Abstract
Background
We investigated the effect of grape seed proanthocyanidins (GSPs) on carbon tetrachloride (CCl4)-induced acute liver injury.
Material/Methods
Sixty SPF KM mice were randomly divided into 6 groups: the control group, CCl4-model group, bifendate group (DDB group), and low-, moderate-, and high-dose GSP groups. The following parameters were measured: serum levels of alanine aminotransferase (ALT); aspartate aminotransferase (AST); tumor necrosis factor (TNF)-α; interleukin-6 (IL-6); high-mobility group box (HMGB)-1; body weight; liver, spleen, and thymus indexes; superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activity; HMGB1 mRNA; malondialdehyde (MDA) content; hepatocyte proliferation; and changes in liver histology.
Results
Compared to the CCl4-model group, decreases in liver index and increases in thymus index significantly increased SOD and GSH-Px activities and reduced MDA content, and higher hepatocyte proliferative activity was found in all GSP dose groups and the DDB group (all P<0.001). Compared with the CCl4-model group, serum TNF-α and IL-6 levels and HMGB 1 mRNA and protein expressions decreased significantly in the high GSP dose group (all P<0.05).
Conclusions
Our results provide strong evidence that administration of GSPs might confer significant protection against CCl4-induced acute liver injury in mice.
MeSH Keywords: Aspartate Aminotransferase, Cytoplasmic; Drug-Induced Liver Injury; Glutathione Peroxidase
Background
Acute liver injury is caused by multiple factors, such as hepatic viruses, excessive alcohol consumption and hepatotoxins (for example, paracetamol, flutamide and carbon tetrachloride), and leads to down-regulation of enzymes that influence drug pharmacokinetics [1,2]. Carbon tetrachloride (CCl4) is an industrial solvent and one of the most potent inducers of acute liver injury, often used in animal studies to replicate human liver injury model [3]. CCl4-induced toxicity depends on the dose and duration of exposure. At low CCl4 doses, transient effects occur, including the loss of Ca2+ sequestration, impaired lipid homeostasis and release of several cytokines. Longer CCl4 exposures alter fatty acid metabolism, and induce fibrosis, cirrhosis and cancer [4]. CCl4 induced hepatotoxicity is the result of reductive dehalogenation reactions catalyzed by hepatic cytochrome P-450, forming unstable trichloromethyl and trichloromethyl peroxyl radicals capable of binding to proteins or lipids, initiating lipid peroxidation and liver damage [5]. Depending on the specific context, treatment of liver diseases involves antiviral drugs, vaccines, N-acetylcysteine, melatonin, 5-aminoisoquinolinone as well as bone marrow stromal stem cells [6–9]. However, these treatments carry adverse side effects, thus safer and more effective drugs are in urgent need [10]. In recent years, focus has shifted towards traditional Chinese medicinal products, such as lignins extracted from Schisandra chinensis, polyphenols in green tea with epigallocatechin-3-gallate and apple polyphenols, for their efficacy and high safety profile in treatment of liver diseases [10–12].
Proanthocyanidins occur naturally in fruits, vegetables, seeds and flowers, and are the major polyphenols present in red wine and grape seeds, with an already wide use as nutritional supplements [13]. Proanthocyanidins improve anti-inflammatory, antioxidant, and anti-carcinogenic activities in human [14]. In particular, grape seed proanthocyanidins (GSPs) mostly contain epicatechin and catechin, and their gallic acid esters [15]. GSPs contain multiple hydroxyl groups, which are important for the beneficial pharmacological and therapeutic properties of GSPs against oxygen free radicals and oxidative stress [16]. GSPs also promote production of nitric oxide (NO), an important signaling molecule involved in multiple physiological pathways, such as regulation of blood pressure and anti-inflammatory mechanisms [17]. Several other beneficial functions of GSPs have been demonstrated by multiple studies in the literature. GSPs play a protective role in high fructose diet induced insulin resistance and in maintaining healthy myocardial activity [15,18]. GSPs have also been demonstrated to be effective anticancer agents in various human cancers, such as colorectal cancer, non-small cell lung cancer, head and neck squamous cancer, cervical cancer and skin cancer [19–23]. Furthermore, GSPs alleviate oxidative damage and inflammatory cytokines release, and promote hypoxia tolerance in hepatic steatosis [17]. Surprisingly, there were few published studies reporting the potential use of GSPs in CCl4-induced acute liver injury. Dai et al. mainly studied the antioxidant properties of proanthocyanidins in reducing CCl4-induced steatosis and liver injury in rats through regulation of CYP2E1 enzyme [24]. However, our study aimed to focus on the effects of GSPs in acute liver injury in the aspect of anti-inflammation, anti-oxidation and anti- lipid peroxidation. To address this issue, our study investigated the experimental outcomes of administration of GSPs in CCl4-induced acute liver injury mouse model, with an aim of providing a novel approach for safe and effective therapy for acute chemical induced hepatic injury in humans.
Material and Methods
Subjects and grouping
Sixty SPE KM mice (average weight, 20±2 g) were obtained from the Laboratory animal center of Liaoning Medical University. Mice were randomly allocated into 6 groups, with 10 mice in each group. The 6 groups were: control group, CCl4-model group, low GSP dose group (50 mg/kg), moderate GSP dose group (250 mg/kg), high GSP dose group (500 mg/kg) and bifendate group (DDB group) (150 mg/kg). CCl4 was purchased from Shanghai Reagent Factory and DDB from Beijing Union Pharmaceutical Factory. This study was conducted in strict adherence with the procedures mentioned in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The ethics committee of laboratory animal center of Liaoning Medical University approved the study protocol, and was carried out in accordance with the Declaration of Helsinki [25].
