Abstract
Fibroblasts lose the ability to replicate in response to growth factors and become unable to express growth-associated immediate-early genes, including c-fos and egr-1, as they become senescent. The serum response factor (SRF), a major transcriptional activator of immediate-early gene promoters, loses the ability to bind to the serum response element (SRE) and becomes hyperphosphorylated in senescent cells. We identify protein kinase C delta (PKCδ) as the kinase responsible for inactivation of SRF both in vitro and endogenously in senescent cells. This is due to a higher level of PKCδ activity as cells age, production of the PKCδ catalytic fragment, and its nuclear localization in senescent but not in low-passage-number cells. The phosphorylation of T160 of SRF by PKCδ in vitro and in vivo led to loss of SRF DNA binding activity. Both the PKCδ inhibitor rottlerin and ectopic expression of a dominant negative form of PKCδ independently restored SRE-dependent transcription and immediate-early gene expression in senescent cells. Modulation of PKCδ activity in vivo with rottlerin or bistratene A altered senescent- and young-cell morphology, respectively. These observations support the idea that the coordinate transcriptional inhibition of several growth-associated genes by PKCδ contributes to the senescent phenotype.
Normal primary human fibroblasts undergo a defined number of population doublings in culture, after which they become morphologically distinct, undergo numerous biochemical alterations, and become unresponsive to mitogens (20, 21). This last characteristic has been studied extensively, but no deficiency in the mitogenic signaling pathways of senescent cells has been clearly defined. Many studies have found that growth factors interact with their receptors normally and that the number of receptors available for ligand binding remains constant with age (13, 37, 42). Conversely, many of the downstream effectors in growth pathways, such as the immediate-early genes c-fos and egr-1, are not induced by serum stimulation in senescent cells (33, 38, 43). Thus, a postreceptor block appears to exist at some point between receptor engagement and growth-associated gene expression within senescent cells.
Previous studies investigating why the expression of c-fos and egr-1 is blocked in senescent cells have focused on examining the posttranslational modifications of serum response factor (SRF), a transcription factor that is responsible for the serum-induced expression of many immediate-early genes (50). SRF was found to be hyperphosphorylated, which correlated with loss of specific DNA binding to the serum response element (SRE) in senescent cells (2). Subsequent work established that diminished SRF binding occurred on all the SREs of the egr-1 promoter, suggesting that a common mechanism inhibited immediate-early gene promoters during senescence (33).
A number of reports have proposed mechanisms to explain the lack of SRF DNA binding activity in senescent cells, including a lack of Elk-1 phosphorylation (47), decreased nuclear localization of ERK1/2 (27), and exclusion of SRF from senescent cell nuclei (10). However, our experiments suggest that SRF is not excluded from the nuclei of primary fibroblasts upon senescence and is independent of ternary complex factor binding at the c-fos promoter. Furthermore, many pathways are believed to influence SRF-SRE-driven transcription, including casein kinase II (25, 31), Jun-associated kinase (26), protein kinase C (PKC) (45), pp90RSK (39), and Rho GTPase/phospatidylinositol-3 kinase (23, 48). Therefore, it appears unlikely that the loss of SRF binding during senescence is a consequence of decreased activity of a single pathway, such as mitogen-activated protein kinase, given the diverse and independent pathways that can target SRF. Although the majority of signaling cascades are associated with activation of transcription factors (50), there is growing evidence that many transcription factors may also be negatively regulated by phosphorylation, including c-Jun, CREB, FKHR-1, NF-AT, and WT-1 (reviewed in reference 52).
Many kinases phosphorylate SRF and enhance DNA binding, but none to date have been found to inhibit DNA binding. To address this possibility, we developed an assay based on SRF binding to the SRE and used it to identify kinases that could regulate SRF binding. With this assay, we show that phosphorylation by a kinase that is activated in senescent cells inhibits SRF binding and that PKC inhibitors restore binding activity. One PKC isoform, PKCδ, has a multifunctional role in various processes, including growth inhibition, differentiation, apoptosis, and tumor suppression (reviewed in reference 16). Although the general functional characteristics of PKCδ are well established, its downstream targets and exact role in many processes are not as well defined. Our study shows that the activity of PKCδ increases in senescent cells and that this results in the hyperphosphorylation and inactivation of SRF. Inactive SRF fails to bind DNA and to act as a transcription factor, resulting in the inhibition of immediate-early gene induction in response to mitogens.
MATERIALS AND METHODS
Cell culture and drug treatment.
Newborn foreskin cells (CRL 1635) human diploid fibroblasts were cultured and passaged to senescence as previously described (51). Stocks of 10-mg/ml rottlerin (Calbiochem) in dimethyl sulfoxide or bistratene A were prepared as indicated (49). Rottlerin treatments were performed on serum-starved cells 4 h prior to serum stimulation. Long-term drug treatment used one application of the drug at the indicated concentration followed by 10 days of observation in culture before harvest. Senescent-cell specific β-galactosidase activity was determined as previously described (9), and stained cells were photographed with a Zeiss Axiovert 35 microscope and a DC120 Kodak digital camera.
Recombinant SRF and mutagenesis.
The pET19b plasmid (a gift from M. Gilman) has an N-terminal histidine tag spliced to the coding region of SRF and was used to generate recombinant SRF protein after induction by isopropylthiogalactopyranoside (IPTG) in the Escherichia coli DE3 strain. Mutagenesis of SRF T160 to A160 was carried out with a Quikchange II mutagenesis kit (Stratagene) with the directions of the manufacturer and primers 5′CTGCGGCGCTACACGGCATTCAGCAAGAGGAAG and 5′CTTCCTCTTGCTGAATGCCGTGTAGCGCCGCAG (bold nucleotides represent mutations). The second mutation in the third position of the T160 codon was to create a BsmI restriction site to facilitate screening of positive clones. Protein purification used a nickel agarose chelating column to purify His-tagged SRF protein (SRF[His]6) from bacterial extracts as described by the manufacturer (QIAGEN). The resulting 1-mg/ml SRF(His)6 stock was used for kinase assays and antibody production.
Nuclear extracts, kinase assays, and EMSA.
Nuclear extracts from young and old Hs68 cells were prepared as previously described (2). These extracts were used to develop a reaction with the kinases present to phosphorylate SRF(His)6 in the presence of ATP. Reactions contained 50 mM HEPES buffer (pH 7.5), 100 mM KCl, 5 mM MgCl2, 5 mM ATP, 250 ng of nuclear protein, and 200 ng of SRF(His)6 and were incubated at 37°C for 45 min. Electrophoretic mobility shift assays (EMSAs) were performed as previously described (33) but were optimized by lowering the MgCl2 concentration to 0.5 mM and the incubation temperature to 4°C to allow measurement of SRF(His)6 binding kinetics consistently. Each shift shown was repeated with different kinase reactions at least three times and gave similar results.
Western blot analyses.
Total cell samples were harvested by applying 2x sodium dodecyl sulfate (SDS) Laemmli sample buffer directly to cell monolayers after three washes with phosphate-buffered saline. Coomassie staining of gels, electrophoresis, transfer to nitrocellulose, and blocking of membranes have been described previously (51). Antibodies for PKCδ (rabbit and goat; Santa Cruz sc-937), Egr-1 (Santa Cruz sc-110), phospho-PKCδ-Thr505 (New England Biolabs 9374), phospho-Rxx(S/T) antibody (New England Biolabs 9621), and phospho-(S/T)-F substrate antibody (New England Biolabs 9621) were incubated at the recommended dilutions with membranes for 1 h at room temperature. The noncommercial polyclonal SRF antibody (SACRC Hybridoma Facility) was used at 1:1,000. Secondary antibodies included anti-rabbit antibody-horseradish peroxidase (Amersham), anti-mouse antibody-horseradish peroxidase (Amersham), and anti-goat antibody-horseradish peroxidase (Amersham), which were used at 1:1,000 and incubated with membranes for 1 h at room temperature. Western blots were visualized by chemiluminescence.
Recombinant kinase reactions and digestions.
Recombinant enzymes used included human PKCδ (Biomol SE-147), human caspase 3 (Biomol SE-169), and casein kinase II (Calbiochem). The kinase reactions contained 5 ng of PKCδ, 50 mM HEPES buffer (pH 7.5), 100 mM KCl, 0.1 mM EGTA, 10 mM MgCl2, 100 μM ATP, and 0.003% Triton. Where indicated, caspase 3 was added to reactions with a 50% dilution of the kinase reaction with 50 mM HEPES buffer (pH 7.5), 100 mM NaCl, 1 mM EDTA, 10 mM MgCl2, 0.1% 3-[(cholamidopropyl)-diethylammonio-1-propanesulfonate, 10% glycerol, and 1 μM dithiothreitol and incubated for 45 min at 30°C. Kinase reactions with SRF(His)6 and preactivated PKCδ used the standard conditions as outlined above except that 0.25 ng of PKCδ or digested PKCδ was added instead of nuclear extracts.
Immunoprecipitations.
