Abstract
Phosphatidylinositol-3,4,5-triphosphate (PtdIns(3,4,5)P3) is one of the most important phosphoinositides and is capable of activating a wide range of proteins through its interaction with their specific binding domains. Localization and activation of these effector proteins regulate a number of cellular functions, including cell survival, proliferation, cytoskeletal rearrangement, intracellular vesicle trafficking, and cell metabolism. Phosphoinositides have been investigated as an important agonist-dependent second messenger in the regulation of diverse physiological events depending upon the phosphorylation status of their inositol group. Dysregulation in formation as well as metabolism of phosphoinositides is associated with various pathophysiological disorders such as inflammation, allergy, cardiovascular diseases, cancer, and metabolic diseases. Recent studies have demonstrated that the impaired metabolism of PtdIns(3,4,5)P3 is a prime mediator of insulin resistance associated with various metabolic diseases including obesity and diabetes. This review examines the current status of the role of PtdIns(3,4,5)P3 signaling in the regulation of various cellular functions and the implications of dysregulated PtdIns(3,4,5)P3 signaling in obesity, diabetes, and their associated complications.
Keywords: PtdIns(3,4,5)P3; PI3K/PTEN equilibria; PH domain-containing proteins; Insulin resistance; Cardiovascular complications; Vascular inflammation
Introduction
Since their discovery in the mid 1950s by Hokin and Hokin [1], phosphoinositides (PI), the phosphorylated derivatives of membrane lipid phosphatidylinositol (PtdIns), have become established as important mediators of a number of signal transduction pathways [2]. They exert their function either indirectly as the precursor of second messengers, such as inositol-1,4,5-triphosphate (Ins(1,4,5)P3) and diacylglycerol (DAG), or directly by interacting with downstream effector protein molecules and thereby orchestrating the spatio-temporal organization of intracellular signal transduction cascades [3-5]. Recent studies demonstrate the role of PI in a variety of physiological processes, including cell proliferation, survival, cytoskeletal regulation, intracellular vesicle trafficking, and cell metabolism [2, 4]. As a result, the enzymes involved in both their formation and their metabolism have become more popular as the subjects of biomedical research, since any alteration to them is linked to the development of pathophysiological disorders [6].
The inositol head group of PtdIns contains five free hydroxyl groups, of which only three have been found to be phosphorylated in mammalian cells [6]. PtdIns constitutes approximately 5-10% of the total lipid in eukaryotic cells membranes, 5% of which is phosphorylated at the 4′-position, and the other 5% of which is phosphorylated at the 4′- and 5′-positions. However, less than 0.25% is phosphorylated at the 3′-position, which indicates specific regulatory functions for these 3′-phosphorylated inositol lipid molecules inside the cell [7]. Phosphatidylinositol 3-kinases (PI3K) are a family of lipid kinases that catalyze the addition of a phosphate group to the 3′-position of the inositol ring of PtdIns or PI, resulting in the formation of four different lipid products: PtdIns(3)P, PtdIns(3,4)P2, PtdIns(3,5)P2, and PtdIns(3,4,5)P3. According to how they use the substrate molecules, the nine members of the PI3K family are grouped into three classes: I, II, and III, as suggested by Domin and Waterfield [8]. Among the four different 3′-phosphorylated inositol lipid products, phosphatidylinositol-3,4,5-triphosphate (PtdIns(3,4,5)P3) is emerging as an important signaling molecule in a myriad of cellular processes with implications in a wide range of human diseases (Fig. 1) [9]. PtdIns(3,4,5)P3 plays a key role in insulin signaling and glucose metabolism; any alteration to this signaling pathway is associated with various metabolic diseases including diabetes and obesity, and their associated complications, such as cardiovascular complications, inflammation, etc [9]. In this review we will discuss the role of PtdIns(3,4,5)P3 in the regulation of insulin signaling and glucose metabolism and the implications of PtdIns(3,4,5)P3 dysregulation in diabetic pathophysiology.
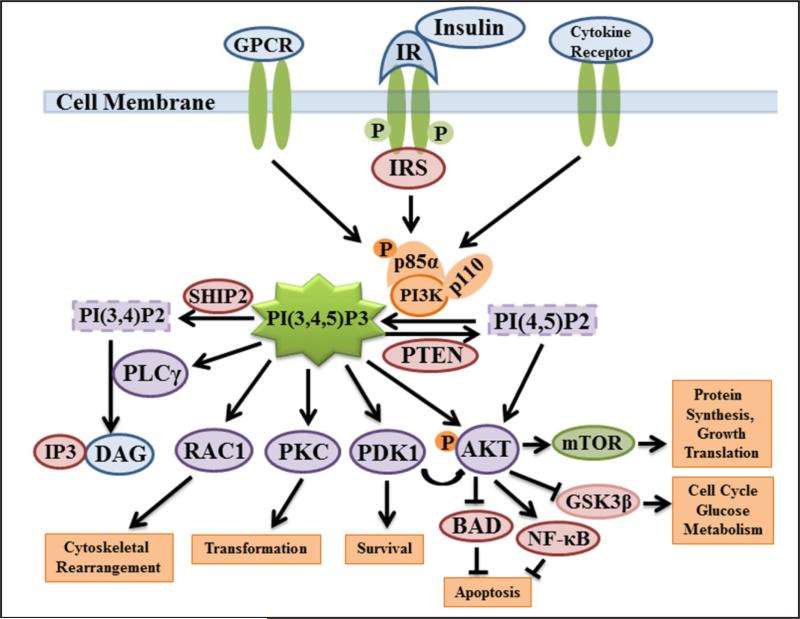
Fig. 1.
The schematic diagram of PtdIns(3,4,5)P3 formation and its downstream signaling. Activation of PI3K either by growth hormone receptor (IRS), G-protein coupled receptor (GPCR), or cytokine receptor phosphorylates PtdIns(4,5)P2 to PtdIns(3,4,5)P3 which recruits PH-domain containing effector molecules, such as AKT, PDK1, PKCZ, small G-protein (RAC1), etc. Activation of these effector protein molecules regulates a myriad of cellular functions, including cell survival, proliferation, cytoskeletal rearrangement, intracellular vesicle trafficking, and cell metabolism. The phosphatases, PTEN and SHIP2 quench PtdIns(3,4,5)P3 formation and its signaling effect.
Regulation of intracellular PtdIns(3,4,5)P3 concentration
The majority of intracellular PtdIns(3,4,5)P3 is synthesized by the phosphorylation of PtdIns(4,5)P2 in response to extracellular stimuli, such as epidermal growth factor (EGF), platelet-derived growth factor (PDGF), insulin, and insulin-like growth factor I (IGF-I) [10]. The class I PI3K are the only enzymes that utilize PtdIns(4,5)P2 as a substrate to synthesize PtdIns(3,4,5)P3. Class I PI3K consist of the p110 catalytic subunit and p85 regulatory subunit. Activation of class IA PI3K (p110α, β, and δ) by growth factor stimulation is regulated either via the interaction of their SH2 domain with the tyrosine-phosphorylated receptor protein kinases, RTK [11], or by the GTP-bound form of the small G protein, Ras [12]. The class IB PI3K (p110γ) can be directly activated via the βγ subunits of the heterotrimeric G proteins [13]. In addition to this, a PtdIns(4,5)P2-independent pathway for PtdIns(3,4,5)P3 synthesis has also been reported [14, 15]. Both Tolias et al. [15] and Zhang et al. [14] reported that PtdIns-4-Phosphate-5-kinases utilize PtdIns(3)P as substrate to form PtdIns(3,4,5)P3 by phosphorylating the 4′- and 5′-positions of the inositol ring in a concerted reaction pathway. However, the contribution of this new pathway to intracellular PtdIns(3,4,5)P3 concentration is still unknown. Under basal conditions, PtdIns(3,4,5)P3 levels in plasma membrane are very low, constituting about 0.0001% of the total plasma membrane lipid content. However, in response to extracellular stimuli, the phosphorylation of PtdIns(4,5)P2 by Class I PI3K causes a rapid increase in PtdIns(3,4,5)P3 concentrations that is 500-fold greater than its basal levels [16]. The low levels of PtdIns(3,4,5)P3 represent its potential regulatory effect on signal transduction pathways.
Dephosphorylation of PtdIns(3,4,5)P3 is essential to prevent its accumulation and constitutive signaling processes. Several PtdIns(3,4,5)P3 phosphatases have been isolated, such as phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and SH2-containing inositol 5-phosphatases (SHIP) [17]. PTEN, a tumor suppressor protein, can dephosphorylate PtdIns(3,4,5)P3 at the 3′-position [18] and SHIP can dephosphorylate it at the 5′-position [19]. Christophe and colleagues reported that PTEN plays an important role in maintaining the basal levels of PtdIns(3,4,5)P3, while SHIP inhibit the stimulus-induced increase in PtdIns(3,4,5)P3 concentration [17]. Therefore, concerted action of PI3K and several phosphatases regulate the PtdIns(3,4,5)P3 levels in the plasma membrane; this tight regulation is critical for the prevention of hyper- and hypo-responsiveness to many extracellular stimuli.
Targets of PtdIns(3f4f5)P3
PtdIns(3,4,5)P3 recruits its effector protein molecules from cytoplasm to the membrane surface to activate, stabilize, and propagate the downstream signaling cascade. The mechanism of action involves direct binding of the inositol head group of PtdIns(3,4,5)P3 to the pleckstrin homology (PH) domain of proteins resulting in their recruitment to the plasma membrane [16]. About 40 of the approximately 250 known PH domain-containing proteins are found to interact with PtdIns(3,4,5)P3 with varying degrees of specificity and affinity. The most commonly studied PtdIns(3,4,5)P3-specific downstream effector protein molecules include protein kinase B/Akt, phosphoinositide-dependent kinase-1 (PDK1), protein kinase ζ (PKCζ), guanine nucleotide-exchange factors, such as GRP1, VAV, and ARNO, and Bruton's tyrosine kinase (Btk). The PH domain of the effector protein molecules consists of approximately 120 amino acid residues arranged into a bowl-like binding pocket so that the inositol head group of PtdIns(3,4,5)P3 can fit easily. The tertiary structure of the binding pocket is highly conserved among different classes of PtdIns(3,4,5)P3-specific effector protein molecules; however, the primary structure varies rapidly, and this variation contributes to the specific affinity for PtdIns(3,4,5)P3 versus other membrane bound PI [20]. The basic amino acid residues of the PH domain create a highly positive electrostatic environment capable of attracting the negatively charged PtdIns(3,4,5)P3 in the interior of PH domain. The arrangement of the phosphate group on the inositol head also plays an important role in binding [16].
The serine/threonine protein kinase B, also known as Akt, is the most well-known target of PtdIns(3,4,5)P3. Binding of PtdIns(3,4,5)P3 to Akt produces conformational changes within the protein resulting in its phosphorylation (at residues Thr308 and Ser473) and full activation [21]. In most cases it has been observed that phosphorylation at the activation loop of these kinases is necessary for activation, but phosphorylation of other residues is also required for full activation. The phosphorylation of Akt on Thr308 is also catalyzed by another PtdIns(3,4,5)P3-dependent protein, PDK1, which is recruited at the plasma membrane and aids in full activation of Akt. Mutation of the PH domain of PDK1 inhibits the full activation of Akt, which prevents the activation of downstream pathways [22]. PDK1 also phosphorylates the activation loop of p70S6K [23] and other PKC family members [24]. PtdIns(3,4,5)P3 causes a marked stimulation of the autophosphorylation of PKCζ, which indicates that PKCζ may be a direct target of PtdIns(3,4,5)P3 [25].
The presence of a PH domain in a wide range of guanine nucleotide-exchange factors for small G protein suggests that PtdIns(3,4,5)P3 fulfills a regulatory function in the activation of these proteins [26]. Membrane localization of the GRP1 family of nucleotide-exchange factors via binding with PtdIns(3,4,5)P3 stimulates their exchange activity toward small G protein, Arf, and Arf5 [27]. PtdIns(3,4,5)P3 also stimulates the exchange activity of another nucleotide-exchange factor, VAV, towards the activation of small G protein, Rac [28]. PDGF-induced binding of GTP to the Rac depends on the activation of PI3K, suggesting a direct effect of PI3K lipid production of the activation of the exchange factor for Rac [29]. The PH domain of Btk was shown to interact with PtdIns(3,4,5)P3 with high affinity [30]. Overexpression of Class I PI3K was shown to stimulate the autophosphorylation of Btk and increase its activity, which was inhibited by a PI3K inhibitor, wortmannin [31, 32]. Mutation in the PH domain of Btk also significantly affects its binding with PtdIns(3,4,5)P3, causing X-linked immunodeficiency in mice [33]. The SH2 domains of the regulatory subunit of Class I PI3K, p85, have also been shown to interact with PtdIns(3,4,5)P3, and the amount of PtdIns(3,4,5)P3 is inversely correlated with the association between PI3K and tyrosine phosphorylated proteins in stimulated cells [34]. PtdIns(3,4,5)P3 was also shown to bind with the SH2 domain of PCLγ to enhance its activity toward PtdIns(4,5)P2 in vitro [35, 36]. Another study reported that the PH domain of PCLγ also binds with PtdIns(3,4,5)P3 and thus mediates the translocation of PCLγ to the plasma membrane in response to extracellular stimuli [37]. Altogether, these studies suggest that PtdIns(3,4,5)P3 plays an important role in activating its effector protein molecules for the regulation of various physiological processes.
