Abstract
We used the budding yeasts Saccharomyces cerevisiae and Torulaspora delbrueckii to examine the evolution of Sir-based silencing, focusing on Sir1, silencers, the molecular topography of silenced chromatin, and the roles of SIR and RNA interference (RNAi) genes in T. delbrueckii. Chromatin immunoprecipitation followed by deep sequencing (ChIP-Seq) analysis of Sir proteins in T. delbrueckii revealed a different topography of chromatin at the HML and HMR loci than was observed in S. cerevisiae. S. cerevisiae Sir1, enriched at the silencers of HMLα and HMRa, was absent from telomeres and did not repress subtelomeric genes. In contrast to S. cerevisiae SIR1's partially dispensable role in silencing, the T. delbrueckii SIR1 paralog KOS3 was essential for silencing. KOS3 was also found at telomeres with T. delbrueckii Sir2 (Td-Sir2) and Td-Sir4 and repressed subtelomeric genes. Silencer mapping in T. delbrueckii revealed single silencers at HML and HMR, bound by Td-Kos3, Td-Sir2, and Td-Sir4. The KOS3 gene mapped near HMR, and its expression was regulated by Sir-based silencing, providing feedback regulation of a silencing protein by silencing. In contrast to the prominent role of Sir proteins in silencing, T. delbrueckii RNAi genes AGO1 and DCR1 did not function in heterochromatin formation. These results highlighted the shifting role of silencing genes and the diverse chromatin architectures underlying heterochromatin.
INTRODUCTION
Heterochromatin-based gene silencing in Saccharomyces cerevisiae and its close relatives among the budding yeasts use the four Sir proteins to bind to nucleosomes throughout specific regions on chromosomes and to block the accessibility of other DNA binding proteins in that region (1–3). In these species, the Sir1 protein is perhaps most enigmatic. In contrast to Sir2, Sir3, and Sir4, which are the structural proteins of heterochromatin necessary for its establishment, maintenance, and inheritance, Sir1's main role in S. cerevisiae seems to be in the establishment of heterochromatin at HMLα and HMRa (4), though it contributes somewhat to the maintenance of heterochromatin (5). sir1Δ cells exhibit a phenotype whereby 50 to 80% of individual cells within the mutant population completely lack silencing at HMLα and HMRa, whereas the remaining cells are fully silenced at these loci. The unsilenced sir1Δ cells express transcripts from the silent mating type loci to the same extent as sir4Δ mutants, are mating defective, and in the case of MATa haploids, lose sensitivity to α-factor (4, 5). Furthermore, individual sir1Δ cells can switch transcriptional states at HML and HMR, switching from unsilenced to silenced once every 250 cell divisions and somewhat more slowly in the reverse direction. Biochemical and structural data revealed that Sir1 directly interacts with Orc1 and Sir4, suggesting that its localization is restricted to the silencers, where it facilitates efficient establishment of silencing (6, 7).
In addition to its bistable mutant phenotype, SIR1 has a dynamic evolutionary history. SIR1 has been duplicated more than once among Saccharomyces yeasts, and some species have lost paralogs, while others have retained them (8). As a result, SIR1 paralogs vary widely among these species in number and in the level of protein sequence similarity between paralogs, which is typically <50%. At one end of the spectrum, Saccharomyces bayanus var. uvarum has four SIR1 paralogs: SIR1 and three Kin-of-SIR1 (KOS1 to KOS3) genes. All four paralogs contribute to silencing in the species (8). At the other end of the spectrum, Kluyveromyces lactis lacks an identifiable SIR1 paralog, and silencing is mediated by SIR2, SIR4, ORC1, and SUM1 (9, 10). Candida glabrata is another yeast that lacks SIR1 yet, like S. cerevisiae, has SIR2, SIR3, and SIR4 orthologs that function in silencing (11). Yeast species seem to have created multiple solutions for establishing gene silencing, with some having no need for a SIR1 gene whereas others have employed up to four SIR1 genes. Analyses of SIR1 orthologs among the species of the clade indicate that KOS3 is the most ancestral form of SIR1 (8).
RNA interference (RNAi) is by far the most common mechanism of gene silencing. Key components of the RNAi machinery include Argonaute, Dicer, and, in most other organisms, an RNA-dependent RNA polymerase (12). RNAi mechanisms involve the production of double-stranded RNAs generated either by DNA-dependent RNA polymerases or by an RNA-dependent RNA polymerase. These double-stranded RNAs are cleaved by Dicer and bound by Argonaute proteins, which use them to direct the modification of DNA and histones occupying sequences complementary to the RNAs bound by the Argonaute protein. RNAi is found widely in plants, animals, and many fungi, including Schizosaccharomyces pombe, but is completely missing from S. cerevisiae.
Torulospora delbrueckii is a budding yeast species evolutionarily well positioned to explore some of the most enigmatic questions concerning the origins of Sir-based silencing, and especially the role of Sir1/Kos3. The species diverged from the Saccharomyces species before the whole-genome duplication and has T. delbrueckii Kos3 (Td-Kos3), the most ancestral form of S. cerevisiae Sir1 (Sc-Sir1). T. delbrueckii also has pre-whole-genome-duplication orthologs of SIR2 and SIR4 and a single gene orthologous to the ORC1-SIR3 gene pair of S. cerevisiae, which we referred to as ORC1-SIR3. In addition, the species has orthologs of key RNAi components: a gene encoding Argonaute, AGO1, and a budding-yeast Dicer-like gene called DCR1. These RNAi-like genes are orthologous to the AGO1 and DCR1 present in Naumovozyma castellii, a species in which they repress transcription of repetitive Ty elements (13). T. delbrueckii thus offers a chance to explore possible connections between, or divergence of, the two major mechanisms of heterochromatic gene silencing.
To date, no one has uncovered a sexual cycle for T. delbrueckii. However, the genome sequence of the T. delbrueckii type strain contains a MAT locus on chromosome (Chr) III, an HMLα locus on the same chromosome, and two HMRa loci (one on chromosome V and the other on chromosome VII) (14). To explore the functions of T. delbrueckii silencing genes, we first created marked strains, protocols, and vectors to allow molecular genetic investigations (A. Ellahi and J. Rine, unpublished data). We then compared the functions of presumptive silencing genes of T. delbrueckii to the functions of their S. cerevisiae orthologs. These experiments offered an unbiased view of the genome-wide function of T. delbrueckii SIR genes, revealing a distinctly different molecular topography of silenced chromatin than is seen in S. cerevisiae. Additionally, we constructed ago1Δ and dcr1Δ single mutants and an ago1Δ dcr1Δ double mutant and performed deep sequencing of mRNAs to uncover all loci that were possibly subject to transcriptional repression by the T. delbrueckii RNAi pathway. The study began with a genome-wide analysis of the roles of Sc-Sir1 in Saccharomyces to set the stage for studies of Td-Kos3 in T. delbrueckii. Collectively, these experiments lead to a new conceptualization of the evolution of Sir1's role in silencing and contribute to an expanded appreciation of the roles of RNAi components. These data provide the most complete picture to date of how the earliest SIR1-containing SIR silencing complex functioned and the evolutionary trajectories it may have followed.
MATERIALS AND METHODS
Identification of SIR1 paralogs.
To identify SIR1 paralogs, the SIR1 protein sequence was used as a BLAST query against sequenced yeast genomes available on the Yeast Gene Order Browser (YGOB). Significant hits included the KOS3 gene in T. delbrueckii (TDEL0E00350), as well as all other previously discovered SIR1 paralogs (8). T. delbrueckii KOS3 itself, when used as a BLAST query against yeast genomes on the YGOB, identified the Zygosaccharomyces rouxii KOS3 gene and the S. bayanus var. uvarum KOS3 gene as the two top matches. Other SIR1 paralogs, including S. cerevisiae SIR1, were among the top 15 matches.
Yeast strains and plasmids.
Yeast strains are listed in Table S1 in the supplemental material. S. cerevisiae strains were generated in the W303 background. Deletion mutants and epitope-tagged alleles of SIR genes were made as previously described, using one-step integration of knockout cassettes (15). T. delbrueckii strains were grown in rich medium (yeast extract-peptone-dextrose [YPD]) at 30°C. Gene disruption in T. delbrueckii required ∼500 bp of sequence identity to the target region. Therefore, knockout cassettes and other tagging constructs were first cloned into plasmids containing 500 bp of sequence identical to the sequences flanking the genomic target and then amplified via PCR and transformed into strains. Transformations for T. delbrueckii were performed using the same lithium acetate-polyethylene glycol (PEG) method used for S. cerevisiae (16).
RNA isolation and qRT-PCR analysis.
Strains of both S. cerevisiae and T. delbrueckii were grown to an A600 of 0.8 to 1.0 at 30°C in YPD medium. RNA was extracted as described previously using the hot acid-phenol method (17, 18). cDNA and quantitative reverse transcriptase (qRT)-PCR analyses were performed as described previously (17). The oligonucleotides used for ACT1 amplification were GCCGGTGACGACGCTCC and CCTCTCTTGGATTGAGCTTCATCACC; the oligonucleotides used for KOS3 amplification were TTGGAGAACTATCGCAGAGAGAGC and TCTCTTTGGCTATTGCGGTTGG.
Chromatin isolation and immunoprecipitation.
All strains were grown in 100 ml YPD medium and harvested in log phase at an A600 of ∼0.7. Cross-linking was performed at 25°C in 1% formaldehyde for 45 min. Chromatin was prepared as previously described (19). Sonication was performed to an average genomic fragment size of 300 to 400 bp. Immunoprecipitation of V5 epitope-tagged Sir1, Td-Kos3, Td-Sir2, and Td-Sir4 was performed overnight at 4°C using 800 μl of chromatin and 75 μl of anti-V5 resin from Sigma (A7345). After several washes, protein and DNA were eluted from beads in Tris-EDTA (TE) buffer plus 1% SDS at 65°C, followed by reversal of cross-linking and then protease treatment. DNA was purified using Qiagen DNA spin columns prior to library preparation. The functions of epitope-tagged SIR alleles in T. delbrueckii were assayed by measuring repression at the silent HMRa1 gene; the function of V5-tagged Sir1 was measured by its ability to complement a sir1Δ mutation.
Library preparation and sequencing.
Chromatin immunoprecipitation (ChIP) libraries were prepared using the Illumina TruSeq DNA Sample Prep kit. Transcriptome sequencing (RNA-Seq) libraries were prepared using the Illumina TruSeq mRNA sample prep kit. One hundred-base-pair paired-end libraries were used to accurately assign reads. A Bioanalyzer instrument (Agilent) was used to quantify all the libraries. The libraries were sequenced on an Illumina HiSeq 2000 machine (see Tables S10 and S11 in the supplemental material for sequence read information for all the libraries).