Young and senescent cells were harvested in radioimmunoprecipitation assay (RIPA) buffer, with addition of 0.1 mg each of leupeptin, aprotinin, and pepstatin per ml. These extracts were quantitated by Bradford assay (Bio-Rad), and equal amounts of protein were used for each subsequent immunoprecipitation within an experiment. Incubation with 20 ng of PKCδ polyclonal antibody (Santa Cruz sc-937) or 0.5 μl of SRF rabbit antiserum was carried out with 500 μg of total cellular protein for 4 h at 4°C. Precipitation was performed with 20 μl of protein A-Sepharose beads, preequilibrated in RIPA buffer, for 1.5 h at 4°C. The resulting precipitate was extensively washed and either resuspended in 2× SDS Laemmli sample buffer for Western blot analysis or kinase buffer for kinase reactions. The standard kinase reaction conditions outlined above were used with 500 ng of SRF(His)6 and 5 μCi of [γ-32P]ATP. Precipitations with the same antibody as the Western blot used 2× SDS sample buffer without reducing agent, followed by SDS-10% polyacrylamide gel electrophoresis (PAGE), nonfixing silver stain (44), and excision of the band corresponding to SRF. The gel slices were then placed in the wells of a new 10% gel and re-resolved. Peptide maps were generated by exposing gel-purified native SRF to 1 U of Glu-C protease for 16 h at 37°C in 100 mM NH4HCO3 and resolution on a 15% Tricine gel (41).
Constructs and transfections.
Constructs with c-fos promoter elements were gifts from R. Prywes and included pSIEGL3 (c-fos SRE and ets), pSIEpm18GL3 (c-fos SRE only), and pSIEpm12GL3 (c-fos ets only), which drive luciferase expression (45). Constructs with human PKCδ were kind gifts from D. Kufe and included pKV, pKV-PKCδ, pKV-PKCδ-CF, and pKV-PKCδ-CF (K-R), which are driven by a simian virus 40 promoter (14).
Young Hs68 fibroblasts were plated in six-well dishes, grown to a cell density of 60%, and serum starved for 1 day prior to transfection, at which point they were 90% confluent. Transfections were carried out with a total of 4 μg of total DNA and 6 μl of Lipofectamine 2000 (Invitrogen) for each 35-mm well, with the manufacturer's protocol. Another 48 h of serum starvation preceded serum stimulation and harvest of the transfected fibroblasts. Sample aliquots were analyzed with the luciferase assay system (Promega) as described by the manufacturer with a Berthhold Technologies luminometer. Transfection efficiency was assessed by cotransfection with a β-galactosidase enzyme assay system (Promega) as described by the manufacturer with a Beckman Biomek 1000 automated plate reader.
RNA isolation and RT-PCR.
Each RNA sample was isolated with Triazol (Gibco-BRL) from a 10-cm dish of Hs68 fibroblasts. egr-1 and c-fos mRNA expression was assessed with reverse transcription-PCR (RT-PCR), as described previously, by the primer dropping method with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control for each reaction (33, 53).
Immunofluorescence microscopy.
Hs68 fibroblasts were fixed for 5 min with 1% paraformaldehyde in phosphate-buffered saline (PBS) and 0.5% Triton X-100 in PBS. Immunofluorescence was performed by incubating with antibody at a 1:50 dilution for 45 min at 37°C in PBS containing 1% bovine serum albumin. After washing in PBS, cells were incubated for 45 min at 37°C with secondary antibody diluted 1:200 (goat anti-rabbit antibody conjugated with indocarbocyanine; Jackson Immunoresearch) and washed with PBS. 4′,6′-Diamidino-2-phenylindole (DAPI) staining was performed with 0.5 ng of DAPI per ml in PBS containing 1% bovine serum albumin for 5 min at 37°C. After mounting the samples, immunofluorescence images were captured with a Leica DM R microscope and a Photometrics 16-bit cooled digital camera.
RESULTS
SRF levels and localization remain constant during cellular senescence.
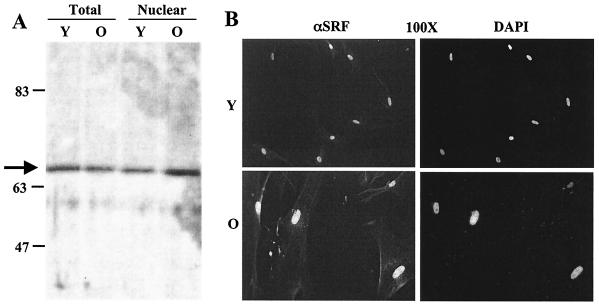
A recent study suggested that decreased binding of SRF in senescent-cell nuclear extracts was due to decreased protein levels of SRF in the nuclei of senescent cells (10). In contrast, a prior report found no difference in cellular localization or concentration of SRF in young and senescent cells (2). In order to address this incongruity, Western blots were performed with equal amounts of protein and two independently derived rabbit polyclonal antibodies. As shown in Fig. 1A, similar amounts of a single 67-kDa band corresponding to the mass of native SRF was detected in both total and nuclear extracts of senescent and young cells. Another polyclonal SRF antibody, R1122, showed similar results (data not shown). In order to avoid potential artifacts due to the preparation of nuclear extracts, we also visualized SRF in situ by indirect immunofluorescence. Staining of individual cells (Fig. 1B) corroborated the results from Western blots, showing nuclear localization of SRF in young and senescent fibroblasts. These data indicate that nuclear SRF levels do not change markedly during cellular senescence in primary fibroblasts.
FIG. 1.
SRF protein levels do not change with cellular age. (A) Western blots used equal amounts of protein from young (Y, 36 mean population doublings) and old (O, 82 mean population doublings) Hs68 fibroblasts. Total (Tot) and nuclear (Nuc) extracts and a polyclonal antibody generated against full-length SRF were used. The arrow identifies a 67-kDa band characteristic of native SRF. (B) Young and senescent primary human diploid fibroblasts were fixed and stained with rabbit anti-SRF followed by goat anti-rabbit antibody-Texas Red and counterstained with DAPI to visualize DNA.
Kinase activity unique to senescent cells inhibits the DNA binding activity of SRF.
A previous study reported that hyperphosphorylation of native SRF in senescent cells correlated with decreased DNA binding activity (2). To examine the effects of phosphorylation, we developed an in vitro kinase assay to test the effects of nuclear extracts on the DNA binding activity of recombinant SRF. SRF was incubated with young and senescent-cell nuclear extracts and used in electrophoretic mobility shift assays (EMSAs), which showed differential binding activity similar to that of previous studies in vivo (Fig. 2A, lanes 1 and 2). The specificity of the SRF-SRE interaction was verified with labeled mutant SRE oligonucleotide, which did not form complexes when incubated with the kinase reactions (Fig. 2A, lanes 3 and 4), while competition with excess unlabeled wild-type SRE effectively decreased the specific SRF-SRE signal (Fig. 2A, lanes 5 and 6). No competition occurred in reactions containing a 100-fold excess of unlabeled mutant SRE (Fig. 2A, lanes 7 and 8) and the complex was supershifted when polyclonal SRF antibody was added (Fig. 2A, lanes 9 and 10). Parallel reactions omitting SRF(His)6 were carried out, and no shift signal from endogenous SRF-SRE complexes was detected (Fig. 2A, lanes 11 to 14) at the concentration of cell nuclear extract used. Therefore, the in vitro kinase assay with SRF(His)6 and nuclear extracts recapitulates the differential binding activity of endogenous proteins previously shown for young and old fibroblasts (33).
FIG. 2.
Kinase activity in senescent-cell nuclear extracts inhibits SRF DNA binding activity. (A) EMSAs with 32P-labeled SRE oligonucleotide and SRF(His)6 previously incubated with nuclear extracts from young (Y) or old (O) nuclear extracts are shown in lanes 1 and 2. Parallel kinase reactions were incubated with mutant (mut) SRE (lanes 3 and 4), competed with a 100-fold excess of unlabeled wild-type (WT) SRE (lanes 5 and 6), or incubated with a 100-fold excess of unlabeled mutant SRE (lanes 7 and 8). Preincubation with polyclonal SRF antibody (αSRF, lanes 9 and 10) supershifted or eliminated the SRF complex. Parallel reactions without SRF(His)6 did not form complexes. (B) SRF(His)6 was used in kinase reactions with equal amounts of young (Y), senescent (O), or a combination of young- and old-cell nuclear extracts (Y/O) and used in SRE EMSAs (lanes 2 to 4). A control (C) kinase reaction with SRF but without nuclear extract is shown in lane 1. Parallel reactions were also done in the presence of the phosphatase inhibitors sodium fluoride (NaF) and sodium vanadate (Na-Van) (lanes 5 to 7). The proportional addition of senescent (Old%) nuclear extracts to young nuclear extracts (Young%) were also used in SRE EMSAs (lanes 8 to 12). (C) Reactions with SRF(His)6 incubated with kinases supplied from equal amounts of young (Y), senescent (O), or a combinations of young and old nuclear extracts (Y/O) with 10 μM ATP were used in SRE EMSAs (lanes 2 to 4). A control (C) reaction with SRF but without nuclear extract is shown in lane 1. Parallel reactions were also carried out in the absence of ATP used in EMSAs (lanes 5 to 8). (D) Data were obtained from SRE EMSAs utilizing SRF kinase reactions in the presence of SRF(His)6 as the substrate and kinases supplied from equal amounts of young (Y) and senescent (O) nuclear extracts. Various amounts of the PKC inhibitors bisinodolylmaleimide II (Bis II; 25, 50, and 100 nM), chelerythrine chloride (CH-Cl; 1.25, 2.5 and 5 μM), rottlerin (Rot; 5, 10, and 20 μM), or dimethyl sulfoxide (DMSO, 1%) vehicle were used in each reaction. Control reactions with SRF but in the absence of nuclear extracts were also followed by EMSA and used to normalize experiments. Histograms show data from scanning densitometry of three independent EMSAs with the average ratio of young and old intensities relative to the control reaction under each drug concentration, with standard deviations shown by error bars.
