ABSTRACT
Clinical activity was observed in metastatic melanoma (MM) patients treated with ipilimumab (IPI) combined with fotemustine (FTM) in the phase II NIBIT-M1 study. Peripheral blood mononuclear cells (PBMCs) and serum were collected from MM patients at pre- and at weeks 12 and 24 post-treatment. A comprehensive phenotypic and functional immunomonitoring of circulating T cells, and the detection of soluble immunoregulatory molecules was carried out and correlated with clinical outcome. The frequency at baseline and along the treatment of circulating T central memory cells expressing activation/differentiation markers, such as CD3+CD4+CD45RO+BTLA+, CD3+CD4+4–1BB or Th17 lymphocytes correlated with the clinical outcome of MM patients. Moreover, either the absence or the presence of soluble NKG2D ligands (ULBP-1 or −2) at baseline in the serum of MM patients enabled to discriminate subjects with long-term survival (median overall survival, (OS) = 33.6 mo for ULBP-1 and −2) from poor survivors (OS = 9.8 or 6.6 mo, respectively). Conversely, no significant association between the levels of soluble MICA, MICB and ULBP-3 and the clinical outcome of patients was observed. An inverse correlation between circulating levels of these molecules at baseline and frequency of either CD3+CD4+CD45RO+BTLA+ or Th17 or CD3+CD4+4–1BB+ T cells occurred in patients with a favorable clinical outcome. The simultaneous monitoring of different immune parameters, though validation in a large cohort of patients is needed, allowed to identify an association between phenotypic and soluble markers representing a possible predictive immunological signature for the clinical activity of IPI plus FTM.
KEYWORDS: Cytotoxic T-lymphocyte Antigen-4 (CTLA-4), Melanoma, NKG2D ligands, T cell subsets
Abbreviations
- BTLA
B and T lymphocyte attenuator
- CTLA-4
Cytotoxic T-Lymphocyte Antigen-4
- DC
Disease Control
- FBS
Fetal Bovine Serum
- FTM
Fotemustine
- ICOS
Inducible T-cell Co-stimulator
- IPI
ipilimumab
- MAA
Melanoma-Associated Antigen
- MICA
MHC (HLA) Class I Chain-Related Gene A
- MICB
MHC (HLA) Class I Chain-Related Gene B
- NKG2D
Natural-Killer Group, Member D
- OR
Objective Response
- OS
Overall Survival
- PD-1
Programmed Cell Death-1
- ULBP-1 or -2
UL16-Binding Protein-1 or -2
Introduction
Monoclonal antibodies (mAbs) that block key immune checkpoint molecules, such as cytotoxic T-lymphocyte antigen 4 (CTLA-4), have shown antitumor effect and modulatory activity on T-cell responses.1,2 These agents showed durable clinical benefit and increased OS,3,4 for patients with advanced melanoma. Since then, the usage of anti-CTLA-4 mAbs (either ipilimumab, IPI or tremelimumab) in several clinical studies or in the context of expanded access programs (EAP) has shown significant increase in OS for MM patients, and promising clinical activity for tumors with other histology.5–8 Of note, long-term clinical responses were achieved by the combination of IPI plus FTM in MM patients, including those with brain metastasis.9,10 However, hallmarks of CTLA-4 blockade therapies are their unknown mechanisms of activity in vivo and that a relatively small subset (≈20%) of patients can achieve a long-term survival.11 Thus, biomarkers predictive of clinical responses from these treatments are needed to optimize patient's selection and for the possible combination with different agents.
Some studies have identified immunological correlates with the clinical efficacy of IPI, although none of them resulted as definitive biomarkers. T cell responses specific for tumor-associated antigens (TAAs) were detected in the circulation of MM patients in association with clinical responses to IPI therapy.12-14 Humoral and T cell responses against TAAs, either pre-existing or induced during the treatment, such as NY-ESO-1, Melan-A/MART-1, MAGE, were found in patients with benefit from IPI.15,16 Moreover, evidences of modification of the balance between effector and regulatory immune responses, involving myeloid derived suppressor cells (MDSCs) and regulatory T cells (Treg), both in the circulation and in tumor infiltrates were reported in association with the clinical activity of IPI.2,17,18 Along this line, the increase of the absolute leukocyte cell count (ALC) or of circulating CD4+ T cells expressing the inducible co-stimulatory molecule (ICOS) have been documented in relation with anti-CTLA-4 mAb therapies.12,19-21 ICOS+ T cells that were augmented by IPI therapy in tumor lesions from prostate cancer patients, represented lymphocytes endowed with antitumor activity.22,23 Moreover, increased levels in the periphery of central memory or effector memory CD4+ and CD8+ T cells have been described in patients undergoing IPI treatment, although there was no correlation with the clinical outcome.24-28
A novel multifaceted immunomonitoring has been set up in the context of the NIBIT-M1 study, with the aim to determine simultaneously phenotypic and functional changes in circulating T cells from MM patients treated with IPI plus FTM. The presence in the baseline serum of MM patients of soluble NKG2D ligands (sNKG2DLs), such as ULBP-1 and ULBP-2, that can play a negative regulatory activity on both T and NK cells, inversely correlated with patients' OS. Moreover, a relationship at baseline between different T cell subsets (central memory either BTLA+ or 4–1BB+ or Th17 T cells) and sNKG2DLs could represent an immunological signature with predictive role for MM patients' clinical outcome.
Material and methods
Melanoma patients
Patients with measurable unresectable stage III or stage IV melanoma with or without brain metastases have been enrolled in a single-arm multi-center phase 2 trial to be treated with IPI 10 mg/kg of i.v. every 3 weeks for a total of 4 doses, then every 12 weeks from week 24, and 100 mg/m2 of i.v. FTM was administered weekly for 3 weeks and then every 3 weeks from week 9 to week 24. These patients could have received a maximum of one previous line of chemotherapy. Response criteria were assessed according the proposed immune-related response criteria, where objective response (OR) included immune-related complete or partial response while disease control (DC) included immune-related confirmed complete, partial or stable disease. For details of the clinical study see Di Giacomo et al, 2012.10 This study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization of Good Clinical Practice and was approved by the Ethics Committee of the participating Italian Institutions. An informed consent was obtained from the enrolled patients.
Biological samples
PBMCs, isolated by Ficoll density gradient centrifugation (Ficoll-Paque PLUS) were cryopreserved from peripheral blood of MM patients, before, at 12 weeks (N = 31) and, when feasible, at 24 weeks (N = 11) post-treatment. Sera were also collected at baseline, at W12 (N = 37) and W24 (N = 23) post-treatment. As control, PBMCs and sera have been collected from healthy donors (HD; N = 10).
Immunofluorescence and flow cytometry analysis
The HLA class I (HLA-A1, -A2, -A3 and –A24) expression by PBMC of MM patients was measured by immunofluorescence (IF) and flow cytometry analysis using the anti-HLA-A1 33642 mAb, anti-HLA-A3 31572 mAb (ABCAM) and anti-HLA-A2 BB7.2 mAb (BD Bioscience). The goat anti-mouse IgG PE conjugated or the goat anti-rabbit IgG FITC (Dako Italia SpA) were used as secondary Abs.
