Abstract
Whether we feel sympathy for another, listen to our heartbeat, experience pain or negotiate, the insular cortex is thought to integrate perceptions, emotions, thoughts, and plans into one subjective image of “our world”. The insula has hence been ascribed an integrative role, linking information from diverse functional systems. Nevertheless, various anatomical and functional studies in humans and non-human primates also indicate a functional differentiation of this region. In order to investigate this functional differentiation as well as the mechanisms of the functional integration in the insula, we performed activation-likelihood-estimation (ALE) meta-analyses of 1,768 functional neuroimaging experiments. The analysis revealed four functionally distinct regions on the human insula, which map to the social-emotional, the sensorimotor, the olfacto-gustatory, and the cognitive network of the brain. Sensorimotor tasks activated the mid-posterior and social-emotional tasks the anterior-ventral insula. In the central insula activation by olfacto-gustatory stimuli was found, and cognitive tasks elicited activation in the anterior-dorsal region. A conjunction analysis across these domains revealed that aside from basic somatosensory and motor processes all tested functions overlapped on the anterior-dorsal insula. This overlap might constitute a correlate for a functional integration between different functional systems and thus reflect a link between them necessary to integrate different qualities into a coherent experience of the world and setting the context for thoughts and actions.
Keywords: Cognitive, Social-emotional, Sensory, BrainMap
Introduction
Experiencing the warmth of a hot cup is likely to make us feel an interpersonal warmth towards others so that we will interpret their intentions as friendlier and kinder (Williams and Bargh 2008). We can also direct awareness to normally unconscious sensory stimuli from within our body, as we are able to detect our heartbeat and compare it to external rhythms (Critchley et al. 2004). Finally it has been shown that patients suffering from borderline personality disorder cannot integrate a feeling of trust into an economic exchange game and hence trend to undermine cooperation and prevent a successful outcome (King-Casas et al. 2008). At first these three examples seem quite unrelated. However, all of them rely on the integration of information from different functional systems in the brain, and throughout all these scenarios, the insular cortex has been discussed as a neural correlate for such integration. This integrative role between cognitive, sensorimotor, social-emotional, and olfacto-gustatory systems has moreover been hypothesized in various other studies analyzing links between sensation, emotion, and cognition (Chen 2007; Craig 2002; Dolan 2002; Frith and Singer 2008; Johansen-Berg and Matthews 2002). The essential role of the insula in the integration between major functional systems has recently led to the proposal that an insular role in function might constitute a correlate of awareness (Craig 2009).
In spite of this evidence for an integrative role of the human insula, cytoarchitectonic and connectivity data in non-human primates indicate a set of anatomically different insular regions (Augustine 1996; Mesulam and Mufson 1985). Mirroring this anatomical data, several functional imaging studies in humans also suggest a functional differentiation of this region. For example, it was reported that sensory processing is located on the posterior aspect of the insula while olfactory-as well as emotion-elicited processing takes place at more anterior locations (Dolan 2002; Dupont et al. 2003; Ostrowsky et al. 2002; Shelley and Trimble 2004; Wager and Barrett 2004). This differentiation has recently been corroborated by first meta-analyses on insular activations (Mutschler et al. 2009; Wager and Barrett 2004). These analyses, however, were more limited in their scopes and could hence not sufficiently address the issue of differentiation and integration in the human insula across a wide range of functional conditions. Thus, although there is already evidence for a functional differentiation in the human insula, its organization and the relationship to the aforementioned integrative role remain largely elusive. More comprehensive data on functional differentiation and functional integration in this region, however, will be an important precondition for understanding the role of the insular lobe and potentially also contribution of its malfunction in neuropsychiatric disorders.
In order to investigate functional integration and differentiation within the insular cortex and its role in different functional systems, we applied a large-scale meta-analysis on 1,768 functional imaging experiments from 13 functional categories, which were frequently reported to activate the insular cortex. We use the term “functional systems” to refer to the functional networks which are employed in cognitive, social-emotional, sensorimotor, or olfacto-gustatory processing (Cabeza and Nyberg 2000; Corbetta et al. 2008; Kandel et al. 1991; Phillips et al. 2003). The term “functional categories” summarizes the tested “brain functions” from the analyzed experiments like attention, empathy, or interoception. For an overview of the tested functional categories and their included experiments, please see Table 1. To address both, a functional integration as well as a functional differentiation within the insula, two different analyses were applied. One was a conventional meta-analysis which yielded all regions involved in a certain category. These regions included multimodal integration regions that were activated in multiple different categories. The other analysis was a modified meta-analysis, which yielded only regions that showed specific effects in a certain category. Thus, multimodal integration regions were not considered. This enabled a differentiation between multimodal integration and specific processing of different functional categories. To further investigate the insular role in different functional systems, we subsumed familial categories to four functional domains (see Table 2), which reflect these described functional systems.
