Abstract
Calorie restriction (CR) increases longevity in many species by unknown mechanisms. The circadian clock was proposed as a potential mediator of CR. Deficiency of the core component of the circadian clock—transcriptional factor BMAL1 (brain and muscle ARNT [aryl hydrocarbon receptor nuclear translocator]-like protein 1)—results in accelerated aging. Here we investigated the role of BMAL1 in mechanisms of CR. The 30% CR diet increased the life span of wild-type (WT) mice by 20% compared to mice on an ad libitum (AL) diet but failed to increase life span of Bmal1−/− mice. BMAL1 deficiency impaired CR-mediated changes in the plasma levels of IGF-1 and insulin. We detected a statistically significantly reduction of IGF-1 in CR vs. AL by 50 to 70% in WT mice at several daily time points tested, while in Bmal1−/− the reduction was not significant. Insulin levels in WT were reduced by 5 to 9%, while Bmal1−/− induced it by 10 to 35% at all time points tested. CR up-regulated the daily average expression of Bmal1 (by 150%) and its downstream target genes Periods (by 470% for Per1 and by 130% for Per2). We propose that BMAL1 is an important mediator of CR, and activation of BMAL1 might link CR mechanisms with biologic clocks.—Patel, S. A., Chaudhari, A., Gupta, R., Velingkaar, N., Kondratov, R. V. Circadian clocks govern calorie restriction–mediated life span extension through BMAL1- and IGF-1-dependent mechanisms.
Keywords: aging, gene expression, glucose, insulin, transcription, food anticipation
Calorie restriction (CR) is a robust intervention that increases longevity across different species, including mammals (1–4). The precise molecular mechanisms of CR are unknown, and multiple theories have been put forward to explain CR-mediated effects on life span and health. Several physiologic systems—such as the mammalian target of rapamycin (mTOR) signaling pathway, the insulin/IGF-1 signaling pathways, and the sirtuin-controlled pathway—are affected by CR in animals and are considered to be potential mediators of CR (5–7). Activation of the NAD-dependent protein deacetylase sirtuin (silent mating type information regulation 2 homolog) 1 (SIRT1) is necessary for the full benefits of CR (8–11). Indeed, several behavioral and physiologic changes induced by CR in wild-type (WT) mice are impaired in SIRT1-null mice: these animals do not demonstrate an increase in daily activity (12). CR has different effects on several metabolic parameters in WT and SIRT1-null mice (13, 14). Finally, there is no increase in the life span of SIRT1-null mice on CR (14).
SIRT1 regulates the activity of many transcription factors. The helix–loop–helix transcription factor BMAL1 (brain and muscle ARNT [aryl hydrocarbon receptor nuclear translocator]-like protein 1) is one of the direct targets of SIRT1 (15). BMAL1 is a component of the circadian clock mechanism (16). The circadian clock is an internal timekeeping system that generates daily rhythms in physiology, metabolism, and behavior (17–20). BMAL1 also has other physiologic functions, such as control of metabolism, glucose homeostasis, antioxidant defense, immune system, and memory formation (21–23). Some of these functions might be linked to the role of BMAL1 as a component of the circadian clock; however, some of them cannot be explained through BMAL1 function in the clock mechanism only. Indeed, BMAL1 deficiency in mice leads to dramatically reduced life span and development of accelerated aging, a phenotype that is unique for BMAL1 deficiency and is not observed in other clock mutants (24).
BMAL1 and its downstream transcriptional targets control signaling pathways implicated in the CR-mediated increase in longevity. BMAL1 regulates antioxidant defense of the organism because BMAL1 deficiency is associated with oxidative stress and development of degenerative diseases (23, 25). Interestingly, BMAL1 is a target of SIRT1, and in turn, BMAL1 may regulate SIRT1 activity through the control of transcription of nicotinamide phosphoribosyl transferase (NAMPT), a rate-limiting enzyme in NAD biosynthesis; thus, BMAL1 and SIRT1 form a feedback loop (26–28). BMAL1 is also involved in the regulation of mTOR signaling (29–31). We hypothesized that BMAL1 may be a part of the mechanism facilitating the beneficial effects of CR in mammals. We studied the effects of CR in Bmal1−/− mice and observed that CR did not increase longevity of these animals, which suggests that BMAL1 is an important component of the CR mechanisms, therefore linking biologic clocks with CR in mammals.
MATERIALS AND METHODS
Animals
All mice were of C57B6/J background. WT mice on this background demonstrated strong beneficial response to CR; 30% CR is one of the most commonly used regimens for longevity experiments (32, 33). Animals were maintained on a 12:12 light:dark cycle with lights on at 7:00 am and were fed an 18% protein rodent diet (Harlan Industries, Indianapolis, IN, USA). The ad libitum (AL) group had unrestricted access to food. CR was started at 3 mo of age; during the first week of CR, animals received a 10% reduction compared to their AL intake and during the second week a 20% reduction; the 30% reduction was started on wk 3 and continued till the end of the experiments. Animals were on 30% CR for 2 mo before tissue collection. For the CR group, food was provided at zeitgeber time (ZT) 14 (2 h after light was off) for longevity experiments, tissue collection, and behavioral experiments. All groups had unrestricted access to water. The CR-adjusted group excluded those that died during the first 40 d of CR. In-cage locomotor activity was studied with an in-cage photobeam activity system (San Diego Instruments, San Diego, CA, USA). Total daily activity for each animal was normalized and set as 24 arbitrary units. The normalization of locomotor activity was necessary because there is a significant difference in the total level of activity between different mice. For every mouse, locomotor activity was recorded for 3 consecutive days. Five male mice of both genotypes were analyzed for AL and CR feeding; all mice were 5 mo of age. All tissue collection experiments were performed for 5 mo old WT and Bmal1−/− male mice. All animal studies were conducted in accordance with the regulations of the Committee on Animal Care and Use (Cleveland State University).
