Abstract
Leptin binds to receptors in multiple hypothalamic nuclei to increase sympathetic nerve activity; however, the neurocircuitry is unclear. Here, using anesthetized male Sprague-Dawley rats, we investigated the role of the paraventricular nucleus of the hypothalamus. Intracerebroventricular injection of leptin slowly increased lumbar sympathetic nerve activity, heart rate, and mean arterial pressure, as well as baroreflex control of lumbar sympathetic nerve activity and heart rate. Inhibition of the paraventricular nucleus with muscimol completely reversed leptin’s effects. Blockade of paraventricular melanocortin 3/4 receptors with SHU9119 or ionotropic glutamate receptors with kynurenate, alone or together, each partially reversed the effects of leptin, implicating increased activation of glutamate and melanocortin 3/4 receptors. Conversely, while blockade of Neuropeptide Y Y1 receptors in the paraventricular nucleus increased lumbar sympathetic nerve activity, mean arterial pressure, and heart rate, these responses were prevented by intracerebroventricular or arcuate nucleus injections of leptin, suggesting that, at least in part, leptin also increases sympathetic nerve activity by suppression of tonic Neuropeptide Y inhibitory inputs from the arcuate nucleus. Injection of the melanocortin 3/4 receptor agonist melanotan-II into the paraventricular nucleus increased lumbar sympathetic nerve activity, mean arterial pressure, and heart rate only after blockade of Neuropeptide Y Y1 receptors. Therefore, we conclude that leptin increases lumbar sympathetic nerve activity in part via increased glutamatergic and α-melanocyte stimulating hormone drive of paraventricular sympathoexcitatory neurons, the latter of which requires simultaneous withdrawal of tonic Neuropeptide Y inhibition.
Keywords: sympathetic nerve activity, male rats, arcuate nucleus, baroreflex, arterial pressure, SHU9119, kynurenate, BIBO 3304
INTRODUCTION
Leptin acts centrally to increase the activity of sympathetic nerves innervating several organs [for review, see 1], including the kidneys (renal sympathetic nerve activity; RSNA), the hindquarters (lumbar SNA; LSNA), brown adipose tissue (BATSNA), and the splanchnic circulation (SSNA).2–4 Multiple hypothalamic nuclei have been identified as sites that initiate the increases in SNA: the ventral medial hypothalamus (VMH), arcuate nucleus (ArcN), dorsal medial hypothalamus (DMH), and lateral hypothalamus (LH).5–10 Leptin has also been shown to increase arterial pressure (AP) when administered into the paraventricular nucleus of the hypothalamus (PVN),8, 11 but increases in SNA have not been observed.5, 8 More recently, the subfornical organ was shown to be required for the action of leptin in plasma to increase RSNA, but not BATSNA.12
While leptin acts in many brain sites to initiate its sympathoexcitatory effects, only limited information is available regarding the neurocircuitry that mediates increased SNA following leptin receptor binding. All hypothalamic sites that respond to leptin send projections to the PVN.13–15 Therefore, the first purpose of the present study was to test if these pathways converge in the PVN, by determining if local inhibition of the PVN, via bilateral nanoinjection of the GABAA agonist, muscimol, completely reverses the effects of leptin to increase LSNA. We infused leptin icv so that multiple nuclei could be targeted, we measured LSNA, since this sympathetic nerve has been shown to be activated by leptin in many hypothalamic sites,8 and we measured baroreflex function so that the effects of leptin could be assessed over a range of arterial pressure levels.
Two potential excitatory inputs to the PVN that may mediate the sympathoexcitatory effects of leptin are α-melanocyte stimulating hormone (α-MSH) and glutamate. Indeed, intracerebroventricular (icv) administration of α-MSH or the MC3/4R agonist melanotan II (MTII) increased SNA,16–19 whereas broad brain inhibition of MC3/4R with icv SHU9119 or Agouti-Related Protein (AgRP) blocked the sympathoexcitatory effects of leptin.16, 17 α-MSH is derived and released from pro-opiomelanocortin (POMC) neurons, which are situated in the ArcN and project to several hypothalamic sites involved in autonomic regulation including the PVN, 14, 20, 21 but the specific role of PVN MC3/4 is unclear. In parallel, the PVN receives glutamatergic inputs from many sites at which leptin increases SNA, including the VMH, DMH, ArcN, and LH.15 Moreover, a subset of ArcN POMC neurons coexpress the glutamate vesicular transporter VGLUT-2,22 and blockade of PVN glutamate receptors reduces the sympathoexcitatory effects of nonspecific chemical stimulation of the ArcN.23 In addition to excitatory inputs, inhibitory ArcN Neuropeptide Y (NPY) neurons project to the PVN.24, 25 ArcN leptin inhibits NPY neurons,26, 27 and NPY inhibits the firing of PVN neurons that are also excited by increases in plasma leptin19 or by α-MSH.24 Therefore, to test the involvement of PVN α-MSH, glutamate and NPY inputs, we performed a series of nanoinjection experiments to block PVN MC3/4R, glutamatergic ionotropic receptors, and NPY Y1 receptors following leptin or aCSF administration.
METHODS
An expanded Methods section is available in the Online Supplement at http://hyper.ahajournals.org.