Cellular functions controlled by PtdIns(3,4,5)P3
With the identification of several targets of PtdIns(3,4,5)P3, many of the PI3K dependent cellular functions can be explained at the molecular level. Localization and activation of PtdIns(3,4,5)P3 dependent effector proteins regulate a number of cellular functions including cell cycle progression, cell survival and apoptosis, cellular growth, cytoskeletal rearrangement, intracellular vesicle trafficking, and chemotaxis.
Cell cycle progression
The family of cyclin proteins plays an important role in the regulation of cell cycle progression via its interaction with cyclin-dependent kinase (CDK) inhibitors including p21Cip1 and p27Kip1 [38]. An increase in cyclin D1 and a reduction in p27klip are required for the cells to transition from the G1 checkpoint to the S phase of interphase of the cell cycle [39]. Binding of PtdIns(3,4,5)P3 to Akt causes a translocation of active Akt to the nucleus where it phosphorylates Forkhead box Class O (FoxO) transcription factors on three different sites and thus promotes the release of FoxO from the nucleus [40]. Binding of export FoxO with 14-3-3 proteins causes the retention of the FoxO/14-3-3 complex in the cytosol, while the absence of FoxO from the nucleus increases the transcription of Cyclin D1 and reduces the transcription of CDK inhibitor p27Kip1, triggering a G1-S phase transition [41]. Activation of Akt again inhibits the activity of glycogen synthase kinase 3β (GSK3β), which also increases the accumulation of cyclin D1 [39].
Cell survival and Apoptosis
The activation of the PI3K/PtdIns(3,4,5)P3/Akt pathway enables one of the most important cell survival signaling circuits in a wide range of cell types [41]. PtdIns(3,4,5)P3 mediated activation of Akt phosphorylates the death promoter BAD at Ser136, leading to its retention in the cytosol via binding to 14-3-3 proteins, and inhibiting its translocation to mitochondria [42, 43]. The anti-apoptotic proteins, Bcl-2 and Bcl-XL, are then released from the mitochondria, resulting in the inhibition of the mitochondrial step in apoptotic activation [43]. Akt can also activate and phosphorylate inhibitory-κB kinase (IKK) at Thr23, which in turn phosphorylates the inhibitor IkB, leading to the release of transcription factor NF-κB. The translocation of NF-κB to the nucleus activates the transcription of the anti-apoptotic proteins [44, 45]. Another important role played by Akt in apoptosis is mediated by the inactivation and cytosolic retention of FoxO transcription factors as mentioned above. FoxO factors stimulate the transcription and synthesis of a variety of proteins involved in apoptosis, including the TNFα family members, TRAIL and FasL, and the pro-apoptotic family members, BIM and PUMA [38].
Cellular growth
Regulation of cellular growth is one of the key functions of PtdIns(3,4,5)P3. Nutrients and growth factors in the extracellular matrix activate the PI3K/ PtdIns(3,4,5)P3 signal transduction pathway, which increases the biosynthesis of various lipids and proteins required for rapid cellular growth. Both Akt and the nutrient sensor mTOR work together to control cell size and growth. PtdIns(3,4,5)P3-activated Akt phosphorylates and inhibits the action of tuberin, which is in a complex with hamartin, known as the tuberous sclerosis complex (TSC) [46]. Once phosphorylated the complex is no longer able to suppress mTOR. Active mTOR binds to eukaryotic initiation factor 3 (eIF3) and phosphorylates its downstream targets, p70S6K and 4EBP1 (eIF4E binding protein) [47]. p70S6K in turn phosphorylates multiple effectors required for cell growth and protein synthesis including ribosomal protein S6, a protein essential for the regulation of cell size [48]. 4EBP1, upon phosphorylation, dissociates from its target eIF4E and increases the cap dependent translation [47]. In addition to the regulation of translation, the action of mTOR has also been linked to the control of the availability of endogenously produced amino acids required for biosynthesis, mitochondrial biogenesis, and de novo lipogenesis [49].
Intracellular vesicle trafficking, cytoskeletal rearrangement, and chemotaxis
It has been postulated that PI3K plays an important role in vesicle recruitment to the plasma membrane based upon the observation that both the PI3K inhibitor and dominant-negative PI3K block the insulin stimulated translocation of glucose transporter 4 (GLUT4) to the plasma membrane [50, 51]. Considerable evidence reports that a signaling pathway necessary for GLUT4 translocation is the one that proceeds from the insulin receptor to the protein kinase Akt via activation of the PI3K/PtdIns(3,4,5)P3 signaling pathway [52]. Activation of Akt stimulates GLUT4 translocation by phosphorylation of its substrate protein AS160, which possesses a GAP (GTPase-activating protein) domain that catalyzes the inactivation of Rab protein [53]. Rab proteins are the small G-proteins that in their GTP-bound form participate in vesicle movement and fusion [54]. The GAP stimulates the GTPase activity of Rab by triggering GTP hydrolysis, thus generating the inactive GDP-bound form of the Rab. Phosphorylation of AS160 by Akt inhibits its GAP activity, subsequently elevating the GTP-bound form of the cognate Rab protein, and thus triggering GLUT4 translocation [55]. When Rab proteins are converted into their GDP-bound form on the acceptor membrane, they are removed from the membrane into the cytosol by GDI (guanine-nucleotide-dissociation inhibitors) [56]. Several Rab proteins that probably participate in insulin stimulated GLUT4 translocation have been identified, including Rab10 in adipocytes [57-59], and Rab8A and Rab14 in muscle cells [60]. Identification of the PtdIns(3,4,5)P3-dependent effector molecule, GRP1, a nucleotide-exchange factor, led to report that PtdIns(3,4,5)P3 mediated localization of GRP1 to the membrane stimulates the nucleotide exchange activity of GRP1 toward Arf1, which provides an explanation for PtdIns(3,4,5)P3 induced regulation of the translocation of intracellular vesicles to the plasma membrane [61, 62]. PtdIns(3,4,5)P3 dependent activation of VAV2 or other Rac exchange factors, followed by binding of Rac to GTP, explain how PI3K mediates the effect of growth factor and Ras-stimulated cytoskeleton arrangement, leading to cell migration [28]. Other PtdIns(3,4,5)P3 dependent effector molecules, Akt and PDK, have also been implicated in the regulation of cell migration and motility. Akt1 has been found to inhibit cell migration via phosphorylation of the actin-binding factor paladin, as well as via the regulation of the nuclear factors of activated T-cells, ERK and TSC2 [63]. PDK−/− cells also show defects in cell migration [64].
Estimation of PtdIns(3,4,5)P3 concentration
The quantitative detection of PtdIns(3,4,5)P3 is an important challenge in cell biology studies [65]. Cellular PtdIns(3,4,5)P3 levels have been quantified by several methods. The classical method for quantitative detection of PtdIns(3,4,5)P3 in cell extract entails labeling cells with [3H] myo-inositol or [32P] phosphate followed by chloroform-methanol lipid extraction, paper chromatography, and autoradiography [66]. In the modern version of this method, radiolabelled lipid extracts are separated by thin-layer chromatography (TLC) and analyzed by reverse-phase high performance liquid chromatography (HPLC), or lipid products are first deacylated and then analyzed by anion exchange HPLC [67, 68]. Although these radioactive methods have the advantage of yielding quantitative results, one of the major problems is that radiotracer labelling of cells is often not very efficient and labelling to isotopic equilibrium can take days. In addition, these radioactive methods are not very practical for analysis in tissue samples.
To avoid this metabolic labeling issue, several non-radioactive detection methods for PtdIns(3,4,5)P3 have been reported. One method is based upon the anion-exchange HPLC separation of the deacylated lipid products followed by conductivity detection [69]. This method measures the changes in resistance between two electrodes, which depends upon the concentration of the deacylated lipid products. Due to the lower sensitivity limit of this conductivity detection method compared with that of the radioactivity based method, another non-radioactive approach has been developed. This new non-radioactive method is based upon electrospray ionization mass spectrometry (ESI-MS) of total extracted lipid upon addition of triethyl ammonium acetate, which improves signal intensity [70-72]. This method can distinguish different PI depending upon their distinct masses; MS analysis also yields information about the type of acyl constituents of the PI. Although this method is very sensitive, quantitative, avoids radioactivity, and allows analysis of PI in tissue samples, it does not distinguish between different isomers and requires sophisticated as well as expensive instruments.
Recently, a protein-lipid overlay assay has been found to be the simplest and fastest way of measuring PtdIns(3,4,5)P3 levels, which involves incubation of PtdIns(3,4,5)P3-binding protein (e.g. GRP1 PH domain probe) with a nitrocellulose membrane that contains aliquots of lipid extract [68, 73]. Since readymade blots of all PI are commercially available, this type of analysis has become highly popular. In a variation of this method, spotting of lipid extract has also been carried out in the wells of microtiter plates instead of on nitrocellulose membranes for high-throughput screens. Although these spot assays are very useful, data from more specific methods are required to confirm results obtained with this method.
Implication of PtdIns(3,4,5)P3 signaling in metabolic disorders and its associated complications
As discussed above, the PtdIns(3,4,5)P3 mediated signaling cascade is a highly ordered and concerted pathway in a variety of cells and tissues. The relatively low abundance and precise control over the levels of PtdIns(3,4,5)P3 ensure the deliberate activation of its downstream effector molecules, which contribute to a wide variety of cellular functions. For this reason, it is not surprising that a significant number of human diseases can be linked to impaired PtdIns(3,4,5)P3 signaling. PtdIns(3,4,5)P3 is a prime mediator of insulin signaling and glucose metabolism and alterations in this pathway are associated with various metabolic disorders including obesity and diabetes, and their associated complications, such as cardiovascular diseases, inflammation, etc.
PtdIns(3,4,5)P3, insulin signaling, obesity, and type 2 diabetes
Obesity and diabetes have emerged as major public health problems throughout the world and are associated with significant, potentially life-threatening co-morbidities. Results from metabolic and epidemiological studies provide strong evidence that the increasing prevalence of obesity is closely associated with the increase in type 2 diabetes [74, 75]. Some experts call this dual epidemic diabesity [76]. Insulin stimulated glucose uptake and metabolism is one of the fundamental regulators of glucose homeostasis in the body. Impaired insulin action or insulin resistance is associated with obesity and type 2 diabetes (T2D). PI3K/PtdIns(3,4,5)P3 signaling plays an important role in the insulin stimulated glucose metabolism pathway [41]. When insulin binds with insulin receptor tyrosine kinase, it increases the phosphorylation of insulin receptor substrates (mainly IRS1 and IRS2) [77]. Mice lacking the IRS2 gene have increased food intake and they develop obesity, fatty liver, and diabetes [78, 79]. Tyrosine phosphorylated IRS proteins bind to the SH2 domains of the p85 regulatory subunit of Class IA PI3K. The catalytic subunit p110 has also been found to be involved in IRS/PI3K metabolic signaling. Mice lacking either p110α or p110β die in early embryogenesis, whereas mice heterozygous for p110α or p110β display impaired glucose metabolism [80, 81]. Peripheral insulin resistance has been suggested to be the product of impaired PI3K signaling in the effector cells [77, 82]. Inhibition of PTEN expression using PTEN antisense oligonucleotides normalized blood glucose levels in ob/ob (obese) mice [83], while overexpression of PTEN resulted in inhibition of PtdIns(3,4,5)P3 production and glucose uptake in 3T3L1 adipocytes [84]. Mice lacking PTEN expression in adipose, muscle, or liver tissue have been found to have an increase in insulin sensitivity and glucose tolerance [85, 86]. SHIP2 heterozygous knockout mice have also demonstrated an increase in insulin sensitivity [87]. Both PTEN and SHIP2 have been implicated as negative regulators of the insulin signaling pathway [88].