URA3 reporter gene assay for silencing.
Cells were grown to saturation overnight in 2 ml of YPD medium containing hygromycin B (to select for plasmids). The cells were then pinned onto plates with three different media: complete supplement mixture (CSM) containing hygromycin B (to assay overall growth), CSM containing hygromycin B and lacking uracil (to select for cells expressing URA3), and CSM containing uracil and 5-fluoroorotic acid (5FOA) to select for cells not expressing URA3 (20). The cells were pinned in a 5-fold dilution series, and the plates were imaged on day 3 of growth.
Data analysis. (i) Chromatin immunoprecipitation followed by deep sequencing (ChIP-Seq).
Reads were mapped, using Bowtie2, to either the S. cerevisiae S288C reference genome or the T. delbrueckii reference genome sequence (14). Duplicate reads were discarded using Picard, and pileup files were generated using Samtools (21). The data were plotted and visualized using custom Python scripts. Statistically significant peaks of enrichment in immunoprecipitation (IP) samples were found by using the MACS peak-calling software (22).
(ii) RNA-Seq.
Data were analyzed as previously described (17). Briefly, Tophat2 was used to map transcripts to their genes of origin. Transcript quantification was performed using Cufflinks (23). DESeq was used to perform tests for differential gene expression (24). The results were filtered for genes that showed differences in expression greater than 2-fold relative to the wild type, with a P value of <0.05 and a false-discovery rate of <10%. Weighted Venn diagrams detailing overlap in gene sets were made using the Matplotlib_venn package in Python.
(iii) Transcription factor binding site analysis.
Putative transcription factor binding sites were identified by the motif-scanning algorithm in MochiView (25).
(iv) GO term analysis.
Gene sets were subjected to gene ontology (GO) term analysis on the Saccharomyces Genome Database website using the “GO Term Finder” tool with default settings and background sets of genes. All significant GO terms with a P value of <0.05 and a false-discovery rate of <10% were included in the final results.
Microarray data accession numbers.
The sequence reads were deposited in the NCBI Sequence Read Archive (SRA) (http://www.ncbi.nlm.nih.gov/sra) under accession numbers SRP055208, SRP065348, SRP065349, SRP065572, and SRP065573.
RESULTS
S. cerevisiae Sir1 localized to the autonomous silencers of HML and HMR-E.
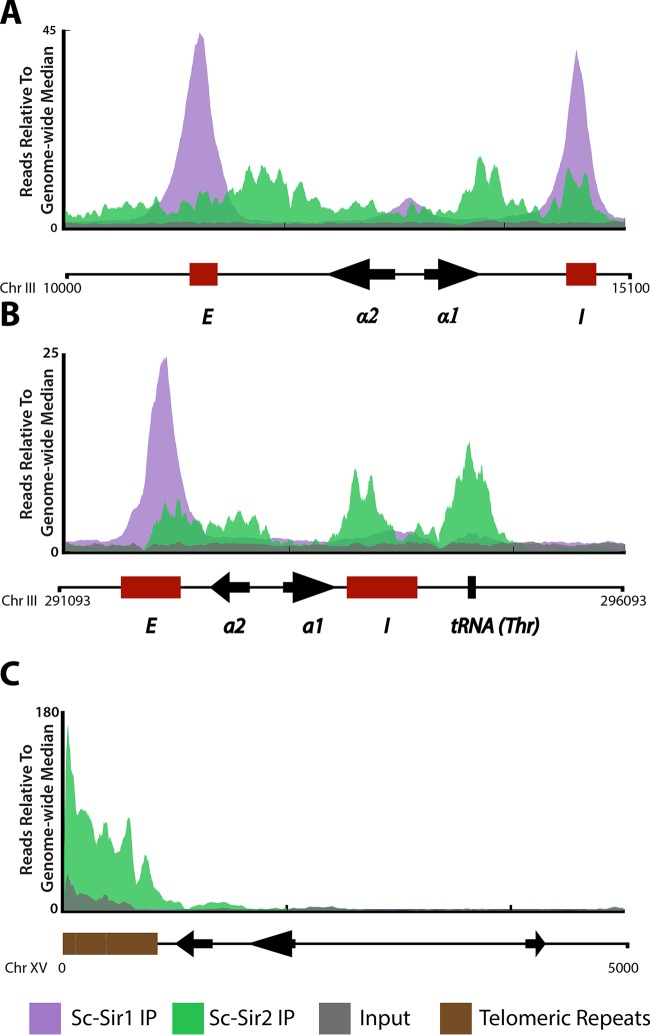
Previous studies of genome-wide Sir protein localization in S. cerevisiae have focused on Sc-Sir2, Sc-Sir3, and Sc-Sir4 (1, 17). To study Sc-Sir1's evolution, we first established the molecular topography of Sc-Sir1 across the S. cerevisiae genome. ChIP-Seq of Saccharomyces Sir1 tagged with three copies of the V5 epitope (Sc-Sir1-3×V5) revealed several important features of Sc-Sir1's genome-wide binding profile. First, Sc-Sir1 displayed a sharp, narrow, largely silencer-restricted binding profile at HML-E, HML-I, and HMR-E (Fig. 1A and B; the no-tag control is shown in Fig. S1 in the supplemental material). This distribution was in agreement with previous ChIP-PCR data suggesting that Sc-Sir1 is restricted to the HMR-E silencer (26). Sc-Sir1's binding profile was strikingly different from previous data on Sc-Sir2, Sc-Sir3, and Sc-Sir4 (Sir2 is shown in green in Fig. 1A and B). The proteins exhibit strong coenrichment in discrete peaks at the pair of silencers flanking HMLα and HMRa, as well as within the silent loci (1). Sc-Sir1 enrichment overlapped Sc-Sir2, Sc-Sir3, and Sc-Sir4 enrichment at three of the silencers and at a smaller peak located in the promoter region of HMLα but not within HMRa (Fig. 1A). Each silencer at HML is sufficient, on its own, for silencing HML (27). At HMR, the E silencer is required for HMR silencing. HMR-I contributes to silencing when the locus is carried on a plasmid but on its own is insufficient to silence HMR and can be deleted from the chromosome with no obvious impact on silencing (28, 29). No Sc-Sir1 enrichment was detected at the HMR-I silencer.
FIG 1.
Sc-Sir1 associates with the silencers of HMLα and HMR-E in S. cerevisiae. ChIP-Seq was performed on V5-tagged Sc-Sir1 protein. Shown are the Sc-Sir1-3×V5 IP enrichment patterns (purple) at various genomic loci, with chromosomal coordinates shown at the bottom of each panel. (A and B) Sc-Sir1 at HMLα (A) and HMRa (B). For comparison, binding of Sc-Sir2 is shown. The E and I silencers are depicted by red boxes, and coding genes are shown by arrows. (C) Sc-Sir2 enrichment (green) at the left arm of chromosome XV, TEL15L. Sc-Sir1 was not enriched at this locus.
S. cerevisiae Sir1 was absent from telomeres.
Telomeres in S. cerevisiae recruit the Sc-Sir2, Sc-Sir3, and Sc-Sir4 proteins through interactions with Rap1 (30). Mutations in SIR2, SIR3, and SIR4 but not SIR1 disrupt transcriptional repression of reporter genes placed adjacent to artificially truncated telomeres (1, 31). These early studies suggested SIR1 has no role in gene silencing near artificial telomeres. However, one study of a URA3 reporter gene at a native telomere (TEL11L) indicated a role for Sir1 in repressing genes at native telomeres (32). Thus, SIR1's role in telomeric and subtelomeric silencing warranted further genome-wide evaluation.
Strikingly, our results showed that the Sc-Sir1 protein was undetectable at all telomeres and subtelomeric regions (TEL15L is shown in Fig. 1C; see Fig. S2 in the supplemental material for all 32 telomeres). The sole exceptions to this rule are the Sc-Sir1 peaks at the silencers of HMLα, which fall within 20 kbp of the left end of chromosome III (Fig. 1A; see Fig. S2 in the supplemental material). In contrast, Sc-Sir2, Sc-Sir3, and Sc-Sir4 are all highly enriched at the telomeres, where they repress ∼6% of subtelomeric genes (Fig. 1C) (1, 17). To test the possibility that Sc-Sir1 binds telomeres transiently, long enough to repress genes but not long enough to be detectably enriched, we performed deep sequencing of mRNAs from wild-type and sir1Δ strains. Genes at HMLα and HMRa were derepressed in the sir1Δ strain, as expected, as were a few genes under a/α control (see Table S2 in the supplemental material). However, consistent with a lack of Sc-Sir1 binding at and/or near telomeres, no subtelomeric genes were derepressed in the sir1Δ mutant.
The T. delbrueckii genome contained KOS3, an ancestral SIR1 paralog.
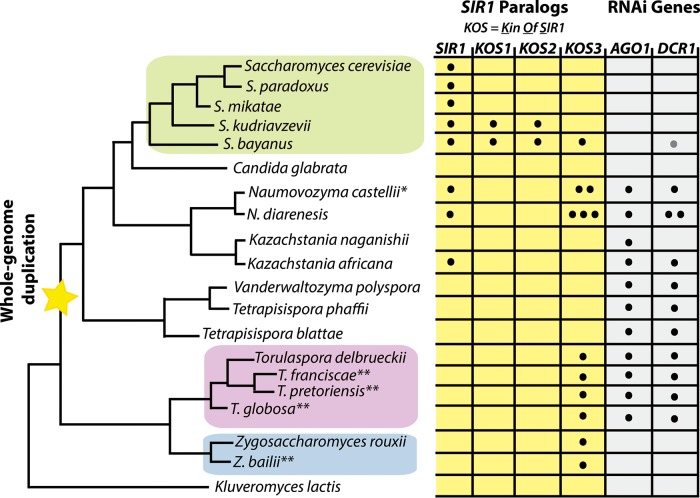
A reconstruction of the evolutionary history of the SIR1 gene (8) yielded two important findings: (i) SIR1 has undergone at least two or three gene duplications among post-whole-genome-duplication yeast species, and (ii) SIR1 itself may also be the product of an internal duplication of a shorter SIR1 paralog called KOS3, first recognized in S. bayanus var. uvarum. This paralog dates back to pre-whole-genome-duplication yeast species (8). T. delbrueckii, like Z. rouxii, has a KOS3 paralog as its only Sir1-related gene (Fig. 2). KOS3 has approximately half the sequence length of SIR1 and aligns best with the C-terminal Orc1-interacting region of Sir1. S. bayanus var. uvarum, N. castellii, and Naumovozyma diarenesis also have KOS3 paralogs of similar size (Fig. 2). The KOS3 paralog in S. bayanus var. uvarum participates in silencing, though its function is partially shared with the other three paralogs in the species (8). All identified SIR1 paralogs are highly divergent at the protein sequence level (8). Similarly, Sc-Sir1 and Td-Kos3 share only 16% protein similarity.