Experimental protocols
All procedures involving rats were approved by the OHSU Animal Care and Use Committee. Briefly, male Sprague-Dawley rats were anesthetized with isoflurane and prepared for icv infusions, PVN or ArcN nanoinjections, and for measurements of mean AP (MAP), heart rate (HR), LSNA, and baroreflex control of LSNA and HR, as previously described.4, 24 Then, a loading dose of α-chloralose (50 mg kg−1) was administered intravenously (iv) over 30 min, while isoflurane was slowly withdrawn, and this was followed by a continuous infusion (25 mg kg−1 h−1) for the duration of the experiment. Protocol 1. What is the role of PVN α-MSH, glutamatergic, or NPY inputs in the effects of leptin? After baseline measurements of LSNA, MAP, HR and baroreflex function, an icv infusion of leptin (3 μg in 3 μL, followed by 5 μg/hr) or the aCSF vehicle commenced. After 60 min, measurements were repeated, and 30 min later one of the following was injected bilaterally into the PVN: aCSF, muscimol, SHU9119 (blocks MC3/4R), kynurenic acid (KYN; blocks ionotropic glutamate receptors), SHU+KYN, or BIBO 3304 (blocks NPY Y1 receptors; NPY1x). After 10 min, final basal and baroreflex measurements were made. Protocol 2. Does ArcN leptin suppress sympathoinhibitory NPY inputs into the PVN? After baseline measurements, leptin (30 ng in 30 nL) or aCSF was injected bilaterally into the ArcN. MAP, HR, and LSNA were continuously recorded for 2 hr, at which time either NPY1x or aCSF was injected bilaterally into the PVN. Measurements continued for a final 30 min. Protocol 3. Is reduced tonic PVN NPY inhibition required for α-MSH excitation of PVN presympathetic neurons? Because presympathetic neurons that are inhibited by NPY are also excited by α-MSH,24 we also determined if the increase in LSNA in response to PVN nanoinjection of the MC3/4R agonist MTII is greater after PVN NPY1 receptor blockade. After baseline measurements of LSNA, MAP, and HR were made, either aCSF or NPY1x was injected into the PVN. Thirty min later, either aCSF or the α-MSH agonist melanotan-II (MTII) was injected into the PVN, and measurements were continued for an additional 30 min.
Statistical Analysis
All data are presented as means ± SEM. Between-group differences were evaluated using one- or two-way ANOVA for repeated measures and the post hoc Newman–Keuls test. P values <0.05 were considered statistically significant.
RESULTS
PVN muscimol completely reverses the effects of icv leptin
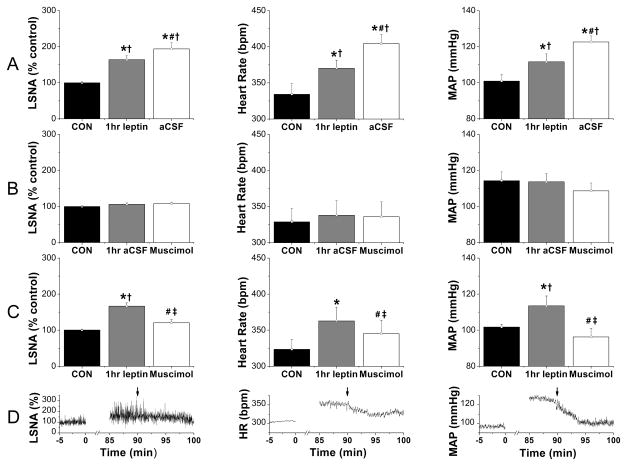
icv leptin increased LSNA, MAP, and HR after 1 hr (Figure 1). Consistent with its slowly developing effects,4, 10 these variables continued to rise (P<0.05, #) in rats receiving PVN aCSF injections 30 min later (Figure 1A), as shown previously in rats not receiving PVN injections.4 PVN muscimol had no effects in icv aCSF treated animals (Figures 1B, S1, and S2). However, in rats receiving icv leptin (Figure 1 C, D), muscimol decreased (P<0.05, #) LSNA, MAP, and HR to values not different from baseline control values or from values in rats receiving icv aCSF and PVN muscimol (Figure 1B).
Figure 1.
LSNA, HR, and MAP were increased 1 hr after icv leptin injection, and these responses were completely reversed by bilateral PVN nanoinjection of muscimol 30 min later. Groups are: A, icv leptin + PVN aCSF (n=7); B, icv leptin + PVN muscimol (n=6); and C, icv aCSF + PVN muscimol (n=3). D, Representative experiment showing icv leptin + PVN muscimol (at arrows). *: P<0.05 compared to baseline control values (CON); #: P<0.05 compared to 1 hr leptin within group; †: P<0.05 compared to icv aCSF at the same time; ‡: P<0.05, icv leptin plus PVN muscimol compared to icv leptin plus PVN aCSF.
