Abstract
Advances in chemical biology have led to selection of synthetic functional nucleic acids for in vivo applications. Discovery of synthetic nucleic acid regulatory elements has been a long-standing goal of chemical biologists. Availability of vast genome level genetic resources has motivated efforts for discovery and understanding of inducible synthetic genetic regulatory elements. Such elements can lead to custom-design of switches and sensors, oscillators, digital logic evaluators and cell–cell communicators. Here, we describe a simple, robust and universally applicable module for discovery of inducible gene regulatory elements. The distinguishing feature is the use of a toxic peptide as a reporter to suppress the background of unwanted bacterial recombinants. Using this strategy, we show that it is possible to isolate genetic elements of non-genomic origin which specifically get activated in the presence of DNA gyrase A inhibitors belonging to fluoroquinolone (FQ) group of chemicals. Further, using a system level genetic resource, we prove that the genetic regulation is exerted through histone-like nucleoid structuring (H-NS) repressor protein. Till date, there are no reports of in vivo selection of non-genomic origin inducible regulatory promoter like elements. Our strategy opens an uncharted route to discover inducible synthetic regulatory elements from biologically-inspired nucleic acid sequences.
INTRODUCTION
Synthetic biology aims to control and decipher structure and operation of biological systems by developing bioengineering tools such as functional genetic circuits. However, designing reliable and predictable gene networks of synthetic origin is difficult due to unexpected behaviour and non-cooperation from RNA and protein synthesis machinery of the host cells (1). Synthetic regulatory elements that dictate the transcriptional control have led to custom-design of switches and sensors, oscillators, digital logic evaluators and cell–cell communicators, etc (2–4). Deciphering the underlying mechanism of such design principles will help tailor the genetic machinery toward applications in biosensing, bioremediation, metabolic engineering and clinical testing (2,5). A prerequisite for the above proposed explorations is to establish basic rules for construction of such genetic circuits. One of the limiting factors in the development of synthetic genetic system is the lack of novel promoter elements which respond to specific stimuli. Combinatorial approaches can facilitate the evolution of desirable genetic regulatory elements by engineering prokaryotic promoters (6–8). Directed evolution of native promoters has been achieved by promoter engineering for isolating constitutive promoters with precise strength and regulation (8). However, there is a dearth of studies which report the isolation of inducible promoter sequences of non-genomic origin. Such inducible regulatory biological ‘parts’ will behave predictably and will allow for the continuous control of gene expression at molecular level. We show here that by using a novel screening strategy, ligand inducible promoters can be discovered via combinatorial engineering.
Bacteria respond to stress by activating specific genes in a highly regulated manner. These responses not only protect bacteria from the offending stress, but also manifest changes in the cell (9–11). Sigma factors (σ) play an important role in controlling the expression of genes which are involved in combating various stresses (12–14). Mechanistically, the stress sigma factors (σE, σ54, σ32, σ24 and σs etc.) bind to cognate regulatory elements and activate the gene expression (14,15). The genetic elements involved in the regulation of stress genes in Escherichia coli have been used to design genetic circuits that can sense the environmental toxicants and also reprogram the bacterial cells by tuning gene expression (16). In the present study, potential of stress responsive promoters was explored by designing a combinatorial library inspired by σE, σ54, σ32, σ24 and σs promoters. Design of the initial library based on stress related sigma factors provided an opportunity for discovery of both constitutive and inducible promoters. An efficient reporter gene is central to the selection of inducible genetic elements. Extant reporter systems based on green fluorescent protein (GFP), yellow fluorescent protein (YFP), luciferase (lux) and β-galactosidase have led to successful isolation of constitutive promoters of varying strengths (8,17–19). As compared to directed evolution of native promoters, combinatorial strategy poses challenge of managing large diversity of clones. In an attempt to isolate both inducible and constitutive promoter elements from a pool of a combinatorial library, we used a GFP based reporter system. However, failure to isolate inducible promoters prompted us to devise a selection strategy based on E. coli toxic peptide (IbsC) as a reporter (20,21). This reporter system efficiently reduced the noise of constitutive promoters. Screening of ∼10 000 clones led to isolation of an inducible genetic element which responded specifically to fluoroquinolone (FQ) category of antibiotics. In silico analysis revealed that the selected DNA sequence is of non-genomic origin. Further, characterization of the regulatory element revealed regular features of prokaryotic promoters like; −10, −35 and spacer sequences. A set of genetic and biochemical experiments were used to investigate the regulatory mechanism of the FQ-responsive genetic element. We have established that the repression of this fluoroquinolone responsive promoter is mediated through a histone-like nulceoid structuring protein (H-NS).
In this study, an in vivo live and dead cell selection strategy has been adopted for the first time to discover an inducible regulatory element of non-genomic origin. The strategy allows minimum background while targeting inducible gene regulatory elements and is applicable to other ligands. This study adds a new dimension to the protein based regulatory elements for engineering inducible synthetic gene circuits.
MATERIAL AND METHODS
Strains and Media
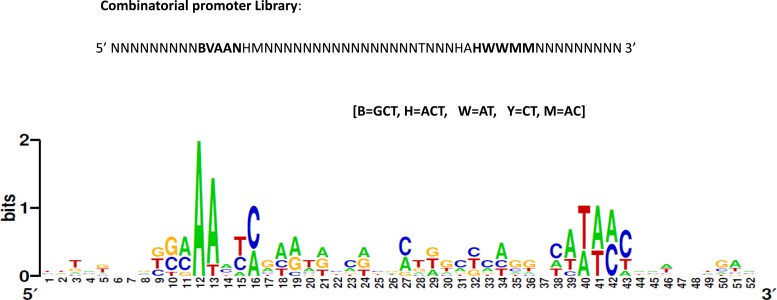
E. coli strains RFM443 (generous gift from Prof. Shimshon Belkin, Hebrew University, Israel), E. coli DH5α and E. coli BL21 DE3 were used for routine transformation experiments. All E. coli knock out strains analyzed in this study originated from the Keio collection (22). Plasmid vector pNYL-MCS11 used in this study is a derivative of pZE21-MCS11 vector (21,23). Assay strains were grown at 37°C with 225 rpm orbital shaking in Luria Bertani (LB) broth. Media was supplemented with antibiotics at the following concentrations: kanamycin 40 μg/ml, ampicillin 100 μg/ml and tetracycline 15 μg/ml, as necessary. All Keio collection knockout strains used in this study were maintained on LB plates contain 15 μg/ml kanamycin. Strains and plasmids used in this study are listed in Supplementary Tables S1 and S2. The degenerate oligonucleotide library is listed in Figure 1. List of other oligonucleotides used in the study are available on request.
Figure 1.
Weblogo diagram of a designed degenerate combinatorial library of E. coli stress promoters.