FIG 2.
SIR1 paralogs and RNAi genes in the family Saccharomycetaceae. Depicted is a phylogenetic tree of budding yeast species in the family Saccharomycetaceae, along with the SIR1 paralogs and RNAi gene paralogs (where applicable; some species, e.g., K. lactis, do not have SIR1 or the RNAi genes AGO1 and DCR1). The number of dots within each box indicates the number of copies of that particular paralog in the genome (e.g., N. castellii has two highly similar KOS3 paralogs). S. cerevisiae contains the defining SIR1 gene, whereas S. bayanus contains four SIR1 genes: SIR1 and three KOS paralogs. KOS3 is the earliest SIR1 paralog, deduced to have originated prior to the whole-genome duplication. T. delbrueckii has the budding yeast orthologs of AGO1 and DCR1. All sequenced species in the Zygosaccharomyces and Torulaspora clades have a KOS3 paralog in their genomes. *, N. castellii also has a fourth SIR1 paralog, KOS4, specific to that species (not shown for simplicity); **, results from additional species (Zygosaccharomyces baillii, Torulaspora francisiae, Torulaspora pretoriensis, and Torulaspora globosa) are unpublished (Devin Scannell, personal communication). The gray dot in the DCR1 gene column for S. bayanus var. uvarum indicates that its DCR1 is a pseudogene.
KOS3 is indispensable for silencing in T. delbrueckii.
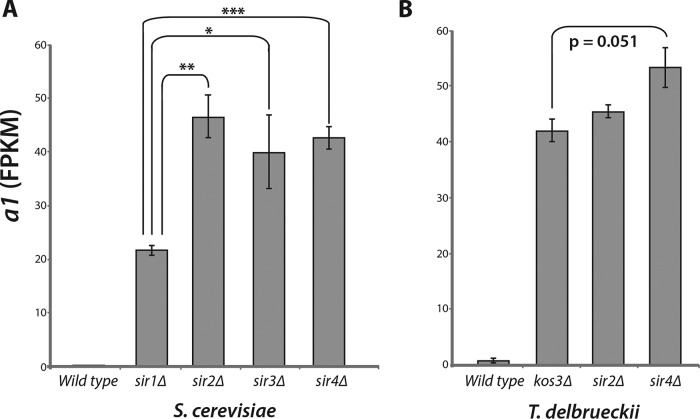
In S. cerevisiae, deletion of SIR1 causes a partial loss of silencing at HMLα and HMRa when evaluated at the population level. At the single-cell level, 50 to 80% of sir1Δ cells lack silencing at HMLα and HMRa, whereas these loci are fully silenced in the remaining cells (5). Thus, expression of HMRa1 in a sir1Δ strain, as measured in bulk RNA from a population of cells, was less than the expression level seen in S. cerevisiae sir2Δ, sir3Δ, or sir4Δ mutants (Fig. 3A).
FIG 3.
T. delbrueckii kos3Δ mutants completely lacked silencing at HMRa. (A) HMRa1 expression in the wild type and four S. cerevisiae silencing mutants: sir1Δ, sir2Δ, sir3Δ, and sir4Δ. Expression was measured from deep sequencing of mRNAs and quantified as FPKM. a1 derepression measured in a population of sir1Δ cells was ∼50% that of the derepression measured in sir2Δ, sir3Δ, and sir4Δ mutants, which completely lack the ability to silence HML and HMR. P values: *, <0.01 to 0.05; **, 0.001 to 0.01; ***, <0.001 (Student's t test). The error bars indicate standard deviations. (B) Chromosome V HMRa1 expression in T. delbrueckii in wild-type, kos3Δ, sir2Δ, and sir4Δ strains. In contrast to the more modest effect seen in the sir1Δ mutant, kos3Δ mutants exhibited as great a silencing defect as sir2Δ or sir4Δ mutants.
To evaluate whether KOS3 was also only partially required for silencing in T. delbrueckii or played a more prominent role, we measured expression of the HMRa1 locus in a MATα strain containing deletion alleles of KOS3, SIR2, or SIR4 (the SIR3 ortholog in T. delbrueckii is ORC1, which appears to be essential [unpublished observation]). In contrast to the partial derepression of HMRa1 seen in S. cerevisiae sir1Δ, T. delbrueckii kos3Δ cells showed complete derepression of HMRa1, indistinguishable from that in sir2Δ and sir4Δ cells (Fig. 3B). Thus, KOS3 played a more central role in silencing in T. delbrueckii than S. cerevisiae SIR1.
T. delbrueckii Kos3 was coenriched with Td-Sir2 and Td-Sir4 at all heterochromatic locations.
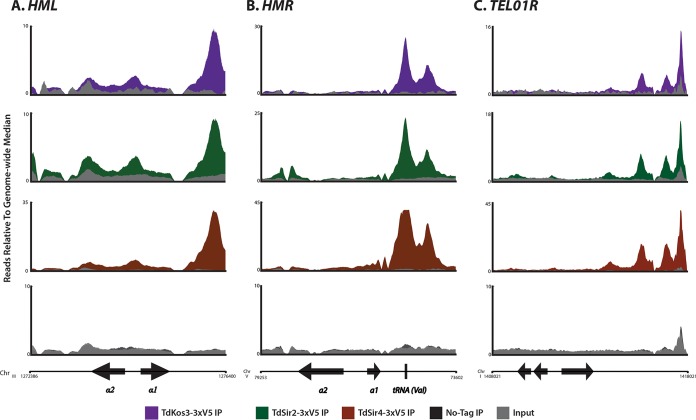
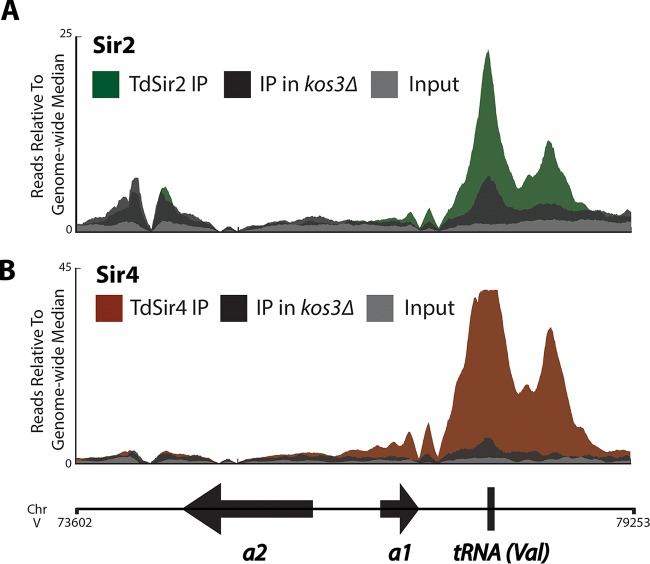
The genome-wide binding profiles of Td-Kos3, Td-Sir2, and Td-Sir4 in T. delbrueckii were striking with respect to the differences in Sir protein distributions in S. cerevisiae. At HMR, Td-Kos3 was most enriched in a pair of close but discrete peaks beginning approximately 670 bp 3′ of HMRa1, which were also the positions most enriched for Td-Sir2 and Td-Sir4 (Fig. 4B). The first of these peaks corresponded to a tRNA-Val gene. The distribution of Td-Kos3, Td-Sir2, and Td-Sir4 at HMLα showed only a single prominent peak of enrichment 770 bp from the 3′ end of HMLα1 (Fig. 4A). At neither HML nor HMR of T. delbrueckii was there evidence of two flanking enrichment peaks analogous to the two silencers flanking the silent mating type loci in S. cerevisiae.
FIG 4.
Enrichment of Kos3, Sir2, and Sir4 in T. delbrueckii at heterochromatic locations. ChIP-Seq of V5-tagged Td-Kos3, Td-Sir2, Td-Sir4, and a no-tag control strain was performed. Shown are the enrichment patterns of the three proteins at HML (A), HMR (B), and a representative telomere, TEL01R (C). The binding pattern of Td-Kos3 mirrored the binding patterns of Td-Sir2 and Td-Sir4 at these loci. The no-tag control immunoprecipitation is also shown. The arrows without labels depict nearby coding genes.
In addition to examining Td-Kos3 binding at HML and HMR, we also interrogated Td-Kos3 enrichment at presumptive telomeres in T. delbrueckii to determine whether it was absent from telomeres, as Sc-Sir1 was in S. cerevisiae. At least 10 out of 16 telomeres showed enrichment of Td-Kos3, Td-Sir2, and Td-Sir4: TEL01L, TEL02L, TEL04L, TEL07L, TEL08L, TEL01R, TEL04R, TEL05R, TEL06R, and TEL08R (Fig. 4C shows TEL01R; see Fig. S3 in the supplemental material for all 16 telomeres). Td-Kos3's presence at telomeric sequences in T. delbrueckii was a marked difference from Sc-Sir1's absence from telomeres. Likewise, many genes within 20 kb of chromosome ends increased in expression in all three T. delbrueckii sir mutants examined (kos3Δ, sir2Δ, and sir4Δ) (see Table S9 in the supplemental material). Thus, similar to its more extensive role in silencing at T. delbrueckii HML and HMR, Td-Kos3 was also required to repress expression of subtelomeric genes.
T. delbrueckii SIR2 had roles outside its functions with KOS3 and SIR4.
We investigated genome-wide functions for T. delbrueckii KOS3, SIR2, and SIR4 by performing mRNA sequencing (mRNA-Seq) in kos3Δ, sir2Δ, and sir4Δ mutants. Overall, 22 genes increased in expression across all three mutants (see Table S9 in the supplemental material). These 22 genes were either at the silent mating type loci or adjacent to the silent mating type loci or were subtelomeric genes within 20 kb of a chromosome end. No centromere-adjacent genes changed expression among this set of mutants. Comparing the overlap between genes across all three sir mutants, we found that the majority of the changes in expression in the kos3Δ and sir4Δ mutants completely overlapped with changes in expression in the sir2Δ mutant, suggesting that KOS3 and SIR4 did not have any function outside their role in the Sir complex (see Fig. S4B in the supplemental material). There were 124 genes that increased specifically in the sir2Δ mutant, however, indicating that like SIR2 in S. cerevisiae, T. delbrueckii SIR2 had roles beyond heterochromatin formation.
