Abstract
Genome-wide association studies (GWAS) have found over 60 loci that confer genetic susceptibility to Type 1 diabetes (T1D). Many of these are defined only by anonymous SNPs: the underlying causative genes, and the molecular bases by which they mediate susceptibility, are not known. Identification of how these variants affect the complex mechanisms contributing to the loss of tolerance is a challenge. We performed systematic analyses to characterize these variants. First, all known genes in strong linkage disequilibrium (LD) (r2 > 0.8) with the reported SNPs for each locus were tested for commonly occurring non-synonymous variations. We found only a total of 22 candidate genes at 16 T1D loci with common non-synonymous alleles. Next, we performed functional studies to examine the effect of non-HLA T1D risk alleles on regulating expression levels of genes in four different cell types: EBV- transformed B cell lines (resting and 6h PMA stimulated); purified CD4+ and CD8+ T cells. We mapped cis-acting expression quantitative trait loci (eQTL) and found 24 non-HLA loci that affected the expression of 31 transcripts significantly in at least one cell type. Additionally, we observed 25 loci that affected 38 transcripts in trans. In summary, our systems genetics analyses defined the effect of T1D risk alleles on levels of gene expression and provide novel insights into the complex genetics of T1D, suggesting most of the T1D risk alleles mediate their effect by influencing expression of multiple nearby genes.
Keywords: Type 1 Diabetes, eQTL, Gene expression, Genome-Wide Association Studies
INTRODUCTION
Type 1 diabetes (T1D) affects approximately 30 million people worldwide (1). It is a complex autoimmune disease causing the destruction of pancreatic β cells. The largest genetic studies of T1D have been carried out by the Type 1 Diabetes Genetics Consortium (T1DGC) (2–4). These and other reports have now defined genetic variants associated with T1D in over 60 different chromosomal regions (see ref 5 for review).
There is a need to identify the causative variants that are in linkage disequilibrium (LD) with the single nucleotide polymorphisms (SNPs) found by such association studies, and to define the molecular bases by which they contribute to disease susceptibility. The challenge of post-genome wide association studies (GWAS) functional studies (6–8) is in finding ways to translate genetic associations into clinically useful information. The strong genetic association of the disease with HLA class II genes of the major histocompatibility complex (MHC) is well established (9) but the identity of the genes associated with many of the non-HLA loci remains largely unknown, especially with respect to those associated SNPs located in non-coding regions of the genome (2, 5). Therefore, this study focuses on characterizing the non-HLA T1D risk loci.
In principle, most genetic variants could plausibly affect biological processes by changing amino acid residues in encoded proteins or by changing their levels of expression in particular tissues. Various DNA sequence repositories allow identification of commonly occurring non-synonymous (missense) variations in genes, and amino-acid substitution polymorphisms could be characterized for their potential to affect biological processes (10). Expression quantitative trait locus (eQTL) analyses can identify genes whose variation in expression is associated with specific SNP markers. For example, sequence variation in promoters or enhancer elements could result in differential cis regulation. Genetic variants can also regulate expression of genes at greater distances from, or on different chromosomes than, the regulatory element, i.e., trans regulation (11). The mechanisms involved in trans regulation could include indirect genetic effects, e.g. by means of variation in encoded proteins such as transcription factors, or by other effects, such as steric (11). Some loci could exert both cis and trans effects.
In the present study, we performed systems genetics (12) analyses of the 55 loci (2, 13–25) (Table I) showing highest evidence of association with T1D, using data generated by the T1DGC (2) and Immunochip projects (13). Additionally, four new SNPs (rs6691977, rs4849135, rs2611215 and rs11954020) that showed strong associations (P < 5 × 10−8) with T1D in (13) were included in our study. SNPs at these loci were assessed for disease gene candidacy. Expression data of 47,323 high-quality transcripts (Illumina, HT-12 V4) were correlated with SNPs reported in T1D loci adjusting for confounding factors such as population structure.
Table I.
List of reported T1D SNPs located in 59 non HLA T1D loci.
| ID | Locus | T1D SNP | CHR | BP | Gene | P-value | Ref. | Tables |
|---|---|---|---|---|---|---|---|---|
| 1 | 1p31.3 | rs2269241 | chr1 | 63881359 | PGM1 | 4.00×10−7 | 2 | II, S |
| 2 | 1p13.2 | rs2476601 | chr1 | 114179091 | PTPN22 | 8.50×10−85 | 2 | II |
| 3 | 1q31.2 | rs2816316 | chr1 | 190803436 | RGS1 | 3.10×10−5* | 2 | |
| 4 | 1q32.1 | rs3024493 | chr1 | 205010591 | IL10 | 1.90×10−9 | 2 | |
| 5 | 2p24.3 | rs1534422 | chr2 | 12558192 | Intergenic | 2.00×10−6 | 2 | IV |
| 6 | 2p23.3 | rs2165738 | chr2 | 24546313 | Intergenic | 4.00×10−6 | 14 | III, S |
| 7 | 2p13.1 | rs363609^ | chr2 | 74756380 | DQX1 | 8.53×10−6^ | 15 | II, III, S |
| 8 | 2q11.2 | rs9653442 | chr2 | 100191799 | AFF3 | 5.00×10−6 | 15 | S |
| 9 | 2q12.1 | rs6543134 | chr2 | 102416890 | IL18RAP | 8.03×10−5* | 16 | III, S |
| 10 | 2q24.2 2q24.2 |
rs1990760 rs3747517 |
chr2 chr2 |
162832297 162837070 |
IFIH1 IFIH1 |
6.60×10−9 4.70×10−7 |
2 2 |
II, IV |
| 11 | 2q33.2 2q33.2 2q33.2 |
rs11571291 rs3087243 rs231727 |
chr2 chr2 chr2 |
204429377 204447164 204449795 |
CTLA4 CTLA4 CTLA4 |
1.19×10−12 1.20×10−15 2.13×10−18 |
17 2 13 |
IV II, S |
| 12 | 2q35 | rs3731865 | chr2 | 218958247 | SLC11A1 | 1.55×10−6 | 18 | III |
| 13 | 3p21.31 | rs11711054 | chr3 | 46320615 | CCR5 | 1.70×10−5* | 2 | IV |
| 14 | 4p15.2 | rs10517086 | chr4 | 25694609 | Intergenic | 4.60×10−10 | 2 | |
| 15 | 4q27 4q27 4q27 |
rs4505848 rs17388568 rs2069763 |
chr4 chr4 chr4 |
123351942 123548812 123596932 |
IL2 IL2 IL2 |
4.70×10−13 3.00×10−6 1.91×10−10 |
2 19 13 |
|
| 16 | 5p13.2 | rs6897932 | chr5 | 35910332 | IL7R | 8.00×10−6 | 15 | II |
| 17 | 6q15 6q15 |
rs597325 rs56297233 |
chr6 chr6 |
91059215 91070750 |
BACH2 BACH2 |
3.38×10−10 5.40×10−8 |
17 2 |
S III |
| 18 | 6q22.32 | rs9388489 | chr6 | 126740412 | C6orf173 | 4.20×10−13 | 2 | III |
| 19 | 6q23.3 6q23.3 |
rs2327832 rs10499194 |
chr6 chr6 |
138014761 138044330 |
TNFAIP3 TNFAIP3 |
1.60×10−4* 3.00×10−4* |
2 2 |
S III, IV |
| 20 | 6q25.3 | rs1738074 | chr6 | 159385965 | TAGAP | 7.59×10−9 | 16 | S |
| 21 | 7p15.2 | rs7804356 | chr7 | 26858190 | Intergenic | 5.30×10−9 | 2 | III, IV |
| 22 | 7p12.2 | rs10272724 | chr7 | 50444707 | IKZF1 | 4.80×10−9 | 20 | III |
| 23 | 9p24.2 9p24.2 |
rs7020673 rs10758593 |
chr9 chr9 |
4281747 4282083 |
GLIS3 GLIS3 |
5.40×10−12 1.18×10−8 |
2 17 |
IV, S S |
| 24 | 10p15.1 | rs12251307 | chr10 | 6163501 | IL2RA | 1.30×10−13 | 2 | IV |
| 25 | 10p15.1 10p15.1 |
rs947474 rs11258747 |
chr10 chr10 |
6430456 6512897 |
PRKCQ PRKCQ |
4.00×10−9 1.20×10−7 |
14 2 |
IV, S |
| 26 | 10p11.22 | rs722988 | chr10 | 33466153 | NRP1 | 4.88×10−8 | 21 | S |
| 27 | 10q23.31 | rs10509540 | chr10 | 90013013 | C10orf59 | 1.30×10−28 | 2 | III, S |
| 28 | 11p15.5 11p15.5 11p15.5 |
rs7928968 rs3842727 rs7111341 |
chr11 chr11 chr11 |
2006875 2141424 2169742 |
INS TH INS |
2.78×10−14 4.89×10−196 4.40×10−48 |
17 13 2 |
IV |
| 29 | 12p13.31 12p13.31 12p13.31 |
rs3764021 rs10466829 rs4763879 |
chr12 chr12 chr12 |
