Significance
Mitochondrial heat-shock protein of 90 kDa (Hsp90) (TRAP1) promotes cell survival and is essential for neoplastic growth. Exploiting human TRAP1 for drug development requires detailed structural and mechanistic understanding. Whereas TRAP1 adopts different conformations associated with distinct nucleotide states, how the TRAP1 dimer senses the bound nucleotide and signals this information to the neighboring subunit remains unknown. We show that unliganded TRAP1 forms a previously unobserved coiled-coil dimer and is found in an autoinhibited state. ATP binding in cis displaces the ATP lid that signals the nucleotide status to the trans subunit. Our findings suggest that human TRAP1 is a ligand-activated molecular chaperone, which couples ATP binding to local changes in structure facilitating dimer closure needed for protein folding.
Keywords: TRAP1, Hsp90, molecular chaperone
Abstract
Heat-shock protein of 90 kDa (Hsp90) is an essential molecular chaperone that adopts different 3D structures associated with distinct nucleotide states: a wide-open, V-shaped dimer in the apo state and a twisted, N-terminally closed dimer with ATP. Although the N domain is known to mediate ATP binding, how Hsp90 senses the bound nucleotide and facilitates dimer closure remains unclear. Here we present atomic structures of human mitochondrial Hsp90N (TRAP1N) and a composite model of intact TRAP1 revealing a previously unobserved coiled-coil dimer conformation that may precede dimer closure and is conserved in intact TRAP1 in solution. Our structure suggests that TRAP1 normally exists in an autoinhibited state with the ATP lid bound to the nucleotide-binding pocket. ATP binding displaces the ATP lid that signals the cis-bound ATP status to the neighboring subunit in a highly cooperative manner compatible with the coiled-coil intermediate state. We propose that TRAP1 is a ligand-activated molecular chaperone, which couples ATP binding to dramatic changes in local structure required for protein folding.
Heat-shock protein of 90 kDa (Hsp90) is a conserved ATP-dependent molecular chaperone (1–4), which together with heat-shock protein of 70 kDa (Hsp70) (5–7) and a cohort of cochaperones (8–10), promotes the late-stage folding of Hsp90 client proteins (11). It is presumed that almost 400 different proteins, including a majority of signaling and tumor promoting proteins, depend on cytosolic Hsp90 for folding (12). Consequently, the ability to inactivate multiple oncogenic pathways simultaneously has made Hsp90 a major target for drug development (13), with several Hsp90 inhibitors currently undergoing clinical trials (14).
Hsp90 chaperones display conformational plasticity in solution (2, 15, 16), with different adenine nucleotides either facilitating or stabilizing distinct Hsp90 dimer conformations (17–19). Interestingly, apo Hsp90 forms a wide-open, V-shaped dimer with the N domains separated by as much as 101 Å (18). This open conformation is markedly distinct from the intertwined, N-terminally closed dimer with ATP bound (20, 21). Because the open-state dimer cannot signal the nucleotide status between neighboring subunits, an intermediate conformation preceding dimer closure must exist, which so far has remained elusive.
Apart from cytosolic Hsp90s, Hsp90 homologs are found in the endoplasmic reticulum, chloroplasts, and mitochondria (Fig. S1) (22). The tumor necrosis factor receptor-associated protein 1 (TRAP1) is the mitochondrial Hsp90 paralog, which prevents apoptosis and protects mitochondria against oxidative damage (23–25). TRAP1 is widely expressed in many tumors (24, 26, 27), but not in mitochondria of most normal tissues (24), benign prostatic hyperplasia (26), or highly proliferating, nontransformed cells (27). Notably, it was found that TRAP1 not only promotes neoplastic growth, but also confers tumorigenic potential on nontransformed cells (27), indicating a major role of TRAP1 in tumorigenesis, although TRAP1’s specific function remains poorly understood (28).
Fig. S1.
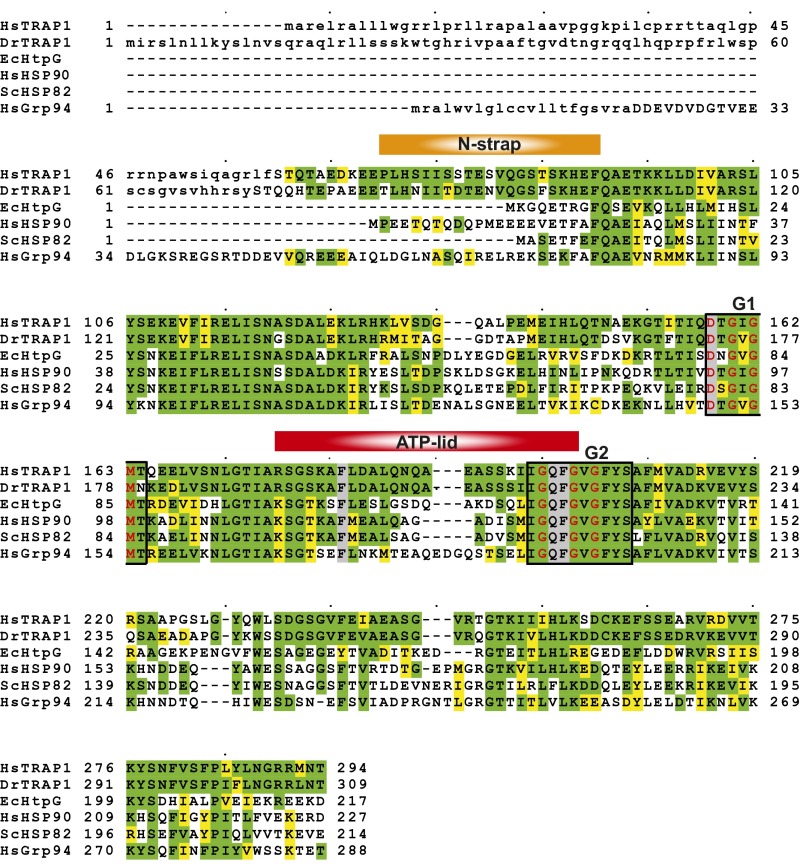
Multiple sequence alignment of the N-terminal nucleotide-binding domain of TRAP1 (mitochondria), HtpG (eubacteria), Hsp90 (cytosolic), and Grp94 (endoplasmic reticulum) from Homo sapiens (Hs), Danio rerio (Dr), E. coli (Ec), and S. cerevisiae (Sc). Residues conserved across sequences of representative members are highlighted in green, and those that are similar are in yellow. Residues not found in the mature protein are shown in lowercase letters. The N strap, ATP lid, and G1 and G2 boxes are marked. Residues that define the G1 and G2 box motifs are in red. Asp158, Phe183, Gln200, and Phe201 are surrounded by a gray box.
TRAP1 is a multidomain protein consisting of an N-terminal or N domain (TRAP1N), a middle domain (TRAP1M), and a C-terminal or C domain (TRAP1C), but it lacks the charged linker found in eukaryotic Hsp90 paralogs. Human TRAP1 is preceded by a mitochondrial localization sequence (MLS) of 59 residues that are cleaved off during import (29). The mature form of TRAP1 is a homodimer held together by TRAP1C, with a second, ATP binding-dependent dimer interface in TRAP1N. The crystal structures of zebrafish TRAP1 (zTRAP1) and zebrafish and human TRAP1NM bound to ADPNP were recently reported (21, 30), and are largely consistent with our current understanding of Hsp90 chaperones. In the ATP-bound state, the N-terminal extension (known as the N strap) straddles the N domain of the neighboring subunit, thereby stabilizing the structure of the closed-state dimer (21, 30). The ordered segment of the N strap significantly lengthens the previously observed β-strand swap and may function as a regulatory element that controls TRAP1 function (16, 21). Interestingly, intact zTRAP1-ADPNP crystallized as an asymmetric dimer that could support a sequential ATP hydrolysis mechanism (31, 32); however, no asymmetric nucleotide binding was observed, and no molecular contacts between cis-bound ADPNP and the N domain of the neighboring subunit were seen in TRAP1 (21, 30) and other known Hsp90 structures (18, 20, 33), leaving open the question of how TRAP1 senses and signals the nucleotide-bound status between subunits.
Here we present atomic structures of human TRAP1N (hTRAP1N) alone and in complex with ADPNP. Unexpectedly, we found that unliganded hTRAP1N forms a previously unobserved coiled-coil dimer that is distinct from the proposed open-state and closed-state conformations (16, 21, 30). Importantly, intact hTRAP1 forms a similar coiled-coil dimer in solution, but only in the absence of ATP. Our findings show that ATP binding triggers a dramatic change in local structure and displaces the ATP lid, which is bound to the ATP-binding pocket, indicating that TRAP1 normally exists in an autoinhibited state. Strikingly, mutations of conserved residues that impair lid binding stimulate the hTRAP1 ATPase activity in a highly cooperative manner, supporting a previously unknown role of the ATP lid in signaling the cis-bound nucleotide status to the trans subunit, which is compatible with the coiled-coil dimer. Finally, we demonstrate that TRAP1 folding requires ATP and the functional cooperation of the mitochondrial Hsp70 chaperone system, supporting the existence of a mitochondrial Hsp90-Hsp70 supercomplex that may present a new target for drug development.
