Abstract
Ruxolitinib, a potent Janus kinase 1/2 inhibitor, resulted in rapid and durable improvements in splenomegaly and disease-related symptoms in the 2 phase III COMFORT studies. In addition, ruxolitinib was associated with prolonged survival compared with placebo (COMFORT-I) and best available therapy (COMFORT-II). We present a pooled analysis of overall survival in the COMFORT studies using an intent-to-treat analysis and an analysis correcting for crossover in the control arms. Overall, 301 patients received ruxolitinib (COMFORT-I, n=155; COMFORT-II, n=146) and 227 patients received placebo (n=154) or best available therapy (n=73). After a median three years of follow up, intent-to-treat analysis showed that patients who received ruxolitinib had prolonged survival compared with patients who received placebo or best available therapy [hazard ratio=0.65; 95% confidence interval (95%CI): 0.46–0.90; P=0.01]; the crossover-corrected hazard ratio was 0.29 (95%CI: 0.13–0.63). Both patients with intermediate-2– or high-risk disease showed prolonged survival, and patients with high-risk disease in the ruxolitinib group had survival similar to that of patients with intermediate-2–risk disease in the control group. The Kaplan-Meier estimate of overall survival at week 144 was 78% in the ruxolitinib arm, 61% in the intent-to-treat control arm, and 31% in the crossover-adjusted control arm. While larger spleen size at baseline was prognostic for shortened survival, reductions in spleen size with ruxolitinib treatment correlated with longer survival. These findings are consistent with previous reports and support that ruxolitinib offers a survival benefit for patients with myelofibrosis compared with conventional therapies. (clinicaltrials.gov identifiers: COMFORT-I, NCT00952289; COMFORT-II, NCT00934544)
Introduction
Myelofibrosis (MF) is a myeloproliferative neoplasm that can present as primary disease or secondary to polycythemia vera (PV) or essential thrombocythemia (ET).1 MF is characterized by fibrosis of the bone marrow, cytopenias, extramedullary hematopoiesis often leading to splenomegaly, and elevated proinflammatory cytokine levels resulting from dysregulation of the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway.2,3 Patients usually present with anemia and splenomegaly and experience a high symptom burden due to debilitating constitutional symptoms (ie. fever, weight loss, and night sweats) and those associated with an enlarged spleen (eg. abdominal pain and early satiety).4,5 Patients with MF have a diminished quality of life6–8 and reduced survival, with median life expectancy ranging from approximately 1.5 to 4 years for patients with high- and intermediate-2–risk disease.9,10
Ruxolitinib is a potent and selective JAK1/JAK2 inhibitor that has shown superiority over conventional therapies for the treatment of MF. In the 2 phase III COntrolled MyeloFibrosis study with ORal JAK inhibitor Treatment (COMFORT) studies, ruxolitinib demonstrated rapid and durable reductions in splenomegaly and improved MF-related symptoms and quality-of-life measures compared with either placebo11,12 or best available therapy (BAT).13,14 In addition, prolonged survival was observed in patients receiving ruxolitinib compared with those receiving placebo in COMFORT-I and BAT in COMFORT-II. However, because patients were allowed to cross over from the control arms of their respective studies to receive ruxolitinib, and a large percentage of them did cross over to receive ruxolitinib treatment after a relatively short time on study (6 months in COMFORT-I or 1 year in COMFORT-II), the survival advantage described in previous reports may not reflect the full magnitude of the survival benefit with ruxolitinib treatment. To a certain degree, these past comparisons were made between patients who received ruxolitinib from the time of randomization and those who started ruxolitinib after a relatively short time on study.
In the current study, the pooled COMFORT-I and COMFORT-II data sets were evaluated for the treatment effect on overall survival (OS) in these 2 pivotal studies, with prolonged follow up in an intent-to-treat (ITT) analysis as well as an analysis accounting for crossover of patients in the control arms. In addition, the prognostic effects of base-line factors on OS and associations between spleen size reductions and OS were analyzed in patients treated with ruxolitinib and BAT.