As previously,4 1 hr icv leptin enhanced baroreflex control of LSNA by increasing (P<0.05) baroreflex gain (BRG), the baroreflex maximum, and the range (e.g. from 102±11 to 212±21% in the Leptin-aCSF group; P<0.05) (Figure S1). Forty min later, 10 min following PVN nanoinjection of aCSF, BRG, maximum and range (to 278±23%) were further increased (Figure S1; P<0.05, #), and the baroreflex minimum was elevated (Figure S1; P<0.05). In contrast, following PVN muscimol, BRG, the maximum and minimum, and the range [from 122±14% to 192±21% (leptin), then to 124±15% (muscimol), P<0.05] were decreased (P<0.05) back to baseline (Figure S1). Also as shown previously,4 leptin altered baroreflex control of HR by increasing HR at any given MAP without altering BRG; that is, the maximum and minimum, and the BP50 were elevated 100 min after leptin (P<0.05). Again, PVN muscimol completely reversed these effects (Figure S2). In contrast, nanoninjections of muscimol outside the PVN did not alter the effects of leptin (Figures S3 and S13).
Blockade of PVN MC3/4R or ionotropic glutamatergic receptors each partially reverses the effects of icv leptin
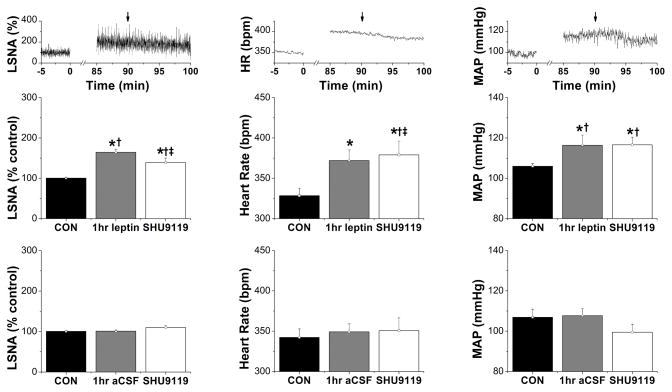
In rats receiving icv aCSF, nanoinjections of SHU9119 or KYN had no effects (Figures 2, 3, and S4–S7). On the other hand, following icv leptin and then PVN SHU9119, basal LSNA, HR and MAP failed to increase further compared to the levels at 60 min and were reduced (P<0.05, ‡), compared to rats given ICV leptin and PVN aCSF (Figure 1A). However, LSNA, HR and MAP remained elevated compared to baseline control values and to rats receiving icv aCSF and SHU9119 (Figure 2).
Figure 2.
LSNA, HR, and MAP were increased 1 hr following icv injection of leptin, and these changes were partially reversed by bilateral nanoinjection of SHU9119 into the PVN 30 min later. Top: Representative experiment showing icv leptin + PVN SHU9119 (at arrows). Groups are: middle, icv leptin + PVN SHU9119 (n=5); bottom, icv aCSF + PVN SHU9119 (n=6). *: P<0.05, compared to baseline control values (CON); †: P<0.05 compared to icv aCSF at the same time; ‡: P<0.05, icv leptin + PVN SHU9119 compared to icv leptin + PVN aCSF (Figure 1A).
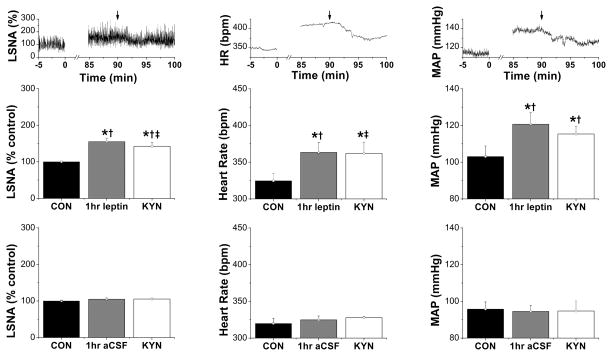
Figure 3.
LSNA, HR, and MAP were increased 1 hr following icv injection of leptin, and these changes were partially reversed by bilateral nanoinjection of KYN into the PVN 30 min later. Top: Representative experiment showing icv leptin + PVN KYN (at arrows). Groups are: middle, icv leptin + PVN KYN (n=6); bottom, icv aCSF + PVN KYN (n=4). *: P<0.05, compared to baseline control values (CON); †: P<0.05 compared to icv aCSF at the same time; ‡: P<0.05, compared to icv leptin plus PVN aCSF (Figure 1A), at the same time.
Similarly, while leptin enhanced baroreflex control of LSNA after 60 min (gain, maximum, minimum, and BP50; P<0.05; Figure S4), these variables did not increase further in animals receiving PVN SHU9119 30 min later. As a result, baroreflex gain and maximum were lower than in rats receiving leptin and PVN aCSF. The ability of leptin to increase baroreflex control of HR was similarly reduced by PVN SHU9119 (Figure S5). Nevertheless, while PVN SHU9119 partially reversed the effects of leptin to enhance baroreflex control of LSNA and HR, LSNA and HR baroreflex function remained elevated compared to pre-leptin baseline values (Figures S4 and S5).
The results were similar when PVN ionotropic glutamate receptors were blocked with KYN (Figures 3, S6, S7). KYN only partially reversed the effects of icv leptin to increase LSNA, HR, and MAP, as well as baroreflex control of LSNA and HR. Moreover, LSNA, HR and MAP, and baroreflex control of LSNA and HR, remained elevated compared to baseline control values and to rats receiving icv aCSF and PVN KYN.