9724895 9767358 9801431 |
NR CLECL1 CD69 |
5.00×10−8 9.19×10−9 1.90×10−11 |
19 17 2 |
III, S III, S |
| 30 | 12q13.2 12q13.2 12q13.2 |
rs705704 rs11171739 rs2292239 |
chr12 chr12 chr12 |
54721679 54756892 54768447 |
ERBB3 ERBB3 ERBB3 |
4.31×10−31 1.00×10−11 2.20×10−25 |
17 19 2 |
III, IV, S III, S |
| 31 | 12q13.3 | rs3809114 | chr12 | 56134906 | NR | 6.90×10−4* | 2 | |
| 32 | 12q14.1 | rs10877012 | chr12 | 56448352 | CYP27B1 | 3.80×10−6 | 22 | II, III, S |
| 33 | 12q24.12 12q24.12 |
rs1265565 rs3184504 |
chr12 chr12 |
110199580 110368991 |
CUX2 SH2B3 |
1.00×10−16 2.80×10−27 |
23 2 |
IV, S II |
| 34 | 12q24.13 | rs17696736 | chr12 | 110971201 | C12orf30 | 1.73×10−13 | 15 | IV, S |
| 35 | 13q32.3 | rs9585056 | chr13 | 98879767 | GPR183 | 5.20×10−9 | 24 | IV |
| 36 | 14q24.1 | rs1465788 | chr14 | 68333352 | Intergenic | 1.80×10−12 | 2 | |
| 37 | 14q32.2 | rs4900384 | chr14 | 97568704 | Intergenic | 3.70×10−9 | 2 | |
| 38 | 14q32.2 | rs56994090 | chr14 | 100376200 | DLK1 | 1.62×10−10 | 25 | |
| 39 | 15q14 15q14 |
rs17574546 rs12908309 |
chr15 chr15 |
36689768 36715969 |
RASGRP1 RASGRP1 |
3.35×10−8 4.31×10−8 |
13 17 |
III IV |
| 40 | 15q25.1 | rs3825932 | chr15 | 77022501 | CTSH | 7.70×10−8 | 2 | II, III, IV |
| 41 | 16p13.13 16p13.13 16p13.13 |
rs12708716 rs12927355 rs416603 |
chr16 chr16 chr16 |
11087374 11102272 11271580 |
CLEC16A DEXI C16orf75 |
2.20×10−16 1.91×10−16 3.00×10−6 |
2 17 14 |
II, III, IV |
| 42 | 16p11.2 16p11.2 |
rs4788084 rs9924471 |
chr16 chr16 |
28447349 28499031 |
IL27 IL27 |
2.60×10−13 1.21×10−11 |
2 17 |
II, III, S II |
| 43 | 16q23.1 16q23.1 |
rs7202877 rs8056814 |
chr16 chr16 |
73804746 73809828 |
Intergenic NR |
3.10×10−15 1.13×10−7 |
2 17 |
|
| 44 | 17p13.1 | rs16956936 | chr17 | 7574417 | Intergenic | 5.00×10−7 | 2 | |
| 45 | 17q12 | rs2290400 | chr17 | 35319766 | ORMDL3 | 5.50×10−13 | 2 | II, III, IV, S |
| 46 | 17q21.2 | rs7221109 | chr17 | 36023812 | Intergenic | 1.30×10−9 | 2 | III, IV, S |
| 47 | 18p11.21 | rs1893217 | chr18 | 12799340 | PTPN2 | 3.60×10−15 | 2 | IV, S |
| 48 | 18q22.2 | rs763361 | chr18 | 65682622 | CD226 | 1.56×10−8 | 16 | II, III, IV |
| 49 | 19p13.2 | rs2304256 | chr19 | 10336652 | TYK2 | 4.13×10−9 | 25 | II, IV, S |
| 50 | 19q13.32 | rs425105 | chr19 | 51900321 | Intergenic | 2.70×10−11 | 2 | IV |
| 51 | 19q13.33 | rs679574 | chr19 | 53897920 | FUT2 | 4.30×10−18 | 16 | IV, S |
| 52 | 20p13 | rs2281808 | chr20 | 1558551 | Intergenic | 1.20×10−11 | 2 | II |
| 53 | 21q22.3 21q22.3 |
rs11203203 rs876498 |
chr21 chr21 |
42709255 42714896 |
UBASH3A UBASH3A |
1.70×10−9 7.06×10−9 |
2 13 |
III, S IV |
| 54 | 22q12.2 | rs5753037 | chr22 | 28911722 | Intergenic | 2.60×10−16 | 2 | III, S |
| 55 | 22q12.3 | rs229541 | chr22 | 35921264 | C1QTNF6 | 2.10×10−7 | 2 | II, S |
| Four newly discovered loci | ||||||||
| 56 | 1q32.1 | rs6691977 | chr1 | 200814959 | NR | 4.30×10−8 | 13 | |
| 57 | 2q13 | rs4849135 | chr2 | 111615079 | NR | 4.40×10−8 | 13 | |
| 58 | 4q32.3 | rs2611215 | chr4 | 166574267 | NR | 1.80×10−11 | 13 | S |
| 59 | 5p13.2 | rs11954020 | chr5 | 35883251 | IL7R | 4.40×10−8 | 13 | |
Ref.: Publication reference. ID: unique T1D loci identifiers. BP: NCBI36 chromosome positions.
rs363609 is in LD with reported SNP rs6546909 (r2 = 0.84) whose p-value is stated.
P-values are derived from Barrett et al. (from Table 2 in (2)). ‘NR’ denotes no gene of interest was reported at the locus.
II, III, IV, S corresponds to Tables where SNPs are featured; II – Non-synonymous LD SNPs, III – cis- interacting genes, or IV – trans- interacting genes, S – Supplementary cis- interacting genes (see Suppl. Table II(i)).
MATERIALS AND METHODS
Study Samples
The Type 1 Diabetes Genetics Consortium (T1DGC) study has been described elsewhere, including phenotypic and extensive genetic characterization of over 4,000 affected sib-pair families (3). Upon joining the T1DGC, family members provided blood samples. Peripheral blood mononuclear cells (PBMC) were isolated and aliquots were used to provide DNA samples; to derive EBV-transformed B lymphoblastoid cell lines (LCL) (26–27); and frozen for later use. EBV-B cells from 202 European subjects from the T1DGC family collection were studied here. These samples consisted of 46 unaffected subjects and the rest were T1D cases. EBV-B cells were either unstimulated, or treated with phorbol-12-myristate-13-acetate (PMA) (28) for 6h (26–27). PMA stimulated samples consisted of 49 unaffected subjects. Cell lines were stimulated on a second occasion to provide a duplicate sample. SNPs were genotyped using the Immunochip (13) platform.
Frozen PBMC samples from 113 T1DGC family members were thawed, cultured overnight, stained and separated into CD4+ and CD8+ T cell populations by flow-sorting. Sufficient RNA was obtained from 102 CD4+ T cell samples and 84 CD8+ T cell samples to perform microarrays. Sex, HLA-DR and autoantibody statuses of the affected subjects are summarized in Suppl. Table I.(i).
Microarray Analyses
After cell culture or flow-sorting, RNA was extracted using TRIZOL® Reagent (Invitrogen) following the manufacturer’s instructions. The RNA quantity was measured by NanoDrop 1000 Spectrophotometer (Thermo Scientific) and RNA quality was checked on Agilent 2100 Bioanalyser (Agilent). Samples with RIN (RNA Integrity Number) number of equal or greater than 8 were biotin labeled using Illumina TotalPrep RNA Amplification kit (Ambion) as per manufacturer’s instructions. The biotin- labelled samples were hybridized onto Illumina HumanHT-12 v4.0 expression beadchips and beadchips were scanned by Beadarray Reader (Illumina) following manufacturer’s instructions. Raw data was finally exported by GenomeStudio software (Illumina) for analysis.
Microarray and eQTL analysis
Genome-wide gene expression values from GenomeStudio (Illumina) for each of 47,323 probes were subjected to background correction using control probe profile, variance stabilizing transformation (VST) and RSN (robust spline normalization) normalization using lumi package (29) in R. We then removed from the analysis 95 transcripts that are ERCC-spike in controls (having gene symbols starting with ‘ERCC’). Four separate gene expression datasets were created. Upon examining initial PCA plots, batch effects were evident. For correction within each cell type, normalized expression data for each gene was centred by batch and centred again after merging batches. The batch correction was validated by PCA analysis (Suppl. Figure 1. (A and B)) and pair plots of PCs 1–4 did not reveal any further batch effects. BLASTn software was to identify probesets with unique sequences.
The data generated in this study have been deposited in the NCBI Gene Expression Omnibus (GEO, Series accession number GSE77350) and is publically accessible via this URL. (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE77350)
To assess association between SNP genotype and gene expression, the MatrixEQTL R (30) package was used. To adjust for unknown confounders in the expression, two correction methods were used and results were compared.
RUV-2 correction
First association of all T1D SNPs with normalized uncorrected data was performed and p-value association of every SNP-gene pair was obtained. For each SNP, the top 5000 associated genes ranked by p-value were excluded and the rest were treated as empirical controls for RUV-2 correction (31–32) using naiveRandRUV method, parameter k set to 20. After correction, the same SNP was tested against the corrected set and p-value association of SNP-gene pair was recorded. This procedure was repeated for all SNPs and finally Benjamini FDR correction was applied to the set of recorded nominal P-values.
