2010;18:1608-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bian L, Zhai DY, Tous E, Rai R, Mauck RL, Burdick JA. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32:6425-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Srivastava R, Brown JQ, Zhu H, McShane MJ. Stable encapsulation of active enzyme by application of multilayer nanofilm coatings to alginate microspheres. Macromol Biosci. 2005;5:717-27. [DOI] [PubMed] [Google Scholar]
- 47. Erickson IE, Huang AH, Sengupta S, Kestle S, Burdick JA, Mauck RL. Macromer density influences mesenchymal stem cell chondrogenesis and maturation in photocrosslinked hyaluronic acid hydrogels. Osteoarthritis Cartilage. 2009;17:1639-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Orth P, Goebel L, Wolfram U, Ong MF, Graber S, Kohn D, et al. Effect of subchondral drilling on the microarchitecture of subchondral bone: analysis in a large animal model at 6 months. Am J Sports Med. 2012;40:828-36. [DOI] [PubMed] [Google Scholar]
- 49. Goulet RW, Goldstein SA, Ciarelli MJ, Kuhn JL, Brown MB, Feldkamp LA. The relationship between the structural and orthogonal compressive properties of trabecular bone. J Biomech. 1994;27:375-89. [DOI] [PubMed] [Google Scholar]
- 50. Xie L, Lin AS, Levenston ME, Guldberg RE. Quantitative assessment of articular cartilage morphology via EPIC-µCT. Osteoarthritis Cartilage. 2009;17:313-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mainil-Varlet P, Van Damme B, Nesic D, Knutsen G, Kandel R, Roberts S. A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am J Sports Med. 2010;38:880-90. [DOI] [PubMed] [Google Scholar]
- 52. Kim M, Erickson IE, Choudhury M, Pleshko N, Mauck RL. Transient exposure to TGF-β3 improves the functional chondrogenesis of msc-laden hyaluronic acid hydrogels. J Mech Behav Biomed Mater. 2012;11:92-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.