Library construction
The nucleotide sequence of promoter regions of stress responsive genes were aligned using online bioinformatics program ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2). Using the program ‘weblogo’ (http://weblogo.berkeley.edu), a consensus oligonucleotide library was designed. While designing, the frequency of different nucleotides at various extant positions in promoter region was taken into consideration. This approach deals with diversifying the stress responsive natural promoter sequence with the objective of evolving a promoter with new characteristics. Multiple sequence alignment of the stress responsive promoters revealed sparse conservation at −10, −35 and the spacer region. Even though alignment did show presence of conserved nucleotide sequence in the −10 and −35 promoter elements, there were always some exceptions (Figure 1 and Supplementary Figure S1). A degenerate −10 and −35 promoter oligonucleotide library was designed. Since the spacer region was A or AT-rich, a bias for both polyA and AT richness was created in the spacer region. In essence, the design of the stress-promoter library appeared to be almost random barring few A or AT-rich regions.
Construction of toxic peptide and GFP-based reporter systems
We developed two reporter systems; based on toxic peptide IbsC and GFP as reporters. For construction of these reporter systems, we used polymerase chain reaction (PCR) to amplify the toxic peptide (ibsC) and GFP encoding fragment from E. coli genome and pPROBE-TT’-GFP plasmid, respectively, by using forward (F-ibsC, F-GFP) and reverse (R-ibsC, R-GFP) primers. Amplified fragment containing BamHI restriction endonuclease sites at their ends were digested with BamHI and ligated into BamHI digested pNYL-MCS11 plasmid vector (11). The constructed reporter plasmid systems were named pNYL-ibsC and pNYL-GFP, respectively. All nucleic acid manipulation enzymes were procured from New England Biolabs, or Fermentas, USA.
Cloning of oligonucleotide library in pNYL-ibsC/GFP vector
Combinatorial oligonucleotide library was synthesized by Integrated DNA Technology, USA. Single stranded oligonucleotide library was annealed with reverse primer complementary to the end of the library sequence. The annealed primer sequence was extended by Klenow fragment of DNA polymerase I and converted into double stranded DNA. Double stranded oligonucleotide library and plasmid vector pNYL-ibsC/GFP were double digested with XhoI and HindIII restriction endonucleases and ligated with XhoI-HindIII digested fragment of pNYL-ibsC/GFP plasmid vectors (Supplementary Figure S2A). Ligated products were transformed in E. coli RFM443 strain and the transformants were selected on LB plate containing 40 μg/ml kanamycin.
Screening of the library clones
For the screening of the library clones, transformed colonies were picked and inoculated individually into 96-well plates containing 200 μl LB broth and 40 μg/ml kanamycin. The inoculated bacterial cultures were incubated at 37°C to an optical density at 600 nm (A600) of 0.3–0.5. The grown cultures were then replica plated in 96 well plates which contained sub-lethal concentration of various stress inducing chemicals like DNA damaging (FQ, Mitomycin C), protein damaging (ethanol) and heavy metals (sodium arsenite, cadmium chloride and lead chloride). Growth pattern of bacterial cells in each replica plate containing various chemicals was monitored at regular time points by following absorbance after an interval of 2 h at 600 nm. The reporter system adopted here negatively selects for the recombinant bacterial cells harboring functional combinatorial regulatory nucleic acid sequences (Supplementary Figure S2B).
Measurement of GFP fluorescence
To quantify the green fluorescence intensity of the GFP, 1 ml of GFP expressing bacterial cells were harvested, washed and re-suspended in 500 μl 1X PBS (phosphate buffered saline). Fluorescence intensities of the re-suspended cells were measured in a black opaque 96 well plate (Corning®, USA) at excitation of 490 nm and emission of 510 nm using the SpectraMax M2e microplate reader (Molecular Device, USA). Fluorescence values were normalized with optical density of the bacterial cells.
Fluorescence microscopy
Fluorescence microscopy was conducted with Nikon Eclipse Ti-E inverted fluorescence microscope (Nikon, Tokyo, Japan). GFP expression was observed using 460–500 band pass excitation filter and long-pass 520 nm barrier filters under 1000X magnification.
Screening of E. coli Keio knockout library
We used Keio collection library for the discovery of repressor proteins. Each Keio knockout strain of E. coli encodes a kanamycin resistant gene. To make compatible selection marker gene, we replaced the kanamycin resistance cassette from reporter plasmid vector pNal::ibsC and pRx::ibsC with ampicillin resistance cassette. Constructed reporter plasmids having ampicillin resistant gene were transformed into selected E. coli deletion mutant strains. Each mutant strain harboring pNal::ibsC-amp and pRx::ibsC-amp reporter plasmid was grown in LB medium containing 15 μg/ml kanamycin and 100 μg/ml ampicillin respectively. The growth assay was carried out in duplicate on two separate 96-well culture plates between the E. coli mutant strain harboring pNal::ibsC-amp plasmid and pRx::ibsC-amp plasmid. Absorbance values at 600 nm (OD600) were normalized against the average OD600 corresponding to positive (μp-WT growth) and negative (μn only media) controls. These values were denoted as ‘Normalized Growth (NG),’ and were calculated by computing values in the equation %NG = (OD600(mutant)-μp/|μn-μp|) × 100.
Cloning and expression of H-NS gene
The genetic locus encoding for H-NS protein was amplified from the genomic DNA of E. coli MG1665 strain by PCR using forward (F-hns) and reverse primer (R-hns) which harbor NcoI and XhoI restriction endonuclease sites at their ends. The amplicon was digested with NcoI and XhoI and ligated into the NcoI/XhoI digested plasmid vector pET28a(+) (Novagen, USA). Ligated product was transformed in E. coli (BL21 DE3) cells [F− ompT gal dcm lon hsdSB(rB− mB−) λ(DE3) pLysS(cmR)] and the transformants were selected on LB plate containing 40 μg/ml kanamycin. Size of the cloned fragment was confirmed by NcoI/XhoI double digestion. The authenticity of the clones was verified by nucleic acid sequence analysis of the cloned fragment.
Protein purification and electrophoretic mobility shift assay (EMSA)
E. coli BL21 DE3 strain harboring pET28a-hns was grown with shaking at 37°C in LB medium to an optical density of 0.3–0.4 (log phase) at 600 nm (A600) and the cells were induced with 0.7 mM IPTG. The bacterial cells were further grown for 4 h. The centrifuged cells from the induced cultures were resuspended in 15 ml of buffer (20 mM Tris–HCl, pH 7.8, 0.5 M NaCl, 10% (v/v) glycerol, 0.1% (v/v) Triton X-100) supplemented with 0.1 mM phenylmethylsulfonyl fluoride (PMSF) at 4°C. The suspension was passed through a French press (1200 bar, 20 000 psi) and centrifuged for 30 min at 15 000 xg. Imidazole-HCl, pH 7.8, was added to the supernatant to give a final concentration of 1 mM and the resultant suspension was applied to a Ni+2-NTA column of 1 ml capacity (Qiagen, USA). The column resin was sequentially washed with about 15 column volumes of 20 mM Tris-HCl, pH 7.8, 0.3 M NaCl, 20 mM imidazole. H-NS protein was eluted in buffer containing 20 mM Tris-HCl, pH 7.8, 0.3 M NaCl and 0.5 M imidazole. The fractions containing H-NS were dialyzed in buffer A (50 mM Tris-HCl, pH 7.5, 1 mM EDTA, 5% glycerol). The dialyzed protein was concentrated at 4°C using Amicon Ultra-15 Centrifugal Filter Units (Millipore, USA) with 10 kDa molecular weight cut off membrane as per manufacturer's instructions. Protein concentrations were determined by using Pierce BCA Protein Assay Kit (Thermo Scientific, USA). Molar concentration of the purified H-NS (15.6 kDa) was calculated on the assumption that the protein existed in monomeric form. These protein samples were used for in vitro studies.