PCA correction
PCs were derived from individual whole expression sets and tested against whole genome Immunochip SNPs (200K). The PCs that showed no or weak genome association (i.e. min SNP-PC association FDR P > 0.001) were chosen as un-associated PCs (33). These PCs were incrementally added in their order of precedence as covariates to assess SNP-gene associations with an aim to maximize the number of significant cis gene detections (at FDR P < 0.001) for the 77 T1D SNPs tested. Based on analysis shown in Suppl. Figure 1 (E and F), the four gene expression datasets were corrected as follows: 7 PCs: 1–6 and 8 were removed from EBV-B basal cell line samples, 3 PCs: 1, 4 and 9 were removed for PMA stimulated EBV-B cell line samples, 4 PCs: 1–4 were removed for CD4+ samples and 2 PCs: 1 and 2 were removed for CD8+ samples.
We compared numbers of cis- and trans- regulated genes detected in each cell type using two methods (Suppl. Table I. (ii)). The RUV-2 method of correction yielded more significant results than PCA methods.
Statistical Analysis
Differential gene expression analysis was performed using the Limma package written for R (34). TDT (sibship) tests were performed using the software package UNPHASED (35–36).
Enrichment Analysis
Candidate gene names were converted to Entrez gene ids and were analysed using the DAVID (37–38) function annotation tool (http://david.abcc.ncifcrf.gov/). Further pathway and network analysis were performed using GATHER (gather.genome.duke.edu) (39) and GENEMANIA (www.genemania.org) (40) respectively.
RESULTS
Systematic evaluation of non-synonymous SNPs in genes in T1D-associated regions
First, we searched for commonly occurring non-synonymous (ns) SNPs in linkage disequilibrium (LD) (r2 > 0.8) with the T1D SNPs (2, 13–25) in the 1000 genomes and HAPMAP (41) CEU datasets. All amino acid substitutions were subject to prediction of the effect of these changes, evaluated as ‘benign’, ‘probably damaging’ or ‘possibly damaging’ by PolyPhen-2 (10). This search returned 25 nsSNPs in strong LD with only 16 of the 60 non-HLA T1D loci. These SNPs occurred in a total of 22 unique genes. The seven potentially damaging effects were found in two genes, SULT1A2 and GSDMB. Prediction status does not affect candidacy per se, so all genes listed in Table II should be evaluated in further studies. In addition, none of the four SNPs recently discovered in (13) were in strong LD (r2 > 0.8) with any nsSNPs. Among the LD SNPs, there were three splice-region variants and one stop-gain variant (summarized in Suppl. Table I.(iii)).
Table II.
Non-synonymous SNPs found in LD (r2 > 0.8) with 15 T1D loci.
| ID | T1D SNPs | Gene | NS variants in LD (r2 > 0.8) |
P- value of T1D SNP |
P-value of best NS SNP |
|---|---|---|---|---|---|
| 1 | rs2269241 | PGM1 | rs11208257 | 3.6×10−7^ | 1.6×10−6^ |
| 2 | rs2476601 | PTPN22 | rs2476601 | 1.35×10−27 | 1.35×10−27 |
| 7 | rs6546909 |
MOGS MRPL53 TTC31 LBX2 |
rs2268416, rs1063588 rs1047911 rs6707475 rs17009998 |
6.3×10−3^ | 8.7×10−3^ |
| 10 | rs1990760 | IFIH1 | rs1990760 | 7.43×10−5 | 7.43×10−5 |
| 11 | rs231727 | CTLA4 | rs231775 | 4.28×10−5 | 4.28×10−5 |
| 16 | rs6897932 | IL7R | rs6897932 | 0.002 | 0.002 |
| 32 | rs10877012 | TSFM | rs1599932 | 0.44 | n/a |
| 33 | rs3184504 | SH2B3 | rs3184504 | 2.11×10−9 | 2.11×10−9 |
| 40 | rs3825932 | CTSH | rs1036938 | 0.19 | 0.13 |
| 41 | rs416603 | TNP2 | rs11640138 | 0.01 | 0.02 |
| 42 | rs4788084, rs9924471 |
APOBR SULT1A2 SH2B1 |
rs180743 rs1059491, rs10797300 rs7498665 |
0.0006, 0.44 | 6.44×10−5 |
| 45 | rs2290400 |
ZPBP2 GSDMB |
rs11557467 rs2305480, rs2305479 |
0.007 | 0.006 |
| 48 | rs763361 | CD226 | rs763361 | 8.07×10−5 | 8.07×10−5 |
| 49 | rs2304256 | TYK2 | rs2304256 | 0.02 | 0.02 |
| 52 | rs2281808 | SIRPG | rs6043409 | 0.0009 | 0.001 |
| 55 | rs229541 | C1QTNF6 | rs229527 | 0.05 | 0.04 |
Next, we searched whether any nsSNPs showed better association with T1D than the reported SNP itself. For this, we performed a transmission disequilibrium test (TDT – sibship test) using UNPHASED (35–36) on a dataset of 2,676 nuclear families with unaffected parents and two or more affected sibs. Results are presented in Table II. Association P-values for SNPs not included in the Immunochip genotyping were derived from (2). At six T1D loci, the nsSNPs were the reported best SNPs. From those nsSNPs that were genotyped by Immunochip, rs7498665 associated with SH2B1 showed slightly better association than the reported rs4788084 (ΔP = 0.1, where ΔP = Pns SNP / Preported SNP). Two other ns SNPs (rs2305480 and rs229527) also showed very small (ΔP > 0.1) improvement in association compared to the reported T1D SNP. Most of the T1D loci did not have associated nsSNPs in nearby genes.
Gene Expression Analyses
EBV-transformed B cell lines (referred to hereafter as EVB-B) were produced from blood samples obtained from T1DGC family members (3). RNA was extracted from 202 available EBV-B cell lines that were cultured under basal conditions and stimulated with PMA. We also purified CD4+ and CD8+ T cells from peripheral blood samples provided by 113 subjects. None of these subjects overlapped with the donors of the 202 EBV samples. After quality control, sufficient high quality RNA to perform microarrays was obtained from 102 CD4+ T cell samples and 84 CD8+ T cell samples. The EVB-B cell samples were derived from both T1D cases and unaffected subjects. The unaffected controls included were first-degree relatives of the subset of case samples and islet autoantibody status was not determined for these unaffected subjects. The details regarding the autoantibody, sex and HLA-DR status of the affected subjects are summarized in Suppl. Table I.(i). As expected, there were no significant differences in the gene expression between cases and unaffected subjects nor between cases and unaffected first-degree relatives (Suppl. Figure 1.C and 1.D) so all samples were used to search for eQTLs.
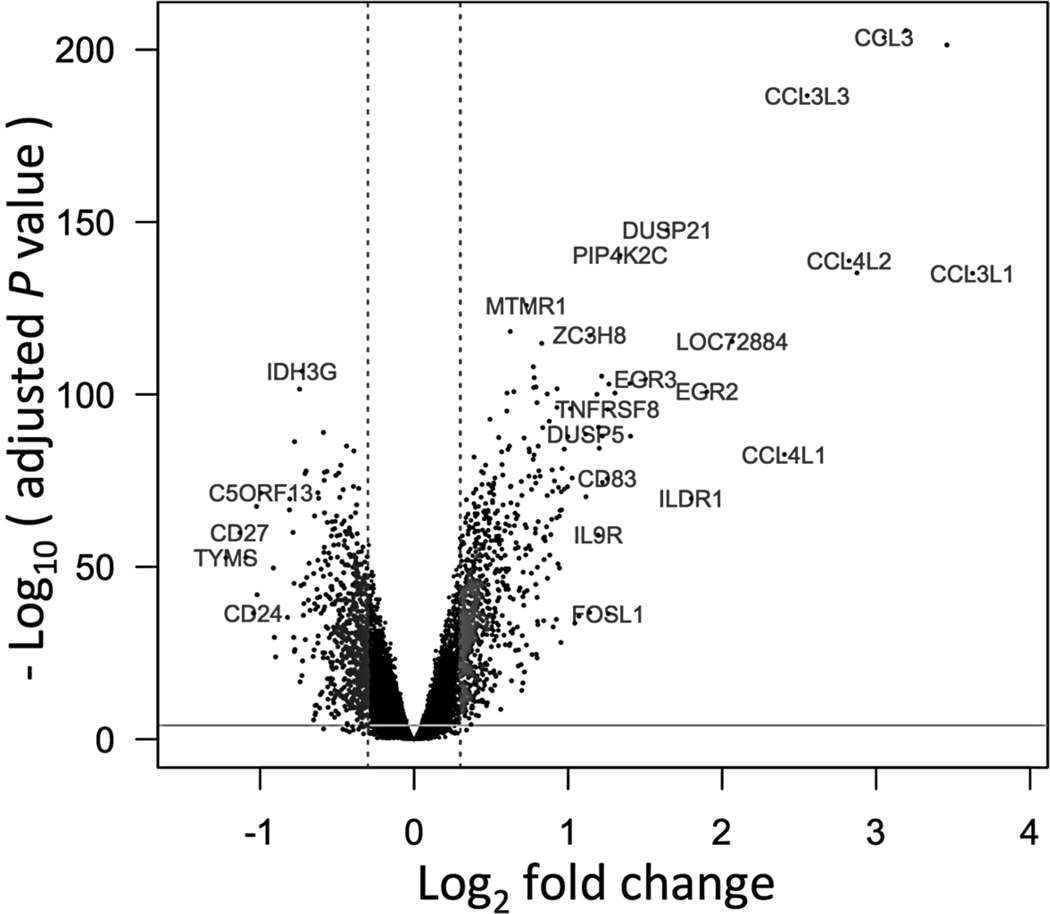
These RNA samples were hybridized to Illumina microarrays (HT-12v4). Data processing was carried out as described in Methods. Batch effects were corrected for each cell type by centering the normalized gene expression data by batch and centering again after merging batches. The batch correction was validated by PCA analysis (Suppl. Figure 1 (A & B)). To eliminate probesets with potential cross-hybridization problems, a BLAST search of each probe sequence was carried out on a custom database of all 47,323 Illumina probeset sequences and 38,500 probes that had a single hit were retained. In doing so, probes associated with two known T1D candidates RPS26 (due to sequence similarity with probes associated with RPS26 pseudogenes) and DEXI (due to sequence similarity with a probe associated with LOC653752) were removed. There were 95 ERCC spike-in controls in the probeset, which were also excluded from analysis. We also performed a search for SNPs within probeset coordinates and excluded any probes that contained SNPs from further analysis. We performed differential expression analysis of unstimulated EBV-B cells and after 6 hour PMA stimulation. The negative log10 (adjusted P-value) of each probe showing differential expression was plotted against the log2 fold change in a ‘volcano plot’ (Figure 1). Adjusted P < 0.0001 was selected as a cut-off for differential expression. A total of 1,465 genes were differentially expressed at this threshold with at least a modest fold change (absolute log2FC > 0.3). Genes with the highest fold changes in expression included CCL3, CCL4, EGR1, EGR2, DUSP21, PIP4K2C, ILDR1 and IL9R.