To examine potential roles that T. delbrueckii SIR2 may have, we performed GO term analysis on the 85 sir2Δ-specific genes that had orthologs in S. cerevisiae. Using the S. cerevisiae functional annotations for these genes, we found 21 genes associated with meiosis and sporulation and 9 genes associated with carbohydrate metabolism (see Table S4 in the supplemental material, asterisks). Since T. delbrueckii SIR2 is the pre-whole-genome duplication ortholog of S. cerevisiae SIR2 and HST1, we also investigated whether, like HST1, T. delbrueckii SIR2 functioned as a promoter-specific repressor by examining whether any genes contained statistically significant Td-Sir2 peaks in their promoters. Of the 124 Td-Sir2-regulated genes, 66 had a Td-Sir2 peak in their promoters (see Table S4 in the supplemental material, ‡).
T. delbrueckii Kos3 bound to the silencers of HMLα and HMRa.
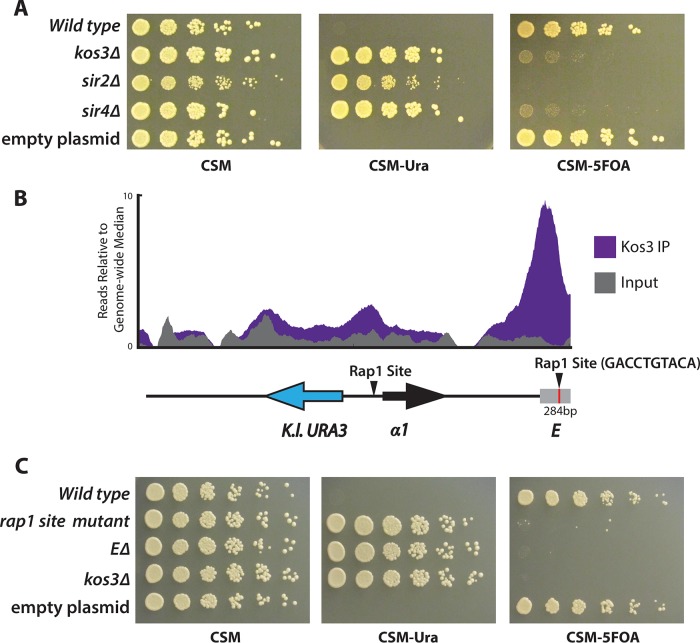
The largely silencer-restricted binding profile of Sc-Sir1 correlated with Sc-Sir1's importance in establishing silencing. To determine whether the regions bound by Td-Kos3 corresponded to the silencers of T. delbrueckii, we created a reporter-based silencing assay using a plasmid containing the entire T. delbrueckii HMLα locus plus 1,000 bp on either side and transformed the plasmid into T. delbrueckii. In this plasmid, the α2 coding region was replaced with K. lactis URA3. Strains auxotrophic for uracil yet containing the plasmid were unable to grow on medium lacking uracil due to silencing of the K. lactis URA3 gene. Deletion of KOS3, SIR2, or SIR4 relieved this repression, leading to URA3 expression and growth on medium lacking uracil (Fig. 5A).
FIG 5.
Kos3 bound to the silencer of HMLα. (A) A plasmid bearing an ∼5-kb fragment of HMLα in which the α2-coding gene had been replaced with the K. lactis URA3 gene was transformed into T. delbrueckii wild-type, kos3Δ, sir2Δ, and sir4Δ strains. T. delbrueckii silencing mutants were able to grow on medium lacking uracil (CSM-Ura) and unable to grow on medium containing 5FOA (CSM-5FOA). (B) Depiction of region E at HML in relation to the α1 gene and the region bound by Td-Kos3. (C) Region E and a putative Rap1 binding site (red line in panel B) were critical for silencing.
To map the silencers at HMLα, we deleted a 284-bp fragment (region E) corresponding to the major Td-Kos3, Td-Sir2, and Td-Sir4 binding peak adjacent to the coding genes and evaluated its impact on URA3 silencing. This deletion completely abolished silencing at HMLα (Fig. 5C). Formally, silencers are defined as cis-acting regulatory sites. Because of the nature of the assay, there was an intact copy of the E region in the chromosome, which nevertheless could not maintain silencing in cells with a deletion of the region on a plasmid-borne HML locus. Therefore, the deleted region contained a silencer for HML, or at least a critical component of one.
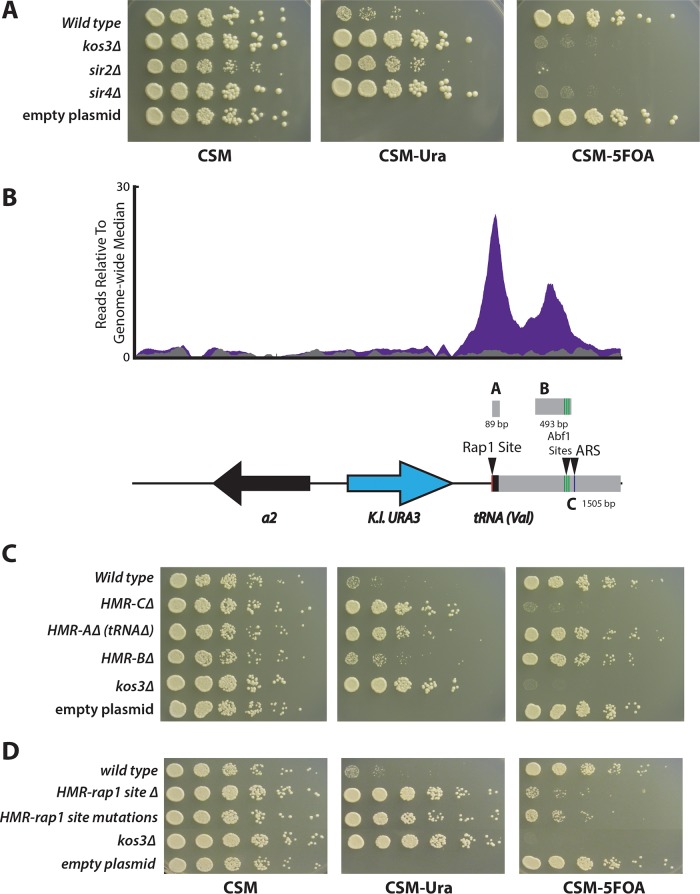
A similar assay was developed to map silencer elements at HMRa by cloning an ∼5-kb fragment containing HMR from T. delbrueckii chromosome V and replacing the a1 coding region with the K. lactis URA3 gene. Silencing of this reporter was also dependent on KOS3, SIR2, and SIR4 (Fig. 6A). The binding profile of Td-Kos3 at HMRa at the putative silencer region showed two peaks, corresponding to regions A and B. Region C included regions A and B and some surrounding sequence (Fig. 6B). Region A was centered on the first peak and contained a valine tRNA gene. Deletion of region A had a modest effect on silencing, resulting in weak growth on medium lacking uracil, but not to the same extent as in the kos3Δ mutant (Fig. 6C). Deletion of region B had slight to almost no effect on silencing, and deletion of region C led to a complete loss of silencing (Fig. 6C). For the reasons described above, the deletion of the C region must have removed all or a critical part of a silencer for HMR.
FIG 6.
Kos3 bound to the silencer of HMRa. A plasmid-based URA3 reporter construct was developed to map silencers at HMRa. (A) Silencing (lack of growth on CSM lacking uracil [CSM-URA]) was dependent on T. delbrueckii SIR genes. CSM-5FOA, CSM containing 5FOA. (B) Depiction of the fragment of HMRa on the plasmid in relation to the region of Td-Kos3 binding (purple). Immediately adjacent to the valine tRNA was a putative Rap1 site (red line). A cluster of three putative Abf1 binding sites was present in region B (green lines), as well as a putative ARS consensus sequence (arrowhead and blue line adjacent to the green lines). (C) Silencing as measured by growth on medium lacking uracil in each of the deletion constructs depicted in panel B. (D) Mutations to the Rap1 binding site adjacent to region A disrupted silencing.
T. delbrueckii silencers contained Rap1 binding sites that were important for silencing.
In S. cerevisiae, the E and I silencers contained combinations of binding sites for Rap1, Abf1, and the origin recognition complex (ORC). The silencers of K. lactis contain binding sites for Reb1 and Ume6, as well as an additional “C box” sequence (33). Since T. delbrueckii lies in a position between S. cerevisiae and K. lactis on the phylogenetic tree, we evaluated whether T. delbrueckii silencers contained binding sites that resembled those of K. lactis or S. cerevisiae, potentially illuminating how this major evolutionary transition of transcription factor binding sites occurred. The T. delbrueckii silencer region E defined by the deletion at HML contained a high-scoring Rap1 DNA binding motif 797 bp away from the 3′ end of the α1 gene: GACCTGTACA. A high-scoring Rap1 site was also found in the promoter region of HML, between the α2 and α1 genes, reminiscent of the Rap1 binding site in the promoter region of HML in S. cerevisiae. To test the importance of the Rap1 binding site within region E, a triple mutant that disrupted the three most conserved base pairs of this Rap1 motif (underlined) (GACCTGTACA to GAAATATACA) was evaluated (Fig. 5C). This mutant diminished silencing to the same extent as deleting the entire E region, suggesting that the Rap1 binding site was a key component of the silencer. A Rap1 binding site was also found in the T. delbrueckii HMR region immediately adjacent to the valine tRNA, residing just outside region A. Disrupting this Rap1 binding site via a complete deletion, or mutating it from CATCCATACA to CATAAATACA, also greatly reduced silencing at HMRa (Fig. 6D).
