Abstract
Direct reprogramming is a promising approach for regenerative medicine whereby one cell type is directly converted into another without going through a multipotent or pluripotent stage. This reprogramming approach has been extensively explored for the generation of functional insulin-secreting cells from non-beta-cells with the aim of developing novel cell therapies for the treatment of people with diabetes lacking sufficient endogenous beta-cells. A common approach for such conversion studies is the introduction of key regulators that are important in controlling beta-cell development and maintenance. In this review, we will summarize the recent advances in the field of beta-cell reprogramming and discuss the challenges of creating functional and long-lasting beta-cells.
Keywords: Direct reprogramming, Cell fate conversion, Beta-cells, Developmental regulators
Introduction
Pancreatic beta-cells play such a central role in regulating blood glucose levels and metabolism that their loss and malfunction lead to diabetes. As of 2014, almost 400 million people in the world suffer from diabetes [1]. Success with strategies to regenerate beta-cells could be of enormous clinical value and has been a key focus of regenerative medicine. Over the years, studies have suggested four major avenues for producing new beta-cells. These include (1) development of beta-cells from putative precursor cells of the adult pancreas, also referred to as neogenesis, (2) replication of existing beta-cells, (3) differentiation from embryonic stem cells or induced pluripotent stem cells (iPS cells), and (4) reprogramming of non-beta to beta-cells. Many excellent reviews have covered the topics of neo-genesis, beta-cell replication, and stem cell-based derivation [2–7]. In this review, we will focus on the recent advances in generating beta-like cells by direct reprogramming.
The term “direct reprogramming” describes direct cell fate conversion from one differentiated cell type into another without going through a multipotent or pluripotent stage [8, 9]. One of the earliest examples of direct reprogramming was the induction of myogenesis by the myogenic master regulator MyoD with the finding that ectopic expression of MyoD directed differentiation of fibroblasts into muscle cells in vitro [10]. The direct reprogramming field has seen rapid advances in recent years. Cells with the characteristics of neurons, cardiomyocytes, vascular cells, and beta-cells have been produced by direct conversion of cultured cells or even cells residing in adult organs [11–13, 14••, 15–22].
One of the first non-beta to beta-cell reprogramming attempts used systemic injection of the transcription factor Pdx1 to direct liver cells toward insulin-producing cells [23]. Since then, generation of insulin+ cells has been reported from various cell populations including pancreatic acinar cells, pancreatic duct cells, pancreatic endocrine alpha- and delta-cells, liver cells, and cells of the gastrointestinal system [11, 14••, 23, 24•, 25••, 26, 27•, 28••] (Fig. 1). Overall, studies of generating beta-like cells by direct reprogramming approaches have focused on starting cell populations of endodermal lineages, which are developmentally related to beta-cells and presumably share epigenetic similarities with beta-cells. Another overarching commonality in beta-cell reprogramming studies is the use of beta-cell master regulators to force cell fate conversion (Fig. 1). Decades of studies on pancreas and beta-cell development have accumulated a great wealth of knowledge about the transcription factors and signaling pathways that govern endocrine and beta-cell fate determination [29–32]. Manipulation of these factors and pathways has since become the dominant method to promote cell fate conversion toward beta-cells. Collectively, these studies have indicated that with appropriate experimental manipulations, some non-beta-cell types can be forced to express insulin and other beta-cell genes. Some studies have documented insulin release and suppression of hyperglycemia in animal models [11, 20, 23, 27•, 28••, 33–39, 40••, 41]. Morphological and ultrastructural remodeling toward beta-cells has also been reported [11, 26, 27•, 37, 39].
Fig. 1.
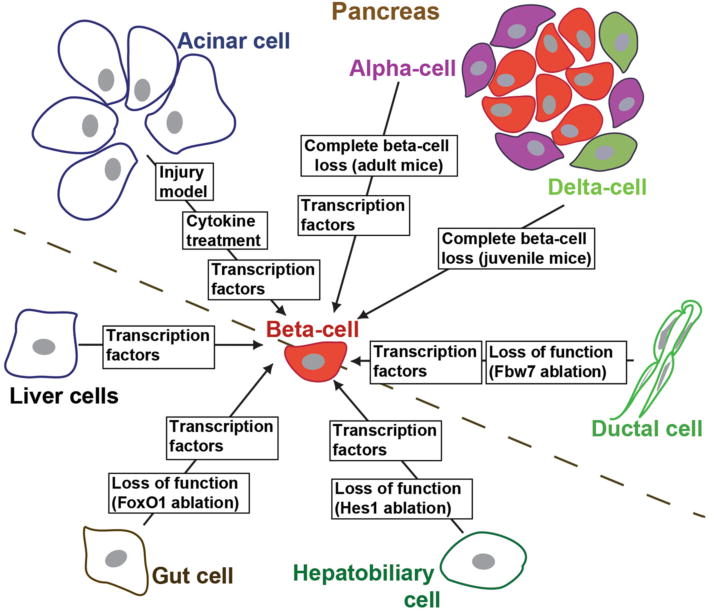
Summary of the parental cell types and induction methods used for direct conversion toward beta-cells
Despite these exciting advances, many challenges remain. For example, it is often unclear whether the converted insulin+ cells have sufficiently extinguished the original cellular program and up-regulated the complete beta-cell program. There is also a lack of understanding on the long-term fate and functionality of the converted beta-like cells, raising the issue of the stability of the acquired cellular state. In this review, we will summarize the current status of the advances and challenges in direct non-beta to beta-cell reprogramming.
Generating Insulin+ Cells by Direct Reprogramming in Animal Models
Liver to Beta-Cell Conversion
Hepatocytes have long been considered a prime target for beta-cell conversion as they display a robust capacity to regenerate and can be easily targeted by systemic delivery of proteins or genetic materials through the blood circulation. Liver is also a permissive site for long-term survival and function of beta-cells as islets delivered into the liver via the portal vein in human islet transplantation can maintain useful insulin production for years [7].
