Abstract
Resting high-frequency heart rate variability (HF-HRV) relates to cardiac vagal control and predicts individual differences in health and longevity, but its functional neural correlates are not well defined. The medial prefrontal cortex (mPFC) encompasses visceral control regions that are components of intrinsic networks of the brain, particularly the default mode network (DMN) and the salience network (SN). Might individual differences in resting HF-HRV covary with resting state neural activity in the DMN and SN, particularly within the mPFC? This question was addressed using fMRI data from an eyes-open, five-minute rest period during which echoplanar brain imaging yielded blood oxygen level dependent (BOLD) time series. Independent components analysis (ICA) yielded functional connectivity estimates defining the DMN and SN. HF-HRV was measured in a rest period outside of the scanner. Midlife (52% female) adults were assessed in two studies (Study 1, N = 107; Study 2, N = 112). Neither overall DMN nor SN connectivity strength was related to HF-HRV. However, HF-HRV related to connectivity of one region within mPFC shared by the DMN and SN; namely, the perigenual anterior cingulate cortex (pgACC), an area with connectivity to other regions involved in autonomic control. In sum, HF-HRV does not seem directly related to global resting state activity of intrinsic brain networks, but rather to more localized connectivity. A mPFC region was of particular interest as connectivity related to HF-HRV was shared by the DMN and SN. These findings may indicate a functional basis for the coordination of autonomic cardiac control with engagement and disengagement from the environment.
Keywords: default mode network, salience network, resting state fMRI, high-frequency heart rate variability
Rest and activity are biobehavioral states that appear both separable and largely non-overlapping in time. From a historical perspective, the contrast between rest and activity was perhaps most clearly evident in early biobehavioral work on the autonomic nervous system. Activation of the sympathetic nervous system was related to task engagement, arousal and the use of metabolic energy; activation of the parasympathetic system with disengagement, rest, relaxation, and the restoration of metabolic energy. For example, Cannon (1929) contrasted the functions of the sympathetic and parasympathetic nerves by drawing on his earlier work and that of Hess. Referring to the cranial/vagal nerve, he suggested that it “…gives the heart opportunity for rest and recuperation by checking its rate in quiet times” (p. 423). The direct opposition of function of the two branches of the autonomic nervous system, however, is no longer accepted (e.g., (Berntson, Norman, Hawkley, & Cacioppo, 2008; J. R. Jennings, 1986). Nevertheless, it remains clear that a leading index of parasympathetic activity, high-frequency heart rate variability (HF-HRV), is typically greater during rest than task engagement. Moreover, this resting index of parasympathetic activity has been widely related to relative cardiovascular and psychological health (Huston & Tracey, 2011; Montano et al., 2009; Thayer, Yamamoto, & Brosschot, 2010). This index is presumptively related to achieving a relaxed/restorative psychophysiological state that is incompatible with chronic and adverse psychological states, such as depression or anxiety. Parenthetically, the expectation that any single central or peripheral index would map directly upon a single psychological state independent of context is likely ill founded, as partial mappings moderated by context seem more likely (Berntson, Cacioppo, & Grossman, 2007).
An apparently similar resting-related brain state has been described for functional brain connections that link particular neural cell groups when the brain is ‘disengaged’ relative to task engaged conditions, termed the Default Mode Network (DMN). The DMN is represented in the correlated neural activity during these resting states, rather than being a network of inactivity, as established by the demonstration of glucose use through positron emission tomography (Raichle et al., 2001). Subsequently, brain areas forming nodes of the DMN were identified variously through observation of deactivation during task performance and a variety of analytic approaches examining correlation among spontaneous activity during a behavioral resting state, e.g., (Greicius, Krasnow, Reiss, & Menon, 2003; S. M. Smith et al., 2009). The anatomy and presumptive functions of the psychophysiological state associated with the DMN have been reviewed previously (e.g., Buckner, Andrews-Hanna, and Schacter (2008)). Areas consistently observed as part of the network include the posterior cingulate cortex, both dorsal and ventral territories of the medial prefrontal cortex, as well as the inferior parietal cortex, lateral temporal cortex and extended hippocampus.
Another established brain network, the so-called salience network (SN), is also relevant to conceptualizations of rest and activity. The SN, additionally termed the ventral attention network, has been hypothesized to mediate a shift away from the resting/default modes characterizing increased HF-HRV and activity in the DMN. The SN is another intrinsic network of the brain that shows high coherence among its components at rest, but it is also identified by the strengthening of connections within the network when activated by an appropriate situation. In the case of the SN, appropriateness is defined by the occurrence of environmental or bodily stimuli, which are thought to be signaled as ‘significant’ within the SN. The SN and an executive control network were identified and separated by Seeley et al. (2007). The SN was observed to be centered on the dorsal anterior cingulate, extending into the perigenual anterior cingulate cortex (pgACC), and orbital fronto-insular cortices, but it also encompassed limbic and brainstem areas. This connectivity, as well as correlation with pre-scan anxiety ratings, implied relevance to autonomic nervous system regulation and interoception; a relationship further developed based on insula connectivity by Menon and Uddin (2010) and Singer, Critchley, and Preuschoff (2009). Some evidence supports the view that DMN activation is switched to SN activation when an interoceptive or environmental stimulus is encoded as significant (Menon & Uddin, 2010; Singer et al., 2009). For example, when participants were aware that they were ‘mind-wandering’ the SN was active, but the DMN was active during mind-wandering and in the absence of this awareness (Hasenkamp, Wilson-Mendenhall, Duncan, & Barsalou, 2012). Other work has shown DMN interplay with SN, e.g., when switching between engagement with self and others (Rilling, Dagenais, Goldsmith, Glenn, & Pagnoni, 2008), orienting to an auditory oddball (Sridharan, Levitin, & Menon, 2008), and inhibiting prepared motor responses (Bonnelle et al., 2012; Ham, Leff, de Boissezon, Joffe, & Sharp, 2013; Jilka et al., 2014). Some have suggested that the SN is active in switching, but not in maintenance of a task-related attentive state (Elton & Gao, 2014). Relevance to HF-HRV is suggested both by the inclusion of known autonomic nervous system control areas in the SN, as well as this vagal marker’s putative role in switching between rest and activity and internal and external focus of attention. Again, HF-HRV generally decreases in amplitude during task engagement (S. W. Porges, 2007) and its resting magnitude is related to vagally-induced slowing of heart rate when orienting to the external environment (Porges & Coles, 1982). Conceptual accounts of processes controlling these vagal reactions have suggested transitions between internal and external focus of attention and transient inhibition of action during action selection (J. Jennings & M. W. van der Molen, 2005; Lacey & Lacey, 1974).
Progress in understanding the DMN and HF-HRV, as well as the SN, might be made if it can be established that these psychophysiological indicators are associated. A strong association between DMN functionality and individual levels of HF-HRV might indicate, for example, that the DMN relates to an anabolic/energy recovery state that includes the enhancement of vagal influence on the heart. Likewise, covariation between the DMN and HF-HRV may reflect coordinated metabolic processes that function to restore/enhance energy. For example, HF-HRV has been postulated to maximize the exchange of oxygen through reduction of pulmonary deadspace (Giardino, Glenny, Borson, & Chan, 2003; Ito et al., 2006; Yasuma & Hayano, 2004), though some questions about this mechanism exits (Sin et al., 2010; Tzeng, Sin, & Galletly, 2009). The sensitivity of vagally-induced heart rate reactions to event salience might further suggest relationships between the SN and HF-HRV, as might the seeming overlap between nodes of the SN and areas related to autonomic control.