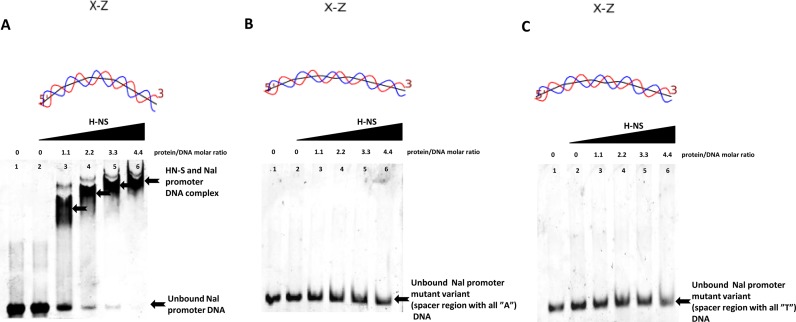
To validate the H-NS binding affinity toward FQ-responsive promoter, EMSA was carried out according to the manufacturer's instructions (Invitrogen; EMSA Kit, USA). Briefly, a 52-bp synthetic promoter sequence (300 ng) was incubated with serially increasing the amount of purified H-NS (0.5 μM, 1 μM, 1.5 μM, 2 μM) in 10X binding buffer (50 mM HEPES, 250 mM KCl, 0.1 mM dithiothreitol, 5% (v/v) glycerol and 0.1 mM ethylenediaminetetraacetic acid, pH 7.4) in 20 μl final reaction volume with protein/DNA molar ratios as shown in Figure 8A. After incubation at 37°C for 30 min, the samples were electrophoresed on 8% non-denaturing polyacrylamide gel at 10 mA, 100 V at 4°C in 0.5X TBE buffer. DNA and protein in the gel were stained using the SYBR Green and SYPRO Ruby dye (Invitrogen; EMSA Kit). The gel was scanned by FLA3000 typhoon scanner (GE Healthcare, USA) and bands were analyzed using ImageJ software. The Kd was determined by employing the non-linear regression analysis using GraphPad Prism software (Version 6.0, San Diego, CA, USA). Kd is the equilibrium binding constant, expressed as concentration of ligand needed to achieve a half-maximum binding at equilibrium.
Figure 8.
EMSA analysis carried out using varying concentrations (0–2 μM) of purified H-NS protein and fixed concentration (300 ng) of FQ-responsive promoter DNA and its variants (A) H-NS protein binding to the Nal promoter in a concentration dependent manner. (B) H-NS protein binding to the ‘all A’ spacer variant of Nal promoter with increasing H-NS concentration. (C) H-NS protein binding to the ‘all T’ spacer region variant of Nal promoter with increasing H-NS concentration. Predicted DNA curvatures are shown on top of each EMSA gel.
Curvature analysis of FQ-responsive genetic element
We used online bioinformatics tool (http://www.lfd.uci.edu/∼gohlke/dnacurve/ http://hydra.icgeb.trieste.it/dna/index.php) that compiles the curvature values and calculates the global 3D structure of a DNA molecule from its nucleotide sequence (24,25).
RESULTS AND DISCUSSION
Selection of constitutive and inducible regulatory elements
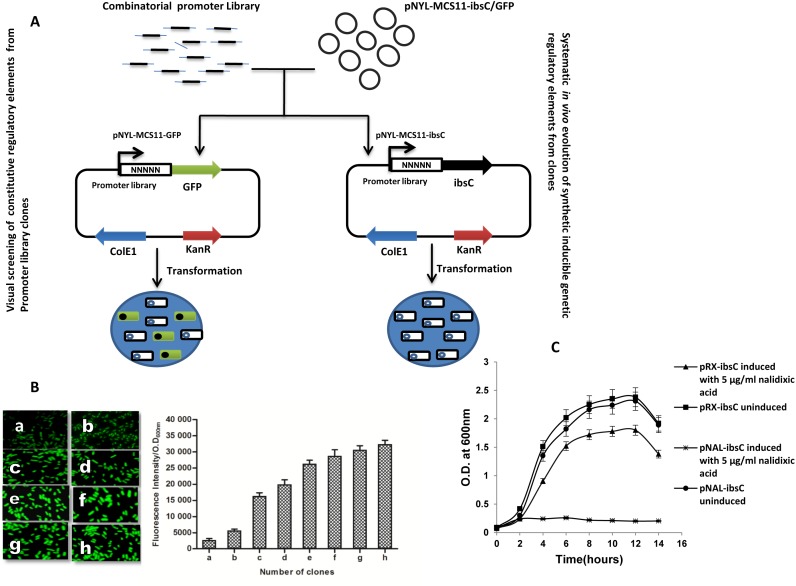
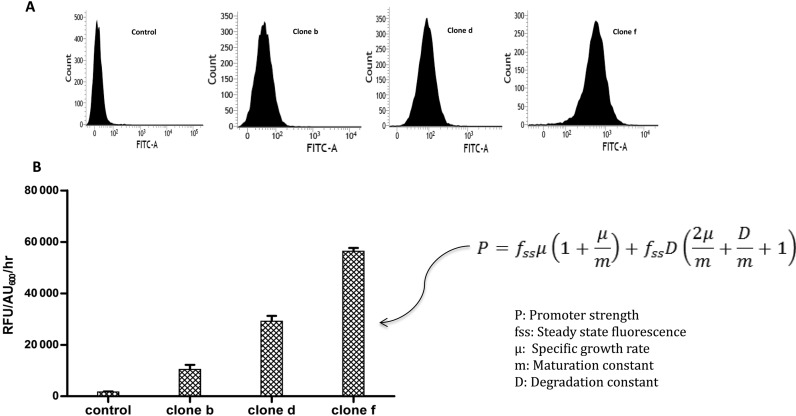
To discover synthetic regulatory elements, a combinatorial promoter library inspired by promoters of stress responsive sigma factors were designed (Figure 1). The library contained large diversity of sequences which provided an opportunity to discover synthetic regulatory elements. Depending on the application, a choice must be made between constitutive and inducible promoters. When steady state expression of a gene is needed, inducible promoters are rarely a practical solution. To screen constitutive promoters from the pool of library, we cloned oligonucleotide library in pNYL::GFP plasmid and visually identified fluorescent E. coli colonies from the LB medium plates. To assess the promoter strength, fluorescence intensities of ∼100 clones were determined using typhoon scanner. The clones were divided into eight different groups of constitutive promoters (a, b, c, d, e, f, g and h) covering a range of promoter strength (Figure 2B and Supplementary Figure S4). We selected three (b, d and f) clones showing variation in the range of GFP fluorescence and calculated their strength metric based on a mathematical model which measures fluorescence by using a dynamic equation balancing GFP production and degradation (26). The selected regulatory elements with varying strengths showed uniform expression levels as measured by flow cytometry (Figure 3A–B). These regulatory elements can be used for metabolic and pathway engineering to optimize flux toward product formation, typically by controlling transcript production at promoter level (6,17,27).