Source of GSPs
GSPs extracted from red grape seeds were obtained from Jianfeng Natural Products R&D Inc. (Tianjin, China). Chemical composition of GSPs was analyzed by high-performance liquid chromatography (HPLC) and the results showed 89.79% total proanthocyanidins, 3.20% total monomeric flavonols and other ingredients.
CCl4 induced acute liver injury
Bean oil (0.2 ml/10 g body weight) was administered to the mice in the control group and the mice in other experimental groups were given 2% CCl4 dissolved in plant oil (0.2ml/10g) by gavage every morning. The administered dose of CCl4 is well-known in the literature to induce characteristic liver injury in mice that functionally resembles the acute liver injury observed in humans [26] (http://d.wanfangdata.com.cn/Periodical_xjykdxxb200711006.aspx). Three hours after CCl4 administration, the low dose, moderate dose and high GSP dose groups were given GSPs by gavage suspended in distilled water at 50, 250, 500 mg/kg body weight, respectively. The dose of GSPs used in this study was selected on the basis of previous studies [27]. The DDB group was given DDB at 150 mg/kg body weight by gavage [28], while the control group and CCl4-model group were given same volume of saline. As per standard protocols, the experimental procedures were continued for 10 days until significantly elevated serum alanine aminotransferase levels were detected [26].
Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), tumor necrosis factor (TNF)-α, interleukin-6 (IL-6), high-mobility group box (HMGB)-1 levels measurement
Blood samples were collected from eye sockets of mice 8 hours after the last drug administration. Serum levels of ALT and AST were used as biomarkers for hepatic damage. ALT and AST levels were determined based on the Reitman-Frankel method using commercially available assay kits (ProDia international, Germany) [29]. ELISA was used to detect serum levels of tumor necrosis factor (TNF)-α, interleukin-6 (IL-6) and high-mobility group box (HMGB)-1. TNF-α and IL-6 kits were obtained from Neobioscience Technology company (Shenzhen, China) and HMGB-l kit was from Elabscience Biotechnology Co., Ltd (Wuhan, China).
Organ index measurement
Mice were weighed 10 h after the last drug administration. Liver, spleen and thymus were removed and the residual blood was rinsed-off with 0.9% sodium chloride solution. After the tissues were dried and weighed, the liver, spleen and thymus index was measured. Liver index=liver weight (g)/mice weight (g) ×100%; spleen index=spleen weight (g)/mice weight (g) ×100%; thymus index=thymus weight (g)/mice weight (g) ×100%.
Liver homogenate preparation
Animals were sacrificed by cervical dislocation and immediately dissected. The root of the hepatic portal vein was clamped, and vessels and ligaments were cut. The operation was handled carefully to avoid liver damage. The liver was taken out and rinsed with saline pre-cooled to 4°C, and the organ was dried with filter paper and weighed after removing adipose and connective tissue. Approximately 1 g of liver tissue was placed into a 20 ml beaker and sliced into small pieces with eye scissors. A 2/3rd volume of saline was transferred into the beaker with a pipette (the total volume of saline at this point was 9 times the weight of the tissue, i.e., 9 ml ice-cold saline was added for 1 g liver tissue). Liver homogenates were obtained by high-speed tissue homogenization using FJ-200S (Shanghai Specimen and Model Factory) at 3000 r/min in an ice bath (10s each time for 3–5 times and with a brief interval of 30 s). The homogenates were centrifuged at 3000 r/min for 15min at 4°C to obtain the supernatants.
Superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) activity and malondialdehyde (MDA) determinations
The supernatants from liver homogenates were used for measurement of SOD and GSH-Px activities, and the MDA content. SOD activity was measured by xanthine oxidase method. GSH-Px activity was measured by 5, 5′-dithiobis-2-nitrobenzoic acid (DTNB) method. MDA was measured using thiobarbituric acid (TBA) colorimetric assay kit. All assay reagents were purchased from Nanjing Jiancheng Bioengineering Institute, Jiangsu, China.
RT-PCR detection
Total RNA of mice livers were extracted using Trizol reagent (Invitrogen, USA) following manufacturer’s instructions. We used 12–18 oligo (dT) primer to obtain cDNA from 20 μl of total RNA sample. HMGB1 fragments were amplified using cDNA as a template. HMGB1 amplification conditions were as follows: 95°C initial denaturation for 12 s, 40 cycles of 95°C denaturation for 30 s, 58 °C annealing for 30 s, 72 °C extension for 45 min, followed by 72°C extension for 12 min. PCR products conducted 1.2% agarose gel electrophoresis and BIO-Rad gel electrophoresis imaging system was used to analyze the area and absorbance value of each electrophoresis band. The β-actin was acted as an internal control. The relative expression level of HMGB-1 was calculated by the ratio of HMGB-1and β-actin (Table 1).
Table 1.
Primer sequences and amplification lengths for HMGB1 and β-actin.
| Primer sequence | Amplification length | |
|---|---|---|
| HMGB1 | F: 5′-GATGGG CAAAGGAGATCCTAAG-3′ | 226bp |
| R: 5′-TCACTTTTTTGTCTC CCCTTTGGG-3′ | ||
| β-actin | F: 5′-CTGTCCCTGTATGCCTCT G-3′ | 289bp |
| R: 5′-CATCGTACTCCTGCTTGCT-3′ |
HMGB1 – high-mobility group box-1; F – forward; R – reverse; bp – base pair.