In view of these open possibilities, the aim of the current research was to test the association between individual differences in HF-HRV and resting state activity in two intrinsic networks of the brain that the literature would appear to implicate in autonomic control: the DMN and SN. To our knowledge, only indirect observations have been previously made of the association between HF-HRV and the DMN (Dhond, Yeh, Park, Kettner, & Napadow, 2008; van Buuren et al., 2009). For example, Ziegler, Dahnke, Yeragani, and Bar (2009) related 4 s averages of heart rate at rest to activation in an area typically included in the DMN, the medial prefrontal cortex, but did not specifically assess network identity or heart rate variability. One exception is Chang et al.’s close examination over time within a rest period of covariation between HF-HRV and brain activation within subjects (Chang et al., 2013). These investigators found within-subject relationships between amygdala and dorsal anterior cingulate seed regions and areas in thalamus, brainstem, putamen, and dorsolateral prefrontal cortex. They did not observe significant between-subject correlations of HF-HRV with regional brain activation among the 35 men studied.
In our larger sample here, our aim was to test ‘trait-like’ relationships between degree of resting HF-HRV and the strength of functional interconnections among areas forming the DMN and SN. Between-subject/trait relations are important due to the literature relating such individual differences in HF-HRV to physical and mental health (Huston & Tracey, 2011; Montano et al., 2009; Thayer et al., 2010). Two hypotheses were entertained. The first suggests that the between-subject strength of connectivity between all DMN nodes would relate positively to the amplitude of resting HF-HRV. The second suggests covariance between connectivity strength of only a subset of nodes of the DMN regions shared with the SN and hence responsible for the putative ‘shifting’ of function away from rest/restoration common to DMN and HF-HRV. Support for the first hypothesis would suggest a common functional process regulating both DMN connectivity and vagal activation. Support for the second hypothesis would suggest that the psychophysiological functions associated with DMN connectivity and HF-HRV are differentially controlled, but share a sensitivity to a shift in orientation away from rest/restoration. Support for the second hypothesis would also be consistent with the SN’s relationship to autonomic response supporting action (Hermans, Henckens, Joels, & Fernandez, 2014; Uddin, 2015) and the known vagal activation during transient focused attention or attentional orienting (Graham & Clifton, 1966; J. R. Jennings & M. W. van der Molen, 2005)
We first test the hypothesis that during a resting behavioral state, connectivity within the DMN network as a whole will directly relate to HF-HRV, such that individual differences in strength of mean connection with the DMN (or SN) should relate to the amplitude of HF-HRV across individuals. Absence of such an overall correlation might suggest that DMN functionality will have differential connectivity, such that some nodes of the network will more directly relate to HF-HRV than other nodes. Selection of which nodes to test in pursuing our second aim was based on areas showing overlapping presence in both our derived DMN and SN. We anticipated such overlap in the medial prefrontal cortex (mPFC), as it is identified as important both as a node in the DMN, in the SN, and as related to HF-HRV (Gianaros and Wager, 2015; Thayer et al.,2012). Anatomically, the mPFC is known to connect to pre-autonomic cell groups in hypothalamus, periaqueductal gray, and brainstem. As importantly, the mPFC is a node in the SN (Seeley et al., 2007). If diffuse attention is a major aspect of the functionality of the DMN, then the overlapping membership of mPFC in the two networks would provide an anatomical site for shifting from DMN activation to SN activation. We anticipated that the connectivity of the mPFC node localized within the DMN via independent component analysis would be positively related to individual differences in resting HF-HRV; and that connectivity of this mPFC region within the SN would similarly be positively related to HF-HRV. Finally, exploratory questions were then asked to examine any statistically strong relationships that would suggest hypotheses for later research. For example, does HF-HRV relate to connectivity of other nodes of the DMN or SN on a voxel-wise basis?
Method
Participants
Data were collected from two projects, the Adult Health and Behavior Project, phase II (AHAB-II) and the Pittsburgh Imaging Project (PIP). Table 1 shows the demographic and general characteristics of the participants. As the table indicates, the samples were quite similar, with some differences in gender distribution that contributed to minor differences in waist circumference and systolic blood pressure. The two groups were aggregated to improve the statistical power. For image quality control, participants who had no more than 5% outliers in the BOLD image time series were selected for finding resting state network. An outlier images was detected when the mean signal intensity was 6 SD outside of the global signal intensity or the movement displacement was more than 2mm from the previous volume. We carried out the outlier identification using Artifact Detection toolbox (http://web.mit.edu/swg/software.htm). 219 participants (107 for AHABII and 112 for PIP) passed quality control (27 individuals failed to pass our threshold), among those passing our threshold, 208 had HF-HRV data (98 for AHAB and 110 for PIP). The combination of the two samples was supported by a two sample Kolmogorov-Smirnoff test asking whether the distribution of log HRV values was different between the two samples. With our standard control for age and gender, the resulting Kolmogorov-Smirnoff Z of 1.25 was not significant (p>.05). Supplementary figure, Figure S1 shows the distribution of HRV for each of the studies.
Table 1.
Demographic Data: AHAB II (n=98) and PIP (n=110)
| Overall | AHABII | PIP | |
|---|---|---|---|
| Age,yrs; M(SD) | 41.19(7.01) | 41.45(7.97) | 40.88(6.04) |
| Gender (M/F)a | 100/108 | 37/61 | 63/47 |
| Race (White/non-White) | 163/45 | 80/18 | 83/27 |
| Education Level (HS/College/Graduate) |
10/112/86 | 4/53/41 | 6/59/45 |
| Yrs of School; M(SD) | 17.65(3.05) | 17.74(3.06) | 17.57(3.06) |
| Smoking(non/former/current Smoker) | 134/50/24 | 60/27/11 | 74/23/13 |
| Waist, inches; M(SD)a | 35.03(5.23) | 34.11(5.27) | 35.85(5.07) |
| BMI, kg/mm2; M(SD) | 26.75(4.93) | 26.22(5.16) | 27.21(4.70) |
| SBP, mmHg; M(SD)a | 119.47(9.79) | 116.32(9.75) | 122.19(9.03) |
| DBP, mmHg; M(SD) | 73.15(8.63) | 72.44(8.47) | 73.76(8.76) |
| Resting HF HRV,HZ- in natural log scale; M(SD) |
5.5(1.47) | 5.72(1.34) | 5.32(1.55) |
| Mean Interbeat Interval, msec; M(SD) | 907.19(145.19) | 902.32(155.17) | 911.53(136.26) |
| BDI, M(SD) | 3.64(3.76) | 3.88(3.76) | 3.44(3.75) |
| STAI; M(SD) | 32.52(8.15) | 32.34(8.79) | 32.67(7.58) |
AHABII and PIP samples are not statistically different, except for a relatively greater number of females in AHABII (chi-square test, p<.05) and greater waist circumference and SBP in PIP relative to AHABII (t-test, p<.05).
To be eligible to participate in AHAB-II, individuals had to be between the ages of 30-54 years and working at least 25 hours per week outside of the home (a sub-study involving this cohort was focused on the association between occupational stress and cardiovascular disease risk). Individuals were excluded from participation if they (a) had a history of cardiovascular disease, schizophrenia or bipolar disorder, chronic hepatitis, renal failure, major neurological disorder, chronic lung disease, or stage 2 hypertension (SBP/DBP ≥ 160/100); (b) consumed alcohol ≥ 5 portions 3-4 times per week; (c) took fish-oil supplements (because of the requirements for another substudy); (d) were prescribed insulin or glucocorticoid, anti-arrhythmic, antihypertensive, lipid-lowering, psychotropic, or prescription weight-loss medications; (e) were pregnant; (f) had less than 8th grade reading skills; or (g) were shift workers. Requirements for PIP were essentially the same, however, the employment, fish oil and shift work requirements were absent. Both studies were approved by the Institutional Review Board of the University of Pittsburgh; all participants provided written informed consent and were paid for their participation. For further sample descriptions see (P. J. Gianaros et al., 2014; Ryan, Sheu, Verstynen, Onyewuenyi, & Gianaros, 2013).