Results
Atomic Structure of Monomeric hTRAP1N in the ATP State.
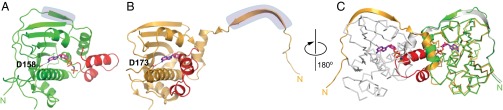
The emerging role of TRAP1 as a potent drug target necessitates atomic structure information of the human protein to exploit this information to develop mitochondrial Hsp90 inhibitors. The 3.3-Å crystal structure of a closed-state human TRAP1NM (hTRAP1NM) dimer bound to ADPNP, which shares close overall similarity with the previously determined crystal structure of the zTRAP1NM-ADPNP dimer (21), was reported recently (30). Conversely, hTRAP1N-ADPNP crystallized as a monomer (Fig. 1A and Fig. S2A), with crystals diffracting to 1.85-Å resolution (Table S1). The absence of a dimer is not the result of TRAP1 truncation, however, given that TRAP1N-ADPNP can also crystallize as an intertwined N-domain dimer (Fig. S3) indistinguishable from that observed with intact zTRAP1-ADPNP (21). The 2Fo-Fc map of monomeric hTRAP1N is of excellent quality (Fig. S4), enabling tracing of all but the first 10 residues of the mature form of hTRAP1N (Fig. S2A). The sole base-specific interaction between hTRAP1N and bound ADPNP is observed with the carboxylate side-chain of Asp158 that is evolutionary conserved (Fig. S1) and makes a hydrogen bond with the exocyclic N6-amine of adenine (Fig. 1A).
Fig. 1.
Structural comparison of the hTRAP1N monomer (green) and the zTRAP1 dimer (gold). The ATP lid is in red, and the N strap is highlighted in purple. ADPNP and Asp158 (Asp173 in zTRAP1) are shown as stick models. (A and B) Crystal structures of hTRAP1N-ADPNP (A) and an equivalent N domain of zTRAP1-ADPNP with the N strap straddling the neighboring subunit (B). Only one N domain is shown. (C) Superposition of the N domains of hTRAP1N-ADPNP and zTRAP1-ADPNP showing that an extended ATP lid would sterically clash with the neighboring subunit (gray) in the closed-state dimer.
Fig. S2.
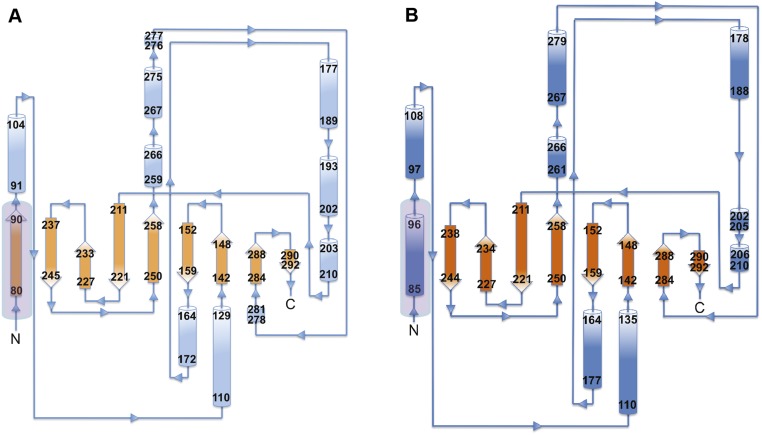
(A) Topology diagram of hTRAP1N-ADPNP. Secondary structure elements with residue numbers are shown as blue cylinders (α-helix) or orange arrows (β-strand). (B) Topology diagram of unliganded hTRAP1N. The N strap (boxed) is now α-helical.
Table S1.
Data collection and refinement statistics
| Statistic | hTRAP1N-ADPNP | apo hTRAP1N |
| Data collection | ||
| Space group | C 2221 | P 3221 |
| Cell dimensions | ||
| a, b, c, Å | 92.57, 143.17, 103.01 | 65.25, 65.25, 233.37 |
| α, β, γ, ° | 90.0, 90.0, 90.0 | 90.0, 90.0, 120.0 |
| Resolution, Å | 29.41–1.85 (1.88–1.85) | 77.79–1.82 (1.85–1.82) |
| Rsym or Rmerge | 0.055 (0.527) | 0.053 (0.486) |
| I/σI | 14.9 (8.4) | 14.0 (3.75) |
| Completeness, % | 98.5 (98.0) | 99.4 (99.6) |
| Redundancy | 7.9 (7.9) | 6.7 (7.0) |
| Refinement | ||
| Resolution, Å | 29.41–1.85 | 77.79–1.82 |
| No. of reflections | 54,569 | 52,606 |
| Rwork/Rfree | 0.176/0.203 | 0.182/0.215 |
| No. of atoms | 4,015 | 3,570 |
| Protein | 3,443 | 3,162 |
| Ligand/ion | 64 | 0 |
| Water | 508 | 408 |
| B factors | 24.7 | 22.8 |
| Protein | 25.7 | 21.6 |
| Ligand/ion | 8.8 | N/A |
| Water | 20.4 | 32.5 |
| rmsd | ||
| Bond lengths, Å | 0.003 | 0.007 |
| Bond angles, ° | 0.656 | 0.835 |
Each dataset was collected from a single crystal. Values in parentheses are for the highest-resolution shell. N/A, not applicable.
Fig. S3.
Crystal structure of the closed-state Dictyostelium discoideum TRAP1N dimer bound to ADPNP, demonstrating that the isolated N domain can adopt a physiological dimer conformation. The DdTRAP1N dimer (magenta/gold) is shown as a ribbon diagram; ADPNP, as a stick model.
Fig. S4.
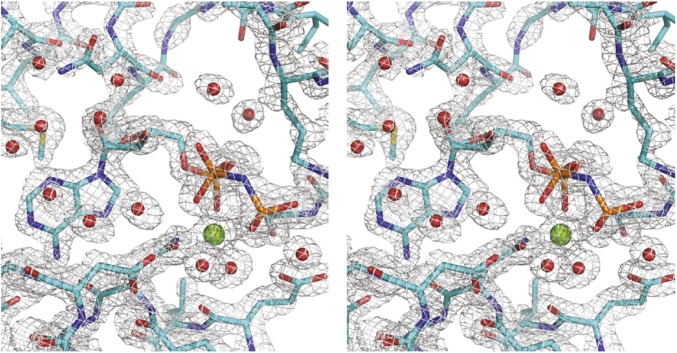
Stereoview of a section of the simulated-annealed composite omit map contoured at the 2.0 σ level. The figure shows the nucleotide-binding pocket of hTRAP1N with bound ADPNP. Ordered water molecules are depicted as red spheres. The bound Mg2+ is shown as a green sphere.
Pairwise comparison of hTRAP1N and the N domain of intact zTRAP1 (PDB ID code 4IPE) (21) shows that the two structures superpose with an rmsd of only 0.7 Å over 148 Cα atoms. However, hTRAP1N crystallizes with the N strap bound in cis (Fig. 1A), as opposed to straddling the N domain in trans (Fig. 1B), but occupies the same binding site in the hTRAP1N monomer as the trans segment in the ADPNP-bound TRAP1 dimer (Fig. 1C). Although this finding is surprising at first glance, the atomic structures of monomeric and dimeric hTRAP1N- and hTRAP1NM-ADPNP complexes are consistent with the notion that hTRAP1-ADPNP can adopt both closed- and open-state dimers, with elevated temperatures favoring the closed-state conformation (16). Interestingly, a cis-bound N-strap conformation also has been observed in crystal structures of hTRAP1NM-inhibitor complexes (30), suggesting that this conformation might be a common feature of monomeric TRAP1.
Another notable difference is the open conformation of the ATP lid (residues 177 to 202), which exposes the bound nucleotide to bulk solvent (Fig. 1A) as opposed to being folded over the nucleotide-binding pocket (Fig. 1B). A similar open-lid conformation was also reported for monomeric Grp94N-nucleotide complexes (34), suggesting that lid closure is not critical for nucleotide binding to Hsp90 chaperones in general. To determine the structural basis for lid closure, we compared the crystal structure of the hTRAP1N-ADPNP monomer with that of the zTRAP1-ADPNP dimer (21). Superposition of the two structures shows that an open-lid conformation is incompatible with the closed-state dimer, because it would sterically clash with the N domain of the neighboring subunit (Fig. 1C). Thus, our structure confirms that lid closure is nonessential for nucleotide binding and is driven largely by steric interference.
Atomic Structure of hTRAP1N Reveals a Coiled-Coil Dimer.