MTT assay to detect hepatocyte proliferation
Freshly isolated hepatocytes were adjusted to 1×106/ml cell density in 10% FBS RPMI-1640 medium. Trypan blue staining to detect live hepatocytes showed more than 95% cell viability. The suspended hepatocytes were seeded into 96-well plates by distributing 100 μl into each well. Each experimental group had five parallel samples and the cells were incubated at 37°C and 5% CO2 under standard cell culture conditions. MTT solution (5 mg/ml) was added into the 96-well plates with 20 μl per well and incubated for 4 h. After the supernatant was discarded, 100 μl dimethyl sulfoxide was added to each well to dissolve blue-purple formazan crystals. The absorbance of each sample was measured by reading the results at 492 nm wavelength using a microplate reader. The absorbance detected can be used to represent the proliferation activity.
Histopathology examination
A small slice of tissue from left lobes of liver from each mouse was taken and fixed with 10% formaldehyde solution and embedded in paraffin wax. Thin sections of the embedded tissue were cut and hematoxylin-eosin stained for histopathological examinations. Images were captured under optical microscope (Olympus BH2, Japan) using Nikon coolpix990 camera at 250× magnification.
Statistical analysis
SPSS 20.0 statistical software was used for data analysis. Measurement data were presented as mean ± standard deviation. The t test was applied to compare groups and q test compared the differences among multiple groups. A P<0.05 was considered as statistically significant.
Results
Serum ALT and AST levels
CCl4-induced acute liver injury model was successfully established, as confirmed by elevated serum ALT and AST levels detected in the experimental groups, compared with the control group, following oral administration of 2% CCl4, (all P<0.01). Serum AST levels in the low, moderate and high GSP dose group were remarkably lower than the CCl4-model group (all P<0.05) and showed a dose-dependent lowering in response to the increasing dose of GSPs, indicating the protective role of GSPs in CCl4-induced acute liver injury. No significant difference in AST level was found between the GSP groups and the DDB group (all P>0.05). Serum ALT levels in the moderate and high GSP dose groups were lower than CCl4-model group (all P<0.05), while the serum ALT level in the low GSP dose group showed no statistical differences compared to CCl4-model group (P>0.05). A similar dose-dependent lowering in serum ALT levels was also observed across the three GSP dose groups, which further confirmed the beneficial role of GSPs in CCl4-induced acute liver injury. In comparison with the DDB group, serum ALT levels were significantly higher in the low and moderate GSP dose group (both P<0.05), while it showed no difference in the high GSP dose group (P>0.05) (Table 2).
Table 2.
Serum ALT and AST levels in the control group, the CCl4-model group, the low, middle, GSP dose group and the DDB group (±s).
| Group | n | AST (U/L) | ALT (U/L) |
|---|---|---|---|
| Control group | 10 | 26.27±6.85 | 27.56±14.72 |
| CCl4-model group | 10 | 143.66±7.41# | 121.34±11.78# |
| Low GSP dose group | 10 | 85.91±6.63#,* | 113.46±12.14# |
| Moderate GSP dose group | 10 | 73.78±6.15#,*,$ | 98.25±16.47#,*,$ |
| High GSP dose group | 10 | 61.62±8.28#,*,$,& | 68.46±12.78#,*,$,& |
| DDB group | 10 | 60.32±6.19#,* | 63.56±14.32#,*,$,& |
CCl4 – carbon tetrachloride; GSP – grape seed proanthocyanidins; DDB – bifendate; ALT – alanine aminotransferase; AST – aspartate aminotransferase;
P<0.01,compared with the control group;
P<0.05, compared with the CCl4-model group;
P<0.05, compared with the low GSP dose group;
P<0.05, compared with the moderate GSP dose group.
Serum TNF-α and IL-6 levels
Compared with the control group, serum TNF-α and IL-6 levels were significantly increased in other three groups (all P<0.05). Serum TNF-α and IL-6 levels in the high GSP dose group and the control group were lower than those in the model group (both P<0.05), significant difference in serum TNF-α level was found between the high GSP dose group and the SAA group (P<0.05), but no significant difference in serum IL-6 level was found between the two groups (P>0.05) (Table 3).
Table 3.
Serum TNF-α and IL-6 levels in the control group, the CCl4-model group, the low, middle, high GSP dose group and the DDB group (n=10).
| Group | TNF-α (pg/mL) | IL-6 (pg/mL) |
|---|---|---|
| Control group | 2132.26±258.69 | 32.58±7.58 |
| CCl4-model group | 6315.26±742.54# | 215.58±24.68# |
| High GSP dose group | 3414.25±419.6#,* | 126.40±54.68#,* |
| DDB group | 3155.6±322.6#,*,$ | 116.24±34.93#,* |
TNF-α – tumor necrosis factor-α; IL-6 – interleukin-6; GSP – grape seed proanthocyanidin; DDB – bifendate;
compared with the control group, P<0.05;
compared with the model group, P<0.05;
compared with the GSP group, P<0.05.
Body weight of mice
Initial weight and final weight of mice at 10 days of CCl4 administration was measured in the six groups. Variance analysis showed no significant differences in weights among the six groups (all P>0.05). Mice in each group showed normal behavior and no adverse reactions were observed (Table 4), indicating the status of health of the mice within the duration of the experiment.
Table 4.