Procedure
Prior to both HRV and MRI scan sessions participants were required to abstain from caffeine, tobacco, or strenuous exercise for at least 3 hours and to abstain from alcohol and taking over the counter medications for at least 12 hours. A rest period of five minutes with eyes opened was administered during the MRI scanning session. This period was identical between studies and occurred prior to tasks that were administered during the remainder of the scanning session. AHAB II subsequently administered tasks related to emotion and reward processing and emotion regulation; PIP subsequently administered cognitive conflict tasks designed to be moderately stressful.
Resting state networks
We applied group probabilistic independent component analysis (ICA) (Beckmann and Smith, 2004 and Beckmann et al., 2005) to extract the networks of interest, namely DMN and SN. Other networks, such as the occipital visual network, resulting from the ICA were also reviewed and compared with publicly reported networks for result verification. ICA is a multivariate exploratory data analysis method that isolates latent factors. Here, the voxel-wise BOLD signal was modeled as a linear mixture of the signals from a set of ‘hidden’ structures (networks), which were characterized by statistically independent non-Gaussian components. The BOLD time series images of the 219 participants were concatenated to construct the spatial-temporal data matrix Y, which is modeled as the product of a mixing matrix X and a set of independent components B namely, Y=XB+e, where the rows of B represent components and e is Gaussian noise. The matrix, B was solved by finding an unmixing matrix W,WY=B, that optimizes the independence of the components. We used FSL’s MEOLODIC toolbox (Beckmann & Smith 2004, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MELODIC), which utilizes the FastICA algorithm to define the non-Gaussianity by negative-entropy and solves the optimization model via fixed-point iteration method (Hyvarinen and Oja 2000).
Individual functional connectivity with the DMN and SN
ICA components (individual functional connectivity maps) were determined by dual regression (Filippini et al., 2009). Specifically, individual temporal mixture weights for all components were estimated first by the regression of individual BOLD time series images on the components attained from the group analysis, and then the individual functional connectivity measures were estimated by the regression of BOLD images on the mixture time series. All the signals were z-transformed before further analysis to avoid tissue variation among participants. The test-retest reliability of ICA metrics using approaches similar to those we employed has been reported as moderately strong (ICC>0.4) (Guo et al., 2012). The individual functional connectivity maps of the DMN component and the SN component were used for the correlation analysis with HRV. The overall strength within the DMN or SN was measured by the mean connectivity strength within the networks.
Seed-based functional connectivity
Associations calculated using the procedures just described identified a region of interest in the mPFC that was common to both DMN and SN (see below). We applied seed based functional connectivity to examine the association of HRV and functional connectivity within this mPFC region-of-interest (ROI). Individual voxel-wise functional connectivity was assessed by a general linear model, with the mean time series in the mPFC ROI as an independent variable. Before the assessment, the time series in each voxel was de-trended, de-spiked, mean-centered, and adjusted for the confounding covariance due to movement, physiological noise, and hemodynamic response using regression. The movement parameters estimated from realignment were used as movement regressors. The physiological noise was modeled by the component-based method (Behzadi et al., 2007), with 3 principal components of BOLD time series from a white matter mask and 2 principal components from a CSF mask. The masks were constructed by using the SPM MNI templates of 95% and 75% probability maps for white matter and CSF respectively; they were further eroded to avoid partial volume effects. The hemodynamic response was modeled by the SPM default hemodynamic response function and its derivative. After the adjustment, the BOLD signals were band-pass filtered (0.008-0.15HZ).
Random Effects and Correlation Analyses
Voxel-wise random effects analysis of connectivity estimates and their correlation with HF-HRV were executed with GLMs implemented in SPM8 and statistically tested at a false positive rate of 0.05 by either family-wise correction threshold implemented in SPM8 or by simulation using 3dClustSim in AFNI (http://afni.nimh.nih.gov), which used a combined threshold of the voxel p-value and a contiguous cluster size threshold.
Heart rate variability
HF-HRV was derived from a modified lead II electrocardiogram in the AHAB study and from beat-by-beat peripheral pulse signals in PIP. In AHAB II, participants were seated in a temperature and sound-controlled chamber. After a 10-minute rest period, two successive 5-minute, resting ECG recordings were obtained. During the first, subjects were instructed to relax and breathe at a comfortable rate. During the second recording period, the subjects' respiratory rate was paced at 11 breaths per minute by playing a high-pitched tone during the inhalation period and a low-pitched tone during the exhalation period. Both the ECG and the derived IBI time series were processed and artifact corrected using automated algorithms and were confirmed by visual inspection of all data. Recording periods were divided into three 90-second epochs and were retained or excluded based on percent artifact (> 20% artifact excluded) and length (> 10% missing excluded). Variables were then averaged across retained epochs (HF was natural log transformed prior to averaging). HRV processing was performed using the band limited variance technique as described in J. J. Allen, Chambers, and Towers (2007). Data from 98 AHAB participants passed quality control and were included in the study. Implementation of this procedure was performed via Christie and Gianaros (2013). The pulse data from PIP was transformed to an IBI series and then processed to derive HF-HRV with the same software. In the PIP study, beat-to-beat pulse intervals (PIs; the time in ms between heart beats) were collected while participants rested inside a mock MRI scanner replica. For the replica protocol, participants were placed on the mock scanner bed and fitted with an appropriately-sized finger cuff for beat-to-beat pulse monitoring using a Finometer® PRO (FMS, Finapres Measurement Systems, Arnhem, Netherlands). Digitized pulse signals were visually inspected and scored offline using locally-developed scripts and with BeatScope® software provided with the Finometer®. Estimates were averaged over the last 5min of an 8-min resting baseline. After quality checking, 101 PIP participants were included in the study. Derivation of HF-HRV from pulse data during behavioral resting states has been successfully validated against electrocardiogram data, e.g. (J.R. Jennings, Westerveldt, & Ackles, 1987). Note that the reclining posture was present during data collection of the PIP study. This posture might alter levels of HF-HRV observed relative to the sitting posture of the AHAB study (Berntson et al., 1994)
Results
Resting State Network- group ICA result
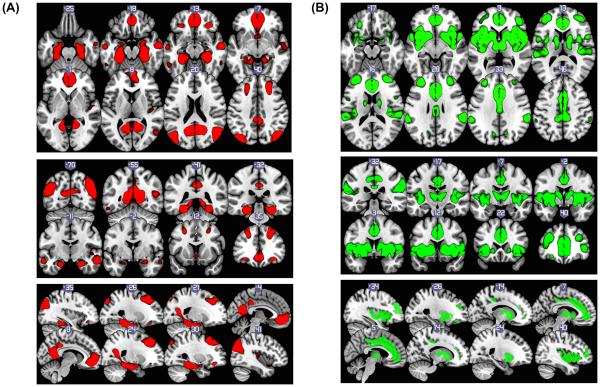
The group ICA revealed 19 components determined by Laplace approximation to the Bayesian evidence of the model order. These 19 components are illustrated in supplemental Figure, Figure S2. Typical resting state networks, such as the visual and somatomotor networks, were readily apparent from visualization. Some variation in networks between studies occurs, so a close examination of the obtained networks was required to appropriately identify the DMN and SN. Figure 1 (A) and (B) shows the DMN and SN networks derived from our sample (these networks are indicated as number 9 and 11 in supplemental Figure 1). By using cross-correlation, our components were compared with the masks generated from two publically available resting state brain network data archives (Yeo et al.,2011: 7 resting networks, Laird., et al.,2011: 20 intrinsic resting networks). Components #9 and #11 closely matched the DMN and SN (for cross-correlation results, see Table S2). Accordingly, the DMN and SN of this sample was determined from component #9 and #11 respectively, with a z-statistic threshold of z > 4 (see Figure 1). A region in the mPFC showed overlapping connectivity in both the DMN and SN (see Figure 2 (A). This region was masked as an ROI for latter analyses in line with our study hypotheses.