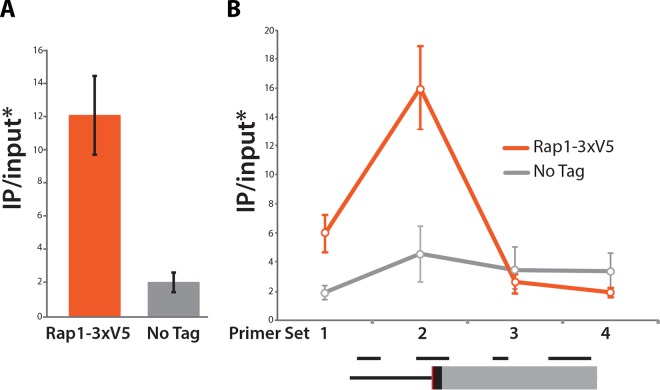
The DNA binding domain of the S. cerevisiae Rap1 protein has been mapped to amino acid residues 358 to 602 (34, 35). Alignment of the Sc-Rap1 and Td-Rap1 protein sequences revealed that the region is highly conserved between the two species, displaying 81% sequence identity, suggesting that Td-Rap1 may bind to the conserved Rap1 binding motifs at the T. delbrueckii silencers. To test directly if Td-Rap1 bound to the silencers, chromatin immunoprecipitation followed by quantitative PCR (qPCR) was performed on tagged Td-Rap1-3×V5. Td-Rap1 was enriched at the silencers of both HML and HMR, most highly in regions that included the conserved Rap1 binding site (Fig. 7).
FIG 7.
Rap1 binds to the silencers of the silent mating type loci in T. delbrueckii. (A) Rap1 was enriched at the E silencer region of HML that included the putative Rap1 binding site. (B) Enrichment of Rap1 along the silencer of the chromosome V HMR, with genomic positions of primer sets 1 to 4 depicted in order below. The Rap1 binding site is shown in red, the tRNA gene is in black, and the C region is in gray. *, all IP-over-input values are relative to SSC1 IP over input. The error bars indicate standard deviations.
In addition to Rap1 binding sites, a motif search also revealed the presence of three putative Abf1 binding sites clustered within region B of HMR (Fig. 6B, green lines under arrowhead), as well as one site within the promoter region of HML (overlapping the putative Rap1 site). Mutations of the highest-scoring of these putative binding sites in the B region, or deletion of all three, had no effect on silencing (data not shown). A search for autonomously replicating sequence (ARS) consensus sequences revealed a potential candidate AT-rich sequence 13 bp in length in the C region of HMR (Fig. 6B, arrowhead marked “ARS”). This C region was also found to have a functional ARS; however, deleting the sequence that may represent this functional ARS had no effect on silencing (data not shown).
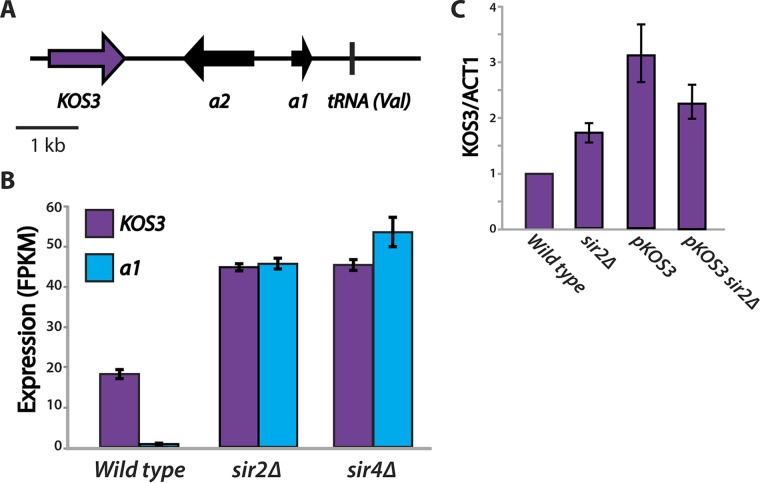
KOS3 expression was autoregulated.
The KOS3 gene itself is located ∼1 kb away from the copy of HMR carried on chromosome V (Fig. 8A). Interestingly, in the sir2Δ and sir4Δ mutants, the expression of KOS3 itself doubled (Fig. 8B). Neither Td-Sir2 nor Td-Sir4 was enriched at the promoter of the KOS3 gene, indicating that these proteins do not directly repress it. Genes adjacent to silent mating type cassettes are often derepressed when losses in silencing occur, presumably because repressive chromatin at the silent locus exerts transcriptional repression on nearby genes; for example, the CHA1 gene adjacent to HML in S. cerevisiae increases in expression in sir mutants (17). When the KOS3 gene was moved from its native location to a plasmid, there was no increase in its expression in a sir2Δ mutant (Fig. 8C). The location of the KOS3 gene and its increased expression when HMR is derepressed suggest that in a wild-type strain, occasional lapses in silencing at HMR would increase the expression of its repressor, KOS3, providing an autoregulatory method of maintaining silencing.
FIG 8.
KOS3 expression was autoregulated by the expression state of the Chr V HMR. (A) The KOS3 gene is located ∼1 kb away from the HMRa2 gene of the Chr V HMR. (B) Derepression at the Chr V HMRa1 gene in sir2Δ and sir4Δ mutants led to a doubling of KOS3 expression. (C) KOS3 expression did not increase in the sir2Δ mutant when the gene was placed on a plasmid. The expression levels shown are relative to a wild-type strain with KOS3 at its native location. The error bars indicate standard deviations.
KOS3 was necessary for efficient recruitment of Sir2 and Sir4 to silenced loci.
In S. cerevisiae, Sc-Sir2, Sc-Sir3, and Sc-Sir4 can be recruited to the silencers of HMR in the absence of Sc-Sir1 (26), presumably due to the interactions between Sc-Rap1 at the silencer and an Sc-Sir2–Sc-Sir4 dimer, which, in turn, recruits Sc-Sir3. These interactions do not require Sc-Sir1 and may allow silencing to be reestablished, albeit inefficiently, in a sir1Δ strain. ChIP-Seq of V5-tagged alleles of SIR2 and SIR4 in kos3Δ strains showed that KOS3 was required for efficient enrichment of Td-Sir2 and Td-Sir4 at HML and HMR and at telomeres (HMRa is shown in Fig. 9; see Fig. S5 in the supplemental material for HMLα and TEL01R).
FIG 9.
T. delbrueckii KOS3 is required for efficient recruitment of Td-Sir2 and Td-Sir4 to HMLα, HMRa, and telomeres. ChIP-Seq was carried out for V5-tagged Td-Sir2 and Td-Sir4 in kos3Δ strains. The enrichment of Td-Sir2 and Td-Sir4 was compared to that of the wild-type strain for KOS3. (A) Enrichment of Td-Sir2 at HMRa in the wild type (green) and in the kos3Δ mutant (black). (B) Enrichment of Td-Sir4 at the same locus in the wild type (brown) and the kos3Δ mutant (black). Signal from input chromatin is also shown. Relevant genomic features on Chr V are shown at the bottom.
Sc-Sir1 and T. delbrueckii Kos3, Sir2, and Sir4 may be enriched at centromeres.
Sc-Sir1 had previously been found at six centromeres (CEN1, CEN2, CEN3, CEN4, CEN11, and CEN16) by locus-specific ChIP, and sir1Δ cac1Δ mutants showed elevated rates of nondisjunction (36). When examining the Sir1 IP track separately from the input track, we saw a consistent underrepresentation of centromere sequences, hinting that centromere DNA was systematically underrecovered in our samples (a representative example is shown in Fig. S6 in the supplemental material). To account for this underrecovery, we plotted Sir1 enrichment in terms of IP over input (IP/input) and compared those values to the IP/input of the no-tag control. This analysis revealed Sc-Sir1 enrichment at all 16 S. cerevisiae centromeres (see Fig. S7 in the supplemental material). ChIP-Seq data sets have been shown to contain certain reproducible but artifactual signals, implying the association of proteins with sequences that they do not actually bind in vivo (37, 38). To rigorously test whether these Sc-Sir1 peaks at centromeres represented ChIP-Seq artifacts, we compared Sc-Sir1 enrichment to enrichment of green fluorescent protein (GFP) tagged with a nuclear localization sequence (GFP-NLS) at centromeres (data from rreference 37). GFP is not expected to bind in a meaningful way to any portion of the yeast genome, yet control experiments showed that it colocalizes with multiple common ChIP-Seq artifacts. Only one centromere, CEN13, showed GFP-NLS IP-over-input enrichment. Thus, although the Sc-Sir1 signal present at that centromere may be spurious (see Fig. S7 in the supplemental material, asterisk), there was no indication of artifactual enrichment at the others. Additionally, despite the presence of Sc-Sir1 at centromere sequences, there was no indication of any Sir-dependent gene silencing adjacent to any centromere (17).
Because we saw Sc-Sir1 enrichment at S. cerevisiae centromeres, we evaluated whether Td-Kos3, Td-Sir2, and Td-Sir4 were present at centromeres in T. delbrueckii. T. delbrueckii, like S. cerevisiae, has point centromeres that have been annotated based on conservation of the centromere DNA elements (CDEI, CDEII, and CDEIII) and by synteny (39). We confirmed the functions of two of these centromeres (T. delbrueckii CEN1 and CEN3) by observing their ability to functionally replace S. cerevisiae CEN6 in the pRS316 vector, allowing strains to maintain the plasmid in the absence of selection in an S. cerevisiae host (centromeres appear to be compatible between the two species). We then examined Td-Kos3, Td-Sir2, and Td-Sir4 enrichment at presumptive T. delbrueckii centromeres in terms of IP/input and detected enrichment of all three proteins at centromeres (see Fig. S8 in the supplemental material). As in S. cerevisiae, we observed no evidence of silencing of genes adjacent to the centromeres.
T. delbrueckii AGO1 and DCR1 had no function in silencing.
Most Saccharomyces yeasts lack the machinery for RNAi, a mechanism of gene silencing found in S. pombe and many other organisms, including plants and animals. The Argonaute and Dicer proteins are required for heterochromatin formation in S. pombe and presumably in all organisms using the RNAi mechanism. Ago1 is a necessary component of the RNA-induced initiation of transcriptional gene silencing (RITS) complex, and Dcr1 cleaves double-stranded RNA into small interfering RNAs (siRNAs) that serve as guide RNAs, directing the heterochromatin machinery to the locus targeted for silencing (40). The N. castellii genome contains an AGO1 ortholog and a DCR1-like gene (DCR1-like because it is not directly orthologous to the S. pombe DCR1, but rather, is a duplicate of RNT1, an RNase specific for double-stranded RNA). N. castellii AGO1 and DCR1 together degrade Ty transcripts (13).