Phenotypic characterization of PBMCs from N=31 patients at baseline and at W12 post-treatment and from N=111 patients at W 24 post-treatment, was done by the multiparametric flow cytometry analysis. Standardized procedures have been applied to optimize the concentration of mAbs to be used and to assess the performance of their combinations. Once the required antibody combinations have been validated, batches of them were prepared and used to provide consistency and to reduce variability of staining. New lots of antibodies have been validated in comparison with that currently used and already titrated. The following mAbs: CD4, CD28, CD134 (OX-40), FITC conjugated; CD69, CD137 (4–1BB), CCR7, CD183, CD272 (BTLA), CD278 (ICOS) PE conjugated; CD45RA ECD conjugated; NKG2D, CD22, CD27, CD56, CD161, CD195 (CCR5) and CD279 (PD-1) allophycocyanin (APC) conjugated; CD4+ APC-Cy7 conjugated; CD196 (CCR6) PercP-Cy5.5 conjugated; CD8+ Pacific Blue conjugated; CD3 Violet 500 conjugated (BD); CD19, CD57, CD62L FITC conjugated; CD69 PE conjugated, CD45RO, PE-Violet 770 conjugated; CD4+ APC-Violet 770 conjugated, CD3 Violet Green conjugated, CD8+ Violet Blue conjugated, CD45RA PerCP-Violet 700 conjugated (Miltenyi Biotec); CD152 FITC conjugated (Cedarlane Labs); IL-12Rβ2 APC, IL23R FITC conjugated (R&D Systems). Lymphocytes were incubated with batches of combinations of Ab (see Table S1 of Supplementary Results) to surface markers at 4°C for 30 min, washed with PBS 5% FBS buffer and fixed with 0.5% paraformaldehyde. Intracellular staining was performed using BD Cytofix/Cytoperm Fixation/Permeabilization Solution Kit (BD) and stained for IL-17 with the IL-17A Alexa-Fluor 700 conjugated mAb (BD).
Data were acquired by BD LSR-Fortessa System flow cytometer (BD); this instrument was accurately calibrated by the usage of 7-color setup beads (BD) to adjust the voltages for each of the detectors. As a result, stained cell samples are properly scaled on fluorescence. The setup beads were also used to adjust spectral overlap, which is subsequently converted to compensation. Moreover, Sphero Ultra Rainbow Calibration Particles (Serotech) designed for routine calibration and for long-term performance tracking of the instrument were used. Cell samples stained by the method being used for testing were prepared and used to optimize fluorescence detector voltages. The acquired data were analyzed with Kaluza Software (Beckman Coulter). The lymphocytes were gated by physical parameters (FSC × SSC) and then gated either on CD3+, CD3+CD4+, CD3+CD8+, CD3+CD4+CD45RA+, CD3+CD4+CD45RO+, CD3+CD8+CD45RA+ or CD3+CD8+CD45RO+ cells (Fig. S1 of Supplementary Results). The surface markers indicated above were evaluated in association with these gated lymphocyte subsets. For Th17 T cell subset the expression of CD183, CD161, CD195, CD196, IL-23R and IL-12Rβ2, which marker association represents a tool to identify Th1/Th17 subset,29 were evaluated in association with either the CD3+CD4+ or CD3+CD8+ T cells expressing IL-17. CD3−CD56+CD16+ was used to identify NK cells, while staining for CD19, CD20 and CD22 was used to identify B cells. For each patient a report of the analysis indicating the gating strategy of lymphocyte populations and a text file with the % of positive cells and the mean of fluorescence intensity for each gated lymphocyte population have been generated. Results are expressed as % of positive cells subtracted of the negative control values. PBMCs from healthy donors (N = 10) represented control samples for these analyses.
Detection of sNKG2DLs in the serum of melanoma patients
sNKG2DL (MICA, MICB, ULBP-1 and ULBP-2) concentrations were determined by commercially available ELISA kits (R&D Systems) in the serum of MM patients at pre-, 12W (N = 37) and 24W (N = 23) post-treatment with IPI plus FTM. For ULBP-3 the commercially available pair antibodies and related reagents (R&D Systems) were used for ELISA assay. A standard curve with determined titrations of the recombinant human proteins allowed to assess sNKG2DL concentrations in the testing sera. Data are represented as pg/mL and are averages of duplicates. Statistical analysis of differences between means of concentration of NKG2DLs at different time points was performed using two-tailed t-test (p < 0.05).
Statistical analysis
Data were analyzed in a descriptive way using mean and standard deviations, absolute frequencies and percentages. This study had an exploratory intent in order to suggest variations in candidate biomarkers possibly associated with OR, DC and OS. OS was also dichotomized in two groups (less or more than 24 mo) to characterize long-term survivors. Differences in means were assessed with the Student t-test (paired or not, as appropriate). Survival curves were estimated by the Kaplan–Meier method and differences were evaluated with the log-rank test.
Under the condition of a limited sample size (N=31 patients at baseline who have been analyzed both for circulating T cell phenotype and for sNKG2DLs in the serum) and in order to better investigate the association between long-term survivors and immunological markers we applied a CART analysis, a recursive partitioning non-parametric method. Also in this case the analysis was merely explorative and hypotheses generating. Due to this aspect no correction for multiple variables analysis was considered. Cut-off of 75th percentile instead of median value was chosen in order to identify a subgroup with fewer but more characterized patients.
Results
Frequency of circulating lymphocyte subsets in MM patients versus HD
The phenotype analysis of circulating T cell subsets was performed at baseline (W1) in MM patients enrolled in the NIBIT-M1 clinical study as compared with samples from HD.
Naïve (CD45RA+) CD4+ or CD8+ T cells expressing either differentiation or activation markers, such as CCR7, BTLA, CTLA-4 and NKG2D, or CD4+CD45RO+ICOS+ were found at higher levels (mean of the % of positive cells) in MM patients versus HD (p < 0.01); representative results are shown in Fig. 1 Panel A. Interestingly, CD3+CD4+ or CD3+CD8+ T cells co-expressing CXCR3, CCR5, CCR6 and IL-23R, representing Th17 cells, were detected at higher levels at baseline in MM patients then in HD (p < 0.001), (24 and 19 versus 2.5 and 0.5 mean of the % of positive cells, respectively; Fig. 1 Panel A). The frequency of these lymphocytes significantly correlated with CD4+ (p = 0.004) and CD8+ (p = 0.06) T cells positive for IL-17, as shown by intracellular staining of these cells with the specific anti-IL-17 mAb. On the other hand, T lymphocytes both CD4+ (p = 0.0001) and CD8+ (p = 0.03) expressing the differentiation molecule CD62L belonging either to naïve or memory subsets were detected at lower levels in patients versus HD, indicating that a general down-modulation of naive prone to differentiation and central memory (Tcm) T cells can occur in MM patients (Fig. 1 Panel A).
Figure 1.