Figure. 1.
Comparison of gene expression in EBV-B cells between basal and 6 hour PMA stimulated samples. Differences (log2 fold change) in gene expression are shown on the X axis; Y axis shows –log10 (adjusted P values).
Parameters for Systems Genetics Analyses
Genotypes of T1DGC subjects were previously determined (2–4, 13) at 77 SNPs in 55 of 60 T1D risk loci (Table I). Based on the risk allele’s code at each T1D SNP, an additive recode [0,1,2] was applied so that the risk allele’s effect on gene expression could be determined. Separate analyses were performed for each of the four expression sets (EBV-B basal, EBV-B 6h PMA stimulated, CD4+ and CD8+). For these analyses, we conservatively defined a cis transcript as being from a gene whose transcription start or end site was located within 1 Mbp from the T1D SNP. A trans regulated transcript was defined as a gene located elsewhere in the genome. For each set, 3,672 cis interactions pairs were tested; ~2.9M trans interactions pairs were tested; and false discovery rate (FDR) P-value corrections were applied separately for cis and trans eQTLs. The MatrixEQTL R package (30) was used to perform these eQTL tests. Due to unknown confounding factors that could limit the power of detecting significantly differentially expressed genes, we performed two methods of correction independently: (a) removing unwanted variation (RUV-2) (31–32); (b) and adding genome wide un-associated expression derived principle components (PCs) as covariates (described in Methods).
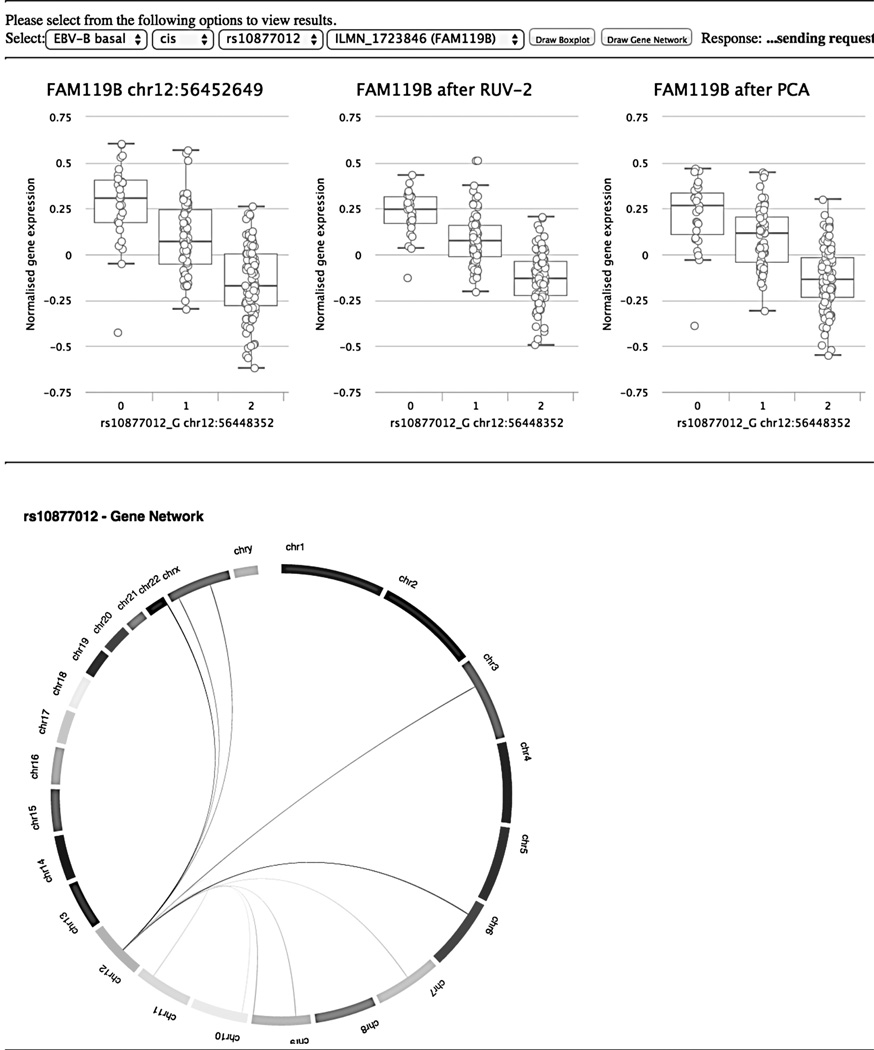
All transcripts with FDR P < 0.05 for each T1D SNP were followed up with enrichment analysis using the DAVID bioinformatics resource (37–38). Additional pathway and network analysis was performed using GATHER (39) and GENEMANIA (40) respectively. The results from these analyses are summarized in Tables III – VII and are described below. Boxplots of eQTL associations can be accessed online through our web resource (42) where we compare effects explained by raw normalized gene expression against RUV-2 and PCA corrected gene expression sets. A screenshot of the user interface is shown in Figure 2.
Table III.
Cis-genes associated with 24 T1D SNPs with minimum False Discovery Rate, FDR P < 0.001.
| ID | T1D SNPs (Effect allele) |
Gene | EBV-B Basal |
EBV-B PMA |
CD4+ | CD8+ |
|---|---|---|---|---|---|---|
| 6 | rs2165738 (C) | ADCY3 | ↓** | ↓*** | ↓**** | ns |
| 7 | rs363609 (C) | INO80B | ns | ↓**** | ns | ns |
| 9 | rs6543134 (C) | IL18R1 | ↓*** | ↓**** | ns | ns |
| 12 | rs3731865 (C) | SLC11A1 | ns | ns | ↑*** | ns |
| 17 | rs56297233 (T) | LYRM2 | ns | ↑*** | ns | ns |
| 18 | rs9388489 (C) | C6orf173 | ns | ns | ↓** | ↓*** |
| 19 | rs10499194 (C) | IFNGR1 | ↑**** | ns | ns | ns |
| 21 | rs7804356 (T) | SKAP2 | ↓*** | ↓*** | ↓*** | ↓** |
| 22 | rs10272724 (T) | IKZF1 | ↑**** | ↑* | ↑**** | ↑** |
| 27 | rs10509540 (T) | C10orf59 | ns | ns | ↑*** | ns |
| 29 | rs3764021 (T) | CLEC2D | ↑**** | ↑**** | ns | ns |
| rs10466829 (T) | CLECL1 | ns | ns | ↓**** | ↓**** | |
| 30 | rs705704 (T) | SUOX | ↓** | ns | ↓**** | ↓* |
| rs2292239 (T) | ERBB3 | ns | ns | ns | ↓**** | |
| 32 | rs10877012 (C) | FAM119B | ↓**** | ↓**** | ↓**** | ↓**** |
| TSFM | ↑**** | ↑* | ns | ns | ||
| XRCC6BP1 | ↑** | ↑*** | ns | ns | ||
| 39 | rs17574546 (C) | RASGRP1 | ↑**** | ↑*** | ns | ns |
| 40 | rs3825932 (C) | CTSH | ↑**** | ↑**** | ns | ns |
| 41 | rs416603 (T) | C16orf75 | ↓**** | ↓**** | ↑**** | ↑** |
| 42 | rs4788084 (C) | LOC728734 | ↓**** | ↓**** | ↓**** | ↓**** |
| SPNS1 | ↓**** | ns | ns | ↓* | ||
| TUFM | ns | ns | ↓*** | ns | ||
| 45 | rs2290400 (T) | ORMDL3 | ↑**** | ↑**** | ↑*** | ↑**** |
| GSDMB | ↑**** | ↑**** | ↑**** | ↑**** | ||
| IKZF3 | ↓**** | ↓**** | ↓** | ↓*** | ||
| ZPBP2 | ↓**** | ↓**** | ns | ns | ||
| 46 | rs7221109 (C) | SMARCE1 | ↓**** | ↓**** | ↓**** | ↓**** |
| 48 | rs763361 (T) | CD226 | ↓**** | ↓**** | ns | ns |
| 53 | rs11203203 (T) | UBASH3A | ↑*** | ns | ns | ns |
| 54 | rs5753037 (T) | MTMR3 | ↓**** | ↓**** | ns | ns |
ID: T1D loci identifiers as in Table I.