The capacity to activate insulin expression in liver cells was first demonstrated by adenovirus-mediated systemic Pdx1 transgene delivery in mice, which resulted in detection of insulin mRNA and protein in hepatocytes and some amelioration of hyperglycemia in diabetic mice [23]. Despite transient expression of the Pdx1-transgene, long-lasting effects on pancreatic markers were observed, likely through auto-induction of the endogenous Pdx1 [33]. Although initial expression of the Pdx1-transgene occurred in 30–60 % of hepatocytes, only 0.1–1 % stained for insulin [23]. Later studies showed that insulin induction could be substantially enhanced by fusing Pdx1 to the transcriptional activation domain of VP-16 [34, 36, 42] or by additional transcription factor delivery/combinations of Pdx1 with NeuroD, Ngn3, Mafa or Pax4 [35, 36, 43]. When using a constitutively active transgene mouse model overexpressing Pdx1 in hepatocytes, the latter expressed a variety of endocrine hormones such as insulin, glucagon, somatostatin, and pancreatic polypeptide, and the liver tissue displayed abnormal lobe structures and multiple cystic lesions [44]. Besides transcription factors, a role for soluble factors has been suggested as betacellulin augmented hepatocyte to beta-cell reprogramming in liver when combined with ectopic NeuroD-expression [37].
In addition to hepatocytes, other cell types in the liver may also serve as substrates of cell fate conversion. For instance, Ngn3/betacellulin delivery to mouse liver led to emergence of islet-like cell clusters in the periportal regions [38]. Putative liver precursor cells, called oval cells, were suggested to be the cells of origin that had converted to pancreatic endocrine cells expressing individual islet hormones in the liver. Gene delivery by a polycistronic vector containing Pdx1, Ngn3, and Mafa in liver led to formation of duct-like structures expressing beta-cell markers [39]. With this polycistronic construct, insulin-producing cells induced from liver cells seem to have evolved from Sox9+ cells [39, 45].
In summary, a small number of transcription factors and soluble factors have been used to convert liver cells, primarily hepatocytes, toward insulin-producing cells. Although it is clearly feasible to generate such cells in the liver, the degree of similarity of these insulin-producing cells to endogenous beta-cells may vary greatly depending on the method used, and it is not yet clear whether cells with a “normal” beta-cell phenotype have been generated. Direct comparison of the different conversion methods will be valuable to identify the best condition for inducing functional insulin-producing cells. Certainly, the long-term stability and functionality of the induced insulin+ cells from liver require further study.
Acinar to Beta-Cell Conversion
Acinar cells are the predominant cell type in the pancreas, secreting digestive enzymes to facilitate breakdown of nutrients. Only a modest fraction of the acinar cell population is required to sustain its exocrine function, which means that large numbers of acinar cells could be available for reprogramming to beta-cells.
Previously, we have shown that transient expression of the three transcription factors Ngn3, Pdx1, and Mafa in adult mouse pancreas rapidly converts acinar cells to beta-like cells within 10 days [11]. Lineage tracing confirmed that mature acinar cells were the predominant target cells converted into insulin-expressing cells after transcription factor delivery. These insulin-expressing cells co-expressed other key beta-cell markers including Nkx6.1, pro-hormone convertase, and Glut2 (Slc2as), but no other islet hormones. Functionally, the induced cells were sufficient to improve but not normalize glycemic control in diabetic mice [11]. The three reprogramming factors play different roles in the acinar conversion [46]: Ngn3 induces delta-specification, thus promoting establishment of a generic endocrine state at the onset of reprogramming by suppressing acinar fate-regulators and activating islet endocrine genes. The primary function of Mafa and Pdx1, in contrast, appears to promote beta-cell specification while simultaneously suppressing alternative cell fates including those of delta- and alpha-cells. Thus, co-expression of all three factors Pdx1, Ngn3, and Mafa is necessary to induce formation of beta-like cells [46]. In a recent work, we assessed the long-term stability and development of induced insulin+ cells from acinar cells in vivo [40••]. Large numbers of beta-like cells were induced with an optimized induction protocol using a polycistronic construct containing Pdx1, Ngn3, and Mafa [40••]. Reprogrammed insulin-producing cells subsequently aggregated to islet-like clusters and persisted throughout the observation period up to 13 months. Detailed analyses over time revealed that DNA methylation changes occurred mainly within 10 days of induction, whereas full maturation as evidenced by DNA network remodeling and acquisition of regulated insulin release required at least two months to resemble endogenous beta-cells [40••]. Thus besides transcription factors, reprogramming success is determined by a maturation process of reprogrammed cells that appears to be assisted by the in vivo environment.
Using a similar polycistronic construct with Pdx1, Ngn3, and Mafa factors, rapid morphological, molecular, and epigenetic changes toward the beta-cell lineage were shown in the pancreatic exocrine cell line AR42j-B13 [47]. However, the reprogrammed cells in this model lacked some of the important beta-cell genes and did not show glucose-stimulated insulin secretion [47]. The difficulty of converting this acinar cell line may be attributed to the genetic and epigenetic changes associated with its immortalized status or the lack of permissive factors assisting a maturation process in an in vivo environment as suggested above.
In a recent study, it was reported that treatment of adult mice with epidermal growth factor (EGF) and ciliary neurotrophic factor (CNTF) through implanted mini-osmotic pumps also led to appearance of numerous beta-like cells in the pancreas of mice made diabetic with alloxan treatment [20]. At least some of the new insulin-producing cells were shown to derive from acinar cells. How EGF and CNTF can force acinar cells to rewire their transcriptome toward a beta-cell-like identity has yet to be elucidated.
A study using Ptf1a lineage tracing demonstrated that Ptf1a+ acinar cells converted into a ductal phenotype upon pancreatic duct ligation, re-expressed Ngn3, and subsequently differentiated into endocrine cells at low frequency, expressing mature beta-cell markers such as Pdx1, Nkx6.1, and Mafa [48]. This study revealed that acinar cell conversion toward an endocrine and beta-cell phenotype is also possible under certain injury conditions. It will be very interesting to define the specific pathways involved, which could be leveraged to enhance the current acinar reprogramming models.
In summary, acinar to beta-cell reprogramming is a promising approach to generate beta-like cells closely resembling true beta-cells with the potential of long-term survival and function.