Table 1.
All 13 categories as defined for analysis
| Category | Papers | Subjects | Experiments | Included paradigms |
|---|---|---|---|---|
| Emotion | 83 | 1,383 | 195 | All paradigms that elicited emotion in the subjects such as induction, imagination or recall of own happiness, fear, anxiety, anger, sadness, or disgust |
| Empathy | 46 | 657 | 120 | All paradigms in which the subjects had to judge emotions in faces or attend to pain in others. This thus involved feeling with the emotions in others, not the more cognitive aspects of “theory of mind” |
| Olfaction | 12 | 175 | 31 | All olfactory stimuli, no stimuli that elicited trigeminal afferents, no gustatory stimuli |
| Gustation | 13 | 162 | 31 | All gustatory tasks. Subjects had to taste tastants compared to a tasteless baseline. No odors |
| Interoception | 43 | 638 | 105 | All visceral sensation, hunger/thirst, sexual arousal, air hunger, changing core temperature or urge to void |
| Pain | 46 | 573 | 79 | All painful stimuli applied to subjects by temperature, electrical or physical stimulation or hypersensitation. Pain was applied to different parts of the subject’s body |
| Somatosensation | 34 | 421 | 46 | All somatosensation, no painful or visceral stimuli. Again, different modalities and bodyparts |
| Motion | 64 | 731 | 125 | All motor tasks, including movement of face, mouth and tongue, and hands/feet irrespective of side |
| Attention | 125 | 1,918 | 264 | Spatial attention, sorting/matching, Stroop/Flanker/Simon/gonogo/Switching tasks, cues |
| Language | 129 | 1,704 | 297 | Semantic, syntactic, phonological, orthographic decisions and listening to language |
| Speech | 51 | 709 | 130 | All motor speech |
| Working memory | 96 | 1,537 | 213 | All short term memory, working memory, n-back tasks, encoding, and recall |
| Memory | 69 | 1,188 | 131 | Memory tasks, recall of information learned previous to the experiment |
| Sum | 811 | 11,796 | 1,768 |
Table 2.
The investigated categories were subsumed to four functional domains, which represent functional systems
| Domain | Category |
|---|---|
| Social-emotional | Emotion and empathy |
| Olfacto-gustatory | Olfaction and gustation |
| Cognitive | Attention, language, speech, working memory, memory |
| Sensorimotor | Interoception, somatosensation, pain, motion |
Methods
We searched the BrainMap database (Fox et al. 2005; Laird et al. 2005b; Laird et al. 2009) and Pubmed for functional neuroimaging experiments investigating paradigms pertaining to 13 functional categories (c.f. Table 1) that are hypothesized to involve the insular cortex. Included studies were normal mapping fMRI or PET experiments (as opposed to the analysis of, e.g., age and gender effects) in healthy subjects and did not comprise pharmacological trials or those involving clinical populations. All studies were whole brain studies. For each category, we only included those contrasts, which clearly aimed at the isolation of the respective function against a high or low level baseline. Thus, for example, “button pressing versus rest” was considered to be a motor task, while “button pressing as a response to attention cues versus random button pressing” was considered to test for attentional responses. Consequently, studies focusing on some kind of functional integration between the categories or multiple components due to unspecific contrasts were not included. All studies obtained from Pubmed were added to the BrainMap database prior to further analysis. By these means, we collected a total of 1,768 experiments from 811 papers, assessing together a population of 11,796 subjects as a basis for the current study (Table 1). A referenced tabular register of all included studies can be found in supplement #1.
Since we hypothesized both a functional integration and a functional differentiation within the insula, the analysis had to serve two different aims. To investigate a functional integration, it had to identify those regions, which were involved in multiple different categories. This was assessed by a conventional meta-analysis for each category which mapped all involved regions, including multimodal integration regions. Subsequently, integration regions were identified by calculating the overlap between the regions which resulted from the conventionally analyzed categories. To achieve the second aim of the analysis, we calculated a modified meta-analysis, which identified those regions that were more active in a certain functional category than in a random selection of tasks. Consequently, it enabled the mapping of those areas which show specific effects to a certain category. Multimodal integration areas, which are active in multiple functional categories, were therefore not incorporated in this second, modified analysis. This mapping of specific effects thus enabled an investigation of a functional differentiation.