Combined blockade of PVN MC3/4R and ionotropic glutamatergic receptors reverses the effects of icv leptin similarly to either alone
Because blockade of PVN MC3/4R and glutamate receptors each only partially reversed the effects of leptin, we next determined if combined blockade normalizes baseline values and baroreflex function following icv leptin. However, administration of SHU9119 followed 5 min later by KYN resulted in a similar reversal of the effects of leptin as either alone (Figures S8 or S9). More specifically, in rats 105 min after icv leptin, while PVN SHU+KYN decreased LSNA, HR, and MAP relative to rats receiving icv leptin and PVN aCSF (Figure 1A), these values remained elevated compared to baseline control values as well as values obtained in control animals receiving icv aCSF and either SHU9119 or KYN in the PVN. Moreover, PVN SHU+KYN did not reduce LSNA, HR, and MAP in icv leptin-infused rats more than either SHU9119 or KYN alone or compared to levels recorded after 60 min of leptin.
icv and ArcN leptin attenuate the sympathoexcitatory effects of blockade of PVN NPY Y1 receptors
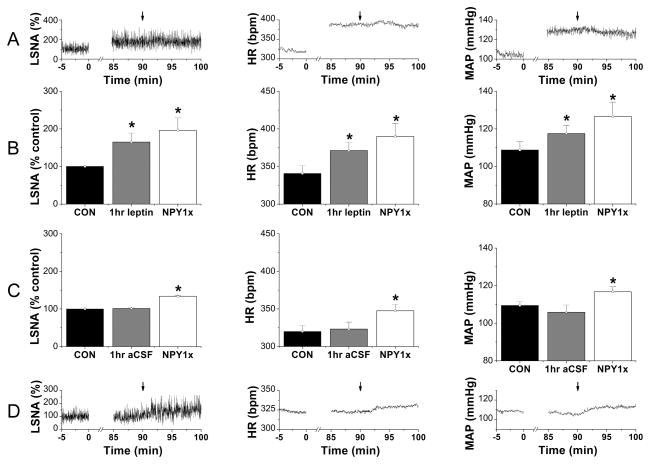
Ninety min after initiating the icv infusion of aCSF, as previously,24 PVN NPY1x increased LSNA, MAP and HR (Figure 4C, D) and baroreflex control of LSNA and HR (Figures S10 and S11). In contrast, after icv leptin, PVN NPY1x failed to further increase LSNA, MAP, and HR (Figure 4A, B). As a result, 10 min after PVN NPY1x, baseline LSNA, MAP, and HR and baroreflex control of LSNA and HR did not differ from values recorded after icv leptin and PVN injections of aCSF (Figure 1A). Similarly, ArcN leptin increased (P<0.05) LSNA, MAP (Figure S12), and HR (from 354±10 to 408±13 bpm), but 2 hr later, neither PVN aCSF nor PVN NPY1x elicited further increases, in contrast to the brisk elevations (P<0.05) in LSNA, MAP and HR (from 319±11 to 343±15 bpm) induced by PVN NPY1x after ArcN aCSF (Figure S12).
Figure 4.
Representative experiment (A) and group data (B) showing that bilateral PVN nanoninjection of NPY1x (at arrows) failed to increase LSNA, HR and MAP 90 min after icv leptin injection (n=6). As a result, the baseline values of LSNA, HR, and MAP (B) were not different from those obtained after PVN nanoinjection of aCSF (Figure 1A). In contrast, 90 min after aCSF injection, PVN NPY1x (n=6) increased LSNA, HR, and MAP both compared to control (CON) values (C) and values immediately prior to the injection (D).
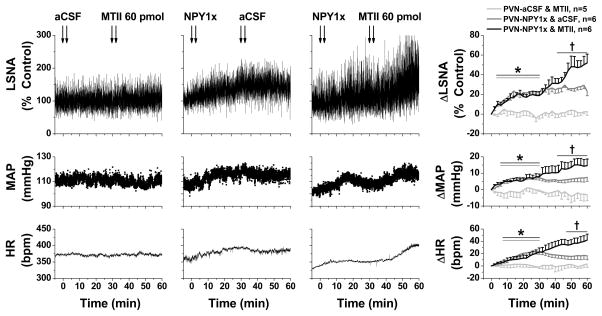
PVN MTII increases LSNA, MAP and HR only after PVN NPY1x
Following PVN aCSF injections, bilateral PVN MTII failed to alter LSNA, MAP or HR (Figure 5). As previously,24 PVN NPY1x induced sympathoexcitatory, pressor and tachycardic effects, which were not altered by PVN aCSF (Figure 5). However, following PVN NPY1x, subsequent PVN MTII produced significant increases in LSNA, MAP and HR (Figure 5).
Figure 5.
Representative experiments (left 3 panels) and grouped data (right) showing that: 1) PVN MTII after PVN aCSF (n=5; light gray symbols) has no effects on LSNA (top), MAP (middle), and HR (bottom); 2) PVN NPY1x (n=6; dark gray symbols) elicits increases in LSNA, MAP, and HR that reach stable values after ~20 min; and 3) PVN MTII after PVN NPY1x (n=6; black symbols) significantly increases LSNA, MAP, and HR. *: P<0.05 compared to time zero (before first injections). †: P<0.05 compared to values just before second PVN injections.