Insulin stimulated glucose uptake in adipocytes and muscle tissues plays an important role in the regulation of body glucose homeostasis. Insulin treatment of fat and muscle cells causes a rapid increase in glucose transport by controlling the amount of GLUT4 translocation from cytoplasm to plasma membrane. Activation of Akt upregulates the glucose uptake mediated by GLUT4 translocation from the intracellular pool to the plasma membrane via the phosphorylation of its substrate protein AS160 containing GAP domain for Rab which are the small G proteins required for membrane trafficking [52, 89]. Overexpression of AS160-4p, a constitutively active form of AS160, inactivates cognate Rab and decreases GLUT4 translocation [55], suggesting it is a negative regulator that is itself inhibited by insulin through the activation of Akt. Activation of cognate Rab by RNAi mediated knockdown of AS160 increases the GLUT4 concentration on the adipocyte surface [90, 91]. However, knockdown of AS160 partially releases the intracellular pool of GLUT4 mobilized by insulin, and careful analysis has shown that other unknown Akt substrate proteins must play an important role in overall GLUT4 regulation by insulin [91-93]. Activation of Akt also promotes glycogen synthesis via activation of glycogen synthase through GSK3 inhibition [94], and transcription of several genes involved in insulin secretion and action mediated by the regulation of the FoxO transcription factor [95]. Mice lacking the protein kinase Akt show insulin resistance and a diabetes mellitus-like syndrome [96]. Our previous study showed reduced levels of PtdIns(3,4,5)P3, downregulation of Akt phosphorylation, and an increase in GLUT2 protein expression in the liver tissues of type 1 and type 2 diabetic rats, but the PtdIns(4,5)P2 levels were unchanged [97]. We also observed that exogenous PtdIns(3,4,5)P3 supplementation increased both glucose uptake and glucose utilization and that the effect is mediated by the activation of the Akt/PKCζ/λ/GLUT4 signaling pathway [98]. Jiang et al. reported that insulin stimulated glucose transport in adipocytes was inhibited by the PI3K inhibitor, wortmannin, but that supplementation with exogenous diC8-PIP3/AM (dioctanoyl-PIP3-acetoxymethyl ester) was capable of overcoming the inhibitory effect of wortmannin in insulin stimulated glucose transport [99]. Genetic mutation of the PH domain of PDK1 inhibits Akt, leading to glucose and insulin intolerance, indicating the critical role played by PtdIns(3,4,5)P3 in glucose homeostasis [22]. The protein kinase Akt has also been identified as an important regulator of renal hypertrophy and apoptosis induced by hyperglycemia [100]. Recent studies in the literature focused on the role of endocannabinoids (EC) in diabetic nephropathy [101]. EC are the endogenous agonists of cannabinoid receptors type 1 and type 2 (CB1 and CB2) and this system participates in the regulation of lipid and glucose metabolism at several levels [101]. The activity of CB2 has been found to be linked with the Akt/MAPK signaling pathway, indicating that the Akt/MAPK signaling pathway plays a role in diabetic nephropathy [100, 102, 103].
In addition to protein kinase B/Akt, impaired activity of atypical protein kinase C (aPKC, including ζ and λ/ι) has also been observed in obese as well as type 2 diabetic patients [104, 105]. The function of aPKC in glucose metabolism is well established [104, 105] and it has been reported that different lipid components, such as phosphatidylinositols, phosphatidic acid, arachidonic acid, and ceramide, can activate aPKC [106, 107]. PtdIns(3,4,5)P3 plays a crucial role in the complete and stable activation of PKCζ [106, 107]. Metformin is a well-known insulin-sensitizing drug widely used in the treatment of type 2 diabetic patients. Farese and colleagues reported that the PtdIns(3,4,5)P3-induced activation of PKCζ is impaired in the muscles of type 2 diabetic or obese humans [104, 105, 108, 109]. However, PtdIns(3,4,5)P3 supplementation increased the PKCζ activity in the muscles of long-term metformin-treated type 2 diabetic subjects compared to that seen in the muscles of diabetic subjects who were off long-term metformin treatment [110]. The improved responsiveness of PKCζ to PtdIns(3,4,5)P3 suggests the important role played by PtdIns(3,4,5)P3 in improving insulin-stimulated aPKC activation during long-term metformin treatment. However, knowledge about the effect of metformin on PtdIns(3,4,5)P3 levels in different tissues of obese as well as diabetic subjects is still lacking and needs to be investigated.
Sirtuins, the mammalian homologue of SIR2, are a group of NAD(+) dependent enzymes that are widely regarded as fuel sensing molecules [111, 112]. SIRT1, the most extensively studied sirtuin, has a profound effect on glucose homeostasis [113, 114]. Decreased SIRT1 expression or activity contributes to the pathogenesis of diseases related to insulin resistance [114]. Inversely, activators of SIRT1 improve insulin sensitivity and ameliorate insulin resistance [113, 115]. Activation of SIRT1 directly regulates the phosphorylation of IRS2 through deacetylation of this substrate, which is a key player in the PI3K/PtdIns(3,4,5) P3 signaling pathway [116]. SIRT1 knockdown in 3T3L1 adipocytes also results in a decrease in tyrosine phosphorylation of IRS1, and serine phosphorylation of AKT (with a subsequent increase in JNK phosphorylation as well as serine phosphorylation of IRS1), followed by inhibition of insulin-stimulated glucose uptake and GLUT4 translocation [117]. Increased in SIRT1 expression of has also been found to downregulate both protein and mRNA levels of PTP1B, which acts as a negative regulator of insulin signaling mainly via the downregulation of IR or IRS1 phosphorylation [118]. These studies demonstrate a positive role for SIRT1 in the regulation of glucose metabolism in an insulin independent manner. In addition to sirtuins, AMP-activated protein kinase (AMPK) is another well-known fuel sensing molecule activated by a decrease in the cell's energy state as reflected by an increase in the AMP/ATP ratio [119, 120]. Studies in humans showed a decrease in AMPK activity in both muscle and adipose tissues of obese, insulin resistant patients [121-123]. A decrease in AMPK activity has also been observed in multiple tissues of animals with some or all of the hallmarks of metabolic syndrome, including obesity and diabetes [124, 125]. A potential mechanism by which AMPK improves insulin sensitivity includes suppression of the inhibitory serine phosphorylation of IRS via regulation of the expression of JNK, IKK, and S6 kinase, an important factor contributing to the development of insulin resistance in obesity and diabetes [119, 126]. Both SIRT1 and AMPK play important roles in the regulation of insulin sensitivity and glucose metabolism via regulating PI3K/PtdIns(3,4,5)P3 signaling mediated by the activation of growth hormone receptor substrate (IRS) [116, 126]. However, the direct effect of these two fuel sensing molecules on the PtdIns(3,4,5)P3 metabolism is not known.
PtdIns(3,4,5)P3 signaling and β-cell response to glucose intolerance
Insulin secreted by the pancreatic β-cells instructs insulin responsive tissues to absorb glucose. Elevated PtdIns(3,4,5)P3 levels in β-cells cause an activation of Akt via PDK1 [127, 128]. Activation of Akt regulates β-cell mass by activating the cell cycle regulators, Cyclin D1, Cyclin D2, p21Clip1 and Cyclin-dependent kinase 4 [129] as well as via regulating various anti-apoptotic genes [130]. IRS-mediated activation of the MAPK signaling pathway through GRB2, a SH2/SH3 containing protein, also stimulates β-cell replication and inhibits apoptotic cell death [131]. Impaired insulin signaling in β-cells causes a decrease in cell proliferation and an increase in apoptosis. Although increased insulin secretion and expansion of the β-cell mass can compensate for the elevated demand for insulin during the initial stages of glucose intolerance in the pathological state of insulin resistance, studies in both diabetic patients and rodents suggest that diabetes results when β-cells no longer proliferate or secrete enough insulin to compensate for insulin resistance [132].
PtdIns(3,4,5)P3 signaling in insulin responsive tissues
Increased insulin resistance in insulin responsive tissues such as liver, muscle, and adipocytes elevates plasma glucose levels. As in β-cells, insulin stimulated upregulation of PtdIns(3,4,5)P3 in hepatocytes also activates Akt and aPKC through PDK1/2. Akt in turn phosphorylates the transcription factor FoxO1 (forkhead box protein O1) which causes its translocation from nucleus to cytoplasm, thus inhibiting its transcriptional activity [133, 134]. In the nucleus FoxO1 activates the promoters of the gluconeogenic enzymes G6Pase (glucose-6-phosphatase) [135] and PEPCK (phosphoenolpyruvate carboxykinase) [136]. Thus PtdIns(3,4,5)P3 signaling normally inhibits gluconeogenesis but impaired insulin signaling causes aberrant hepatic glucose production and increased blood glucose levels. In addition to the impaired gluconeogenesis, increased fat storage in the liver is another contributing event in the development of T2D. The transcription factors SREBP (sterol regulatory element binding proteins) play an important role in cell metabolism by regulating synthesis of fatty acids, triglycerides, and cholesterol [137]. Activation of aPKC in hepatocytes activates the transcription of SREBP1c, while Akt stimulates the processing of SREBP1c, which is required for its activity [138, 139]. SREBP1c in turn transactivates FAS, promotes fat storage within the liver, and thus substantially reduces overall insulin sensitivity [140, 141]. The importance of oxidative stress in the pathogenesis of diabetes has become increasingly apparent over the past few years [142]. High levels of ROS can cause severe damage to DNA, proteins, and lipids, which could lead to cell death and organ dysfunction [143-146]. Using liver tissue from streptozotocin-induced type 1 diabetic rats, Martinovic et al. reported that activation of JNK and Akt/JNK signaling pathways determines the extent of DNA damage [147].
In muscle, PtdIns(3,4,5)P3-mediated activation of Akt and aPKC stimulates the translocation of GLUT4 from cytoplasm to the plasma membrane, which allows increased glucose uptake [89] followed by elevated glycogenesis [94]. Impaired PtdIns(3,4,5)P3 signaling results in decreased levels of GLUT4 translocation and reduced glucose uptake leading to diabetic pathophysiology [148]. PtdIns(3,4,5)P3 signaling plays a profound role in adipogenesis. The effector molecule Akt plays an important role in adipocyte differentiation [149]. An RNAi-mediated decrease in Akt1 inhibited the differentiation of 3T3L1 cells into adipocytes [150], whereas constitutively active Akt can promote the differentiation of 3T3L1 cells, even in the absence of other inputs [151]. Furthermore, mouse embryonic fibroblasts (MEF) lacking Akt1 also displayed an inability to differentiate into adipocytes [152]. Activated Akt regulates the activities of several downstream target proteins involved in adipogenesis and lipogenesis [153-155]. Phosphorylation of the transcription factor GATA2 by Akt increases adipose tissue differentiation and reduces adipose tissue related inflammation in high-fat diet fed mice [156]. Constitutive activation of Akt promotes the nuclear localization of SREBP1, the expression of lipogenic genes, and the production of various classes of lipids (unsaturated and saturated fatty acids, phosphatidylcholine, and phosphatidylglycerol) [154]. Porstmann et al. reported that inhibition of mTOR1 by rapamycin blocks Akt-induced nuclear accumulation of SREBP1 and the expression of lipogenic genes [157].
In addition to the insulin responsive tissues, insulin resistance in brain is also associated with obesity and diabetes [158]. Neuron-specific knockout mice models of IR or its receptor shows obese, physically inactive, and insulin resistant phenotypes [159, 160]. Besides saturated free fatty acids, elevated levels of leptin plays an important role with regard to impaired insulin action [161]. Elevated leptin concentrations decrease insulin-mediated phosphorylation of IR and Akt in both human primary astrocytes and brain tissues of mice, which leads to a decline in locomotor activity [162]. Thus approaches that keep leptin levels in the brain within the physiological range may be beneficial in promoting physical activity and weight loss.
Role ofPtdIns(3,4,5)P3 in the inhibition of insulin signaling
In addition to the stimulation of insulin signaling [41], PtdIns(3,4,5)P3 also initiates cellular events that can cause the inhibition of insulin signaling [163, 164]. PtdIns(3,4,5)P3 mediated recruitment of O-linked-β-N-acetylglucosamine transferase (O-GlcNAc, OGT) from the nucleus to the plasma membrane activates OGT, which catalyzes the addition of GlcNAc to the insulin signaling intermediates downstream of PtdIns(3,4,5)P3, leading to the inhibition of the insulin signaling pathways [163]. Mitochondrial protein prohibitin (PHB) has also been shown to inhibit insulin signaling via its interaction with PtdIns(3,4,5)P3 [164]. Studies have shown that the central nervous system (CNS) can also influence insulin action and glucose homeostasis via regulation of feeding behavior and energy expenditure in response to hormones and various nutrients [165]. Leptin and insulin are two well-known fuel sensors that can inhibit food intake and stimulate energy expenditure via their hypothalamic receptors, which leads to the activation of the neurons that express proopiomelanocortin (POMC), a key hypothalamic neuronal subset for energy homeostasis [166]. The intracellular signaling mechanism of these two hormones converges at the PI3K pathway. Enhanced PtdIns(3,4,5)P3 signaling in POMC neurons of POMC cell-restricted PTEN knockout mice caused hyperphagia and sexually dimorphic diet-sensitive obesity [167]. As discussed above, any mechanism related to decreased or increased PtdIns(3,4,5)P3 production or expression can result in obesity and diabetic phenotypes.
PtdIns(3,4,5)P3 signaling and cardiovascular disease
Metabolic disorder is one of the major contributors to the prevalence of cardiovascular disease [168, 169]. Excessive body weight, insulin resistance, and glucose intolerance have been found to be directly associated with a number of cardiovascular risk factors including hypertension, impaired glycemic control, dyslipidemia, and hemostatic and rheological factors [170]. These changes lead to an increased risk for coronary heart disease, stroke, and cardiovascular death. A significant increase in cardiovascular morbidity and mortality has been observed among obese people compared to that in normal-weight subjects [171]. Dysregulated PtdIns(3,4,5)P3 signaling plays an important role in the development of cardiac pathophysiology including cell survival, hypertrophy, contractility, and metabolism [172]. Both PI3K and PTEN are expressed throughout the cardiac tissue, which includes cardiomyocytes, fibroblasts, endothelial cells, and vascular smooth muscle cells [173]. Class IA PI3K isoforms (p110α and p110β) have been found to regulate physiologic growth of cardiac tissue via activation of RTK [173, 174], whereas class IB PI3K isoform (p110γ) is required for the contractility function of myocardium [174]. Overactivation of PI3K or loss of the PTEN mediated increase in PtdIns(3,4,5)P3 signaling induces various pathological disorders, including myocardial hypertrophy and decreased contractility, thus impairing normal cardiac function [173, 174]. In contrast, activation of PtdIns(3,4,5)P3 signaling has been shown to mimic the cardio-protective nature of ischemic preconditioning [175, 176]. Enhanced PI3K (p110α) activity in response to exercise has been shown to delay or prevent the progression of heart disease [177].