Statistical analysis
At least 3 animals for every time point, for each feeding type, and for each genotype were used for all experiments. Data are shown as averages ± sd. SPSS Statistics 20 (IBM, Armonk, NY, USA) and GraphPad Prism 5.04 (GraphPad Software, La Jolla, CA, USA) software were used for statistical analysis. Effects of genotype (Bmal1−/− vs. WT), feeding type (AL vs. CR), and time of day were tested for significance. To assess the effects that genotype, feeding, and time of the day on plasma glucose, insulin, and IGF-1 levels and for IGF-1 protein and mRNA levels in the liver, analysis was performed by 3-way ANOVA followed by post hoc analysis with Bonferroni correction. Two-way repeated-measures ANOVA followed by post hoc analysis using Bonferroni correction was used for analysis of the effect of genotype, feeding, and time of the day on daily locomotion. Two-way repeated ANOVA followed by post hoc analysis using Bonferroni correction was used for analysis of the effect of feeding and time of the day on circadian clock gene expression. The Student's t test was used for analysis in other cases. Thirty-six Bmal1−/− mice (both genders) were on 30% CR, 18 Bmal1−/− mice (both genders) were on the AL diet, 27 WT mice (both genders) were on 30% CR, and 41 WT mice (both genders) were on the AL diet. If mice needed to be killed, according to veterinarian recommendation as a result of morbidity or severe pathology, these mice were counted as alive before the day of death and as missing after the day of death. Because the treatment was started with mice 3 mo of age (90 d), we excluded from consideration all animals that had died before the beginning of the treatment. For the CR-adjusted group, animals that had died during first 40 d after the start of CR were excluded from analysis. The log rank test was used for analysis of longevity experiments. P < 0.05 was considered to be a statistically significant difference.
Analysis of protein expression
RIPA buffer with Protease/Phosphatase Inhibitor Cocktail (#5872; Cell Signaling Technology, Danvers, MA, USA) was used for tissue extract preparations. Total protein concentration was determined using the Bio-Rad DC protein assay kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s protocol. Staining of the same membranes for β-actin was used for normalization. Anti-IGF-1 ab 36532 (Abcam, Cambridge, MA, USA) and anti-β-actin (Sigma-Aldrich, St. Louis, MO, USA) antibodies were used for Western blot analysis.
Analysis of mRNA expression
Total RNA was isolated using TriZol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. RNA quantification was performed using quantitative real-time PCR with SYBR Green mix (Bio-Rad); relative mRNA abundance was calculated using the comparative δCt method with ribosomal 18S rRNA as standard. The following primers were used for the analysis of expression of the following: 18S rRNA, F 5′-GCT TAA TTT GAC TCA ACA CGG GA-3′; 18S rRNA R, 5′-AGC TAT CAA TCT GTC AAT CCT GTC-3′; Per1, F 5′-AGG TGG CTT TCG TGT TGG-3′; Per1, R 5′-CAA TCG ATG GAT CTG CTC TGA-3′; Bmal1, F 5′-CCA AGA AAG TAT GGA CAC AGA CAA A-3′; Bmal1, R 5′-GCA TTC TTG ATC CTT CCT TGG T-3′; Per2, F 5′-AAT CTT CCA ACA CTC ACC CC-3′; Per2, R 5′-AAT CTT CCA ACA CTC ACC CC-3′; Clock, F 5′-CCT TCT GCC GTA GCC CTA GT-3′; Clock, R 5′-CCC ATA AGG ATC CCC AGG CA-3′.
Measurement of plasma IGF-1, insulin, and glucose levels
Plasma samples were collected at 6 time points (ZT2, ZT6, ZT10, ZT14, ZT18, and ZT22) from 3 animals per time point. Plasma IGF-1 level was determined using the RayBio Mouse IGF-1 ELISA kit (RayBiotech, Norcross, GA, USA) according to the manufacturer’s protocol. Sandwich ELISA with 2 different antibodies and streptavidin–biotin horseradish peroxidase system with manufacturer-provided TMB substrate was used for detection of both IGF-1 and insulin. The detection limit for IGF-1 measurement is 4 pg/ml and for insulin is 5 U/ml. Blood glucose level was measured by True Test result kit by Nipro Diagnostics (Fort Lauderdale, FL, USA), according to the manufacturer’s protocol. The detectable range of glucose is 20 to 600 mg/dl.
RESULTS
Bmal1−/− mice have normal behavioral response to 30% CR
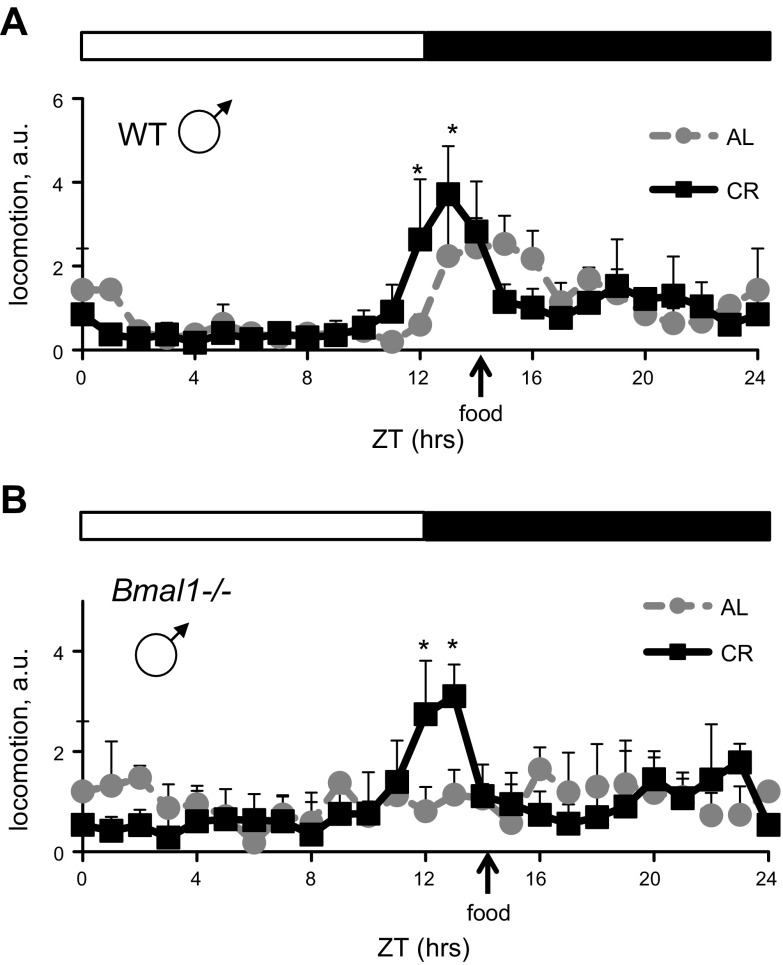
WT mice subjected to CR demonstrate robust increase in locomotion around the time of feeding (food anticipation) (34). We investigated whether Bmal1−/− mice have defects in CR-induced food anticipation. We monitored in cage locomotion for 3 d. Results of behavioral experiments are presented in Fig. 1. The 3-way interaction of genotype × diet × time was statistically significant (F = 2.688, P = 0.015; Supplemental Table 1). For both WT and Bmal1−/− mice, the increase in locomotion at ZT12 and ZT13 was statistically significant (P < 0.05, post hoc analysis using Bonferroni correction), which was an indication of food anticipation. Thus, BMAL1 is not necessary for the CR-mediated increase in locomotion before the feeding time.