Analysis
For each category the reported coordinates for functional activations were analyzed for topographic convergence using the activation likelihood estimation (ALE) method. The idea behind this approach is that reported foci are not treated as single points but as localization probability distributions centered at the given coordinates, which were modeled by three-dimensional Gaussean functions (Laird et al. 2005a). Thus, the reported maxima are assigned a spatial profile representing the probability for their true location based on an empirical model for the spatial uncertainty associated with neuroimaging results. As this uncertainty (and thus the applied FWHM) is directly dependent on the number of subjects, it was calculated individually for each experiment based on empirical estimates of between-subject variability (see Eickhoff et al. 2009). Subsequently, an ‘activation likelihood estimate’ (ALE), given by the union of the probabilities associated with the different foci, was calculated for each voxel.
Conventional ALE analysis mapping
The ALE map for any given category indicates the convergence of the individual studies of that category at each voxel but does not yet allow distinguishing random convergence (noise) from true clustering of reported activation. To this end, the significance of the convergence across individual experiments reflected by the ALE map was statistically evaluated to identify those regions, where ALE scores were higher than could be expected by chance. The null-hypothesis, against which a particular ALE map was tested, represented the case of no convergence between the single experiments of the respective category (Eickhoff et al. 2009), that is, no spatial association between the different individual experiments. To assess this hypothesis, an independently sampled random voxel was drawn from each experiment, and an ALE score was calculated from these and recorded. This procedure was iterated 2 × 1010 times (1 × 105 times for each of the ~200,000 voxel per volume) to generate a null-distribution, against which the ALE map can then be compared (Eickhoff et al. 2009). ALE probability maps were then thresholded at p < 0.05 (cluster level corrected for multiple comparisons).
Mapping the specific functional processing
We also tested whether the convergence across experiments contained in a particular category was higher than across an equally large random sample of experiments from the BrainMap database. By this means we were able to account for the presence of multimodal integration areas that integrate and relay information from different functional categories. These areas consequently show no specific effects for a certain category and are thus not incorporated in this analysis. The null hypothesis, against which the ALE maps were tested, represented the case that no region showed a higher convergence between the tested experiments than across an equally large random sample of neuroimaging experiments. To assess this hypothesis, we used all experiments of the BrainMap database (altogether 7,156 at the time of calculation). From this sample, the same number of experiments as in the subset of studies that is being tested was drawn randomly. An ALE map was then calculated across this randomly drawn subset of experiments and stored. This procedure was again iterated 105 times to generate the null-distribution, against which the actual ALE map was tested. The resulting ALE probability maps were then thresholded at p < 0.05 (cluster level corrected for multiple comparisons).
Evaluation and interpretation
For the identification of multimodal integration regions, we used the results from the conventional ALE, as they comprised all regions involved in a category. Since these multimodal integration regions are not specific for one certain category but involved in several, we calculated the overlap between all regions. To this means, we calculated two different conjunctions. The first conjunction comprised all 13 categories and assumed that there was an area which was active in all categories. The second conjunction comprised all categories except somatosensation and motion. This second conjunction was calculated as the analyzed somatosensory and motor tasks did not need cognitive or emotional evaluation. It could therefore be assumed, that these somatosensory and motor tasks would be executed without any need for a functional integration with other brain systems.
For the investigation of a functional differentiation it has to be kept in mind that different functional categories can be grouped to the same functional systems. For example, both attention and working memory tasks employ—among other regions—roughly a fronto-parietal network, which is commonly found to be activated in cognitive tasks (Cabeza and Nyberg 2000; Corbetta et al. 2008; Kandel et al. 1991). This cognitive network differs from a social-emotional network, which is employed by processing emotional stimuli and empathy (Phillips et al. 2003). To investigate a functional differentiation, we sorted familial categories to four functional domains (c.f. Table 2), which represent the respective functional systems. For each functional domain a modified analysis was calculated and the results mapped on the insula. Thus, we were able to identify those regions that were specifically activated by processing a certain domain.