Methods
COMFORT-I and COMFORT-II are randomized phase III studies comparing ruxolitinib with placebo or BAT, respectively, in patients with International Prognostic Scoring System (IPSS) intermediate-2– or high-risk primary MF (PMF), post-PV MF (PPV-MF), or post-ET MF (PET-MF) by World Health Organization and International Working Group for Myelofibrosis Research and Treatment (IWG-MRT) criteria.15,16 COMFORT-I was conducted at 89 clinical sites in the United States, Canada, and Australia, and COMFORT-II was conducted at 56 clinical sites in 9 countries across Europe (Online Supplementary Appendix). The study designs and patients’ populations have been described previously.11,13 For each patient, the starting dose of ruxolitinib was determined based on base-line platelet count (15 or 20 mg twice daily [bid]) and was individually titrated over the course of treatment (5–25 mg bid) to optimize safety and efficacy. In COMFORT-II, investigator-selected BAT included any commercially available agent (as monotherapy or in combination) or no therapy, and could be changed at any time. In each study, patients were allowed to cross over from the control arm to the ruxolitinib arm upon protocol-defined progressive splenomegaly, defined as a 25% or more increase in spleen volume from baseline or on-study nadir in COMFORT-I and -II, respectively. At the time of this analysis, all ongoing patients in the control arms of both studies had crossed over to ruxolitinib. The dates of data cutoff were January 25, 2013, for COMFORT-I and December 1, 2012, for COMFORT-II, with a follow up of approximately three years in each study.
Both studies were designed by Incyte Corporation, approved by the institutional review boards of the respective institutions, and conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained for all patients. The data were analyzed and interpreted by the sponsor’s clinical and statistical teams in collaboration with the study investigators. An independent data and safety monitoring board reviewed the trial data and made recommendations regarding the continuation of the study.
The primary end point in both studies was the proportion of patients who achieved a 35% or more reduction from baseline in spleen volume, as assessed by magnetic resonance imaging (MRI) or computed tomography scanning at week 24 (COMFORT-I) or week 48 (COMFORT-II). Spleen length was assessed by physical examination. OS was a secondary end point and was defined as the interval between the dates of the first dose and death in COMFORT-I and as the interval between the dates of randomization and death from any cause in COMFORT-II. A meta-analysis of OS across both studies was pre-specified in the protocol of COMFORT-I and was carried out using an ITT analysis. The crossover-corrected treatment effect was estimated using a rank-preserving structural failure time (RPSFT) model (Online Supplementary Figure S1).17,18 OS was evaluated by the degree of spleen size reduction achieved at week 24 in a landmark analysis.19 The reverse Kaplan-Meier method was used to analyze censoring patterns in each study. A multivariate Cox regression model was used to assess treatment effect, with adjustment for selected patient base-line characteristics (for additional information see Supplementary Appendix).
Results
Patients
Overall, 301 patients were randomized to ruxolitinib (COMFORT-I, n=155; COMFORT-II, n=146), and 227 patients were randomized to placebo (n=154) or BAT (n=73). The patient populations were generally similar across studies.11,13 In the combined ruxolitinib group, 162 patients (54%) had high-risk MF and 138 (46%) had intermediate-2–risk MF at baseline by IPSS criteria compared with 135 (59%) and 91 (40%) patients in the combined control group, respectively; 2 patients did not have defined IPSS risk status. The median duration of follow up was 34.3 months in COMFORT-I and 34.7 months in COMFORT-II. The patient disposition (Online Supplementary Figure S2), demographic and base-line characteristics, and individual study results for the 3-year analyses have been described in detail.14,20 At the time of this analysis, all ongoing patients originally randomized to the control arms had crossed over to ruxolitinib [72% (n=111) of placebo-treated patients and 62% (n=45) of BAT-treated patients] with a Kaplan-Meier estimated time to crossover of 41 weeks in COMFORT-I and 75 weeks in COMFORT-II.
Overall survival
After three years of follow up, ITT analysis showed that the hazard ratio (HR) for overall survival favored patients who were randomized to receive ruxolitinib in COMFORT-I compared with patients who received placebo (HR=0.69; 95%CI: 0.46–1.03; log rank P=0.067), and those who were randomized to ruxolitinib in COMFORT-II had prolonged survival compared with patients who received BAT (HR=0.48; 95%CI: 0.28–0.85; stratified log rank P=0.009). There was no significant difference in survival between the COMFORT-I and COMFORT-II patient populations (HR=1.1; 95%CI: 0.8–1.6; P=0.54).