Figure 1.
BMAL1 deficiency did not affect CR-mediated increase in locomotion around the feeding time. In-cage locomotion of WT (A) and Bmal1−/− (B) male mice. Mice were subjected to following diets: AL, gray circles, gray dotted lines; 30% CR, black squares, solid black lines. Each graph represents average normalized activity per hour and se (3 consecutive days for every animal, 5 mice per group). a.u., arbitrary units of normalized daily locomotor activity; total daily activity was set as 24 a.u. Zero and 24 h time points were double plotted. *Statistically significant increase in locomotor activity before feeding (food anticipation) for CR group compared to AL group. Light and dark bars represent light and dark phase of day. ZT0 is time when light is on and ZT12 is time when light is off. Food for CR group was provided at ZT14.
BMAL1 is necessary for life span extension on 30% CR
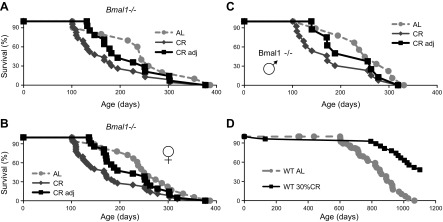
WT and Bmal1−/− mice were subjected to CR starting at 3 mo of age. For longevity experiments, food for CR groups was provided at ZT14 (2 h after light was off, which is a normal physiologic feeding time for mice). At this age, Bmal1−/− mice do not demonstrate any signs of accelerated aging; they are indistinguishable from WT mice by gross appearance and body weight. At the start of the experiment, average body weights were as follows: WT males, 27.3 ± 2.1 g; Bmal1−/− males, 26.4 ± 3.3 g; WT females, 20.7 ± 1.1 g; and Bmal1−/− females, 20.6 ± 1.6 g (Supplemental Fig. 1). Animals of both genotypes consumed the same amount of food (3.4 ± 0.31 g for males and 3.1 ± 0.23 for females). The beneficial effect of 30% CR on life span of WT mice was in agreement with the previously published data for C57B6 mice; while the experiment is still in progress, we observed a significant life span extension in the CR group of WT mice compared to the AL group (Fig. 2D). The effect of 30% CR on life span of Bmal1−/− mice is shown on Fig. 2A. Because gender might affect the outcome of CR, we checked the longevity of males (13 mice) and females (23 mice) separately. As evidenced by data presented in Supplemental Fig. 2, we did not detect any effect of gender on survival. Previously, we reported that gender does not affect the life span of Bmal1−/−; therefore, we combined male and female data for longevity analysis. In contrast to the effects in WT mice, 30% CR did not extend life span of Bmal1−/− mice (Fig. 2A); moreover, we observed a reduction in the life span of Bmal1−/− mice. The reduction in life span of Bmal1−/− on 30% CR was statistically significant. Average life span of Bmal1−/− mice on the AL diet was 8 mo but was only 6 mo on the 30% CR diet. We also noticed that a significant number of animals died during the early stages of CR, when, most likely, metabolic changes had to occur in order to permit the animal to adapt to a novel feeding regimen. About 40% of Bmal1−/− animals (15 of 36) died within 40 d from the start of CR, while only 1 animal died in the WT group. Again, we did not detect any effect of gender: 6 of 13 males and 9 of 23 females died during the adaptation period. When we excluded from the analysis the Bmal1−/− mice that died during the adaptation period (shown in Fig. 2A–C as the CR-adjusted group), we still did not detect any increase in the life span on CR: the average life span of this group was about 7.5 mo. The difference in the life span between CR-adjusted and AL groups was not statistically significant. Thus, BMAL1 plays an important role in preventing death during the metabolic adaptation to CR, and BMAL1 is necessary for CR-induced life span extension. Hence, BMAL1 is a necessary mediator of the beneficial CR effects on life span.
Figure 2.
Circadian clock protein BMAL1 is necessary for life span extension in response to CR. A) Kaplan-Meier survival curves of Bmal1−/− mice on AL (n = 18, gray circles), 30% CR (n = 36, dark gray diamonds), and CR-adjusted (n = 21, black squares) feeding. Mice that died during first 3 wk of 30% CR were excluded from analysis. Mice of both genders were used. Difference between survival curves of AL and CR is statistically significant by log rank test; no statistically significant difference between AL and CR-adjusted groups was observed. B) Kaplan-Meier survival curves of female Bmal1−/− mice on AL (n = 9, gray circles), 30% CR (n = 23, dark gray diamonds), and CR-adjusted (n = 14 black squares) feeding. Mice that died during first 3 wk of 30% CR were excluded from analysis. Difference between survival curves of AL and CR is statistically significant according to log rank test; no statistically significant difference between AL and CR-adjusted groups was observed. C) Kaplan-Meier survival curves of male Bmal1−/− mice on AL (n = 9, gray circles), 30% CR (n = 13, dark gray diamonds), and CR-adjusted (n = 9 black squares) feeding. Mice that died during first 3 wk of 30% CR were excluded from analysis. Difference between survival curves of AL and CR is statistically significant according to log rank test; no statistically significant difference between AL and CR-adjusted groups was observed. D) Kaplan-Meier survival curves of WT mice on AL (n = 73, gray circles and gray dotted line) or CR (n = 31; black squares and solid black line) feeding. Mice of both genders were used. *Difference between survival curves of AL and CR statistically significant according to log rank test.