For evaluation of insular activation a macroanatomical mask of the insular cortex in MNI-space was created and used for masking the resulting activation from the ALE meta-analysis. The mask comprised the gray matter of the insular cortex, which is limited by the anterior, superior and inferior limiting sulci, the extreme capsule, and the CSF (Mesulam and Mufson 1985; von Economo and Koskinas 1925; Zilles 2004). Activations within this mask were then projected onto a single subject template, from which temporal lobe and operculum were removed to gain a free view on the insular cortex (see Fig. 1). Thus, the topographic relationships of the resulting activations to each other and to anatomical landmarks of the insular cortex could be investigated. Activations were also assigned histologically using the SPM Anatomy Toolbox (Eickhoff et al. 2005). The latter approach was important in order to eliminate activation of, e.g., the parietal operculum spilling over into the insular mask.
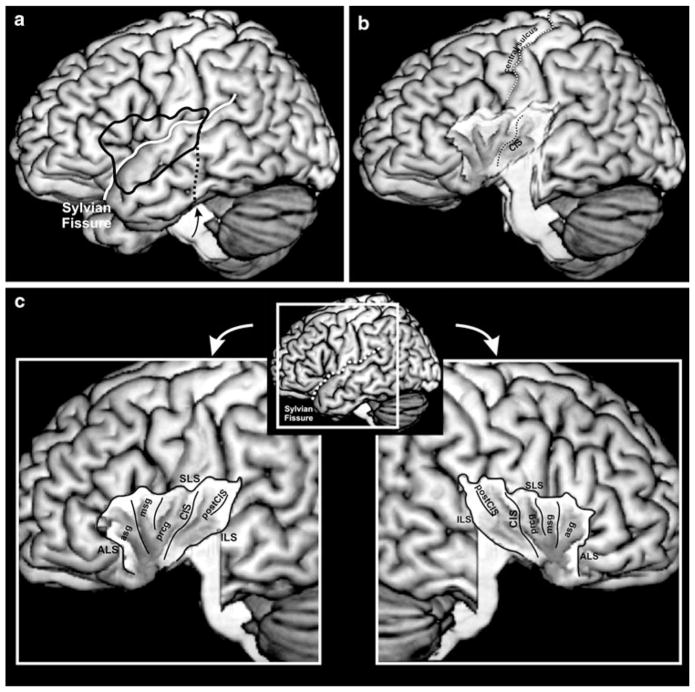
Fig. 1.
To gain a free view on the insula, temporal lobe and opercula were removed (a, b). c visualization of the insular cortex, view on the left side left, on the right side right. This visualization will be used to display the results. ILS inferior limiting sulcus, SLS superior limiting sulcus, ALS anterior limiting sulcus, CIS central insular sulcus, postCIS postcentral insular sulcus, prcg precentral gyrus, msg middle short gyrus, asg anterior short gyrus
Results
As outlined above, we investigated the functional organization of the human insula by conventional ALE analysis of activations associated with different cognitive, sensori-motor, and social-emotional processes (cf. Table 2). Moreover, by using the modified variant of the ALE algorithm, we could delineate those insular regions, which were specific to a particular process. Throughout all analyses (Figs. 2, 3, 4, 5, maxima provided in Table 3), we observed that specific effects were much more confined as compared with the broader regions that were associated with a particular function per se. This observation already indicates that although functional segregation, i.e., specialization is present, integration of information across functional domains is also happening in the insular cortex.
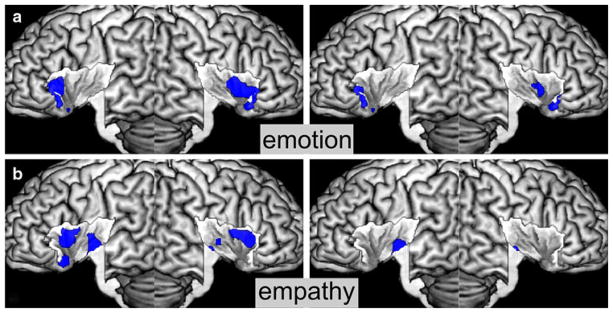
Fig. 2.