Pancreatic Alpha- and Delta-Cell to Beta-Cell Conversion
Pancreatic alpha- and delta-cells are derived from Ngn3+ progenitors, which also give rise to beta-cells. The major physiological role of alpha-cells is secretion of glucagon that counteracts the action of insulin on blood glucose levels. Delta-cells secrete somatostatin, which suppresses insulin-, glucagon-, and pancreatic exocrine secretions; however, its physiological role is not yet clearly understood.
During development, the transcription factors Arx and Pax4 play opposing roles in alpha- and beta-cell subtype specification, with Arx promoting differentiation toward alpha-lineage [49] and Pax4 promoting differentiation toward beta- and delta-lineages [50]. Constitutive loss of Pax4 or ectopic expression of Arx in early pancreatic precursor cells or insulin-expressing cells led to loss of beta-cells and a concomitant increase in the number of alpha-cells [50, 51]. Conversely, constitutive ectopic expression of Pax4 in early pancreatic precursor cells or glucagon-expressing cells resulted in a loss of alpha- and a dramatic increase in insulin-containing cells [18, 41]. It was reported that progenitor cells expressing Ngn3 activated in the ductal lining were responsible for replenishment of glucagon+ cells that eventually converted into beta-cells [18, 41]. However, the newly generated beta-cells were not fully functional, as after a transient hypoglycemic stage animals became hyperglycemic [41]. Another transcription factor involved in alpha- to beta-cell reprogramming is Pdx1. While Pdx1-overexpression neither induced acinar to beta-cell [46] nor alpha- to beta-cell reprogramming [52], forced expression of Pdx1 in Ngn3+ endocrine progenitor cells resulted in their reprogramming to beta-cells following a stage of expression of both glucagon and insulin [52]. This is consistent with Pdx1’s role of repressing the alpha-cell program and maintaining beta-cell identity and function [53]. Also, forced expression of the transcription factor HNF4a in the pancreatic alpha-cell line alpha TC1-9 suppressed glucagon expression and induced key beta-cell markers, enabling these cells to secrete insulin in a glucose-regulated manner [54].
Besides ectopic expression of transcription factors, severe beta-cell loss has been described as another stimulus for alpha- to beta-cell reprogramming: Near complete beta-cell loss by diphtheria-toxin-induced acute selective near-total beta-cell ablation triggered spontaneous alpha- to beta-cell reprogramming [14••]. Shortly after beta-cell ablation, islet remnants contained mostly alpha-cells. This was followed by emergence of islet cells expressing both glucagon and insulin, a feature found throughout the observation period of up to 10 months. By lineage tracing methods, 32–81 % of the new insulin+ cells were shown to originate from alpha-cells. It is possible that such conversion depends upon genetic ablation of beta-cells or other unknowns as another study using the beta-cell toxin streptozocin (STZ) and morphometric quantification of beta- and alpha-cells found no evidence for such a conversion [55].
Using the same diphtheria toxin model of extreme beta-cell loss as described in [14••], an age-dependency of beta-cell reprogramming was shown [24•]. While senescence did not alter alpha-cell plasticity, somatostatin-producing delta-cells replenished beta-cells after ablation before puberty. Beta-cell reconstitution after ablation in juveniles followed a defined sequence of events: delta-cells dedifferentiated as evidenced by loss of somatostatin-expression, replicated as shown by increased Ki67-positivity, and then about half of the progeny activated Ngn3-expression before becoming insulin+. In contrast to alpha- to beta-cell conversion, bihormonal cells were rarely found.
In summary, conversion of alpha- and delta-cells to insulin+ cells can occur by either ectopic expression of specific transcription factors or by near-complete genetic ablation of beta-cells, whereby the cell-of-origin is dependent on the age of the animals. It will be important to determine whether conversion between islet cell types shown in these experimental ablation models can occur spontaneously in any form of diabetic state. Defining the signals that trigger the cell conversion after beta-cell loss should become a fruitful area of investigation.
Pancreatic Ductal to Beta-Cell Conversion
Pancreatic duct cells secrete fluids that enhance the flux of digestive enzymes to the intestine and provide HCO3 for neutralization of gastric acid, thereby providing an optimal pH environment for digestive enzymes. The concept of beta-cell regeneration from ducts has been built upon many observations including the finding of insulin+ cells within or “budding” from the ductal epithelium [56]. Early in pancreatic development, pancreatic buds are composed of multipotent progenitors; later the buds branch out, the branches constituting of endocrine and duct progeny and the tip of multipotent progenitors that later restrict to an exocrine fate [57]. Beta-cell regeneration from ductal epithelium plays an important role in the fetal and neonatal period [58, 59]. In contrast, conflicting data exist on beta-cell generation from duct cells in the adult stage. While some studies using injury models (partial pancreatectomy in rodents [60, 61], pancreatic duct ligation [58, 62–64]) have suggested that postnatal beta-cells can arise from ducts, other studies have provided negative results [65–68]. In contrast to the above-mentioned injury models, a recent study showed that genetic deletion of an ubiquitin ligase, Fbw7, induced pancreatic ductal cells to reprogram into alpha-, delta-, and beta-cells by regulating Ngn3 protein stability [25••]. Also, direct reprogramming of ductal cells toward beta-like phenotypes has been assessed in in vitro studies on primary adult human pancreatic ductal cells [21, 69]. Thus, it seems that adult pancreatic ductal cells may indeed have the potential to give rise to endocrine cells. Future studies may uncover other genes, pathways, and conditions that will help unlock this endocrine potential.
Biliary to Beta-Cell Conversion
The hepatobiliary system consists of the liver, gall bladder, and bile ducts and is involved in the production and transportation of bile besides the manifold metabolic functions of the liver. Extrahepatic biliary duct epithelial cells have been shown to contain endocrine pancreatic hormone-producing cells that arise from the liver domain [70, 71]. It has been reported that in Hes1-deficient mice ectopic endocrine cells could arise from biliary cells [26]. These mice displayed morphological abnormalities of the biliary system such as gallbladder agenesis and severe hypoplasia of extrahepatic bile ducts, but at the same time Ptf1a and Ngn3 were ectopically expressed in the developing biliary epithelium with subsequent differentiation into the pancreatic exocrine, endocrine, and duct cells [26, 72]. These studies indicate that Hes1 determines biliary organogenesis by preventing the pancreatic differentiation program during embryogenesis.