In this pooled analysis, 24% of patients (71 of 301) had died in the ruxolitinib group compared with 33% of patients (76 of 227) in the control group, representing a reduction in the risk of death with ruxolitinib by 35% in an ITT analysis (HR=0.65; 95%CI: 0.46–0.90; P=0.01) (Figure 1A). Improvement in survival was evident in the ruxolitinib group independent of IPSS risk categories (Figure 1B). Survival estimates for patients in the ruxolitinib group with high-risk MF were similar to those for lower-risk patients (intermediate-2) in the control group. Survival estimates beyond treatment discontinuations were similar for both treatment arms for patients who discontinued either study due to disease progression or for any reason.
Figure 1.
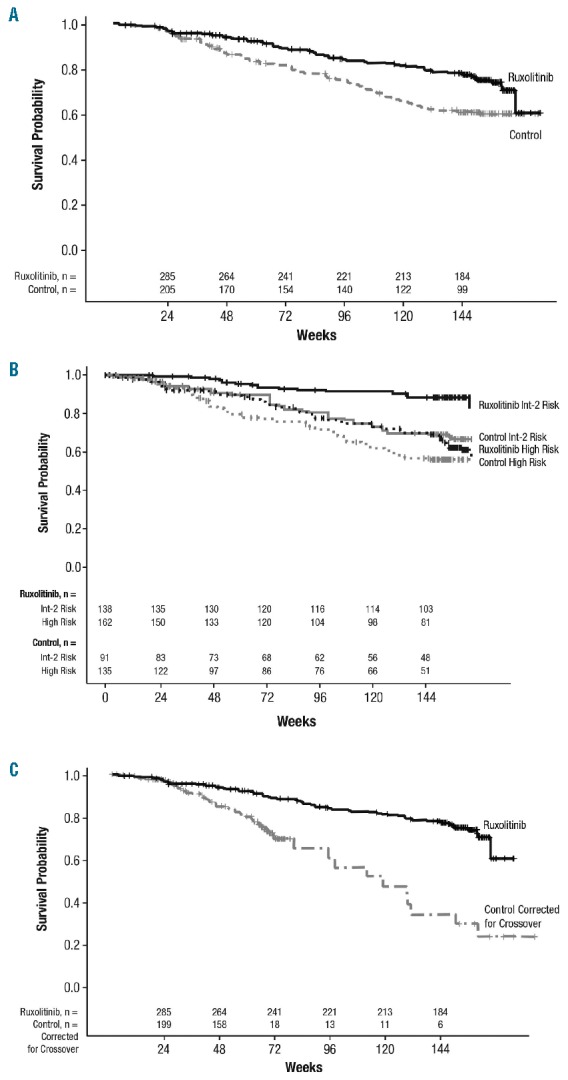
Kaplan-Meier analysis of overall survival by (A) intent to treat,a (B) International Prognostic Scoring System risk status,b and (C) correcting for crossover from the control arms (rank-preserving structural failure time analysis).c aHR: 0.65; 95%CI: 0.46–0.90; P=0.01. bHR: 0.47; 95%CI: 0.33–0.67; P<0.0001. cHR: 0.29; 95%CI: 0.13–0.63; P=0.01.
For correction of crossover from the control arm of each study, OS was analyzed using the RPSFT method (Online Supplementary Figure S1). The RPSFT method maintains the original randomized group definitions and thus preserves the validity of between-group comparisons by providing a randomization-based estimate of treatment effect corrected for the bias introduced by crossover. This approach is expected to project survival estimates for patients in the control arm to mirror survival as if they had not crossed over to receive investigational treatment and thus allows a closer approximation of the true incremental survival benefit between the 2 treatment arms. With the RPSFT analysis correcting for crossover from the control arm of each study, the HR was 0.29 (95%CI: 0.13–0.63) (Figure 1C). Kaplan-Meier estimate of OS at week 144 was 78% in the ruxolitinib arm, 61% in the ITT control arm, and 31% in the RPSFT-adjusted control arm. This corresponds to a 17%–47% absolute risk reduction with ruxolitinib treatment compared with control at week 144.