Insular activations by the social-emotional domain. a Activations by emotion, b activations by empathy. Conventional results are displayed on the left, specific results on the right
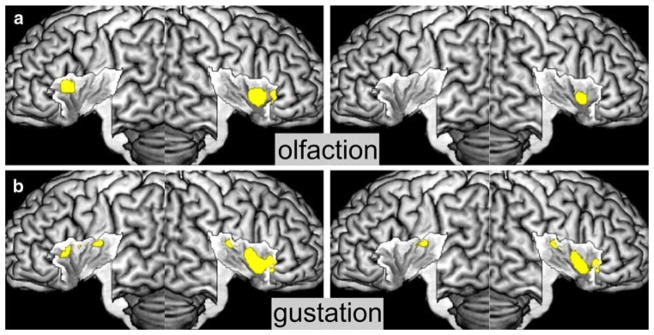
Fig. 3.
Insular activations by the chemical sensory domain. a Activations by olfaction, b activations by gustation. Conventional results are displayed on the left, specific results on the right
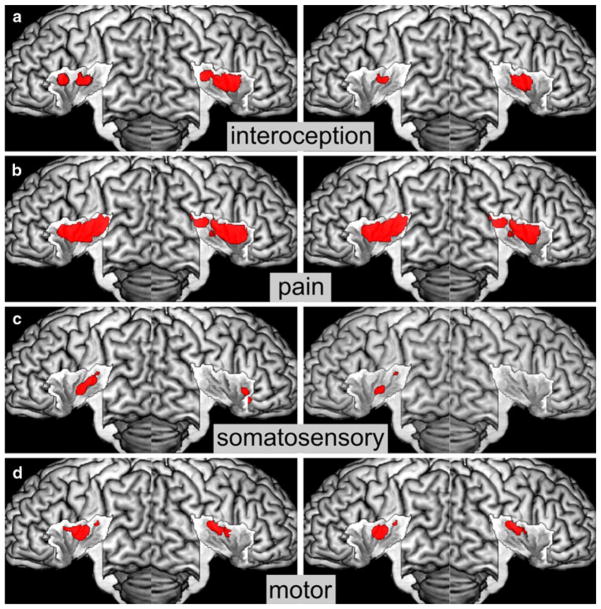
Fig. 4.
Insular activations by the sensorimotor domain. a Activations by interoception, b activations by pain, c activations by somatosensation, d activations by motion. Conventional results are displayed on the left, specific results on the right
Fig. 5.
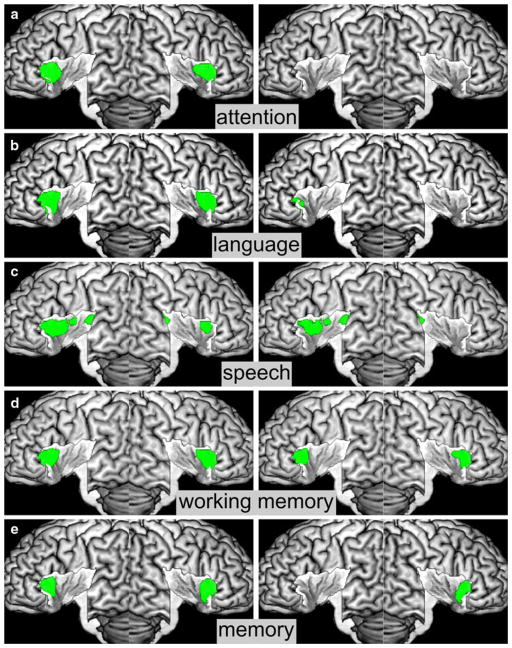
Insular activations by the cognitive domain. a Activations by attention, b activations by language, c activations by speech d activations by working memory, e activations by memory. Conventional results are displayed on the left, specific results on the right
Table 3.