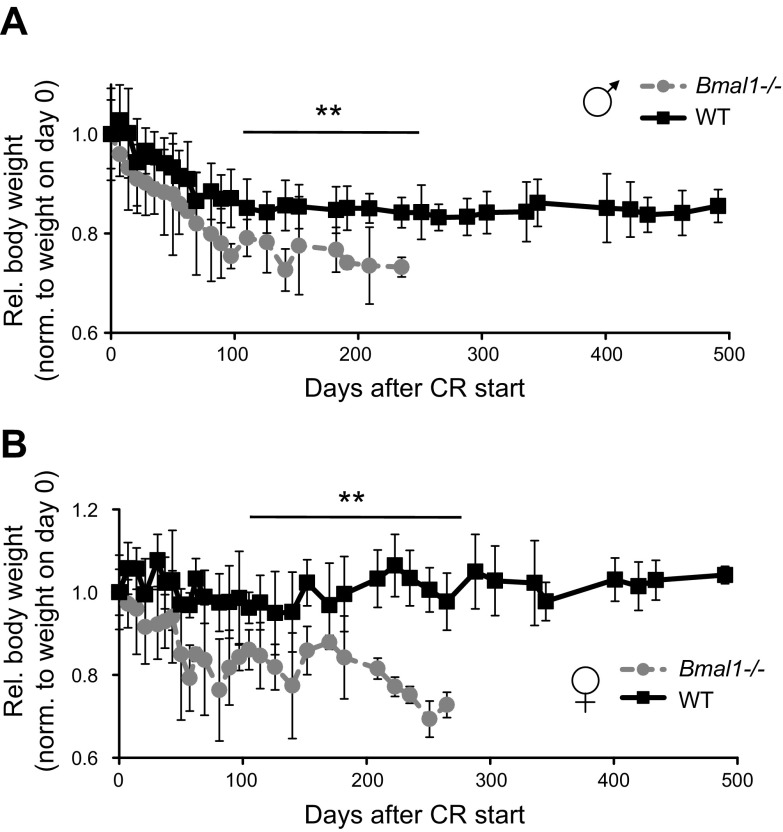
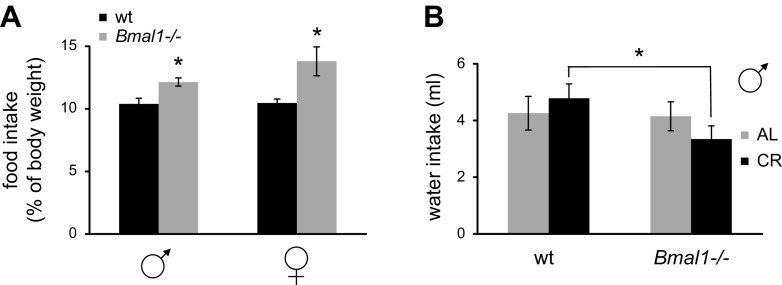
As expected, 30% CR significantly affected body weight of both WT and Bmal1−/− mice (Fig. 3A, B; Supplemental Fig. 1). WT mice demonstrated some reduction in body weight during the first few weeks of CR, after which their body weight stabilized and did not change during the rest of the experiment; the stabilized weight was about 85% of the original weight for males and about 90% of the original weight for females. In contrast, the body weight of Bmal1−/− mice demonstrated dramatic reduction compared to WT mice. Importantly, because the average food consumption was the same for both genotypes at the start of the experiment, Bmal1−/− mice actually consumed more food relative to their body weight (Fig. 4A). Daily food consumption for WT males and females equaled about 10% of their body weight, while for Bmal1−/− males this was about 12% and for Bmal1−/− females about 14% of their body weight. This difference between genotypes was statistically significant. We also monitored water consumption and found that Bmal1−/− mice on 30% CR consumed about 30% less water than WT (Fig. 4B), whereas there was no significant difference in water consumption between the genotypes when mice had constant access to food. The cause for the reduced water consumption is unknown; however, the phenomenon may contribute to the observed difference in body weight.
Figure 3.
Thirty percent CR affects body weight of WT and Bmal1−/− mice. A) Changes in body weights of male WT mice (n = 16, black squares and solid black line) and Bmal1−/− mice (n = 13, gray circles and gray dotted line). B) Changes in body weights for female WT (n = 14, black squares and solid black line) and Bmal1−/− (n = 19, gray circles and gray dotted line) mice. Mouse weights were normalized to weight of animals at start of experiment. **Statistically significant difference between genotypes detected at indicated age range.
Figure 4.
Daily food and water consumptions in WT and Bmal1−/− mice on 30% CR. A) Relative daily food intake of WT (black bars) and Bmal1−/− (light gray bars) for male and female mice on 30% CR. Relative food intake was calculated by dividing daily food consumption by mouse body weight. *Statistically significant difference between genotypes (P < 0.05). B) Daily water consumption of WT and Bmal1−/− mice on AL (light gray bars) or 30% CR (black bars) feeding. *Difference between genotypes statistically significant (P < 0.05).