Following notations are used: FDR: **** P < 0.0001, *** P < 0.001, ** P < 0.01, * P < 0.05, ns – not significant, ↓ risk (effect) allele reduces expression, ↑ risk (effect) allele increases expression (determined using beta coefficient)
Table VII.
Significant enrichment terms found using DAVID bioinformatics resource.
| ID | T1D SNP | Enrichment Type | Enriched Term | FDR P |
|---|---|---|---|---|
| 2 | rs2476601 | GOterm BP | Antigen processing and presentation of peptide antigen via MHC class I | 0.017 |
| 10 | rs1990760 | PIR Keywords | Acetylation | 0.00065 |
| 15 | rs17388568 | PIR Keywords | Phosphoprotein | 0.025 |
| 22 | rs10272724 | PIR Keywords | Cytoplasm | 0.032 |
| PIR Keywords | Phosphoprotein | 0.047 | ||
| 29 | rs10466829 | PIR Keywords | Lectin | 0.002 |
| GOterm MF | Sugar binding | 0.0096 | ||
| PIR Keywords | Signal-anchor | 0.041 | ||
| GOterm MF | Carbohydrate binding | 0.045 | ||
| 33 | rs3184504 | Interpro | HIN-200/IF120x | 0.0031 |
| 34 | rs17696736 | GOterm BP | Response to virus | 0.00049 |
| PIR Keywords | Antiviral defence | 0.0058 | ||
| 41 | rs416603 | Biocarta | IL-10 Anti-inflammatory Signalling Pathway | 0.0021 |
| KEGG Pathway | Intestinal immune network for IgA production | 0.034 | ||
| 42 | rs9924471 | PIR Keywords | Nucleus | 0.0069 |
| 51 | rs679574 | Smart | IGc1 | 0.0031 |
| KEGG Pathway | Antigen processing and presentation | 0.0088 | ||
| KEGG Pathway | Viral myocarditis | 0.011 | ||
| Interpro | Immunoglobulin/major histocompatibility complex, conserved site | 0.017 | ||
| KEGG Pathway | Graft-versus-host disease | 0.021 | ||
| KEGG Pathway | Allograft rejection | 0.024 |
ID: T1D loci identifiers as in Table I.
PIR: Protein Information Resource, GO: Gene Ontology, BP: Biological process, MF: Molecular Function
Figure. 2.
Screen capture of the Web interface for browsing box-plots and gene networks. URL: http://www.sysgen.org/T1DGCSysGen/
Effect of T1D-associated non-HLA SNPs on neighboring gene expression in EBV-B Cell lines
We examined cis genes in EBV-B basal cell line samples at various FDR P value thresholds. At P < 0.001, 15 T1D SNPs were associated with differences in expression of 20 genes (Table III). Using lower thresholds of adjusted P [< 0.05], an additional 13 T1D SNPs affected the expression of a further 20 genes (Suppl. Table II.(i)). Hence, 28 T1D SNPs were found to be associated with changes in a total of 40 significant cis genes. Of these, three SNPs (rs10877012, rs4788084 and rs2290400) showed strong cis effects with multiple nearby genes that were either up- or down- regulated by the corresponding risk allele. In testing the four newly discovered T1D SNPs, (13) we observed that the risk allele associated with rs2611215 reduced expression of TMEM192 (FDR P = 0.008) (Suppl. Table II.(i)).
Next, we tested 6h PMA stimulated EBV-B cell line samples. Results confirmed the cis effects associated with 22 of 40 candidate genes identified in unstimulated EBV-B cells (at minimum FDR P < 0.05) and the effect directions were consistent. IFNGR1, SUOX, SPNS1 and UBASH3A were among genes that showed regulatory effects in basal cells but not after PMA stimulation. In addition, 17 T1D SNP genotypes significantly regulated the expression of 16 new candidate genes (FDR P < 0.05). Of these, genes INO80B and LYRM2 were detected highly significant at FDR P < 0.001 (Table III). The expression of candidate genes IKZF1 and TSFM showed decreased association with their corresponding T1D SNPs after stimulation, compared to basal condition (refer to 42). The rest of these results are presented in Suppl. Table II.(i).
In summary, 31 T1D SNPs affected the expression of a total of 38 candidate cis genes, 22 of which had shown evidence of cis effects in unstimulated EBV-B cells while the remaining nine showed association after PMA stimulation, thus suggesting genes that may play a role after immune activation.
Effect of T1D-associated non-HLA SNPs on neighboring gene expression in CD4+ and CD8+ T cells
Tests of CD4+ T cell samples revealed 16 T1D SNP genotypes regulated the expression of 20 genes significantly. Of these genes, eleven (SMARCE1, LOC728734, SUOX, FAM119B, C16ORF75, GSDMB, IKZF1, ADCY3, ORMDL3, SKAP2 and IKZF3) were found to be cis regulated in both EBV-B and CD4+ T cells by the same T1D SNPs (Table III). In particular, the risk allele of rs2290400 (T) affected nearby genes ORMDL3, GSDMB and IKZF3 similar to that observed in EBV-B cells. The effect directions between the cell types for the 11 shared genes were consistent, except for gene C16ORF75 where the risk allele increased expression in CD4+ cells but decreased it in EBV-B cells (42). We also noted expression of candidate gene SUOX showed a clear increase in the significance of association (i.e. lower p-value) with T1D risk allele rs705704 (T) in the CD4+ cells compared to EBV-B cells. In addition, there were nine newly identified candidate genes associated with nine T1D SNPs. Five of these SNPs had showed cis effects in the EBV-B cells, but had affected a different set of genes. Among these 9 new candidate genes, CLECL1 was the most significantly associated (Table III). The cis genes detected at lower FDR thresholds of 0.01 and 0.05 are presented in the Suppl. Table II.(i). These results suggest that the effects of the T1D risk SNPs on gene expression vary between cell types.
Finally, we performed analyses of the CD8+ T cell samples and identified 17 T1D SNP genotypes regulated the expression of 19 genes across all samples tested. Excepting ADCY3, ten candidate genes were found cis regulated in EBV-B, CD4+ T cells and CD8+ cells. Thirteen of the 19 candidate genes were cis regulated in both CD4+ and CD8+ T cells and the effect directions were consistent. The remaining six that were neither differentially regulated in EBV-B cells nor in CD4+ cells were associated with six T1D SNPs in CD8+ cells. Of these, T1D SNP rs2292239 regulated the expression of candidate gene ERBB3 most significantly (FDR P < 0.001) (Table III). The rest of the results are presented in Suppl. Table II.(i).
In summary, 24 T1D SNP genotypes regulated the expression of 31 candidate genes highly significantly at FDR P < 0.001 (Table III). Using lower FDR adjusted P-value thresholds (P < 0.05), 43 T1D SNP genotypes regulated the expression of 71 candidate genes. Using even lesser stringent suggestive threshold of nominal un-adjusted P < 0.001 for evidence of cis effect, we could define up to 85 candidate genes that were affected by 50 T1D SNPs in the four cell types tested.
T1D-associated SNPs associated with changes in expression of distant genes
Next, we investigated whether T1D loci showed trans-regulatory effects. After performing ~2.9M tests for each cell type and appropriate statistical correction, we identified 38 genes that were highly significantly associated with 25 T1D SNPs at FDR P < 0.001 (Table IV). Five of these SNPs (rs1534422, rs1990760, rs11571291, rs9585056 and rs425105) did not show any cis effect on nearby genes in the cell types tested. Trans-regulated genes shared between B and T cells were detected at only one T1D locus (defined by T1D SNP rs705704) and the effect direction was consistent. Except for ZMYM5, GRAMD1B and LOC389386, all significant trans genes were detected in the EBV-B cells. Upon characterizing the function of 38 trans genes in DAVID (37–38), we identified two clusters: CD276, ST6GAL1, CCL5 and IRF8 were associated with immune response and a further two genes (ID2 and IRF8) were associated with immune system and hemopoietic (lymphoid) organ development. Eight T1D SNPs (Table IV: highlighted in bold) showed highly significant cis as well as trans regulatory interactions in one or more cell types tested, suggesting co-regulation between cis and trans genes. We describe tests for meaningful relationships between these genes in the next section.
Table IV.