The histological locations of PVN and ArcN nanoinjections are illustrated in Figures S13 and S14.
DISCUSSION
The purpose of the present study was to begin to delineate the neurocircuitry by which brain leptin increases LSNA and its baroreflex regulation. Our major new findings are: 1) PVN inhibition with muscimol completely reversed the effects of icv leptin to increase LSNA, MAP, HR and baroreflex control of LSNA and HR; 2) Inhibition of PVN MC3/4R and ionotropic glutamate receptors each partially reversed the effects of leptin, and the effects of combined MC3/4R and glutamate receptor inhibition was similar to either alone; 3) The sympathoexcitatory, pressor and tachycardic effects of blockade of PVN NPY Y1 receptors were eliminated by prior icv or ArcN leptin administration; and 4) PVN nanoinjection of the α-MSH agonist MTII increased SNA only after blockade of PVN NPY Y1 receptors. Collectively, these data indicate that in male rats icv leptin increases LSNA in part via increased α-MSH and glutamatergic drive of PVN presympathetic neurons and by suppression of tonic NPY inhibition. Moreover, our data suggest that the removal of tonic NPY inhibition is required to unmask the increased α-MSH excitation of PVN presympathetic neurons.
Leptin has been shown to activate a network of sites to increase SNA,1 similarly to its effects on energy balance.14 We confirm that leptin administered icv, as well as into the ArcN, increases SNA, HR and MAP3–5, 8, 10 and that icv leptin enhances baroreflex control of LSNA and HR.4 Since bilateral inhibition of the PVN with muscimol completely reversed the effects of leptin, we conclude that all sites (accessed and stimulated by icv leptin) must converge either in or proximal to the PVN. The results also confirm the involvement of α-MSH,17–19 and further show that MC3/4R in the PVN are specifically engaged in males as we recently demonstrated in females in protestrus.28
A major finding, however, was that unlike PVN muscimol, PVN SHU9119 only partially reversed the sympathoexcitatory effects of leptin. Because the present dose of PVN SHU9119 is similar to those used to significantly alter various aspects of energy balance29, 30 and is sufficient to completely reverse comparable increases in SNA evoked by iv insulin31 (which acts solely in the ArcN,32, 33 unlike leptin), additional excitatory mechanisms must be involved. Indeed, we found that KYN also reduced the stimulatory effects of leptin on LSNA and baroreflex function, implicating a glutamatergic input to the PVN in the leptin responses. Nevertheless, like SHU9119, while our dose of KYN largely reversed the sympathoexcitatory effects of iv insulin,34 it only partially reduced the effects of leptin. Moreover, the combined effects of SHU9119 and KYN did not produce a greater effect than either alone. One interpretation of these results is that the sympathoexcitatory actions of PVN glutamate and α-MSH converge, such that one mechanism occludes the other. However, previous studies demonstrated that MC3/4R agonists like MTII act postsynaptically to excite PVN RVLM-projecting neurons,24, 35, 36 and that MTII does not alter presynaptic release of glutamate.35 Therefore, it is unlikely that SHU9119 blocked the effects of KYN by preventing MC3/4R enhancement of glutamate release. In addition, the sympathoexcitatory effects of PVN injection of N-Methyl-D-aspartic acid are not altered by PVN SHU9119,31 suggesting that the drug does not hinder postsynaptic effects of glutamate. Alternatively, glutamatergic and α-MSH inputs may have converged to elicit a maximal or ceiling level of sympathoexcitation via these combined mechanisms.
The finding that combined MC3/4R and glutamate receptor inhibition only partially reversed the effects of icv leptin suggests that yet other excitatory mechanisms contribute. For example, leptin, by activating MC3/4R or glutamatergic metabotropic receptors, may trigger cellular signaling pathways that lead to events that are not rapidly reversed. In support, the sympathoexcitatory effects of leptin are well known to be slowly developing (e.g. Figure 1) and to evoke cellular signaling.37, 38 Additionally, leptin may act directly in the PVN, since previous studies demonstrated that injection of leptin into the PVN increases arterial pressure.8, 11 Future experiments are required to test these possibilities.
Another major goal was to investigate whether decreased NPY inhibition is involved in leptin-induced sympathoexcitation. Because icv leptin administration markedly inhibited the actions of PVN NPY1x to increase MAP and basal and baroreflex control of LSNA and HR, we conclude that, like local PVN NPY1x, leptin decreases tonic NPY inhibition of SNA, HR and MAP, thereby contributing to its sympathoexcitatory effects. NPY inputs to PVN arise not only from the ArcN, but also from the brainstem.39, 40 Therefore, the finding that ArcN leptin similarly suppressed PVN NPY inhibition further indicates that the ArcN-to-PVN population of NPY neurons can contribute to leptin-induced sympathoexcitation and that leptin does not suppress NPY neuronal activity via binding in other hypothalamic sites. Finally, NPY and α-MSH inputs converge onto PVN presympathetic neurons, since 1) these neurons express NPY Y1 and MC4 receptors,24, 36 2) the increases in SNA and MAP following PVN NPY1x are prevented by prior inhibition of MC3/4R,24 and 3) α-MSH excites and NPY inhibits single PVN-to-RVLM neurons.24 Our present finding that PVN administration of a low dose of MTII41 elicits sympathoexcitation only after prior blockade of NPY Y1R suggests that the simultaneous disinhibition of PVN presympathetic neurons through withdrawal of NPY tone is required for the excitatory effect of leptin-induced increases in α-MSH to prevail.