The downstream targets of PtdIns(3,4,5)P3 signaling, such as Akt, GSK3, etc., also play an important role in cardiac complications [178, 179]. All three Akt isoforms are expressed in cardiac tissue; among them, Akt1 and Akt2 are the most prevalent [180, 181]. Activation of Akt in myocardium is required for cell growth, metabolism, and inhibition of apoptosis [182]. Cardiac tissue specific knockdown of Akt1 reduced the number of cardiomyocytes and decreased the overall heart size, but no effect was observed on contractility [183]. In contrast, either overexpression or increased Akt activity can cause cardiac hypertrophy and abnormal contractility [183-185]. Both isoforms of GSK3 (α and β) are expressed in cardiac tissue [179]. GSK3β has been found to be a regulator of important transcription factors, particularly the nuclear factors of activated T-cells, CREB, and the Jun family of proteins [179]. Constitutively active GSK3β has been shown to act as a hypertrophic restraint in the heart [186]; induction of hypertrophy by increased PtdIns(3,4,5)P3 signaling has been linked to the inhibition of GSK3β [179]. Additional cardiac pathologies, such as diabetic cardiomyopathy [187], adrinmycin-induced cardiomyopathy [188], chronic β-adrenergic receptor stimulation [189], or pressure overload induced hypertrophy [190], have also been shown to involve an alteration in PI3K/Akt signaling.
High glucose treatment increases endothelial cell apoptosis [191], permeability [192, 193], and monocyte-endothelial cell (EC) adhesion [193, 194]. PI3K/Akt signaling in endothelial cells regulates cell survival, proliferation, microvascular permeability, and angiogenesis [195-197]. Varma et al. reported that high glucose-induced inhibition of the PI3K/Akt pathway causes endothelial cell proliferative dysfunction [198]. Using human umbilical vein endothelial cells (HUVEC) and THP-1 monocytes, our study demonstrates that high glucose treatment decreased intracellular PtdIns(3,4,5)P3 levels and increased the expression of adhesion molecules such as ICAM-1 (intercellular adhesion molecule 1) in endothelial cells and CD11a (a subunit of lymphocyte function-associated antigen 1, LFA-1) in monocytes, and adhesion of monocytes to endothelial cells (EC) [199]. Treatment with a specific inhibitor of PtdIns(3,4,5)P3, PIT-1, also increased the expression of adhesion molecules and monocyte-EC adhesion. Exogenous PIP3 supplementation, however, restored the loss in intracellular PtdIns(3,4,5)P3 levels and downregulated the expression of adhesion molecules as well as monocyte-EC adhesion in HG-treated cells, suggesting a causal role for PtdIns(3,4,5)P3 in the regulation of adhesion molecules and the adhesion of monocytes to endothelial cells treated with high glucose. Overall, the PtdIns(3,4,5)P3 signaling pathway has been shown to exert multiple effects on the regulation of cardiac pathophysiology.
PtdIns(3,4,5)P3 signaling and inflammation
Both obesity and diabetes are associated with excessive production of the pro-inflammatory cytokines seen in chronic low-grade inflammation. Progression of insulin resistance exacerbates inflammation. Class IA PI3Kα and PI3Kβ are expressed ubiquitously, whereas class IA PI3Kδ and class IB PI3Kγ are predominantly found in cells of hematopoietic lineage [200]. Because of their restricted expression, it has been suggested that these isoforms are critical regulators in different populations of immune cells such as neutrophils, mast cells, macrophages, T-cells, B-cells, and NK cells [201]. PI3Kδ and PI3Kγ mediated production of PtdIns(3,4,5)P3 has been found to be an important target in a range of inflammatory and autoimmune diseases as well as in transplantation and hematological malignancies [201, 202]. In response to various extracellular stimuli, different cellular receptors such as antigen, cytokine, or chemokine receptors activate PtdIns(3,4,5)P3 signaling in the immune system [203]. PtdIns(3,4,5)P3 then recruits and activates GEF for Rac and Arf GTPases, resulting in actin cytoskeletal rearrangement and directional cellular movement [204]. Several studies reported the role of PI3Kγ as the primary isoform responsible for cellular migration in response to chemoattractants in the early steps of inflammation [205, 206]. Using a selective PI3Kδ inhibitor, IC87114, Sadhu et al. also reported the essential role played by PI3Kδ-mediated amplification of PtdIns(3,4,5)P3 levels, which leads to neutrophil polarization and directional movement [207]. The role played by PTEN phosphorylation and stability has also been found to be essential for proper inflammatory cell migration, suggesting the importance of PtdIns(3,4,5)P3 signaling [208]. The downstream effector molecules of PtdIns(3,4,5)P3 signaling, including PDK1 and Akt, also regulate the production of reactive oxygen species at the site of inflammation via the regulation of various NADPH oxidase family proteins [209, 210]. Increased activation of neutrophils and macrophages has been associated with various diseases including atherosclerosis, lupus, and rheumatoid arthritis, suggesting that impaired PtdIns(3,4,5)P3 signaling plays a role in the development of these inflammatory diseases [211, 212].
Allergy and inflammation have been found to involve both mast cells and macrophages, followed by invasion of the inflamed area by effector cells such as monocytes, neutrophils, and mast cell precursors [213]. Mast cells express a high affinity receptor for IgE on their surface and, in response to allergens, this IgE receptor is crosslinked followed by phosphorylation of immune-receptor tyrosine-based activation motifs (ITAM) on the receptor β and γ chains. Class I PI3K are then activated via binding to the phosphorylated ITAM. Production of PtdIns(3,4,5)P3 initiates the activation of Btk and PLCγ resulting in the opening of plasma membrane Ca2+ channels and granule release [214]. Activation of PI3Kδ regulates the initial degranulation and release of cytotoxic molecules [215], whereas PI3Kγ regulates the subsequent degranulation [216]. PTEN deficiency in mast cells, both as the result of genetic deletion or shRNA-mediated knockdown of PTEN, induces mastocytosis and increases allergic responses in mice [217, 218]. Increased PtdIns(3,4,5)P3 signaling in mast cells has been implicated in a variety of immune disorders including allergic diseases, asthma, autoimmunity, and mastocytosis, suggesting that PtdIns(3,4,5)P3 regulation is a promising therapy for mast cell mediated diseases [219].
T-cells and B-cells play an important role in adaptive immune responses. PI3Kδ and PI3Kγ have been found to be essential for the development and function of both T-cells and B-cells [220]. It has been suggested that PI3Kγ mediates chemotaxis in T-cells, whereas PI3Kδ is responsible for the migration of B-cells [221]. Several studies reported that both increased and decreased PtdIns(3,4,5)P3 signaling have been associated with the development of autoimmunity, suggesting that PtdIns(3,4,5)P3 plays a role in the regulation of the functions of T-cells [203, 222, 223]. The evidence discussed above provides information supporting the importance of PtdIns(3,4,5)P3 signaling in immune function and diseases. Targeting the appropriate PI3K isoform could be a therapeutic tool for the treatment of various inflammatory diseases; several isoform specific inhibitors are currently under clinical investigation [202]. Impaired wound healing is a serious complication of diabetes, which diminishes physical activity, and, in some cases, leads to limb amputation [224]. Would healing is a complex multistage process that includes different phases: inflammation, formation of granulation tissue, production of new structures, and tissue remodeling [225]. These processes are all regulated by the secretion of various pro-inflammatory cytokines (IL-1, IL-6, IL-10, and TNF-α) [226-228], chemokines [229, 230], and growth factors [226] that, upon binding to their specific receptor, activate various signaling pathways and transcription factors, such as Akt, STAT (signal transducer and activator of transcription), and NF-kB [231, 232]. Supplementation with undenatured whey protein, a cysteine rich protein, restored the activation of Akt, STAT3, and NF-kB and improved the closure of diabetic wounds in diabetic mice compared to those in control mice [233].
Conclusion
PtdIns(3,4,5)P3 has been established as an important second messenger in a variety of cellular processes, which has implications for a wide range of diseases. Activation of various PI3K isoforms and expression of different phosphatases such as PTEN and SHIP2 have emerged as important regulators of intracellular PtdIns(3,4,5)P3 levels. Following formation, PtdIns(3,4,5)P3 recruits various effector protein molecules such as PDK1, Akt, PKCζ, Btk, etc. at the inner plasma membrane, where they are able to exert effects on a number of cellular functions including cell survival, proliferation, cytoskeletal rearrangement, intracellular vesicle trafficking, and cell metabolism. Impaired PtdIns(3,4,5)P3 signaling has been found to be associated with various metabolic diseases such as diabetes and obesity, and their associated complications, including cardiovascular diseases and inflammation. Overall, the PtdIns(3,4,5)P3 signaling pathway has multiple effects on the regulation and pathophysiology of various human diseases. Although significant progress has been made in understanding the role of PtdIns(3,4,5)P3 signaling in human diseases, further studies need to be performed on the activities of PI3K isoforms and the expression of PtdIns(3,4,5)P3 phosphatases in various tissues among different patient populations. The information gained from these studies will aid in the development of target specific inhibitors; particular care should be taken to avoid the any kind of toxicity following exposure to various inhibitors. Additionally, the expression as well as the regulation of PtdIns(3,4,5)P3 effector molecules remains to be fully characterized under normal as well as disease conditions. Understanding the precise mechanism of PtdIns(3,4,5)P3 signaling in organ pathophysiology will be helpful in the development of potential therapeutic targets for the treatment of associated human diseases.
Acknowledgements
The authors are supported by grants from NCCAM of the National Institute of Health RO1 AT007442 and the Malcolm Feist Endowed Chair in Diabetes. This study is also funded by a fellowship from the Malcolm Feist Cardiovascular Research Endowment, LSU Health Sciences Center, Shreveport. The authors thank Ms Georgia Morgan for excellent editing of this manuscript.
Footnotes
Disclosure Statement
The authors declare that they have no conflict of interest.