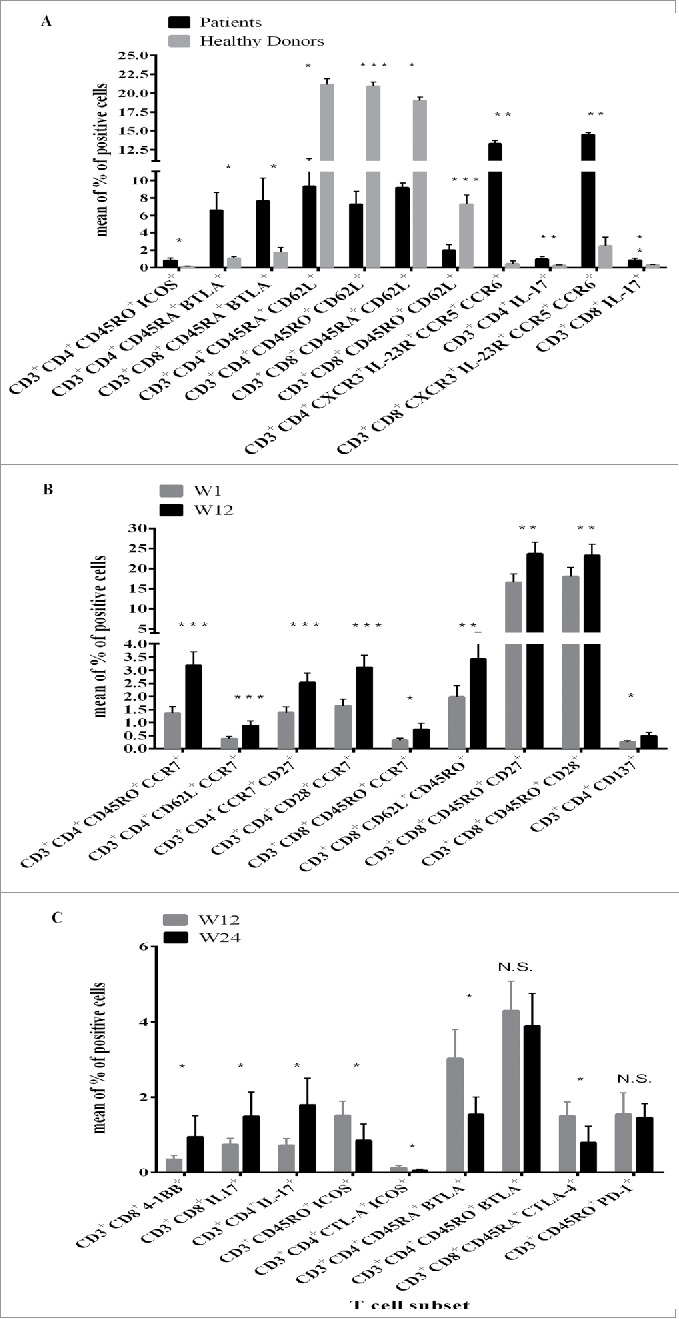
Frequency of T cell subsets in PBMCs of MM patients at baseline as compared with HD or in relation with IPI plus FTM treatment. The phenotype characterization of T cell subsets was performed by IF and cytofluorimetric analysis (see Table S1 and Material and Methods) on PBMCs from MM patients or HDs. The lymphocytes were gated by physical parameters (FSC × SSC) and then gated either on CD3+, CD3+CD4+, CD3+CD8+, CD3+CD4+CD45RA+, CD3+CD4+CD45RO+, CD3+CD8+CD45RA+ or CD3+CD8+CD45RO+ cells (Fig. S1). The surface markers were evaluated in association with these gated lymphocyte subsets. For Th17 T cell subset the expression of CXCR3, CD161, CCR5, CCR6, IL-23R 2 or of the intracellular staining for IL-17 were evaluated in association with either the CD3+CD4+ or CD3+CD8+ gated cells. Results were measured as % of positive cells subtracted of the negative control values. Panel A. PBMCs from N = 31 MM patients (black bar) enrolled in the NIBIT-M1 clinical study and from N = 10 HDs (gray bar) were analyzed. In the figure the mean of the % of positive cells and the standard error (SE) are represented. Panel B: The changes in the frequency of T cell subsets in MM patients were analyzed at baseline (W1; gray bar) and along with therapeutic treatment (W12; black bar). Results are shown as the mean of the % of positive cells and the related standard error (SE). Panel C: Changes in terms of the mean of the % of positive cells for defined T cell subsets at W12 (gray bar) versus W24 (black bar) post-treatment in MM patients are shown. The standard error for each bar is represented a swell. For all the result differences in means were assessed with the Student t-test. *: p < 0.03; **: p < 0.004; ***: p < 0 .0001; N.S.: not significant.
Phenotype analysis of peripheral blood lymphocytes of treated patients
The frequency of circulating T lymphocyte subsets in MM patients in the course of treatment was assessed by the comparison between: (i) PBMCs at W1 and W12; (ii) PBMCs at W12 and W24.
Changes in T cell phenotype subsets were observed along with IPI plus FTM treatment in PBMCs of MM patients. An increase in the frequency in the peripheral blood of both CD4+ and CD8+ central memory T cells (CD3+CD45RO+CCR7+ and/or CD62L+) was observed at W12 post-IPI treatment as compared with baseline (p < 0 .003) (Fig. 1 Panel B). Moreover, these circulating T cells co-expressed the co-stimulatory molecules CD27 and CD28 (Fig. 1 Panel B), supporting the hypothesis that antigen specific expansion and maturation of T cells occurred in vivo in association with the treatment. The terminally differentiation marker CD57 was not detected on the analyzed circulating T cell subsets (data not shown). On the other hand, a significant decrease of the frequency of circulating naive (CD45RA+) T lymphocytes co-expressing or not CD27, CD28 and BTLA (p < 0 .001) was found in PBMCs of MM patients at W12 post-treatment versus W1 (data not shown). In addition, CD3+CD4+4–1BB+ T cells were increased at W12, slightly in term of the % of positive cells but with statistical significance (p = 0.04).
Even though the collection of PBMCs at W24 post treatment was limited to 11 MM patients, a significant increase versus W12 in terms of frequency of CD3+CD8+4–1BB+ (p = 0.01) and of both CD4+ (p = 0.04) and CD8+(p = 0.07) IL-17+ T lymphocytes was detected (Fig. 1 Panel C). In addition, CD3+CD4+CD45RA+ BTLA+ T cells, that were higher in MM at baseline as compared with HD, were decreased (p = 0.01; Fig. 1 Panel C) at W24 post-treatment. Similarly, the frequency of other circulating T cell subsets belonging to the “naïve” compartment, such as CD3+CD8+CD45RA+CTLA-4+ (Fig. 1 Panel C) and CD3+CD45RA+PD-1+ (data not shown) were decreased at W24. On the other hand, the frequency of ‘memory’ T cells co-expressing BTLA or PD-1 was not significantly changed at W24 versus W12 (Fig. 1 Panel C). The frequency of circulating CD3+CD45RO+CTLA-4+ICOS+ and CD3+CD4+CTLA-4+ICOS+ at W24 versus W12 following the treatment was reduced.
The presence of NK and B cells in PBMCs was determined in concomitance with T cell subsets mentioned above; however, no significant changes were detected along with the therapeutic regimen or at baseline as compared with HD.
T cell subsets and clinical outcome of MM patients
The correlation among the frequency of multiple T cell subsets identified by IF and cytofluorimetric analysis and clinical responses were analyzed at baseline and at W12 post-treatment in MM patients. In Tables 1–3 are reported all the T cell subsets that showed any association with clinical responses.
Table 1.
Circulating T cell subsets associated at baseline with clinical responses to IPI plus FTM.
| T cell subset | DC | Meana | Standard Error | p |
|---|---|---|---|---|
| CD3+CD4+CD45RO+CD62L+ | Yes | 10.04 | 2,07 | 0.01 |
| No | 2.87 | 1,21 | ||
| CD3+CD8+CD45RO+CD62L+ | Yes | 2.63 | 0.62 | 0.05 |
| No | 0.96 | 0.38 | ||
| CD3+ICOS+NKG2D+ | Yes | 0.10 | 0.034 | 0.02 |
| No | 0.01 | 0.004 | ||
| CD3+CD45RO+ICOS+ | Yes | 1.55 | 0.64 | 0.05 |
| No | 0.20 | 0.10 | ||
| T cell subset | OR | Meana | Standard Error | P |
| CD3+CD4+CD45RO+CD69+ | Yes | 3.2 | 0.91 | 0.03 |
| No | 0.92 | 0.36 | ||
| CD3+CD4+CD45RO+CD62L+ | Yes | 11.03 | 2.33 | 0.02 |
| No | 4.15 | 1.59 | ||
| CD3+CD4+CD45RO+CCR7+ | Yes | 1.91 | 0.47 | 0.04 |
| No | 0.91 | 0.17 | ||
| CD3+CD4+CD28+CCR7+ | Yes | 2.22 | 0.48 | 0.05 |
| No | 1.16 | 0.20 | ||
| CD3+CD4+CXCR3+CCR6+CD161+ | Yes | 2.06 | 0.62 | 0.04 |
| No | 0.60 | 0.22 |
DC: disease control; OR: overall response; a: mean of the percentage of positive cells; number of patients with DC =19; number of patients without DC = 12; number of patients with =OR = 14; number of patients without OR = 17.