Weight gain of the mice after 10 days of CCl4 treatment (±s).
| Group | n | Initial weight (g) | Final weight (g) | Gain in weight (g) |
|---|---|---|---|---|
| Control group | 10 | 23.10±2.78 | 31.80±3.32 | 8.70±0.54 |
| CCl4-model group | 10 | 24.10±2.69 | 33.10±2.65 | 9.00±0.04 |
| Low GSP dose group | 10 | 24.20±2.70 | 33.10±3.14 | 8.90±0.44 |
| Moderate GSP dose group | 10 | 24.30±2.80 | 33.3±2.81 | 9.00±0.01 |
| High GSP dose group | 10 | 23.50±2.74 | 32.4±2.92 | 8.90±0.18 |
| DDB group | 10 | 23.00±1.70 | 32.10±2.05 | 9.10±0.35 |
| F | 0.287 | |||
| P | 1.278 |
CCl4 – carbon tetrachloride; GSP – grape seed proanthocyanidins; DDB – bifendate.
Liver, spleen and thymus indexes of mice
Compared with the control group, significantly increased liver index and decreased thymus index was found in CCl4-model group (both P<0.05), while the liver index and thymus index showed no significant differences within the GSP dose groups and the DDB group (P>0.05). In comparison with the CCl4-model group, a remarkably decreased liver index and increased thymus index was found in all the GSP dose groups and the DDB group (all P<0.001), indicating the protective role of GSPs. However, no difference in spleen index was found among the six groups (all P>0.05) (Table 5).
Table 5.
Comparison of liver, splenic and thymus index in the control group, the CCl4-model group, the low, middle and high GSP dose group and the DDB group (±s).
| Group | n | Liver index (×10−3) | Splenic index (×10−3) | Thymus index (×10−3) |
|---|---|---|---|---|
| Control group | 10 | 55.96±6.41 | 3.45±0.77 | 4.78±0.66 |
| CCl4-model group | 10 | 64.13±5.01* | 3.83±0.83 | 3.03±0.47* |
| Low GSP dose group | 10 | 53.32±3.71# | 3.80±0.65 | 4.35±0.28# |
| Moderate dose GSP | 10 | 54.97±5.65# | 3.72±0.63 | 4.55±0.28# |
| High GSP dose group | 10 | 55.34±3.94# | 3.52±0.59 | 4.53±0.28# |
| DDB group | 10 | 55.43±3.94# | 3.50±0.65 | 4.50±0.19# |
CCl4 – carbon tetrachloride; GSP – grape seed proanthocyanidins; DDB – bifendate;
P<0.05 compared with the control group;
P<0.01 compared with the CCl4-model group.
SOD, GSH-Px activities and MDA content in liver tissue
In comparison with the control group, decreased SOD and GSH-Px activity, and increased MDA content was found in the five experimental groups (all P<0.01). On the other hand, in comparison with CCl4-model group, the SOD and GSH-Px activities were significantly increased, and MDA content were significantly decreased in all the GSP dose groups and the DDB group (all P<0.05). A clear dose-dependent increase in SOD activity, and decrease in MDA content was observed with increasing GSP dose (all P<0.05), while GSH-Px activity showed a significant increase only in the high GSP dose group (both P<0.05), and not in the low and moderate GSP dose groups (P>0.05). Interestingly, higher SOD activity was observed in DDB group compared to the low GSP dose group (P<0.05), but no such differences were found between the moderate and high GSP dose groups and the DDB group (all P>0.05). GSH-Px activity in the DDB group was also higher than the low and moderate GSP dose groups (both P<0.05), but its activity was comparable with high GSP dose group (P>0.05). The MDA content in the DDB group was lower than low and moderate GSP dose groups (both P<0.05), but no such difference was found between the DDB group and the high GSP dose group (P>0.05) (Table 6).
Table 6.
SOD and GSH-Px activities and MDA content in liver tissue (±s).
| Group | n | SOD (U/mg pro) | MDA (U/mg pro) | GSH-Px (U/mg pro) |
|---|---|---|---|---|
| Control group | 10 | 267.43±33.52 | 3.74±1.74 | 203.92±35.01 |
| CCl4-model group | 10 | 86.51±25.25# | 13.56±2.71# | 110.69±23.26# |
| Low GSP dose group | 10 | 134.74±44.82#,* | 10.73±1.41#,* | 140.29±25.41#,* |
| Moderate dose GSP group | 10 | 195.68±34.88#,*,$ | 9.03±1.64#,*,$ | 141.96±23.13#,* |
| High GSP dose group | 10 | 228.14±21.06#,*,$,& | 6.33±1.07#,*,$,& | 174.38±28.34#,*,$,& |
| DDB group | 10 | 213.47±25.12#,*,$ | 5.98±1.58#,*,$,& | 175.08±24.73#,*,$,& |
CCl4 – carbon tetrachloride; GSP – grape seed proanthocyanidins; DDB – bifendate; SOD – Superoxide dismutase; MDA – malondialdehyde; GSH-Px – glutathione peroxidase; U/mg pro – nmol min−1mg−1protein;
P<0.01, compared with the control group;
P<0.05, compared with the CCl4-model group;
P<0.05, compared with the low GSP dose group;
P<0.05, compared with the moderate GSP dose group.
HMGB 1 mRNA and protein expression
Compared with the control group, HMGB 1 mRNA and protein expression increased significantly in the model group (both P<0.05); compared with the model group, HMGB 1 mRNA and protein expression decreased significantly in the high GSP dose group (both P<0.05), while no differences in HMGB 1 mRNA expression and protein was found between the high GSP dose group and SAA group (both P>0.05) (Table 7).
Table 7.