References
- 1.Hokin MR, Hokin LE. Enzyme secretion and the incorporation of P32 into phospholipides of pancreas slices. J Biol Chem. 1953;203:967–977. [PubMed] [Google Scholar]
- 2.Sasaki T, Sasaki J, Sakai T, Takasuga S, Suzuki A. The physiology of phosphoinositides. Biol Pharm Bull. 2007;30:1599–1604. doi: 10.1248/bpb.30.1599. [DOI] [PubMed] [Google Scholar]
- 3.Payrastre B, Missy K, Giuriato S, Bodin S, Plantavid M, Gratacap M. Phosphoinositides: key players in cell signalling, in time and space. Cell Signal. 2001;13:377–387. doi: 10.1016/s0898-6568(01)00158-9. [DOI] [PubMed] [Google Scholar]
- 4.Toker A. Phosphoinositides and signal transduction. Cell Mol Life Sci. 2002;59:761–779. doi: 10.1007/s00018-002-8465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blind RD, Sablin EP, Kuchenbecker KM, Chiu HJ, Deacon AM, Das D, Fletterick RJ, Ingraham HA. The signaling phospholipid PIP3 creates a new interaction surface on the nuclear receptor SF-1. Proc Natl Acad Sci U S A. 2014;111:15054–15059. doi: 10.1073/pnas.1416740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pendaries C, Tronchere H, Plantavid M, Payrastre B. Phosphoinositide signaling disorders in human diseases. FEBS Lett. 2003;546:25–31. doi: 10.1016/s0014-5793(03)00437-x. [DOI] [PubMed] [Google Scholar]
- 7.Rameh LE, Cantley LC. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274:8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 8.Domin J, Waterfield MD. Using structure to define the function of phosphoinositide 3-kinase family members. FEBS Lett. 1997;410:91–95. doi: 10.1016/s0014-5793(97)00617-0. [DOI] [PubMed] [Google Scholar]
- 9.Riehle RD, Cornea S, Degterev A. Role of phosphatidylinositol 3,4,5-trisphosphate in cell signaling. Adv Exp Med Biol. 2013;991:105–139. doi: 10.1007/978-94-007-6331-9_7. [DOI] [PubMed] [Google Scholar]
- 10.Hawkins PT, Jackson TR, Stephens LR. Platelet-derived growth factor stimulates synthesis of PtdIns(3,4,5) P3 by activating a PtdIns(4,5)P2 3-OH kinase. Nature. 1992;358:157–159. doi: 10.1038/358157a0. [DOI] [PubMed] [Google Scholar]
- 11.Cantley LC, Auger KR, Carpenter C, Duckworth B, Graziani A, Kapeller R, Soltoff S. Oncogenes and signal transduction. Cell. 1991;64:281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- 13.Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Loijens JC, Boronenkov IV, Parker GJ, Norris FA, Chen J, Thum O, Prestwich GD, Majerus PW, Anderson RA. Phosphatidylinositol-4-phosphate 5-kinase isozymes catalyze the synthesis of 3-phosphatecontaining phosphatidylinositol signaling molecules. J Biol Chem. 1997;272:17756–17761. doi: 10.1074/jbc.272.28.17756. [DOI] [PubMed] [Google Scholar]
- 15.Tolias KF, Rameh LE, Ishihara H, Shibasaki Y, Chen J, Prestwich GD, Cantley LC, Carpenter CL. Type I phosphatidylinositol-4-phosphate 5-kinases synthesize the novel lipids phosphatidylinositol 3,5-bisphosphate and phosphatidylinositol 5-phosphate. J Biol Chem. 1998;273:18040–18046. doi: 10.1074/jbc.273.29.18040. [DOI] [PubMed] [Google Scholar]
- 16.Rosen SA, Gaffney PR, Spiess B, Gould IR. Understanding the relative affinity and specificity of the pleckstrin homology domain of protein kinase B for inositol phosphates. Phys Chem Chem Phys. 2012;14:929–936. doi: 10.1039/c1cp22240f. [DOI] [PubMed] [Google Scholar]
- 17.Blero D, Payrastre B, Schurmans S, Erneux C. Phosphoinositide phosphatases in a network of signalling reactions. Pflugers Arch. 2007;455:31–44. doi: 10.1007/s00424-007-0304-5. [DOI] [PubMed] [Google Scholar]
- 18.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 19.Damen JE, Liu L, Rosten P, Humphries RK, Jefferson AB, Majerus PW, Krystal G. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-triphosphate 5-phosphatase. Proc Natl Acad Sci U S A. 1996;93:1689–1693. doi: 10.1073/pnas.93.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He J, Haney RM, Vora M, Verkhusha VV, Stahelin RV, Kutateladze TG. Molecular mechanism of membrane targeting by the GRP1 PH domain. J Lipid Res. 2008;49:1807–1815. doi: 10.1194/jlr.M800150-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 22.Bayascas JR, Wullschleger S, Sakamoto K, Garcia-Martinez JM, Clacher C, Komander D, van Aalten DM, Boini KM, Lang F, Lipina C, Logie L, Sutherland C, Chudek JA, van Diepen JA, Voshol PJ, Lucocq JM, Alessi DR. Mutation of the PDK1 PH domain inhibits protein kinase B/Akt, leading to small size and insulin resistance. Mol Cell Biol. 2008;28:3258–3272. doi: 10.1128/MCB.02032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pullen N, Dennis PB, Andjelkovic M, Dufner A, Kozma SC, Hemmings BA, Thomas G. Phosphorylation and activation of p70s6k by PDK1. Science. 1998;279:707–710. doi: 10.1126/science.279.5351.707. [DOI] [PubMed] [Google Scholar]
- 24.Le Good JA, Ziegler WH, Parekh DB, Alessi DR, Cohen P, Parker PJ. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 25.Nakanishi H, Brewer KA, Exton JH. Activation of the zeta isozyme of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1993;268:13–16. [PubMed] [Google Scholar]
- 26.Musacchio A, Gibson T, Rice P, Thompson J, Saraste M. The PH domain: a common piece in the structural patchwork of signalling proteins. Trends Biochem Sci. 1993;18:343–348. doi: 10.1016/0968-0004(93)90071-t. [DOI] [PubMed] [Google Scholar]
- 27.DiNitto JP, Delprato A, Gabe Lee MT, Cronin TC, Huang S, Guilherme A, Czech MP, Lambright DG. Structural basis and mechanism of autoregulation in 3-phosphoinositide-dependent Grp1 family Arf GTPase exchange factors. Mol Cell. 2007;28:569–583. doi: 10.1016/j.molcel.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han J, Luby-Phelps K, Das B, Shu X, Xia Y, Mosteller RD, Krishna UM, Falck JR, White MA, Broek D. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- 29.Hawkins PT, Eguinoa A, Qiu RG, Stokoe D, Cooke FT, Walters R, Wennstrom S, Claesson-Welsh L, Evans T, Symons M, et al. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- 30.Salim K, Bottomley MJ, Querfurth E, Zvelebil MJ, Gout I, Scaife R, Margolis RL, Gigg R, Smith CI, Driscoll PC, Waterfield MD, Panayotou G. Distinct specificity in the recognition of phosphoinositides by the pleckstrin homology domains of dynamin and Bruton's tyrosine kinase. EMBO J. 1996;15:6241–6250. [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Wahl MI, Eguinoa A, Stephens LR, Hawkins PT, Witte ON. Phosphatidylinositol 3-kinase-gamma activates Bruton's tyrosine kinase in concert with Src family kinases. Proc Natl Acad Sci U S A. 1997;94:13820–13825. doi: 10.1073/pnas.94.25.13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scharenberg AM, El-Hillal O, Fruman DA, Beitz LO, Li Z, Lin S, Gout I, Cantley LC, Rawlings DJ, Kinet JP. Phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3)/Tec kinase-dependent calcium signaling pathway: a target for SHIP-mediated inhibitory signals. EMBO J. 1998;17:1961–1972. doi: 10.1093/emboj/17.7.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukuda M, Kojima T, Kabayama H, Mikoshiba K. Mutation of the pleckstrin homology domain of Bruton's tyrosine kinase in immunodeficiency impaired inositol 1,3,4,5-tetrakisphosphate binding capacity. J Biol Chem. 1996;271:30303–30306. doi: 10.1074/jbc.271.48.30303. [DOI] [PubMed] [Google Scholar]
- 34.Rameh LE, Chen CS, Cantley LC. Phosphatidylinositol (3,4,5)P3 interacts with SH2 domains and modulates PI 3-kinase association with tyrosine-phosphorylated proteins. Cell. 1995;83:821–830. doi: 10.1016/0092-8674(95)90195-7. [DOI] [PubMed] [Google Scholar]
- 35.Bae YS, Cantley LG, Chen CS, Kim SR, Kwon KS, Rhee SG. Activation of phospholipase C-gamma by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:4465–4469. doi: 10.1074/jbc.273.8.4465. [DOI] [PubMed] [Google Scholar]
- 36.Rameh LE, Rhee SG, Spokes K, Kazlauskas A, Cantley LC, Cantley LG. Phosphoinositide 3-kinase regulates phospholipase Cgamma-mediated calcium signaling. J Biol Chem. 1998;273:23750–23757. doi: 10.1074/jbc.273.37.23750. [DOI] [PubMed] [Google Scholar]
- 37.Falasca M, Logan SK, Lehto VP, Baccante G, Lemmon MA, Schlessinger J. Activation of phospholipase C gamma by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Vos KE, Coffer PJ. The extending network of FOXO transcriptional target genes. Antioxid Redox Signal. 2011;14:579–592. doi: 10.1089/ars.2010.3419. [DOI] [PubMed] [Google Scholar]
- 39.Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339–345. [PubMed] [Google Scholar]
- 40.Furukawa-Hibi Y, Kobayashi Y, Chen C, Motoyama N. FOXO transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxid Redox Signal. 2005;7:752–760. doi: 10.1089/ars.2005.7.752. [DOI] [PubMed] [Google Scholar]
- 41.Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta. 2008;1784:159–185. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 42.del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 43.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 44.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 45.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 46.Pan D, Dong J, Zhang Y, Gao X. Tuberous sclerosis complex: from Drosophila to human disease. Trends Cell Biol. 2004;14:78–85. doi: 10.1016/j.tcb.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 48.Fenton TR, Gout IT. Functions and regulation of the 70kDa ribosomal S6 kinases. Int J Biochem Cell Biol. 2011;43:47–59. doi: 10.1016/j.biocel.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 49.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheatham B, Vlahos CJ, Cheatham L, Wang L, Blenis J, Kahn CR. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haruta T, Morris AJ, Rose DW, Nelson JG, Mueckler M, Olefsky JM. Insulin-stimulated GLUT4 translocation is mediated by a divergent intracellular signaling pathway. J Biol Chem. 1995;270:27991–27994. doi: 10.1074/jbc.270.47.27991. [DOI] [PubMed] [Google Scholar]
- 52.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5:237–252. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Miinea CP, Sano H, Kane S, Sano E, Fukuda M, Peranen J, Lane WS, Lienhard GE. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem J. 2005;391:87–93. doi: 10.1042/BJ20050887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 55.Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y, Deng Y, Zhang J, Yang L, Xie X, Xu T. GDI-1 preferably interacts with Rab10 in insulin-stimulated GLUT4 translocation. Biochem J. 2009;422:229–235. doi: 10.1042/BJ20090624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sano H, Roach WG, Peck GR, Fukuda M, Lienhard GE. Rab10 in insulin-stimulated GLUT4 translocation. Biochem J. 2008;411:89–95. doi: 10.1042/BJ20071318. [DOI] [PubMed] [Google Scholar]
- 58.Sano H, Eguez L, Teruel MN, Fukuda M, Chuang TD, Chavez JA, Lienhard GE, McGraw TE. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 2007;5:293–303. doi: 10.1016/j.cmet.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 59.Chen Y, Wang Y, Zhang J, Deng Y, Jiang L, Song E, Wu XS, Hammer JA, Xu T. Lippincott-Schwartz J: Rab10 and myosin-Va mediate insulin-stimulated GLUT4 storage vesicle translocation in adipocytes. J Cell Biol. 2012;198:545–560. doi: 10.1083/jcb.201111091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ishikura S, Bilan PJ, Klip A. Rabs 8A and 14 are targets of the insulin-regulated Rab-GAP AS160 regulating GLUT4 traffic in muscle cells. Biochem Biophys Res Commun. 2007;353:1074–1079. doi: 10.1016/j.bbrc.2006.12.140. [DOI] [PubMed] [Google Scholar]
- 61.Klarlund JK, Guilherme A, Holik JJ, Virbasius JV, Chawla A, Czech MP. Signaling by phosphoinositide-3,4,5-trisphosphate through proteins containing pleckstrin and Sec7 homology domains. Science. 1997;275:1927–1930. doi: 10.1126/science.275.5308.1927. [DOI] [PubMed] [Google Scholar]
- 62.Klarlund JK, Rameh LE, Cantley LC, Buxton JM, Holik JJ, Sakelis C, Patki V, Corvera S, Czech MP. Regulation of GRP1-catalyzed ADP ribosylation factor guanine nucleotide exchange by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:1859–1862. doi: 10.1074/jbc.273.4.1859. [DOI] [PubMed] [Google Scholar]
- 63.Chin YR, Toker A. Akt isoform-specific signaling in breast cancer: uncovering an anti-migratory role for palladin. Cell Adh Migr. 2011;5:211–214. doi: 10.4161/cam.5.3.15790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Primo L, di Blasio L, Roca C, Droetto S, Piva R, Schaffhausen B, Bussolino F. Essential role of PDK1 in regulating endothelial cell migration. J Cell Biol. 2007;176:1035–1047. doi: 10.1083/jcb.200607053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rusten TE, Stenmark H. Analyzing phosphoinositides and their interacting proteins. Nat Methods. 2006;3:251–258. doi: 10.1038/nmeth867. [DOI] [PubMed] [Google Scholar]