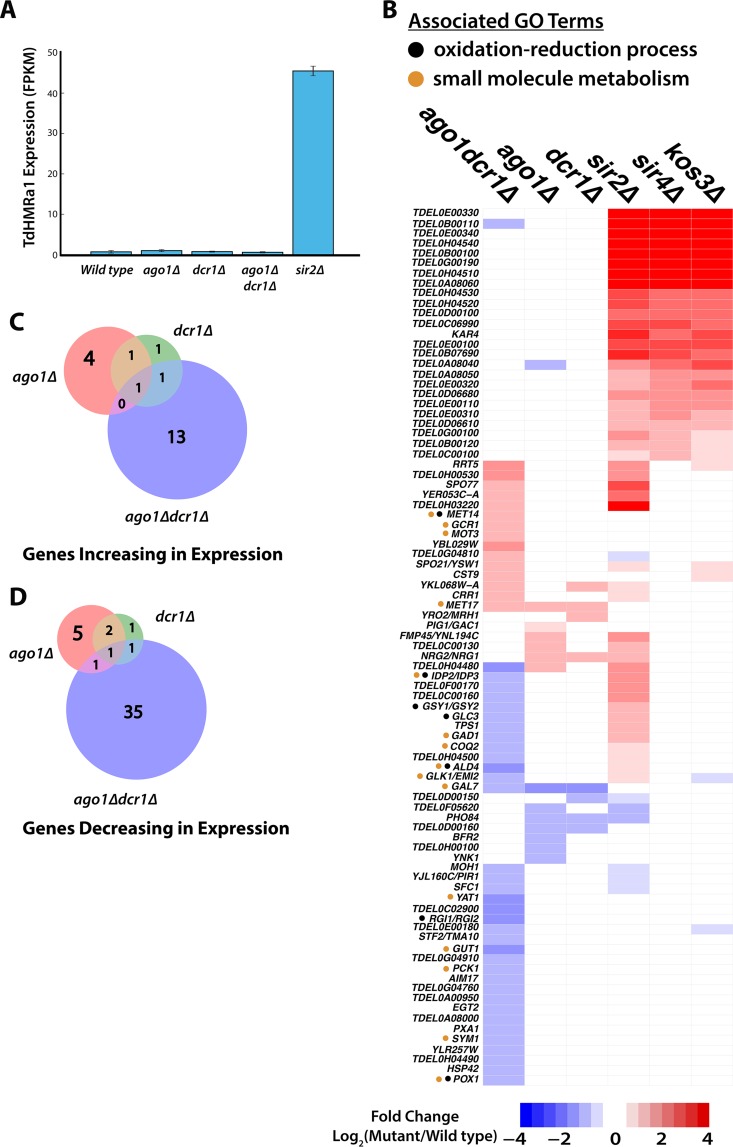
The T. delbrueckii genome also contains an AGO1- and a DCR1-like gene, orthologous to those of N. castellii. Given that AGO1 and DCR1 repress Ty elements in N. castellii, we tested whether the AGO1 and DCR1 genes functioned in silencing in T. delbrueckii by deep sequencing of mRNAs in T. delbrueckii ago1Δ and dcr1Δ mutants and ago1Δ dcr1Δ double mutants. These mutants displayed no defect in transcriptional repression of HML or HMR or of any genes near telomeres (Fig. 10A and B), and thus, these genes displayed no overlap in function with the SIR genes. Additionally, no genes showed a clear signal of derepression in the RNAi mutants—i.e., no genes went from 0 fragments per kilobase per million (FPKM) in the wild type to an FPKM of >0 in the mutant. Overall, 15 genes significantly changed in expression in the ago1Δ mutant, 9 in the dcr1Δ mutant, and 53 in the ago1Δ dcr1Δ double mutant (Fig. 10B; see Tables S6 to S8 in the supplemental material). Among the genes changing in expression in RNAi mutants, little to no overlap was seen among these gene sets (Fig. 10C and D). The most striking observation was that the double mutant had a bigger impact on the expression of genes than either of the single mutants (discussed below). For the genes that had S. cerevisiae orthologs, we performed GO term analysis for the ago1Δ dcr1Δ double mutant and found that several genes were associated with oxidation-reduction processes and/or small-molecule metabolism, indicating a possible coordinating role in metabolic function (Fig. 10B, black and orange dots).
FIG 10.
RNAi does not contribute to silencing in T. delbrueckii. (A) Expression of HMRa1 in the wild type, ago1Δ and dcr1Δ mutants, an ago1Δ dcr1Δ double mutant, and a sir2Δ mutant. Repression of a1 was maintained in all three RNAi mutants. The error bars indicate standard deviations. (B) Heat map displaying significant changes in expression of genes across the three RNAi mutants, as well as the 22 genes that increased in expression across all three sir mutants. All expression changes were filtered for genes that increased or decreased in expression more than 2-fold relative to the wild type and showed a false-discovery rate of <10%. For genes with orthologs in S. cerevisiae, the three-letter gene name is shown. Whole-genome duplicates are labeled with the names of both S. cerevisiae duplicates (e.g., “RGI1/RGI2” represents the pre-whole-genome-duplication ancestor of these two genes in T. delbrueckii). (C and D) Weighted Venn diagrams of overlapping genes increasing and decreasing, respectively, in expression relative to the wild type in each of the RNA mutants and the double mutant.
DISCUSSION
In this study, we exploited four opportunities provided by T. delbrueckii to explore theme and variation in the evolution of gene silencing. Specifically, T. delbrueckii, as a pre-whole-genome-duplication ascomycete, has one of the oldest versions of the SIR1 gene, perhaps the most enigmatic of all budding yeast silencing genes. We explored the functional trajectory of this gene from its earliest recognized appearance in T. delbrueckii to its reduced role in S. cerevisiae. Interestingly, we found that although the overall function of SIR1 in the formation of heterochromatin has remained constant, its precise role in that process has evolved considerably. The effect of deleting SIR1 on silencing in S. cerevisiae is relatively minor on a cell population basis. In contrast, in T. delbrueckii, deletion of KOS3 completely abolished silencing. Second, in addition to having the oldest SIR-silencing components, T. delbrueckii also has genes orthologous to budding yeast AGO1 and DCR1, whose function(s) in T. delbrueckii is not known. Third, the silencer composition of the only other preduplication species examined, K. lactis, differs from that of S. cerevisiae. Hence, T. delbrueckii offered the chance to explore whether the S. cerevisiae composition originated before or after the whole-genome duplication event. Finally, T. delbrueckii offered the opportunity to explore to what extent unusual features of the molecular topography of silenced chromatin were intrinsic to the mechanism of silencing.
Sc-Sir1 is associated with silencers, except for the HMR-I silencer.
Sc-Sir1 clearly bound to three of the four silencers in S. cerevisiae: it was strikingly enriched at HML-E, HML-I, and HMR-E but not at HMR-I. It bound to the silencers that are sufficient on their own to maintain silencing (27). Sc-Sir1 directly interacts with Orc1, a component of the origin of recognition complex, and this interaction likely brings Sc-Sir1 to the silencer (7, 41). However, the ORC presumably associates with all four silencers, as an ARS consensus sequence is present at each one, and all four are capable of functioning as an origin of replication when on plasmids. Moreover, both HMR-E and HMR-I are origins of replication in their chromosomal context (42, 43). Therefore, it is perplexing that Sir1 enrichment was absent from HMR-I. Interestingly, HMR-I lacks a Rap1 binding site. It is possible that Sc-Rap1 stabilizes the interactions between Sc-Sir1, ORC, and Sc-Sir4 and that Sc-Sir1's absence is due to Sc-Rap1's absence at this silencer.
Td-Kos3 is essential for silencing, whereas Sc-Sir1 is not.
Two observations emphasize the importance of Kos3 in silencing: (i) T. delbrueckii kos3Δ strains exhibited a complete loss of silencing at HML, HMR, and telomeres and (ii) in the absence of Td-Kos3, enrichment of Td-Sir2 and Td-Sir4 at these positions was greatly reduced. In S. cerevisiae, Sc-Sir1 and Sc-Sir4 interact (6). Sc-Rap1 is also present at the silencer, and the interactions between Sc-Rap1 and Sc-Sir4 and between Sc-Rap1 and Sc-Sir3 are well documented (44). Therefore, in addition to the interaction between Sc-Sir1 and Sc-Sir4, interactions between Sc-Rap1 and Sc-Sir4 and between Sc-Rap1 and Sc-Sir3 boost the efficiency with which silencing proteins associate with the silencer in S. cerevisiae. Td-Rap1 bound silencers in T. delbrueckii and contributed to silencing the adjacent loci. The absence of a Sir3 paralog and/or the lack of a Td-Sir4–Td-Rap1 interaction in T. delbrueckii may explain why Td-Kos3 is essential for silencing in that species: Td-Kos3 may be the primary protein mediating an interaction between Td-Sir4/Td-Sir2 and the silencer.
Td-Kos3 functions at telomeres, whereas Sc-Sir1 does not.
Early studies of telomeric silencing in S. cerevisiae found no role for Sir1 in the “telomere position effect,” as measured by reporter genes adjacent to synthetic telomeres. Our ChIP-Seq data for Sc-Sir1 and RNA-Seq data for the sir1Δ mutant corroborated these early observations and extended them to all telomeres. We saw no Sc-Sir1 protein enrichment at or near telomeres (except for at HMLα), and no subtelomeric genes were derepressed in the sir1Δ mutant. In contrast, Td-Kos3 bound to at least 10 out of 16 telomeric and subtelomeric sequences in T. delbrueckii, where its enrichment pattern closely matched that of Td-Sir2 and Td-Sir4. These data suggest that the ancestral SIR1 was once a part of a core silencing complex composed of Td-Orc1/Td-Kos3/Td-Sir4/Td-Sir2, functionally equivalent to the Sc-Sir2/Sc-Sir3/Sc-Sir4 complex. For the five telomeres where Td-Kos3 was absent, Td-Sir2 and Td-Sir4 were also absent. It may be that the genome assembly for these five telomeres is less complete; sequencing using longer genomic inserts (>1 kb) would be required to fully assemble the remaining five telomeres and to assess whether Td-Kos3, Td-Sir2, and Td-Sir4 are present at those ends, as well.