Trans-genes associated with 25 T1D SNPs with minimum FDR P < 0.001.
| ID | T1D SNPs (Effect allele) |
Gene | EBV-B Basal |
EBV-B PMA |
CD4+ | CD8+ |
|---|---|---|---|---|---|---|
| 5 | rs1534422 (C) | ST6GAL1 | ↓**** | ns | ns | ns |
| 10 | rs1990760 (T) | LOC643997 | ↓*** | ↓** | ns | ns |
| 11 | rs11571291 (T) | TMUB2 | ns | ↓*** | ns | ns |
| 13 | rs11711054 (C) | GRAMD1B | ns | ns | ↑*** | ns |
| 19 | rs10499194 (C) | TUBB6 | ↓*** | ↓*** | ns | ns |
| 21 | rs7804356 (T) | POLA2 | ns | ↑*** | ns | ns |
| RRP15 | ns | ↓*** | ns | ns | ||
| SEC61G | ns | ↓*** | ns | ns | ||
| SLC39A8 | ↑*** | ↑** | ns | ns | ||
| TYMS | ns | ↑*** | ns | ns | ||
| 23 | rs7020673 (C) | FHL3 | ns | ↓*** | ns | ns |
| PLCB2 | ns | ↑*** | ns | ns | ||
| 24 | rs12251307 (C) | DERA | ns | ↑*** | ns | ns |
| 25 | rs947474 (C) | MEIS2 | ↑*** | ↑* | ns | ns |
| 28 | rs3842727 (T) | CD276 | ns | ↓*** | ns | ns |
| ID2 | ↑* | ↑**** | ns | ns | ||
| 30 | rs705704 (T) | IP6K2 | ↑**** | ↑**** | ↑**** | ↑**** |
| LOC389386 | ns | ns | ↑**** | ↑**** | ||
| LOC728873 | ↑**** | ns | ns | ns | ||
| LOC92659 | ↑* | ↑* | ↑**** | ↑**** | ||
| MIR130A | ↑**** | ↑**** | ↑**** | ↑**** | ||
| MIR1471 | ↑* | ↑* | ↑*** | ↑**** | ||
| 33 | rs1265565 (T) | ZMYM5 | ns | ns | ↓*** | ns |
| 34 | rs17696736 (C) | IRF8 | ↑** | ↑*** | ns | ns |
| NCOA7 | ↑* | ↑**** | ns | ns | ||
| 35 | rs9585056 (C) | AHCTF1 | ns | ↓*** | ns | ns |
| 39 | rs12908309 (C) | FAHD1 | ↓* | ↓**** | ns | ns |
| RNF13 | ↓*** | ns | ns | ns | ||
| 40 | rs3825932 (C) | PCK2 | ns | ↑*** | ns | ns |
| 41 | rs416603 (T) | SORL1 | ns | ↑*** | ns | ns |
| 45 | rs2290400 (T) | TEX9 | ↓* | ↓**** | ns | ns |
| 46 | rs7221109 (C) | USP14 | ns | ↑*** | ns | ns |
| 47 | rs1893217 (C) | MCM3AP | ns | ↓*** | ns | ns |
| 48 | rs763361 (T) | P2RY11 | ↓* | ↓**** | ns | ns |
| 49 | rs2304256 (C) | ZNF280D | ↑*** | ns | ns | ns |
| 50 | rs425105 (T) | CCL5 | ↑** | ↑*** | ns | ns |
| 51 | rs679574 (C) | EIF5A | ↓* | ↑*** | ns | ns |
| 53 | rs876498 (T) | CAT | ns | ↓*** | ns | ns |
ID: T1D loci identifiers as in Table I.
Following notations are used: FDR: **** P < 0.0001, *** P < 0.001, ** P < 0.01, * P < 0.05, ns – not significant, ↓ risk allele reduces expression, ↑ risk allele increases expression. T1D SNPs highlighted also showed strong cis-regulatory effects at FDR P < 0.001.
In summary, in addition to the loci that affected genes in cis, we could identify five loci that exclusively affected genes in trans. Of the T1D loci that were not associated with expression changes in any of the four cell types, three loci contained non-synonymous SNPs defined in Table II. The trans regulatory effects detected at lower threshold levels are presented in Suppl. Table II (ii) and Suppl. Table III.
Enrichment analysis of genes associated with T1D susceptibility
We investigated the function of the genes whose expression was changed by individual risk SNPs. The DAVID enrichment analysis software (37–38) tests whether sets of genes are enriched for terminology referenced by UniProt Protein Information Resource (PIR) keywords, Gene Ontology (GO) and KEGG Pathways. First, we performed analysis to explore for enrichment between the highly significant (FDR P < 0.001) cis and trans gene candidates for the eight T1D SNPs highlighted in Table IV. For three of these SNPs, the candidate genes shared a common keyword (Table V). Second, using the list of 86 candidate genes derived from Tables II – IV, we performed pathway and enrichment analysis using GATHER (39) and we report results obtained with high confidence (unadjusted P < 0.001) in Table VI. In these results, we found that the cytokine-cytokine receptor interaction pathway received the highest significance. Third, we performed network analysis using GENEMANIA (40) for the same list of 86 candidate genes. The significant functional findings are presented in Table VI. The full GENEMANIA report can be accessed online (www.sysgen.org/T1DGCSysGen/genemania.pdf). Finally, we analyzed the list of cis and trans genes detected at FDR P < 0.05 for every T1D SNP separately. We identified 21 enrichment terms (excluding Gene Ontology cellular component terms) that were significantly enriched at Benjamini P < 0.05 for 10 T1D SNPs. These results are summarized in Table VII and below.
Table V.
Enrichment terms shared between cis genes and trans genes in association with three T1D SNPs.
| ID | T1D SNP | Genes | Enrichment term |
|---|---|---|---|
| 19 | rs10499194 | IFNGR1 (cis), TUBB6 | Cytoskeletal part (GO:0044430) |
| 21 | rs7804356 | SKAP2 (cis), POLA2, RRP15, SLC39A8, TYMS | Phosphoprotein (PIR Keywords) |
| 48 | rs763361 | CD226 (cis), PRRY11 | Receptor (PIR Keywords) |
ID: T1D loci identifiers as in Table I.
Table VI.
Network and Pathway analysis of the list of candidate genes identified in Tables II–IV using GATHER and GENEMANIA (Unadjusted P < 0.001).
| Annotation Type | Annotation | P-value |
|---|---|---|
| GATHER Gene Annotation and Pathway Analysis | ||
| Chromosome | 2p13 (TTC31, MOGS, INO80B, LBX2, MRPL53) | < 0.0001 |
| Transcription factor binding sites | c-Ets-2 binding sites | 0.0009 |
| KEGG Pathways | path:hsa04060: Cytokine-cytokine receptor interaction | 0.0005 |
| path:hsa00920: Sulfur metabolism | 0.0007 | |
| Gene Ontology | GO:0009607 [4]: response to biotic stimulus | 0.0003 |
| GO:0006952 [5]: defense response | 0.0004 | |
| GO:0006955 [4]: immune response | 0.0004 | |
| GO:0009613 [5]: response to pest, pathogen or parasite | 0.0009 | |
| GO:0043207 [5]: response to external biotic stimulus | 0.0009 | |
| GENEMANIA Network analysis | ||
| Functions | regulation of leukocyte activation | 5.4×10−5 |
| regulation of lymphocyte activation | 6.8×10−5 | |
| regulation of cell activation | ||
| T cell activation | 0.0002 | |
| mononuclear cell proliferation | 0.0006 | |
| positive regulation of cell activation | ||
| lymphocyte proliferation | ||
| positive regulation of leukocyte activation | ||
| regulation of T cell activation | ||
| leukocyte proliferation | 0.0008 | |
| regulation of lymphocyte proliferation | ||
| regulation of mononuclear cell proliferation | ||
The term ‘Lectin’ was highly enriched for the T1D locus defined by rs10466829 since it affected expression of five c-type lectin genes (CLEC1A, CLEC2B, CLEC2D, CLECL1 and CD69) in the cell types tested. The T1D locus defined by rs17696736 was highly enriched for ‘response to virus’ and ‘anti-viral defense’ due to changes in expression of 7 trans genes (EIF2AK2, IFI16, IFNGR1, MX1, MX2, PLSCR1 and STAT1). Furthermore, genes MX1 and MX2 are also known inflammatory and immune response genes. In addition, the T1D SNP rs416603 showed significant enrichment for ‘IL10-anti inflammatory signaling pathway’ and ‘intestinal immune network IgA production pathway’ through its regulation of three genes (IL10, IL10RA and STAT5A). We also noted that two risk SNPs (rs2476601 and rs679574) showed association in trans with genes in the MHC (HLA-F, G, H, and DRB4), which gave positive enrichment for terms such ‘antigen processing and presentation’. These results provide insights into the functions of genes whose expression is affected by the T1D loci.
Validation of trans-regulatory gene interactions
To confirm our results, we searched using the blood eQTL browser (43) for the trans regulatory associations we identified at significance threshold FDR P < 0.05. Since not all T1D SNPs may be present in this browser, we allowed a 100Kb window for the search of the expression SNP. Two trans genes were validated: UBE2L6 (EBV-B +/− PMA) associated with rs3184504 and STAT1 (EBV-B basal) with rs17696736. Secondly, we searched in the trans regulatory interactions reported by Fairfax et al. (44) and validated a further three gene interactions reported in their study: LOC728823, IP6K2 and LOC389386 all associated with the T1D SNP rs705704. Although many cis gene effects were clearly defined from our datasets, validating trans genes poses a challenge warranting further investigation.
DISCUSSION
Our results provide a potential molecular basis for disease association at 46 of the 59 identified T1D loci (Table I). Sixteen of these loci contained non-synonymous SNPs in strong LD with the T1D SNP. Thirty-six of the loci showed cis effects on 75 nearby genes. The remainder showed statistically significant trans regulatory interactions that were substantiated by significant enrichment results (Tables V–VII). These candidate genes can be the focus for further studies. For example, a systems genetics study (45) into candidate gene CTSH whose expression was affected by T1D SNP rs3825932, supported its product as a novel therapeutic target.
Onengut-Gumuscu et al. (13) recently confirmed several previously reported T1D associated SNPs (2, 5) in addition to the identification of four additional new T1D risk SNPs of which one SNP (rs2611215) had high significance (P = 1.817 × 10−11) while P values of the rest only just exceeded the significance threshold (P < 5 × 10−8). This study found that the associated SNPs localized to enhancer sequences active in thymus, T and B cells, and CD34+ stem cells. Of the four new T1D associated SNPs (13) we were able to establish likely candidacy for rs2611215 as TMEM192.