Table 2.
Relationship between the frequency at W12 versus baseline of circulating T cell subsets and clinical responses of MM patients.
| T cell subset | DC | Meana | Standard Error | P |
|---|---|---|---|---|
| CD3+CD45RO+BTLA+ | Yes | 258.54 | 20.97 | 0.03 |
| No | −24.54 | 115.63 | ||
| CD3+CD4+CD45RO+BTLA+ | Yes | 75.08 | 23.14 | 0.05 |
| No | −21.50 | 26.96 | ||
| CD3+CD4+CXCR3+CCR5+ CCR6+IL-23R+ | Yes | 95.06 | 56.69 | 0.05 |
| No | −25.76 | 17.49 | ||
| CD3+BTLA+CTLA-4+ | Yes | 20.70 | 81.77 | 0.03 |
| No | −27.44 | 35.13 | ||
| T cell subset | OR | Meana | Standard Error | P |
| CD3+ CD4+CD45RO+ICOS+ | Yes | 319.91 | 101.46 | 0.05 |
| No | 96.05 | 51.78 | ||
| CD3+CD4+CD45RO+BTLA+ | Yes | 81.49 | 31.51 | 0.05 |
| No | 1.62 | 24.11 | ||
| CD3+CD8+CD45RO+BTLA+ | Yes | 67.90 | 25.59 | 0.05 |
| No | −4.92 | 22.79 | ||
| CD3+CD45RO+PD-1+ | Yes | 256.87 | 101.18 | 0.05 |
| No | 38.96 | 36.48 | ||
| CD3+CD8+CD45RO+PD-1+ | Yes | 170.57 | 72.80 | 0.05 |
| No | 21.14 | 30.78 |
DC: disease control; OR: objective response; a relative difference of the mean of the % of positive cells between baseline and W12 in PBMCs from melanoma patients; number of patients with DC = 19; number of patients without DC = 12; number of patients with = OR = 14; number of patients without OR = 17.
Table 3.
Relationship between the frequency either at baseline or at W12 of circulating T cell subsets and OS of MM patients.
| T cell subset | OS | Meana Baseline | Standard Error | P |
|---|---|---|---|---|
| CD3+CD4+CD137+ | < 24 mo. | 0.17 | 0.20 | 0.04 |
| > 24 mo. | 0.36 | 0.29 | ||
| CD3+CD4+CD45RO+BTLA+ | < 24 mo. | 2.27 | 0.58 | 0.05 |
| > 24 mo. | 6.64 | 2.25 | ||
| CD3+CD8+CD45RA+CTLA-4+ | < 24 mo. | 2.06 | 0.55 | 0.02 |
| > 24 mo. | 0.55 | 0.14 | ||
| CD3+CD4+IL-17+ | < 24 mo. | 0.34 | 0.09 | 0.02 |
| > 24 mo. | 1.74 | 0.60 | ||
| CD3+CD8+IL-17+ | < 24 mo. | 0.38 | 0.10 | 0.02 |
| > 24 mo. | 1.41 | 0.44 | ||
| T cell subset | OS | Meana W12 | Standard Error | P |
| CD3+ CD45RO+CD62L+ | < 24 mo. | 4.39 | 1.55 | 0.02 |
| > 24 mo. | 12.36 | 3.18 | ||
| CD3+ CD4+CD45RO+CD62L+ | < 24 mo. | 5.40 | 1.82 | 0.02 |
| > 24 mo. | 14.34 | 3.24 | ||
| CD3+CD4+CD45RA+BTLA+ | < 24 mo. | 4.63 | 1.28 | 0.01 |
| > 24 mo. | 1.08 | 0.99 | ||
| CD3+CD8+CD45RA+CTLA-4+ | < 24 mo. | 2.04 | 0.52 | 0.007 |
| > 24 mo. | 0.41 | 0.11 |
The association between the frequency of circulating T cell subsets and long-term survival (OS>24 mo) of MM patients has been analyzed and the statistically significant results have been reported in the table. a: mean of the percentage of positive cells in PBMCs from melanoma patients; mo.: months.
Patients with highest values at baseline of Tcm (CD3+CD45RO+CD62L+ and/or CCR7+), both CD4+ and CD8+, showed favorable clinical outcome in terms of disease control (DC; n = 19) or overall response (OR; n = 14) (Table 1). Along this line, circulating CD4+ Tcm subpopulations expressing CD69 (p = 0.03), CD62L (p = 0.02), CCR7 (P = 0.04) or both CD28 and CCR7 (p = 0.05) were found with higher frequency at baseline of patients with positive OR or DC (Table 1). The frequency at baseline of CD4+T cells expressing co-stimulatory receptors, such as ICOS or both ICOS and NKG2D (p = 0.05 or 0.02, respectively) was higher in the circulation of MM patients with DC (Table 1). At the same time point higher values (mean of % of positive cells = 2.06 versus 0.6) of a subset of Th17 T cells (CD3+CD4+CXCR3+CD161+CCR6+) were detected in PBMCs of the subgroup of patients with OR (p = 0.04; Table 1) versus not responders (N = 17). Of note, T cells belonging to Th17 subset (CD3+CD4+CXCR3+CCR5+CCR6+IL-23R+) were significantly (p = 0.05) increased (95.06 relative difference of the % of positive cells) at W12 versus baseline in patients with DC (Table 2).
A striking enrichment (75–82 relative difference of the mean of the % of positive cells; p = 0.05) at W12 post treatment of CD3+CD4+CD45RO+BTLA+ T cells was detected in association with MM patients with either DC or OR while increase in the frequency of CD3+CD8+CD45RO+BTLA+ T cells was found only in relation with OR (p = 0.05; Table 2). Along this line, T cells co-expressing the activation markers BTLA and CTLA-4 were significantly increased at W12 in relation with DC (p = 0.03). Other T cell subsets, CD8+CD45RO+PD-1+ and CD4+CD45RO+ICOS+ were increased (8 and 3-fold increase in the mean of the % of positive cells, respectively; p = 0.05) at W12 along with IPI plus FTM treatment in relationship with OR of MM patients (Table 2). Notably, the frequency at baseline of circulating T cell subsets, such as CD3+CD4+4–1BB+ (P = 0.04), CD3+CD4+CD45RO+BTLA+ (p = 0.05) or Th17 (p = 0.02) T cells discriminated patients with long-term survival (>24 mo) from poorer survivors (Table 3). On the other hand, T cell subsets belonging from the naïve compartment (CD45RA+), including CD3+CD45RA+CD27+/CD28+ (data not shown) and CD3+CD8+CD45RA+CTLA-4+ T cells (p = 0.02; Table 3), were down-modulated in patients with improved survival (OS > 24 mo). These circulating T cell subpopulations, such as CD3+CD8+CD45RA+CTLA-4+ (p = 0.007) and CD3+CD4+CD45RA+BTLA+ (p = 0.01), were also detected with lower values (mean of the % of positive cells) at W12 post-treatment in patients with improved OS while at the same time point higher frequencies of central memory T cells, e.g., CD3+CD4+CD45RO+CD62L+ (p = 0.02), were observed in patients with better OS (Table 3).
The correlation between the frequency of T cell subsets at W24 post-treatment and clinical responses was not evaluated to avoid a possible bias in the analysis, since biological samples from this time point were available only from patients with a better clinical outcome in the course of the treatment.
Correlation between serum levels of sNKG2DLs and clinical outcome
The presence of sULBP-1 was detected at high levels (199–12700 pg/mL) in the baseline serum of 7 MM patients and, for 3 of them, in the course of the treatment with IPI plus FTM the absence or decreased levels of this protein were found (Fig. S2 Panel A). This molecule was either augmented (N = 4) or detected in additional patients (N = 3) (1,155–10,964 pg/mL) at W12 post-treatment. Soluble ULBP-1 was detected in the serum at W24 (562–10,336 pg/mL) of 5 MM patients with, for 3 of them, increased serum levels as compared with W12 (Fig. S2 Panel A) time point.