Figure 1.
(A) Default Mode Network (threshold at z>4 from component #9); included regions of posterior cingulate (BA31,23), hippocampus, amygdala, medial orbital frontal gyrus (BA10,24,25, and 32), mid frontal gyrus(BA8,9) L/R mid temporal gyrus (BA20,37), L/R inferior orbital frontal gyrus (BA11,47), temporal lobe, parietal lobe and angular gyrus (B) Salience Network (threshold at z>4 from component #11) includes L/R insula(BA13,22,47), L/R mid inferior frontal gyrus (BA10), thalamus and L/R putamen and caudate.
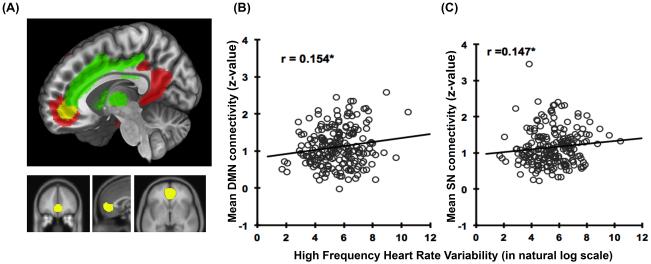
Figure 2.
(A) mPFC region of interest (yellow), which is the intersection of default mode network (red) and salience network (green). Total volume: 13032 mm3, center of mass (mm): (1,46,-3), x: -14 to 14 mm, y: 30 to 60 mm, and z: -14 to 18mm. (B) Correlation of mean DMN connectivity in the ROI and HF-HRV. (C) Correlation of mean SN in the ROI and HF-HRV (n=207).
Does HRV associate with global measures of DMN or SN connectivity?
The global connectivity within each network was assessed by the mean connectivity over all voxels within the DMN and SN. The global DMN connectivity between subjects ranged from 0.42 to 2.24 (z-statistics) with a mean = 1.03 and SD = 0.30. Global SN connectivity between subjects ranged from 0.34 to 2.48 with mean = 0.97 and SD=0.31. The DMN connectivity within subject variability (SD) ranged from 0.5 to 2.48; the within subject SN connectivity SD ranged from 0.37 to 2.19. The range of between subject connectivity values was judged sufficient to identify relationships with between subject variations in HF-HRV. The divergence in within subject variability suggested, however, that regions varied in connectivity and did so differently in different individuals, i.e., overall mean connectivity may be a limited measure. In fact, the correlations between HF-HRV and global DMN or SN were not significant (DMN: r=0.08, p=0.26; SN: r=0.06, p=0.37). This suggests that diffuse or aggregate connectivity strength of these two intrinsic networks does not relate to HF-HRV.
The connectivity of individual regions within either the DMN or SN did show modest relationships with HF-HRV. A mPFC region (BA 32, perigenual anterior cingulate) showed a significant positive correlation with HF-HRV in its connectivity within both the DMN (r=.27) and SN (r=.29). A region in the insula showed a similar (r=.26) correlation, but only with the DMN. Positive correlations of essentially the same magnitude with SN connectivity were shown by the posterior cingulate, insula, precentral gyrus, thalamus and superior occipital gyrus. These relations and the details of the voxel-wise regression results are shown in supplementary table S3. These areas are comparable to those identified previously as showing associations of cerebral blood flow to HF-HRV by Allen et al. (2015). Correlations were of comparable magnitude with the connectivity of each of the areas, i.e. no area showed a relationship that was statistically stronger than any other area. The dual relationship of the mPFC area with SN and DMN was novel, however, and further examined.
mPFC connectivity and HF-HRV
A region in the mPFC, largely encompassing the perigenual portion of the anterior cingulate cortex (pgACC), extending into Broadmann area 10, showed significant connectivity strength with both the DMN and SN (z-score >4, see Figure 2 (A), supplementary table S3). To further explore this region, mean DMN connectivity and mean SN connectivity were extracted from this region for each participant. The aim of this was to see if variability in the network connectivities of this area common to the DMN and SN related to HF-HRV. Participants’ mean DMN connectivity in the ROI ranged from 0.2 to 2.60. The mean SN connectivity in the ROI ranged from 0.23 to 3.50 . One outlier that was 3SD from the mean was excluded from further analysis. The connectivity with DMN and SN in this mPFC region was correlated (r = 0.27, p<0.001). The connectivity strengths with DMN and SN in this mPFC region were also associated with HF-HRV (see Figure 2 (B) and (C); r=0.157, p=0.024 for DMN and r=0.147 p=0.035 for SN). Heart rate, as opposed to HF-HRV, was not significantly related to the connectivity of this mPFC region, although partialling out the effect of heart rate for the correlation of HF-HRV and the DMN did reduce the correlation slightly to r=0.115 for DMN and r=0.139 for SN. Heart rate was examined as a simpler measure than HF-HRV and one that has been interpreted as less specific to vagal control.
Result of seed based connectivity with the mPFC seed
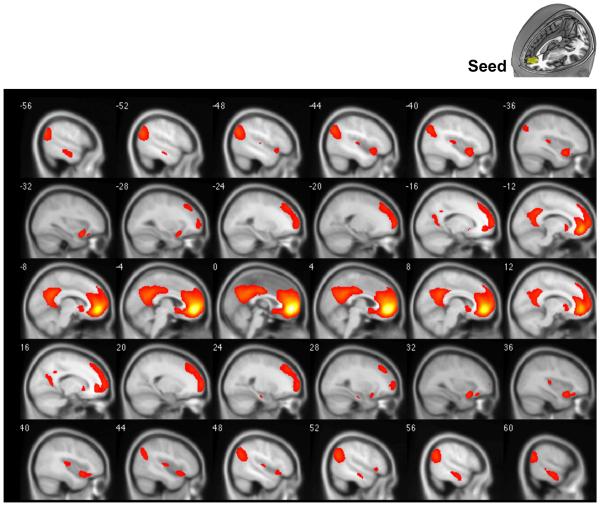
Given the relationships between DMN and SN connectivities of the mPFC and HF-HRV, we then tested the functional connectivity of this specific region. The whole brain voxel-wise correlation map with the seed is shown in Figure 3. The result shows that mPFC connectivity is observed not only with anticipated regions within both DMN and SN, but also with frontal lobe regions, such as the lateral prefrontal cortex. Regions showing significant resting state connectivity with this region (r>0.25, FWE p<0.05) include the posterior cingulate, bilateral insula (anterior and posterior), medial temporal lobe, superior frontal lobe, caudate, and amygdala.
Figure 3.
Functional connectivity map with mPFC seed. Threshold at correlation coefficient r >0.25. Regions include areas in DMN: medial frontal lobe (BA8,9,10,11), anterior cingulate (BA24, 24,32), posterior cingulate (BA31,23) and L/R parahippocampus; in SN: L/R insula (BA13,47), L/R superior temporal gyrus. It also includes areas outside the DMN and SN, such as superior frontal gyrus.
Do areas exhibiting seed-based connectivity with the mPFC also relate to HRV?