An important conclusion from our study is that the cell type was important in characterizing T1D SNP function, i.e. eQTLs are cell type-specific. For example, the candidate gene ERBB3 was highly significantly cis regulated in CD8+ T cells but its variation effect was largely undetectable in other cell types. The risk allele associated with rs4788084 reduced expression of candidate gene TUFM exclusively in the CD4+ cells. Similarly, CLECL1 did not show any effect in EBV-B cell lines but showed highly significant effects in both T cell types tested. Among the weakly detected effects, there was evidence that suggested the risk allele associated with rs231727 reduced expression in cis of a well known candidate (CTLA4) exclusively in the CD8+ cells (unadjusted P=0.0003, FDR P = 0.04) (Suppl. Table II. (i)). Our CD4+/ CD8+ cell type data also assisted in mapping candidate genes at otherwise anonymous T1D SNPs; the most significant of these candidates included SLC11A1 (rs3731865), C6Orf173 (rs9388489) and C10orf59 (rs10509540).
Sixteen transcripts (twelve in cis, four in trans) were significantly associated with T1D SNPs in both EBV-B and the T cell types tested. Of these, a novel uncharacterized cis transcript LOC728734 (nuclear pore complex interacting protein family, member B8) was identified to be associated with T1D SNP rs4788084 (chr 16p11.2) where the risk allele decreased expression in all four cell types. The effect direction of cis and trans regulation by T1D SNPs on genes detected across multiple cell types were found consistent for all SNPs except C16ORF75. We also noted that probes associated with candidate genes DEXI and RPS26 also showed strong cis regulatory effect in association with T1D risk SNPs rs12708716 and rs705704, respectively, in one or more cell types. However, due to quality control procedures relevant to cross hybridisation problems described in the previous section, these probes were excluded from further analysis. Non-synonymous SNPs may also affect gene expression in trans. We found two examples of these: rs1990760 (chr 2q24.2) in IFIH1 also affected the expression of LOC643997 in trans; similarly rs2304256 (chr 19p13.2) in TYK2 also affected the expression of ZNF280D in trans.
Pathway analysis identified the “Cytokine-cytokine receptor interaction” pathway with highest confidence. The “Sulfur metabolism” pathway also scored high significance because two genes SUOX (cis) and SULT1A2 (non-synonymous) involved in this pathway were identified as candidates in this study. It is also well known that sulfur plays an important role in insulin production (see 46, for review). Furthermore, DAVID enrichment analysis of locus specific cis and trans transcript perturbations revealed significant enrichment of 48 category terms in 15 of the T1D regions at FDR P < 0.05. Among the best enriched terms were ‘response to virus’, ‘acetylation’, ‘lectin’ and ‘IL10-anti-inflamatory pathway’. From the enrichment analysis for genes associated with each T1D SNP, upon examination T1D risk SNP rs17696736 (chr 12q24.12) was notably associated with ‘response to virus’ and ‘antiviral defence’ due to trans genes that are involved in pro-inflamatory response (such as MX1, MX2) in the salmonella infection pathway (KEGG Pathway: 05132). In contrast, chemokine gene CCL5 was highly significantly associated with diabetes loci associated with T1D SNP rs425105 (chr 19q13.32). These results support evidence found in a recent work (47) suggesting salmonella and chemokine vaccines can prove clinically useful in diabetes management and prevention.
In conclusion, our results confirm systems genetics (12) as a powerful tool for investigating the genetic architecture of complex diseases such as T1D. Many genes were identified whose expression levels were influenced by SNPs associated with T1D susceptibility. These nsSNPs, cis and trans regulated genes we identified are important candidates for further investigation. So that other researchers can extend the work reported here, we have implemented a web interface (42) allowing users to browse boxplots for the eQTL interactions reported below.
Supplementary Material
Acknowledgments
This research utilizes resources provided by the Type 1 Diabetes Genetics Consortium, a collaborative clinical study sponsored by NIDDK, National Institute of Allergy and Infectious Diseases, National Human Genome Research Institute, National Institute of Child Health and Human Development and Juvenile Diabetes Research Foundation International.
This work was supported by Program Grants 53000400 and 37612600 from the National Health and Medical Research Council of Australia, by the Diabetes Research Foundation (WA) and by grant 1DP3DK085678 from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). R.R. is supported by MACA Ride to Conquer Cancer in association with Harry Perkins Institute of Medical research. B.O.B. is supported by the Deutsche Forschungsgemeinschaft and a grant from the Boehringer Ingelheim Ulm University BioCenter.
ABBREVIATIONS
- T1D
Type 1 Diabetes
- HLA
Human Leukocyte Antigen
- MHC
Major histocompatibility complex
- eQTL
expression Qualitative trait locus
- eSNP
expression Single Nucleotide Polymorphism
- TDT
Transmission Disequilibrium Test of Association
- T1DGC
Type 1 Diabetes Genetics Consortium
- LD
Linkage Disequilibrium
- PBMC
Peripheral blood mononuclear cells
- EBV
Epstein-Barr virus
- PMA
phorbol. 12-myristate 13-acetate
- GWAS
Genome Wide Association Study
- WGA
Whole Genome Association
Footnotes
Online Resource: http://www.sysgen.org/T1DGCSysGen/
REFERENCES
- 1.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 2.Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, Julier C, Morahan G, Nerup J, Nierras C, Plagnol V, Pociot F, Schuilenburg H, Smyth DJ, Stevens H, Todd JA, Walker NM, Rich SS. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rich SS, Akolkar B, Concannon P, Erlich H, Hilner JE, Julier C, Morahan G, Nerup J, Nierras C, Pociot F, Todd JA. Overview of the Type I Diabetes Genetics Consortium. Genes Immun. 2009;10(Suppl 1):S1–S4. doi: 10.1038/gene.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morahan G, Mehta M, James I, Chen WM, Akolkar B, Erlich HA, Hilner JE, Julier C, Nerup J, Nierras C, Pociot F, Todd JA, Rich SS. Tests for genetic interactions in type 1 diabetes: linkage and stratification analyses of 4,422 affected sib-pairs. Diabetes. 2011;60:1030–1040. doi: 10.2337/db10-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morahan G. Insights into type 1 diabetes provided by genetic analyses. Curr Opin Endocrinol Diabetes Obes. 2012;19:263–270. doi: 10.1097/MED.0b013e328355b7fe. [DOI] [PubMed] [Google Scholar]
- 6.Editorial. On beyond GWAS. Nat Genet. 2010;42:551. doi: 10.1038/ng0710-551. [DOI] [PubMed] [Google Scholar]
- 7.Freedman ML, Monteiro AN, Gayther SA, Coetzee GA, Risch A, Plass C, Casey G, De Biasi M, Carlson C, Duggan D, James M, Liu P, Tichelaar JW, Vikis HG, You M, Mills IG. Principles for the post-GWAS functional characterization of cancer risk loci. Nat Genet. 2011;43:513–518. doi: 10.1038/ng.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Kirkman MS, Lernmark A, Metzger BE, Nathan DM. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34:e61–e99. doi: 10.2337/dc11-9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morahan G, Varney M. The Genetics of Type 1 Diabetes. In: Mehra NK, Kaur G, McCluskey J, Christiansen FT, Claas FHJ, editors. The HLA Complex in Biology and Medicine: A Resource Book. 1st. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2010. pp. 205–218. [Google Scholar]
- 10.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veyrieras JB, Kudaravalli S, Kim SY, Dermitzakis ET, Gilad Y, Stephens M, Pritchard JK. High-resolution mapping of expression-QTLs yields insight into human gene regulation. PLoS Genet. 2008;4:e1000214. doi: 10.1371/journal.pgen.1000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morahan G, Williams RW. Systems genetics: the next generation in genetics research? Novartis Found Symp. 2007;281:181–188. doi: 10.1002/9780470062128.ch15. discussion 188-91, 208-9. [DOI] [PubMed] [Google Scholar]