ULBP-2 and ULBP-3 were also found as soluble form, although with variable levels (88–2,694 and 87–4,867 pg/mL, respectively), in baseline serum of 7 and 16 patients, respectively (Fig. S2 Panel B and C, respectively). Modulation of the post-treatment levels of sULBP-2 and sULBP-3 were also observed (Fig. S2 Panels B and C). sMICA and sMICB were not detectable in the serum of most of MM patients (data not shown).
The serum of all HD was negative for MICA and MICB while only for 2/10 of these subjects 44 and 50 pg/mL of sULBP-1 and 2, respectively were found (data not shown).
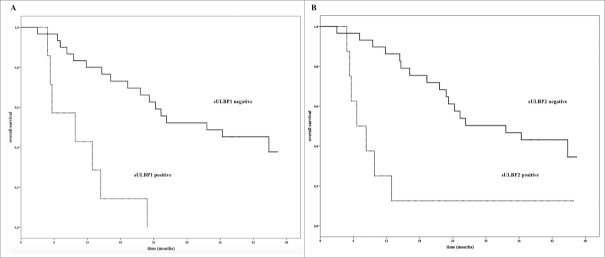
Interestingly, as shown in Figure 2, the absence or the presence at baseline serum of either soluble ULBP-1 (Panel A) or ULBP-2 (Panel B) could distinguish patients according to their OS. Median OS = 33.6 mo for absence of sULBP-1 (P < 0.0001) or sULBP-2 (P < 0.001) versus median OS of 9.8 mo or 6.6 mo, respectively for detectable soluble molecules in the serum.
Figure 2.
Kaplan–Meier plots of OS of MM patients in relation with the levels at baseline of soluble ULBP-1 and ULBP-2 The detection (gray line) or not (black line) sULBP-1 (Panel A) or sULBP-2 (Panel B) in the serum of baseline from MM patients (N = 37) could discriminate patients with long term survival (median OS = 33.6 mo) from poor survivors (median OS = 9.8 or 6.6 mo, respectively); ULBP-1: p < 0.0001; ULBP-2: p < 0.001, mo.: months.
These data indicate that the presence of sULBP-1 or -2 in the serum at baseline of MM patients can affect the clinical activity of the therapeutic combination of IPI plus FTM.
The relationship between T cell subsets and sNKG2DLs correlates with clinical outcome of MM patients
The analysis of correlation between immunological parameters and OS of MM patients undergoing IPI plus FTM treatment lead to the identification of T cell subsets relevant for the clinical outcome of these patients. Notably, MM patients with long-term survival (OS > 24 mo) displayed at baseline PBMCs high frequency of either CD3+CD4+4–1BB+ (p = 0.04), or CD3+CD4+CD45RO+BTLA+ (p = 0.05) or Th17 (p = 0.02) T cell subpopulations (Table 3). The multivariate and CART analysis was applied to assess any association among multiple biological factors and patients' clinical responses. These analyses allowed to identify, for the first time, a strict relationship between the levels of sNKG2DLs in the baseline serum of MM patients and the frequency of defined circulating T cell subsets.
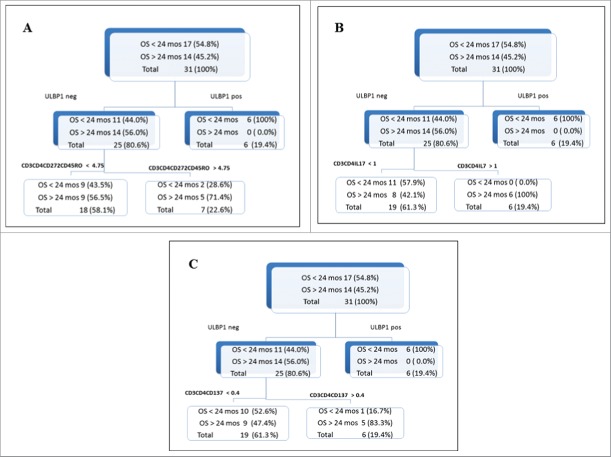
MM patients, as shown by the tree plots in Fig. 3, were divided in long-term survivors (>24 mo; N = 14) versus poor survivor (<24 mo; N = 17). All patients (N = 6) with presence of sULBP-1 in baseline serum had shorter survival (<24 mo; Fig. 3 Panel A upper right box). Within the subpopulation of patients with negative levels of sULBP-1 the 56% (N = 14) were long-term survivors while the 44 % of them (N = 11) showed poor survival (see upper left box in Fig. 3 Panel A). Notably, 71.4 % of patients with high frequency at baseline of circulating CD3+CD4+CD45RO+BTLA+ T cells (% of positive cells >4.75, representing the 75th percentile) (see lower right box Fig. 3 Panel A) showed absence of sULBP-1 in the serum and belong to long-term survivors. On the other hand lower frequency of this T cell subset in the peripheral blood (% of positive cells <4.75, representing the 75th percentile; lower left box of Fig. 3 Panel A) did not impact on OS of patients. The subpopulation of subjects with negative sULBP-1 and high frequency of circulating CD3+CD4+ cells belonging to Th17 T cells (% of positive cells >1, representing the 75th percentile) showed OS >24 mo (100 % of patients; Fig. 3 Panel B, upper left and lower right boxes). On the other hand, lower levels of this T cell subset (% of positive cells <1) did not discriminate patients with long-term OS from poorer survivors (lower left box of Fig.3 Panel B). Similar behavior was observed for the correlation of absence of sULBP-1 and the frequency of CD3+CD4+4–1BB+ T cells in the peripheral blood of MM patients (cut-off value 0.4, representing the 75th percentile), with 83.3% of patients with high frequency of this T cell subset and absence of sULBP-1 showing better OS (>24 mo; Fig. 3 Panel C).
Figure 3.
CART analysis of relationship between multiple immunological markers and OS of MM patients from NIBIT-M1 study CART analysis, a recursive partitioning non parametric method was applied to assess any association among OS of MM patients and multiple immunological parameters, such as the presence at baseline of sNKG2DLs and the frequency of circulating T cell subsets (CD3+CD4+CD45RO+BTLA+, CD3+CD4+4–1BB, and Th17). The values of the 75th percentile of % of positive T cells were used to discriminate patients' groups. Patients were also divided in two groups based on the presence or the absence of sULBP-1 and sULBP-2 in the serum at baseline.
The relationship at baseline between sULBP-2 and the frequency of either CD3+CD4+4–1BB+, or CD3+CD4+CD45RO+BTLA+, or Th17 T cells was found to impact on OS of MM patients in a similar manner as sULBP-1 (data not shown).
Discussion
MM patients could benefit from the treatment with IPI plus FTM in the context of the phase II NIBIT-M1 trial.9 The long-term follow-up analysis of these MM patients showed a median follow-up of 39.9 mo and a median OS of 12.9 mo, with 2- or 3-y survival for 33.4% and 28.5%, respectively.10
The prospective multi-parametric immunomonitoring that was carried out for a group of MM patients enrolled in the NIBIT-M1 study allowed to identify novel immunological parameters associated with the clinical activity of the treatment.
Interestingly, the frequency of different T cell subsets varied in the peripheral blood of MM patients as compared with HD. Either Th17 or CD4+ICOS+ CD45RO+ T cells were found at higher levels at baseline in PBMCs from MM than HD. These baseline differences between circulating T cell subsets may affect the responsiveness of antitumor T cell-mediated responses in the course of immunotherapy treatments and thus impact on the circulating T cell repertoire associated with the treatment with IPI plus FTM.