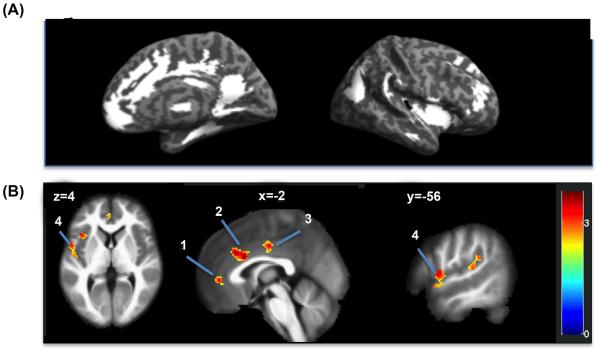
Figure 4 shows regions in DMN and SN where the connectivity with the mPFC seed positively correlated with HF-HRV (threshold at voxel-wise p<0.01) tested at a false positive rate of alpha α < 0.05. These included the pgACC, dorsal ACC, mid ACC, and left superior temporal gyrus extending into the left insula. Table 2 summarizes the cluster sizes, the peak z-values, and the coordinates of these regions. The significance levels were corrected for multiple comparisons in the search region that combined DMN and SN (# of voxels = 27959) using simulation by 3dClustSim. Based on the data variance, the simulation result indicated that a combination of a height threshold of p<0.01 and contiguous cluster size of 151 voxels or more would achieve a false positive rate of α=0.05. An area typically related to autonomic control, the anterior insula, was also identified but failed to pass our significance threshold. This area was further identified in the whole brain analysis described in the supplementary material (see Figure S3 (B) x=42). The whole brain exploratory results for the correlation between mPFC connectivity and HF-HRV are reported in the supplemental figure, Figure S3 and supplemental tables, Table S2 and Table S3.
Figure 4.
(A) ROI Search regions (white): combined DMN and SN, # of voxels=27959, voxel size=2mm3(B) Regions in which the connectivity with mPFC seed at rest associated with high frequency heart rate variability (threshold at T>2.34, p<.01); the associations were tested with a false positive detection rate of α =0.05 (corrected threshold using 3dClustSim) The regions shown are 1) pgACC, 2) dACC, 3) MCC, and 4) left temporal gyrus extending into the left insula. See Table 2 for detailed coordinates.
Table 2.
Regions in which the connectivity with mPFC seed correlated with high frequency heart rate variability (Search regions: DMN and SN)
| Cluster Size | Peak coordinates | Peak Z-value | ||||
|---|---|---|---|---|---|---|
| Threshold at T=2.34, p=0.01 |
X | y | Z | |||
| Cingulate Gyrus (dACC) | BA24, 32 | 401 | −2 | 16 | 28 | 3.76 |
| Cingulate Gyrus (MCC) | BA24 | 157 | 0 | −12 | 40 | 3.42 |
| Cingulate Gyrus (pgACC) | BA32, 24 | 152 | −4 | 46 | 2 | 3.39 |
| Left Inferior Frontal | ||||||
| Gyrus; Superior Temporal Gyrus, Insula |
BA22, BA44 |
221 | −58 | 8 | 4 | 3.34 |
Discussion
A primary aim of the present study was to examine whether HF-HRV covaried with DMN connectivity. Despite the seemingly similar psychophysiological states implied by their conceptual descriptions, our results failed to show any overall association between HF-HRV and DMN. Individual levels of HF-HRV failed to correlate with mean levels of connectivity within the DMN. We did observe, however, associations between connectivity and a number of regions previously known to relate to HRV (B. Allen, Jennings, Thayer, Gianaros, & Manuck, 2015; Beissner, Meissner, Bar, & Napadow, 2013; P.J. Gianaros & Wager, 2015; Wager et al., 2009). Positive associations were observed between connectivity of the mPFC and dACC, pgACC and marginally, anterior insula; while negative associations were seen, marginally, with the brainstem (see supplemental material). In a sample of 27 adults, Kano et al. (2014) reported a negative association between a putative indicator of cardiac vagal activity derived from a phase shift analysis of inter-beat intervals and resting blood flow in the cerebellum and a more dorsal cingulate region assessed via arterial spin labeling, not resting state fMRI BOLD connectivity. This work emphasized the importance of the cerebellum and ACC, but otherwise seems only marginally relevant to our finding. In short, connectivity within the DMN overall is unrelated to individual differences in HF-HRV, but a limited number of regions do show a shared neural substrate between these resting states.
Our results do little to clarify the similarity or differences of psychological states related to DMN or HF-HRV. The psychological state related to either HF-HRV or the DMN has not been definitively established. More attempts to understand the functional significance of the DMN have been made (Anticevic et al., 2012; Laird et al., 2013; Leech & Sharp, 2014; Northoff, Qin, & Nakao, 2010; Qin & Northoff, 2011). Andrews-Hanna (2012), see also (Buckner et al., 2008), reviews these studies and alternative views that the DMN reflects an idling, metabolic recovery state, passive attention to the external environment or attention to internalized, self-directed thought. Complementary reviews also establish that preceding conditions/experience modulate the DMN and that level of DMN activation can modulate subsequent task activation (Northoff et al., 2010). Finally, recent work suggests that modest levels of DMN activity may facilitate relative routine behavior, albeit behavior requiring attention to the environment and simple responding (Esterman, Noonan, Rosenberg, & Degutis, 2013). The literature clearly suggests a role for internal mentation, but it is unclear that the resting/default brain state is composed solely of such mentation—a role for metabolic recovery or diffuse attention has not been excluded. Our results suggest, however, that to the extent HF-HRV reflects vagal metabolic rest and recovery, the DMN is not primarily regulating such rest/recovery.
Despite a voluminous literature on resting HRV, little empirical evidence exists on how this measure relates to concurrent psychological states or processes as opposed to trait or clinical assessments of depression or anxiety, e.g., (Kemp, Quintana, Felmingham, Matthews, & Jelinek, 2012; Nahshoni et al., 2004; Wang et al., 2013). Measures of mood concurrently or preceding resting HRV measures show weak positive relationships with positive/happy moods (Ingjaldsson, Laberg, & Thayer, 2003; Pieper, Brosschot, van der Leeden, & Thayer, 2010) and inverse relationships with negative moods/prior experience (Dywan, Mathewson, Choma, Rosenfeld, & Segalowitz, 2008; Fabes & Eisenberg, 1997; Ingjaldsson et al., 2003; T. W. Smith et al., 2011). A somewhat different type of state, inferred rather than subjectively expressed—flexibility, behavioral regulation or readiness to react to the environment—has been related to higher HF-HRV (Beauchaine, 2001; Butler, Wilhelm, & Gross, 2006). Engagement in virtually any task typically reduces HF-HRV (Stephen W. Porges, 2007). Increased HF-HRV has been observed during emotional self-regulation, perhaps, particularly in social situations (Butler et al., 2006; Stephen W. Porges, 2007). This observation is potentially related to the interpretations reviewed above for the DMN.1 It is important to note, however, that there are few studies and even fewer with substantial sample sizes that relate positive affective and social experiences to HF-HRV. Accordingly, more research is clearly needed. Beyond this, the literature appears mute on whether the biologically based view of vagal activation as restorative/relaxing is related to any perception of heightened restoration/relaxation during periods of high amplitude HF-HRV. The available empirical evidence appears to suggest that high amplitude HF-HRV is associated with a positive mood, absence of negative affect, and an alert readiness to engage with the physical and social environment.
Switching, SN, DMN, and HF-HRV
Regions seemingly sharing correlations between DMN connectivity and HF-HRV can be characterized as both related to autonomic nervous system control and to areas in the SN (Seeley et al., 2007). Initial work on the SN and a model of the functioning of this network (Menon & Uddin, 2010) suggested that the SN functioned to detect significant environmental events and switch between DMN function and active processing of events in an executive control network, e.g. switching to executive control after an error (T. E. Ham et al., 2013). The SN was linked to autonomic nervous system function in that both affect and orienting to the environment relate to sensitivity to environmental challenges. Our extraction of the SN in our results showed that one area related to HF-HRV was also jointly observed in both the SN and the DMN, the pgACC within the mPFC. Interestingly, this area, when subjected to Transcranial Magnetic Stimulation, has been shown to induce switching between DM and executive control networks (Chen et al., 2013); related work supports the same interpretation based on inferences from a Granger causality model (Goulden et al., 2014). The possible further significance of this area is suggested by the observed relationships between a similar cingulate area and the cortisol response to stress in adolescents (Thomason, Hamilton, & Gotlib, 2011) and relationships between post traumatic stress severity and SN/DMN connectivity strength (Sripada et al., 2012).