Interestingly, the neuronal circuitry by which insulin increases SNA is remarkably similar to leptin. Although insulin acts solely in the ArcN,31–33 like leptin, it increases SNA via a neuronal pathway that includes the PVN31, 32 and the RVLM.24, 42 Both α-MSH and glutamatergic inputs into the PVN are involved.31, 34 Because insulin and leptin are often increased together, such as after a meal or in insulin resistant individuals, these parallel pathways may converge and amplify sympathoexcitatory effects. Moreover, because the effects of leptin4 and insulin31, 33, 43 to increase the activity of various sympathetic nerves are diverse, relative increases in leptin versus insulin may homeostatically and differentially engage increased sympathetic outflow to several organ beds.
Perspectives
Obesity increases SNA, in particular to the hindlimb and kidney, and elevations in leptin and hypothalamic melanocortin activity are widely viewed as essential.44 Remarkably, however, the potential role of NPY in obesity-induced increases in SNA and MAP is completely unexplored. In rats and mice, diet-induced obesity decreases NPY expression in the PVN/ArcN or NPY mRNA levels in the ArcN.45–50 Moreover, inbred obesity-prone rats exhibit decreases in NPY/AgRP processes in PVN.51 These data coupled with our current findings that a decrease in tonic NPY inhibition is required for leptin-induced increases in PVN MC3/4R drive of sympathetic outflow suggests that obesity also decreases tonic NPY inhibition of SNA, which unmasks the well-described increased α-MSH excitation. This information thus underscores the need for more research into the import of PVN NPY in obesity-induced sympathoexcitation.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is new?
Inhibition of the PVN with muscimol completely reverses the effects of icv leptin to increase LSNA, HR, MAP and baroreflex control of LSNA and HR.
Blockade of PVN MC3/4R and glutamate ionotropic receptors, alone or together, only partially reverses the effects of icv leptin to increase LSNA, HR, MAP and baroreflex control of LSNA and HR.
icv or arcuate injections of leptin prevent the normal increases in LSNA, MAP and HR following blockade of NPY Y1 receptors.
PVN nanoinjections of the MC3/4R agonist, MTII, increase LSNA, MAP, and HR only after blockade of PVN NPY Y1 receptors.
What is relevant?
Obesity increases leptin, which has been shown to contribute to the sympathoexcitation and eventual hypertension. Recognition of the PVN as a site of convergence in the neurocircuitry may influence future experimental or treatment strategies.
These are the first data implicating PVN NPY in leptin’s sympathoexcitatory actions, which may lead to novel pharmacological approaches to treat excess SNA in conditions like obesity.
While increased activation of MC3/4R is widely considered a major factor underlying obesity-induced sympathoexcitation, this excitation may require simultaneous withdrawal of NPY inhibition, a novel hypothesis that requires investigation.
Summary
While leptin acts in many hypothalamic sites to increase SNA, the PVN is a node of convergence in the neurocircuity via increased α-MSH and glutamatergic excitatory inputs, as well as suppression of tonic inhibitory NPY inputs.
Acknowledgments
The authors thank Rubing Xing and Dasuni Wickramaratne for histological sectioning.
SOURCES OF FUNDING.
This study was supported in part by a National Institutes of Health grant HL088552, a Grant-in-Aid from the American Heart Association (12GRNT11550018), the Medical Research Foundation of Oregon, and by a NIH CTSA (OHSU) Strategic Investment (UL1TR000128).
Footnotes
DISCLOSURES.
None.