Along with IPI plus FTM treatment (at W12 post-treatment) the frequency in the circulation of memory T cells, either CD4+ or CD8+, expressing the differentiation markers CD62L and/or CCR7 and either co-stimulatory or activatory molecules (e.g., CD27, CD28, BTLA, PD-1) were increased. Along this line, Th17 T cells were up regulated as well in the course of the treatment, showing that augmentation of circulating ‘antigen experienced’ T cells occurred in relationship with the treatment. Similar observations were previously reported for IPI treatment of MM patients, though no association with the patients' clinical outcome was found.24-28 The detailed phenotypic analysis carried out in PBMCs of patients enrolled in the NIBIT-M1 study allowed to identify for the first time multiple circulating T cell subsets, including memory/activated and Th17 T cells, which frequency in the circulation either at baseline or at W12 post-treatment was associated with the clinical outcome of MM patients. The analysis of the frequency at W24 post-treatment of T cell subpopulations in PBMCs of MM patients was informative for the identification of T lymphocytes persisting in the circulation in the course of treatment. However, since biological samples from this time point belong mostly to patients with better clinical outcome, no correlation of these data with clinical responses was determined. The lack in this study of a control group treated with chemotherapy alone prevented to determine its possible contribution to the observed immunological modulations. Nevertheless, CD3+CD45RO+CD4+ICOS+ T cells were shown to be increased at W12 post-treatment and to correlate with clinical responses in terms of DC and OS in these MM patients (Fig. 1).10 This observation is in line with previous reports showing that this T cell subpopulation was upregulated both in the peripheral blood and at tumor site of MM patients following IPI treatment.19,22,23,28,30-31 The evidence that IPI treatment of MM patients can upregulate the frequency in the periphery of memory/activated T cell subsets was also observed in our study.24-28 Taken together these observations corroborate the hypothesis that changes of the frequency of circulating T cell subsets from patients enrolled in the NIBIT-M1 study resulted from the activity of the checkpoint blockade agent and were not affected by chemotherapy.
IPI can modulate the tumor milieu by decreasing the frequency both in the peripheral blood and at tumor site of immune cells endowed with regulatory functions, such as Tregs and MDSCs.32–34 Moreover, the frequency of these cells in the circulation at baseline of cancer patients has been shown to act as a possible predictor of clinical outcome for IPI regimens.32,35,36 Unfortunately, the limited availability of PBMCs from these patients prevented to evaluate the frequency of circulating Tregs and MDSCs in these MM patients; this analysis will be objective of future studies.
The frequency at baseline or along with treatment of few circulating T cell subsets, such as CD3+CD4+CD45RO+BTLA+, CD3+CD4+4–1BB+ and Th17 T cells correlated with favorable clinical outcome and OS of MM patients. These evidences further support the hypothesis that the treatment can promote the enrichment of activated and differentiated T lymphocytes, possibly including those stimulated by TAAs. It has been previously shown that the expression of inhibitory receptors, such as PD-1 and BTLA, on human CD8+ T cells correlated with the differentiation status and was not associated with their ‘exhaustion’ . 37 Furthermore, T cells isolated from tumor lesions expressing either PD-1 or BTLA identified clonally expanded tumor reactive T lymphocytes that, following T cell adoptive administrations into MM patients, mediated tumor regression.38,39 Albeit future studies need to be performed to characterize ex vivo the functional role of the T cell subsets we have found to be associated with the clinical outcome of MM patients, they could represent candidate predictive or early on treatment biomarkers for IPI plus FTM treatment.
The characterization of MAA and/or tumor recognition by T cells from PBMCs of 23 HLA typed MM patients of the NIBIT-M1 study was assessed. Heterogeneous pattern of MAA reactivity and/or antitumor responses was detected by ex vivo isolated PBMCs (Table S2 and Fig. S3 of Supplementary material). Baseline reactivity by T cells against single or multiple MAAs was detected (Table S2 and Fig. S3). Moreover, a modulation along with the treatment of this pattern of antigen or tumor recognition occurred (Table S2 and Fig. S3). The limited number of evaluated patients prevented to obtain definitive results; nevertheless, an association was found among the presence of circulating T cells reactive either at baseline or at W12 against allogeneic HLA-matched melanoma cell lines (p = 0.07) or MART-1 or Tyrosinase (p = 0.001 and 0.01, respectively) and OS or OR of MM patients (data not shown). The association between NY-ESO-1 specific T cell responses and clinical benefit to immune checkpoint blockade agents has been reported.12,13,15,40 The differences between the conclusions of previous reports and ours may lie in the ex vivo, without any in vitro antigen stimulation of T cells, analysis we have applied to prevent any perturbation of the circulating T cell repertoire. Recently, the detection of T cells reacting against patient-specific mutated antigens has been related with clinical responses to IPI.41–43 The determination of the repertoire of T cells reactive with both differentiation/cancer-testis and mutated TAAs along with the therapeutic usage of immunomodulatory agents need to be further dissected in MM patients. In addition, it is worthy to monitor T cell responses against tumor cells, which in our study represented a possible immunological parameter associated with the clinical outcome of patients.
NKG2DLs represent indicator of cellular stress and are over-expressed by tumor cells.44 Moreover, the release of NKG2DLs in the soluble form by tumor cells represents one well-known mechanism of tumor escape from immunity through the engagement of NKG2D receptor on NK and T cells and the resulting attenuation of their antitumor activity.44,45 The detection of soluble NKG2DLs has been documented in the serum of tumor patients with different histological origins, with, in some cases, a prognostic role.44–46 An interesting observation of a role of the NKG2D pathway in the clinical activity of the combination of vaccination plus anti-CTLA-4 mAb was reported.47 In this context our results showed that the absence versus the presence in the baseline serum of sULBP-1 or -2 could discriminate MM patients with improved (median 33.6 mo) or poor (median 9.8 or 6.6 mo, respectively) OS. To our knowledge, this is the first study reporting the role of sNKG2DLs as possible predictive markers for the clinical outcome of MM patients in the context of IPI regimens. A preliminary analysis showed that the predictive role of sNKG2DLs was independent from the serum levels of LDH, that is a well-assessed prognostic marker for MM patients. Although, we could not evaluate for the NIBIT-M1 study a control group of MM patients, the role of sNKG2DLs in relation with the clinical outcome has been determined in an independent large cohort of MM treated with IPI alone in the context of EAP studies (Maccalli C et al., manuscript in preparation). Recently, it has been reported that soluble CD25 (IL-2Rα) in baseline serum represented an independent indicator of OS for MM patients undergoing IPI therapy, highlighting the need to further investigate the role of soluble molecules as biomarkers for this therapy.48
Notably, we could identify at baseline an inverse correlation between the absence of sNKG2DLs in the serum and the enrichment of few circulating T cell subsets (e.g., CD3+CD4+CD45RO+BTLA+, CD3+CD4+4–1BB+, and Th17) in MM patients with clinical responses to IPI plus FTM regimen.
NKG2D, that represents the receptor of MICA, MICB and ULBPs, was homogeneously express by T lymphocytes (65–90 % of positive CD8+ T cells; data not shown). Higher frequency of T lymphocytes co-expressing ICOS and NKG2D was detected at baseline in patients with better clinical responses (DC), however we could not find any statistically significant association with the levels of sNKG2DLs in the serum of these patients.