Future work may more clearly implicate the insula’s relationship the mPFC area sharing connectivity with the DMN and SN. We observed connectivity to the insula, but this area was only marginally related to HF-HRV. Relatively posterior areas of the insula appear to be closely related to visceral sensory function; while more anterior areas may be more related to emotional salience and attention control—interpretations drawn from brain resting state connectivity as well as active engagement of these areas (Cauda et al., 2012; Cauda et al., 2011; Craig, 2009).
Limitations
Although benefitting from a large sample size formed from two studies, the studies did differ somewhat in methodology. Assessment of networks and HRV was done separately in the two studies. However, the similarity of results suggested that combination of the samples was appropriate. The benefits of added power and resolution also appeared to outweigh any true effect of the methodological differences between studies. Heart rate variability was assessed in seated postures in AHAB, but reclining in PIP. This may have led to somewhat different levels of HF-HRV between the studies although individual differences in level are less likely to be altered by posture. The PIP study derived heart rate variability from a pulse signal. Despite validation of such measurement, the use of electrocardiographic signals is more common and likely slightly more precise. As noted, measures of heart rate variability were not concurrent with brain measures. Though concurrent measurement would aid interpretation of the concurrence of brain and cardiac measures, heart rate variability in the literature is uniformly assessed under conditions similar to those used in the current studies. To the extent that individual differences in HRV are trait differences, the current measures generalize better to the extant literature than would HRV measures from the scanner environment. Questions about the psychological state related to either DMN or HF-HRV were not directly addressed with the current data; our brain data were collected without an explicit interest in perceptions related to resting states. The other limitation of the study was that the data were acquired from a 5 minute scan. Although estimated functional connectivity has been found to be stable with a 5 minute-acquisition (see VanDijk et al., 2010), recent studies (Birm et al., 2013, Gozaler-Casilo et al., 2014) suggested that 10 minutes of acquisition or more could produce more stable results. Connectivity estimates derived from ICA were, however, shown to have occasion-to-occasion test-retest repeatability using a 6.5 minute scan (ICC>0.4, Zuo et al., 2010).
Conclusions
The brain control of resting states represented by the DMN and HF-HRV is largely separate and non-overlapping. Functional orientation away from psychophysiological states related to DMN and HF-HRV may, however, share regulation by a mPFC area common to both the DMN and SN and related to HF-HRV. Specific assessment during such switching of the relevant brain areas as well as the heart rate responses is necessary to support this interpretation.
Supplementary Material
Acknowledgements
We gratefully acknowledge the support of NIH grants R01 HL101959 (JRJ), PO1 HL040962 (SBM), and R01 HL089850 (PJG). We also acknowledge the concept and earlier analysis of some of the issues addressed in this paper in the dissertation work of Dr. V. Egizio (Egizio, 2014).
Footnotes
We thank Dr. Julian Thayer for assistance with the literature on this issue.
References
- Allen B, Jennings JR, Gianaros PG, Thayer J, Manuck SM. Resting High-Frequency Heart Rate Variability is related to Resting Brain Perfusion. Psychophysiology. 2015;52(2):277–287. doi: 10.1111/psyp.12321. http://dx.doi.org/10.1111/psyp.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JJ, Chambers AS, Towers DN. The many metrics of cardiac chronotropy: a pragmatic primer and a brief comparison of metrics. Biological Psychology. 2007;74(2):243–262. doi: 10.1016/j.biopsycho.2006.08.005. http://dx.doi.org/10.1016/j.biopsycho.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR. The brain's default network and its adaptive role in internal mentation. Neuroscientist. 2012;18(3):251–270. doi: 10.1177/1073858411403316. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends in Cognitive Sciences. 2012;16(12):584–592. doi: 10.1016/j.tics.2012.10.008. doi: http://dx.doi.org/10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP. Vagal tone, development, and Gray's motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13(2):183–214. doi: 10.1017/s0954579401002012. doi: http://dx.doi.org/10.1017/S0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23(2):137–152. doi: 10.1109/TMI.2003.822821. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bar KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. Journal of Neuroscience. 2013;33(25):10503–10511. doi: 10.1523/JNEUROSCI.1103-13.2013. doi: http://dx.doi.org/10.1523/JNEUROSCI.1103-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Norman GJ, Hawkley LC, Cacioppo JT. Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology. 2008;45(4):643–652. doi: 10.1111/j.1469-8986.2008.00652.x. doi: http://dx.doi.org/10.1111/j.1469-8986.2008.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn RM, Molloy EK, Patriat R, Parker T, Meier TB, Kirk GR, Prabhakaran V. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage. 2013;83:550–558. doi: 10.1016/j.neuroimage.2013.05.099. doi:10.1016/j.neuroimage.2013.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, Sharp DJ. Salience network integrity predicts default mode network function after traumatic brain injury. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(12):4690–4695. doi: 10.1073/pnas.1113455109. doi: http://dx.doi.org/10.1073/pnas.1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. http://dx.doi.org/10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43(6):612–622. doi: 10.1111/j.1469-8986.2006.00467.x. doi: http://dx.doi.org/10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Cannon WB. Organization for physiological homeostasis. Physiological Reviews. 1929;9(3):399–431. [Google Scholar]
- Cauda F, Costa T, Torta DM, Sacco K, D'Agata F, Duca S, Vercelli A. Meta-analytic clustering of the insular cortex: characterizing the meta-analytic connectivity of the insula when involved in active tasks. NeuroImage. 2012;62(1):343–355. doi: 10.1016/j.neuroimage.2012.04.012. doi: http://dx.doi.org/10.1016/j.neuroimage.2012.04.012 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. NeuroImage. 2011;55(1):8–23. doi: 10.1016/j.neuroimage.2010.11.049. doi: 2012.04.012http://dx.doi.org/10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Chang C, Metzger CD, Glover GH, Duyn JH, Heinze HJ, Walter M. Association between heart rate variability and fluctuations in resting-state functional connectivity. NeuroImage. 2013;68:93–104. doi: 10.1016/j.neuroimage.2012.11.038. doi: http://dx.doi.org/10.1016/j.neuroimage.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Oathes DJ, Chang C, Bradley T, Zhou ZW, Williams LM, Etkin A. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(49):19944–19949. doi: 10.1073/pnas.1311772110. doi: http://dx.doi.org/10.1073/pnas.1311772110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie IC, Gianaros PJ. PhysioScripts: an extensible, open source platform for the processing of physiological data. Behavior Research Methods. 2013;45(1):125–131. doi: 10.3758/s13428-012-0233-x. doi: http://dx.doi.org/10.3758/s13428-012-0233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhond RP, Yeh C, Park K, Kettner N, Napadow V. Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain. 2008;136(3):407–418. doi: 10.1016/j.pain.2008.01.011. doi: http://dx.doi.org/10.1016/j.pain.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dywan J, Mathewson KJ, Choma BL, Rosenfeld B, Segalowitz SJ. Autonomic and electrophysiological correlates of emotional intensity in older and younger adults. Psychophysiology. 2008;45(3):389–397. doi: 10.1111/j.1469-8986.2007.00637.x. doi: http://dx.doi.org/10.1111/j.1469-8986.2007.00637.x. [DOI] [PubMed] [Google Scholar]