References
- 1.Harlan SM, Rahmouni K. Neuroanatomical determinants of the sympathetic nerve responses evoked by leptin. Clin Auton Res. 2013;23:1–7. doi: 10.1007/s10286-012-0168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J Clin Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunbar JC, Hu Y, Lu H. Intracerebroventricular leptin increases lumbar and renal sympathetic nerve activity and blood pressure in normal rats. Diabetes. 1997;46:2040–2043. doi: 10.2337/diab.46.12.2040. [DOI] [PubMed] [Google Scholar]
- 4.Li B, Shi Z, Cassaglia PA, Brooks VL. Leptin acts in the forebrain to differentially influence baroreflex control of lumbar, renal, and splanchnic sympathetic nerve activity and heart rate. Hypertension. 2013;61:812–819. doi: 10.1161/HYPERTENSIONAHA.111.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marsh AJ, Fontes MA, Killinger S, Pawlak DB, Polson JW, Dampney RA. Cardiovascular responses evoked by leptin acting on neurons in the ventromedial and dorsomedial hypothalamus. Hypertension. 2003;42:488–493. doi: 10.1161/01.HYP.0000090097.22678.0A. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, Jones JC, Rhodes C, Munzberg H. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J Neurosci. 2011;31:1873–1884. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enriori PJ, Sinnayah P, Simonds SE, Garcia RC, Cowley MA. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci. 2011;31:12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montanaro MS, Allen AM, Oldfield BJ. Structural and functional evidence supporting a role for leptin in central neural pathways influencing blood pressure in rats. Exp Physiol. 2005;90:689–696. doi: 10.1113/expphysiol.2005.030775. [DOI] [PubMed] [Google Scholar]
- 9.Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circulation Research. 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahmouni K, Morgan DA. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension. 2007;49:647–652. doi: 10.1161/01.HYP.0000254827.59792.b2. [DOI] [PubMed] [Google Scholar]
- 11.Shih CD, Au LC, Chan JY. Differential role of leptin receptors at the hypothalamic paraventricular nucleus in tonic regulation of food intake and cardiovascular functions. J Biomed Sci. 2003;10:367–378. doi: 10.1159/000071156. [DOI] [PubMed] [Google Scholar]
- 12.Young CN, Morgan DA, Butler SD, Mark AL, Davisson RL. The brain subfornical organ mediates leptin-induced increases in renal sympathetic activity but not its metabolic effects. Hypertension. 2013;61:737–744. doi: 10.1161/HYPERTENSIONAHA.111.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawchenko PE, Swanson LW. The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol. 1983;218:121–144. doi: 10.1002/cne.902180202. [DOI] [PubMed] [Google Scholar]
- 14.Myers MG, Jr, Munzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: More complicated than a simple arc. Cell Metab. 2009;9:117–123. doi: 10.1016/j.cmet.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulrich-Lai YM, Jones KR, Ziegler DR, Cullinan WE, Herman JP. Forebrain origins of glutamatergic innervation to the rat paraventricular nucleus of the hypothalamus: Differential inputs to the anterior versus posterior subregions. J Comp Neurol. 2011;519:1301–1319. doi: 10.1002/cne.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunbar JC, Lu H. Leptin-induced increase in sympathetic nervous and cardiovascular tone is mediated by proopiomelanocortin (pomc) products. Brain Res Bull. 1999;50:215–221. doi: 10.1016/s0361-9230(99)00197-5. [DOI] [PubMed] [Google Scholar]
- 17.Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 1999;33:542–547. doi: 10.1161/01.hyp.33.1.542. [DOI] [PubMed] [Google Scholar]
- 18.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23:5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang ZH, Felder RB. Melanocortin receptors mediate the excitatory effects of blood-borne murine leptin on hypothalamic paraventricular neurons in rat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R303–R310. doi: 10.1152/ajpregu.00504.2003. [DOI] [PubMed] [Google Scholar]
- 20.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 21.King CM, Hentges ST. Relative number and distribution of murine hypothalamic proopiomelanocortin neurons innervating distinct target sites. PLoS One. 2011;6:e25864. doi: 10.1371/journal.pone.0025864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mercer AJ, Hentges ST, Meshul CK, Low MJ. Unraveling the central proopiomelanocortin neural circuits. Front Neurosci. 2013;7:19. doi: 10.3389/fnins.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawabe T, Kawabe K, Sapru HN. Effect of barodenervation on cardiovascular responses elicited from the hypothalamic arcuate nucleus of the rat. PLoS One. 2012;7:e53111. doi: 10.1371/journal.pone.0053111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cassaglia PA, Shi Z, Li B, Reis WL, Clute-Reinig NM, Stern JE, Brooks VL. Neuropeptide y acts in the paraventricular nucleus to suppress sympathetic nerve activity and its baroreflex regulation. J Physiol. 2014;592:1655–1675. doi: 10.1113/jphysiol.2013.268763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawabe T, Kawabe K, Sapru HN. Cardiovascular responses to chemical stimulation of the hypothalamic arcuate nucleus in the rat: Role of the hypothalamic paraventricular nucleus. PLoS One. 2012;7:e45180. doi: 10.1371/journal.pone.0045180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jobst EE, Enriori PJ, Cowley MA. The electrophysiology of feeding circuits. Trends Endocrinol Metab. 2004;15:488–499. doi: 10.1016/j.tem.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Varela L, Horvath TL. Leptin and insulin pathways in pomc and agrp neurons that modulate energy balance and glucose homeostasis. EMBO Rep. 2012;13:1079–1086. doi: 10.1038/embor.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Z, Brooks VL. Leptin differentially increases sympathetic nerve activity and its baroreflex regulation in female rats: Role of oestrogen. J Physiol. 2015;593:1633–1647. doi: 10.1113/jphysiol.2014.284638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giraudo SQ, Billington CJ, Levine AS. Feeding effects of hypothalamic injection of melanocortin 4 receptor ligands. Brain Res. 1998;809:302–306. doi: 10.1016/s0006-8993(98)00837-3. [DOI] [PubMed] [Google Scholar]