Insular maxima for each analyzed category
| Category | Conventional analysis
|
Specific analysis
|
||||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | T | x | y | z | T | |
| Emotion | −31 | 24 | −4 | 6.13 | −31 | 24 | −6 | 5.77 |
| −30 | 12 | 10 | 2.25 | 28 | 17 | −15 | 4.42 | |
| 42 | 15 | −3 | 4.96 | 42 | 8 | −6 | 3.32 | |
| 39 | 7 | 0 | 4.54 | 44 | 10 | −4 | 3.03 | |
| 28 | 17 | −15 | 4.42 | |||||
| Empathy | −40 | 15 | 3 | 5.38 | −35 | −11 | −7 | 4.69 |
| −31 | 16 | −19 | 4.04 | |||||
| −41 | −7 | 4 | 3.25 | |||||
| 39 | 19 | 3 | 5.11 | |||||
| 37 | 8 | 7 | 4.76 | |||||
| 46 | −6 | −1 | 3.37 | |||||
| Olfaction | −30 | 16 | 5 | 4.10 | 42 | 13 | −4 | 5.96 |
| 42 | 13 | −4 | 5.96 | |||||
| Gustation | −35 | 20 | 5 | 3.67 | −33 | 0 | 12 | 3.07 |
| −36 | 10 | 11 | 3.60 | 42 | 26 | −6 | 4.68 | |
| −33 | 0 | 11 | 3.09 | 44 | 9 | 1 | 4.15 | |
| 44 | 24 | −5 | 4.79 | 39 | 12 | −7 | 4.10 | |
| 44 | 9 | 2 | 4.17 | 47 | 7 | 6 | 3.22 | |
| 40 | 12 | −6 | 4.12 | |||||
| Interoception | −43 | −3 | 6 | 5.94 | −43 | −3 | 6 | 5.94 |
| −38 | 15 | 7 | 4.99 | 41 | 2 | 3 | 5.18 | |
| 41 | 2 | 3 | 5.18 | |||||
| 38 | 15 | 7 | 4.76 | |||||
| 35 | −13 | 9 | 3.56 | |||||
| Pain | −39 | −18 | 13 | 6.73 | −39 | −18 | 13 | 6.73 |
| −44 | 2 | 7 | 6.66 | −44 | 2 | 7 | 6.66 | |
| 34 | −20 | 14 | 5.58 | 42 | 9 | −4 | 3.97 | |
| Somatosensation | −37 | −11 | 5 | 3.50 | −37 | −2 | −4 | 3.34 |
| −39 | 0 | −4 | 3.46 | |||||
| −38 | −4 | 1 | 3.10 | |||||
| 41 | 21 | −5 | 3.39 | |||||
| Motion | −29 | 12 | 9 | 3.85 | – | – | – | – |
| −41 | −13 | 13 | 3.57 | |||||
| Attention | −35 | 18 | 7 | 6.35 | – | – | – | – |
| −33 | 18 | −5 | 6.22 | |||||
| 36 | 19 | 3 | 7.98 | |||||
| Language | −33 | 20 | 3 | 8.13 | – | – | – | – |
| −35 | 22 | 2 | 8.13 | |||||
| 36 | 20 | −1 | 7.84 | |||||
| Speech | −34 | 20 | 7 | 8.13 | −34 | 20 | 7 | 8.13 |
| 36 | 18 | 5 | 5.10 | |||||
| Working memory | −30 | 24 | 3 | 8.13 | −32 | 24 | 4 | 8.13 |
| −33 | 20 | 1 | 8.13 | −32 | 20 | 1 | 8.13 | |
| 36 | 21 | 1 | 7.78 | −34 | 21 | 1 | 8.12 | |
| 34 | 21 | 1 | 7.69 | |||||
| 39 | 21 | 1 | 7.57 | |||||
| Memory | −32 | 22 | 3 | 6.27 | 35 | 21 | 0 | 7.07 |
| 36 | 17 | −7 | 5.56 | |||||
Results by the conventional analysis are given on the right, results by the specific analysis on the left. All co-ordinates are given in MNI space
Insular localization of functional categories
Emotion
Activation by emotional tasks such as induction, imagination, or recall of own emotions converged on the whole anterior insula. It extended onto the central part on the right hemisphere. After testing for specific effects evoked by emotional processing, only the anterior-ventral insula bilaterally and a small cluster on the right central region remained significant (see Fig. 2a).
Empathy
Activation by paradigms tapping into empathy like judging emotions in faces was observed on the anterior-dorsal insula bilaterally, on the left anterior-ventral region, and bilaterally on the posterior ventral part. When testing for specific effects of empathic processing we found significant effects in the posterior ventral part of the insula, in particular on the left hemisphere (see Fig. 2b).
Olfaction
Activation by olfactory stimulation was found on the anterior dorsal insula on both sides, extending onto the central insula on the right hemisphere. This right central cluster also remained significant after testing for specific effects, while left hemispheric effects were not seen any more (see Fig. 3a).
Gustation
We observed bilateral activation by gustatory stimuli on the anterior dorsal insula as well as on the dorsal mid-insula. In particular on the right hemisphere, these activations comprised the whole anterior insula. When tested for specific effects of gestation, activation was only observed on the right anterior and the bilateral mid-dorsal insula (see Fig. 3b).