In order to distinguish whether senescent cells lacked an activator or contained dominant negative activity for SRF DNA binding, in vitro kinase reactions combining both young and senescent-cell nuclear extracts were assessed for binding activity. These reactions combined an equal amount of young and old cell nuclear extracts and suggested that the lower levels of SRE binding are due to a dominant factor in senescent extracts (Fig. 2B, lanes 2 to 4). Phosphatase inhibitors did not restore senescent-cell-specific binding (Fig. 2B, lanes 5 to 7), suggesting that the dominant activity in old cells is not a phosphatase. Additionally, different proportions of senescent and young cell nuclear extracts combined with SRF(His)6 in a kinase reaction confirmed that a proportional downregulation of SRF-SRE binding occurred as the percentage of old cell extract increased (Fig. 2B, lanes 8 to 12). These data suggest that a titratable activity exists in senescent cells that is capable of modulating SRF-SRE binding activity.
To determine whether the dominant activity in senescent cells was due to a kinase, we tested for binding activity in the presence and absence of ATP. Kinase reactions were optimized and used 10 μM ATP, no ATP, or 10 μM ATP with phosphatase inhibitors. The results in Fig. 2C show that differential SRE binding was maintained in the presence of ATP (lanes 1 to 4) but not in the absence of ATP (lanes 5 to 8) compared to control reactions. Addition of phosphatase inhibitors with ATP augmented binding in young-cell extracts but not in old-cell extracts (data not shown). These data suggest that a factor unique to senescent cells is dominant over activating kinases in young cells, sensitive to the absence of ATP, and not affected by phosphatase inhibitors. SRF hyperphosphorylation during senescence (2) and the data from Fig. 2 are consistent with the idea that a kinase inhibits SRF DNA binding in senescent cells.
Inhibitory kinase activity in senescent-cell nuclear extracts is sensitive to PKC inhibitors.
Preliminary experiments with the inhibitors bisinodolylamaleimide II, chelerythrine chloride, and rottlerin in kinase reactions followed by SRE EMSAs revealed that inhibitors of PKC influenced SRF-SRE binding reproducibly (Fig. 2D). The histogram of EMSA densitometry results shows that the presence of bisinodolylamaleimide II demonstrated no significant changes in the differential binding seen between young and senescent nuclear extracts (Fig. 2D, lanes 2 to 4). This inhibitor is selective for conventional PKC isoforms in the nanomolar range and inhibits novel isoforms at concentrations 10 times higher (28). Control lanes represent the binding activity of SRF in the absence of nuclear extract and were used to normalize reactions between experiments (Fig. 2D, lane 1). Conversely, the general PKC inhibitor chelerythrine chloride works by competitive inhibition with the phosphoacceptor of all PKC isoforms at the concentrations used (22). The histogram data showing the EMSA results with chelerythrine chloride demonstrated that an increase in drug concentration paralleled the restoration of SRF-SRE binding in senescent nuclear extracts and did not affect binding in young-cell nuclear extracts (Fig. 2D, lanes 5 to 7). These data suggest that a novel isoform of PKC was active in senescent-cell nuclear extracts that was able to phosphorylate SRF and lead to inhibition of DNA binding.
Considering the many potential PKC phosphorylation sites in the primary sequence of SRF (50) and that PKCδ activity is associated with cellular arrest (reviewed in reference 7), we asked whether PKCδ could affect SRF binding in senescent cells. Rottlerin inhibits PKCδ selectively, with an effect 10 times greater than for other PKC isoforms, and acts as a competitive inhibitor of ATP (17). The histogram of the EMSA results with rottlerin showed that this compound was capable of restoring SRF-SRE binding activity in senescent-cell nuclear extracts with a threshold of 20 μM (Fig. 2D, lanes 8 to 10). The EMSAs indicate that SRF DNA binding was restored in reactions containing SRF(His)6 and senescent-cell extract at 20 μM rottlerin (lanes 1 versus 10). Conversely, SRF(His)6 incubated with young-cell nuclear extract was only modestly sensitive to rottlerin (Fig. 2D, lanes 1 versus 8 to 10). This parallels the results obtained with chelerythrine chloride and demonstrates that PKCδ is likely the kinase which affects SRF-SRE binding in senescent-cell nuclear extracts.
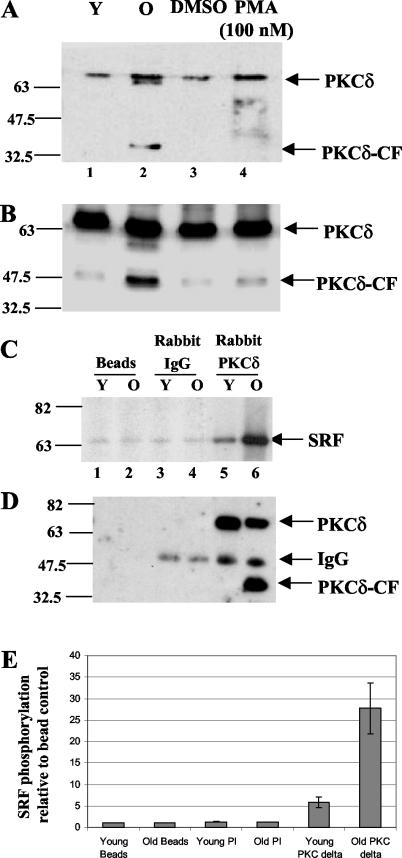
Kinase reactions in the presence of [γ-32P]ATP were performed with recombinant SRF and nuclear extracts from young and senescent cells. Reactions were carried out in the presence of dimethyl sulfoxide vehicle, rottlerin, or bistratene A. Bistratene A is a compound that selectively activates PKCδ in various cellular systems including fibroblasts, HL60 cells, and intestinal epithelial cells (12, 15, 49). As shown in Fig. 3A, the majority of SRF phosphorylation seen during incubation with senescent-cell extracts was inhibited by rottlerin and moderately enhanced by the presence of bistratene A (Fig. 3A, compare lanes 3, 7, and 11). Conversely, kinase activity in young cell nuclear extracts was relatively insensitive to either drug (Fig. 3A, compare lanes 2, 6, and 10). Mixing extracts from young and old cells showed an intensity of phosphorylation comparable to that of reactions containing senescent-cell extract, indicating that the same kinases are likely being affected (Fig. 3A, compare lanes 4, 8, and 12).
FIG. 3.
Specific PKCδ kinase inhibitors and activators modulate SRF DNA binding activity. (A) In vitro kinase reactions with SRF(His)6 and kinases supplied from equal amounts of young (Y), senescent (O), or a combination of young- and old-cell nuclear extracts (Y/O) were performed in the presence of [γ-32P]ATP. Control (C) reactions with SRF but without nuclear extract were also incubated in the presence of [γ-32P]ATP (lanes 1, 5, and 9). Parallel sets of reactions were carried out in the presence of either 20 μM rottlerin (lanes 5 to 8) or 50 nM bistratene A (lanes 9 to 12), which inhibit and activate PKCδ, respectively (B). In vitro kinase reactions performed in parallel without radiolabel were used with labeled SRE EMSAs (lanes 13 to 24).
Kinase reactions with rottlerin and bistratene A as outlined above were performed in parallel without radioisotope and used in SRE EMSAs. The resulting shift intensities of SRE binding inversely reflected the phosphorylation status of SRF (Fig. 4B, lanes 13 to 24). The SRE binding ability of SRF(His)6 treated with senescent-cell nuclear extract was restored when rottlerin was present in the reaction (Fig. 4B, lanes 15 versus 19), even when combinations of young and old nuclear extracts were used in reactions (Fig. 4B, lanes 16 versus 20). Conversely, bistratene A moderately depressed SRE binding in old-cell extracts (Fig. 4B, compare lanes 15 and 16 with 23 and 24). The SRF-SRE binding activity of reactions incubated with senescent-cell extract progressively diminished as the concentration of bistratene A was increased from 35 to 75 nM, but reactions with young-cell extract remained relatively insensitive to the activator (data not shown). These data provide evidence that the kinase which hyperphosphorylates SRF is inhibited by rottlerin and activated by bistratene A, leading to the modulation of SRF-SRE binding affinity in vitro.
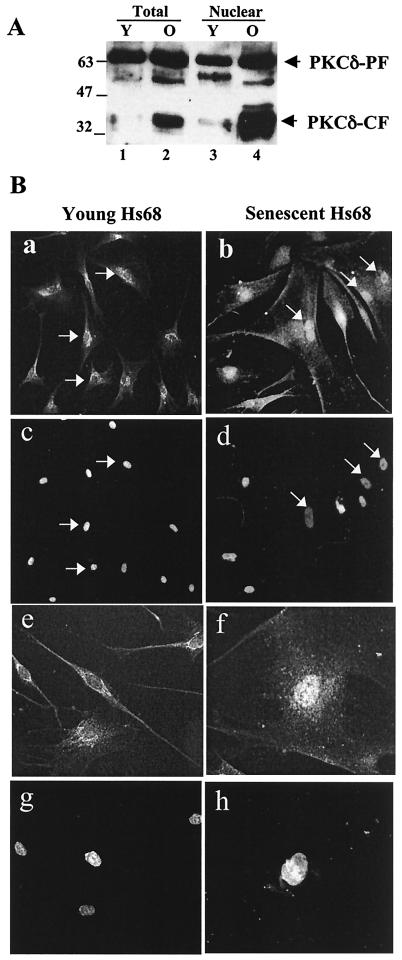
FIG. 4.