Figure 2.
Schematic diagram illustrating the cloning strategy of promoter library (A) Cloning of combinatorial promoter library in pNYL-GFP/IbsC vector harboring GFP and toxic peptide IbsC as reporter genes. (B) A panel of synthetic constitutive promoter showing a wide range of promoter activity. (C)Toxic peptide IbsC based screening led to discovery of a clone which showed inducible nature with 5 μg/ml of nalidixic acid (sub-growth inhibitory concentration of nalidixic acid toward E. coli RFM 443), and growth profile (kinetic) analysis of the cells containing the isolated promoter in the presence of nalidixic acid.
Figure 3.
Calculation of selected synthetic constitutive promoters strength covering wide range of fluorescence intensity (clone b, d and f). (A) All of the selected promoters have a uniform expression level on a single-cell level, as measured by GFP signal as illustrated by representative flow cytometry histogram. (B) Histogram and mathematical model showing a dynamic equation balancing GFP production and degradation for calculation of the promoter strength.
Development of inducible synthetic genetic networks has been hampered due to scarcity of synthetic inducible regulatory elements. A combinatorial oligonucleotide library based on stress regulatory elements was used to screen inducible promoters. Selection of inducible regulatory elements from the pool of oligonucleotide library using GFP as a positive selection marker proved to be difficult due to large background and time required for screening. We overcame this hurdle by developing a live and dead in vivo selection strategy which was based on a toxic peptide as reporter. This reporter system proved to be a powerful selection tool as we were able to minimize the background of constitutive promoters in the library. Specifically, ibsC based reporter system has been adopted which encodes for a toxic peptide in the opposite strand of sibC small RNA in E. coli genome (20,21). This reporter system provides advantage over the positive selection system because the expression of the toxic peptide leads to killing of the bacterial cells which eventually screens out the background clones harboring constitutive promoters. We cloned the combinatorial library sequences upstream of ibsC gene as pNYL::ibsC in E. coli RFM443 strain (Figure 2A). This strategy ensured that following transformation, the surviving cells harbor either inducible genetic elements or non-functional sequences. Due to strong bactericidal effect of the peptide, the reporter system selects against leaky and constitutive promoters. To discover the inducible regulatory elements from this pool, replica plates of about 10 000 clones from the library were screened with chemicals from following categories – DNA damaging, protein damaging and heavy metals at sub-lethal concentrations (Supplementary Figure S2A–B).
This screening strategy led to the discovery of a clone harboring nalidixic acid responsive genetic element. Nalidixic acid responsive recombinant plasmid was isolated from the clone and re-transformed in the host strain E. coli RFM443 strain to confirm the effect of the cloned DNA fragment. Further, the growth kinetics of E. coli RFM443 strain harboring reporter plasmid and a control plasmid (pRx::ibsC) were studied in presence of 5 μg/ml nalidixic acid. It was observed that the growth profile of uninduced pNal::ibsC and pRx::ibsC was nearly similar but 5 μg/ml of nalidixic acid was able to induce the expression of toxic peptide which resulted in inhibition of growth of pNal::ibsC harboring E. coli cells (Figure 2C). Though there was some reduction in the growth of induced pRx::ibsC cells attributable to the antibacterial effect of nalidixic acid at 5 μg/ml concentration, no growth was visible for pNal::ibsC containing cells. Notably, this is the first report where a toxic peptide has been successfully used for the discovery of inducible regulatory elements from a diverse pool of sequences. Nucleotide sequence analysis of the selected genetic element through promEC [http://margalit.huji.ac.il/promec] and BLAST with E. coli genome database did not return any sequences bearing significant homology with any known promoters in specific and whole genome in general; thus showing that the selected sequence was novel and of non-genomic origin (Supplementary Figure S5).
Specificity of the inducible regulatory element
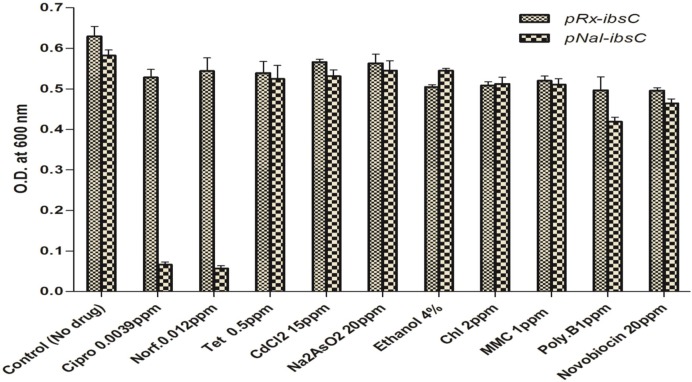
E. coli cells harboring the pNal::ibsC and pRx::ibsC were tested in the presence of sub-lethal concentration of known stress inducing chemicals (cadmium chloride, sodium aresenite, ethanol, norfloxacin, ciprofloxacin, tetracycline, mitomycin-C, chloramphenicol, polymyxin and novobiocin). Sub-lethal concentration of ciprofloxacin and norfloxacin (which are DNA gyrase A inhibitors) induced the expression of toxic peptide IbsC resulting in the cell death, however, other chemical species (cadmium chloride, sodium arsenite, ethanol, tetracycline, chloramphenicol, polymixin and novobiocin) were unable to induce the nalidixic acid responsive genetic element (Figure 4). This observation suggested that the nalidixic acid responsive genetic element is highly specific toward FQ group of antibiotics.
Figure 4.
Specificity of Screened FQ responsive promoter. Response of Nal inducible promoter to various stress inducing agents. E. coli RFM443 cells harboring reporter plasmid pNal::ibsC and pRx::ibsC were treated with sub-lethal concentration of different chemical ligands [cadmium chloride (CdCl2), sodium aresenite (Na2AsO2), ethanol, norfloxacin (Norf), ciprofloxacin (Cipro), tetracycline (Tet), chloramphenicol (Chl), Mitomycin C (MMC), Novobiocin and polymyxin B (Poly-B)] which trigger stress response in the cell. Ciprofloxacin and norfloxacin (DNA GyrA inhibitors) activated pNal::ibsC reporter system and resulted in cell death. Other chemical ligands (cadmium chloride, sodium arsenite, ethanol, tetracycline, chloramphenicol, MMC and poly-B) having different mode of action were unable to induce similar response in test and control plasmids (pRx::ibsC).