Interoception
Activation by interoceptive tasks such as listening to one’s own heartbeat or suppressing the urge to void was found on the central and anterior dorsal insula bilaterally. Among these effects, those observed in the central insula were bilaterally shown to be specific to interoceptive processing (see Fig. 4a).
Pain
Extended activation by painful stimuli was found on virtually the entire insula and extended onto the parietal operculum. Interestingly, even after testing for specific effects, extended activation on the central and posterior insula was observed (see Fig. 4b).
Somatosensation
Somatosensory stimuli evoked activation on the left central region of the insula and its right anterior dorsal part. As most studies examined somatosensory stimulation of the right hand, however, this lateralization must be seen cautiously and should warrant further investigation. Testing for specificity revealed only activation on the left central insula (see Fig. 4c).
Motor
Activation by active motor tasks was found bilaterally on the central insula, close to the representation of somato-sensory stimuli reported above but more bilateral. Observed activation after testing for specific effects might have been rather due to spillover effects from opercular areas and basal ganglia, as maxima of activation were located in these directly neighboring structures rather than in the insula. This observation indicates that the insular region identified by the conventional analysis might not only be specifically engaged in motor tasks, but also in other functional categories (see Fig. 4d).
Attention
Convergent activation by tasks tapping into attentional processes, e.g., Stroop-, Simon-, Go/NoGo-tasks, or spatial attention, was found bilaterally on the anterior-dorsal insula. However, there was no significant effect to be found when testing for activation that is specific to attentional tasks, indicating that regions sustaining these functions are also engaged in other cognitive or sensory processes as well (see Fig. 5a).
Language
Activations by language processing, e.g., lexical decision making or semantic judgments, were found on the anterior dorsal insula bilaterally. After testing for specific effects, no maximum of activation was found on the insula. Rather, we only observed spillover effects from the left frontal operculum on the anterior dorsal insula on this hemisphere (see Fig. 5b).
Speech
Activations by active (overt) speech were located on the anterior and posterior parts of the dorsal insula bilaterally. However, it has to be noted that activation on the posterior insula seems to be caused by spillover effects, in particular from the neighboring primary auditory cortex (areas Te1.0 and TE1.1, see Morosan et al. 2001). This interpretation is in line with the observation that among the implicated regions, only the left anterior insula remained significant after testing for specific effects (see Fig. 5c).
Working memory
Activations by working memory tasks, such as n-back or Sternberg paradigms, were found bilaterally on the anterior-dorsal insula. Moreover, activation in these regions also remained significant after testing for specific effects (see Fig. 5d).
Memory
Activations by episodic or short-term memory retrieval were found bilaterally on the anterior-dorsal insula in a similar location as the activations associated with working memory. However, in contrast to the above, only the effects on the right anterior-dorsal insula were specific to memory paradigms (see Fig. 5e).
Functional integration
All analyses revealed a considerably smaller extent of activation when comparing the results from the conventional and the modified ALE. This indicates that the insular cortex is engaged in functional integration between different functional categories. To map those regions where different categories are integrated (i.e. where results from the conventional ALE overlap), we calculated two conjunction analyses over the single categories (see “Methods”). The first conjunction over all 13 analyzed categories revealed no overlap throughout the whole brain. The second analysis over all investigated categories except somatosensation and motion revealed an overlap on the anterior-dorsal insula (see Fig. 6). No other overlap was observed throughout the whole brain.
Fig. 6.