T. delbruekii SIR2 has roles in addition to silencing.
SIR2 in S. cerevisiae has other roles in the cell, in addition to its role in heterochromatin formation at telomeres and the silent mating type loci, such as suppression of recombination at rDNA repeats and life span regulation (45, 46). Our RNA-Seq data suggested that even in T. delbrueckii, SIR2 regulates many genes and likely performs functions other than silencing, as there were 146 expression changes that were specific to the sir2Δ mutant (124 genes increased and 22 decreased in expression). T. delbrueckii SIR2 is the pre-whole-genome-duplication ancestor of the S. cerevisiae SIR2 and HST1 duplicates; thus, T. delbrueckii SIR2 may also repress genes that in S. cerevisiae are repressed by HST1. S. cerevisiae Hst1, in complex with Sum1 and Rfm1, functions in promoter-specific repression of middle-sporulation genes (47). K. lactis SIR2, another pre-whole-genome-duplication ortholog of S. cerevisiae SIR2 and HST1, possesses functions of both S. cerevisiae SIR2 and HST1 (9, 48). Interestingly, T. delbrueckii orthologs of two middle-sporulation genes repressed by Hst1 in S. cerevisiae were derepressed in the T. delbrueckii sir2Δ mutant: SPS4 and DIT1. Many other orthologs of meiotic genes were also derepressed (see the 21 marked genes in Table S4 in the supplemental material), and six of them had Sir2 peaks in their promoters: DIT2, SPO19, SPS101, SPS2, SPS4, and IME2. The presence of promoter-specific Sir2 peaks suggests that, like K. lactis SIR2, T. delbrueckii SIR2 is capable of acting as both a promoter-specific repressor and a long-range, promoter-independent repressor of gene expression.
Silencer conservation and diversity among budding yeasts.
Pairs of silencers flank both HML and HMR in S. cerevisiae, which are all bound by Sc-Sir2, Sc-Sir3, and Sc-Sir4 and, as shown here, with the exception of HMR-I, by Sc-Sir1. A single prominent site bound by Td-Kos3, Td-Sir2, and Td-Sir4 adjacent to HML and a close pair of sites adjacent to one side of HMR mediated silencing of these loci in T. delbrueckii. Although the analysis of these binding sites has only just begun, these sites are, in fact, silencers. A Rap1 binding motif was clearly critical for silencing at both loci, and the Rap1 protein itself associated with regions that included this binding motif. The HMR silencer supported autonomous replication of a plasmid, implying the existence of an origin of replication and, thus, an ORC binding site. Abf1 binding site motifs were also evident. Individual mutations to the putative Abf1 binding sites and the putative ARS had no effect on silencing. While this result might suggest that these binding sites do not contribute to silencing, it is possible that, as in S. cerevisiae, they have partially redundant roles in facilitating transcriptional repression. As in S. cerevisiae, mutating the two sites simultaneously may be required to disrupt repression (49). Further analysis will be required to map more precisely the functional elements of the silencer, but already there are notable differences between the structure of silenced chromatin in T. delbrueckii and that in S. cerevisiae, pointing to alternative means by which silencing can occur.
In K lactis, Reb1 substitutes for the Rap1 protein in silencer function (50), even though Rap1 is critical for telomeric gene silencing (51). In T. delbrueckii, Rap1 sites were clearly important for silencer function, and Td-Rap1 bound to the silencer regions of both HML and the chromosome V HMR. Thus, the substitution of Reb1 for Rap1 was not associated with the whole-genome duplication. It is possible that the elevated substitution rate at silencers drives the diversification of transcription factor binding sites at silencers and silencer binding proteins (52). It is curious that the Sir proteins themselves (with the exception of Sir2) are also rapidly evolving. Whatever the driver of this rapid evolution may be, the result is that hemiascomycete species have a variable repertoire of Sir proteins with differing numbers of Sir1 paralogs. Selection may be imposed on whichever set of protein-protein interactions results in the successful recruitment of the Sir2-Sir4 dimer (Sir2, being the catalytic component, is the member that can deacetylate H4K16Ac and ultimately repress the locus). Rapid protein evolution may have strengthened some protein-protein interactions and weakened others. Thus, species that require multiple Sir1 paralogs, like S. bayanus var. uvarum, may be those in which Rap1 or Sir3 is insufficient to stably recruit Sir2-Sir4. Species that lack a SIR1 paralog entirely may be those in which silencer-bound proteins have evolved a higher affinity for the Sir2-Sir4 dimer, obviating the need for Sir1.
Presence of Sc-Sir1 and Td-Kos3 at centromeres.
Heterochromatin is characteristically assembled at centromeres of eukaryotes, including S. pombe, yet in Saccharomyces and other organisms with point centromeres, heterochromatin is not found at centromeres, and no genes near centromeres were derepressed in sir mutants in S. cerevisiae or T. delbruekii. Earlier work established that the Sc-Sir1 protein of S. cerevisiae is present at some centromeres, where it contributes to proper chromosome segregation, along with the chromatin assembly factor (CAF) complex (36). We found some enrichment of Sc-Sir1 at all but one centromere. All three Sir proteins in T. delbrueckii (Td-Kos3, Td-Sir2, and Td-Sir4) were found at all eight centromeres in the organism. In both species, the enrichment of IP reads over input for the centromere regions did not reach statistical significance, as assessed by MACS. However, MACS is designed to detect peaks created by an enrichment of IP reads relative to input at a particular genomic region, not peaks created by greater underenrichment in the input sample. Viewing the data in terms of IP over input clearly showed peaks at the centromeres. Unfortunately, we have been unable to express GFP in T. delbrueckii and hence were unable to use this established metric to evaluate whether these peaks represented biological or artifactual associations. One interpretation of these data is that Td-Kos3 in T. delbruekii, like Sc-Sir1, plays some conserved role in centromere function. Whether the other Sir proteins with a ChIP-Seq enrichment signal at a subset of centromeres represent some latent centromere function of these proteins, the vestigial presence of silencing proteins at centromeres, or a new class of ChIP-Seq artifacts awaits further study.
Role of RNAi in T. delbrueckii.
Our RNA-Seq data for ago1Δ and dcr1Δ mutants of T. delbrueckii revealed that AGO1 and DCR1 did not function in silencing at HML, HMR, or telomeres. Thus, if these proteins contribute to RNAi function in T. delbrueckii, RNAi must have a role other than in heterochromatin function. Of the 77 genes found to significantly change in expression across all candidate RNAi mutants, ∼32% are genes of unknown function that have no ortholog in S. cerevisiae. Moreover, budding yeast DCR1 is not directly orthologous to S. pombe DCR1, but rather, a duplicate of RNT1 that encodes an RNase involved in the processing of rRNA transcripts (53). Therefore, DCR1 may have inherited a separate set of interaction partners and functional constraints from its RNT1 ancestor and may be on a different evolutionary trajectory from AGO1. Additionally, the AGO1 and DCR1 genes of N. castellii that repress Ty elements are thought to mediate repression at the posttranscriptional level, not at the epigenetic level, via interactions with chromatin-modifying enzymes (such as histone deacetylases and demethylases). Furthermore, Candida albicans DCR1, an ortholog of both the T. delbrueckii and N. castellii DCR1 genes, functions in rRNA and spliceosomal RNA processing, strengthening the possibility of an RNA-processing function for T. delbrueckii DCR1 (54). As of yet, there exists no evidence tying budding yeast RNAi genes to any chromatin factors involved in the establishment or maintenance of heterochromatin, although there are many direct interactions between chromatin modifiers and DCR1 and AGO1 in S. pombe (12).
Argonaute itself has had a complex evolutionary journey. Eukaryotic Argonaute proteins bind short RNA guide molecules to target transcripts. Prokaryotic Argonaute proteins, however, can bind DNA and may participate in genome defense against mobile elements (55). Budding yeast Argonaute copurifies with small interfering RNAs generated by Dicer, which suggests that it functions like other eukaryotic Argonaute proteins (13). However, other binding properties of budding yeast Argonaute have yet to be explored. Little overlap was observed in gene sets between ago1Δ and dcr1Δ mutants; however, the 48 genes whose expression is altered only in the ago1Δ dcr1Δ double mutant imply that the two proteins may share overlapping functions. The overlapping functions must not be ones that the proteins carry out together; rather, based upon the unique phenotype of the double mutant, either must be able to contribute to the function in the absence of the other.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by an NSF predoctoral fellowship to A.E. and by a grant from the National Institutes of Health to J.R. (GM31105). We thank the Vincent J. Coates Genomics Sequencing Laboratory at the University of California, Berkeley, supported by National Institutes of Health S10 Instrumentation grants S10-RR029668 and S10-RR027303.
We also thank Devin Scannell for generously providing Torulaspora strains, Peter Combs and Jackie Villalta for sequencing reagents, and members of the Rine laboratory for thoughtful discussion, especially Oliver Zill and Debbie Thurtle.
Funding Statement
National Science Foundation (NSF) provided funding to Aisha Ellahi through a Graduate Research Fellowship.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.01013-15.