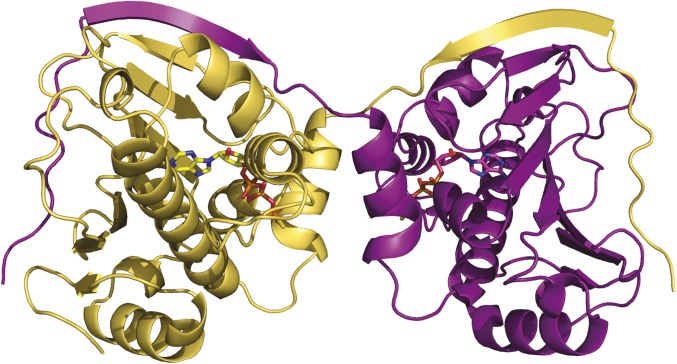
In addition to the ATP-bound state, we report the first, to our knowledge, atomic structure of unliganded hTRAP1N at 1.82-Å resolution (Table S1). Unexpectedly, the crystal structure reveals a previously unobserved coiled-coil dimer in the asymmetric unit of the crystal (Fig. 2A), which is markedly distinct from the proposed open- and closed-state conformations (16, 21, 30). The coiled-coil dimer interface is stabilized by an extensive network of hydrogen bonds formed between the N-terminal helices from each subunit, and occludes 1,765 Å2 of solvent accessible area. The latter is 38 Å2 more than the closed-stated zTRAP1N-ADPNP dimer (1,727 Å2) as calculated with PISA (35) over the corresponding range of residues (zTRAP1N residues 97 to 309), not taking into account an additional 1,218 Å2 owing to contributions of N-strap residues (zTRAP1N residues 85 to 96), which are disordered in hTRAP1N.
Fig. 2.
Structural comparison of the coiled-coil hTRAP1N dimer (blue/magenta) and the closed-state zTRAP1-ADPNP dimer (gold/pink). (A) Crystal structure of the unliganded hTRAP1N dimer. Phe183 is shown as CPK model. (B) Superposition of unliganded hTRAP1N (blue) onto the structure of the zTRAP1-ADPNP dimer, illustrating the local structural rearrangements in hTRAP1N on nucleotide binding. (1) ATP binding displaces the ATP lid from the nucleotide-binding pocket, concomitant with (2) the N strap undergoing a structural transition from an α-helix to a β-strand that straddles the neighboring subunit and stabilizes the closed-state dimer. (3) Owing to steric interference, the ATP lid must fold over the nucleotide-binding pocket trapping the bound nucleotide.
In addition to the previously unobserved dimer interface, it is immediately evident that each hTRAP1N monomer adopts a more extended, solvent-exposed conformation than previously observed ADPNP-bound complexes (21, 30) (Fig. 1A). Perhaps most strikingly, the N strap no longer forms a β-strand as seen in the ADPNP-bound state, but instead forms an α-helix (Fig. 2B and Fig. S2B) and pairs up with the helical N strap of the other subunit to form a zipper-like, coiled-coil dimer (Fig. 2A). The coiled-coil interface is further stabilized by interactions between Phe183 in the ATP lid of the trans subunit and Phe90, Glu93, and Thr94 of the cis subunit.
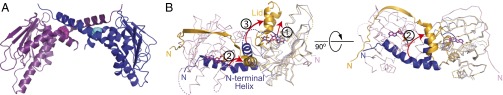
The coiled-coil dimer structure was unexpected, given the common presumption that Hsp90N is a monomer in the absence of nucleotide (36), a conformation supported by the apo structure of full-length bacterial Hsp90 (18), which shows a wide-open, V-shaped dimer conformation. Consistently, superposing unliganded hTRAP1N onto the equivalent domain of zTRAP1-ADPNP (21) and TRAP1 homologs (18, 20, 33) shows that the helical N strap will sterically clash with the neighboring N domain in the closed-state dimer (Fig. 2B and Fig. S5 A and B), whereas in the proposed open state, the N domains are too far apart to contact each other.
Fig. S5.
Pairwise structural alignment of unliganded hTRAP1N with known crystal structures of full-length Hsp90. Only the N domains are shown. (A) Zebrafish TRAP1-ADPNP dimer (PDB ID code 4IPE) (21). (B) Yeast Hsp90-ATP dimer (PDB ID code 2CG9) (20). (C) Canine Grp94-ADPNP (PDB ID code 2O1U) (33). (D) Bacterial Hsp90-apo (HtpG) (PDB ID code 2IOQ) (18).
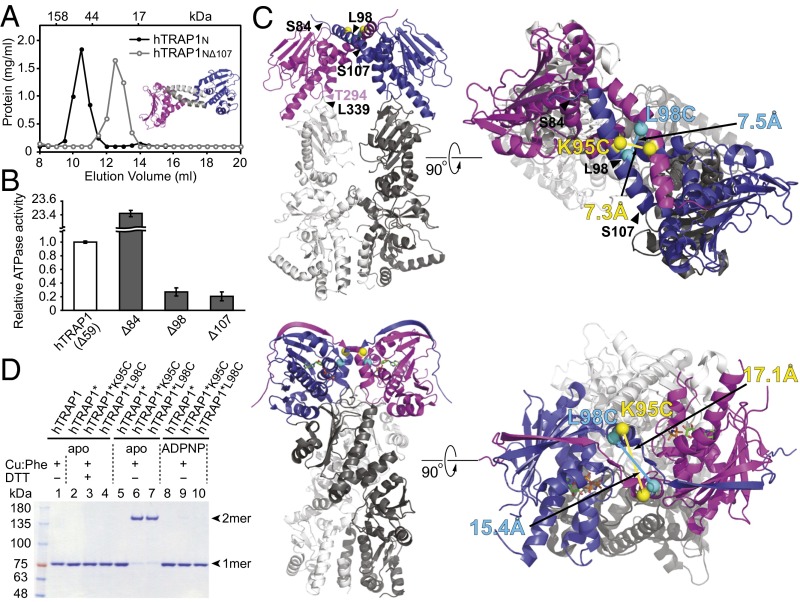
To exclude the possibility that the coiled-coil dimer results from crystal packing interactions, we determined the oligomeric state of hTRAP1N in solution. Consistent with the crystal structure, we found that unliganded hTRAP1N is a dimer, whereas an N-terminally truncated hTRAP1N variant lacking the N-terminal α-helix (hTRAP1NΔ107) is monomeric (Fig. 3A).
Fig. 3.
Human TRAP1N and hTRAP1 form a coiled-coil dimer. (A) Size-exclusion chromatogram of hTRAP1N (black curve) and hTRAP1NΔ107 (gray curve). (Inset) The hTRAP1N dimer, with the N-terminal helices deleted in hTRAP1NΔ107 shown in gray. (B) Relative ATPase activities of hTRAP1 (Δ59) and N-terminal truncated hTRAP1 mutants lacking the first 84 (Δ84), 98 (Δ98), or 107 (Δ107) residues. (C) In silico model of the intact coiled-coil hTRAP1 dimer (Top) and the crystal structure of the closed-state zTRAP1-ADPNP dimer (21) (Bottom). TRAP1N is shown in blue and magenta; TRAP1MC, in different shades of gray. Introduced Cys sites are marked by spheres. Corresponding Cys pairs are shown in the same color, with distances between Cα atoms indicated. (D) Disulfide cross-linking of hTRAP1 and Cys-containing hTRAP1* variants without or with ADPNP. Cross-linked dimers can be monomerized with 100 mM DTT (+DTT). Bands corresponding to hTRAP1 monomers (1mer) and dimers (2mer) are indicated.
It has been reported that an N-strap truncation (hTRAP1Δ84) stimulates ATPase activity by ∼30-fold compared with the mature form of hTRAP1 (hTRAP1Δ59) (16) (Fig. 3B). Strikingly, we found that further deletion of the N-terminal α-helix (hTRAP1Δ107) or only the helical N strap (hTRAP1Δ98) nearly abolished the ATPase activity of hTRAP1 (Fig. 3B). Our findings demonstrate that the helical N strap has an essential regulatory function and is required for formation of the coiled-coil dimer that may represent an intermediate conformation preceding dimer closure.
The N-Terminal α-Helices of Intact hTRAP1 Form a Coiled Coil in the Absence of Nucleotide.