- Egizio VB. The relationship between the default mode resting state neural network, respiratory sinus arrhythmia, and self-focused cognition: An empirical analysis. Dissertation Abstracts International: Section B: The Sciences and Engineering. 2014;75 (3-B(E)) [Google Scholar]
- Elton A, Gao W. Divergent task-dependent functional connectivity of executive control and salience networks. Cortex. 2014;51:56–66. doi: 10.1016/j.cortex.2013.10.012. doi: http://dx.doi.org/10.1016/j.cortex.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Esterman M, Noonan SK, Rosenberg M, Degutis J. In the zone or zoning out? Tracking behavioral and neural fluctuations during sustained attention. Cerebral Cortex. 2013;23(11):2712–2723. doi: 10.1093/cercor/bhs261. doi: http://dx.doi.org/10.1093/cercor/bhs261. [DOI] [PubMed] [Google Scholar]
- Fabes RA, Eisenberg N. Regulatory control and adults' stress-related responses to daily life events. Journal of Personality and Social Psychology. 1997;73(5):1107–1117. doi: 10.1037//0022-3514.73.5.1107. doi: http://dx.doi.org/10.1037/0022-3514.73.5.1107. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Marsland AL, Kuan DC, Schirda BL, Jennings JR, Sheu LK, Manuck SB. An inflammatory pathway links atherosclerotic cardiovascular disease risk to neural activity evoked by the cognitive regulation of emotion. Biological Psychiatry. 2014;75(9):738–745. doi: 10.1016/j.biopsych.2013.10.012. doi: http://dx.doi.org/10.1016/j.biopsych.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Wager TD. Brain body pathways linking psychological stress and physical health. Current Directions in Psychological Science. 2015;24:313–321. doi: 10.1177/0963721415581476. doi:10.1177/0963721415581476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino ND, Glenny RW, Borson S, Chan L. Respiratory sinus arrhythmia is associated with efficiency of pulmonary gas exchange in healthy humans. American Journal of Physiology - Heart & Circulatory Physiology. 2003;284(5):H1585–1591. doi: 10.1152/ajpheart.00893.2002. [DOI] [PubMed] [Google Scholar]
- Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, Mullins PG. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. NeuroImage. 2014;99:180–190. doi: 10.1016/j.neuroimage.2014.05.052. doi: http://dx.doi.org/10.1016/j.neuroimage.2014.05.052. [DOI] [PubMed] [Google Scholar]
- Graham FK, Clifton RK. Heart-rate change as a component of the orienting response. Psychological Bulletin. 1966;65(5):305–320. doi: 10.1037/h0023258. doi: http://dx.doi.org/10.1037/h0023258. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CC, Kurth F, Zhou J, Mayer EA, Eickhoff SB, Kramer JH, Seeley WW. One-year test-retest reliability of intrinsic connectivity network fMRI in older adults. Neuroimage. 2012;61(4):1471–1483. doi: 10.1016/j.neuroimage.2012.03.027. doi: http://dx.doi.org/10.1016/j.neuroimage.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham T, Leff A, de Boissezon X, Joffe A, Sharp DJ. Cognitive control and the salience network: an investigation of error processing and effective connectivity. Journal of Neuroscience. 2013;33(16):7091–7098. doi: 10.1523/JNEUROSCI.4692-12.2013. doi: http://dx.doi.org/10.1523/JNEUROSCI.4692-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham TE, de Boissezon X, Leff A, Beckmann C, Hughes E, Kinnunen KM, Sharp DJ. Distinct frontal networks are involved in adapting to internally and externally signaled errors. Cerebral Cortex. 2013;23(3):703–713. doi: 10.1093/cercor/bhs056. doi: http://dx.doi.org/10.1093/cercor/bhs056. [DOI] [PubMed] [Google Scholar]
- Hasenkamp W, Wilson-Mendenhall CD, Duncan E, Barsalou LW. Mind wandering and attention during focused meditation: a fine-grained temporal analysis of fluctuating cognitive states. NeuroImage. 2012;59(1):750–760. doi: 10.1016/j.neuroimage.2011.07.008. doi: http://dx.doi.org/10.1016/j.neuroimage.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Hermans EJ, Henckens MJ, Joels M, Fernandez G. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends in Neurosciences. 2014;37(6):304–314. doi: 10.1016/j.tins.2014.03.006. doi: http://dx.doi.org/10.1016/j.tins.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. Journal of Internal Medicine. 2011;269(1):45–53. doi: 10.1111/j.1365-2796.2010.02321.x. http://dx.doi.org/10.1111/j.1365-2796.2010.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvarinen A, Oja E. Independent component analysis: algorithms and applications. Neural Networks. 2000;13(4-5):411–430. doi: 10.1016/s0893-6080(00)00026-5. http://dx.doi.org/10.1016/S0893-6080(00)00026-5 [DOI] [PubMed] [Google Scholar]
- Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: Relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological Psychiatry. 2003;54(12):1427–1436. doi: 10.1016/s0006-3223(02)01926-1. doi: http://dx.doi.org/10.1016/S0006-3223%2802%2901926-1. [DOI] [PubMed] [Google Scholar]
- Ito S, Sasano H, Sasano N, Hayano J, Fisher JA, Katsuya H. Vagal nerve activity contributes to improve the efficiency of pulmonary gas exchange in hypoxic humans. Experimental Physiology. 2006;91(5):935–941. doi: 10.1113/expphysiol.2006.034421. [DOI] [PubMed] [Google Scholar]
- Jennings J, van der Molen MW. Preparation for Speeded Action as a Psychophysiological Concept. Psychological Bulletin. 2005;131(3):434–459. doi: 10.1037/0033-2909.131.3.434. doi: http://dx.doi.org/10.1037/0033-2909.131.3.434. [DOI] [PubMed] [Google Scholar]
- Jennings JR. Bodily Changes during Attending. In: Coles MGH, Donchin E, Porges SW, editors. Psychophysiology:Systems, Processes, and Applications. Guilford; New York: 1986. pp. 269–289. [Google Scholar]
- Jennings JR, van der Molen MW. Preparation for Speeded Action as a Psychophysiological Concept. Psychological Bulletin. 2005;131(3):434–459. doi: 10.1037/0033-2909.131.3.434. doi: http://dx.doi.org/10.1037/0033-2909.131.3.434. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Westerveldt WW, Ackles PK. On plethysmographic measures of heart rate responses. In: Jennings JR, Ackles PK, Coles MGH, editors. Advances in psychophysiology: A research annual. Vol. 2. Jessica Kingsley Publishers; London, England: 1987. pp. 233–240. [Google Scholar]
- Jilka SR, Scott G, Ham T, Pickering A, Bonnelle V, Braga RM, Sharp DJ. Damage to the Salience Network and interactions with the Default Mode Network. Journal of Neuroscience. 2014;34(33):10798–10807. doi: 10.1523/JNEUROSCI.0518-14.2014. doi: http://dx.doi.org/10.1523/JNEUROSCI.0518-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Coen SJ, Farmer AD, Aziz Q, Williams SCR, Alsop DC, O’Gorman RL. Physiological and psychological individual differences influence resting brain function measured by ASL perfusion. Brain Structure Function. 2014;219(5):1673–1684. doi: 10.1007/s00429-013-0593-8. S. J. A. D. doi: 10.1007/s00429-013-0593-8 24. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Felmingham KL, Matthews S, Jelinek HF. Depression, comorbid anxiety disorders, and heart rate variability in physically healthy, unmedicated patients: Implications for cardiovascular risk. PLoS ONE. 2012;7(2) doi: 10.1371/journal.pone.0030777. doi: http://dx.doi.org/10.1371/journal.pone.0030777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey BC, Lacey JI. Cardiovascular psychophysiology: Current issues in response mechanisms, biofeedback and methodology. AldineTransaction; US; New Brunswick, NJ: 1974. Studies of heart rate and other bodily processes in sensorimotor behavior; pp. 538–564. [Google Scholar]