REFERENCES
- 1.Thurtle DM, Rine J. 2014. The molecular topography of silenced chromatin in Saccharomyces cerevisiae. Genes Dev 28:245–258. doi: 10.1101/gad.230532.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steakley DL, Rine J. 2015. On the mechanism of gene silencing in Saccharomyces cerevisiae. G3 (Bethesda) 5:1751–1763. doi: 10.1534/g3.115.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grunstein M, Gasser SM. 2013. Epigenetics in Saccharomyces cerevisiae. Cold Spring Harb Perspect Biol 5:a017491–a017491. doi: 10.1101/cshperspect.a017491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pillus L, Rine J. 1989. Epigenetic inheritance of transcriptional states in S. cerevisiae. Cell 59:637–747. doi: 10.1016/0092-8674(89)90009-3. [DOI] [PubMed] [Google Scholar]
- 5.Dodson AE, Rine J. 2015. Heritable capture of heterochromatin dynamics in Saccharomyces cerevisiae. Elife 4:e05007. doi: 10.7554/eLife.05007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bose ME, McConnell KH, Gardner-Aukema KA, Ller UM, Weinreich M, Keck JL, Fox CA. 2004. The origin recognition complex and Sir4 protein recruit Sir1p to yeast silent chromatin through independent interactions requiring a common Sir1p domain. Mol Cell Biol 24:774–786. doi: 10.1128/MCB.24.2.774-786.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu H-C, Stillman B, Xu R-M. 2005. Structural basis for origin recognition complex 1 protein-silence information regulator 1 protein interaction in epigenetic silencing. Proc Natl Acad Sci U S A 102:8519–8524. doi: 10.1073/pnas.0502946102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallagher JEG, Babiarz JE, Teytelman L, Wolfe KH, Rine J. 2009. Elaboration, diversification and regulation of the Sir1 family of silencing proteins in Saccharomyces. Genetics 181:1477–1491. doi: 10.1534/genetics.108.099663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hickman MA, Rusche LN. 2009. The Sir2-Sum1 complex represses transcription using both promoter-specific and long-range mechanisms to regulate cell identity and sexual cycle in the yeast Kluyveromyces lactis. PLoS Genet 5:e1000710. doi: 10.1371/journal.pgen.1000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hickman MA, Rusche LN. 2010. Transcriptional silencing functions of the yeast protein Orc1/Sir3 subfunctionalized after gene duplication. Proc Natl Acad Sci U S A 107:19384–19389. doi: 10.1073/pnas.1006436107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Las Peñas A, Pan S, Castaño I, Alder J, Cregg R, Cormack BP. 2003. Virulence-related surface glycoproteins in the yeast pathogen Candida glabrata are encoded in subtelomeric clusters and subject to RAP1- and SIR-dependent transcriptional silencing. Genes Dev 17:2245–2258. doi: 10.1101/gad.1121003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grewal S. 2010. RNAi-dependent formation of heterochromatin and its diverse functions. Curr Opin Genet Dev 20:134–141. doi: 10.1016/j.gde.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drinnenberg IA, Weinberg DE, Xie KT, Mower JP, Wolfe KH, Fink GR, Bartel DP. 2009. RNAi in budding yeast. Science 326:544–550. doi: 10.1126/science.1176945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon JL, Armisen D, Proux-Wera E, OhEigeartaigh SS, Byrne KP, Wolfe KH. 2011. Evolutionary erosion of yeast sex chromosomes by mating-type switching accidents. Proc Natl Acad Sci U S A 108:20024–20029. doi: 10.1073/pnas.1112808108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longtine MS, McKenzie A III, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953–961. [DOI] [PubMed] [Google Scholar]
- 16.Gietz DR. 2014. Yeast transformation by the LiAc/SS Carrier DNA/PEG method. Methods Mol Biol 1205:1–12. doi: 10.1007/978-1-4939-1363-3_1. [DOI] [PubMed] [Google Scholar]
- 17.Ellahi A, Thurtle D, Rine J. 2015. The chromatin and transcriptional landscape of native Saccharomyces cerevisiae telomeres and subtelomeric domains. Genetics 200:505–521. doi: 10.1534/genetics.115.175711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collart MA, Oliviero S. 2001. Preparation of yeast RNA, p 13.12.1–13.12.5. In Current protocols in molecular biology. John Wiley & Sons, Inc., New York, NY. [DOI] [PubMed] [Google Scholar]
- 19.Aparicio OM, Geisberg JV, Sekinger E, Yang A, Moqtaderi Z, Struhl K. 2005. Chromatin immunoprecipitation for determining the association of proteins with specific genomic sequences in vivo. Curr Protoc Mol Biol Chapter 21:Unit 21.3. doi: 10.1002/0471142727.mb2103s69. [DOI] [PubMed] [Google Scholar]
- 20.Boeke JD, Trueheart J, Natsoulis G, Fink GR. 1987. 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol 154:164–175. doi: 10.1016/0076-6879(87)54076-9. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Liu T, Meyer C, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS. 2008. Model-based analysis of ChIP-Seq (MACS). Genome Biol 9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. 2012. Differential gene and transcript expression analysis of RNA-Seq experiments with TopHat and Cufflinks. Nat Protoc 7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anders S, Huber W. 2010. Differential expression analysis for sequence count data. Genome Biol 11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Homann OR, Johnson AD. 2010. MochiView: versatile software for genome browsing and DNA motif analysis. BMC Biol 8:49. doi: 10.1186/1741-7007-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rusche LN, Kirchmaier AL, Rine J. 2002. Ordered nucleation and spreading of silenced chromatin in Saccharomyces cerevisiae. Mol Biol Cell 13:2207–2222. doi: 10.1091/mbc.E02-03-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahoney DJ, Broach JR. 1989. The HML mating-type cassette of Saccharomyces cerevisiae is regulated by two separate but functionally equivalent silencers. Mol Cell Biol 9:4621–4630. doi: 10.1128/MCB.9.11.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brand AH, Breeden L, Abraham J, Sternglanz R, Nasmyth K. 1985. Characterization of a “silencer” in yeast: a DNA sequence with properties opposite to those of a transcriptional enhancer. Cell 41:41–48. doi: 10.1016/0092-8674(85)90059-5. [DOI] [PubMed] [Google Scholar]
- 29.Abraham J, Nasmyth KA, Strathern JN, Klar AJ, Hicks JB. 1984. Regulation of mating-type information in yeast. negative control requiring sequences both 5′ and 3′ to the regulated region. J Mol Biol 176:307–331. doi: 10.1016/0022-2836(84)90492-3. [DOI] [PubMed] [Google Scholar]
- 30.Moretti P, Freeman K, Coodly L, Shore D. 1994. Evidence that a complex of SIR proteins interacts with the silencer and telomere-binding protein RAP1. Genes Dev 8:2257–2269. doi: 10.1101/gad.8.19.2257. [DOI] [PubMed] [Google Scholar]
- 31.Aparicio OM, Billington BL, Gottschling DE. 1991. Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell 66:1279–1287. doi: 10.1016/0092-8674(91)90049-5. [DOI] [PubMed] [Google Scholar]
- 32.Pryde FE, Louis EJ. 1999. Limitations of silencing at native yeast telomeres. EMBO J 18:2538–2550. doi: 10.1093/emboj/18.9.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barsoum E, Sjostrand JOO, Astrom SU. 2010. Ume6 is required for the MATa/MATα cellular identity and transcriptional silencing in Kluyveromyces lactis. Genetics 184:999–1011. doi: 10.1534/genetics.110.114678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feldmann E, De Bona P, Galletto R. 2015. The wrapping loop and Rap1 C-terminal (RCT) domain of yeast Rap1 modulate access to different DNA binding modes. J Biol Chem 290:11455–11466. doi: 10.1074/jbc.M115.637678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.König P, Giraldo R, Chapman L, Rhodes D. 1996. The crystal structure of the DNA-binding domain of yeast RAP1 in complex with telomeric DNA. Cell 85:125–136. doi: 10.1016/S0092-8674(00)81088-0. [DOI] [PubMed] [Google Scholar]
- 36.Sharp JA, Krawitz DC, Gardner KA, Fox CA, Kaufman PD. 2003. The budding yeast silencing protein Sir1 is a functional component of centromeric chromatin. Genes Dev 17:2356–2361. doi: 10.1101/gad.1131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teytelman L, Thurtle DM, Rine J, van Oudenaarden A. 2013. Highly expressed loci are vulnerable to misleading ChIP localization of multiple unrelated proteins. Proc Natl Acad Sci U S A 110:18602–18607. doi: 10.1073/pnas.1316064110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park D, Lee Y, Bhupindersingh G, Iyer VR. 2013. Widespread misinterpretable ChIP-seq bias in yeast. PLoS One 8:e83506. doi: 10.1371/journal.pone.0083506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Byrne KP, Wolfe KH. 2005. The Yeast Gene Order Browser: combining curated homology and syntenic context reveals gene fate in polyploid species. Genome Res 15:1456–1461. doi: 10.1101/gr.3672305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reyes-Turcu FE, Grewal SIS. 2012. Different means, same end—heterochromatin formation by RNAi and RNAi-independent RNA processing factors in fission yeast. Curr Opin Genet Dev 22:156–163. doi: 10.1016/j.gde.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Triolo T, Sternglanz R. 1996. Role of interactions between the origin recognition complex and SIR1 in transcriptional silencing. Nature 381:251–253. doi: 10.1038/381251a0. [DOI] [PubMed] [Google Scholar]
- 42.Fox C, Loo S, Rivier DH, Foss M, Rine J. 1993. A transcriptional silencer as a specialized origin of replication that establishes functional domains of chromatin. Cold Spring Harb Symp Quant Biol 58:443–455. doi: 10.1101/SQB.1993.058.01.051. [DOI] [PubMed] [Google Scholar]
- 43.Rivier DH, Ekena JL, Rine J. 1999. HMR-I is an origin of replication and a silencer in Saccharomyces cerevisiae. Genetics 151:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo K, Vega-Palas M, Grunstein M. 2002. Rap1-Sir4 binding independent of other Sir, yKu, or Histone interactions initiates the assembly of telomeric heterochromatin in yeast. Genes Dev 16:1528–1539. doi: 10.1101/gad.988802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith JS, Boeke JD. 1997. An unusual form of transcriptional silencing in yeast ribosomal DNA. Genes Dev 11:241–254. doi: 10.1101/gad.11.2.241. [DOI] [PubMed] [Google Scholar]
- 46.Lin S-J, Defossez P, Guarente L. 2000. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 47.Xie J, Pierce M, Gailus-Durner V, Wagner M, Winter E, Vershon AK. 1999. Sum1 and Hst1 repress middle sporulation-specific gene expression during mitosis in Saccharomyces cerevisiae. EMBO J 18:6448–6454. doi: 10.1093/emboj/18.22.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Froyd CA, Rusche LN. 2011. The duplicated deacetylases Sir2 and Hst1 subfunctionalized by acquiring complementary inactivating mutations. Mol Cell Biol 31:3351–3365. doi: 10.1128/MCB.05175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brand AH, Micklem G, Nasmyth K. 1987. A yeast silencer contains sequences that can promote autonomous plasmid replication and transcriptional activation. Cell 51:709–719. doi: 10.1016/0092-8674(87)90094-8. [DOI] [PubMed] [Google Scholar]
- 50.Sjöstrand JOO, Kegel A, Aström SU. 2002. Functional diversity of silencers in budding yeasts. Eukaryot Cell 1:548–557. doi: 10.1128/EC.1.4.548-557.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gurevich R, Smolikov S, Maddar H, Krauskopf A. 2003. Mutant telomeres inhibit transcriptional silencing at native telomeres of the yeast Kluyveromyces lactis. Mol Genet Genomics 268:729–738. [DOI] [PubMed] [Google Scholar]
- 52.Teytelman L, Eisen MB, Rine J. 2008. Silent but not static: accelerated base-pair substitution in silenced chromatin of budding yeasts. PLoS Genet 4:e1000247. doi: 10.1371/journal.pgen.1000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Catala M, Tremblay M, Samson E, Conconi A, Abou Elela S. 2008. Deletion of Rnt1p alters the proportion of open versus closed rRNA gene repeats in yeast. Mol Cell Biol 28:619–629. doi: 10.1128/MCB.01805-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bernstein DA, Vyas VK, Weinberg DE, Drinnenberg IA, Bartel DP, Fink GR. 2012. Candida albicans Dicer (CaDcr1) is required for efficient ribosomal and spliceosomal RNA maturation. Proc Natl Acad Sci U S A 109:523–528. doi: 10.1073/pnas.1118859109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swarts DC, Makarova K, Wang Y, Nakanishi K, Ketting RF, Koonin EV, Patel DJ, van der Oost J. 2014. The evolutionary journey of Argonaute proteins. Nat Struct Mol Biol 21:743–753. doi: 10.1038/nsmb.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.