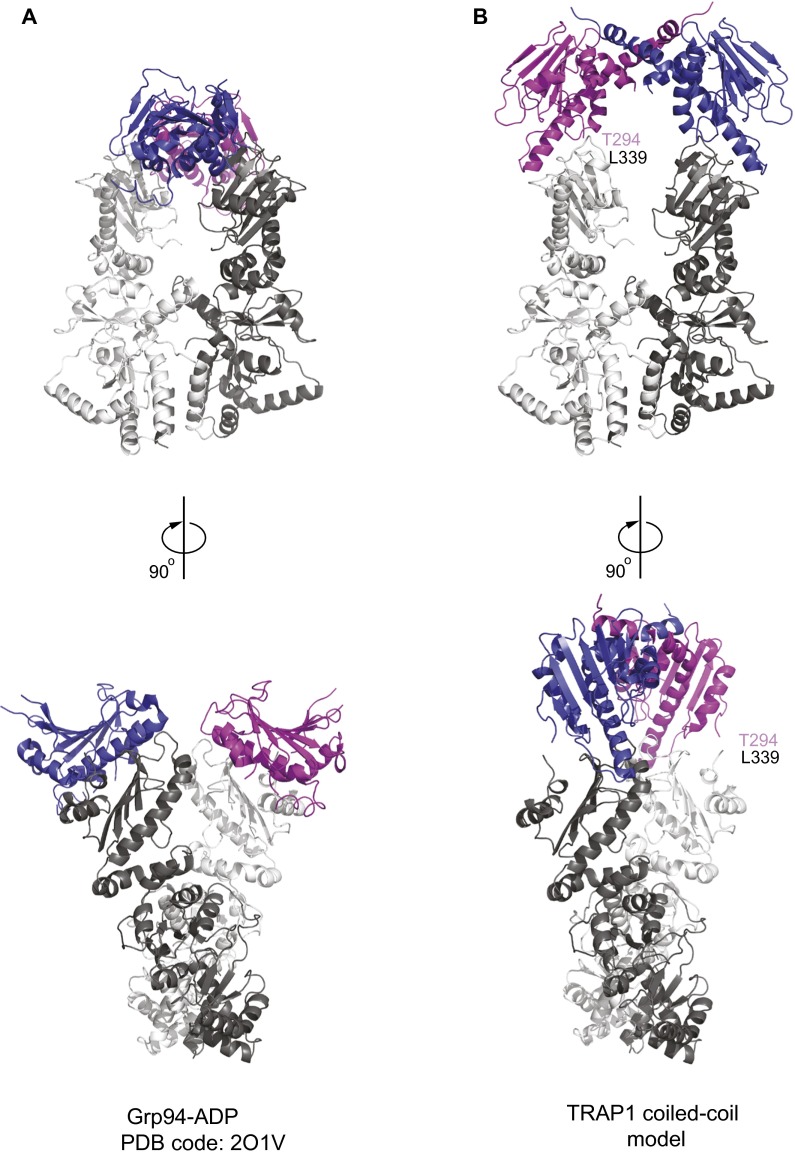
Our attempts to crystallize intact hTRAP1 without nucleotide were unsuccessful. Thus, to generate an in silico model for hTRAP1, we examined previously reported structures of full-length Hsp90 chaperones to identify an Hsp90MC conformation compatible with the coiled-coil hTRAP1N dimer structure. Because the structure of the coiled-coil intermediate has not been observed previously, it seemed unlikely that we would find a compatible structure. Fortuitously, several crystal structures were compatible with the imposed twofold restraint. We found that the X-ray structures of Grp94 in complex with ADP (PDB ID code 2O1V) or ADPNP (PDB ID code 2O1U) (33) fit best. The two structures are nearly identical to each other and superpose with an rmsd of only 0.5 Å over all Cα atoms. In our model, the C termini of hTRAP1N (Thr294 and Thr294′) are positioned to their respective N termini of Grp94MC (Leu339 and Leu339′) with an equal distance of only 4.3 Å (Fig. 3C, Top and Fig. S6).
Fig. S6.
Crystal structure of Grp94-ADP and composite model of the coiled-coil hTRAP1 dimer. The N domains are shown in blue and magenta, and the middle and C domains are in different gray scales. (A) X-ray structure of the Grp94-ADP complex (PDB ID code 2O1V) (33). (B) Composite model of intact TRAP1 generated from crystal structures of the unliganded hTRAP1N and Grp94MC-ADP dimers.
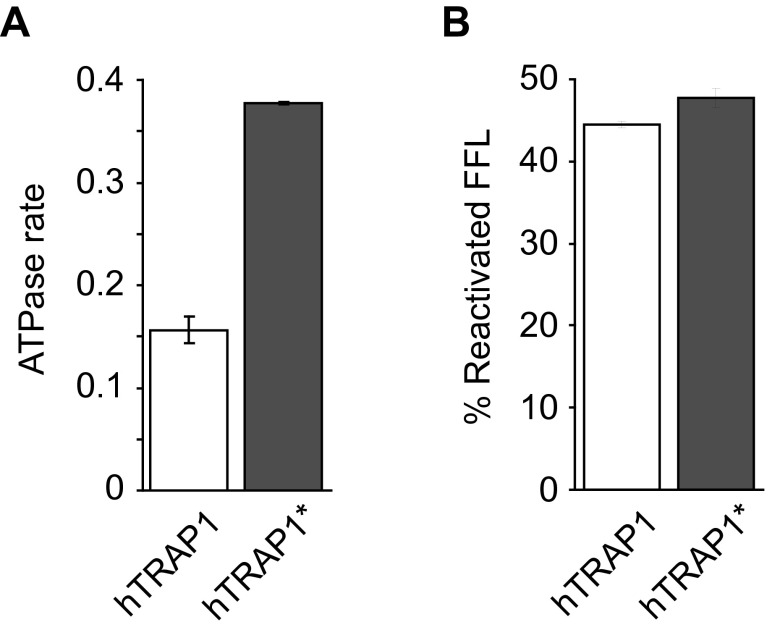
To validate our model, we wished to probe the coiled-coil dimer interface of intact hTRAP1 in solution. Because a coiled-coil dimer is observed only in the unliganded state, we engineered hTRAP1 variants featuring a Cys in the helical N strap, which potentially could form a disulfide cross-link in the absence of nucleotide. In the crystal structure, Lys95 and Leu98 are near the cross-point of the coiled coil with Cα distances of 7.3 Å (Lys95-Lys95′) and 7.5 Å (Leu98-Leu98′), compatible with disulfide bond formation (Fig. 3C, Top), as opposed to 17.1 Å and 15.4 Å in the ADPNP-bound state (21) (Fig. 3C, Bottom). Thus, we generated hTRAP1 mutants by replacing Lys95 or Leu98 with Cys in a Cys-free hTRAP1 variant (hTRAP1*), which is fully functional (Fig. S7). Notably, hTRAP1*K95C and hTRAP1*L98C formed a cross-linked dimer only in the absence of nucleotide (Fig. 3D, lanes 6 and 7), but not in the presence of ADPNP (Fig. 3D, lanes 9 and 10). The cross-linking reaction is reversible with DTT (Fig. 3D, compare lanes 6 and 7 with lanes 3 and 4), and no cross-linked products were observed with hTRAP1 (Fig. 3D, lane 1), underscoring the specificity of the cross-linking reaction. Taken together, our findings lead us to conclude that unliganded hTRAP1 adopts a similar coiled-coil dimer in solution as observed with hTRAP1N in the crystal.
Fig. S7.
The cysteine-free hTRAP1* variant is functional. (A) ATPase rate of hTRAP1 and hTRAP1* expressed in µmol ATP hydrolyzed per min per µmol protein. (B) Coupled-chaperone assay with heat-denatured FFL. Recovered FFL activities by hTRAP1 or hTRAP1* together with the mitochondrial Hsp70 system were measured after 120 min and are expressed relative to native FFL. Averages of three independent measurements ± SD are shown.
The ATP Lid Functions as a Key Regulatory Element.
The crystal structure of TRAP1 confirmed that the nucleotide-binding domain shares the canonical Bergerat fold (37), which features several conserved motifs, including the G1 box and G2 box that flank the ATP lid (Fig. S1). It was initially proposed that the lid represents an ATP-binding motif (37) that folds over the nucleotide-binding pocket to stabilize bound ATP in GHKL (gyrase, Hsp90, histidine kinase, MutL) ATPases (38, 39). Although deletion of the lid did not affect nucleotide binding to Hsp90, lidless yeast Hsp90 lacks ATPase activity and cannot rescue an hsp82−/hsc82− yeast strain (40), underscoring the importance of the ATP lid to Hsp90 function.