HMGB 1 mRNA and protein in the control group, the CCl4-model group, high GSP dose group and the DDB group (n=10).
| Group | HMGB 1 mRNA | HMGB 1 protein (pg/mL) |
|---|---|---|
| Control group | 0.62±0.09 | 4.11±0.74 |
| CCl4-model group | 1.38±0.31# | 32.26±12.15# |
| High GSP dose group | 0.93±0.12#,* | 16.40±4.67#,* |
| DDB group | 0.94±0.13#,* | 15.24±4.23#,* |
HMGB 1 – high-mobility group box-1; CCl4 – carbon tetrachloride; GSP – grape seed proanthocyanidin; DDB – bifendate;
compared with the control group, P<0.05;
compared with the model group, P<0.05.
Hepatocyte proliferative activity
Hepatocyte proliferative activities in the control group, the CCl4-model group, the low, moderate and high GSP dose groups and the DDB group and positive control group DDB were 0.399±0.019, 0.293±0.011, 0.300±0.008, 0.337±0.025, 0.357±0.010 and 0.351±0.009, respectively. Compared with the control group, a significantly reduced hepatocyte proliferation was observed in the five experimental groups (all P<0.01). Compared with the CCl4-model group, all GSP dose groups and DDB group showed significantly higher hepatocyte proliferation activity (all P<0.05). Hepatocyte proliferative activity increased with increasing GSP in a dose-dependent manner. The hepatocyte proliferative activity in DDB group was higher than the low and moderate GSP dose groups (both P<0.05), but showed no significant difference with high GSP dose group (P>0.05).
Histopathological observations
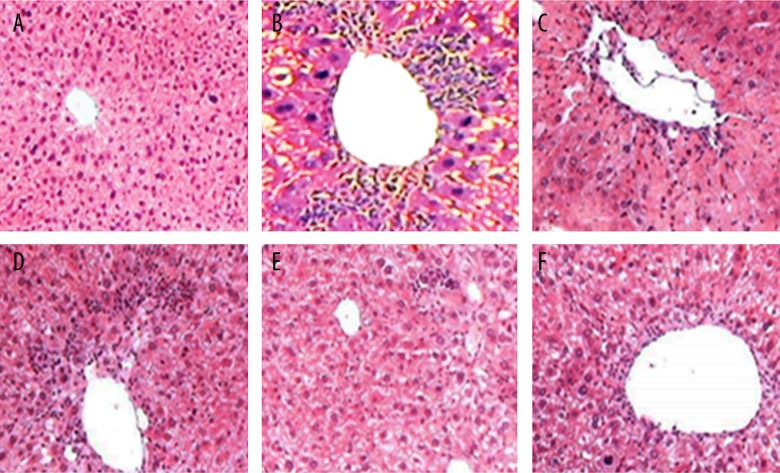
Histological assessment of liver tissue was used to verify the protective role of GSP in CCl4 induced acute liver injury. Control mice showed normal tissue structure with complete hepatic lobules, orderly arranged hepatic cords, with no abnormality in hepatic sinusoid and portal area, no inflammatory cell infiltration and normal hepatocyte morphology (Figure 1A). In the CCl4-model group, swollen hepatocytes, cytoplasmic relaxation, dark stained nuclei and obvious punctate or focal necrosis were observed, and neutrophil and lymphocyte infiltration was found in portal area (Figure 1B). Compared with the CCl4-model group, reduced tissue damage and decreased punctate and focal necrosis was observed in the low GSP dose group and the DDP group (Figure 1C, 1D). Importantly, the tissue structure appeared normal in the moderate GSP dose group and the high GSP dose group, with rare necrotic hepatocytes and markedly decreased infiltration of inflammatory cell types. (Figure 1E, 1F).
Figure 1.
Representative images of histological examination results of mice liver tissues stained with hematoxylin and eosin (×250) (A the control Group; B the CCl4-model group; C the low GSP dose group; D the DDB group; E the moderate GSP dose group; F the high GSP dose group). Note: CCl4 – carbon tetrachloride; GSP – grape seed proanthocyanidins; DDB – bifendate.
Discussion
CCl4-induced liver injury simulates acute liver injury in humans and is used as an experimental model to investigate hepatoprotective role of natural products and drugs [10]. Epigallocatechin-3-gallate from green tea significantly reduces the severity of CCl4-induced liver injury and prevents progression of liver fibrosis by decreasing oxidative stress and pro-inflammatory responses [12]. Dioscin also protects against chemically induced acute liver injury due to its inhibitory effects on lipid peroxidation, necrosis and apoptosis, and the suppression of inflammatory cytokine production [11]. Schisandra chinensis pollen extract protects against acute hepatotoxicity by strongly increasing antioxidant activity and inhibiting lipid peroxidation [5]. Our study investigated the protective effect of GSPs in CCl4 induced acute liver injury model in mice. A previous study showed that GSPs protect against reperfusion-induced injury in cardiomyocytes via reducing or scavenging free radicals [30]. GSPs have remarkable beneficial effects in cancers. In head and neck squamous cell carcinoma, GSPs down-regulated epidermal growth factor receptor expression, suppressed NF-kB activation and inhibited epithelial-to-mesenchymal transition [31]. Further, GSPs showed a significant therapeutic potential in preventing pancreatic beta-cell death in type 2 diabetes mellitus by regulating endoplasmic reticulum stress [13]. Therefore, GSPs appear to have extraordinarily beneficial activities in diverse disease settings.