The FQ specific response tempted us to test the efficacy of this regulatory element as a whole-cell biosensor specific to FQ. To improve the sensitivity of FQ-responsive element, we transformed the pNal::ibsC plasmid in wild type E. coli BW25113 cells and deletion mutant of the major efflux pump E. coli BW25113 ΔtolC. TolC is a known efflux pump for FQ category of antibiotics. E. coli cells lacking tolC gene displayed nearly 3-fold enhanced response with FQ as compared to wild-type E. coli BW25113 cells. Further, the genetic element was inducible at 1.0 μg/ml of nalidixic acid as compared to 5 μg/ml in the latter case (Supplementary Figure S6A–B).
Inducible regulatory elements are required for controlling gene expression continuously for processes like expression of recombinant proteins, development of biosensors for environmental monitoring and discovery of novel antibacterial compounds with predefined mode of action (28,29). FQ-responsive regulatory elements discovered in this study can be optimized for some of the above processes. For example, toxic peptide based reporter system (pNal::ibsC) can be used as a tool to discover gyrase A inhibitors from small molecule libraries of synthetic and natural origin. Further, it can be easily adapted as a biosensor for detecting presence of antibiotics of fluoroquinolone category in aquatic ecosystems, potable water, food items and environmental samples (30).
Architecture of FQ-responsive genetic element
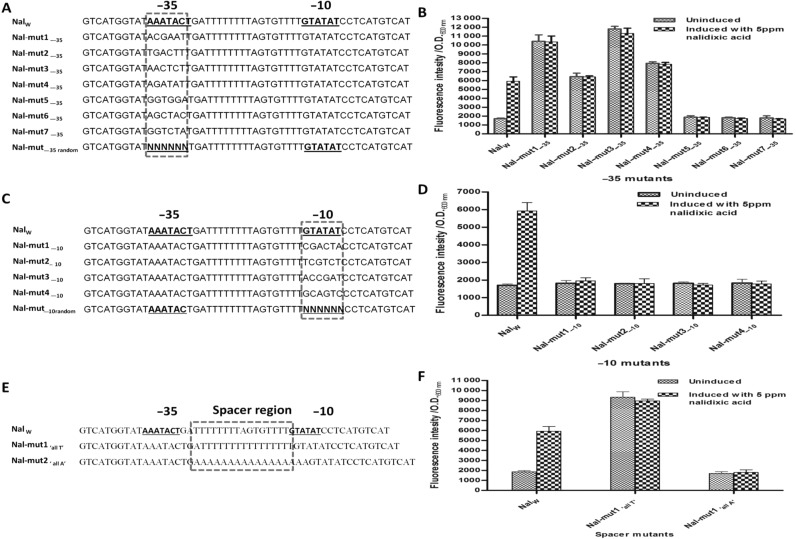
Since the homology based bioinformatics searches could not reveal significant homologs with the selected non-genomic origin nucleotide sequences, it was assumed that the promoter selected is novel and has been derived through in vivo selection. In E. coli, a typical promoter is recognized by RNA polymerase based on its −10 (TATAAT) and −35 (TAGACA) consensus regions which are separated by a stretch of ∼15–16 spacer nucleotides (28,29) except for σ54 promoters that contain −12 and −24 signature consensus sequence. The putative −10 TATA box (3′-TATATG-5′) and −35 box (3′-CATAAA-5′) consensus sequences of FQ-responsive genetic element were identified using prokaryotic promoter prediction software, PePPER (31). Interestingly, the spacer region in the selected sequence was predominantly rich with thymine nucleotides (T). In order to ascertain if the putative −10 and −35 sequences conform to their conventional positions, we replaced the toxic peptide reporter with GFP and tested the response of the FQ-responsive genetic element (shown as NalW in Figure 5). The enhanced GFP fluorescence in the presence of 5 μg/ml nalidixic acid confirmed that the response was not limited to expression of toxic peptide only. Following this, two partially random oligonucleotide libraries at −35 (Figure 5A and B) and −10 (Figure 5C and D) were synthesized and cloned in GFP reporter plasmid to study the regulatory role of −10 and −35 regions in FQ-responsive element. Variation of sequence at putative −10 and −35 boxes led to either total abrogation of promoter activity (Nal-mut5, Nal-mut6 and Nal-mut7) likely due to lack of interaction with RNA polymerase or the promoter lost its inducibility and turned constitutive (Nal-mut1, Nal-mut2, Nal-mut3 and Nal-mut4). The results of the mutants with extreme activities are summarized in Figure 5A–D. Further, the role of spacer region was investigated by synthesizing two mutant variants; one with all thymine ‘all T’ and another with all adenine ‘all A’ (Figure 5E). Interestingly, the loss in the regulation through nalidixic acid occurred for ‘all T’ variants as inducible nature of promoter was converted into constitutive nature. The ‘all A’ spacer mutant lost the promoter like activity as no GFP protein could be detected under uninduced or induced conditions (Figure 5E and F). Taken together, it appears that the nucleotide sequence of the spacer, −10 and −35 regions of the selected promoter are important for gene regulation which may be indirect effect of FQ on modulating the H-NS binding on promoter.
Figure 5.
Architecture analysis of FQ responsive promoter. Partial random library of isolated promoter were synthesized and cloned in GFP reporter plasmid (pNYL-GFP) to study −10, −35 and the spacer regions. (A–D) Variation in spacing between the −35 and −10 regions of the promoter affects the promoter activity and shows varying GFP response. (E–F) The spacer region showed extremities in regulation. A variation in three nucleotides in wild-type spacer region to convert it into ‘all T’ caused loss in the regulation through nalidixic acid as the inducible nature was converted into constitutive nature. ‘All A’ mutant lost the promoter like activity as it was not able to give any fluorescence response under uninduced or induced conditions.