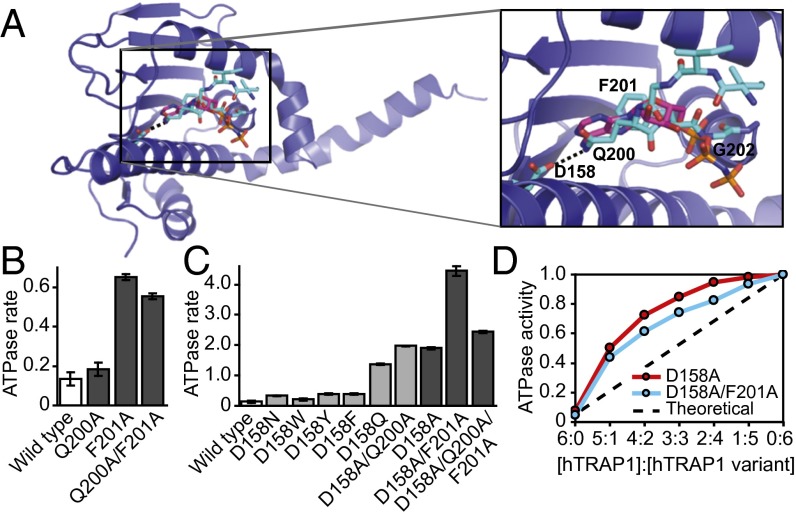
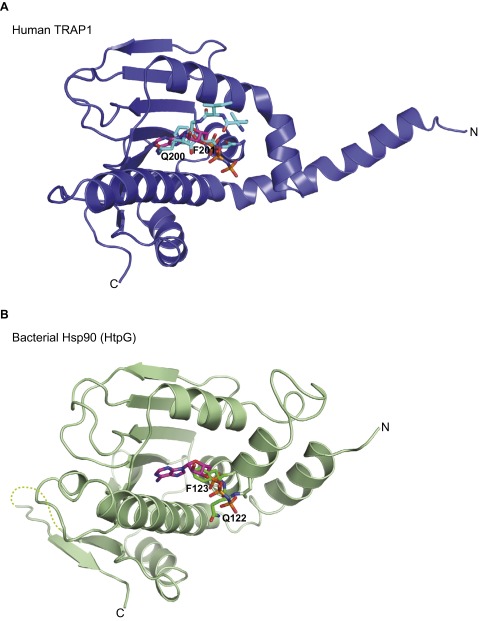
In the crystal structure of hTRAP1N-ADPNP, the ATP lid is folded away from the nucleotide-binding pocket, consistent with the notion that the lid is dispensable for ATP binding. Interestingly, in the crystal structure of unliganded hTRAP1N, the ATP lid folds over the nucleotide-binding pocket. Closer inspection reveals that Gln200, Phe201, and Gly202 of the G2 box overlapped with the ribose ring and the α-phosphate of the bound nucleotide (Fig. 4A), effectively competing with nucleotide binding (Fig. S8A). A similar G2 box motif interaction was also observed in the crystal structure of full-length bacterial Hsp90 in the apo conformation (Fig. S8B) (18), indicating that mitochondrial and bacterial Hsp90 may normally exist in an autoinhibited state. Interestingly, in hTRAP1, the amine group of the Gln200 side chain superposed with the exocyclic N6-amine of adenine in the ADPNP-bound state, and formed a hydrogen bond with the carboxylate side chain of Asp158 (Fig. 4A).
Fig. 4.
Conserved residues of the ATP-lid G2 box motif compete with ATP for binding to the ATP-binding pocket. (A) Superposition of unliganded and ADPNP-bound hTRAP1N. Only ADPNP of the hTRAP1N-ADPNP complex is shown. (B and C) ATPase rates, expressed in µmol ATP hydrolyzed per min per µmol protein of hTRAP1 and hTRAP1 variants carrying a mutation in Gln200 or Phe201 (B) and/or Asp158 (C). Averages of three independent measurements ± SD are shown. (D) Relative ATPase activities of mixtures of hTRAP1 and hTRAP1D158A (red), and hTRAP1 and hTRAP1D158A/F201A heterodimers (cyan) at indicated ratios. The dashed line represents the theoretical, linear relationship if the stimulated ATPase activities resulted from an increase in ATPase activity in only one subunit.
Fig. S8.
The ATP lid of Hsp90 chaperones binds to the ATP-binding pocket in the absence of nucleotide. ADPNP and conserved Gln and Phe residues of the ATP-lid G2 motif in mitochondrial (TRAP1) and bacterial Hsp90 (HtpG) are shown as stick models. Only ADPNP of the nucleotide-bound complex is shown. (A) Superposition of hTRAP1N and hTRAP1N-ADPNP. (B) Superposition of full-length bacterial Hsp90 (PDB ID code 2IOQ) (18) and hTRAP1N-ADPNP. Only the N domain of bacterial Hsp90 is shown.
Because the ATP lid has been implicated in the regulation of Hsp90 ATPase activity (40, 41), we reasoned that the conserved G2 box motif might function as a sensor of nucleotide binding, providing a means to signal the cis-bound nucleotide status to the neighboring subunit. Consistent with a coordinated ATP hydrolysis mechanism (21, 31), hTRAP1 variants impaired in lid binding should stimulate ATPase activity by signaling a constitutive cis-bound nucleotide status to the trans subunit.
To test this idea, we mutated Asp158, Gln200, and Phe201 either alone or in combination. We found that our engineered hTRAP1 variants retained ATPase activity (Fig. 4 B and C), including an hTRAP1 variant featuring a mutation of Asp158 to Asn. Although in principle, an Asn is compatible with ATP binding, our finding differs from previous observations with cytosolic Hsp90 (42, 43). Surprisingly, hTRAP1Q200A/F201A showed only 4.1-fold higher ATPase activity than hTRAP1, slightly less than that observed with hTRAP1F201A alone (Fig. 4B), indicating that lid binding is driven largely by van der Waals interactions. Interestingly, we found that the ATPase activity of TRAP1D158N was increased only slightly (2.5-fold), whereas a mutation of Asp158 to Ala stimulated ATPase activity by 14.6-fold (Fig. 4C), suggesting that the stimulated ATPase activity of hTRAP1 variants depends on the nature of the introduced substitution. The greatest stimulation was observed with hTRAP1D158A/F201A, which increased ATPase activity by 33.0-fold, significantly greater than the increases seen with hTRAP1D158A/Q200A/F201A triple mutant (18.1-fold) or any of the single mutants alone (Fig. 4C).
To determine whether the stimulation of ATPase activity resulted from cooperative interactions between hTRAP1 subunits, we generated heterodimers of hTRAP1 and hTRAP1D158A and of hTRAP1 and hTRAP1D158A/F201A by mixing wild-type and mutant subunits in defined molar ratios, keeping the total protein amount constant. We would expect to observe a linear relationship if the stimulated ATPase activity resulted from an increase in activity within one subunit, and a nonlinear relationship if stimulation occurred between neighboring subunits. As shown in Fig. 4D, we observed a clear nonlinear relationship with both the hTRAP1/hTRAP1D158A and hTRAP1/hTRAP1D158A/F201A heterodimers. Thus, mutations that impair lid binding in cis stimulate the ATPase activity in trans. Taken together, our findings suggest that the ATP lid competes with nucleotide binding and is displaced by ATP, providing a means to signal the cis-bound nucleotide status to the neighboring subunit that must be in close physical proximity, a conformation that is compatible with the coiled-coil dimer.
TRAP1 Cooperates with Mitochondrial Chaperones in Folding.
To determine the significance of the unliganded and ATP-bound TRAP1 conformation, we developed an assay to monitor the ATP-dependent hTRAP1 chaperone activity. It is known that cytosolic Hsp90 requires Hsp70 and a cohort of cochaperones to promote the folding of Hsp90 clients (1, 10). Interestingly, hTRAP1 cannot functionally replace cytosolic Hsp90 in vitro (29, 44), suggesting the need for other factors to reconstitute TRAP1-dependent folding.
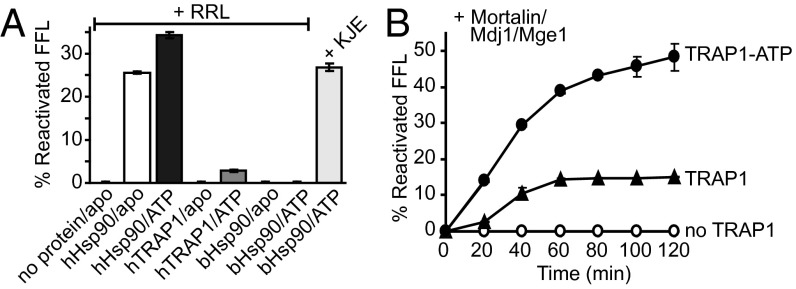
Rabbit reticulocyte lysate (RRL) provides a rich source of proteins, and can substitute for Hsp70 and essential cochaperones to reconstitute active folding by cytosolic Hsp90 in vitro (45). Although RRL facilitated hHsp90-dependent folding of heat-denatured firefly luciferase (FFL), no recovered FFL activity was observed when hHsp90 was replaced with either hTRAP1 or bacterial Hsp90 (Fig. 5A). However, bacterial Hsp90 could recover FFL activity when RRL was replaced with the bacterial Hsp70 system (Fig. 5A).
Fig. 5.
TRAP1 requires the mitochondrial Hsp70 system for protein folding. Averages of three independent measurements ± SD are shown. (A) Reactivation of heat-denatured FFL after 120 min by hHsp90, hTRAP1, or bHsp90 together with RRL or the bacterial Hsp70 system (KJE). Recovered FFL activities are expressed relative to native FFL. (B) Reactivation of heat-denatured FFL without hTRAP1 (no TRAP1), with hTRAP1 (TRAP1), or with hTRAP1 and 5 mM ATP (TRAP1-ATP), and together with Mortalin/Mdj1/Mge1. FFL recovery was monitored every 20 min for 120 min.