In this study, we investigated the potential of GSPs to protect against chemically induced liver injury. In order to achieve this, CCl4 induced acute liver injury model in mice was successfully established, as shown by the elevated serum ALT and AST levels after CCl4 treatment [32]. Histopathological observations showing swollen hepatocyte morphology, cytoplasmic relaxation, dark stained nuclei, punctate or focal necrosis, and neutrophil and lymphocyte infiltration in the CCl4-model group further confirmed that the liver injury was successfully induced by CCl4. Serum AST and ALT levels decreased in the GSP groups compared with the CCl4-model group, indicating the active protective effects of daily doses of GSPs. The protective effects of GSPs were further confirmed by significant reductions in liver tissue damage and decreased punctate and focal necrosis in the GSP groups. MTT assay to detect hepatocyte proliferation also showed that compared to the CCl4-model group, all GSP dose groups and DDB group showed significantly higher hepatocyte proliferative activity, which increased in a dose-dependent manner with increasing GSP dose. Consistent with our results, Zhong et al. found that GSPs inhibit hepatocyte damage induced by ethanol and CCl4, and stimulate normal hepatocyte proliferation [33].
Compared with the control group, serum TNF-α and IL-6 levels were significantly increased in other three groups. Serum TNF-α and IL-6 levels in the GSP group were lower than those in the model group, suggesting the anti-inflammatory role of GSPs on the ALI. TNF-α was identified as an endogenous pyrogen and a key component of an inflammatory response and one of the most potent pro-inflammatory cytokines released by innate immune cells [34]. IL-6 is secreted by the white blood cells, such as macrophages and endothelial cells and any inflammatory stimulus can increase the IL-6 concentrations [35].
Interestingly, apple polyphenols prevented increase in body weight [10]. However, we did not detect any significant changes in mouse body weight within the experimental period in our study after CCl4 and GSP treatments. Compared with the control group, a significantly increased liver index was observed in the CCl4-model group, with swollen and deep colored liver, indicating liver injury. Compared to the CCl4-model group, a remarkable decreased in liver index and increased thymus index were found in all the GSP groups. GSPs also displayed protective effects in organs important in immune function through its beneficial effects on the thymus. Thymus is an important organ that facilitates the maturation of T lymphocytes, macrophages and lymphocytes in preparation for their critical roles in immune system [36]. We did not observe differences in spleen index among the six groups.
Our study also obtained preliminary evidence of the potential mechanisms involved in the protection conferred by GSPs against the liver injury induced by CCl4. Our result showed significantly elevated SOD and GSH-Px activities and decreased MDA content after administration of GSPs. Liver contains high endogenous expression of various antioxidant enzymes, such as SOD and GSH-Px, which are the primary defense mechanisms against reactive oxygen species [37]. SOD removes superoxides and protects cells from oxidative damage [38], and GSH-Px protects the structural integrity of cell membranes [39]. GSPs increased SOD and GSH-Px activities after CCl4 treatment, suggesting that GSPs might augment the expression of antioxidant enzymes and increase the antioxidant capacity. Increased MDA content in cells leads to the deactivation of many lipids and proteins, which may further lead to DNA damage [40]. By down-regulating MDA, GSPs prevents lipid peroxidation, which is the main cause of CCl4-induced liver injury.
Another result of our study showed HMGB 1 mRNA and protein expression increased significantly in the model group compared with the control group, suggesting the cell inflammation caused by D-Gal in mice livers; compared with the model group, HMGB 1 mRNA and protein expression decreased significantly in the GSP group, further indicating the anti-inflammatory role of GSPs on the ALI. HMGB1 is a highly conserved non-histone nuclear protein and constitutively expressed by nucleated cells and facilitates gene transcription, repair and recombination, and the biological activity of the HMGB1 depends on its location and post-translational modification [41]. Intracellular HMGB1 binds DNA and modulates chromosomal architecture; and once cells were activated and injured, HMGB1 can translocate outside of the cells [42]. Once released from the cell, HMGB1 binds to the transmembrane receptors, cytokines, and other exogenous molecules, and is known as one of the key endogenous damage-associated molecular pattern molecules and can trigger an array of inflammatory responses [43]. HMGB1 is an important pro-inflammatory molecule and has been reported to play a role in response to pro-inflammatory diseases such as acute lung injury, epithelial barrier dysfunction and arthritis in mice and arthritis, sepsis, disseminated intravascular coagulation, acute liver failure, acute appendicitis and other inflammatory disorders in human [44].
Conclusions
Our results showed that GSP protects against acute oxidative hepatotoxicity induced by CCl4 in mice. We also obtained preliminary evidence that this protective effect of GSPs may mainly involve free radical scavenging, preserving the organs important for immune function, and increasing the antioxidant capacity and inhibition of lipid peroxidation. However, the obtained GSPs in our study contain other ingredients, such as monomeric flavonols, which might also affect the injured liver. Thus, we need to exclude their effects in our further studies. Taken together, our results show that GSPs are an abundant source of polyphenolic antioxidants and they are safe and effective for treatment of acute chemically-induced liver injury.
Acknowledgements
We would like to thank our researchers for their hard work and reviewers for their valuable advice.
Footnotes
Source of support: Departmental sources
Competing interests
All authors in our study have no conflict of interest.