- 30.Blevins JE, Morton GJ, Williams DL, Caldwell DW, Bastian LS, Wisse BE, Schwartz MW, Baskin DG. Forebrain melanocortin signaling enhances the hindbrain satiety response to cck-8. American journal of physiology. Regulatory, integrative and comparative physiology. 2009;296:R476–484. doi: 10.1152/ajpregu.90544.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension. 2011;57:435–441. doi: 10.1161/HYPERTENSIONAHA.110.160671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cassaglia PA, Hermes SM, Aicher SA, Brooks VL. Insulin acts in the arcuate nucleus to increase lumbar sympathetic nerve activity and baroreflex function in rats. J Physiol. 2011;589:1643–1662. doi: 10.1113/jphysiol.2011.205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luckett BS, Frielle JL, Wolfgang L, Stocker SD. Arcuate nucleus injection of an anti-insulin affibody prevents the sympathetic response to insulin. Am J Physiol Heart Circ Physiol. 2013;304:H1538–H1546. doi: 10.1152/ajpheart.00081.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stocker SD, Gordon KW. Glutamate receptors in the hypothalamic paraventricular nucleus contribute to insulin-induced sympathoexcitation. J Neurophysiol. 2015;113:1302–1309. doi: 10.1152/jn.00764.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye ZY, Li DP. Activation of the melanocortin-4 receptor causes enhanced excitation in presympathetic paraventricular neurons in obese zucker rats. Regul Pept. 2011;166:112–120. doi: 10.1016/j.regpep.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Ghamari-Langroudi M, Srisai D, Cone RD. Multinodal regulation of the arcuate/paraventricular nucleus circuit by leptin. Proc Natl Acad Sci US A. 2011;108:355–360. doi: 10.1073/pnas.1016785108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Intracellular mechanisms involved in leptin regulation of sympathetic outflow. Hypertension. 2003;41:763–767. doi: 10.1161/01.HYP.0000048342.54392.40. [DOI] [PubMed] [Google Scholar]
- 38.Rahmouni K, Sigmund CD, Haynes WG, Mark AL. Hypothalamic erk mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes. 2009;58:536–542. doi: 10.2337/db08-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, Polak JM. Colocalization of neuropeptide y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol. 1985;241:138–153. doi: 10.1002/cne.902410203. [DOI] [PubMed] [Google Scholar]
- 40.Chronwall BM, DiMaggio DA, Massari VJ, Pickel VM, Ruggiero DA, O’Donohue TL. The anatomy of neuropeptide-y-containing neurons in rat brain. Neuroscience. 1985;15:1159–1181. doi: 10.1016/0306-4522(85)90260-x. [DOI] [PubMed] [Google Scholar]
- 41.Li P, Cui BP, Zhang LL, Sun HJ, Liu TY, Zhu GQ. Melanocortin 3/4 receptors in paraventricular nucleus modulate sympathetic outflow and blood pressure. Exp Physiol. 2013;98:435–443. doi: 10.1113/expphysiol.2012.067256. [DOI] [PubMed] [Google Scholar]
- 42.Bardgett ME, McCarthy JJ, Stocker SD. Glutamatergic receptor activation in the rostral ventrolateral medulla mediates the sympathoexcitatory response to hyperinsulinemia. Hypertension. 2010;55:284–290. doi: 10.1161/HYPERTENSIONAHA.109.146605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan DA, Balon TW, Ginsberg BH, Mark AL. Nonuniform regional sympathetic nerve responses to hyperinsulinemia in rats. Am J Physiol. 1993;264:R423–R427. doi: 10.1152/ajpregu.1993.264.2.R423. [DOI] [PubMed] [Google Scholar]
- 44.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: Role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285:17271–17276. doi: 10.1074/jbc.R110.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Beck B. Neuropeptide y in normal eating and in genetic and dietary-induced obesity. Philos Trans R Soc Lond B Biol Sci. 2006;361:1159–1185. doi: 10.1098/rstb.2006.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen MJ, Jovanovska V, Morris MJ. Adaptive responses in hypothalamic neuropeptide y in the face of prolonged high-fat feeding in the rat. J Neurochem. 2004;88:909–916. doi: 10.1046/j.1471-4159.2003.02217.x. [DOI] [PubMed] [Google Scholar]
- 48.Lin S, Storlien LH, Huang XF. Leptin receptor, npy, pomc mrna expression in the diet-induced obese mouse brain. Brain Res. 2000;875:89–95. doi: 10.1016/s0006-8993(00)02580-4. [DOI] [PubMed] [Google Scholar]
- 49.la Fleur SE, van Rozen AJ, Luijendijk MC, Groeneweg F, Adan RA. A free-choice high-fat high-sugar diet induces changes in arcuate neuropeptide expression that support hyperphagia. Int J Obes (Lond) 2010;34:537–546. doi: 10.1038/ijo.2009.257. [DOI] [PubMed] [Google Scholar]
- 50.Lee AK, Mojtahed-Jaberi M, Kyriakou T, Astarloa EA, Arno M, Marshall NJ, Brain SD, O’Dell SD. Effect of high-fat feeding on expression of genes controlling availability of dopamine in mouse hypothalamus. Nutrition. 2010;26:411–422. doi: 10.1016/j.nut.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB. Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab. 2008;7:179–185. doi: 10.1016/j.cmet.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.