Although perplexing on first sight, it has been reported that RRLs mostly lack functional mitochondria (46). Thus, we reasoned that our inability to reconstitute TRAP1-dependent protein folding with RRL might be due to the lack of one or more components of the mitochondrial chaperone system. Indeed, we found that hTRAP1 reactivated heat-denatured FFL when RRL was replaced with the mitochondrial Hsp70 system (Fig. 5B). Importantly, hTRAP1’s ability to reactivate FFL requires ATP during heat denaturation, whereas the presence of unliganded hTRAP1 and the absence of hTRAP1 resulted in very low and no recovered FFL activity, respectively (Fig. 5B).
Discussion
Our findings provide mechanistic insight into how ATP-binding–induced local changes in TRAP1 structure facilitate dimer closure, and suggest that TRAP1 is a ligand (ATP)-activated molecular chaperone. Although our results reconcile disparate structural and biochemical observations, they also reveal nuances specific to mitochondrial Hsp90. For instance, we find that an hTRAP1 variant featuring an Asp158-to-Asn mutation behaves differently from that reported for cytosolic Hsp90s (42, 43). Although an Asn in place of an Asp is not unprecedented among GHKL ATPases (47), it indicates that the hTRAP1 ATPase activity is fine-tuned differently from other Hsp90s. Moreover, the inability to functionally interact with known Hsp90 cochaperones suggests that TRAP1 is dependent on other regulatory elements integral to TRAP1, such as the N strap (16) and the ATP lid, whose function is uncovered here.
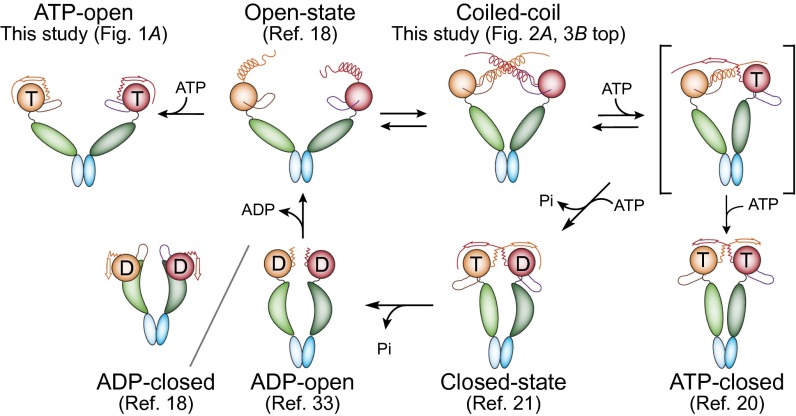
Our findings suggest that TRAP1-ATP can in principle adopt two distinct conformations: a closed-state dimer (ATP closed) with the N strap swapped between subunits (21, 30) and an open-state dimer (ATP open) with the N strap bound in cis (Fig. 6), which is consistent with the observed conformational plasticity of Hsp90 in solution (2, 15, 16). However, we propose that the ATP open state does not promote protein folding.
Fig. 6.
Schematic of the hTRAP1 cycle. TRAP1 subunits are shown in different hues and colored red (TRAP1N), green (TRAP1M), and blue (TRAP1C). “T” and “D” indicate bound ATP and ADP, respectively.
More importantly, our findings support a role of the ATP lid as a sensor of nucleotide binding. ATP binding in cis displaces the ATP lid that in turn signals the nucleotide status to the trans subunit. To do so, the N domains must be in close physical proximity, a conformation that is fully compatible with our coiled-coil intermediate dimer. The role of the Gln200 side chain is perhaps most intriguing. In the crystal structure of unliganded hTRAP1N, the Gln200 side chain mimics the bound nucleotide and contributes toward lid binding by forming a hydrogen bond with the Asp158 side chain (Fig. 4A). Although an inhibitory lid conformation also has been observed with full-length bacterial Hsp90 (18), the interaction between the conserved Gln200 and Asp158 side chains is new. Consistent with a nucleotide sensor function, hTRAP1 variants carrying a mutation in the conserved Asp158 or Phe201, which impairs lid-binding, show highly stimulated ATPase activity (Fig. 4 B and C). On the other hand, substituting Gln200 with Ala has a negligible effect on ATPase activity, and hTRAP1 variants carrying an additional mutation in Asp158, Phe201, or both exhibit similar or reduced ATPase activity compared with those that do not feature the Gln200 mutation (Fig. 4 B and C). Taken together, these findings point to an additional role for Gln200 in the ATPase cycle. In the crystal structure of zTRAP1-ADPNP (PDB ID code 4IPE-A) (21), the equivalent glutamine forms water-mediated interactions with the main chain of a β-bridge featuring the ATP sensor (Arg402) (48). Thus, we speculate that, in addition to lid binding, Gln200 is needed to position the ATP-sensor loop required for full ATPase activity.
Finally, we have demonstrated that TRAP1-dependent protein folding requires ATP (Fig. 5B). ATP hydrolysis may be needed for substrate release, as has been reported for cytosolic Hsp90 (49). In addition to ATP, TRAP1-dependent protein folding also requires the functional cooperation of the mitochondrial Hsp70 system. The latter supports a direct physical interaction, as was recently reported for cytosolic Hsp90 chaperones (5–7, 50), and opens up new avenues for drug development by targeting the specific interaction between mitochondrial chaperones.
Experimental Procedures
Cloning, mutagenesis, and protein expression and purification procedures are described in SI Experimental Procedures.
Crystallization.
Human TRAP1N crystals were grown at 14 °C from hanging drops. Then 2 μL of hTRAP1N (25 mg/mL) in 30 mM Tris⋅HCl pH 8.5, 0.15 M NaCl, and 1 mM tris(2-carboxyethyl)phosphine (TCEP) was mixed with an equal volume of reservoir solution consisting of 100 mM Hepes pH 7.5, 100 mM CaCl2, 23% (wt/vol) PEG 3350, 4% (vol/vol) isopropanol, and 0.4 μL of 1 M LiCl. hTRAP1N crystals were harvested in reservoir solution containing 5% (vol/vol) PEG400, but reducing the PEG 3350 concentration to 21% (wt/vol), and flash frozen in liquid nitrogen. hTRAP1N-ADPNP was prepared by incubating hTRAP1N (55 mg/mL) in 50 mM Tris⋅HCl pH 7.6, 0.2M NaCl, and 1 mM TCEP with 5 mM ADPNP and 10 mM MgCl2 for 30 min on ice. The sample was mixed with an equal volume of 100 mM sodium citrate pH 5.6, 22% (wt/vol) PEG4000, and 5% (vol/vol) isopropanol. Crystals were harvested in reservoir solution containing 5% (vol/vol) glycerol and flash frozen.
X-ray Crystallographic Analysis and Refinement.
Complete datasets were collected at the SBC-ID19 beamline (Table S1) and processed using HKL3000 (51). The crystal structure of hTRAP1N-ADPNP was determined by molecular replacement using PHENIX (52) and yeast cytosolic Hsp90N (PDB ID code 2CG9) (20) as the search model. The crystal structure of the coiled-coil hTRAP1N dimer was determined using the structure of hTRAP1N-ADPNP as a search model. Structures were refined using PHENIX (52) and REFMAC5 (53), with 5% of the data excluded from refinement for cross-validation purposes, which was interspersed by several rounds of manual model building in Coot (54). Water molecules were fitted automatically. The refined structures have excellent stereochemical properties, with none of the residues in generously allowed or disallowed regions of the Ramachandran plot.
In Silico Modeling.
To generate a model of intact hTRAP1, the twofold axis of the coiled-coil hTRAP1N dimer was aligned with that of intact Hsp90 chaperone structures to identify a compatible Hsp90MC conformation. The coiled-coil hTRAP1N dimer was then rotated around the twofold axis until the C termini of hTRAP1N were positioned closest to the N termini of Grp94MC avoiding any steric clash.
Analytical Size Exclusion Chromatography.
The oligomeric states of hTRAP1N and hTRAP1NΔ107 (25 mg/mL) were determined at 4 °C in 50 mM Tris⋅HCl pH 7.5, 100 mM NaCl, and 1 mM DTT on a Superdex 75 10/300 GL column (GE Healthcare).
Disulfide Cross-Linking.
hTRAP1, hTRAP1*, or Cys-containing hTRAP1* variant at 0.1 mg/mL was preincubated for 60 min on ice in 40 mM Tris⋅HCl pH 7.5, 50 mM KCl, 5 mM MgCl2, and 0.2 mM TCEP without or with 5 mM ADPNP, followed by incubation for 10 min at 37 °C. The addition of TCEP was necessary to prevent nonspecific cross-linking of Cys-containing hTRAP1* variants. Disulfide bond formation was facilitated by incubating the sample with 100 µM copper-o-phenanthroline (Cu:Phe) for 5 min at 23 °C and then quenching with 20 mM EDTA (final). Cross-linked products were analyzed by nonreducing SDS/PAGE (3–8%) and Coomassie blue staining.