A 40-kDa band corresponding to the catalytic fragment of PKCδ is present in senescent cells. Western blots and indirect immunofluorescence used a polyclonal antibody (sc-937) which detects both full-length PKCδ and catalytic fragment (PKCδ-CF) of PKCδ. (A) Western blot of whole-cell (Total) and nuclear extracts collected from both young (Y) and old (O) quiescent cells. (B) PKCδ immunofluorescence with the sc-937 PKCδ antibody on young (a) and senescent (b) cells (c and d) DAPI staining of panels a and b, respectively. White arrows indicate nuclei in a typical field. A 400× magnification is also shown for young (e) and senescent (f) cells, with DAPI counterstaining (g and h, respectively).
PKCδ catalytic fragment is enriched in senescent cells.
A Western blot was used to establish PKCδ localization and protein levels in whole-cell and nuclear extracts from young and old fibroblasts. No dramatic change in the amount of endogenous PKCδ was observed between young and old cells, but an intense 40-kDa band was seen almost exclusively in senescent cells (Fig. 4A). This 40-kDa band corresponds well to a previously described proteolytic cleavage product comprised of the PKCδ catalytic fragment (PKCδ-CF) (3, 14). Since SRF is activated in response to mitogen stimulation, we assessed whether serum stimulation altered accumulation of PKCδ-CF. The amounts of PKCδ or PKCδ-CF were not sensitive to rottlerin, but the CF accumulated slightly in old cells 90 min after serum stimulation (data not shown). This accumulation is consistent with the activity inhibiting immediate-early gene expression (33).
Since SRF localizes exclusively to the nucleus, any kinase which potentially phosphorylates SRF would also have to be nuclear. Thus, we examined young and old fibroblasts by indirect immunofluorescence with a PKCδ antibody that detects both PKCδ and PKCδ-CF. Both young and old cells showed immunolocalization of PKCδ to the perinuclear region, while senescent cells also showed more intense nuclear staining (Fig. 4B), suggesting that PKCδ accumulates in senescent-cell nuclei. This increased intensity is likely due to the presence of both PKCδ and PKCδ-CF, consistent with the Western blot of the nuclear fractions (Fig. 4A).
Senescent cells have high endogenous levels of PKCδ activity.
To determine if PKCδ activity was altered in aging human fibroblasts, lysates from young, old, and 100 mM phorbol myristate acetate-treated young fibroblasts (as a positive control) were blotted with anti-phospho-PKCδ. This antibody detects phosphorylation at Thr 505 of PKCδ by phosphoinositide-dependent protein kinase 1 (PDK-1) and is an indicator of PKC activation (29). The phosphorylation of this site was significantly higher in senescent than in young cells (Fig. 5A, lanes 1 and 2), was comparable to the increase seen after phorbol myristate acetate stimulation in young cells (Fig. 5A, lane 4), and was not due to differences in the amount of PKCδ protein present (Fig. 5B). Additionally, the antibody detected a phosphorylated PKCδ-CF only in senescent cells.
FIG. 5.
PKCδ activity is elevated during senescence (A) Phospho-Thr-505 Western blot of total lysates of young (Y) and old (O) cells harvested after serum starvation for 48 h or when stimulated for 0.5 h with 100 nM phorbol myristate acetate. (B) The samples used in panel A were used in a PKCδ Western blot (sc-937) as a loading control for the phosphorylation-specific Western blot. (C) Lysates from young and old fibroblasts were precipitated with anti-PKCδ antibody (sc-937), nonspecific rabbit immunoglobulin G, or beads. Kinase reactions were performed with aliquots of the immunoprecipitations described in panel B, [γ-32P]ATP, and SRF(His)6. (D) Precipitated PKCδ was visualized by a Western blot with polyclonal goat anti-PKCδ (lanes 1 to 6). (E) Histogram of data obtained from scanning densitometry of three independent immunoprecipitation kinase reactions as described for panel C. Error bars indicate standard deviations.
We next wanted to test PKCδ activity in young- and senescent-cell nuclear extracts and determine whether SRF was a potential substrate. A kinase reaction with an aliquot of each immunoprecipitation, SRF(His)6 and [γ-32P]ATP demonstrated much higher phosphorylation of SRF by PKCδ from senescent cells (Fig. 5C, lanes 5 and 6). The nonspecific immunoglobulin G and bead controls did show some background phosphorylation (Fig. 5C, lanes 1 to 4), which is likely due to nonspecific absorption of kinases onto the beads. A Western blot with a goat polyclonal antibody verified that the amount of PKCδ immunoprecipitated was similar in young and old cells (Fig. 5D, lanes 5 and 6). The increased phosphorylation of SRF by PKCδ in immunoprecipitations from senescent-cell extracts was consistently observed in three independent experiments (Fig. 5E). Together, the phosphorylation-specific Western blot and immunoprecipitation kinase assay demonstrate that PKCδ activity is increased in senescent cells and that SRF is a substrate of PKCδ in vitro.
Recombinant PKCδ phosphorylates SRF and modulates its DNA binding activity.
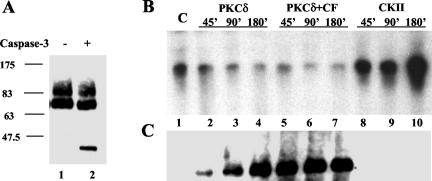
Next we assessed whether phosphorylation of SRF by PKCδ was able to inhibit its DNA binding activity. In order to recapitulate conditions in senescent cells, which contain both PKCδ and PKCδ-CF, recombinant PKCδ was digested with caspase 3. After optimization of caspase cleavage, a fraction enriched for PKCδ-CF was generated (Fig. 6A) and shown to be active in phosphorylating SRF (data not shown). These fractions were used in subsequent reactions to address whether PKCδ could directly affect SRF DNA binding activity. Recombinant casein kinase II was used as a positive control because it has been reported to activate SRF DNA binding (32). PKCδ activity was capable of reducing SRF DNA binding activity with a magnitude correlated to the duration of the kinase reaction (Fig. 6B, lanes 2 to 7). Additionally, casein kinase II activity promoted SRF binding proportionally to the length of the kinase reaction (Fig. 6B, lanes 8 to 10). Thus, direct phosphorylation of SRF by PKCδ is capable of downregulating SRF DNA binding activity.
FIG. 6.
Recombinant PKCδ inhibits SRF DNA binding activity. (A) A preparative digest of PKCδ was performed by incubation for 3 h at 37°C with recombinant caspase 3. A PKCδ Western blot shows the liberation of the PKCδ catalytic fragment (PKCδ-CF). These preparative fractions were used in subsequent phosphorylation analyses with PKCδ. (C) Phosphorylation-specific Western blot and SRE EMSA of in vitro kinase assays with activated PKCδ (lanes 2 to 4), caspase-cleaved PKCδ (lanes 5 to 7), and recombinant casein kinase II (lanes 8 to 10). The kinases were used to phosphorylate SRF(His)6 in a time course of 45 to 180 min at 37°C. The control (lane 1) used SRF(His)6 alone under the same conditions without any kinase. Reaction products were used in SRE EMSAs (B) or in a Western blot with a phosphoserine/threonine-phenylalanine (Phe +1) antibody (C).
An antibody that recognizes phospho-serine/threonine (S/T) with a phenylalanine (F) at the +1 position was obtained to examine possible sites of phosphorylation on SRF. PKCδ substrates show a predominance of target consensus sequences that meet the F +1 requirement. As shown in Fig. 6C, the S/T-F phospho-site is only detected on SRF after phosphorylation by PKCδ, while casein kinase II phosphorylation is not detected by this antibody. The S/T-F phosphorylation on SRF correlates with inhibition of SRF DNA binding (compare Fig. 6B and C). Furthermore, the rate of phosphorylation by PKCδ is much slower than the rate when PKCδ-CF is present (compare lanes 2 to 4 with 5 to 7). This implies that SRF is a better substrate for PKCδ-CF than for PKCδ but that both are able phosphorylate SRF on a Phe +1 site that inhibits SRF DNA binding. A Phe +1 site in SRF that is the closest to a PKCδ consensus (34) and is required for DNA binding (35) was narrowed down to T160.
Mutation of SRF Thr160 to Ala160 blocks the negative regulation of SRF by PKCδ phosphorylation.
Given that SRF was phosphorylated on a site consistent with T160, we next generated a mutant form of SRF with this site changed to alanine. The mutant form of SRF (A160) no longer acted as a robust substrate for PKCδ (Fig. 7A), although it still acted as a substrate for casein kinase II, indicating that the mutation does not affect another known kinase which targets SRF (compare lanes 3 and 4). Although this site does not account for the total phosphorylation of SRF by PKCδ (Fig. 7A, lanes 1 and 2), it does account for the Western blot signal with antibodies that detect either phospho-Rxx(S/T) or (S/T)F motifs, since SRF A160 treated with PKCδ was undetectable (Fig. 7A, compare lanes 1 and 2 in the three panels). T160 is the only site in SRF that is part of the epitope for both antibodies, and considering that neither antibody detects phosphorylation of SRF A160 after PKCδ treatment, it is consistent with both of these antibodies' specifically detecting T160 phosphorylation of SRF. Most importantly, treating SRF A160 with PKCδ did not affect its DNA binding activity (Fig. 7B, lanes 4 versus 6), while casein kinase II increased the binding activity of the mutant, indicating that DNA binding activity can still be regulated in the mutant protein (Fig. 7A, lane 5). Note the basal DNA binding activity of the A160 mutant SRF was also decreased compared to the wild-type level (Fig. 7B, lanes 1 versus 4), which is consistent with a previous study that used a truncated form of SRF A160 in an EMSA (35). Together, these observations indicate that PKCδ phosphorylates and negatively modulates SRF DNA binding activity through T160.