To convert adult biliary cells toward beta-cells, transcription factors Pdx1, NeuroD, or Pdx1-VP16 were ectopically expressed in intrahepatic biliary epithelial cells cultured in a novel collagen matrix, whereupon cells started to express beta-cell genes as well as other islet hormones including glucagon and somatostatin [73]. Similarly, primary mouse gall bladder epithelial cells transfected with Ngn3, Pdx1, and Mafa and cultured in media supplemented with retinoic acid and a notch inhibitor resulted in induction of pancreatic endocrine genes [74]. However, the reprogrammed insulin+ cells from the gall bladder were not fully mature, as they had a polyhormonal phenotype and were not responsive to glucose stimulation [74].
In summary, forced expression of transcription factors can also lead to some adoption of a beta-like phenotype by cultured hepatobiliary cells. The maturation of these cells, however, seems to be incomplete.
Gut Enteroendocrine to Beta-Cell conversion
Gastrointestinal endocrine cells release different gastrointestinal hormones that regulate digestive actions, metabolism, and a myriad of other functions. The intestinal epithelium is an attractive tissue for beta-cell conversion as adult intestine stem cells residing in the crypts have the potential of self-renewal and for generating a variety of cell types, including hormone-producing enteroendocrine cells [75].
During embryonic development, it has been shown that overexpression of Ptf1a in transgenic Xenopustadpoles leads to formation of ectopic pancreatic tissues from the stomach and duodenum [76]. Whether a similar strategy could be used in adult gastrointestinal tissue transformation is not clear. Using the IEC-6 (Immature rat intestinal stem cells) cell line as a model, two groups have reported formation of insulin-containing cells by either sequential transfection with Pdx-1 and exposure to betacellulin, or transfection with Pdx-1 and the LIM homeodomain transcription factor Islet-1 [77, 78]. However, the induced insulin+ cells did not display glucose-stimulated insulin secretion. Recently, it was reported that transient expression of combined Ngn3, Pdx1, and Mafa transcription factors in adult mouse intestine promoted the conversion of intestinal crypt cells into endocrine cells, which coalesced into “neo-islets” below the crypt base [27•]. These cells showed some level of glucose-responsiveness and partially rescued STZ-treated animals from hyperglycemia. This study suggests that adult intestinal cells could be induced to become beta-like cells with appropriate reprogramming factors.
In addition to the gain-of-function approaches to induce gut cell conversion toward beta-cells, FoxO1 ablation in Ngn3+ gut endocrine progenitor cells led to formation of insulin+ cells in the gut that exhibited glucose-stimulated insulin secretion [28••]. Although these induced insulin+ cells from the gut are individually short-lived, their continuous generation from the gut epithelium allowed for long-term suppression of hyperglycemia in animal models [28••]. These studies provided an exciting new direction of generating functional insulin+ cells from the pool of enteroendocrine cells with the potential advantage of the constantly self-renewing nature of the gut stem cells.
Generating Insulin+ Cells by Direct Reprogramming Using Human Tissues
Liver to Beta-Cell Conversion
Similar to animal models, the first attempt of converting non-beta-cells to beta-cells with human tissues was carried out with liver cells. Pdx1-VP16 transfection of the human liver cell line HepG2 resulted in cells that resembled both pancreatic exocrine and endocrine cells [42]. Hepatocytes from adult and fetal liver were cultured and infected with a recombinant adeno- or lentivirus containing rat Pdx1, whereupon they started to produce insulin, store it in granules and release it in response to glucose [79, 80]. When transplanted under the renal capsule of diabetic immunodeficient mice, the reprogrammed liver cells ameliorated hyperglycemia [79, 80]. The reprogramming yield could substantially be increased when human liver cells were transfected with the three pancreatic transcription factors Pdx1, Pax4, and Mafa as opposed to only Pdx1 [43]. Although some change of phenotype has been found with reprogramming of human hepatocytes, no reports show that it is possible to generate insulin-producing cells that have a close resemblance to true beta-cells.
Pancreatic Alpha-Cell to Beta-Cell Conversion
Based on distinct histone methylation signatures between human alpha- and beta-cells, treatment of cultured pancreatic islets with a histone methyltransferase inhibitor led to co-localization of glucagon and insulin or Pdx1, respectively, in human islets and co-localization of glucagon and insulin in mouse islets by immunohistochemistry. Although the conversion from alpha- to beta-cell is far from complete, this study provided an important proof-of principle that epigenetic manipulation could significantly facilitate conversion between human islet cell phenotypes [81].
Pancreatic Ductal to Beta-Cell Conversion
Adenoviral transduction with the four transcription factors Pdx1, Ngn3, MafA, and Pax4 of human exocrine-enriched cells that had undergone epithelial-to-mesenchymal transition resulted in conversion to alpha-like cells expressing high levels of glucagon but little insulin [82]. Reprogramming toward beta-like cells was enhanced by using unpassaged freshly cultured exocrine cells in combination with Chromatin-modifying agents and was suggested to occur from ductal cells as no evidence of acinar origin was found with lineage tracing [82]. The resultant cells secreted insulin in response to glucose and ameliorated hyperglycemia when grafted into immune-deficient STZ-induced diabetic mice. Reprogramming of FACS-sorted adult human pancreatic duct cells to beta-like insulin-secreting cells has also been accomplished with a combination of adenoviruses expressing Pdx1, Ngn3, MafA, and Pax6 instead of Pax4 [21]. These studies provide evidence that direct reprogramming of human pancreatic duct cells with defined transcription factors might serve as a new strategy for beta-cell replacement therapy in diabetes.