ATPase Assay.
ATPase activity was determined at 30 °C by measuring the amount of inorganic phosphate released after 30 min using the malachite green calorimetric assay (55) in refolding buffer (30 mM Hepes pH 7.5, 50 mM KCl, 5 mM MgCl2, and 2 mM DTT) containing 5 µM hTRAP1 or hTRAP1 variants and 2 mM ATP. For mixing experiments, hTRAP1 and hTRAP1 variants were mixed at indicated ratios, keeping the total protein amount constant. Heterodimers were incubated for 15 min at 22 °C before the assay.
Chaperone Assay.
Chaperone activity was measured by monitoring the recovery of heat-denatured FFL using a coupled-chaperone assay consisting of Hsp90 chaperones and untreated RRL (Promega), or the bacterial [DnaK/DnaJ/GrpE (KJE)] or mitochondrial Hsp70 system (Mortalin/Mdj1/Mge1). In brief, 167 nM FFL (Promega) was mixed with 20 µM hTRAP1, hHsp90, or bacterial Hsp90 (bHsp90) in the absence or presence of 5 mM ATP in denaturing buffer (30 mM Tris⋅HCl pH 7.5 and 2 mM DTT), and heat-denatured for 5 min at 45 °C. The samples were cooled on ice for 5 min and then diluted 10-fold in refolding buffer containing 50% RRL or 4 µM KJE with 5 mM ATP, or 4 µM Mortalin/Mdj1/Mge1 and an ATP-regenerating system (20 mM creatine phosphate, 0.12 mg/mL creatine kinase, and 5 mM ATP). Recovery of FFL activity was measured at 30 °C every 20 min over 120 min using an LS55 fluorescence spectrophotometer (PerkinElmer).
SI Experimental Procedures
Cloning and Site-Directed Mutagenesis.
Human TRAP1N (residues Ser60 to Thr294) and hTRAP1NΔ107 (residues Gln108 to Thr294) were cloned by PCR into pTYB12 (New England BioLabs). The mature form of hTRAP1 (residues Ser60 to His704) (29), hTRAP1Δ84 (residues Thr85 to His704), hTRAP1Δ98 (residues Asp99 to His704), and hTRAP1Δ107 (residues Glu108 to His704) were cloned by PCR into pPROEX HTb. Cys-free hTRAP1C261S/C501S/C527A/C573R (hTRAP1*) and variants thereof were generated by QuikChange site-directed mutagenesis (Agilent). Human and Escherichia coli Hsp90 were amplified by PCR and cloned into pPROEX HTb. Saccharomyces cerevisiae Mdj1 (residues Asn56 to Asn511) and Mge1 (residues Ser45 to Asn228) were amplified from yeast genomic DNA (BY4741) without their predicted MLS, and then cloned into pET24d (Mdj1Δ55) and pPROEX HTb (Mge1Δ44). All pPROEX HTb constructs feature an N-terminal TEV protease cleavable His6-tag.
Protein Expression and Purification.
Human TRAP1N and hTRAP1NΔ107 with an N-terminal chitin-binding domain followed by an intein tag were overexpressed overnight at 16 °C in E. coli BL21 codon plus (DE3)-RIL cells. Protein expression was induced with 0.5 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) at OD600 ∼0.5. Soluble proteins were purified on a chitin column, followed by on-column cleavage in 20 mM Tris⋅HCl pH 8.5, 1 M NaCl, and 50 mM DTT at 23 °C following the manufacturer’s protocol. The eluted protein was dialyzed at 4 °C against 30 mM Tris⋅HCl pH 8.5, 5% (vol/vol) glycerol, and 2 mM β-mercaptoethanol (βME), and then further purified by anion-exchange chromatography.
Human TRAP1 was bacterially overexpressed at 25 °C after induction with 0.4 mM IPTG at OD600 ∼0.7. Intact hTRAP1 was purified from the cleared supernatant by Ni-NTA affinity chromatography, followed by TEV cleavage and removal of the liberated His-tag and His-TEV. The sample in 0.5 M ammonium sulfate (final) was loaded onto a hydrophobic interaction column, eluted using a linear gradient, and dialyzed against 25 mM Tris⋅HCl pH 7.5, 100 mM KCl, and 6 mM βME. Human TRAP1 mutants and hHsp90 were expressed and purified similarly, except for hTRAP1* and variants that were dialyzed against 40 mM Tris⋅HCl pH 7.5, 100 mM KCl, 10% (vol/vol) glycerol, and 0.2 mM TCEP. E. coli Hsp90 was bacterially overexpressed at 30 °C by IPTG induction and then purified by affinity chromatography. The eluted protein was treated with His-TEV protease and dialyzed against 25 mM Tris⋅HCl pH 7.5, 100 mM NaCl, 5% (vol/vol) glycerol, and 5 mM βME.
His6-tagged human Mortalin was coexpressed with yeast Hep148–174, and purified as described previously (56). Yeast Mdj1Δ55-His6 (Mdj1-His6) and yeast His6-Mge1Δ44 (His6-Mge1) were overexpressed in E. coli and purified at 4 °C. The E. coli DnaK system was purified as described previously (57).
Acknowledgments
We thank Drs. D. Toft, S. Felts, J. Silberg, J. Barral, and E. Craig for expression constructs. Use of the Structural Biology Center (SBC) beamlines at the Advanced Photon Source was supported by the US Department of Energy, Office of Biological and Environmental Research (Contract DE-AC02-06CH11357). This work was supported by the National Institutes of Health (Grants R01 GM111084 and R01 GM104980) and the Welch Foundation (Grant Q-1530). J.L. was a Welch fellow.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.S. is a guest editor invited by the Editorial Board.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 5F5R and 5F3K).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1516167113/-/DCSupplemental.
References
- 1.Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- 2.Krukenberg KA, Street TO, Lavery LA, Agard DA. Conformational dynamics of the molecular chaperone Hsp90. Q Rev Biophys. 2011;44(2):229–255. doi: 10.1017/S0033583510000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Buchner J. Structure, function, and regulation of the hsp90 machinery. Biomed J. 2013;36(3):106–117. doi: 10.4103/2319-4170.113230. [DOI] [PubMed] [Google Scholar]
- 4.Mayer MP, Le Breton L. Hsp90: Breaking the symmetry. Mol Cell. 2015;58(1):8–20. doi: 10.1016/j.molcel.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Genest O, Hoskins JR, Camberg JL, Doyle SM, Wickner S. Heat shock protein 90 from Escherichia coli collaborates with the DnaK chaperone system in client protein remodeling. Proc Natl Acad Sci USA. 2011;108(20):8206–8211. doi: 10.1073/pnas.1104703108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamoto H, et al. Physical interaction between bacterial heat shock protein (Hsp) 90 and Hsp70 chaperones mediates their cooperative action to refold denatured proteins. J Biol Chem. 2014;289(9):6110–6119. doi: 10.1074/jbc.M113.524801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirschke E, Goswami D, Southworth D, Griffin PR, Agard DA. Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles. Cell. 2014;157(7):1685–1697. doi: 10.1016/j.cell.2014.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young JC, Barral JM, Ulrich Hartl F. More than folding: Localized functions of cytosolic chaperones. Trends Biochem Sci. 2003;28(10):541–547. doi: 10.1016/j.tibs.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Ishida M, et al. Biochemical characterization and cooperation with co-chaperones of heat shock protein 90 from Schizosaccharomyces pombe. J Biosci Bioeng. 2013;116(4):444–448. doi: 10.1016/j.jbiosc.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Röhl A, Rohrberg J, Buchner J. The chaperone Hsp90: Changing partners for demanding clients. Trends Biochem Sci. 2013;38(5):253–262. doi: 10.1016/j.tibs.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: Emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11(7):515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 12.Taipale M, et al. Quantitative analysis of HSP90–client interactions reveals principles of substrate recognition. Cell. 2012;150(5):987–1001. doi: 10.1016/j.cell.2012.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: Are we there yet? Clin Cancer Res. 2012;18(1):64–76. doi: 10.1158/1078-0432.CCR-11-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jhaveri K, Taldone T, Modi S, Chiosis G. Advances in the clinical development of heat shock protein 90 (Hsp90) inhibitors in cancers. Biochim Biophys Acta. 2012;1823(3):742–755. doi: 10.1016/j.bbamcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krukenberg KA, Förster F, Rice LM, Sali A, Agard DA. Multiple conformations of E. coli Hsp90 in solution: Insights into the conformational dynamics of Hsp90. Structure. 2008;16(5):755–765. doi: 10.1016/j.str.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Partridge JR, et al. A novel N-terminal extension in mitochondrial TRAP1 serves as a thermal regulator of chaperone activity. eLife. 2014;3:e03487. doi: 10.7554/eLife.03487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prodromou C, et al. The ATPase cycle of Hsp90 drives a molecular “clamp” via transient dimerization of the N-terminal domains. EMBO J. 2000;19(16):4383–4392. doi: 10.1093/emboj/19.16.