References
- 1.Xie Y, Hao H, Kang A, et al. Integral pharmacokinetics of multiple lignan components in normal, CCl4-induced hepatic injury and hepatoprotective agents pretreated rats and correlations with hepatic injury biomarkers. J Ethnopharmacol. 2010;131:290–99. doi: 10.1016/j.jep.2010.06.038. [DOI] [PubMed] [Google Scholar]
- 2.Manso G, Thole Z, Salgueiro E, et al. Spontaneous reporting of hepatotoxicity associated with antiandrogens: data from the Spanish pharmacovigilance system. Pharmacoepidemiol Drug Saf. 2006;15:253–59. doi: 10.1002/pds.1168. [DOI] [PubMed] [Google Scholar]
- 3.Chen M, Huang W, Wang C, et al. High-mobility group box 1 exacerbates CCl(4)-induced acute liver injury in mice. Clin Immunol. 2014;153:56–63. doi: 10.1016/j.clim.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Cui Y, Yang X, Lu X, et al. Protective effects of polyphenols-enriched extract from Huangshan Maofeng green tea against CCl4-induced liver injury in mice. Chem Biol Interact. 2014;220:75–83. doi: 10.1016/j.cbi.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Cheng N, Ren N, Gao H, et al. Antioxidant and hepatoprotective effects of Schisandra chinensis pollen extract on CCl4-induced acute liver damage in mice. Food Chem Toxicol. 2013;55:234–40. doi: 10.1016/j.fct.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Sheikh IA, Luksic M, Ferstenberg R, et al. Spice/K2 synthetic marijuana-induced toxic hepatitis treated with N-acetylcysteine. Am J Case Rep. 2014;15:584–88. doi: 10.12659/AJCR.891399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colak C, Parlakpinar H, Ozer MK, et al. Investigating the protective effect of melatonin on liver injury related to myocardial ischemia-reperfusion. Med Sci Monit. 2007;13(11):BR251–54. [PubMed] [Google Scholar]
- 8.Mota-Filipe H, Sepodes B, McDonald MC, et al. The novel PARP inhibitor 5-aminoisoquinolinone reduces the liver injury caused by ischemia and reperfusion in the rat. Med Sci Monit. 2002;8(11):BR444–53. [PubMed] [Google Scholar]
- 9.Chen XW, Zhu DJ, Ju YL, Zhou SF. Therapeutic effect of transplanting magnetically labeled bone marrow stromal stem cells in a liver injury rat model with 70%-hepatectomy. Med Sci Monit. 2012;18(10):BR375–82. doi: 10.12659/MSM.883476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Li Y, Wang F, Wu C. Hepatoprotective effects of apple polyphenols on CCl4-induced acute liver damage in mice. J Agric Food Chem. 2010;58:6525–31. doi: 10.1021/jf903070a. [DOI] [PubMed] [Google Scholar]
- 11.Lu B, Xu Y, Xu L, et al. Mechanism investigation of dioscin against CCl4-induced acute liver damage in mice. Environ Toxicol Pharmacol. 2012;34:127–35. doi: 10.1016/j.etap.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 12.Tipoe GL, Leung TM, Liong EC, et al. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology. 2010;273:45–52. doi: 10.1016/j.tox.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Ding Y, Zhang Z, Dai X, et al. Grape seed proanthocyanidins ameliorate pancreatic beta-cell dysfunction and death in low-dose streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats partially by regulating endoplasmic reticulum stress. Nutr Metab (Lond) 2013;10:51. doi: 10.1186/1743-7075-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou M, Wei L, Sun Z, et al. Production and transcriptional regulation of proanthocyanidin biosynthesis in forage legumes. Appl Microbiol Biotechnol. 2015;99:3797–806. doi: 10.1007/s00253-015-6533-1. [DOI] [PubMed] [Google Scholar]
- 15.Wang XH, Huang LL, Yu TT, et al. Effects of oligomeric grape seed proanthocyanidins on heart, aorta, kidney in DOCA-salt mice: role of oxidative stress. Phytother Res. 2013;27:869–76. doi: 10.1002/ptr.4793. [DOI] [PubMed] [Google Scholar]
- 16.Ding Y, Dai X, Jiang Y, et al. Functional and morphological effects of grape seed proanthocyanidins on peripheral neuropathy in rats with type 2 diabetes mellitus. Phytother Res. 2014;28:1082–87. doi: 10.1002/ptr.5104. [DOI] [PubMed] [Google Scholar]
- 17.Song X, Xu H, Feng Y, et al. Protective effect of grape seed proanthocyanidins against liver ischemic reperfusion injury: particularly in diet-induced obese mice. Int J Biol Sci. 2012;8:1345–62. doi: 10.7150/ijbs.4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yogalakshmi B, Bhuvaneswari S, Sreeja S, Anuradha CV. Grape seed proanthocyanidins and metformin act by different mechanisms to promote insulin signaling in rats fed high calorie diet. J Cell Commun Signal. 2014;8:13–22. doi: 10.1007/s12079-013-0210-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nandakumar V, Singh T, Katiyar SK. Multi-targeted prevention and therapy of cancer by proanthocyanidins. Cancer Lett. 2008;269:378–87. doi: 10.1016/j.canlet.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaur M, Tyagi A, Singh RP, et al. Grape seed extract upregulates p21 (Cip1) through redox-mediated activation of ERK1/2 and posttranscriptional regulation leading to cell cycle arrest in colon carcinoma HT29 cells. Mol Carcinog. 2011;50:553–62. doi: 10.1002/mc.20739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma SD, Meeran SM, Katiyar SK. Proanthocyanidins inhibit in vitro and in vivo growth of human non-small cell lung cancer cells by inhibiting the prostaglandin E(2) and prostaglandin E(2) receptors. Mol Cancer Ther. 2010;9:569–80. doi: 10.1158/1535-7163.MCT-09-0638. [DOI] [PubMed] [Google Scholar]