Gut Enteroendocrine to Beta-Cell Conversion
Following upon the animal studies, the role of FoxO1 in converting gut enteroendocrine cells toward beta-cells was tested with embryonic stem cell-derived human gut tissues. FoxO1 was shown to be expressed in human gut endocrine progenitor and serotonin-producing cells. Using gut organoids, FoxO1-inhibition by dominant-negative mutant or lentivirus-encoded shRNA resulted in generation of insulin+ cells that expressed markers of mature beta-cells such as Mafa or Ucn3, and released C-peptide in response to secretagogues [83]. Also, overexpression of Ngn3, Pdx1, and Mafa in organoids derived from human pluripotent stem cells enabled a beta-cell-like conversion similar to the one observed in the mouse model [27•]. Currently, it is unknown, whether the two approaches described, FoxO1 loss-of-function and Ngn3, Pdx1, Mafa gain-of-function act through similar mechanisms or even the same cell type, and how close the products are or compare to each other.
Conclusion
There are many potential ways to derive beta-like cells from different sources: Replication of beta-cells is the main process for beta-cell expansion in postnatal mice [84, 85], after beta-cell loss [86], and in pregnancy [87]. Beta-cell neogenesis, thought to originate from exocrine cells, contributes to beta-cell expansion during the neonatal period [58, 59] and potentially during adult life. Beta-cells derived in vitro from embryonic and induced pluripotent stem cells are a potentially unlimited source of replacement cells, and progress is being made in improving differentiation and functionality in vitro [88••, 89••]. While intense work continues on multiple fronts, direct reprogramming between differentiated cell types offers a promising alternative strategy for beta-cell regeneration. The reprogramming approach offers a potentially rapid method at deriving patient-specific beta-cells with low risk of tumor formation.
Numerous challenges have to be addressed to achieve greater success with direct reprogramming. Reprogrammed cells should ideally adopt a stable and mature phenotype without any hybrid characteristics [90]. In the case of acinar to beta-cell reprogramming, an initial transition through an immature state carries a risk of hypoglycemia [40••]. However, with further maturation, the reprogrammed cells assumed a long-term stable phenotype similar to true beta-cells [40••]. Tumor formation, which is a safety issue with iPS-cell reprogramming, has not yet been reported in direct reprogramming and might not be a problem because cells are interconverted directly between differentiated cell types without going through a multipotent or pluripotent state. The direct reprogramming approach relies heavily on ectopic expression of transcription factors using viral delivery vehicles. To facilitate translation of the direct reprogramming approach toward clinics, it is important to identify safer strategies to reprogram cells, such as the use of small molecules, protein transduction, and RNA transduction, with non-viral delivery methods.
Thus, while many challenges remain, rapid and promising progress has been made in recent years on generating beta-cells with the direct reprogramming approach. A solid foundation of knowledge on different reprogramming aspects is now in place. We are optimistic that the reprogramming approach will make rapid advances in the near future and prove to be a serious contender for cell replacement therapies for diabetes.
Acknowledgments
Harvard University has received grants from the Juvenile Diabetes Research Foundation, National Institute of Diabetes, Digestive and Kidney Diseases and Harvard Stem Cell Institute not related to this article.
Footnotes
Conflict of Interest Claudia Cavelti-Weder, Weida Li, Adrian Zumsteg, Marianne Stemann, Takatsugu Yamada, Susan Bonner-Weir, Gordon Weir, and Qiao Zhou declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Claudia Cavelti-Weder, Email: claudia.cavelti-weder@usb.ch, Section on Islet Cell and Regenerative Biology, Joslin Diabetes Center, Boston, MA, USA.
Weida Li, Email: weidali@mcb.harvard.edu, Department of Stem Cell and Regenerative Biology, Harvard University, Cambridge, MA, USA.
Adrian Zumsteg, Email: adrian.zumsteg@gmail.com, Department of Stem Cell and Regenerative Biology, Harvard University, Cambridge, MA, USA.
Marianne Stemann, Email: marianne.stemann@bric.ku.dk, Section on Islet Cell and Regenerative Biology, Joslin Diabetes Center, Boston, MA, USA.
Takatsugu Yamada, Email: highnet@naramed-u.ac.jp, Section on Islet Cell and Regenerative Biology, Joslin Diabetes Center, Boston, MA, USA.
Susan Bonner-Weir, Email: susan-bonner-weir@joslin.harvard.edu, Section on Islet Cell and Regenerative Biology, Joslin Diabetes Center, Boston, MA, USA.
Gordon Weir, Email: gordon.weir@joslin.harvard.edu, Section on Islet Cell and Regenerative Biology, Joslin Diabetes Center, Boston, MA, USA.
Qiao Zhou, Email: qiao_zhou@harvard.edu, Department of Stem Cell and Regenerative Biology, Harvard University, Cambridge, MA, USA.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.http://www.idf.org/diabetesatlas/update-2014
- 2.Bonner-Weir S, Guo L, Li WC, Ouziel-Yahalom L, Lysy PA, Weir GC, et al. Islet neogenesis: a possible pathway for beta-cell replenishment. Rev Diabet Stud. 2012;9(4):407–416. doi: 10.1900/RDS.2012.9.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hebrok M. Generating beta cells from stem cells-the story so far. Cold Spring Harb Perspect Med. 2012;2(6):a007674. doi: 10.1101/cshperspect.a007674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nostro MC, Keller G. Generation of beta cells from human pluripotent stem cells: potential for regenerative medicine. Semin Cell Dev Biol. 2012;23(6):701–710. doi: 10.1016/j.semcdb.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pagliuca FW, Melton DA. How to make a functional beta-cell. Development. 2013;140(12):2472–2483. doi: 10.1242/dev.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiesser JV, Wells JM. Generation of beta cells from human pluripotent stem cells: are we there yet? Ann N Y Acad Sci. 2014;1311:124–137. doi: 10.1111/nyas.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weir GC, Bonner-Weir S. Islet beta cell mass in diabetes and how it relates to function, birth, and death. Ann N Y Acad Sci. 2013;1281:92–105. doi: 10.1111/nyas.