- 66.Hokin LE, Hokin MR. Phosphoinositides and protein secretion in pancreas slices. J Biol Chem. 1958;233:805–810. [PubMed] [Google Scholar]
- 67.Dove SK, Cooke FT, Douglas MR, Sayers LG, Parker PJ, Michell RH. Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature. 1997;390:187–192. doi: 10.1038/36613. [DOI] [PubMed] [Google Scholar]
- 68.Guillou H, Stephens LR, Hawkins PT. Quantitative measurement of phosphatidylinositol 3,4,5-trisphosphate. Methods Enzymol. 2007;434:117–130. doi: 10.1016/S0076-6879(07)34007-X. [DOI] [PubMed] [Google Scholar]
- 69.Nasuhoglu C, Feng S, Mao J, Yamamoto M, Yin HL, Earnest S, Barylko B, Albanesi JP, Hilgemann DW. Nonradioactive analysis of phosphatidylinositides and other anionic phospholipids by anion-exchange high-performance liquid chromatography with suppressed conductivity detection. Anal Biochem. 2002;301:243–254. doi: 10.1006/abio.2001.5489. [DOI] [PubMed] [Google Scholar]
- 70.Han X, Gross RW. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: a bridge to lipidomics. J Lipid Res. 2003;44:1071–1079. doi: 10.1194/jlr.R300004-JLR200. [DOI] [PubMed] [Google Scholar]
- 71.Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422:198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 72.Hsu FF, Turk J. Characterization of phosphatidylinositol, phosphatidylinositol-4-phosphate, and phosphatidylinositol-4,5-bisphosphate by electrospray ionization tandem mass spectrometry: a mechanistic study. J Am Soc Mass Spectrom. 2000;11:986–999. doi: 10.1016/S1044-0305(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 73.Dowler S, Kular G, Alessi DR. Protein lipid overlay assay. Sci STKE. 2002;2002:pl6. doi: 10.1126/stke.2002.129.pl6. [DOI] [PubMed] [Google Scholar]
- 74.Golay A, Ybarra J. Link between obesity and type 2 diabetes. Best Pract Res Clin Endocrinol Metab. 2005;19:649–663. doi: 10.1016/j.beem.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 75.Hussain AH, MZI. Claussen B, Asghar S. Type 2 Diabetes and obesity: A review. Journal of Diabetology. 2010;2:1–7. [Google Scholar]
- 76.Kalra S. Diabesity. J Pak Med Assoc. 2013;63:532–534. [PubMed] [Google Scholar]
- 77.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 78.Tobe K, Suzuki R, Aoyama M, Yamauchi T, Kamon J, Kubota N, Terauchi Y, Matsui J, Akanuma Y, Kimura S, Tanaka J, Abe M, Ohsumi J, Nagai R, Kadowaki T. Increased expression of the sterol regulatory elementbinding protein-1 gene in insulin receptor substrate-2(−/−) mouse liver. J Biol Chem. 2001;276:38337–38340. doi: 10.1074/jbc.C100160200. [DOI] [PubMed] [Google Scholar]
- 79.Masaki T, Chiba S, Noguchi H, Yasuda T, Tobe K, Suzuki R, Kadowaki T, Yoshimatsu H. Obesity in insulin receptor substrate-2-deficient mice: disrupted control of arcuate nucleus neuropeptides. Obes Res. 2004;12:878–885. doi: 10.1038/oby.2004.106. [DOI] [PubMed] [Google Scholar]
- 80.Foukas LC, Claret M, Pearce W, Okkenhaug K, Meek S, Peskett E, Sancho S, Smith AJ, Withers DJ, Vanhaesebroeck B. Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006;441:366–370. doi: 10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
- 81.Brachmann SM, Ueki K, Engelman JA, Kahn RC, Cantley LC. Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice. Mol Cell Biol. 2005;25:1596–1607. doi: 10.1128/MCB.25.5.1596-1607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shulman GI. Unraveling the cellular mechanism of insulin resistance in humans: new insights from magnetic resonance spectroscopy. Physiology (Bethesda) 2004;19:183–190. doi: 10.1152/physiol.00007.2004. [DOI] [PubMed] [Google Scholar]
- 83.Butler M, McKay RA, Popoff IJ, Gaarde WA, Witchell D, Murray SF, Dean NM, Bhanot S, Monia BP. Specific inhibition of PTEN expression reverses hyperglycemia in diabetic mice. Diabetes. 2002;51:1028–1034. doi: 10.2337/diabetes.51.4.1028. [DOI] [PubMed] [Google Scholar]
- 84.Ono H, Katagiri H, Funaki M, Anai M, Inukai K, Fukushima Y, Sakoda H, Ogihara T, Onishi Y, Fujishiro M, Kikuchi M, Oka Y, Asano T. Regulation of phosphoinositide metabolism, Akt phosphorylation, and glucose transport by PTEN (phosphatase and tensin homolog deleted on chromosome 10) in 3T3-L1 adipocytes. Mol Endocrinol. 2001;15:1411–1422. doi: 10.1210/mend.15.8.0684. [DOI] [PubMed] [Google Scholar]
- 85.Kurlawalla-Martinez C, Stiles B, Wang Y, Devaskar SU, Kahn BB, Wu H. Insulin hypersensitivity and resistance to streptozotocin-induced diabetes in mice lacking PTEN in adipose tissue. Mol Cell Biol. 2005;25:2498–2510. doi: 10.1128/MCB.25.6.2498-2510.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stiles B, Wang Y, Stahl A, Bassilian S, Lee WP, Kim YJ, Sherwin R, Devaskar S, Lesche R, Magnuson MA, Wu H. Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity [corrected]. Proc Natl Acad Sci U S A. 2004;101:2082–2087. doi: 10.1073/pnas.0308617100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Suwa A, Kurama T, Shimokawa T. SHIP2 and its involvement in various diseases. Expert Opin Ther Targets. 2010;14:727–737. doi: 10.1517/14728222.2010.492780. [DOI] [PubMed] [Google Scholar]
- 88.Vinciguerra M, Foti M. PTEN and SHIP2 phosphoinositide phosphatases as negative regulators of insulin signalling. Arch Physiol Biochem. 2006;112:89–104. doi: 10.1080/13813450600711359. [DOI] [PubMed] [Google Scholar]
- 89.Thong FS, Dugani CB, Klip A. Turning signals on and off: GLUT4 traffic in the insulin-signaling highway. Physiology (Bethesda) 2005;20:271–284. doi: 10.1152/physiol.00017.2005. [DOI] [PubMed] [Google Scholar]
- 90.Larance M, Ramm G, Stockli J, van Dam EM, Winata S, Wasinger V, Simpson F, Graham M, Junutula JR, Guilhaus M, James DE. Characterization of the role of the Rab GTPase-activating protein AS160 in insulinregulated GLUT4 trafficking. J Biol Chem. 2005;280:37803–37813. doi: 10.1074/jbc.M503897200. [DOI] [PubMed] [Google Scholar]
- 91.Eguez L, Lee A, Chavez JA, Miinea CP, Kane S, Lienhard GE, McGraw TE. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab. 2005;2:263–272. doi: 10.1016/j.cmet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 92.Bai L, Wang Y, Fan J, Chen Y, Ji W, Qu A, Xu P, James DE, Xu T. Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab. 2007;5:47–57. doi: 10.1016/j.cmet.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 93.Gonzalez E, McGraw TE. Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol Biol Cell. 2006;17:4484–4493. doi: 10.1091/mbc.E06-07-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–776. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 95.Barthel A, Schmoll D, Unterman TG. FoxO proteins in insulin action and metabolism. Trends Endocrinol Metab. 2005;16:183–189. doi: 10.1016/j.tem.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 96.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 97.Manna P, Jain SK. Decreased hepatic phosphatidylinositol-3,4,5-triphosphate (PIP3) levels and impaired glucose homeostasis in type 1 and type 2 diabetic rats. Cell Physiol Biochem. 2012;30:1363–1370. doi: 10.1159/000343325. [DOI] [PubMed] [Google Scholar]
- 98.Manna P, Jain SK. PIP3 but not PIP2 increases GLUT4 surface expression and glucose metabolism mediated by AKT/PKCzeta/lambda phosphorylation in 3T3L1 adipocytes. Mol Cell Biochem. 2013 doi: 10.1007/s11010-013-1714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jiang T, Sweeney G, Rudolf MT, Klip A, Traynor-Kaplan A, Tsien RY. Membrane-permeant esters of phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:11017–11024. doi: 10.1074/jbc.273.18.11017. [DOI] [PubMed] [Google Scholar]
- 100.Rane MJ, Song Y, Jin S, Barati MT, Wu R, Kausar H, Tan Y, Wang Y, Zhou G, Klein JB, Li X, Cai L. Interplay between Akt and p38 MAPK pathways in the regulation of renal tubular cell apoptosis associated with diabetic nephropathy. Am J Physiol Renal Physiol. 2010;298:F49–61. doi: 10.1152/ajprenal.00032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Di Marzo V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia. 2008;51:1356–1367. doi: 10.1007/s00125-008-1048-2. [DOI] [PubMed] [Google Scholar]
- 102.Jenkin KA, McAinch AJ, Briffa JF, Zhang Y, Kelly DJ, Pollock CA, Poronnik P, Hryciw DH. Cannabinoid receptor 2 expression in human proximal tubule cells is regulated by albumin independent of ERK1/2 signaling. Cell Physiol Biochem. 2013;32:1309–1319. doi: 10.1159/000354529. [DOI] [PubMed] [Google Scholar]
- 103.Romero-Sandoval EA, Horvath R, Landry RP, DeLeo JA. Cannabinoid receptor type 2 activation induces a microglial anti-inflammatory phenotype and reduces migration via MKP induction and ERK dephosphorylation. Mol Pain. 2009;5:25. doi: 10.1186/1744-8069-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Beeson M, Sajan MP, Dizon M, Grebenev D, Gomez-Daspet J, Miura A, Kanoh Y, Powe J, Bandyopadhyay G, Standaert ML, Farese RV. Activation of protein kinase C-zeta by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: amelioration by rosiglitazone and exercise. Diabetes. 2003;52:1926–1934. doi: 10.2337/diabetes.52.8.1926. [DOI] [PubMed] [Google Scholar]
- 105.Kim YB, Kotani K, Ciaraldi TP, Henry RR, Kahn BB. Insulin-stimulated protein kinase C lambda/zeta activity is reduced in skeletal muscle of humans with obesity and type 2 diabetes: reversal with weight reduction. Diabetes. 2003;52:1935–1942. doi: 10.2337/diabetes.52.8.1935. [DOI] [PubMed] [Google Scholar]
- 106.Xiao H, Liu M. Atypical protein kinase C in cell motility. Cell Mol Life Sci. 2013;70:3057–3066. doi: 10.1007/s00018-012-1192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hirai T, Chida K. Protein kinase Czeta (PKCzeta): activation mechanisms and cellular functions. J Biochem. 2003;133:1–7. doi: 10.1093/jb/mvg017. [DOI] [PubMed] [Google Scholar]
- 108.Beeson M, Sajan MP, Daspet JG, Luna V, Dizon M, Grebenev D, Powe JL, Lucidi S, Miura A, Kanoh Y, Bandyopadhyay G, Standaert ML, Yeko TR, Farese RV. Defective Activation of Protein Kinase C-z in Muscle by Insulin and Phosphatidylinositol-3,4,5,-(PO(4))(3) in Obesity and Polycystic Ovary Syndrome. Metab Syndr Relat Disord. 2004;2:49–56. doi: 10.1089/met.2004.2.49. [DOI] [PubMed] [Google Scholar]
- 109.Vollenweider P, Menard B, Nicod P. Insulin resistance, defective insulin receptor substrate 2-associated phosphatidylinositol-3′ kinase activation, and impaired atypical protein kinase C (zeta/lambda) activation in myotubes from obese patients with impaired glucose tolerance. Diabetes. 2002;51:1052–1059. doi: 10.2337/diabetes.51.4.1052. [DOI] [PubMed] [Google Scholar]
- 110.Luna V, Casauban L, Sajan MP, Gomez-Daspet J, Powe JL, Miura A, Rivas J, Standaert ML, Farese RV. Metformin improves atypical protein kinase C activation by insulin and phosphatidylinositol-3,4,5-(PO4)3 in muscle of diabetic subjects. Diabetologia. 2006;49:375–382. doi: 10.1007/s00125-005-0112-4. [DOI] [PubMed] [Google Scholar]
- 111.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 113.Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 114.Liang F, Kume S, Koya D. SIRT1 and insulin resistance. Nat Rev Endocrinol. 2009;5:367–373. doi: 10.1038/nrendo.2009.101. [DOI] [PubMed] [Google Scholar]
- 115.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang J. The direct involvement of SirT1 in insulin-induced insulin receptor substrate-2 tyrosine phosphorylation. J Biol Chem. 2007;282:34356–34364. doi: 10.1074/jbc.M706644200. [DOI] [PubMed] [Google Scholar]
- 117.Yoshizaki T, Milne JC, Imamura T, Schenk S, Sonoda N, Babendure JL, Lu JC, Smith JJ, Jirousek MR, Olefsky JM. SIRT1 exerts anti-inflammatory effects and improves insulin sensitivity in adipocytes. Mol Cell Biol. 2009;29:1363–1374. doi: 10.1128/MCB.00705-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 119.Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 120.Richter EA, Ruderman NB. AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J. 2009;418:261–275. doi: 10.1042/BJ20082055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sriwijitkamol A, Coletta DK, Wajcberg E, Balbontin GB, Reyna SM, Barrientes J, Eagan PA, Jenkinson CP, Cersosimo E, DeFronzo RA, Sakamoto K, Musi N. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes. 2007;56:836–848. doi: 10.2337/db06-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.De Filippis E, Alvarez G, Berria R, Cusi K, Everman S, Meyer C, Mandarino LJ. Insulin-resistant muscle is exercise resistant: evidence for reduced response of nuclear-encoded mitochondrial genes to exercise. Am J Physiol Endocrinol Metab. 2008;294:E607–614. doi: 10.1152/ajpendo.00729.2007. [DOI] [PubMed] [Google Scholar]