- Laird AR, Eickhoff SB, Rottschy C, Bzdok D, Ray KL, Fox PT. Networks of task co-activations. NeuroImage. 2013;80:505–514. doi: 10.1016/j.neuroimage.2013.04.073. doi: http://dx.doi.org/10.1016/j.neuroimage.2013.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, Fox PT. Behavioral interpretations of intrinsic connectivity networks. J. Cogn. Neurosci. 2011;23(12):4022–4037. doi: 10.1162/jocn_a_00077. doi:10.1162/jocn_a_00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137:12–32. doi: 10.1093/brain/awt162. Pt 1. doi: http://dx.doi.org/10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure & Function. 2010;214(5-6):655–667. doi: 10.1007/s00429-010-0262-0. doi: http://dx.doi.org/10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano N, Porta A, Cogliati C, Costantino G, Tobaldini E, Casali KR, Iellamo F. Heart rate variability explored in the frequency domain: A tool to investigate the link between heart and behavior. Neuroscience and Biobehavioral Reviews. 2009;33(2):71–80. doi: 10.1016/j.neubiorev.2008.07.006. doi: http://dx.doi.org/10.1016/j.neubiorev.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Nahshoni E, Aravot D, Aizenberg D, Sigler M, Zalsman G, Strasberg B, Weizman A. Heart Rate Variability in Patients With Major Depression. Psychosomatics: Journal of Consultation and Liaison Psychiatry. 2004;45(2):129–134. doi: 10.1176/appi.psy.45.2.129. doi: http://dx.doi.org/10.1176/appi.psy.45.2.129. [DOI] [PubMed] [Google Scholar]
- Northoff G, Qin P, Nakao T. Rest-stimulus interaction in the brain: a review. Trends in Neurosciences. 2010;33(6):277–284. doi: 10.1016/j.tins.2010.02.006. doi: http://dx.doi.org/10.1016/j.tins.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Pieper S, Brosschot JF, van der Leeden R, Thayer JF. Prolonged cardiac effects of momentary assessed stressful events and worry episodes. Psychosomatic Medicine. 2010;72(6):570–577. doi: 10.1097/PSY.0b013e3181dbc0e9. doi: http://dx.doi.org/10.1097/PSY.0b013e3181dbc0e9. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. doi: http://dx.doi.org/10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Coles M. Individual differences in respiratory-heart period coupling and heart period responses during two attention-demanding tasks. Physiological Psychology. 1982;10(2):215–220. http://dx.doi.org/10.3758/BF03332939. [Google Scholar]
- Qin P, Northoff G. How is our self related to midline regions and the default-mode network? NeuroImage. 2011;57(3):1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. doi: http://dx.doi.org/10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA. A default mode of brain function. Proc. Natl.Acad. Sci. U.S.A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. http://dx.doi.org/10.1073/pnas.98.2.676 al., e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl V, Bienkowska K, Strobel C, Tahmasian M, Grimmer T, Forster S, Drzezga A. Local activity determines functional connectivity in the resting human brain: a simultaneous FDG-PET/fMRI study. Journal of Neuroscience. 2014;34(18):6260–6266. doi: 10.1523/JNEUROSCI.0492-14.2014. doi: http:// dx.doi.org/10.1523/JNEUROSCI.0492-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling JK, Dagenais JE, Goldsmith DR, Glenn AL, Pagnoni G. Social cognitive neural networks during in-group and out-group interactions. NeuroImage. 2008;41(4):1447–1461. doi: 10.1016/j.neuroimage.2008.03.044. doi: http://dx.doi.org/10.1016/j.neuroimage.2008.03.044 25. [DOI] [PubMed] [Google Scholar]
- Ryan JP, Sheu LK, Verstynen TD, Onyewuenyi IC, Gianaros PJ. Cerebral blood flow links insulin resistance and baroreflex sensitivity. PLoS ONE [Electronic Resource] 2013;8(12):e83288. doi: 10.1371/journal.pone.0083288. doi: http://dx.doi.org/10.1371/journal.pone.0083288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. http://dx.doi.org/10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin PY, Webber MR, Galletly DC, Ainslie PN, Brown SJ, Willie CK, Tzeng YC. Interactions between heart rate variability and pulmonary gas exchange efficiency in humans. Experimental Physiology. 2010;95(7):788–797. doi: 10.1113/expphysiol.2010.052910. doi: http://dx.doi.org/10.1113/expphysiol.2010.052910. [DOI] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends in Cognitive Sciences. 2009;13(8):334–340. doi: 10.1016/j.tics.2009.05.001. doi: http://dx.doi.org/10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE. Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences, USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. http://dx.doi.org/10.1073/pnas.0905267106 al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TW, Cribbet MR, Nealey-Moore JB, Uchino BN, Williams PG, MacKenzie J, Thayer JF. Matters of the variable heart: Respiratory sinus arrhythmia response to marital interaction and associations with marital quality. Journal of Personality and Social Psychology. 2011;100(1):103–119. doi: 10.1037/a0021136. doi: http://dx.doi.org/10.1037/a0021136. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. doi: http://dx.doi.org/10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, Liberzon I. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosomatic Medicine. 2012;74(9):904–911. doi: 10.1097/PSY.0b013e318273bf33. doi: http://dx.doi.org/10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. International Journal of Cardiology. 2010;141(2):122–131. doi: 10.1016/j.ijcard.2009.09.543. http://dx.doi.org/10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Ahs F, Fredrikson M, Sollers JJ, 3rd, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012;36(2):747–56. doi: 10.1016/j.neubiorev.2011.11.009. http://dx.doi.org/10.1016/j.neubiorev.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Hamilton JP, Gotlib IH. Stress-induced activation of the HPA axis predicts connectivity between subgenual cingulate and salience network during rest in adolescents. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2011;52(10):1026–1034. doi: 10.1111/j.1469-7610.2011.02422.x. doi: http://dx.doi.org/10.1111/j.1469-7610.2011.02422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng YC, Sin PY, Galletly DC. Human sinus arrhythmia: inconsistencies of a teleological hypothesis. American Journal of Physiology - Heart & Circulatory Physiology. 2009;296(1):H65–70. doi: 10.1152/ajpheart.00716.2008. doi: http://dx.doi.org/10.1152/ajpheart.00716.2008. [DOI] [PubMed] [Google Scholar]
- Uddin LQ. Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience. 2015;16(1):55–61. doi: 10.1038/nrn3857. doi: http://dx.doi.org/10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- van Buuren M, Gladwin TE, Zandbelt BB, van den Heuvel M, Ramsey NF, Kahn RS, Vink M. Cardiorespiratory effects on default-mode network activity as measured with fMRI. Human Brain Mapping. 2009;30(9):3031–3042. doi: 10.1002/hbm.20729. doi: http://dx.doi.org/10.1002/hbm.20729 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. doi:10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao X, O'Neil A, Turner A, Liu X, Berk M. Altered cardiac autonomic nervous function in depression. BMC Psychiatry. 2013;13:187. doi: 10.1186/1471-244X-13-187. http://dx.doi.org/10.1186/1471-244X-13-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiech K, Lin CS, Brodersen KH, Bingel U, Ploner M, Tracey I. Anterior insula integrates information about salience into perceptual decisions about pain. Journal of Neuroscience. 2010;30(48):16324–16331. doi: 10.1523/JNEUROSCI.2087-10.2010. doi: http://dx.doi.org/10.1523/JNEUROSCI.2087-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuma F, Hayano J. Respiratory sinus arrhythmia: why does the heartbeat synchronize with respiratory rhythm? Chest. 2004;125(2):683–690. doi: 10.1378/chest.125.2.683. http://dx.doi.org/10.1378/chest.125.2.683. [DOI] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49(3):2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. doi:10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler G, Dahnke R, Yeragani VK, Bar KJ. The relation of ventromedial prefrontal cortex activity and heart rate fluctuations at rest. European Journal of Neuroscience. 2009;30(11):2205–2210. doi: 10.1111/j.1460-9568.2009.07008.x. doi: http://dx.doi.org/10.1111/j.1460-9568.2009.07008.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.