FIG. 7.
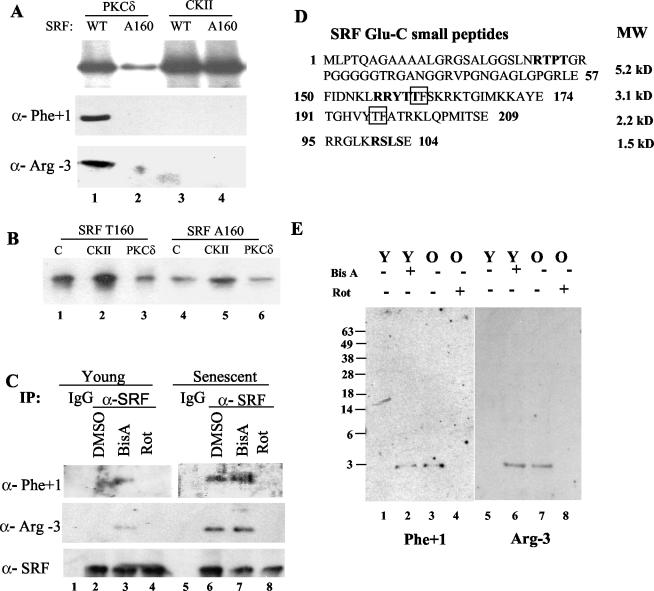
PKCδ phosphorylates both native and recombinant SRF on T160, and mutation of this site blocks SRF inactivation. (A) The mutant form of SRF, A160, and wild-type SRF were subjected to PKCδ and casein kinase II treatment for 90 min. Kinase reactions with [γ-32P]ATP, SRF (wild type or A160), and PKCδ or casein kinase II were performed, and the results are shown in the top panel. Parallel reactions without radiolabel were analyzed by Western blotting with the Phe +1 or Arg −3 phosphorylation-specific antibodies and are shown in the bottom panels. (B) Parallel unlabeled reactions were also used in SRF-SRE EMSAs. Control (C) reactions in the EMSA used SRF T160 or A160 but were not treated with kinase. (C) Native SRF phospho-analysis was performed with young- and senescent-cell extracts treated with dimethyl sulfoxide (DMSO), rottlerin (Rot), or bistratene A (BisA) before harvesting. An immunoprecipitation with the SRF polyclonal was followed by resolution by SDS-10% PAGE and transfer. Western blots of native SRF used anti-SRF (αSRF), phospho-S/T Phe +1 (anti-Phe +1), or phospho-S/T Arg −3 (anti-Arg-3) antibodies. (D) Small peptides of SRF which are generated by Glu-C digestion and contain the consensus sequence for the anti-phospho-Phe +1 antibody (S/T-F, boxed) or anti-phospho-Arg −3 antibody (RxxS/T, bold). (E) Peptide analysis was carried out by immunoprecipitating SRF from young (Y) and senescent (O) cell extracts after vehicle, bistratene A (BisA), or rottlerin (Rot) treatment, silver staining, isolation from gels, and digestion with Glu-C. The resulting peptides were resolved on a 15% Tricine gel and Western blotted with phospho-S/T Phe +1 (Phe +1) or phospho-S/T Arg −3 (Arg-3) antibodies.
SRF T160 is phosphorylated in senescent cells and is modulated by a PKCδ-specific activator and inhibitor.
Increased PKCδ activity and differential localization were seen in senescent cells, and a likely mechanism to account for inhibition of SRF DNA binding through T160 was established. We next asked whether SRF was phosphorylated on this site in vivo. An SRF immunoprecipitation with young and senescent cells was performed after treatment with vehicle, rottlerin, or bistratene A. The immunoprecipitated protein was gel purified and Western blotted with antibodies that detect either phospho-Rxx(S/T) or (S/T)F motifs. An SRF Western blot acted as a control to demonstrate that comparable amounts of SRF were being precipitated and accessed in each lane. Both Phe +1 and Arg −3 phosphorylation-specific antibodies clearly detect SRF precipitated from senescent cells but not young cells (Fig. 7C, compare lanes 2 and 6). Additionally, bistratene A leads to visible phosphorylation in young cells (lane 3, compare panels), and rottlerin blocks phosphorylation in senescent cells (lane 8, compare panels).
Although the phosphorylation-specific antibodies only have overlapping motifs at T160, these motifs also occur elsewhere in the protein and therefore could lead to ambiguous results with full-length SRF. Complete digestion of native SRF with Glu-C enabled identification of the peptide fragment containing the T160 residue with phosphorylation-specific antibodies in Western blots. Figure 7D shows candidate phosphorylatable peptides, including T160. Both antibodies detected the same 3.1-kDa (25-amino-acid) peptide containing T160 in native SRF (Fig. 7E). Additionally, bistratene A treatment led to this phosphorylation in young cells (Fig. 7E, lanes 2 and 6), and rottlerin inhibited the phosphorylation in senescent cells (Fig. 7E, lanes 4 and 8). Therefore, SRF T160 is phosphorylated directly by PKCδ in vitro (Fig. 7A) and is also phosphorylated in vivo in senescent cells but not in young cells (Fig. 7E). These data provide strong evidence that SRF T160 is phosphorylated in vivo by PKCδ.
Ectopic expression of PKCδ leads to inhibition of SRE-driven transcription.
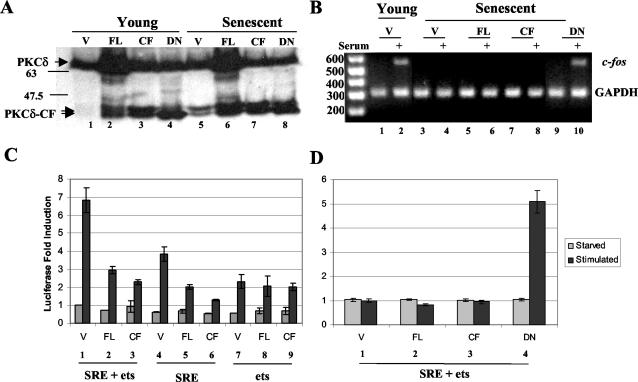
In order to address whether PKCδ affects SRE transcription by an independent method in the absence of kinase inhibitors that have been reported to have nonspecific effects (6, 46), full-length PKCδ (PKCδ-FL) and PKCδ-CF were ectopically expressed in both young and senescent cells. Figure 8A shows that the PKCδ constructs produced PKCδ proteins of the expected molecular weights and that overexpression of PKCδ-FL also resulted in the production of PKCδ-CF (lanes 2 and 6). Serum induction of endogenous immediate-early gene transcripts was restored by expression of dominant negative PKCδ. RT-PCR showed that dominant negative PKCδ was able to restore c-fos (Fig. 8B, lane 10) and egr-1 (data not shown) induction in response to serum.
FIG. 8.
Ectopic expression of PKCδ affects SRE-dependent transcription and endogenous immediate-early gene expression. (A) A PKCδ Western blot (sc-937) with extracts of young and senescent cells previously transfected with the pkV vector (V), PKCδ-FL (FL), PKCδ-CF (CF), or dominant negative PKCδ-CF(K-R) (DN). (B) Ectopic expression of PKCδ constructs and RT-PCR analysis of endogenous c-fos transcript levels in young and senescent fibroblast RNA. Glyceraldehhyde-3-phosphate dehydrogenase (GAPDH) served as an internal control for loading, amplification, efficiency, and RNA integrity. (C and D) Ectopic expression of PKCδ constructs and SRE-driven reporter expression in young (C) and senescent (D) fibroblasts. Young and senescent fibroblasts were transfected with equal amounts of a c-fos luciferase reporter and PKCδ expression constructs. Luciferase activity was measured before and after serum induction for each cotransfection to assess c-fos SRE- plus ets-, SRE-, or ets-dependent transcription in vivo. The histograms show the effect of induction relative to each serum-starved transfected construct for three independent trials. Error bars indicate standard deviations.
The PKCδ constructs were also cotransfected with luciferase reporter constructs harboring the SRE or SRE plus ets sites of the c-fos promoter (Fig. 8C and D). SRE-driven expression was significantly inhibited by the presence of both PKCδ-FL and PKCδ-CF when the reporters were activated by serum addition (Fig. 8C, bars 1 versus 2 and 3 and bars 4 versus 5 and 6). Conversely, the activity of the ets sequence alone was not significantly affected by the presence of PKCδ (Fig. 8C, bars 7 to 9). PKCδ-CF (Fig. 8C, bars 3 and 6) was modestly more effective at inhibiting SRE-dependent transcription than PKCδ-FL (Fig. 8C, bars 2 and 5), which corroborates the results of the in vitro EMSA (Fig. 6).