BMAL1 regulates CR-mediated effects on insulin and IGF-1 levels
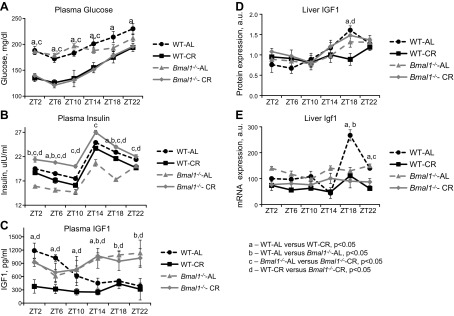
CR significantly affects glucose, insulin, and IGF-1 levels in WT mice (35, 36); therefore, we next studied these parameters in Bmal1−/− mice maintained on AL and CR regimens. Results of statistical analysis are presented in Supplemental Table 2. Similar to the effect in WT mice, CR resulted in reduced levels of glucose in the blood of Bmal1−/− mice (Fig. 5A). The reduced levels of plasma glucose were comparable for both genotypes (the difference was significant for the diets but not for the genotypes); we thus concluded that Bmal1−/− mice had a normal response to CR for this parameter. At the same time, we found that in contrast to Bmal1−/− mice maintained on the AL regimen, which had reduced levels of insulin, in agreement with previously published data (37), Bmal1−/− mice maintained on a CR regimen demonstrated increased insulin levels (Fig. 5B). Thus, in spite of the reduced levels of plasma glucose, Bmal1−/− mice had an increased level of insulin, which may indicate different insulin sensitivity between the genotypes on the CR regimen. Next, plasma and liver IGF-1 levels were measured. In the AL WT groups, the level of plasma IGF-1 significantly varied across the day, with the highest levels at ZT2 to ZT6 and the lowest levels at ZT14. In Bmal1−/− animals, IGF-1 did not significantly change across the day (Fig. 5C). In WT mice, CR resulted in significant reduction in plasma IGF-1 levels compared to the AL group (Fig. 5C and Supplemental Table 2). In Bmal1−/− mice, the effect of CR was not statistically significant (Fig. 5C). Thus, BMAL1 deficiency resulted in a strong suppression of CR-mediated reduction in the plasma levels of IGF-1. At the same time, the effect of CR on the levels of IGF-1 expressed in the liver were comparable between the genotypes, the difference between the AL and CR groups was statistically significant at ZT18 for WT (Fig. 5D); the effect of CR was thus dependent on the time of day. There were also time-of-day-dependent effects of diet and genotype on IGF-1 mRNA level in the liver; a statistically significant reduction was observed on CR in WT mice at ZT18 and ZT22 (Fig. 5E).
Figure 5.
Circadian clock protein BMAL1 is necessary for reduction in plasma IGF-1 level in response to CR. A–C) Daily profiles of (A) glucose, (B) insulin, and (C) IGF-1 in plasma of WT and Bmal1−/− mice on AL and CR. D) Daily profiles of liver IGF-1 protein level of WT and Bmal1−/− mice on AL and CR. E) Daily profiles of liver IGF-1 mRNA level of WT and Bmal1−/− mice on AL and CR. Three male mice of each genotype and on each feeding regimen were studied per time point. A–E) WT mice on AL feeding (black circles, black dashed lines); WT mice on CR feeding (black squares, black solid lines); Bmal1−/− mice on AL feeding (gray triangles, gray dashed lines); Bmal1−/− mice on CR feeding (gray diamonds, gray solid lines). aStatistically significant difference (P < 0.05) between WT mice on AL and CR feeding. bStatistically significant difference (P < 0.05) between WT and Bmal1−/− mice on AL feeding. cStatistically significant difference (P < 0.05) between Bmal1−/− mice on AL and CR feeding. dStatistically significant difference (P < 0.05) between WT and Bmal1−/− mice on CR feeding.
CR affects expression of Bmal1 and BMAL1 transcriptional targets
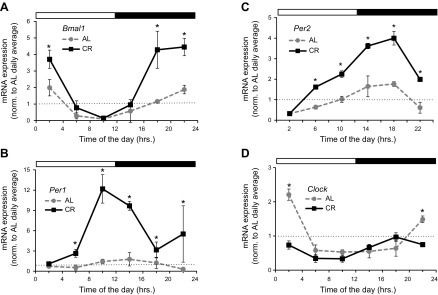
Next we decided to study whether CR has any effects on the expression and transcriptional activity of BMAL1. BMAL1 is a transcription factor that, in complex with another transcription factor, CLOCK (or its paralog, neuronal PAS domain protein 2 [NPAS2], in the CNS and cardiovascular system), drives the expression of its target genes (38–40). Analysis of Bmal1 expression in the liver tissue of WT mice maintained on different diets is presented in Fig. 6A. CR significantly induced Bmal1 mRNA at several time points during the day (Supplemental Table 3). Expressions of 2 well-known BMAL1 targets, Per1 and Per2, were also significantly induced at several time points in the liver of CR mice (Fig. 6B, C). The daily average expression of Bmal1, Per1, and Per2 was increased (Supplemental Fig. 3A). The increase in daily average expression was also accompanied by a dramatic increase in the amplitude of rhythms for Bmal1, Per1, and Per2 (Supplemental Fig. 3B). Interestingly, 30% CR did not increase the expression of Clock gene (Fig. 6D), whereas in contrast, the daily average and amplitude were even reduced on CR (Supplemental Fig. 3).
Figure 6.
CR increases expression of Bmal1 and BMAL1 transcriptional target genes. mRNA expression of Bmal1 (A), 2 BMAL1 transcriptional target genes; Per1 (B) and Per2 (C), and Clock gene (D) have been studied in liver of mice subjected to following feeding regimens: AL, gray circles and gray dotted line; 30% CR, black squares and solid black lines. At least 3 male mice were used for every time point in both groups. Light and dark bars represent light and dark phase of day. ZT0 is time when light is on and ZT12 is time when light is off. Food for CR group was provided at ZT14. *Statistically significant (P < 0.05) with AL group at this time point.
DISCUSSION
The beneficial effects of CR on longevity have been reported in different organisms, including humans. Different physiologic systems were proposed as important mediators of CR. Biologic clock proteins such as BMAL1 and PER2 have been implicated in the control of aging in different organisms. Interestingly, it was demonstrated that both these proteins are targets of SIRT1 (15, 41). Both Bmal1- and Sirt1-null mice have significantly reduced life span (42, 43). We investigated the effect of CR in Bmal1−/− mice. We found that 30% CR did not extend longevity of Bmal1−/− mice (Fig. 2A–C), while the same intervention significantly increased the life span of WT mice (Fig. 2D). Even more, 30% CR resulted in reduced longevity of Bmal1−/− mice. One of the known adverse effects of CR is elevated mortality during the first week of CR: as reported previously, when CR was applied to mice caught in wilderness, a significant number of animals died during the adaptation period; animals that survived this period lived longer than the AL-fed group (43). The cause of this difference in adaptation is unknown; our data demonstrate that BMAL1 is critically important for metabolic adaptation to CR. It will be interesting to study whether natural variations in biologic rhythms of BMAL1 expression and activity could be responsible for the differential adaptation to CR.