Identification of regulatory protein involved in the regulation of FQ-responsive promoter
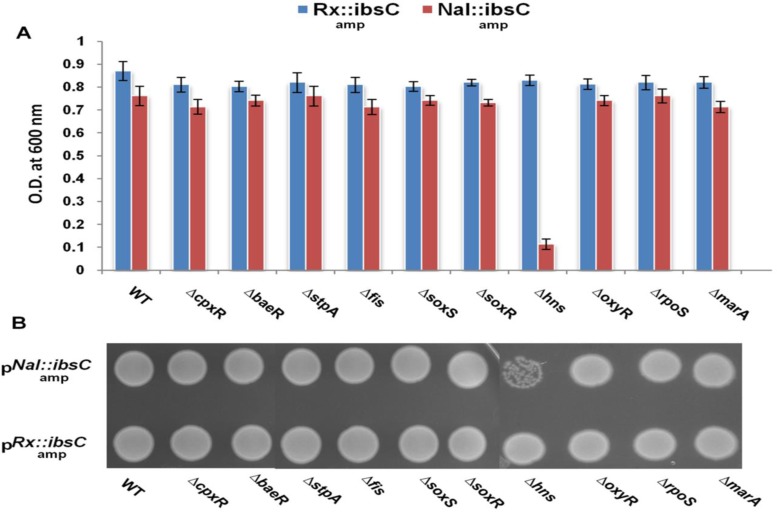
Inducible nature of FQ-responsive promoter (Nal) prompted us to investigate its regulatory mechanism. We hypothesized that the regulation of FQ inducible promoter might be mediated by a repressor or an activator protein. To explore the regulatory pathway of the promoter, we used a genetic strategy involving over-expressed ASKA and Keio knock out library of E. coli (22,32). We selected a panel of previously reported E. coli regulatory proteins which play a significant role in regulation of stress response (33,34). To identify the activator protein involved in the regulation of Nal-promoter, pNal::ibsC-amp (test) and pRx::ibsC-amp (control) were co-transformed separately in the selected ASKA library overexpressors. The co-transformed E. coli cells harboring pNal::ibsC-amp and pCA24N ASKA overexpressor plasmids were induced with IPTG during the early log phase. No noticeable difference between the growth of induced cells was observed which ruled out the role of inducer protein in regulation of the FQ-responsive promoter (data not shown). Further on, we focused on the possibility of repression based gene regulation of this promoter. To investigate the repressor proteins, we used Keio knockout library and transformed pNal::ibsC-amp and pRx::ibsC-amp fusion system in the selected panel of deletion mutants of E. coli stress regulators (Supplementary Figure S7). Out of a battery of selected stress-related regulatory protein knockout strains, we observed that the deletion mutant of hns gene of E. coli strain harboring pNal::ibsC plasmid showed scanty colonies with retarded growth due to expression of toxic peptide IbsC; whereas, the control pRx::ibsC harboring E. coli cells did not show any significant growth difference (Figure 6A–B). We hypothesize that the absence of cognate repressor likely led to the expression of IbsC toxic peptide leading to the growth retardation of Δhns knockout strain.
Figure 6.
Growth pattern of various stress related gene regulator knock out strains of E. coli harboring pNal::ibsC-amp and pRx::ibsC-amp fusion system in liquid and solid growth medium. (A) The growth assay of E. coli mutant strains in liquid LB media. A600 measurements were normalized against the average A600 corresponding to positive and negative controls. (B) The growth assay of E. coli mutant strains on LB agar. Null mutant of hns harboring pNal::ibsC plasmid showed scanty colonies and a retarded growth in broth and plate due to expression of toxic peptide IbsC in E. coli cells whereas, the control pRx::ibsC cells did not show significant growth difference.
In Gram-negative bacteria, H-NS is one of a few repressor proteins that can act by binding to the region located downstream of the transcriptional start site (35,36). H-NS is a nucleoid associated protein which preferentially binds to the AT-rich sequences. It is also known to bind to the horizontally acquired foreign DNA sequences (36,37). The genetic evidence from the above experimental results suggested that H-NS plays a pivotal role in regulation of FQ-responsive promoter likely by binding and repressing the promoter.
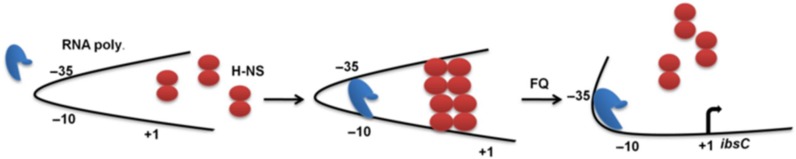
The mechanism of regulation of the selected FQ-responsive promoter may be hypothesized on the basis of global role of H-NS as stress repressor coupled with FQ mediated stress response in E. coli. Our experimental data are also in line with this hypothesis. H-NS binds preferentially to DNA with intrinsic curvature (38,39). H-NS contains an amino-terminal oligomerization domain, a carboxyl-terminal nucleic-acid-binding domain and a flexible linker that connects these two functional modules. Due to its structural features, H-NS forms dimers and has the ability to create DNA–protein–DNA bridges both between separate DNA strands and between different portions of the same DNA strand (36,40). H-NS–DNA nucleoprotein structures can impede the movement of RNA polymerase and thus H-NS can repress transcription through its DNA-binding and bridging activities (35,41). The bridging activity of H-NS makes the protein sensitive to the structure of DNA, as a result of which it binds to the promoter DNA leading to transcription repression. In our case, sub-lethal concentration of FQ inhibits the strand resealing DNA activity of gyrase A but not the nicking activity. Consequently, this concentration of fluoroquinolone induces topological changes in the DNA strands. We hypothesize that the FQ-induced relaxation of DNA alters the intrinsic curvature of promoter DNA that leads to the inability of H-NS to remain bound to the promoter leading to the turning-on of the promoter. Our proposed model in Figure 7 is in agreement with the repressive H-NS promoter complex based gene regulation proposed in recent studies (35,36,39,42).
Figure 7.
A model illustrating the repression mechanism of FQ-responsive promoter by H-NS. Fluoroquinolone induces relaxation of DNA and changes the intrinsic curvature of promoter DNA. The curvature change releases promoter bound H-NS leading to the turning-on of the promoter.
Confirmation of H-NS binding to FQ-responsive promoter
To investigate whether H-NS regulates the FQ-responsive promoter directly, we purified recombinant H-NS protein (Supplementary Figure S3) and performed EMSA. H-NS protein was able to bind to the promoter in a concentration dependent manner (Figure 8A). In order to determine the binding affinity of selected regulatory element with H-NS protein, the data from gel-shift experiments were analyzed. The apparent binding constant (Kd) of H-NS interaction with the selected regulatory element was calculated to be 399 nM. It is well documented that H-NS binds to DNA in a sequence-independent fashion, yet it shows a strong preference for regions of intrinsic curvature (43,44). Architecture analysis of Nal promoter variants at −10, −35 and spacer regions already established that the selected genetic element is a unique inducible promoter. Interestingly it has been observed that the inactive and constitutive variants of spacer regions (‘all A’ and ‘all T’, respectively) failed to show any binding in the mobility shift assays (Figure 8B and C). We used DNA curve software (http://www.lfd.uci.edu/∼gohlke/dnacurve/) to assess the bending region(s) of FQ-responsive promoter (Nal) and their variants (Supplementary Figure S8) (24,25). We observed that the Nal variants had lost the curvature nature which affected the H-NS repressor binding (Figure 8B and C). The DNA sequence of FQ-responsive promoter showed an accentuated curvature, possibly favoring the interaction with H-NS (Figure 8A). Analysis of other stress related E. coli promoters (grpE and recA) revealed that the curvature of these promoter sequences was insignificant (data not shown). Repression was most effective at spacer regions of the promoter because repressor protein was able to bind at spacer regions hence interfering with RNA polymerase binding (23,45). Also, the in silico analysis of variants of spacer region (‘all A’ and ‘all T’) was found not to favor the intrinsic curvature. Taken together, the in vitro and in silico analysis data indicate that the possible reason for the inducible nature of FQ-responsive promoter is the curvature attained by the selected nucleotide sequence.