These transfection experiments demonstrate that PKCδ can significantly inhibit SRF-SRE binding independent of pharmacological agents and does not act through a ternary complex factor- or ets-related mechanism. Consistent with a role for PKCδ in cellular aging, the ectopic expression of PKCδ in senescent fibroblasts did not affect the loss of SRE-dependent transcription (Fig. 8D, bars 1 to 3). Conversely, the expression of the dominant negative mutant of PKCδ-CF was capable of restoring the SRE-dependent serum response in senescent fibroblasts (Fig. 8D, bar 4). The results in Fig. 8 corroborate the observation of increased basal PKCδ activity in senescent cells (Fig. 5), further supporting the idea that PKCδ has an active role in suppressing the SRF-SRE association and SRE-dependent transcription.
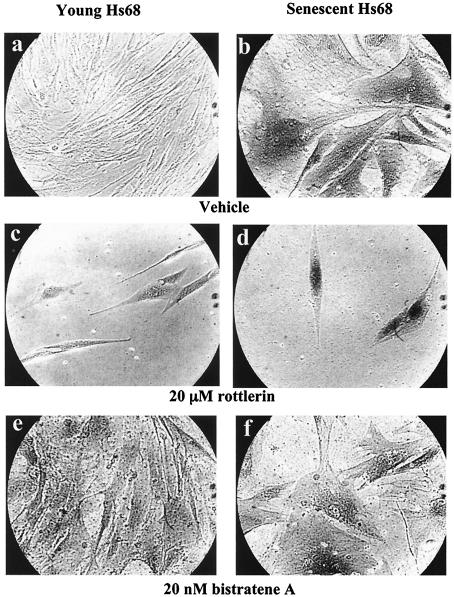
Rottlerin and bistratene A affect primary fibroblast morphology.
Since the PKCδ activator and inhibitor affected SRF phosphorylation and binding affinity in vivo, experiments with these drugs in live cells were undertaken. Senescence was assessed by the use of an acidic β-galactosidase assay (9) after drug treatment of both young and senescent cells. Rottlerin had little effect on young-cell morphology (Fig. 9c). The low cell density of rottlerin-treated cells was due to drug-induced growth arrest, even at 5 μM (results not shown). Old cells treated with rottlerin appeared to regain the phenotypic characteristics of young cells but still stained blue in the β-galactosidase assay (Fig. 9d). Bistratene A caused a transient effect on the morphology of both young and senescent cells, causing them to round up and lose definition (data not shown). Within a few days, the cells recovered from this state if the concentration of bistratene A was sufficiently low. Young cells recovered from the treatment for 10 days at low concentrations (10 nM, data not shown) or developed a morphology typical of senescent cells at moderate concentrations (20 nM) (Fig. 9e). Senescent cells remained relatively unchanged by the bistratene A treatment and still stained blue in the β-galactosidase assay (Fig. 9f). These studies indicate that chronic activation of PKCδ is sufficient to elicit changes typical of cell senescence. However, rottlerin did not restore proliferative competence (data not shown) despite inducing a morphology typical of young cells.
FIG. 9.
PKCδ activity alters senescent-cell morphology. (a to f). Newly plated young (a, c, and e) or senescent (b, d, and f) fibroblasts were treated with dimethyl sulfoxide, 20 μM rottlerin, or 20 nM bistratene A for 10 days in culture, fixed, and assayed for acidic β-galactosidase. Images show typical fields of cells at 100× magnification.
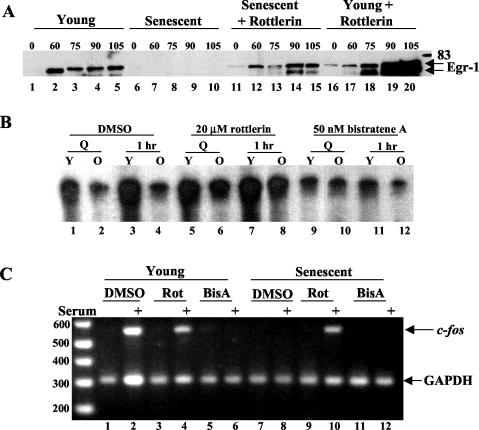
PKCδ inhibitor restores endogenous immediate-early gene expression in senescent cells.
To test whether PKCδ could modulate endogenous immediate-early gene expression, Egr-1 was analyzed after serum stimulation in the absence and presence of rottlerin. As shown in Fig. 10A, rottlerin restored expression of Egr-1 in old cells and enhanced expression in young cells (lanes 11 to 20 versus 1 to 10), suggesting that rottlerin blocked a kinase that negatively impinges on the egr-1 promoter. A similar pattern was seen for c-Fos protein induction (data not shown). Examination of the endogenous SRF binding activity of cells treated with rottlerin and bistratene A showed that these drugs modulated SRF-SRE complex formation in vivo (Fig. 10B), similar to the results seen with recombinant SRF (Fig. 3). Rottlerin restored SRF binding in senescent cells (Fig. 10B lanes 5 to 8), and bistratene A augmented the inhibition of SRF DNA binding (Fig. 10B lanes 9 to 12). Additionally, RT-PCR confirmed that induction of endogenous c-fos (Fig. 10C, lane 10) and egr-1 (data not shown) transcripts were restored in the presence of rottlerin in serum-stimulated senescent cells. However, rottlerin had little effect on c-fos (Fig. 10C, lane 4) or egr-1 (data not shown) serum-induced expression in young cells. These results corroborate those obtained with a dominant negative form of PKCδ (Fig. 8B).
FIG. 10.
Rottlerin restores immediate-early gene expression in senescent fibroblasts. (A) Young and senescent Hs68 cells were serum starved for 48 h prior to stimulation by serum for 60 to 105 min. All cells were treated for 4 h before harvest with 20 μM rottlerin (lanes 11 to 20) or dimethyl sulfoxide vehicle (lanes 1 to 10). Total cellular extract was harvested and used in an Egr-1 Western blot. (B) Young (Y) and senescent (O) Hs68 fibroblasts were serum starved for 48 h prior to stimulation by serum for 60 min. Cells were treated 4 h before harvest with 20 μM rottlerin, 50 nM bistratene A, or dimethyl sulfoxide vehicle. Nuclear extracts from these cells were harvested and used in an SRF-SRE EMSA (C). RNA isolated from parallel plates of cells (described for B) and used as the substrate in RT-PCRS to detect c-fos transcript levels. Glyceraldehhyde-3-phosphate dehydrogenase (GAPDH) served as an internal control for loading, amplification, efficiency, and RNA integrity.
The dramatic restoration of immediate-early gene expression and SRF-SRE binding is consistent with PKCδ's suppressing the c-fos and egr-1 promoters in both young and senescent serum-stimulated cells. Considering that the main promoter element contributing to the serum-induced expression of these genes is the SRE, these data provide strong support for the contention that SRF is a physiologically relevant substrate of PKCδ.
DISCUSSION
Previous studies have shown that the block in c-fos and egr-1 expression in senescent cells is correlated with an inability of SRF to bind to the SRE of immediate-early promoters and that SRF was hyperphosphorylated during senescence. Since these initial observations, we have established that the decline in SRF binding is not due to a reduction of protein levels or nuclear localization in primary fibroblasts. With in vitro assays, we found that the senescent-cell-specific inhibitory activity is a kinase that is sensitive to inhibitors and activators of PKCδ. PKCδ levels appear to be constant with cell age, but PKCδ activity is elevated in senescent cells and appears concomitantly with a 40-kDa PKCδ-CF which is enriched in the nucleus. We also show that SRF is a substrate of PKCδ and that this phosphorylation correlates with the inhibition of SRF-SRE binding.
Mutation of the residue that PKCδ targets (T160) prevents negative regulation, and T160 is phosphorylated in vivo as cells age. Modulation of PKCδ in vivo by both kinase inhibitors and ectopic expression influenced SRE-dependent transcription and was ternary complex factor and ets independent. Activation of PKCδ by bistratene A led to inhibition of SRF binding and to a senescent-cell morphology in young cells. Conversely, immediate-early gene expression and SRF DNA binding activity could be restored in senescent cells by inhibiting PKCδ activity with rottlerin or by ectopic expression of a dominant negative PKCδ, although the restoration was not sufficient to reactivate cell proliferation.
The loss of SRF-DNA binding during senescence is not due to differences in SRF protein level or localization. However, nuclear extracts of senescent cells have a higher basal phosphorylation activity on recombinant SRF, consistent with a previously noted hyperphosphorylation of SRF in aging cells (2). This kinase activity in the nuclear extracts of senescent cells was shown to be responsible for inhibiting SRF DNA binding activity and was dominant when mixtures of young- and senescent-cell nuclear extracts were used. Thus, a kinase activity specific to senescent cells is responsible for the downregulation of SRF DNA binding activity.
Rottlerin was capable of restoring SRF binding activity and immediate-early gene induction in senescent cells, while bistratene A inhibited serum-induced immediate-early gene induction and reduced SRF binding in vitro and in vivo (Fig. 2 and 10). The same compounds affect the hyperphosphorylation of SRF by senescent-cell but not by young-cell nuclear extracts (Fig. 3A). Additionally, SRF T160, which we show to be a direct target of PKCδ, is phosphorylated only in senescent cells and is modulated by rottlerin and bistratene A in vivo (Fig. 7). This supports the idea that PKCδ is responsible for the inhibitory effects on SRF-SRE binding activity during senescence. However, several studies have questioned whether these compounds selectively target PKCδ (6, 46), raising the possibility that they both affected SRF-SRE binding through pathways distinct from PKCδ.