In conclusion, the multifaceted immunological monitoring of MM patients enrolled in the NIBIT-M1 study that we have carried out represents a useful tool to evaluate simultaneously different parameters both at baseline and in relation with the therapeutic treatment. We have identified for the first time that the relationship between T cell-mediated immune responses and the absence of sNKG2DLs can represent candidate immunological signature with possible predictive role for clinical outcome of MM patients in the course of IPI plus FTM treatment. These results support further investigations for validation in large cohort of MM patients in the context of therapeutic immune checkpoint blockade agents, including groups of appropriate control patients. Along this line the phase III NIBIT-M2 trial, comparing the treatment with FTM versus the combination of IPI and FTM or IPI and anti-PD-1 mAb in MM patients with brain metastases is ongoing (Eudra CT number 2012–004301–27).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
Acknowledgment
We acknowledge the following NIBIT investigators who collaborated to this study by providing biological samples of MM patients: Dr. Massimo Guidoboni's (Immunotherapy and Cell Therapy Unit, IRCCS IRST, Meldola Italy), Dr. Licia Rivoltini's (Unit of Immunotherapy of Human Tumors Department of Experimental Oncology and Molecular Medicine, Istituto Nazionale Tumori, Milan, Italy) and Dr. Pier Francesco Ferrucci's (Medical Oncology of Melanoma Unit, Division of Medical Oncology, European Institute of Oncology, Milan, Italy) groups.
Supplemental Material
Supplemental Material may be downloaded here: publisher's website
References
- 1.Hoos A, Ibrahim R, Korman A, Abdallah K, Berman D, Shahabi V, Chin K, Canetta R, Humphrey R. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol 2010; 37:533-46; PMID:21074069; http://dx.doi.org/ 10.1053/j.seminoncol.2010.09.015 [DOI] [PubMed] [Google Scholar]
- 2.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med 2009; 206:1717-25; PMID:19581407; http://dx.doi.org/ 10.1084/jem.20082492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al.. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:20525992; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maio M, Grob J-J, Aamdal S, Bondarenko I, Robert C, Thomas L, Garbe C, Chiarion-Sileni V, Testori A, Chen TT et al.. Five-year survival rates for treatment-naïve patients with advanced melanoma who received ipilimumab plus dacabarzine in a phase III trial. J Clin Oncol 2015; 33:1191-6; PMID:25713437; http://dx.doi.org/ 10.1200/JCO.2014.56.6018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ott PA, Hodi FS, Robert C. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res 2013; 19:5300-9; PMID:24089443; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-0143 [DOI] [PubMed] [Google Scholar]
- 6.Rijavec E, Genova C, Barletta G, Burrafato G, Biello F, Dal Bello MG, Coco S, Truini A, Alama A, Boccardo F et al.. Ipilimumab in non-small cell lung cancer and small-cell lung cancer: new knowledge on a new therapeutic strategy. Expert Opin Biol Ther 2014; 14:1007-17; PMID:24702205; http://dx.doi.org/ 10.1517/14712598.2014.907786 [DOI] [PubMed] [Google Scholar]
- 7.Schweizer MT, Drake CG. Immunotherapy for prostate cancer: recent developments and future challenges. Cancer Metastasis Rev 2014; 33:641-55; PMID:24477411; http://dx.doi.org/ 10.1007/s10555-013-9479-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calabrò L, Danielli R, Sigalotti L, Maio M. Clinical studies with anti-CTLA-4 antibodies in non-melanoma indications. Semin Oncol 2010; 37:460-7; PMID:25965359; http://dx.doi.org/22894884 10.1053/j.seminoncol.2010.09.006 [DOI] [PubMed] [Google Scholar]
- 9.Di Giacomo AM, Ascierto PA, Pilla L, Santinami M, Ferrucci PF, Giannarelli D, Marasco A, Rivoltini L, Simeone E, Nicoletti SV et al.. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm phase 2 trial. Lancet Oncol 2012; 13:879-86; PMID:22894884; http://dx.doi.org/ 10.1016/S1470-2045(12)70324-8 [DOI] [PubMed] [Google Scholar]
- 10.Di Giacomo AM, Ascierto PA, Queirolo P, Pilla L, Ridolfi R, Santinami M, Testori A, Simeone E, Guidoboni M, Maurichi A et al.. Three-year follow-up of advanced melanoma patients who received ipilimumab plus fotemustine in the Italian Network for Tumor Biotherapy (NIBIT)-M1 phase II study. Ann Oncol 2015;26:798-803; PMID:25538176; http://dx.doi.org/ 10.1093/annonc/mdu577 [DOI] [PubMed] [Google Scholar]
- 11.Wolchok JD, Weber J, Maio M, Neyns B, Harmankaya K, Chin K, Cykowski L, de Pril V, Humphrey R, Lebbé C. Four-year survival rates for patients with metastatic melanoma who received ipilimumab in phase II clinical trials. Ann Oncol 2013; 24:2174-80; PMID:23666915; http://dx.doi.org/ 10.1093/annonc/mdt161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan J, Gnjatic S, Li H, Powel S, Gallardo HF, Ritter E, Ku GY, Jungbluth AA, Segal NH, Rasalan TS et al.. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc Natl Acad Sci USA 2008; 105:20410-5; PMID:19074257; http://dx.doi.org/ 10.1073/pnas.0810114105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callahan MK, Wolchok JD, James P. Allison. anti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy Semin Oncol 2010; 37:473-84; PMID:21074063; http://dx.doi.org/ 10.1053/j.seminoncol.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan J, Ginsberg B, Page D, Li Y, Rasalan T, Gallardo HF, Xu Y, Adams S, Bhardwaj N, Busam K et al.. CTLA-4 blockade increases antigen-specific CD8(+) T cells in pre vaccinated patients with melanoma: three cases. Cancer Immunol Immunother 2011; 60:1137-46; PMID:21465316; http://dx.doi.org/ 10.1007/s00262-011-1011-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, Xu Y, Pogoriler E, Terzulli SL, Kuk D et al.. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipi. Proc Natl Acad Sci USA 2011; 108:16723-8; PMID:21933959; http://dx.doi.org/ 10.1073/pnas.1110814108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber JS, Hamid O, Chasalow SD, Wu DY, Parker SM, Galbraith S, Gnjatic S, Berman D. Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma. J Immunother 2012; 35:89-97; PMID:22130166; http://dx.doi.org/ 10.1097/CJI.0b013e31823aa41c [DOI] [PubMed] [Google Scholar]
- 17.Tarhini AA, Edington H, Butterfield LH, Lin Y, Shuai Y, Tawbi H, Sander C, Yin Y, Holtzman M, Johnson J et al.. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One 2014; 9:e87705; PMID:24498358; http://dx.doi.org/ 10.1371/journal.pone.0087705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N, Leyvraz L, Michielin O, Romano E, Speiser DE. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother 2014; 63:247-57; PMID:24357148; http://dx.doi.org/ 10.1007/s00262-013-1508-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carthon BC, Wolchok JD, Yuan J, Kamat A, Ng Tang DS, Sun J, Ku G, Troncoso P, Logothetis CJ, Allison JP et al.. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a pre surgical clinical trial. Clin Cancer Res 2010; 16:2861-71; PMID:20460488; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-0569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Giacomo AM, Calabrò L, Danielli R, Fonsatti E, Bertocci E, Pesce I, Fazio C, Cutaia O, Giannarelli D, Miracco C et al.. Long-term survival and immunological parameters in metastatic melanoma patients who responded to ipilimumab 10 mg/kg within an expanded access program. Cancer Immunol Immunother 2013; 62:1021-8; PMID:23591982; http://dx.doi.org/ 10.1007/s00262-013-1418-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calabrò L, Morra A, Fonsatti E, Cutaia O, Amato G, Giannarelli D, Di Giacomo AM, Danielli R, Altomonte M, Mutti L et al.. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol 2013; 14:1104-11; PMID:24035405; http://dx.doi.org/22020206 10.1016/S1470-2045(13)70381-4 [DOI] [PubMed] [Google Scholar]