CONCLUSION
Development of customized gene regulatory elements using DNA sequences of non-genomic origin presents an exciting challenge. Such non-genomic sequences bearing no homology with the bacterial genome can prove to be more stable due to lack of recombination with genomic sequences. Use of GFP-based reporter system for isolation of inducible genetic regulatory elements from a pool of diverse oligonucleotide library proved to be complicated. The library screening and background issues associated with conventional reporters were resolved by developing a simple, robust and universally applicable screening methodology. This led to the isolation of a novel regulatory element which specifically responded to fluoroquinolones (DNA gyrase A inhibitors). The methodology is a noise-free selection strategy and opens a new route to discover ligand-responsive novel gene regulatory elements from diverse oligonucleotide sequences. The FQ-responsive regulatory sequence contained attributes of a promoter. We used genetic and biochemical assays to confirm and validate that regulation of the selected genetic elements occurs through the transcriptional silencer, H-NS. This study also establishes and confirms findings by other groups that H-NS protein preferentially regulates curved and acquired functional DNA sequences. Live and dead selection strategy developed in the present study opens opportunity to isolate custom ligand based synthetic riboswitches from random nucleotide libraries (46,47).
In summary, our approach allowed (i) Isolation of a ligand specific regulatory element based on live and dead screening methodology controlled by expression of a toxic peptide, (ii) application of FQ-responsive promoter in tolC null-mutant of E. coli for sensitive and specific detection of DNA gyrase A inhibitors, (iii) identification of regulatory protein using a combination of E. coli overexpressors and knockouts and (iv) establishment of the fact that the isolated promoter is regulated by an H-NS mediated repression via fluoroquinolone category of drugs.
Supplementary Material
Acknowledgments
This work was supported by Faculty Initiation Grant (FIG) of Indian Institute of Technology Roorkee (IITR) through Ministry of Human Resources and Development (MHRD), Government of India to NKN. We thank Prof. Shimshon Belkin, Hebrew University Israel for providing E. coli RFM443 strain.
Footnotes
Present address: Dr Naveen Kumar Navani, Department of Biotechnology, Indian Institute of Technology Roorkee, Roorkee, Uttarakhand 247667, India.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Ministry of Human Resources and Development (MHRD); Government of India (to N.K.N. and R.P., respectively). The open access publication charge for this paper has been waived by Oxford University Press - NAR.
Conflict of interest statement. None declared.
REFERENCES
- 1.Szybalski W., Skalka A. Nobel prizes and restriction enzymes. Gene. 1978;4:181–182. doi: 10.1016/0378-1119(78)90016-1. [DOI] [PubMed] [Google Scholar]
- 2.Mukherji S., van Oudenaarden A. Synthetic biology: understanding biological design from synthetic circuits. Nat. Rev. Genet. 2009;10:859–871. doi: 10.1038/nrg2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khalil A.S., Collins J.J. Synthetic biology: applications come of age. Nat. Rev. Genet. 2010;11:367–379. doi: 10.1038/nrg2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arpino J.A., Hancock E.J., Anderson J., Barahona M., Stan G.-B.V., Papachristodoulou A., Polizzi K. Tuning the dials of synthetic biology. Microbiology. 2013;159:1236–1253. doi: 10.1099/mic.0.067975-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu T.K., Khalil A.S., Collins J.J. Next-generation synthetic gene networks. Nat. Biotechnol. 2009;27:1139–1150. doi: 10.1038/nbt.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young E., Alper H. Synthetic biology: tools to design, build, and optimize cellular processes. J. Biomed. Biotechnol. 2010;2010 doi: 10.1155/2010/130781. doi:10.1155/2010/130781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos C.N.S., Stephanopoulos G. Combinatorial engineering of microbes for optimizing cellular phenotype. Curr. Opin. Chem. Biol. 2008;12:168–176. doi: 10.1016/j.cbpa.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Alper H., Fischer C., Nevoigt E., Stephanopoulos G. Tuning genetic control through promoter engineering. Proc. Natl. Acad. Sci. U.S.A. 2005;102:12678–12683. doi: 10.1073/pnas.0504604102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz N., Silhavy T.J. Sensing external stress: watchdogs of the Escherichia coli cell envelope. Curr. Opin. Microbiol. 2005;8:122–126. doi: 10.1016/j.mib.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Heimann J.D. The extracytoplasmic function (ECF) sigma factors. Adv. Microbial Physiol. 2002;46:47–110. doi: 10.1016/s0065-2911(02)46002-x. [DOI] [PubMed] [Google Scholar]
- 11.Aertsen A., Michiels C.W. Stress and how bacteria cope with death and survival. Crit. Rev. Microbiol. 2004;30:263–273. doi: 10.1080/10408410490884757. [DOI] [PubMed] [Google Scholar]
- 12.Boor K.J. Bacterial stress responses: what doesn't kill them can make them stronger. PLoS Biol. 2006;4:18–20. doi: 10.1371/journal.pbio.0040023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alba B.M., Gross C.A. Regulation of the Escherichia coli σE dependent envelope stress response. Mol. Microbiol. 2004;52:613–619. doi: 10.1111/j.1365-2958.2003.03982.x. [DOI] [PubMed] [Google Scholar]
- 14.Wösten M. Eubacterial sigma factors. FEMS Microbiol. Rev. 1998;22:127–150. doi: 10.1111/j.1574-6976.1998.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhou D., Yang R. Global analysis of gene transcription regulation in prokaryotes. Cell. Mol. Life Sci. 2006;63:2260–2290. doi: 10.1007/s00018-006-6184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Meer J.R., Belkin S. Where microbiology meets microengineering: design and applications of reporter bacteria. Nat. Rev. Microbiol. 2010;8:511–522. doi: 10.1038/nrmicro2392. [DOI] [PubMed] [Google Scholar]