Independent confirmation of data generated with inhibitors was provided by ectopic expression of PKCδ, which was also capable of inhibiting SRE-dependent transcription in young cells (Fig. 8C). Furthermore, expression of dominant negative PKCδ restored SRE-dependent transcription and endogenous immediate-early gene expression in senescent cells (Fig. 8B and D). This mechanism was corroborated by the observation that SRF is a substrate of PKCδ (Fig. 5 and 6) and that phosphorylation by PKCδ leads to inhibition of SRF DNA binding (Fig. 6 and 7). In addition, a general PKC inhibitor also increased the binding of SRF in senescent cells (Fig. 2D). These studies indicate that PKCδ is capable of directly phosphorylating SRF and that this event prevents SRF from interacting with the SRE both in vitro and in vivo.
Phosphorylation of an S/T-F site in SRF is correlated with loss of its DNA binding activity (Fig. 7). Such a site exists in the DNA binding region of the MADS box of SRF (Thr160) and has been shown to be phosphorylated in a myogenic system by calmodulin kinase II (CaMKII) (11). Although other S/T-F sites exist in SRF, the primary sequence surrounding T160 has the closest consensus to a PKCδ substrate (34). Mutation of this site demonstrated that it is the main residue which is phosphorylated by PKCδ in vitro and is the target of negative regulation by PKCδ (Fig. 7). Furthermore, this site is shown to be phosphorylated in vivo and to be modulated by drugs that affect PKCδ activity (Fig. 7). Additionally, previous mutational studies (35) and the crystal structure of the SRF-SRE interaction (36) indicate that this residue is important for the DNA binding activity of SRF. Given its pivotal location in coordinating SRF DNA binding (36), a likely mechanism is that it causes a steric repulsion between the phosphodiester backbone of the SRE and the phospho-T160 SRF, preventing protein-DNA interactions.
PKCδ has a higher basal activity in senescent cells, as measured by Thr-505 PKCδ phosphorylation and a PKCδ immunoprecipitation kinase assay with SRF as a substrate (Fig. 5). Additionally, PKCδ-CF, which is only produced upon activation of PKCδ, is highly enriched in senescent cells (Fig. 4), consistent with the sensitivity of senescent-cell nuclear extracts to the presence of drugs that modulate PKCδ activity (Fig. 2, 3, and 9) and the restoration of immediate-early gene expression by ectopic expression of dominant negative PKCδ (Fig. 8). Constitutive activation of PKCδ may be linked to a lack of Src or Fyn activity impinging upon PKCδ during senescence, since Src family kinases are able to both inactivate and lead to the degradation of PKCδ (4, 8), and we have previously shown that caveolar structure is lost during senescence, leading to the loss of Fyn localization to these structures (51).
Both PKCδ-FL and PKCδ-CF are found in the senescent-cell nucleus (Fig. 4), consistent with reports of an activated 40-kDa catalytic fragment that has been observed after caspase 3 digestion during apoptosis (14). Previous reports identified DNA-dependent protein kinase as a physiological substrate of nuclear PKCδ-CF that is degraded upon phosphorylation (3) and show that an increased leupeptin-sensitive protease activity consistent with caspase 3 exists in senescent fibroblasts (5, 24). Consistent with these observations, DNA-dependent protein kinase activity has been reported to be modified in senescent cells, mainly due to reduced levels of the protein (40). Thus, PKCδ-CF in senescent cells may represent an active soluble kinase that is no longer constrained for available substrates by membrane attachment. The presence of the catalytic fragment of this kinase in the nucleoplasm of senescent cells places it in the right location to directly phosphorylate SRF and to play a role in the process of cell senescence.
This study has confirmed that a kinase activity is responsible for the inhibition of SRF binding activity. Subsequently, it was established that PKCδ is capable of phosphorylating SRF and that this phosphorylation event inhibits SRF-SRE binding activity. Experiments in vivo with PKCδ-specific drugs or independent studies with ectopic expression in the absence of inhibitors establish that both immediate-early gene expression and senescent cell morphology are affected by PKCδ. Thus, it would appear that several aspects of the senescent phenotype can be directly modulated by altering the kinase activity of PKCδ. However, this modulation cannot completely reverse the senescent phenotype, implying that some aspects may be irreversible and that PKCδ is part of the development of a larger “senescence program” most likely initiated by the erosion of telomeric sequences (18, 19, 30).
Acknowledgments
We thank the SACRC hybridoma facility at the University of Calgary for producing polyclonal SRF antibody and acknowledge reagents kindly provided as gifts from D. Watters (bistratene A), M. Gilman (pET19b SRF construct), M. Greenberg (R1122 SRF antibody), R. Prywes (SIE, pm18, and pm12 constructs), and D. Kufe (pKV PKCδ constructs). Special thanks go to S. Benchimol (Medical Biophysics, University of Toronto) for allowing the manuscript to be revised and completed in his laboratory.
REFERENCES
- 1.Abate, C., D. R. Marshak, and T. Curran. 1991. Fos is phosphorylated by p34cdc2, cAMP-dependent protein kinase and protein kinase C at multiple sites clustered within regulatory regions. Oncogene 6:2179-2185. [PubMed] [Google Scholar]
- 2.Atadja, P. W., K. F. Stringer, and K. T. Riabowol. 1994. Loss of serum response element-binding activity and hyperphosphorylation of serum response factor during cellular aging. Mol. Cell. Biol. 14:4991-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bharti, A., S. K. Kraeft, M. Gounder, P. Pandey, S. Jin, Z. M. Yuan, S. P. Lees-Miller, R. Weichselbaum, D. Weaver, L. B. Chen, D. Kufe, and S. Kharbanda. 1998. Inactivation of DNA-dependent protein kinase by protein kinase Cδ: implications for apoptosis. Mol. Cell. Biol. 18:6719-6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blake, R. A., P. Garcia-Paramio, P. J. Parker, and S. A. Courtneidge. 1999. Src promotes PKCδ degradation. Cell Growth Differ. 10:231-241. [PubMed] [Google Scholar]
- 5.Carlin, C., P. D. Phillips, K. Brooks-Frederich, B. B. Knowles, and V. J. Cristofalo. 1994. Cleavage of the epidermal growth factor receptor by a membrane-bound leupeptin-sensitive protease active in nonionic detergent lysates of senescent but not young human diploid fibroblasts. J. Cell Physiol. 160:427-434. [DOI] [PubMed] [Google Scholar]
- 6.Davies, S. P., H. Reddy, M. Caivano, and P. Cohen. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dempsey, E. C., A. C. Newton, D. Mochly-Rosen, A. P. Fields, M. E. Reyland, P. A. Insel, and R. O. Messing. 2000. Protein kinase C isozymes and the regulation of diverse cell responses. Am. J. Physiol. Lung Cell. Mol. Physiol. 279:L429-L438. [DOI] [PubMed] [Google Scholar]
- 8.Denning, M. F., A. A. Dlugosz, D. W. Threadgill, T. Magnuson, and S. H. Yuspa. 1996. Activation of the epidermal growth factor receptor signal transduction pathway stimulates tyrosine phosphorylation of protein kinase C delta. J. Biol. Chem. 271:5325-5331. [DOI] [PubMed] [Google Scholar]
- 9.Dimri, G. P., X. Lee, G. Basile, M. Acosta, G. Scott, C. Roskelley, E. E. Medrano, M. Linskens, I. Rubelj, O. Pereira-Smith, et al. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 92:9363-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding, W., S. Gao, and R. E. Scott. 2001. Senescence represses the nuclear localization of the serum response factor and differentiation regulates its nuclear localization with lineage specificity. J. Cell Sci. 114:1011-1018. [DOI] [PubMed] [Google Scholar]
- 11.Fluck, M., F. W. Booth, and M. N. Waxham. 2000. Skeletal muscle CaMKII enriches in nuclei and phosphorylates myogenic factor SRF at multiple sites. Biochem. Biophys. Res. Commun. 270:488-494. [DOI] [PubMed] [Google Scholar]
- 12.Frey, M. R., O. Leontieva, D. J. Watters, and J. D. Black. 2001. Stimulation of protein kinase C-dependent and -independent signaling pathways by bistratene A in intestinal epithelial cells. Biochem. Pharmacol. 61:1093-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerhard, G. S., P. D. Phillips, and V. J. Cristofalo. 1991. EGF- and PDGF-stimulated phosphorylation in young and senescent WI-38 cells. Exp. Cell Res. 193:87-92. [DOI] [PubMed] [Google Scholar]