- 22.Prasad R, Katiyar SK. Bioactive phytochemical proanthocyanidins inhibit growth of head and neck squamous cell carcinoma cells by targeting multiple signaling molecules. PLoS One. 2012;7:e46404. doi: 10.1371/journal.pone.0046404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaid M, Prasad R, Singh T, et al. Grape seed proanthocyanidins reactivate silenced tumor suppressor genes in human skin cancer cells by targeting epigenetic regulators. Toxicol Appl Pharmacol. 2012;263:122–30. doi: 10.1016/j.taap.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai N, Zou Y, Zhu L, et al. Antioxidant properties of proanthocyanidins attenuate carbon tetrachloride (CCl4)-induced steatosis and liver injury in rats via CYP2E1 regulation. J Med Food. 2014;17:663–69. doi: 10.1089/jmf.2013.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MPN World Medical Association publishes the Revised Declaration of Helsinki. Natl Med J India. 2014;27:56. [PubMed] [Google Scholar]
- 26.Kim HY, Kim JK, Choi JH, et al. Hepatoprotective effect of pinoresinol on carbon tetrachloride-induced hepatic damage in mice. J Pharmacol Sci. 2010;112:105–12. doi: 10.1254/jphs.09234fp. [DOI] [PubMed] [Google Scholar]
- 27.Zhao YM, Gao LP, Zhang HL, et al. Grape seed proanthocyanidin extract prevents DDP-induced testicular toxicity in rats. Food Funct. 2014;5:605–11. doi: 10.1039/c3fo60486a. [DOI] [PubMed] [Google Scholar]
- 28.Ye S, Li X, Shao Y. Protective effect of purple sweet potato flavonoids on CCL4-induced acute liver injury in mice. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2013;42:649–53. doi: 10.3785/j.issn.1008-9292.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 30.Pataki T, Bak I, Kovacs P, et al. Grape seed proanthocyanidins improved cardiac recovery during reperfusion after ischemia in isolated rat hearts. Am J Clin Nutr. 2002;75:894–99. doi: 10.1093/ajcn/75.5.894. [DOI] [PubMed] [Google Scholar]
- 31.Sun Q, Prasad R, Rosenthal E, Katiyar SK. Grape seed proanthocyanidins inhibit the invasiveness of human HNSCC cells by targeting EGFR and reversing the epithelial-to-mesenchymal transition. PLoS One. 2012;7:e31093. doi: 10.1371/journal.pone.0031093. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Ki SH, Park O, Zheng M, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong JY, Cong HQ, Zhang LH. Inhibitory effects of grape procyanidins on free radical-induced cell damage in rat hepatocytes in vitro. World J Gastroenterol. 2007;13:2752–55. doi: 10.3748/wjg.v13.i19.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bach E, Nielsen RR, Vendelbo MH, et al. Direct effects of TNF-alpha on local fuel metabolism and cytokine levels in the placebo-controlled, bilaterally infused human leg: increased insulin sensitivity, increased net protein breakdown, and increased IL-6 release. Diabetes. 2013;62:4023–29. doi: 10.2337/db13-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bacci MR, Leme RC, Zing NP, et al. IL-6 and TNF-alpha serum levels are associated with early death in community-acquired pneumonia patients. Braz J Med Biol Res. 2015;48:427–32. doi: 10.1590/1414-431X20144402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toker A, Engelbert D, Garg G, et al. Active demethylation of the Foxp3 locus leads to the generation of stable regulatory T cells within the thymus. J Immunol. 2013;190:3180–88. doi: 10.4049/jimmunol.1203473. [DOI] [PubMed] [Google Scholar]
- 37.Pamplona R, Costantini D. Molecular and structural antioxidant defenses against oxidative stress in animals. Am J Physiol Regul Integr Comp Physiol. 2011;301:R843–63. doi: 10.1152/ajpregu.00034.2011. [DOI] [PubMed] [Google Scholar]
- 38.Crawford A, Fassett RG, Coombes JS, et al. Glutathione peroxidase, superoxide dismutase and catalase genotypes and activities and the progression of chronic kidney disease. Nephrol Dial Transplant. 2011;26:2806–13. doi: 10.1093/ndt/gfq828. [DOI] [PubMed] [Google Scholar]
- 39.Gutierrez-Salinas J, Garcia-Ortiz L, Morales Gonzalez JA, et al. In vitro effect of sodium fluoride on malondialdehyde concentration and on superoxide dismutase, catalase, and glutathione peroxidase in human erythrocytes. ScientificWorldJournal. 2013;2013:864718. doi: 10.1155/2013/864718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buldak L, Labuzek K, Buldak RJ, et al. Metformin affects macrophages’ phenotype and improves the activity of glutathione peroxidase, superoxide dismutase, catalase and decreases malondialdehyde concentration in a partially AMPK-independent manner in LPS-stimulated human monocytes/macrophages. Pharmacol Rep. 2014;66:418–29. doi: 10.1016/j.pharep.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Rickenbacher A, Jang JH, Limani P, et al. Fasting protects liver from ischemic injury through Sirt1-mediated downregulation of circulating HMGB1 in mice. J Hepatol. 2014;61:301–8. doi: 10.1016/j.jhep.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 42.Gu Q, Guan H, Shi Q, et al. Curcumin attenuated acute Propionibacterium acnes-induced liver injury through inhibition of HMGB1 expression in mice. Int Immunopharmacol. 2015;24:159–65. doi: 10.1016/j.intimp.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Harris HE, Andersson U, Pisetsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;8:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 44.Zhou RR, Liu HB, Peng JP, et al. High mobility group box chromosomal protein 1 in acute-on-chronic liver failure patients and mice with ConA-induced acute liver injury. Exp Mol Pathol. 2012;93:213–19. doi: 10.1016/j.yexmp.2012.05.006. [DOI] [PubMed] [Google Scholar]