- 22.Sharma P, Wagner K, Wolchok JD, Allison JP. Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer 2011; 11:805-12; PMID:22020206; http://dx.doi.org/ 10.1038/nrc3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu T, He Q, Sharma P. The ICOS/ICOSL pathway is required for optimal antitumor responses mediated by anti-CTLA-4 therapy. Cancer Res 2011; 71:5445-54; PMID:21708958; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-1138 [DOI] [PubMed] [Google Scholar]
- 24.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ et al.. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA 2003; 100:8372-7; PMID:12826605; http://dx.doi.org/ 10.1073/pnas.1533209100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE et al.. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol 2005; 23:6043-53; PMID:16087944; http://dx.doi.org/ 10.1200/JCO.2005.06.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maker AV, Phan GQ, Attia P, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Haworth LR, Levy C et al.. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte-associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol 2005; 12:1005-16; PMID:16283570; http://dx.doi.org/ 10.1245/ASO.2005.03.536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maker AV, Yang JC, Sherry RM, Topalian SL, Kammula US, Royal RE, Hughes M, Yellin MJ, Haworth LR, Levy C et al.. Intrapatient dose escalation of anti-CTLA-4 antibody in patients with metastatic melanoma. J Immunother 2006; 29:455-63; PMID:16799341; http://dx.doi.org/ 10.1097/01.cji.0000208259.73167.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber JS, Hamid O, Chasalow SD, Wu DY, Parker SM, Galbraith S, Gnjatic S, Berman D. Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma. J Immunother 2012; 35:89-97; PMID:22130166; http://dx.doi.org/ 10.1097/CJI.0b013e31823aa41c [DOI] [PubMed] [Google Scholar]
- 29.Ji Y, Zhang W. Th17 cells: positive or negative role in tumor? Cancer Immunol Immunother 2010; 59:979-87; PMID:20352428; http://dx.doi.org/ 10.1007/s00262-010-0849-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W, Yu D, Sarnaik AA, Yu B, Hall M, Morelli D, Zhang Y, Zhao X, Weber JS. Biomarkers on melanoma patient T cells associated with ipilimumab treatment. J Transl Med 2012; 10:146; PMID:22788688; http://dx.doi.org/ 10.1186/1479-5876-10-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu T, He Q, Sharma P. The ICOS/ICOSL pathway is required for optimal antitumor responses mediated by anti-CTLA-4 therapy. Cancer Res 2011; 71:5445-54; PMID:21708958; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-1138 [DOI] [PubMed] [Google Scholar]
- 32.Tarhini AA, Butterfield LH, Shuai Y, Gooding WE, Kalinski P, Kirkwood JM. Differing patterns of circulating regulatory T cells and myeloid-derived suppressor cells in metastatic melanoma patients receiving anti-CTLA-4 antibody and interferon-α or TLR-9 agonist and GM-CSF with peptide vaccination. J Immunother 2012; 35:702-10; PMID:23090079; http://dx.doi.org/ 10.1097/CJI.0b013e318272569b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menard C, Ghiringhelli F, Roux S, Chaput N, Mateus C, Grohmann U, Caillat-Zucman S, Zitvogel L, Robert C. CTLA-4 blockade confers lymphocyte resistance to regulatory T-cells in advanced melanoma: surrogate marker of efficacy of tremelimumab? Clin Cancer Res 2008; 14:5242-9; PMID:18698043; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-4797 [DOI] [PubMed] [Google Scholar]
- 34.Simeone E, Gentilcore G, Giannarelli D, Grimaldi AM, Caracò C, Curvietto M, Esposito A, Paone M, Palla M, Cavalcanti E et al.. Immunological and biological changes during ipilimumab treatment and their potential correlation with clinical response and survival in patients with advanced melanoma. Cancer Immunol Immunother 2014; 63:675-83; PMID:24695951; http://dx.doi.org/ 10.1007/s00262-014-1545-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weide B, Martens A, Zelba H, Stutz C, Derthovanessian E, Di Giacomo AM, Maio M, Sucker A, Schilling B, Schadendorf D et al.. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY-ESO-1 or Melan-A-specific T cells. Clin Cancer Res 2014; 20:1601-9; PMID:24323899; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-2508 [DOI] [PubMed] [Google Scholar]
- 36.Pico de Coana Y, Poschlke I, Gentilcore G, Mao Y, Nystrom M, Hansson J, Masucci GV, Kiessling R. Ipilimumab treatment results in an early decrease in the frequency of circulating granulocytic myeloid-derived suppressor cells as well as their Arginase 1 production. Cancer Immunol Res. 2013 Sep; 1(3):158-62. doi: 10.1158/2326-6066.CIR-13-0016 Epub 2013 Aug 2. Erratum in: Cancer Immunol Res. 2013 Dec;1(6):438.] http://dx.doi.org/10.1007/s00262-014-1545-8 [DOI] [PubMed] [Google Scholar]
- 37.Legat A, Speiser DE, Pircher H, Zehn D, Fuertes Marraco SA. Inhibitory receptor expression depends more dominantly on differentiation and activation than “Exhaustion” of human CD8 T cells. Front Immunol 2013; 4:1-15; PMID:23355837; http://dx.doi.org/ 10.3389/fimmu.2013.00455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Radvanyi LG, Bernatchez C, Zhang M, Fox PS, Miller P, Chacon J, Wu R, Lizee G, Mahoney S, Alvarado G et al.. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res 2012; 18:6758-70; PMID:23032743; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Wunderlich JR, Mixon A, Farid S, Dudley ME, Hanada K et al.. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J Clin Invest 2014; 124:2246-59; PMID:24667641; http://dx.doi.org/ 10.1172/JCI73639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitano S, Tsuji T, Liu C, Hirschhorn-Cymerman D, Kyi C, Mu Z, Allison JP, Gnjatic S, Yuan JD, Wolchok JD. Enhancement of tumor-reactive cytotoxic CD4+ T cell responses after ipilimumab treatment in four advanced melanoma patients. Cancer Immunol Res 2013; 1:235-44; PMID:24396833; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Rooij N, van Buuren MM, Philips D, Velds A, Toebes M, Heemskerk B, van Dijk LJ, Behjati S, Hilkmann H, El Atmioui D et al.. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol 2013; 31:e439-42; PMID:24043743; http://dx.doi.org/ 10.1200/JCO.2012.47.7521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kvistborg P, Philips D, Kelderman S, Hageman L, Ottensmeier C, Joseph-Pietras D, Welters MJ, van der Burg S, Kapiteijn E, Michielin O et al.. Anti-CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med 2014; 6:254ra128; PMID:25232180; http://dx.doi.org/25409260 10.1126/scitranslmed.3008918 [DOI] [PubMed] [Google Scholar]
- 43.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS et al.. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med 2014;371: 2189-99; PMID:25409260; http://dx.doi.org/ 10.1056/NEJMoa1406498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maccalli C, Scaramuzza S, Parmiani G. TNK (NKG2D+ CD8+ or CD4+ T lymphocytes) in the control of human tumors. Cancer Immunol Immunother 2009; 58:801-7; PMID:19089424; http://dx.doi.org/ 10.1007/s00262-008-0635-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paschen A, Baingo J, Schadendorf D. Expression of stress ligands of the immuno-receptor NKG2D in melanoma: regulation and clinical significance. Eur J Cell Biol 2014; 93:49-54; PMID:24629838; http://dx.doi.org/ 10.1016/j.ejcb.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 46.Baragano Raneros A, Suarez-Alvarez A, Lopez-Larrea C. Secretory pathways generating immunosuppressive NKG2D ligands. New targets for therapeutic intervention. Onco Immunol 2014; 3:e28497-9; PMID:2505021516754847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jinushi M, Hodi FS, Dranoff G. Therapy-induced antibodies to MHC class I-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity. Proc Natl Acad Sci USA 2006; 103:9190-5; PMID:16754847; http://dx.doi.org/ 10.1073/pnas.0603503103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hannani D, Vétizou M, Enot D, Rusakiewicz S, Chaput N, Klatzmann D, Desbois M, Jacquelot N, Vimond N, Chouaib S et al.. Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res 2015; 25:208-24; PMID:25582080; http://dx.doi.org/ 10.1038/cr.2015.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.