CR does not extend the longevity of Sirt1-null mice (13, 14); thus, it is tempting to speculate that BMAL1 and SIRT1 can be components of the same mechanism that mediates life span extension by CR. However, we found that several behavioral (Fig. 1) and physiologic (Fig. 5) changes induced by CR in WT mice can also be detected in Bmal1−/− animals, indicating that BMAL1 is not involved in all beneficial effects of CR. The conclusions on the role of BMAL1 in the beneficial effects of CR on longevity must be taken with caution because Bmal1−/− mice developed a premature aging phenotype and have a short life span, which might contribute to the failure of CR in this model. Importantly, at the starting point of the CR (3 mo of age), Bmal1−/− mice are very similar with WT mice in terms of gross appearance, food consumption, and body weight. Interestingly, treatment with rapamycin increases the life span of these mice (30), suggesting that longevity in these mice can be affected by another antiaging intervention.
CR induces dramatic reduction of the plasma levels of glucose, insulin, and IGF-1 and leads to increased insulin sensitivity in WT mice (35, 36). We observed that in Bmal1−/− mice CR resulted in a comparable reduction in blood glucose level. Thus, while it was demonstrated that BMAL1 is involved in the control of glucose homeostasis (21), it is not necessary for the CR-mediated reduction in plasma glucose. At the same time, we found that CR did not reduce the plasma level of insulin in Bmal1−/− mice; moreover, the insulin level in the CR group of Bmal1−/− mice was increased compared to the AL group, which, together with the data on reduced glucose levels, indicates reduced insulin sensitivity in Bmal1−/− animals. Increased insulin sensitivity is implicated in life span extension by CR (35). As previously reported, BMAL1 regulates insulin secretion by pancreatic islets (37); in agreement with that, we observed reduced plasma insulin levels in Bmal1−/− mice maintained on an AL diet compared to the WT AL group (Fig. 5). This difference in the effect of BMAL1 deficiency on insulin requires further studies on the role of BMAL1 in pancreatic islet functions. Thus, BMAL1 is involved in insulin production and most likely in insulin sensitivity under conditions of CR, which warrants further study of BMAL1 functions. Finally, BMAL1 deficiency led to significant defects in the regulation of plasma IGF-1. It is well known that the plasma IGF-1 level is significantly affected by nutrients, but molecular mechanisms of reduced level of plasma IGF-1 on CR are not well studied. We did not observe significant reductions of circulating IGF-1 in the blood of Bmal1−/− mice (the difference was significant only at 1 time point), compared to dramatic reductions in WT mice (Fig. 5). mRNA expression of Igf1 in the liver was comparable between genotypes under both AL and CR conditions, and it was equally suppressed under CR compared to AL in both WT and Bmal1−/− mice. The effect of CR on the level of the IGF-1 protein in the liver was complicated; CR led to elevated levels of the IGF-1 protein in the liver of WT animals at ZT14 and ZT18 and to reduced levels at ZT6. CR also affected levels of IGF-1 protein in the liver of Bmal1−/− mice; the difference was significant at ZT18 (Fig. 5). These data suggest that in WT mice, CR might suppress plasma IGF-1 levels through control of IGF-1 production and secretion (a dramatic reduction in plasma IGF-1 has been observed, while the effect on liver IGF-1 was moderate and at some time points opposite to the effect on plasma IGF-1), and that BMAL1 deficiency leads to impaired control of secretion of the IGF-1 protein. We also cannot exclude the possibility that CR might affect the turnover rate of plasma IGF-1. Thus, BMAL1 may be involved in control of IGF-1 secretion and/or turnover.
We observed that 30% CR results in a significant increase in the expression of the circadian clock genes Bmal1, Per1, and Per2. Per1 and Per2 are direct transcriptional targets of the BMAL1/CLOCK complex (16). Interestingly, we did not observe induction of Clock gene expression, which suggests that the effect of CR is not universal for all clock genes. One of the possible effects of CR is an increase in the activity of the BMAL1/CLOCK transcriptional complex; the observed induction of the BMAL1/CLOCK downstream targets is in agreement with that. Alternatively, it is possible that rhythmic feeding increases synchronization of individual cellular oscillators in a tissue. In this case, we would expect an increase in the amplitude of expression without any significant effect on daily average expression, which is not the case. However, we cannot completely rule out this interpretation because it is possible that CR has different effects on clock gene expression during different times of the day. This selective regulation may occur through some epigenetic mechanisms that have been recently proposed as regulators of the circadian transcriptome (44). Regardless of the particular mechanism involved, our data demonstrate that CR significantly increases the expression of BMAL1 target genes, suggesting that CR may directly affect BMAL1 transcriptional activity, thus supporting the importance of BMAL1 for the full effects of CR.
CR leads to increased expression and activation of BMAL1 through circadian clock–dependent or –independent mechanisms. CR also activates SIRT1, which may regulate CLOCK and BMAL1. BMAL1 contributes to CR-mediated regulation of circulating IGF-1 levels. IGF-1 is important for CR-induced increase in longevity (35, 36). Another pathway considered to be critical for CR mechanisms is the mTOR complex 1 (mTORC1) signaling pathway (45). Previously we demonstrated that BMAL1 is a negative regulator of mTORC1 (30). Therefore, it will be important to study whether the effects of CR on mTORC1 activity are impaired in Bmal1−/− mice.