- 123.Gauthier MS, O'Brien EL, Bigornia S, Mott M, Cacicedo JM, Xu XJ, Gokce N, Apovian C, Ruderman N. Decreased AMP-activated protein kinase activity is associated with increased inflammation in visceral adipose tissue and with whole-body insulin resistance in morbidly obese humans. Biochem Biophys Res Commun. 2011;404:382–387. doi: 10.1016/j.bbrc.2010.11.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yu X, McCorkle S, Wang M, Lee Y, Li J, Saha AK, Unger RH, Ruderman NB. Leptinomimetic effects of the AMP kinase activator AICAR in leptin-resistant rats: prevention of diabetes and ectopic lipid deposition. Diabetologia. 2004;47:2012–2021. doi: 10.1007/s00125-004-1570-9. [DOI] [PubMed] [Google Scholar]
- 125.Saha AK, Avilucea PR, Ye JM, Assifi MM, Kraegen EW, Ruderman NB. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun. 2004;314:580–585. doi: 10.1016/j.bbrc.2003.12.120. [DOI] [PubMed] [Google Scholar]
- 126.Chopra I, Li HF, Wang H, Webster KA. Phosphorylation of the insulin receptor by AMP-activated protein kinase (AMPK) promotes ligand-independent activation of the insulin signalling pathway in rodent muscle. Diabetologia. 2012;55:783–794. doi: 10.1007/s00125-011-2407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Okada T, Liew CW, Hu J, Hinault C, Michael MD, Krtzfeldt J, Yin C, Holzenberger M, Stoffel M, Kulkarni RN. Insulin receptors in beta-cells are critical for islet compensatory growth response to insulin resistance. Proc Natl Acad Sci U S A. 2007;104:8977–8982. doi: 10.1073/pnas.0608703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kandel ES, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res. 1999;253:210–229. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- 129.Fatrai S, Elghazi L, Balcazar N, Cras-Meneur C, Krits I, Kiyokawa H, Bernal-Mizrachi E. Akt induces betacell proliferation by regulating cyclin D1, cyclin D2, and p21 levels and cyclin-dependent kinase-4 activity. Diabetes. 2006;55:318–325. doi: 10.2337/diabetes.55.02.06.db05-0757. [DOI] [PubMed] [Google Scholar]
- 130.Wrede CE, Dickson LM, Lingohr MK, Briaud I, Rhodes CJ. Protein kinase B/Akt prevents fatty acid-induced apoptosis in pancreatic beta-cells (INS-1). J Biol Chem. 2002;277:49676–49684. doi: 10.1074/jbc.M208756200. [DOI] [PubMed] [Google Scholar]
- 131.Skolnik EY, Lee CH, Batzer A, Vicentini LM, Zhou M, Daly R, Myers MJ, Jr., Backer JM, Ullrich A, White MF, et al. The SH2/SH3 domain-containing protein GRB2 interacts with tyrosine-phosphorylated IRS1 and Shc: implications for insulin control of ras signalling. EMBO J. 1993;12:1929–1936. doi: 10.1002/j.1460-2075.1993.tb05842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sachdeva MM, Stoffers DA. Minireview: Meeting the demand for insulin: molecular mechanisms of adaptive postnatal beta-cell mass expansion. Mol Endocrinol. 2009;23:747–758. doi: 10.1210/me.2008-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 134.Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nakae J, Kitamura T, Silver DL, Accili D. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest. 2001;108:1359–1367. doi: 10.1172/JCI12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Altomonte J, Richter A, Harbaran S, Suriawinata J, Nakae J, Thung SN, Meseck M, Accili D, Dong H. Inhibition of Foxo1 function is associated with improved fasting glycemia in diabetic mice. Am J Physiol Endocrinol Metab. 2003;285:E718–728. doi: 10.1152/ajpendo.00156.2003. [DOI] [PubMed] [Google Scholar]
- 137.Shao W, Espenshade PJ. Expanding roles for SREBP in metabolism. Cell Metab. 2012;16:414–419. doi: 10.1016/j.cmet.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Du X, Kristiana I, Wong J, Brown AJ. Involvement of Akt in ER-to-Golgi transport of SCAP/SREBP: a link between a key cell proliferative pathway and membrane synthesis. Mol Biol Cell. 2006;17:2735–2745. doi: 10.1091/mbc.E05-11-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yellaturu CR, Deng X, Cagen LM, Wilcox HG, Mansbach CM, 2nd, Siddiqi SA, Park EA, Raghow R, Elam MB. Insulin enhances post-translational processing of nascent SREBP-1c by promoting its phosphorylation and association with COPII vesicles. J Biol Chem. 2009;284:7518–7532. doi: 10.1074/jbc.M805746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Matsumoto M, Ogawa W, Akimoto K, Inoue H, Miyake K, Furukawa K, Hayashi Y, Iguchi H, Matsuki Y, Hiramatsu R, Shimano H, Yamada N, Ohno S, Kasuga M, Noda T. PKClambda in liver mediates insulininduced SREBP-1c expression and determines both hepatic lipid content and overall insulin sensitivity. J Clin Invest. 2003;112:935–944. doi: 10.1172/JCI18816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sajan MP, Standaert ML, Rivas J, Miura A, Kanoh Y, Soto J, Taniguchi CM, Kahn CR, Farese RV. Role of atypical protein kinase C in activation of sterol regulatory element binding protein-1c and nuclear factor kappa B (NFkappaB) in liver of rodents used as a model of diabetes, and relationships to hyperlipidaemia and insulin resistance. Diabetologia. 2009;52:1197–1207. doi: 10.1007/s00125-009-1336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med. 2011;50:567–575. doi: 10.1016/j.freeradbiomed.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jain SK, McVie R, Duett J, Herbst JJ. Erythrocyte membrane lipid peroxidation and glycosylated hemoglobin in diabetes. Diabetes. 1989;38:1539–1543. doi: 10.2337/diab.38.12.1539. [DOI] [PubMed] [Google Scholar]
- 144.Jain SK. Hyperglycemia can cause membrane lipid peroxidation and osmotic fragility in human red blood cells. J Biol Chem. 1989;264:21340–21345. [PubMed] [Google Scholar]
- 145.Jain SK, Levine SN, Duett J, Hollier B. Elevated lipid peroxidation levels in red blood cells of streptozotocintreated diabetic rats. Metabolism. 1990;39:971–975. doi: 10.1016/0026-0495(90)90310-9. [DOI] [PubMed] [Google Scholar]
- 146.Jain SK, Shohet SB. Calcium potentiates the peroxidation of erythrocyte membrane lipids. Biochim Biophys Acta. 1981;642:46–54. doi: 10.1016/0005-2736(81)90136-x. [DOI] [PubMed] [Google Scholar]
- 147.Martinovic V, Grigorov I, Bogojevic D, Petrovic A, Jovanovic S, Ilic M, Ivanovic Matic S. Activation level of JNK and Akt/ERK signaling pathways determinates extent of DNA damage in the liver of diabetic rats. Cell Physiol Biochem. 2012;30:723–734. doi: 10.1159/000341452. [DOI] [PubMed] [Google Scholar]
- 148.Tremblay F, Lavigne C, Jacques H, Marette A. Defective insulin-induced GLUT4 translocation in skeletal muscle of high fat-fed rats is associated with alterations in both Akt/protein kinase B and atypical protein kinase C (zeta/lambda) activities. Diabetes. 2001;50:1901–1910. doi: 10.2337/diabetes.50.8.1901. [DOI] [PubMed] [Google Scholar]
- 149.Yun SJ, Kim EK, Tucker DF, Kim CD, Birnbaum MJ, Bae SS. Isoform-specific regulation of adipocyte differentiation by Akt/protein kinase Balpha. Biochem Biophys Res Commun. 2008;371:138–143. doi: 10.1016/j.bbrc.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 150.Xu J, Liao K. Protein kinase B/AKT 1 plays a pivotal role in insulin-like growth factor-1 receptor signaling induced 3T3-L1 adipocyte differentiation. J Biol Chem. 2004;279:35914–35922. doi: 10.1074/jbc.M402297200. [DOI] [PubMed] [Google Scholar]
- 151.Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 152.Baudry A, Yang ZZ, Hemmings BA. PKBalpha is required for adipose differentiation of mouse embryonic fibroblasts. J Cell Sci. 2006;119:889–897. doi: 10.1242/jcs.02792. [DOI] [PubMed] [Google Scholar]
- 153.Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 154.Porstmann T, Griffiths B, Chung YL, Delpuech O, Griffiths JR, Downward J, Schulze A. PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene. 2005;24:6465–6481. doi: 10.1038/sj.onc.1208802. [DOI] [PubMed] [Google Scholar]
- 155.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 156.Menghini R, Marchetti V, Cardellini M, Hribal ML, Mauriello A, Lauro D, Sbraccia P, Lauro R, Federici M. Phosphorylation of GATA2 by Akt increases adipose tissue differentiation and reduces adipose tissuerelated inflammation: a novel pathway linking obesity to atherosclerosis. Circulation. 2005;111:1946–1953. doi: 10.1161/01.CIR.0000161814.02942.B2. [DOI] [PubMed] [Google Scholar]
- 157.Porstmann T, Santos CR, Griffiths B, Cully M, Wu M, Leevers S, Griffiths JR, Chung YL, Schulze A. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 2008;8:224–236. doi: 10.1016/j.cmet.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 159.Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- 160.Lin X, Taguchi A, Park S, Kushner JA, Li F, Li Y, White MF. Dysregulation of insulin receptor substrate 2 in beta cells and brain causes obesity and diabetes. J Clin Invest. 2004;114:908–916. doi: 10.1172/JCI22217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV. Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet. 1996;348:159–161. doi: 10.1016/s0140-6736(96)03173-x. [DOI] [PubMed] [Google Scholar]
- 162.Sartorius T, Heni M, Tschritter O, Preissl H, Hopp S, Fritsche A, Lievertz PS, Gertler A, Berthou F, Taouis M, Staiger H, Haring HU, Hennige AM. Leptin affects insulin action in astrocytes and impairs insulin-mediated physical activity. Cell Physiol Biochem. 2012;30:238–246. doi: 10.1159/000339060. [DOI] [PubMed] [Google Scholar]
- 163.Yang X, Ongusaha PP, Miles PD, Havstad JC, Zhang F, So WV, Kudlow JE, Michell RH, Olefsky JM, Field SJ, Evans RM. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- 164.Ande SR, Mishra S. Prohibitin interacts with phosphatidylinositol 3,4,5-triphosphate (PIP3) and modulates insulin signaling. Biochem Biophys Res Commun. 2009;390:1023–1028. doi: 10.1016/j.bbrc.2009.10.101. [DOI] [PubMed] [Google Scholar]
- 165.Stefater MA, Seeley RJ. Central nervous system nutrient signaling: the regulation of energy balance and the future of dietary therapies. Annu Rev Nutr. 2010;30:219–235. doi: 10.1146/annurev.nutr.012809.104723. [DOI] [PubMed] [Google Scholar]
- 166.Niswender KD, Schwartz MW. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Front Neuroendocrinol. 2003;24:1–10. doi: 10.1016/s0091-3022(02)00105-x. [DOI] [PubMed] [Google Scholar]
- 167.Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Munzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R, Alber J, Belgardt BF, Koch L, Seibler J, Schwenk F, Fekete C, Suzuki A, Mak TW, Krone W, Horvath TL, Ashcroft FM, Bruning JC. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest. 2006;116:1886–1901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Poirier P, Eckel RH. Obesity and cardiovascular disease. Curr Atheroscler Rep. 2002;4:448–453. doi: 10.1007/s11883-002-0049-8. [DOI] [PubMed] [Google Scholar]
- 169.Chrostowska M, Szyndler A, Hoffmann M, Narkiewicz K. Impact of obesity on cardiovascular health. Best Pract Res Clin Endocrinol Metab. 2013;27:147–156. doi: 10.1016/j.beem.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 170.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068. [DOI] [PubMed] [Google Scholar]
- 171.Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, Taskinen MR, Groop L. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 172.Oudit GY, Penninger JM. Cardiac regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc Res. 2009;82:250–260. doi: 10.1093/cvr/cvp014. [DOI] [PubMed] [Google Scholar]
- 173.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 174.Crackower MA, Oudit GY, Kozieradzki I, Sarao R, Sun H, Sasaki T, Hirsch E, Suzuki A, Shioi T, Irie-Sasaki J, Sah R, Cheng HY, Rybin VO, Lembo G, Fratta L, Oliveira-dos-Santos AJ, Benovic JL, Kahn CR, Izumo S, Steinberg SF, Wymann MP, Backx PH, Penninger JM. Regulation of myocardial contractility and cell size by distinct PI3K-PTEN signaling pathways. Cell. 2002;110:737–749. doi: 10.1016/s0092-8674(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 175.Murphy E, Tong H, Steenbergen C. Preconditioning: is the Akt-ion in the PI3K pathway? J Mol Cell Cardiol. 2003;35:1021–1025. doi: 10.1016/s0022-2828(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 176.Siddall HK, Warrell CE, Yellon DM, Mocanu MM. Ischemia-reperfusion injury and cardioprotection: investigating PTEN, the phosphatase that negatively regulates PI3K, using a congenital model of PTEN haploinsufficiency. Basic Res Cardiol. 2008;103:560–568. doi: 10.1007/s00395-008-0735-y. [DOI] [PubMed] [Google Scholar]