Assessment of base-line covariates
The following base-line factors were evaluated for their prognostic effect upon OS, irrespective of treatment: age (per 5 years), age over 65 years, sex, MF subtype (PMF/secondary MF), IPSS risk category, JAK2 V617F mutation status, base-line palpable spleen length (per cm below left costal margin), base-line spleen volume (per 5 dL), baseline hemoglobin (Hb; per 10 g/L), Hb <10 g/dL (yes/no), base-line white blood cell count (WBC; per 5×109/L), WBC >25×109/L (yes/no), base-line platelet count (per 50×109/L), presence of constitutional symptoms, and presence of 1% or more circulating blasts (yes/no). A set of Cox models was fitted to the data with 1 to n covariates at a time.21,22 Covariates that assessed the same parameter on a continuous or discrete scale were not included in the same model [eg. either base-line Hb as continuous (g/L) or binary (< 10 g/dL, yes/no) but not together]. Goodness of fit was evaluated with the Akaike information criterion (AIC), and the models were ordered according to minimizing AIC. The covariates most often included in the top 1000 models were considered for the final model, and treatment effect was estimated with adjustment for these covariates. The covariates considered were those identified as potential source of bias (ie. study) or known to have impact on MF prognosis.
Seven base-line factors (Hb, WBC, age, MF subtype, sex, spleen size, and platelet count) were identified as prognostic for survival, irrespective of treatment. When adjustments were made for these prognostic base-line characteristics and treatment was controlled for, larger base-line spleen volume, higher base-line WBC, and increased age correlated with incremental increases in the risk of death (Figure 2). The risk of death was 1.14 times higher for each additional 5 dL in spleen volume at baseline (HR=1.14; 95%CI: 1.07–1.21; Online Supplementary Figure S3). Higher base-line Hb, classification of PPV/PET-MF (vs. PMF), female sex (vs. male), and higher base-line platelet count correlated with incremental decreases in the risk of death (Figure 2). In this analysis, JAK2 mutation status was not a significant prognostic factor for survival (HR=0.91; 95%CI: 0.61–1.36; P=0.64). The HR for the treatment effect in the presence of the identified base-line covariates was 0.64 (95%CI: 0.46–0.85).
Figure 2.
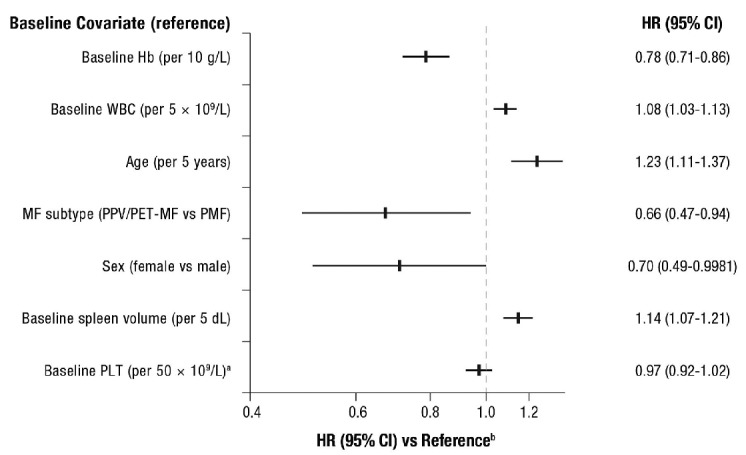
Overall survival by base-line covariates.a Patients were required to have platelet counts (PLT) ≥ 100×109/L at baseline. bAdjusted for prognostic base-line characteristics and controlled for treatment. Hazard ratio (HR) > 1 indicates an increased risk of death. Higher Hb level, secondary MF subtype, female sex, and higher platelet count were associated with better prognosis while higher base-line WBC, age, and spleen volume were associated with worse prognosis independently of treatment. For the following continuous covariates the risk of death was incrementally lower on each unit increase (Hb, platelet count) or incrementally higher (WBC, age, and spleen volume). Hb: hemoglobin; MF: myelofibrosis; PET: post–essential thrombocythemia; PPV: post–polycythemia vera; WBC: white blood cell count.