Our study also puts forward several important questions that ought to be addressed. BMAL1 is a component of the circadian clock, which was proposed to be a contributor to the beneficial effects of CR (46). Induction of expression of several clock genes argues that activation of the clock mechanisms might be important for CR. At the same time, BMAL1 has clock-independent metabolic functions; thus, the observed induction of Per1 and Per2 expression can be a biomarker of the increased BMAL1 expression and activity, while other downstream targets of BMAL1 are functionally important for the CR mechanisms. At this stage, it is too early to discuss if the role of BMAL1 in CR depends on circadian clock mechanisms or is circadian clock independent. Future experiments on the effects of CR in other circadian clock mutants will help address this question. It is also important to note that although we clearly observed the role of BMAL1 in CR-mediated longevity, the effect of CR on clock gene expression was studied only in the liver, and we do not know if it has the same effect in other tissues. However, independent of the outcome of these future studies, our data suggest that BMAL1 is an important target of CR, and the BMAL1-controlled pathways ought to be considered for the understanding of CR mechanisms and for developing antiaging strategies.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health/National Institute on Aging Grant 1R01AG039547, funds from the Center for Gene Regulation in Health and Disease (Cleveland State University) to R.V.K., and a Dissertation Research Award (Cleveland State University) to S.P. The authors thank J. Holcomb (Department of Mathematics, Cleveland State University), for help with statistical analysis. The authors also thank 2 anonymous reviewers for their helpful suggestions. The authors declare no conflicts of interest.
Glossary
- AL
ad libitum
- BMAL1
brain and muscle ARNT (aryl hydrocarbon receptor nuclear translocator)-like protein 1
- CR
calorie restriction
- mTORC1
mammalian target of rapamycin complex 1
- NAMPT
nicotinamide phosphoribosyl transferase
- NPAS2
neuronal PAS domain protein 2
- Sirt1
sirtuin (silent mating type information regulation 2 homolog) 1
- WT
wild-type
- ZT
zeitgeber time
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Taormina G., Mirisola M. G. (2014) Calorie restriction in mammals and simple model organisms. BioMed Res. Int. 2014, 308690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontana L., Partridge L., Longo V. D. (2010) Extending healthy life span—from yeast to humans. Science 328, 321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravussin E., Redman L. M., Rochon J., Das S. K., Fontana L., Kraus W. E., Romashkan S., Williamson D. A., Meydani S. N., Villareal D. T., Smith S. R., Stein R. I., Scott T. M., Stewart T. M., Saltzman E., Klein S., Bhapkar M., Martin C. K., Gilhooly C. H., Holloszy J. O., Hadley E. C., Roberts S. B.; CALERIE Study Group (2015) A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J. Gerontol. A Biol. Sci. Med. Sci. 70, 1097–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Redman L. M., Ravussin E. (2011) Caloric restriction in humans: impact on physiological, psychological, and behavioral outcomes. Antioxid. Redox Signal. 14, 275–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallinetti J., Harputlugil E., Mitchell J. R. (2013) Amino acid sensing in dietary-restriction-mediated longevity: roles of signal-transducing kinases GCN2 and TOR. Biochem. J. 449, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y. (2014) Molecular links between caloric restriction and Sir2/SIRT1 activation. Diabetes Metab. J. 38, 321–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gesing A., Al-Regaiey K. A., Bartke A., Masternak M. M. (2014) Growth hormone abolishes beneficial effects of calorie restriction in long-lived Ames dwarf mice. Exp. Gerontol. 58, 219–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mercken E. M., Hu J., Krzysik-Walker S., Wei M., Li Y., McBurney M. W., de Cabo R., Longo V. D. (2014) SIRT1 but not its increased expression is essential for lifespan extension in caloric-restricted mice. Aging Cell 13, 193–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nogueira L. M., Lavigne J. A., Chandramouli G. V. R., Lui H., Barrett J. C., Hursting S. D. (2012) Dose-dependent effects of calorie restriction on gene expression, metabolism, and tumor progression are partially mediated by insulin-like growth factor-1. Cancer Med. 1, 275–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitada M., Kume S., Takeda-Watanabe A., Tsuda S., Kanasaki K., Koya D. (2013) Calorie restriction in overweight males ameliorates obesity-related metabolic alterations and cellular adaptations through anti-aging effects, possibly including AMPK and SIRT1 activation. Biochim. Biophys. Acta 1830, 4820–4827 [DOI] [PubMed] [Google Scholar]
- 11.Kennedy B. K., Gotta M., Sinclair D. A., Mills K., McNabb D. S., Murthy M., Pak S. M., Laroche T., Gasser S. M., Guarente L. (1997) Redistribution of silencing proteins from telomeres to the nucleolus is associated with extension of life span in S. cerevisiae. Cell 89, 381–391 [DOI] [PubMed] [Google Scholar]
- 12.Chen D., Steele A. D., Lindquist S., Guarente L. (2005) Increase in activity during calorie restriction requires Sirt1. Science 310, 1641 [DOI] [PubMed] [Google Scholar]
- 13.Chen D., Bruno J., Easlon E., Lin S. J., Cheng H. L., Alt F. W., Guarente L. (2008) Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 22, 1753–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boily G., Seifert E. L., Bevilacqua L., He X. H., Sabourin G., Estey C., Moffat C., Crawford S., Saliba S., Jardine K., Xuan J., Evans M., Harper M. E., McBurney M. W. (2008) SirT1 regulates energy metabolism and response to caloric restriction in mice. PLoS One 3, e1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakahata Y., Kaluzova M., Grimaldi B., Sahar S., Hirayama J., Chen D., Guarente L. P., Sassone-Corsi P. (2008) The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134, 329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunger M. K., Wilsbacher L. D., Moran S. M., Clendenin C., Radcliffe L. A., Hogenesch J. B., Simon M. C., Takahashi J. S., Bradfield C. A. (2000) Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103, 1009–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green C. B., Takahashi J. S., Bass J. (2008) The meter of metabolism. Cell 134, 728–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt T., Sassone-Corsi P. (2007) Riding tandem: circadian clocks and the cell cycle. Cell 129, 461–464 [DOI] [PubMed] [Google Scholar]