- 177.McMullen JR, Amirahmadi F, Woodcock EA, Schinke-Braun M, Bouwman RD, Hewitt KA, Mollica JP, Zhang L, Zhang Y, Shioi T, Buerger A, Izumo S, Jay PY, Jennings GL. Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signaling in dilated and hypertrophic cardiomyopathy. Proc Natl Acad Sci U S A. 2007;104:612–617. doi: 10.1073/pnas.0606663104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chaanine AH, Hajjar RJ. AKT signalling in the failing heart. Eur J Heart Fail. 2011;13:825–829. doi: 10.1093/eurjhf/hfr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sugden PH, Fuller SJ, Weiss SC, Clerk A. Glycogen synthase kinase 3 (GSK3) in the heart: a point of integration in hypertrophic signalling and a therapeutic target? A critical analysis. Br J Pharmacol. 2008;153(Suppl 1):S137–153. doi: 10.1038/sj.bjp.0707659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–2104. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- 181.DeBosch B, Sambandam N, Weinheimer C, Courtois M, Muslin AJ. Akt2 regulates cardiac metabolism and cardiomyocyte survival. J Biol Chem. 2006;281:32841–32851. doi: 10.1074/jbc.M513087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hers I, Vincent EE, Tavare JM. Akt signalling in health and disease. Cell Signal. 2011;23:1515–1527. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 183.Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, Cantley LC, Izumo S. Akt/protein kinase B promotes organ growth in transgenic mice. Mol Cell Biol. 2002;22:2799–2809. doi: 10.1128/MCB.22.8.2799-2809.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, Russo MA, Gu Y, Dalton N, Chung C, Latronico MV, Napoli C, Sadoshima J, Croce CM, Ross J., Jr. Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:12333–12338. doi: 10.1073/pnas.172376399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cook SA, Matsui T, Li L, Rosenzweig A. Transcriptional effects of chronic Akt activation in the heart. J Biol Chem. 2002;277:22528–22533. doi: 10.1074/jbc.M201462200. [DOI] [PubMed] [Google Scholar]
- 186.Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, Richardson JA, Hill JA, Olson EN. Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2002;99:907–912. doi: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res. 2006;98:596–605. doi: 10.1161/01.RES.0000207406.94146.c2. [DOI] [PubMed] [Google Scholar]
- 188.Kim KH, Oudit GY, Backx PH. Erythropoietin protects against doxorubicin-induced cardiomyopathy via a phosphatidylinositol 3-kinase-dependent pathway. J Pharmacol Exp Ther. 2008;324:160–169. doi: 10.1124/jpet.107.125773. [DOI] [PubMed] [Google Scholar]
- 189.Oudit GY, Crackower MA, Eriksson U, Sarao R, Kozieradzki I, Sasaki T, Irie-Sasaki J, Gidrewicz D, Rybin VO, Wada T, Steinberg SF, Backx PH, Penninger JM. Phosphoinositide 3-kinase gamma-deficient mice are protected from isoproterenol-induced heart failure. Circulation. 2003;108:2147–2152. doi: 10.1161/01.CIR.0000091403.62293.2B. [DOI] [PubMed] [Google Scholar]
- 190.Carnevale D, Lembo G. PI3Kgamma in hypertension: a novel therapeutic target controlling vascular myogenic tone and target organ damage. Cardiovasc Res. 2012;95:403–408. doi: 10.1093/cvr/cvs166. [DOI] [PubMed] [Google Scholar]
- 191.Liu B, Bhat M, Nagaraj RH. AlphaB-crystallin inhibits glucose-induced apoptosis in vascular endothelial cells. Biochem Biophys Res Commun. 2004;321:254–258. doi: 10.1016/j.bbrc.2004.06.151. [DOI] [PubMed] [Google Scholar]
- 192.Dang L, Seale JP, Qu X. Reduction of high glucose and phorbol-myristate-acetate-induced endothelial cell permeability by protein kinase C inhibitors LY379196 and hypocrellin A. Biochem Pharmacol. 2004;67:855–864. doi: 10.1016/j.bcp.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 193.Kim JA, Berliner JA, Natarajan RD, Nadler JL. Evidence that glucose increases monocyte binding to human aortic endothelial cells. Diabetes. 1994;43:1103–1107. doi: 10.2337/diab.43.9.1103. [DOI] [PubMed] [Google Scholar]
- 194.Nandy D, Janardhanan R, Mukhopadhyay D, Basu A. Effect of hyperglycemia on human monocyte activation. J Investig Med. 2011;59:661–667. doi: 10.231/JIM.0b013e31820ee432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 196.Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci U S A. 2000;97:1749–1753. doi: 10.1073/pnas.040560897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.McGinn S, Saad S, Poronnik P, Pollock CA. High glucose-mediated effects on endothelial cell proliferation occur via p38 MAP kinase. Am J Physiol Endocrinol Metab. 2003;285:E708–717. doi: 10.1152/ajpendo.00572.2002. [DOI] [PubMed] [Google Scholar]
- 198.Varma S, Lal BK, Zheng R, Breslin JW, Saito S, Pappas PJ, Hobson RW, 2nd, Duran WN. Hyperglycemia alters PI3k and Akt signaling and leads to endothelial cell proliferative dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H1744–1751. doi: 10.1152/ajpheart.01088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Manna P, Jain SK. Effect of PIP3 on adhesion molecules and adhesion of THP-1 monocytes to HUVEC treated with high glucose. Cell Physiol Biochem. 2014;33:1197–1204. doi: 10.1159/000358688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat Rev Immunol. 2003;3:317–330. doi: 10.1038/nri1056. [DOI] [PubMed] [Google Scholar]
- 201.Blunt MD, Ward SG. Pharmacological targeting of phosphoinositide lipid kinases and phosphatases in the immune system: success, disappointment, and new opportunities. Front Immunol. 2012;3:226. doi: 10.3389/fimmu.2012.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Blunt MD, Ward SG. Targeting PI3K isoforms and SHIP in the immune system: new therapeutics for inflammation and leukemia. Curr Opin Pharmacol. 2012;12:444–451. doi: 10.1016/j.coph.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 203.Kashiwada M, Lu P, Rothman PB. PIP3 pathway in regulatory T cells and autoimmunity. Immunol Res. 2007;39:194–224. doi: 10.1007/s12026-007-0075-2. [DOI] [PubMed] [Google Scholar]
- 204.Hawkins PT, Stephens LR, Suire S, Wilson M. PI3K signaling in neutrophils. Curr Top Microbiol Immunol. 2010;346:183–202. doi: 10.1007/82_2010_40. [DOI] [PubMed] [Google Scholar]
- 205.Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287:1049–1053. doi: 10.1126/science.287.5455.1049. [DOI] [PubMed] [Google Scholar]
- 206.Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, Wakeham A, Itie A, Bouchard D, Kozieradzki I, Joza N, Mak TW, Ohashi PS, Suzuki A, Penninger JM. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 207.Sadhu C, Masinovsky B, Dick K, Sowell CG, Staunton DE. Essential role of phosphoinositide 3-kinase delta in neutrophil directional movement. J Immunol. 2003;170:2647–2654. doi: 10.4049/jimmunol.170.5.2647. [DOI] [PubMed] [Google Scholar]
- 208.Vemula S, Shi J, Hanneman P, Wei L, Kapur R. ROCK1 functions as a suppressor of inflammatory cell migration by regulating PTEN phosphorylation and stability. Blood. 2010;115:1785–1796. doi: 10.1182/blood-2009-08-237222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Condliffe AM, Davidson K, Anderson KE, Ellson CD, Crabbe T, Okkenhaug K, Vanhaesebroeck B, Turner M, Webb L, Wymann MP, Hirsch E, Ruckle T, Camps M, Rommel C, Jackson SP, Chilvers ER, Stephens LR, Hawkins PT. Sequential activation of class IB and class IA PI3K is important for the primed respiratory burst of human but not murine neutrophils. Blood. 2005;106:1432–1440. doi: 10.1182/blood-2005-03-0944. [DOI] [PubMed] [Google Scholar]
- 210.Perisic O, Wilson MI, Karathanassis D, Bravo J, Pacold ME, Ellson CD, Hawkins PT, Stephens L, Williams RL. The role of phosphoinositides and phosphorylation in regulation of NADPH oxidase. Adv Enzyme Regul. 2004;44:279–298. doi: 10.1016/j.advenzreg.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 211.Fung-Leung WP. Phosphoinositide 3-kinase delta (PI3Kdelta) in leukocyte signaling and function. Cell Signal. 2011;23:603–608. doi: 10.1016/j.cellsig.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 212.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 213.Williams CM, Galli SJ. The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. J Allergy Clin Immunol. 2000;105:847–859. doi: 10.1067/mai.2000.106485. [DOI] [PubMed] [Google Scholar]
- 214.Rommel C, Camps M, Ji H. PI3K delta and PI3K gamma: partners in crime in inflammation in rheumatoid arthritis and beyond? Nat Rev Immunol. 2007;7:191–201. doi: 10.1038/nri2036. [DOI] [PubMed] [Google Scholar]
- 215.Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E, Fox R, Bruce I, Walker C, Sawyer C, Okkenhaug K, Finan P, Vanhaesebroeck B. Essential role for the p110delta phosphoinositide 3-kinase in the allergic response. Nature. 2004;431:1007–1011. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- 216.Laffargue M, Calvez R, Finan P, Trifilieff A, Barbier M, Altruda F, Hirsch E, Wymann MP. Phosphoinositide 3-kinase gamma is an essential amplifier of mast cell function. Immunity. 2002;16:441–451. doi: 10.1016/s1074-7613(02)00282-0. [DOI] [PubMed] [Google Scholar]
- 217.Furumoto Y, Charles N, Olivera A, Leung WH, Dillahunt S, Sargent JL, Tinsley K, Odom S, Scott E, Wilson TM, Ghoreschi K, Kneilling M, Chen M, Lee DM, Bolland S, Rivera J. PTEN deficiency in mast cells causes a mastocytosis-like proliferative disease that heightens allergic responses and vascular permeability. Blood. 2011;118:5466–5475. doi: 10.1182/blood-2010-09-309955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Furumoto Y, Brooks S, Olivera A, Takagi Y, Miyagishi M, Taira K, Casellas R, Beaven MA, Gilfillan AM, Rivera J. Cutting Edge: Lentiviral short hairpin RNA silencing of PTEN in human mast cells reveals constitutive signals that promote cytokine secretion and cell survival. J Immunol. 2006;176:5167–5171. doi: 10.4049/jimmunol.176.9.5167. [DOI] [PubMed] [Google Scholar]
- 219.Shenker BJ, Ali H, Boesze-Battaglia K. PIP3 regulation as promising targeted therapy of mast-cell-mediated diseases. Curr Pharm Des. 2011;17:3815–3822. doi: 10.2174/138161211798357926. [DOI] [PubMed] [Google Scholar]
- 220.Okkenhaug K, Fruman DA. PI3Ks in lymphocyte signaling and development. Curr Top Microbiol Immunol. 2010;346:57–85. doi: 10.1007/82_2010_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Reif K, Okkenhaug K, Sasaki T, Penninger JM, Vanhaesebroeck B, Cyster JG. Cutting edge: differential roles for phosphoinositide 3-kinases, p110gamma and p110delta, in lymphocyte chemotaxis and homing. J Immunol. 2004;173:2236–2240. doi: 10.4049/jimmunol.173.4.2236. [DOI] [PubMed] [Google Scholar]
- 222.Ohashi PS. T-cell signalling and autoimmunity: molecular mechanisms of disease. Nat Rev Immunol. 2002;2:427–438. doi: 10.1038/nri822. [DOI] [PubMed] [Google Scholar]
- 223.Okkenhaug K, Bilancio A, Farjot G, Priddle H, Sancho S, Peskett E, Pearce W, Meek SE, Salpekar A, Waterfield MD, Smith AJ, Vanhaesebroeck B. Impaired B and T cell antigen receptor signaling in p110delta PI 3-kinase mutant mice. Science. 2002;297:1031–1034. doi: 10.1126/science.1073560. [DOI] [PubMed] [Google Scholar]
- 224.Silhi N. Diabetes and wound healing. J Wound Care. 1998;7:47–51. doi: 10.12968/jowc.1998.7.1.47. [DOI] [PubMed] [Google Scholar]
- 225.Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 226.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 227.Siqueira MF, Li J, Chehab L, Desta T, Chino T, Krothpali N, Behl Y, Alikhani M, Yang J, Braasch C, Graves DT. Impaired wound healing in mouse models of diabetes is mediated by TNF-alpha dysregulation and associated with enhanced activation of forkhead box O1 (FOXO1). Diabetologia. 2010;53:378–388. doi: 10.1007/s00125-009-1529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Peranteau WH, Zhang L, Muvarak N, Badillo AT, Radu A, Zoltick PW, Liechty KW. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Invest Dermatol. 2008;128:1852–1860. doi: 10.1038/sj.jid.5701232. [DOI] [PubMed] [Google Scholar]
- 229.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:513–521. [PubMed] [Google Scholar]
- 230.Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127:998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 231.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol. 2007;178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 232.Seitz O, Schurmann C, Hermes N, Muller E, Pfeilschifter J, Frank S, Goren I. Wound healing in mice with high-fat diet-or ob gene-induced diabetes-obesity syndromes: a comparative study. Exp Diabetes Res. 2010;2010:476969. doi: 10.1155/2010/476969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Badr G. Supplementation with undenatured whey protein during diabetes mellitus improves the healing and closure of diabetic wounds through the rescue of functional long-lived wound macrophages. Cell Physiol Biochem. 2012;29:571–582. doi: 10.1159/000338511. [DOI] [PubMed] [Google Scholar]