Overall survival by spleen size reduction
Associations between spleen volume and length reductions at week 24 and OS by treatment are shown in Figure 3A and B, respectively. OS was evaluated by the degree of spleen volume reduction achieved at week 24 (≥10% to <25%, ≥25% to <35%, ≥35% to <50%, and ≥50%), and HRs were calculated compared with patients who achieved a less than 10% reduction from baseline or who had no assessment at week 24. All models were adjusted for base-line characteristics: age, sex, MF subtype, Hb, WBC, platelet count, and spleen volume. All spleen volume reduction categories in the ruxolitinib group were associated with better prognosis compared with the less than 10% reduction category (Figure 3A). Similarly, patients who achieved palpable spleen length reductions of 25% or more with ruxolitinib treatment (including those who had reductions of ≥25% to <50%, ≥50% to <75%, and ≥75% to no longer palpable) had prolonged survival compared with patients who had no change or an increase in spleen length (Figure 3B). An analysis of spleen response by IWG-MRT criteria23 (ie, ≥50% reduction in palpable spleen length for base-line spleen more than 10 cm or 100% reduction for base-line spleen 10 cm or less) showed a similar trend; however, it did not reach nominal significance (P=0.09 in the model adjusted for base-line covariates). There was no association seen between spleen reduction and survival in the combined control arm, possibly because very few patients experienced a spleen response. Each 10% reduction from baseline in spleen length at week 24 was associated with a 9% reduction in the risk of death for ruxolitinib-treated patients (HR=0.91; 95%CI: 0.84–0.99; P=0.02), but this was not seen for patients in the combined control group (HR=0.95; 95%CI: 0.87–1.04; P=0.27) in the models with adjustment for the study effect and IPSS risk group.
Figure 3.
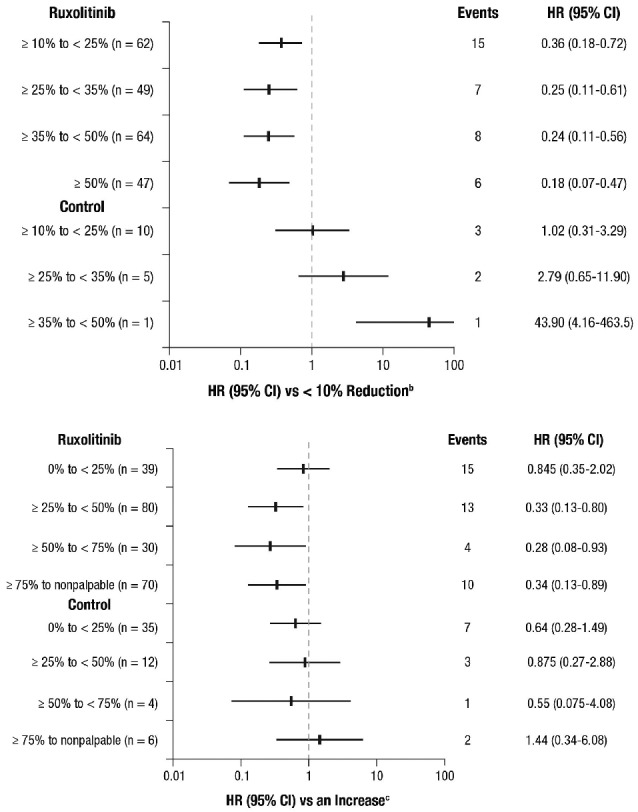
Correlation of (A) spleen volume reduction and (B) spleen length reduction at week 24 with overall survival (landmark analysis at 24 weeksa). aIncludes patients known to be alive at week 24. bCategory includes patients with a <10% reduction from baseline in spleen volume at week 24 or no assessment (ruxolitinib, n=64; control, n=189); among these patients, there were 26 deaths (events) in the pooled ruxolitinib group and 63 deaths in the control group. cCategory includes patients with no change or an increase from baseline in palpable spleen length at week 24 or no assessment (ruxolitinib, n=23; control, n=95); among these patients, there were 8 deaths (events) in the pooled ruxolitinib group and 28 deaths in the control group. HR: hazard ratio.