- 19.Dibner C., Schibler U., Albrecht U. (2010) The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 72, 517–549 [DOI] [PubMed] [Google Scholar]
- 20.Froy O. (2011) Circadian rhythms, aging, and life span in mammals. Physiology (Bethesda) 26, 225–235 [DOI] [PubMed] [Google Scholar]
- 21.Rudic R. D., McNamara P., Curtis A. M., Boston R. C., Panda S., Hogenesch J. B., Fitzgerald G. A. (2004) BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2, e377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondratova A. A., Kondratov R. V. (2012) The circadian clock and pathology of the ageing brain. Nat. Rev. Neurosci. 13, 325–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musiek E. S., Lim M. M., Yang G., Bauer A. Q., Qi L., Lee Y., Roh J. H., Ortiz-Gonzalez X., Dearborn J. T., Culver J. P., Herzog E. D., Hogenesch J. B., Wozniak D. F., Dikranian K., Giasson B. I., Weaver D. R., Holtzman D. M., Fitzgerald G. A. (2013) Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J. Clin. Invest. 123, 5389–5400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondratov R. V., Kondratova A. A., Gorbacheva V. Y., Vykhovanets O. V., Antoch M. P. (2006) Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 20, 1868–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pekovic-Vaughan V., Gibbs J., Yoshitane H., Yang N., Pathiranage D., Guo B., Sagami A., Taguchi K., Bechtold D., Loudon A., Yamamoto M., Chan J., van der Horst G. T. J., Fukada Y., Meng Q. J. (2014) The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 28, 548–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang H. C., Guarente L. (2013) SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 153, 1448–1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsey K. M., Yoshino J., Brace C. S., Abrassart D., Kobayashi Y., Marcheva B., Hong H. K., Chong J. L., Buhr E. D., Lee C., Takahashi J. S., Imai S., Bass J. (2009) Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324, 651–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakahata Y., Sahar S., Astarita G., Kaluzova M., Sassone-Corsi P. (2009) Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324, 654–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jouffe C., Cretenet G., Symul L., Martin E., Atger F., Naef F., Gachon F. (2013) The circadian clock coordinates ribosome biogenesis. PLoS Biol. 11, e1001455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khapre R. V., Kondratova A. A., Patel S., Dubrovsky Y., Wrobel M., Antoch M. P., Kondratov R. V. (2014) BMAL1-dependent regulation of the mTOR signaling pathway delays aging. Aging (Albany, N.Y.) 6, 48–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cornu M., Oppliger W., Albert V., Robitaille A. M., Trapani F., Quagliata L., Fuhrer T., Sauer U., Terracciano L., Hall M. N. (2014) Hepatic mTORC1 controls locomotor activity, body temperature, and lipid metabolism through FGF21. Proc. Natl. Acad. Sci. USA 111, 11592–11599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blackwell B. N., Bucci T. J., Hart R. W., Turturro A. (1995) Longevity, body weight, and neoplasia in ad libitum–fed and diet-restricted C57BL6 mice fed NIH-31 open formula diet. Toxicol. Pathol. 23, 570–582 [DOI] [PubMed] [Google Scholar]
- 33.Liao C. Y., Rikke B. A., Johnson T. E., Diaz V., Nelson J. F. (2010) Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell 9, 92–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luby, M.D., Hsu, C.T., Shuster, S.A., Gallardo, C.M., Mistlberger, R.E., King, O.D., and Steele A.D. (2012) Food anticipatory activity behavior of mice across a wide range of circadian and non-circadian intervals. PLoS One 7, e37992 [DOI] [PMC free article] [PubMed]
- 35.Piper M. D., Bartke A. (2008) Diet and aging. Cell Metab. 8, 99–104 [DOI] [PubMed] [Google Scholar]
- 36.Brown-Borg H. M. (2007) Hormonal regulation of longevity in mammals. Ageing Res. Rev. 6, 28–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marcheva B., Ramsey K. M., Buhr E. D., Kobayashi Y., Su H., Ko C. H., Ivanova G., Omura C., Mo S., Vitaterna M. H., Lopez J. P., Philipson L. H., Bradfield C. A., Crosby S. D., JeBailey L., Wang X., Takahashi J. S., Bass J. (2010) Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466, 627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gekakis N., Staknis D., Nguyen H. B., Davis F. C., Wilsbacher L. D., King D. P., Takahashi J. S., Weitz C. J. (1998) Role of the CLOCK protein in the mammalian circadian mechanism. Science 280, 1564–1569 [DOI] [PubMed] [Google Scholar]
- 39.Hogenesch J. B., Gu Y. Z., Jain S., Bradfield C. A. (1998) The basic–helix–loop–helix–PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc. Natl. Acad. Sci. USA 95, 5474–5479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McNamara P., Seo S. B., Rudic R. D., Sehgal A., Chakravarti D., FitzGerald G. A. (2001) Regulation of CLOCK and MOP4 by nuclear hormone receptors in the vasculature: a humoral mechanism to reset a peripheral clock. Cell 105, 877–889 [DOI] [PubMed] [Google Scholar]
- 41.Asher G., Gatfield D., Stratmann M., Reinke H., Dibner C., Kreppel F., Mostoslavsky R., Alt F. W., Schibler U. (2008) SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134, 317–328 [DOI] [PubMed] [Google Scholar]
- 42.McBurney M. W., Yang X., Jardine K., Hixon M., Boekelheide K., Webb J. R., Lansdorp P. M., Lemieux M. (2003) The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol. Cell. Biol. 23, 38–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng H.-L., Mostoslavsky R., Saito S., Manis J. P., Gu Y., Patel P., Bronson R., Appella E., Alt F. W., Chua K. F. (2003) Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc. Natl. Acad. Sci. USA 100, 10794–10799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eckel-Mahan K. L., Patel V. R., de Mateo S., Orozco-Solis R., Ceglia N. J., Sahar S., Dilag-Penilla S. A., Dyar K. A., Baldi P., Sassone-Corsi P. (2013) Reprogramming of the circadian clock by nutritional challenge. Cell 155, 1464–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blagosklonny M. V. (2010) Revisiting the antagonistic pleiotropy theory of aging: TOR-driven program and quasi-program. Cell Cycle 9, 3151–3156 [DOI] [PubMed] [Google Scholar]
- 46.Froy O., Miskin R. (2010) Effect of feeding regimens on circadian rhythms: implications for aging and longevity. Aging (Albany, N.Y.) 2, 7–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.