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graf T. Historical origins of transdifferentiation and reprogramming. Cell Stem Cell. 2011;9(6):504–516. doi: 10.1016/j.stem.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Vierbuchen T, Wernig M. Direct lineage conversions: unnatural but useful? Nat Biotechnol. 2011;29(10):892–907. doi: 10.1038/nbt.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455(7213):627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142(3):375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14••.Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, Chera S, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature. 2010;464(7292):1149–1154. doi: 10.1038/nature08894. This is the first study to show that alpha-cells can convert towards beta-cells after genetic ablation of majority of endogenous beta-cells in adult mouse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caiazzo M, Dell’Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476(7359):224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- 16.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485(7400):599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginsberg M, James D, Ding BS, Nolan D, Geng F, Butler JM, et al. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFbeta suppression. Cell. 2012;151(3):559–575. doi: 10.1016/j.cell.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Hasani K, Pfeifer A, Courtney M, Ben-Othman N, Gjernes E, Vieira A, et al. Adult duct-lining cells can reprogram into beta-like cells able to counter repeated cycles of toxin-induced diabetes. Dev Cell. 2013;26(1):86–100. doi: 10.1016/j.devcel.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Guo Z, Zhang L, Wu Z, Chen Y, Wang F, Chen G. In Vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell. 2014;14(2):188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baeyens L, Lemper M, Leuckx G, De Groef S, Bonfanti P, Stange G, et al. Transient cytokine treatment induces acinar cell reprogramming and regenerates functional beta cell mass in diabetic mice. Nat Biotechnol. 2014;32(1):76–83. doi: 10.1038/nbt.2747. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Lee J, Sugiyama T, Liu Y, Wang J, Gu X, Lei J, Markmann JF, Miyazaki S, Miyazaki J, Szot GL, Bottino R, Kim SK. Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. Elife. 2013;2:e00940. doi: 10.7554/eLife.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su Z, Niu W, Liu ML, Zou Y, Zhang CL. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun. 2014;5:3338. doi: 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6(5):568–572. doi: 10.1038/75050. [DOI] [PubMed] [Google Scholar]
- 24•.Chera S, Baronnier D, Ghila L, Cigliola V, Jensen JN, Gu G, Furuyama K, Thorel F, Gribble FM, Reimann F, Herrera PL. Diabetes recovery by age-dependent conversion of pancreatic δ-cells into insulin producers. Nature. 2014;514(7523):503–507. doi: 10.1038/nature13633. Using the same method as in reference 14, this study showed that extreme genetic ablation of beta-cells before puberty harnesses new beta-cells from conversion of delta-cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Sancho R, Gruber R, Gu G, Behrens A. Loss of Fbw7 reprograms adult pancreatic ductal cells into alpha, delta, and beta cells. Cell Stem Cell. 2014;15(2):139–153. doi: 10.1016/j.stem.2014.06.019. In contrast to injury models with conflicting results regarding the ductal origin of beta-cells in the adult, this study showed that genetic deletion of Fbw7 led to conversion of adult pancreatic ductal cells to alpha-, delta-, and beta-cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sumazaki R, Shiojiri N, Isoyama S, Masu M, Keino-Masu K, Osawa M, et al. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat Genet. 2004;36(1):83–87. doi: 10.1038/ng1273. [DOI] [PubMed] [Google Scholar]
- 27•.Chen YJ, Finkbeiner SR, Weinblatt D, Emmett MJ, Tameire F, Yousefi M, et al. De novo formation of insulin-producing “neo-beta cell islets” from intestinal crypts. Cell Rep. 2014;6(6):1046–1058. doi: 10.1016/j.celrep.2014.02.013. This study shows that gain-of-function by Ngn3, Pdx1, and Mafa promoted the conversion of intestinal cells into beta-like cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Talchai C, Xuan S, Kitamura T, DePinho RA, Accili D. Generation of functional insulin-producing cells in the gut by Foxo1 ablation. Nat Genet. 2012;44(4):406–412. S1. doi: 10.1038/ng.2215. This is the first demonstration that genetic ablation of FoxO1 can induce formation of insulin+ cells from the endocrine progenitors of mouse intestine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wells JM, Melton DA. Vertebrate endoderm development. Annu Rev Cell Dev Biol. 1999;15:393–410. doi: 10.1146/annurev.cellbio.15.1.393. [DOI] [PubMed] [Google Scholar]
- 30.Murtaugh LC, Melton DA. Genes, signals, and lineages in pancreas development. Annu Rev Cell Dev Biol. 2003;19:71–89. doi: 10.1146/annurev.cellbio.19.111301.144752. [DOI] [PubMed] [Google Scholar]
- 31.Gittes GK. Developmental biology of the pancreas: a comprehensive review. Dev Biol. 2009;326(1):4–35. doi: 10.1016/j.ydbio.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 32.Avolio F, Pfeifer A, Courtney M, Gjernes E, Ben-Othman N, Vieira A, et al. From pancreas morphogenesis to beta-cell regeneration. Curr Top Dev Biol. 2013;106:217–238. doi: 10.1016/B978-0-12-416021-7.00006-7. [DOI] [PubMed] [Google Scholar]
- 33.Ber I, Shternhall K, Perl S, Ohanuna Z, Goldberg I, Barshack I, et al. Functional, persistent, and extended liver to pancreas transdifferentiation. J Biol Chem. 2003;278(34):31950–31957. doi: 10.1074/jbc.M303127200. [DOI] [PubMed] [Google Scholar]
- 34.Imai J, Katagiri H, Yamada T, Ishigaki Y, Ogihara T, Uno K, et al. Constitutively active PDX1 induced efficient insulin production in adult murine liver. Biochem Biophys Res Commun. 2005;326(2):402–409. doi: 10.1016/j.bbrc.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 35.Kaneto H, Matsuoka TA, Nakatani Y, Miyatsuka T, Matsuhisa M, Hori M, et al. A crucial role of MafA as a novel therapeutic target for diabetes. J Biol Chem. 2005;280(15):15047–15052. doi: 10.1074/jbc.M412013200. [DOI] [PubMed] [Google Scholar]