Discussion
In the COMFORT studies, ruxolitinib was shown to provide a survival benefit compared with placebo and BAT.11,12,14,20 A meta-analysis with the combined data of the 2 studies was prospectively planned in the protocol of COMFORT-I to derive a more precise estimation of the HR of the treatment effect. In this ITT analysis, patients who were randomized to ruxolitinib in the COMFORT studies had significantly prolonged survival compared with patients who were randomized to either placebo or BAT, thus confirming earlier reports. As described in these reports, there was no apparent trend in any treatment arm with regard to causes of death and no increased risk of death-related specific events, including infection, inflammation, bleeding events, or events from cardiovascular causes. In addition, this analysis showed that ruxolitinib conferred a relative survival benefit that was independent of the defined risk categories. A survival benefit with ruxolitinib was seen for patients with intermediate-2– or high-risk MF compared with placebo and BAT; moreover, patients with high-risk disease in the ruxolitinib group appeared to have risk of death similar to that of patients with intermediate-2–risk disease in the control group.
Overall survival was analyzed using an ITT method based on an individual patient data pooling approach that combined observations from both COMFORT studies. Treatment groups were defined according to the treatment assignment at randomization. This approach was selected to allow covariates to be taken into consideration in the models, recognizing the fact that pooling of individual patient data can be susceptible to generating potentially biased results and might, therefore, lead to misleading interpretations.24 Such imbalances might be caused by differences in base-line characteristics or by differences in the numbers of patients recruited in each arm. However, because the inclusion and exclusion criteria were similar for both COMFORT studies, in particular for those criteria related to disease and patients’ characteristics that could influence treatment effect, the 2 study populations were comparable. In addition, the on-treatment behavior of the individual control groups had similar efficacy and safety profiles.25
As reported previously,14 there was an apparent imbalance in the proportions of patients who were lost to follow up between the BAT and ruxolitinib arms of COMFORT-II. However, this imbalance did not reach nominal significance when assessed by reverse Kaplan-Meier analysis (P=0.12) (Online Supplementary Figure S4); this imbalance was not observed in the COMFORT-I study. Analyses of base-line and post-base-line characteristics (Online Supplementary Table S1) of these patients suggest minor differences between the 2 groups.
The designs of the COMFORT studies allowed for crossover from the control arms, limiting the interpretation of the magnitude of the survival advantage in the individual analyses. Median exposure to ruxolitinib for patients randomized to placebo was 104 weeks and for patients randomized to BAT was 56 weeks. The consequences of this crossover are apparent in the OS analyses of COMFORT-I, in which the HRs at 1, 2, and 3 years were progressively less favorable to ruxolitinib (HR=0.50, 0.58, and 0.69, respectively)11,12,20 and were of borderline significance at the 3-year mark (95%CI: 0.46–1.03; P=0.067). Because patients crossed over from placebo to ruxolitinib at a median of 41 weeks and all patients were receiving ruxolitinib after 80 weeks, the 3-year analysis (median follow up) was comparing the OS of patients who commenced on ruxolitinib three years before the analysis (ruxolitinib arm) with that of patients who began receiving ruxolitinib approximately one year later in the placebo arm. Longer follow up of COMFORT-II and the consequential longer exposure to ruxolitinib in the BAT arm will also likely have a similar effect on the ITT analysis of OS; however, because crossover occurred later in COMFORT-II (median time to crossover, 75 weeks) relative to COMFORT-I, this effect was not yet observed at the 3-year analysis, as reflected by the different HRs for survival (COMFORT-I: HR 0.69, P=0.067; COMFORT-II: HR 0.48, P=0.009). Here, we used the RPSFT method to evaluate the impact of crossover on the OS analysis; RPSFT is an accepted method to correct for crossover in randomized trials and has been used in other studies.17,18 The RPSFT analysis supports the hypothesis that the crossing over of placebo- or BAT-treated patients to ruxolitinib may have led to underestimating the actual effect of ruxolitinib on survival.