- 14.Ghayur, T., M. Hugunin, R. V. Talanian, S. Ratnofsky, C. Quinlan, Y. Emoto, P. Pandey, R. Datta, Y. Huang, S. Kharbanda, H. Allen, R. Kamen, W. Wong, and D. Kufe. 1996. Proteolytic activation of protein kinase C delta by an ICE/CED 3-like protease induces characteristics of apoptosis. J. Exp. Med. 184:2399-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffiths, G., B. Garrone, E. Deacon, P. Owen, J. Pongracz, G. Mead, A. Bradwell, D. Watters, and J. Lord. 1996. The polyether bistratene A activates protein kinase C-delta and induces growth arrest in HL60 cells. Biochem. Biophys. Res. Commun. 222:802-808. [DOI] [PubMed] [Google Scholar]
- 16.Gschwendt, M. 1999. Protein kinase C delta. Eur. J. Biochem. 259:555-564. [DOI] [PubMed] [Google Scholar]
- 17.Gschwendt, M., H. J. Muller, K. Kielbassa, R. Zang, W. Kittstein, G. Rincke, and F. Marks. 1994. Rottlerin, a novel protein kinase inhibitor. Biochem. Biophys. Res. Commun. 199:93-98. [DOI] [PubMed] [Google Scholar]
- 18.Harley, C. B. 2002. Telomerase is not an oncogene. Oncogene 21:494-502. [DOI] [PubMed] [Google Scholar]
- 19.Harley, C. B., A. B. Futcher, and C. W. Greider. 1990. Telomeres shorten during ageing of human fibroblasts. Nature 345:458-460. [DOI] [PubMed] [Google Scholar]
- 20.Hayflick, L. 1965. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 37:614-636. [DOI] [PubMed] [Google Scholar]
- 21.Hayflick, L., and P. S. Moorhead. 1961. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25:585-621. [DOI] [PubMed] [Google Scholar]
- 22.Herbert, J. M., J. M. Augereau, J. Gleye, and J. P. Maffrand. 1990. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 172:993-999. [DOI] [PubMed] [Google Scholar]
- 23.Hill, C. S., J. Wynne, and R. Treisman. 1995. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell 81:1159-1170. [DOI] [PubMed] [Google Scholar]
- 24.Ikebe, T., E. Jimi, M. Beppu, H. Takeuchi, H. Nakayama, and K. Shirasuna. 2000. Aging-dependent proteolysis of NF-kappaB in human fibroblasts. J. Cell Physiol. 182:247-255. [DOI] [PubMed] [Google Scholar]
- 25.Janknecht, R., R. A. Hipskind, T. Houthaeve, A. Nordheim, and H. G. Stunnenberg. 1992. Identification of multiple SRF N-terminal phosphorylation sites affecting DNA binding properties. EMBO J. 11:1045-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Janknecht, R., and T. Hunter. 1997. Activation of the Sap-1a transcription factor by the c-Jun N-terminal kinase (JNK) mitogen-activated protein kinase. J. Biol. Chem. 272:4219-4224. [DOI] [PubMed] [Google Scholar]
- 27.Kim, K., K. Nose, and M. Shibanuma. 2000. Significance of nuclear relocalization of ERK1/2 in reactivation of c-fos transcription and DNA synthesis in senescent fibroblasts. J. Biol. Chem. 275:20685-20692. [DOI] [PubMed] [Google Scholar]
- 28.Kiss, Z., H. Phillips, and W. H. Anderson. 1995. The bisindolylmaleimide GF 109203X, a selective inhibitor of protein kinase C, does not inhibit the potentiating effect of phorbol ester on ethanol-induced phospholipase C-mediated hydrolysis of phosphatidylethanolamine. Biochim. Biophys. Acta 1265:93-95. [DOI] [PubMed] [Google Scholar]
- 29.Le Good, J. A., W. H. Ziegler, D. B. Parekh, D. R. Alessi, P. Cohen, and P. J. Parker. 1998. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science 281:2042-2045. [DOI] [PubMed] [Google Scholar]
- 30.Levy, M. Z., R. C. Allsopp, A. B. Futcher, C. W. Greider, and C. B. Harley. 1992. Telomere end-replication problem and cell aging. J. Mol. Biol. 225:951-960. [DOI] [PubMed] [Google Scholar]
- 31.Manak, J. R., and R. Prywes. 1993. Phosphorylation of serum response factor by casein kinase II: evidence against a role in growth factor regulation of fos expression. Oncogene 8:703-711. [PubMed] [Google Scholar]
- 32.Marais, R. M., J. J. Hsuan, C. McGuigan, J. Wynne, and R. Treisman. 1992. Casein kinase II phosphorylation increases the rate of serum response factor-binding site exchange. EMBO J. 11:97-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyyappan, M., K. Wheaton, and K. T. Riabowol. 1999. Decreased expression and activity of the immediate-early growth response (Egr-1) gene product during cellular senescence. J. Cell Physiol. 179:29-39. [DOI] [PubMed] [Google Scholar]
- 34.Nishikawa, K., A. Toker, F. J. Johannes, Z. Songyang, and L. C. Cantley. 1997. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J. Biol. Chem. 272:952-960. [DOI] [PubMed] [Google Scholar]
- 35.Nurrish, S. J., and R. Treisman. 1995. DNA binding specificity determinants in MADS-box transcription factors. Mol. Cell. Biol. 15:4076-4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pellegrini, L., S. Tan, and T. J. Richmond. 1995. Structure of serum response factor core bound to DNA. Nature 376:490-498. [DOI] [PubMed] [Google Scholar]
- 37.Phillips, P. D., E. Kuhnle, and V. J. Cristofalo. 1983. [125I]EGF binding ability is stable throughout the replicative life-span of WI-38 cells. J. Cell Physiol. 114:311-316. [DOI] [PubMed] [Google Scholar]
- 38.Riabowol, K., J. Schiff, and M. Z. Gilman. 1992. Transcription factor AP-1 activity is required for initiation of DNA synthesis and is lost during cellular aging. Proc. Natl. Acad. Sci. USA 89:157-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivera, V. M., C. K. Miranti, R. P. Misra, D. D. Ginty, R. H. Chen, J. Blenis, and M. E. Greenberg. 1993. A growth factor-induced kinase phosphorylates the serum response factor at a site that regulates its DNA-binding activity. Mol. Cell. Biol. 13:6260-6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salminen, A., M. Helenius, T. Lahtinen, P. Korhonen, T. Tapiola, H. Soininen, and V. Solovyan. 1997. Down-regulation of Ku autoantigen, DNA-dependent protein kinase, and poly(ADP-ribose) polymerase during cellular senescence. Biochem. Biophys. Res. Commun. 238:712-716. [DOI] [PubMed] [Google Scholar]
- 41.Schagger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 42.Sell, C., A. Ptasznik, C. D. Chang, J. Swantek, V. J. Cristofalo, and R. Baserga. 1993. IGF-1 receptor levels and the proliferation of young and senescent human fibroblasts. Biochem. Biophys. Res. Commun. 194:259-265. [DOI] [PubMed] [Google Scholar]
- 43.Seshadri, T., and J. Campisi. 1990. Repression of c-fos transcription and an altered genetic program in senescent human fibroblasts. Science 247:205-209. [DOI] [PubMed] [Google Scholar]
- 44.Shevchenko, A., A. Loboda, W. Ens, B. Schraven, K. G. Standing, H. Schagger, G. von Jagow, and D. W. Cleveland. 2001. Archived polyacrylamide gels as a resource for proteome characterization by mass spectrometry. Electrophoresis 22:1194-1203. [DOI] [PubMed] [Google Scholar]
- 45.Soh, J. W., E. H. Lee, R. Prywes, and I. B. Weinstein. 1999. Novel roles of specific isoforms of protein kinase C in activation of the c-Fos serum response element. Mol. Cell. Biol. 19:1313-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soltoff, S. P. 2001. Rottlerin is a mitochondrial uncoupler that decreases cellular ATP levels and indirectly blocks protein kinase Cδ tyrosine phosphorylation. J. Biol. Chem. 276:37986-37992. [DOI] [PubMed] [Google Scholar]
- 47.Tresini, M., A. Lorenzini, L. Frisoni, R. G. Allen, and V. J. Cristofalo. 2001. Lack of Elk-1 phosphorylation and dysregulation of the extracellular regulated kinase signaling pathway in senescent human fibroblast. Exp. Cell Res. 269:287-300. [DOI] [PubMed] [Google Scholar]
- 48.Wang, Y., M. Falasca, J. Schlessinger, S. Malstrom, P. Tsichlis, J. Settleman, W. Hu, B. Lim, and R. Prywes. 1998. Activation of the c-fos serum response element by phosphatidyl inositol 3-kinase and rho pathways in HeLa cells. Cell Growth Differ. 9:513-522. [PubMed] [Google Scholar]
- 49.Watters, D., B. Garrone, G. Gobert, S. Williams, R. Gardiner, and M. Lavin. 1996. Bistratene A causes phosphorylation of talin and redistribution of actin microfilaments in fibroblasts: possible role for PKC-delta. Exp. Cell Res. 229:327-335. [DOI] [PubMed] [Google Scholar]
- 50.Wheaton, K., P. Atadja, and K. Riabowol. 1996. Regulation of transcription factor activity during cellular aging. Biochem. Cell. Biol. 74:523-534. [DOI] [PubMed] [Google Scholar]
- 51.Wheaton, K., K. Sampsel, F. M. Boisvert, A. Davy, S. Robbins, and K. Riabowol. 2001. Loss of functional caveolae during senescence of human fibroblasts. J. Cell Physiol. 187:226-235. [DOI] [PubMed] [Google Scholar]
- 52.Whitmarsh, A. J., and R. J. Davis. 2000. Regulation of transcription factor function by phosphorylation. Cell. Mol. Life Sci. 57:1172-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wong, H., W. D. Anderson, T. Cheng, and K. T. Riabowol. 1994. Monitoring mRNA expression by polymerase chain reaction: the “primer-dropping” method. Anal. Biochem. 223:251-258. [DOI] [PubMed] [Google Scholar]