- 36.Kaneto H, Nakatani Y, Miyatsuka T, Matsuoka TA, Matsuhisa M, Hori M, et al. PDX-1/VP16 fusion protein, together with NeuroD or Ngn3, markedly induces insulin gene transcription and ameliorates glucose tolerance. Diabetes. 2005;54(4):1009–1022. doi: 10.2337/diabetes.54.4.1009. [DOI] [PubMed] [Google Scholar]
- 37.Kojima H, Fujimiya M, Matsumura K, Younan P, Imaeda H, Maeda M, et al. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med. 2003;9(5):596–603. doi: 10.1038/nm867. [DOI] [PubMed] [Google Scholar]
- 38.Yechoor V, Liu V, Espiritu C, Paul A, Oka K, Kojima H, et al. Neurogenin3 is sufficient for transdetermination of hepatic progenitor cells into neo-islets in vivo but not transdifferentiation of hepatocytes. Dev Cell. 2009;16(3):358–373. doi: 10.1016/j.devcel.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banga A, Akinci E, Greder LV, Dutton JR, Slack JM. In vivo reprogramming of Sox9+ cells in the liver to insulin-secreting ducts. Proc Natl Acad Sci USA. 2012;109(38):15336–15341. doi: 10.1073/pnas.1201701109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Li W, Cavelti-Weder C, Zhang Y, Clement K, Donovan S, Gonzalez G, et al. Long-term persistence and development of induced pancreatic beta cells generated by lineage conversion of acinar cells. Nat Biotechnol. 2014;32(12):1223–1230. doi: 10.1038/nbt.3082. This is the first study on the long-term fate and development of lineage-converted beta cells. [DOI] [PubMed] [Google Scholar]
- 41.Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138(3):449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horb ME, Shen CN, Tosh D, Slack JM. Experimental conversion of liver to pancreas. Curr Biol. 2003;13(2):105–115. doi: 10.1016/s0960-9822(02)01434-3. [DOI] [PubMed] [Google Scholar]
- 43.Berneman-Zeitouni D, Molakandov K, Elgart M, Mor E, Fornoni A, Dominguez MR, et al. The temporal and hierarchical control of transcription factors-induced liver to pancreas transdifferentiation. PLoS ONE. 2014;9(2):e87812. doi: 10.1371/journal.pone.0087812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyatsuka T, Kaneto H, Kajimoto Y, Hirota S, Arakawa Y, Fujitani Y, et al. Ectopically expressed PDX-1 in liver initiates endocrine and exocrine pancreas differentiation but causes dysmorphogenesis. Biochem Biophys Res Commun. 2003;310(3):1017–1025. doi: 10.1016/j.bbrc.2003.09.108. [DOI] [PubMed] [Google Scholar]
- 45.Yang Y, Akinci E, Dutton JR, Banga A, Slack JM. Stage specific reprogramming of mouse embryo liver cells to a beta cell-like phenotype. Mech Dev. 2013;130(11–12):602–612. doi: 10.1016/j.mod.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li W, Nakanishi M, Zumsteg A, Shear M, Wright C, Melton DA, et al. In vivo reprogramming of pancreatic acinar cells to three islet endocrine subtypes. eLife. 2014;3:e01846. doi: 10.7554/eLife.01846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akinci E, Banga A, Greder LV, Dutton JR, Slack JM. Reprogramming of pancreatic exocrine cells towards a beta (beta) cell character using Pdx1, Ngn3 and MafA. Biochem J. 2012;442(3):539–550. doi: 10.1042/BJ20111678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan FC, Bankaitis ED, Boyer D, Xu X, Van de Casteele M, Magnuson MA, et al. Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development. 2013;140(4):751–764. doi: 10.1242/dev.090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, et al. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 2003;17(20):2591–2603. doi: 10.1101/gad.269003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386(6623):399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- 51.Collombat P, Hecksher-Sorensen J, Krull J, Berger J, Riedel D, Herrera PL, et al. Embryonic endocrine pancreas and mature beta cells acquire alpha and PP cell phenotypes upon Arx misexpression. J Clin Invest. 2007;117(4):961–970. doi: 10.1172/JCI29115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang YP, Thorel F, Boyer DF, Herrera PL, Wright CV. Context-specific alpha- to-beta-cell reprogramming by forced Pdx1 expression. Genes Dev. 2011;25(16):1680–1685. doi: 10.1101/gad.16875711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao T, McKenna B, Li C, Reichert M, Nguyen J, Singh T, et al. Pdx1 maintains beta cell identity and function by repressing an alpha cell program. Cell Metab. 2014;19(2):259–271. doi: 10.1016/j.cmet.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sangan CB, Jover R, Heimberg H, Tosh D. In vitro reprogramming of pancreatic alpha cells towards a beta cell phenotype following ectopic HNF4 alpha expression. Mol Cell Endocrinol. 2014;399C:50–59. doi: 10.1016/j.mce.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 55.Cavelti-Weder C, Shtessel M, Reuss JE, Jermendy A, Yamada T, Caballero F, et al. Pancreatic duct ligation after almost complete beta-cell loss: exocrine regeneration but no evidence of beta-cell regeneration. Endocrinology. 2013;154(12):4493–4502. doi: 10.1210/en.2013-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonner-Weir S, Li WC, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A. Beta-cell growth and regeneration: replication is only part of the story. Diabetes. 2010;59(10):2340–2348. doi: 10.2337/db10-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Q, Law AC, Rajagopal J, Anderson WJ, Gray PA, Melton DA. A multipotent progenitor domain guides pancreatic organogenesis. Dev Cell. 2007;13(1):103–114. doi: 10.1016/j.devcel.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 58.Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci USA. 2008;105(50):19915–19919. doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakamura K, Minami K, Tamura K, Iemoto K, Miki T, Seino S. Pancreatic beta-cells are generated by neogenesis from non-beta-cells after birth. Biomed Res. 2011;32(2):167–174. doi: 10.2220/biomedres.32.167. [DOI] [PubMed] [Google Scholar]
- 60.Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE. A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes. 1993;42(12):1715–1720. doi: 10.2337/diab.42.12.1715. [DOI] [PubMed] [Google Scholar]
- 61.Li WC, Rukstalis JM, Nishimura W, Tchipashvili V, Habener JF, Sharma A, et al. Activation of pancreatic-duct-derived progenitor cells during pancreas regeneration in adult rats. J Cell Sci. 2010;123(Pt 16):2792–2802. doi: 10.1242/jcs.065268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van de Casteele M, Leuckx G, Baeyens L, Cai Y, Yuchi Y, Coppens V, et al. Neurogenin 3(+) cells contribute to beta-cell neogenesis and proliferation in injured adult mouse pancreas. Cell Death Dis. 2013;4:e523. doi: 10.1038/cddis.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang RN, Klöppel G, Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia. 1995;38(12):1405–1411. doi: 10.1007/BF00400600. [DOI] [PubMed] [Google Scholar]