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiau AK, Harris SF, Southworth DR, Agard DA. Structural analysis of E. coli hsp90 reveals dramatic nucleotide-dependent conformational rearrangements. Cell. 2006;127(2):329–340. doi: 10.1016/j.cell.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 19.Southworth DR, Agard DA. Species-dependent ensembles of conserved conformational states define the Hsp90 chaperone ATPase cycle. Mol Cell. 2008;32(5):631–640. doi: 10.1016/j.molcel.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali MM, et al. Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature. 2006;440(7087):1013–1017. doi: 10.1038/nature04716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavery LA, et al. Structural asymmetry in the closed state of mitochondrial Hsp90 (TRAP1) supports a two-step ATP hydrolysis mechanism. Mol Cell. 2014;53(2):330–343. doi: 10.1016/j.molcel.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson JL. Evolution and function of diverse Hsp90 homologs and cochaperone proteins. Biochim Biophys Acta. 2012;1823(3):607–613. doi: 10.1016/j.bbamcr.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Pridgeon JW, Olzmann JA, Chin LS, Li L. PINK1 protects against oxidative stress by phosphorylating mitochondrial chaperone TRAP1. PLoS Biol. 2007;5(7):e172. doi: 10.1371/journal.pbio.0050172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang BH, et al. Regulation of tumor cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network. Cell. 2007;131(2):257–270. doi: 10.1016/j.cell.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 25.Montesano Gesualdi N, et al. Tumor necrosis factor-associated protein 1 (TRAP-1) protects cells from oxidative stress and apoptosis. Stress. 2007;10(4):342–350. doi: 10.1080/10253890701314863. [DOI] [PubMed] [Google Scholar]
- 26.Leav I, et al. Cytoprotective mitochondrial chaperone TRAP-1 as a novel molecular target in localized and metastatic prostate cancer. Am J Pathol. 2010;176(1):393–401. doi: 10.2353/ajpath.2010.090521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sciacovelli M, et al. The mitochondrial chaperone TRAP1 promotes neoplastic growth by inhibiting succinate dehydrogenase. Cell Metab. 2013;17(6):988–999. doi: 10.1016/j.cmet.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasola A, Neckers L, Picard D. Mitochondrial oxidative phosphorylation TRAP(1)ped in tumor cells. Trends Cell Biol. 2014;24(8):455–463. doi: 10.1016/j.tcb.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Felts SJ, et al. The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties. J Biol Chem. 2000;275(5):3305–3312. doi: 10.1074/jbc.275.5.3305. [DOI] [PubMed] [Google Scholar]
- 30.Lee C, et al. Development of a mitochondria-targeted Hsp90 inhibitor based on the crystal structures of human TRAP1. J Am Chem Soc. 2015;137(13):4358–4367. doi: 10.1021/ja511893n. [DOI] [PubMed] [Google Scholar]
- 31.Richter K, Muschler P, Hainzl O, Buchner J. Coordinated ATP hydrolysis by the Hsp90 dimer. J Biol Chem. 2001;276(36):33689–33696. doi: 10.1074/jbc.M103832200. [DOI] [PubMed] [Google Scholar]
- 32.Mishra P, Bolon DN. Designed Hsp90 heterodimers reveal an asymmetric ATPase-driven mechanism in vivo. Mol Cell. 2014;53(2):344–350. doi: 10.1016/j.molcel.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dollins DE, Warren JJ, Immormino RM, Gewirth DT. Structures of GRP94-nucleotide complexes reveal mechanistic differences between the hsp90 chaperones. Mol Cell. 2007;28(1):41–56. doi: 10.1016/j.molcel.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Immormino RM, et al. Ligand-induced conformational shift in the N-terminal domain of GRP94, an Hsp90 chaperone. J Biol Chem. 2004;279(44):46162–46171. doi: 10.1074/jbc.M405253200. [DOI] [PubMed] [Google Scholar]
- 35.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372(3):774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 36.Scheibel T, et al. The charged region of Hsp90 modulates the function of the N-terminal domain. Proc Natl Acad Sci USA. 1999;96(4):1297–1302. doi: 10.1073/pnas.96.4.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergerat A, et al. An atypical topoisomerase II from Archaea with implications for meiotic recombination. Nature. 1997;386(6623):414–417. doi: 10.1038/386414a0. [DOI] [PubMed] [Google Scholar]
- 38.Wigley DB, Davies GJ, Dodson EJ, Maxwell A, Dodson G. Crystal structure of an N-terminal fragment of the DNA gyrase B protein. Nature. 1991;351(6328):624–629. doi: 10.1038/351624a0. [DOI] [PubMed] [Google Scholar]
- 39.Ban C, Junop M, Yang W. Transformation of MutL by ATP binding and hydrolysis: A switch in DNA mismatch repair. Cell. 1999;97(1):85–97. doi: 10.1016/s0092-8674(00)80717-5. [DOI] [PubMed] [Google Scholar]
- 40.Richter K, et al. Intrinsic inhibition of the Hsp90 ATPase activity. J Biol Chem. 2006;281(16):11301–11311. doi: 10.1074/jbc.M510142200. [DOI] [PubMed] [Google Scholar]
- 41.Vaughan CK, Piper PW, Pearl LH, Prodromou C. A common conformationally coupled ATPase mechanism for yeast and human cytoplasmic HSP90s. FEBS J. 2009;276(1):199–209. doi: 10.1111/j.1742-4658.2008.06773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Panaretou B, et al. ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J. 1998;17(16):4829–4836. doi: 10.1093/emboj/17.16.4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obermann WM, Sondermann H, Russo AA, Pavletich NP, Hartl FU. In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J Cell Biol. 1998;143(4):901–910. doi: 10.1083/jcb.143.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson BD, et al. Hsp90 chaperone activity requires the full-length protein and interaction among its multiple domains. J Biol Chem. 2000;275(42):32499–32507. doi: 10.1074/jbc.M005195200. [DOI] [PubMed] [Google Scholar]
- 45.Schumacher RJ, et al. ATP-dependent chaperoning activity of reticulocyte lysate. J Biol Chem. 1994;269(13):9493–9499. [PubMed] [Google Scholar]
- 46.Gasko O, Danon D. Deterioration and disappearance of mitochondria during reticulocyte maturation. Exp Cell Res. 1972;75(1):159–169. doi: 10.1016/0014-4827(72)90532-0. [DOI] [PubMed] [Google Scholar]
- 47.Wei H, Ruthenburg AJ, Bechis SK, Verdine GL. Nucleotide-dependent domain movement in the ATPase domain of a human type IIA DNA topoisomerase. J Biol Chem. 2005;280(44):37041–37047. doi: 10.1074/jbc.M506520200. [DOI] [PubMed] [Google Scholar]
- 48.Cunningham CN, Southworth DR, Krukenberg KA, Agard DA. The conserved arginine 380 of Hsp90 is not a catalytic residue, but stabilizes the closed conformation required for ATP hydrolysis. Protein Sci. 2012;21(8):1162–1171. doi: 10.1002/pro.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young JC, Hartl FU. Polypeptide release by Hsp90 involves ATP hydrolysis and is enhanced by the co-chaperone p23. EMBO J. 2000;19(21):5930–5940. doi: 10.1093/emboj/19.21.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Genest O, Hoskins JR, Kravats AN, Doyle SM, Wickner S. Hsp70 and Hsp90 of E. coli directly interact for collaboration in protein remodeling. J Mol Biol. 2015;427(24):3877–3889. doi: 10.1016/j.jmb.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minor W, Cymborowski M, Otwinowski Z, Chruszcz M. HKL-3000: The integration of data reduction and structure solution from diffraction images to an initial model in minutes. Acta Crystallogr D Biol Crystallogr. 2006;62(Pt 8):859–866. doi: 10.1107/S0907444906019949. [DOI] [PubMed] [Google Scholar]
- 52.Adams PD, et al. PHENIX: Building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr. 2002;58(Pt 11):1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- 53.Murshudov GN, et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr. 2011;67(Pt 4):355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 55.Carter SG, Karl DW. Inorganic phosphate assay with malachite green: An improvement and evaluation. J Biochem Biophys Methods. 1982;7(1):7–13. doi: 10.1016/0165-022x(82)90031-8. [DOI] [PubMed] [Google Scholar]
- 56.Zhai P, Stanworth C, Liu S, Silberg JJ. The human escort protein Hep binds to the ATPase domain of mitochondrial hsp70 and regulates ATP hydrolysis. J Biol Chem. 2008;283(38):26098–26106. doi: 10.1074/jbc.M803475200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sielaff B, Tsai FTF. The M-domain controls Hsp104 protein remodeling activity in an Hsp70/Hsp40-dependent manner. J Mol Biol. 2010;402(1):30–37. doi: 10.1016/j.jmb.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]