- 17.Mijakovic I., Petranovic D., Jensen P.R. Tunable promoters in systems biology. Curr. Opin. Biotechnol. 2005;16:329–335. doi: 10.1016/j.copbio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Hammer K., Mijakovic I., Jensen P.R. Synthetic promoter libraries–tuning of gene expression. Trends Biotechnol. 2006;24:53–55. doi: 10.1016/j.tibtech.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 19.De Mey M., Maertens J., Lequeux G.J., Soetaert W.K., Vandamme E.J. Construction and model-based analysis of a promoter library for E. coli: an indispensable tool for metabolic engineering. BMC Biotechnol. 2007;7:34. doi: 10.1186/1472-6750-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fozo E.M., Kawano M., Fontaine F., Kaya Y., Mendieta K.S., Jones K.L., Ocampo A., Rudd K.E., Storz G. Repression of small toxic protein synthesis by the Sib and OhsC small RNAs. Mol. Microbiol. 2008;70:1076–1093. doi: 10.1111/j.1365-2958.2008.06394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mok W.W., Navani N.K., Barker C., Sawchyn B.L., Gu J., Pathania R., Zhu R.D., Brown E.D., Li Y. Identification of a toxic peptide through bidirectional expression of small RNAs. Chembiochem. 2009;10:238–241. doi: 10.1002/cbic.200800591. [DOI] [PubMed] [Google Scholar]
- 22.Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K.A., Tomita M., Wanner B.L., Mori H. Construction of Escherichia coli K-12 in-frame, single gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2006;2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lutz R., Bujard H. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res. 1997;25:1203–1210. doi: 10.1093/nar/25.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodsell D.S., Dickerson R.E. Bending and curvature calculations in B-DNA. Nucleic Acids Res. 1994;22:5497–5503. doi: 10.1093/nar/22.24.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulanovsky L., Bodner M., Trifonov E.N., Choder M. Curved DNA: design, synthesis, and circularization. Proc. Natl. Acad. Sci. U.S.A. 1986;83:862–866. doi: 10.1073/pnas.83.4.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leveau J.H., Lindow S.E. Predictive and interpretive simulation of green fluorescent protein expression in reporter bacteria. J. Bacteriol. 2001;183:6752–6762. doi: 10.1128/JB.183.23.6752-6762.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen P.R., Hammer K. Artificial promoters for metabolic optimization. Biotechnol. Bioeng. 1998;58:191–195. [PubMed] [Google Scholar]
- 28.Hawley D.K., McClure W.R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983;11:2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harley C.B., Reynolds R.P. Analysis of E. coli pormoter sequences. Nucleic Acids Res. 1987;15:2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham D.W., Larive C.K., Lydy M., deNoyelles F.J. Final report: fate and effects of fluoroquinolone antibacterial agents in aquatic ecosystems. 2004. cfpub.epa.gov/ncer_abstracts/index.cfm/fuseaction/display.highlight/abstract/1064/report/F cfpub.epa.gov/ncer_abstracts/index.cfm/fuseaction/display.highlight/abstract/1064/report/F (15 June 2015, date last accessed)
- 31.de Jong A., Pietersma H., Cordes M., Kuipers O.P., Kok J. PePPER: a webserver for prediction of prokaryote promoter elements and regulons. BMC Genomics. 2012;13:299–308. doi: 10.1186/1471-2164-13-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitagawa M., Ara T., Arifuzzaman M., Ioka-Nakamichi T., Inamoto E., Toyonaga H., Mori H. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res. 2006;12:291–299. doi: 10.1093/dnares/dsi012. [DOI] [PubMed] [Google Scholar]
- 33.Weber H., Polen T., Heuveling J., Wendisch V.F., Hengge R. Genome-wide analysis of the general stress response network in Escherichia coli: σS-dependent genes, promoters, and sigma factor selectivity. J. Bacteriol. 2005;187:1591–1603. doi: 10.1128/JB.187.5.1591-1603.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cases I., De Lorenzo V. Promoters in the environment: transcriptional regulation in its natural context. Nat. Rev. Microbiol. 2005;3:105–118. doi: 10.1038/nrmicro1084. [DOI] [PubMed] [Google Scholar]
- 35.Stoebel D.M., Free A., Dorman C.J. Anti-silencing: overcoming H-NS-mediated repression of transcription in Gram-negative enteric bacteria. Microbiology. 2008;154:2533–2545. doi: 10.1099/mic.0.2008/020693-0. [DOI] [PubMed] [Google Scholar]
- 36.Dorman C.J. H-NS: a universal regulator for a dynamic genome. Nat. Rev. Microbiol. 2004;2:391–400. doi: 10.1038/nrmicro883. [DOI] [PubMed] [Google Scholar]
- 37.Williams R.M., Rimsky S. Molecular aspects of the E. coli nucleoid protein, H-NS: a central controller of gene regulatory networks. FEMS Microbiol. Lett. 1997;156:175–185. doi: 10.1111/j.1574-6968.1997.tb12724.x. [DOI] [PubMed] [Google Scholar]
- 38.Owen-Hughes T.A., Pavitt G.D., Santos D.S., Sidebotham J.M., Hulton C.S., Hinton J.C., Higgins C.F. The chromatin-associated protein H-NS interacts with curved DNA to influence DNA topology and gene expression. Cell. 1992;71:255–265. doi: 10.1016/0092-8674(92)90354-f. [DOI] [PubMed] [Google Scholar]
- 39.Thei Dame R., Wyman C., Goosen N. Structural basis for preferential binding of H-NS to curved DNA. Biochimie. 2001;83:231–234. doi: 10.1016/s0300-9084(00)01213-x. [DOI] [PubMed] [Google Scholar]
- 40.Spurio R., Falconi M., Brandi A., Pon C.L., Gualerzi C.O. The oligomeric structure of nucleoid protein H-NS is necessary for recognition of intrinsically curved DNA and for DNA bending. EMBO J. 1997;16:1795–1805. doi: 10.1093/emboj/16.7.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dillon S.C., Dorman C.J. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat. Rev. Microbiol. 2010;8:185–195. doi: 10.1038/nrmicro2261. [DOI] [PubMed] [Google Scholar]
- 42.Dorman C.J. H-NS, the genome sentinel. Nat. Rev. Microbiol. 2007;5:157–161. doi: 10.1038/nrmicro1598. [DOI] [PubMed] [Google Scholar]
- 43.Rimsky S., Zuber F., Buckle M., Buc H. A molecular mechanism for the repression of transcription by the H-NS protein. Mol. Microbiol. 2001;42:1311–1323. doi: 10.1046/j.1365-2958.2001.02706.x. [DOI] [PubMed] [Google Scholar]
- 44.Rimsky S. Structure of the histone-like protein H-NS and its role in regulation and genome superstructure. Curr. Opin. Microbiol. 2004;7:109–114. doi: 10.1016/j.mib.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Cox R.S., Surette M.G., Elowitz M.B. Programming gene expression with combinatorial promoters. Mol. Syst. Biol. 2007;3:145. doi: 10.1038/msb4100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma V., Nomura Y., Yokobayashi Y. Engineering complex riboswitch regulation by dual genetic selection. J. Am. Chem. Soc. 2008;130:16310–16315. doi: 10.1021/ja805203w. [DOI] [PubMed] [Google Scholar]
- 47.Topp S., Gallivan J.P. Random walks to synthetic riboswitches a high throughput selection based on cell motility. Chembiochem. 2008;9:210–213. doi: 10.1002/cbic.200700546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.