- 64.Xu X, D’Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132(2):197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 65.Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43(1):34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 66.Kopinke D, Murtaugh LC. Exocrine-to-endocrine differentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC Dev Biol. 2010;10:38. doi: 10.1186/1471-213X-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, et al. Sox9 + ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138(4):653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Solar M, Cardalda C, Houbracken I, Martin M, Maestro MA, De Medts N, et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell. 2009;17(6):849–860. doi: 10.1016/j.devcel.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 69.Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, et al. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA. 2000;97(14):7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dutton JR, Chillingworth NL, Eberhard D, Brannon CR, Hornsey MA, Tosh D, et al. Beta cells occur naturally in extrahepatic bile ducts of mice. J Cell Sci. 2007;120(Pt 2):239–245. doi: 10.1242/jcs.03330. [DOI] [PubMed] [Google Scholar]
- 71.Eberhard D, Tosh D, Slack JM. Origin of pancreatic endocrine cells from biliary duct epithelium. Cell Mol Life Sci. 2008;65(21):3467–3480. doi: 10.1007/s00018-008-8427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fukuda A, Kawaguchi Y, Furuyama K, Kodama S, Horiguchi M, Kuhara T, et al. Ectopic pancreas formation in Hes1 -knockout mice reveals plasticity of endodermal progenitors of the gut, bile duct, and pancreas. J Clin Invest. 2006;116(6):1484–1493. doi: 10.1172/JCI27704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nagaya M, Katsuta H, Kaneto H, Bonner-Weir S, Weir GC. Adult mouse intrahepatic biliary epithelial cells induced in vitro to become insulin-producing cells. J Endocrinol. 2009;201(1):37–47. doi: 10.1677/JOE-08-0482. [DOI] [PubMed] [Google Scholar]
- 74.Hickey RD, Galivo F, Schug J, Brehm MA, Haft A, Wang Y, et al. Generation of islet-like cells from mouse gall bladder by direct ex vivo reprogramming. Stem Cell Res. 2013;11(1):503–515. doi: 10.1016/j.scr.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154(2):274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 76.Jarikji ZH, Vanamala S, Beck CW, Wright CV, Leach SD, Horb ME. Differential ability of Ptf1a and Ptf1a-VP16 to convert stomach, duodenum and liver to pancreas. Dev Biol. 2007;304(2):786–799. doi: 10.1016/j.ydbio.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kojima H, Nakamura T, Fujita Y, Kishi A, Fujimiya M, Yamada S, et al. Combined expression of pancreatic duodenal homeobox 1 and islet factor 1 induces immature enterocytes to produce insulin. Diabetes. 2002;51(5):1398–1408. doi: 10.2337/diabetes.51.5.1398. [DOI] [PubMed] [Google Scholar]
- 78.Yoshida S, Kajimoto Y, Yasuda T, Watada H, Fujitani Y, Kosaka H, et al. PDX-1 induces differentiation of intestinal epithelioid IEC-6 into insulin-producing cells. Diabetes. 2002;51(8):2505–2513. doi: 10.2337/diabetes.51.8.2505. [DOI] [PubMed] [Google Scholar]
- 79.Sapir T, Shternhall K, Meivar-Levy I, Blumenfeld T, Cohen H, Skutelsky E, et al. Cell-replacement therapy for diabetes: generating functional insulin-producing tissue from adult human liver cells. Proc Natl Acad Sci U S A. 2005;102(22):7964–7969. doi: 10.1073/pnas.0405277102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zalzman M, Gupta S, Giri RK, Berkovich I, Sappal BS, Karnieli O, et al. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proc Natl Acad Sci USA. 2003;100(12):7253–7258. doi: 10.1073/pnas.1136854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bramswig NC, Everett LJ, Schug J, Dorrell C, Liu C, Luo Y, et al. Epigenomic plasticity enables human pancreatic alpha to beta cell reprogramming. J Clin Invest. 2013;123(3):1275–1284. doi: 10.1172/JCI66514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lima MJ, Muir KR, Docherty HM, Drummond R, McGowan NW, Forbes S, et al. Suppression of epithelial-to-mesenchymal transitioning enhances ex vivo reprogramming of human exocrine pancreatic tissue toward functional insulin-producing beta-like cells. Diabetes. 2013;62(8):2821–2833. doi: 10.2337/db12-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bouchi R, Foo KS, Hua H, Tsuchiya K, Ohmura Y, Sandoval PR, et al. FOXO1 inhibition yields functional insulin-producing cells in human gut organoid cultures. Nat commun. 2014;5:4242. doi: 10.1038/ncomms5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429(6987):41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 85.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12(5):817–826. doi: 10.1016/j.devcel.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 86.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest. 2007;117(9):2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parsons JA, Brelje TC, Sorenson RL. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology. 1992;130(3):1459–1466. doi: 10.1210/endo.130.3.1537300. [DOI] [PubMed] [Google Scholar]
- 88••.Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O’Dwyer S, Quiskamp N, Mojibian M, Albrecht T, Yang YH, Johnson JD, Kieffer TJ. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32(11):1121–1133. doi: 10.1038/nbt.3033. These two studies identified in vitro differentiation conditions for generating functional beta cells from human pluripotent stem cells. [DOI] [PubMed] [Google Scholar]
- 89••.Pagliuca FW, Millman JR, Gurtler M, Segel M, Van Dervort A, Ryu JH, et al. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159(2):428–439. doi: 10.1016/j.cell.2014.09.040. These two studies identified in vitro differentiation conditions for generating functional beta cells from human pluripotent stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou Q, Melton DA. Extreme makeover: converting one cell into another. Cell Stem Cell. 2008;3(4):382–388. doi: 10.1016/j.stem.2008.09.015. [DOI] [PubMed] [Google Scholar]