A potential confounding factor to interpretation of the survival advantage lies in the assumption that patients who were randomized to ruxolitinib may have received more supportive care, whereas treating investigators had more experience administering medications in the BAT arm and were more versed in the safety profiles of these agents. The focus on patients receiving an investigational agent may have led to comorbidities being more rapidly identified and treated, potentially affecting survival outcomes. Because of the designs of each study (open label vs. double blind), this would have an impact only on findings from COMFORT-II but not those from COMFORT-I. Also, assessments per protocol were independent of the treatment arms, thus ensuring that there were no major imbalances in the care/follow-up pattern while on treatment, and that after crossover, all patients would likely receive the same supportive care while on ruxolitinib.
In this analysis, several base-line clinical features and demographic characteristics were prognostic for survival. Larger base-line spleen volume, higher base-line WBC, increased age, lower base-line Hb level, PMF subtype, male sex, and lower base-line platelet count correlated with an increased risk of death when adjustments were made for other important base-line characteristics. Older age, leukocytosis, and anemia are recognized prognostic indicators on the IPSS and Dynamic International Prognostic Scoring System.9,10 There was no trend among the causes of death between men and women to account for the difference in survival observed; it is likely a composite of multiple factors that these studies were not specifically designed to evaluate. Although the above factors were shown to be prognostic in this analysis, a post hoc subgroup analysis of COMFORT-I26 and a similar analysis of COMFORT-II27 demonstrated that ruxolitinib treatment benefited each subgroup compared with placebo and BAT separately.
Spleen length reductions were correlated with improved survival in a previous analysis of patients who received ruxolitinib treatment at The University of Texas MD Andersen Cancer Center in a phase I/II study (n=107); patients who achieved spleen length reductions of 50% or more from baseline had a significantly prolonged survival compared with patients who achieved less than 25% reductions.28 Here we observed a similar improvement in survival for patients who had larger spleen size reductions, both by volume as assessed by MRI and palpable spleen length, compared with patients who had increased splenomegaly or no change from baseline. The positive correlation of greater on-treatment spleen size reduction with a reduced risk of death that was observed in the combined ruxolitinib group was not observed for patients in the combined control group, thus precluding use of spleen size reduction as a general surrogate marker for survival, independent of treatment. Because symptoms were assessed differently in each of the COMFORT studies, a pooled analysis of the impact of symptomatic improvement with ruxolitinib was not possible in this analysis.
These findings are consistent with previously reported observations11,14 and support the concept that ruxolitinib offers a survival benefit for patients with MF compared with other conventional treatment options. The survival benefit observed with ruxolitinib may be a composite derivative of multiple treatment effects (eg. reduction in spleen size, improvement in cytokine-mediated constitutional symptoms, and improvement in nutritional status11,12) and warrants further exploration.
Acknowledgments
Editorial assistance was provided by John Togneri, PhD, and was funded by Novartis. All authors contributed to the drafting and approval of the manuscript.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
This research is supported in part by the MD Anderson Cancer Center Support grant CA016672.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Tefferi A. Essential thrombocythemia, polycythemia vera, and myelofibrosis: current management and the prospect of targeted therapy. Am J Hematol. 2008;83(6):491–497. [DOI] [PubMed] [Google Scholar]
- 2.Vannucchi AM. Management of myelofibrosis. Hematology Am Soc Hematol Educ Program. 2011;2011:222–230. [DOI] [PubMed] [Google Scholar]
- 3.Vainchenker W, Delhommeau F, Constantinescu SN, Bernard OA. New mutations and pathogenesis of myeloproliferative neoplasms. Blood. 2011;188(7):1723–1735. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Wahab O, Levine RL. Primary myelofibrosis: update on definition, pathogenesis, and treatment. Ann Rev Med. 2009;60:233–245. [DOI] [PubMed] [Google Scholar]