- 13.Onengut-Gumuscu S, Chen WM, Burren O, Cooper NJ, Quinlan AR, Mychaleckyj JC, Farber E, Bonnie JK, Szpak M, Schofield E, Achuthan P, Guo H, Fortune MD, Stevens H, Walker NM, Ward LD, Kundaje A, Kellis M, Daly MJ, Barrett JC, Cooper JD, Deloukas P, Todd JA, Wallace C, Concannon P, Rich SS. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat Genet. 2015;47:381–386. doi: 10.1038/ng.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper JD, Smyth DJ, Smiles AM, Plagnol V, Walker NM, Allen JE, Downes K, Barrett JC, Healy BC, Mychaleckyj JC, Warram JH, Todd JA. Meta-analysis of genome-wide association study data identifies additional type 1 diabetes risk loci. Nat Genet. 2008;40:1399–1401. doi: 10.1038/ng.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, Bailey R, Nejentsev S, Field SF, Payne F, Lowe CE, Szeszko JS, Hafler JP, Zeitels L, Yang JH, Vella A, Nutland S, Stevens HE, Schuilenburg H, Coleman G, Maisuria M, Meadows W, Smink LJ, Healy B, Burren OS, Lam AA, Ovington NR, Allen J, Adlem E, Leung HT, Wallace C, Howson JM, Guja C, C. Ionescu-Tîrgovişte, Genetics of Type 1 Diabetes in Finland. Simmonds MJ, Heward JM, Gough SC, Wellcome Trust Case Control Consortium. Dunger DB, Wicker LS, Clayton DG. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39:857–864. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smyth DJ, Plagnol V, Walker NM, Cooper JD, Downes K, Yang JH, Howson JM, Stevens H, McManus R, Wijmenga C, Heap GA, Dubois PC, Clayton DG, Hunt KA, van Heel DA, Todd JA. Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med. 2008;359:2767–2777. doi: 10.1056/NEJMoa0807917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradfield JP, Qu HQ, Wang K, Zhang H, Sleiman PM, Kim CE, Mentch FD, Qiu H, Glessner JT, Thomas KA, Frackelton EC, Chiavacci RM, Imielinski M, Monos DS, Pandey R, Bakay M, Grant SF, Polychronakos C, Hakonarson H. A genome-wide meta-analysis of six type 1 diabetes cohorts identifies multiple associated loci. PLoS Genet. 2011;7:e1002293. doi: 10.1371/journal.pgen.1002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang JH, Downes K, Howson JM, Nutland S, Stevens HE, Walker NM, Todd JA. Evidence of association with type 1 diabetes in the SLC11A1 gene region. BMC Med Genet. 2007;12:59. doi: 10.1186/1471-2350-12-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swafford AD, Howson JM, Davison LJ, Wallace C, Smyth DJ, Schuilenburg H, Maisuria-Armer M, Mistry T, Lenardo MJ, Todd JA. An allele of IKZF1 (Ikaros) conferring susceptibility to childhood acute lymphoblastic leukemia protects against type 1 diabetes. Diabetes. 2011;60:1041–1044. doi: 10.2337/db10-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evangelou M, Smyth DJ, Fortune MD, Burren OS, Walker NM, Guo H, Onengut-Gumuscu S, Chen WM, Concannon P, Rich SS, Todd JA, Wallace C. A method for gene-based pathway analysis using genomewide association study summary statistics reveals nine new type 1 diabetes associations. Genet Epidemiol. 2014;38:661–670. doi: 10.1002/gepi.21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey R, Cooper JD, Zeitels L, Smyth DJ, Yang JH, Walker NM, Hyppönen E, Dunger DB, Ramos-Lopez E, Badenhoop K, Nejentsev S, Todd JA. Association of the vitamin D metabolism gene CYP27B1 with type 1 diabetes. Diabetes. 2007;56:2616–2621. doi: 10.2337/db07-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J, Ellinghaus D, Franke A, Howie B, Li Y. 1000 Genomes-based imputation identifies novel and refined associations for the Wellcome Trust Case Control Consortium phase 1 Data. Eur J Hum Genet. 2012;20:801–805. doi: 10.1038/ejhg.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinig M, Petretto E, Wallace C, Bottolo L, Rotival M, Lu H, Li Y, Sarwar R, Langley SR, Bauerfeind A, Hummel O, Lee YA, Paskas S, Rintisch C, Saar K, Cooper JD, Buchan R, Gray EE, Cyster JG, Cardiogenics Consortium. Erdmann J, Hengstenberg C, Maouche S, Ouwehand WH, Rice CM, Samani NJ, Schunkert H, Goodall AH, Schulz H, Roider HG, Vingron M, Blankenberg S, Münzel T, Zeller T, Szymczak S, Ziegler A, Tiret L, Smyth DJ, Pravenec M, Aitman TJ, Cambien F, Clayton D, Todd JA, Hubner N, Cook SA. A trans-acting locus regulates an anti-viral expression network and type 1 diabetes risk. Nature. 2010;467:460–464. doi: 10.1038/nature09386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wallace C, Smyth DJ, Maisuria-Armer M, Walker NM, Todd JA, Clayton DG. The imprinted DLK1-MEG3 gene region on chromosome 14q32.2 alters susceptibility to type 1 diabetes. Nat Genet. 2010;42:68–71. doi: 10.1038/ng.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burster T, Boehm BO. Processing and presentation of (pro)-insulin in the MHC class II pathway: the generation of antigen-based immunomodulators in the context of type 1 diabetes mellitus. Diabetes Metab Res Rev. 2010;26:227–238. doi: 10.1002/dmrr.1090. [DOI] [PubMed] [Google Scholar]
- 27.Rosinger S, Nutland S, Mickelson E, Varney MD, Boehm BO, Olsem GJ, Hansen JA, Nicholson I, Hilner JE, Perdue LH, Pierce JJ, Akolkar B, Nierras C, Steffes MW. Collection and processing of whole blood for transformation of peripheral blood mononuclear cells and extraction of DNA: the Type 1 Diabetes Genetics Consortium. Clin Trials. 2010;7:S65–S74. doi: 10.1177/1740774510373493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nomura N, Nomura M, Sugiyama K, Hamada J. Phorbol 12-myristate 13-acetate (PMA)-induced migration of glioblastoma cells is mediated via p38MAPK/Hsp27 pathway. Biochem Pharmacol. 2007;74:690–701. doi: 10.1016/j.bcp.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Du P, Kibbe WA, Lin SM. lumi: a pipeline for processing Illumina microarray. Bioinformatics. 2008;24:1547–1548. doi: 10.1093/bioinformatics/btn224. [DOI] [PubMed] [Google Scholar]
- 30.Shabalin AA. Matrix eQTL: ultra fast eQTL analysis via large matrix operations. Bioinformatics. 2012;28:1353–1358. doi: 10.1093/bioinformatics/bts163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Risso D, Ngai J, Speed TP, Dudoit S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat Biotechnol. 2014;32:896–902. doi: 10.1038/nbt.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacob L, Gagnon-Bartsch JA, Speed TP. Correcting gene expression data when neither the unwanted variation nor the factor of interest are observed. Biostatistics. 2015 doi: 10.1093/biostatistics/kxv026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flutre T, Wen X, Pritchard J, Stephens M. A Statistical Framework for Joint eQTL Analysis in Multiple Tissues. PLoS Genet. 2013;9:e1003486. doi: 10.1371/journal.pgen.1003486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- 35.Dudbridge F. Likelihood-based association analysis for nuclear families and unrelated subjects with missing genotype data. Hum Hered. 2008;66:87–98. doi: 10.1159/000119108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dudbridge F, Holmans PA, Wilson SG. A flexible model for association analysis in sibships with missing genotype data. Ann Hum Genet. 2011;75:428–438. doi: 10.1111/j.1469-1809.2010.00636.x. [DOI] [PubMed] [Google Scholar]
- 37.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 38.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang JT, Nevins JR. GATHER: A Systems Approach to Interpreting Genomic Signatures. Bioinformatics. 2006;22:2926–2933. doi: 10.1093/bioinformatics/btl483. [DOI] [PubMed] [Google Scholar]
- 40.Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT, Maitland A, Mostafavi S, Montojo J, Shao Q, Wright G, Bader GD, Morris Q. The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38:214–220. doi: 10.1093/nar/gkq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. http://www.hapmap.org/hapmart.html.en. [Google Scholar]
- 42. http://www.sysgen.org/T1DGCSysGen/ [Google Scholar]
- 43.Westra HJ, Peters MJ, Esko T, Yaghootkar H, Schurmann C, Kettunen J, Christiansen MW, Fairfax BP, Schramm K, Powell JE, Zhernakova A, Zhernakova DV, Veldink JH, Van den Berg LH, Karjalainen J, Withoff S, Uitterlinden AG, Hofman A, Rivadeneira F, 't Hoen PA, Reinmaa E, Fischer K, Nelis M, Milani L, Melzer D, Ferrucci L, Singleton AB, Hernandez DG, Nalls MA, Homuth G, Nauck M, Radke D, Volker U, Perola M, Salomaa V, Brody J, Suchy-Dicey A, Gharib SA, Enquobahrie DA, Lumley T, Montgomery GW, Makino S, Prokisch H, Herder C, Roden M, Grallert H, Meitinger T, Strauch K, Li Y, Jansen RC, Visscher PM, Knight JC, Psaty BM, Ripatti S, Teumer A, Frayling TM, Metspalu A, van Meurs JB, Franke L. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet. 2013;45:1238–1243. doi: 10.1038/ng.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fairfax BP, Makino S, Radhakrishnan J, Plant K, Leslie S, Dilthey A, Ellis P, Langford C, Vannberg FO, Knight JC. Genetics of gene expression in primary immune cells identifies cell type-specific master regulators and roles of HLA alleles. Nat Genet. 2012;44:502–510. doi: 10.1038/ng.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Floyel T, Brorsson C, Nielsen LB, Miani M, Bang-Berthelsen CH, Friedrichsen M, Overgaard AJ, Berchtold LA, Wiberg A, Poulsen P, Hansen L, Rosinger S, Boehm BO, Ram R, Nguyen Q, Mehta M, Morahan G, Concannon P, Bergholdt R, Nielsen JH, Reinheckel T, von Herrath M, Vaag A, Eizirik DL, Mortensen HB, Storling J, Pociot F. CTSH regulates beta-cell function and disease progression in newly diagnosed type 1 diabetes patients. Proc Natl Acad Sci U S A. 2014;111:10305–10310. doi: 10.1073/pnas.1402571111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallace JL, Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov. 2015;14:329–345. doi: 10.1038/nrd4433. [DOI] [PubMed] [Google Scholar]
- 47.Husseiny MI, Rawson J, Kaye A, Nair I, Todorov I, Hensel M, Kandeel F, Ferreri K. An oral vaccine for type 1 diabetes based on live attenuated Salmonella. Vaccine. 2014;32:2300–2307. doi: 10.1016/j.vaccine.2014.02.070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.