- 5.Mesa RA, Schwager S, Radia D, et al. The Myelofibrosis Symptom Assessment Form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk Res. 2009;33(9):1199–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiladjian J, Gisslinger H, Passamonti F, et al. Health-related quality of life and symptom burden in patients with myelofibrosis in the COMFORT-II study. J Clin Oncol. 2012; 30(15 suppl) [abstract 6626]. [Google Scholar]
- 7.Emanuel RM, Dueck AC, Geyer HL, et al. Myeloproliferative neoplasm (MPN) symptom assessment form total symptom score: prospective international assessment of an abbreviated symptom burden scoring system among patients with MPNs. J Clin Oncol. 2012;30(33):4098–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geyer HL, Scherber RM, Dueck AC, et al. Distinct clustering of symptomatic burden among myeloproliferative neoplasm patients: retrospective assessment in 1470 patients. Blood. 2014;123(24):3803–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–2901. [DOI] [PubMed] [Google Scholar]
- 10.Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115(9):1703–1708. [DOI] [PubMed] [Google Scholar]
- 11.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verstovsek S, Mesa RA, Gotlib J, et al. Efficacy, safety and survival with ruxolitinib in patients with myelofibrosis: results of a median 2-year follow-up of COMFORT-I. Haematologica. 2013;98(12):1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–798. [DOI] [PubMed] [Google Scholar]
- 14.Cervantes F, Vannucchi AM, Kiladjian JJ, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013;122(25):4047–4053. [DOI] [PubMed] [Google Scholar]
- 15.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. [DOI] [PubMed] [Google Scholar]
- 16.Barosi G, Mesa RA, Thiele J, et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia. 2008;22(2):437–438. [DOI] [PubMed] [Google Scholar]
- 17.Robins JM, Tsiatis AA. Correcting for noncompliance in randomized trials using rank preserving structural failure time models. Commun Stat Theory Methods. 1991; 20(8):2609–2631. [Google Scholar]
- 18.Korhonen P, Zuber E, Branson M, et al. Correcting overall survival for the impact of crossover via a rank-preserving structural failure time (RPSFT) model in the RECORD-1 trial of everolimus in metastatic renal-cell carcinoma. J Biopharm Stat. 2012;22(6): 1258–1271. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1(11):710–719. [DOI] [PubMed] [Google Scholar]
- 20.Verstovsek S, Mesa RA, Gotlib J, et al. Long-term outcomes of ruxolitinib therapy in patients with myelofibrosis: 3-year update from COMFORT-I. Blood. 2013;122(21) [abstract 396]. [Google Scholar]
- 21.Mucciardi AN, Gose EE. A comparison of seven techniques for choosing subsets of pattern recognition properties. IEEE Trans Comput. 1971;C-20(9):1023–1031. [Google Scholar]
- 22.Cover TM, Van Campenhout JM.On the possible orderings in the measurement selection problem. IEEE Trans Syst Man Cybern. 1977;SMC-7(9):657–661. [Google Scholar]
- 23.Tefferi A, Cervantes F, Mesa R, et al. Revised response criteria for myelofibrosis: International Working Group-Myeloproliferative Neoplasms Research and Treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood. 2013;122(8):1395–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altman DG, Deeks JJ. Meta-analysis, Simpson’s paradox, and the number needed to treat. BMC Med Res Methodol. 2002;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mesa RA, Kiladjian JJ, Verstovsek S, et al. Comparison of placebo and best available therapy for the treatment of myelofibrosis in the phase 3 COMFORT studies. Haematologica. 2014;99(2):292–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verstovsek S, Mesa RA, Gotlib J, et al. The clinical benefit of ruxolitinib across patient subgroups: analysis of a placebo-controlled, phase III study in patients with myelofibrosis. Br J Haematol. 2013;161(4):508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison C, Kiladjian JJ, Gisslinger H, et al. Ruxolitinib provides reductions in splenomegaly across subgroups: an analysis of spleen response in the COMFORT-II study. Blood. 2011;118(21):129 [abstract 279].21505189 [Google Scholar]
- 28.Verstovsek S, Kantarjian HM, Estrov Z, et al. Long-term follow-up outcomes of 107 patients with advanced myelofibrosis receiving the JAK1/JAK2 inhibitor ruxolitinib: survival advantage in comparison to a matched historical control group of patients. Blood. 2012;120(6):1202–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]