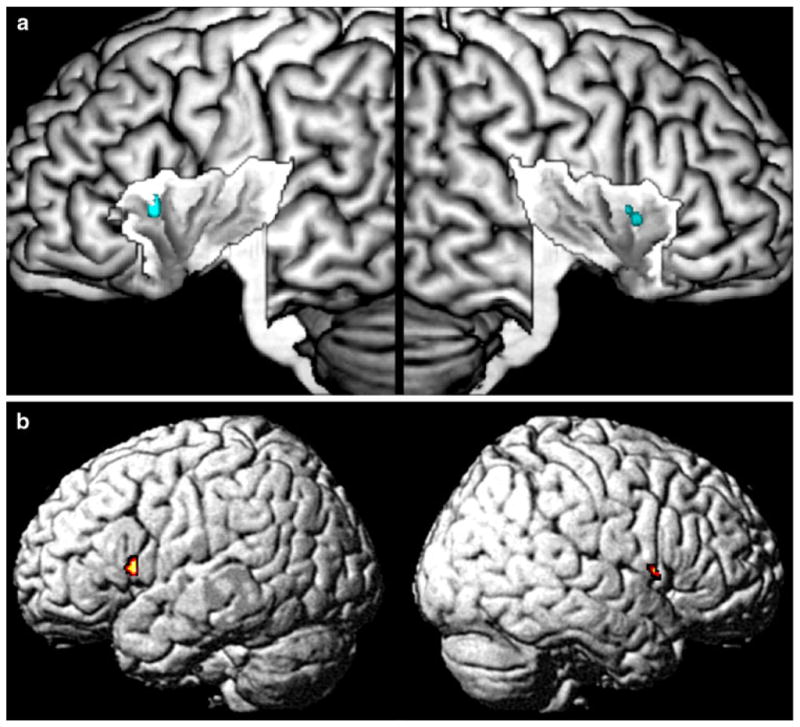
Overlap of all functional categories except somatosensation and motion. All other eleven categories overlap on the anterior-dorsal part of the insula (a), indicating functional integration between them. b Throughout the whole brain the anterior-dorsal insula was the only region that participated in the processing of all eleven categories
Functional differentiation
To investigate a functional differentiation of the insula the 13 investigated functional categories were sorted into four functional domains (cf. Table 2, methods). The modified analysis of every functional domain revealed a functional segregation of the insular cortex (see Fig. 7). The mid-posterior insula was activated by tasks from the sensori-motor domain. This region extended from posterior insula to the posterior short insular gyrus. The anterior-ventral insula was specific to social-emotional functions, i.e., emotional processing and empathy. This region was located around the insular pole bilaterally. The anterior-dorsal insula processed tasks from the cognitive domain. It comprised the dorsal part of the anterior and middle short gyrus. Finally, for the olfacto-gustatory domain a region on the right middle insular gyrus was revealed to be specific.
Fig. 7.
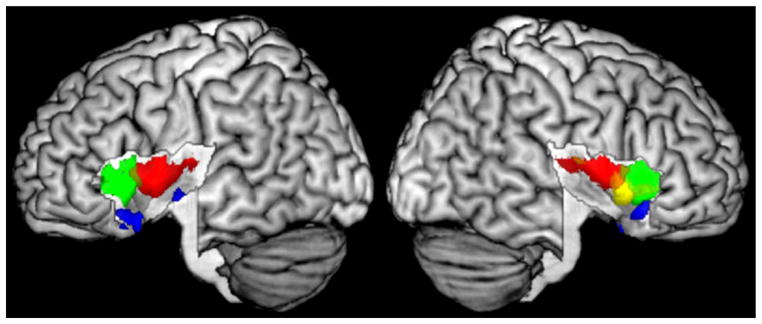
Functional differentiation of the insula by functional domains. Red sensorimotor, green cognitive, yellow chemical sensory, blue social-emotional
Discussion
The present results indicate a functional map of the human insula based on a large-scale coordinate-based meta-analysis of published functional neuroimaging studies. Using this approach, we could show a differentiation of the insular cortex into four functional regions. These regions were defined by a sensorimotor, a cognitive, a social-emotional, and an olfacto-gustatory domain. Throughout the whole brain activations by these domains reflected the previously described social-emotional, cognitive, and sen-sorimotor networks as well as to a network processing olfacto-gustatory stimuli (Corbetta et al. 2008; Kandel et al. 1991; Phillips et al. 2003). Apart from this functional differentiation, a conjunction analysis of investigated functional categories revealed the anterior dorsal insula to be involved in the processing of all investigated categories except somatosensation and motion. This finding points the anterior-dorsal insula to act as a multimodal integration region.
A functional differentiation of the insular cortex was already indicated by anatomical data reported in non-human primates. Cytoarchitectonically, the non-human primate insula can be divided into an anterior-basal agranular, allocortical part, a posterior granular part, and a dysgranular part which is located between these and covers the anterior-dorsal and central insula (Augustine 1996; Mesulam and Mufson 1982). This parcellation is also reflected by differences in connectivity as investigated by invasive tracing studies in these species. In monkeys, the anterior-basal part shows dense connections to limbic areas like the amygdala, periamygdaloid, and entorhinal cortices as well as to the temporal pole (Fudge et al. 2005; Hoistad and Barbas 2008; Mesulam and Mufson 1985; Stefanacci and Amaral 2002). Furthermore, the anterior insula also participates in a structurally connected network formed together with the orbitofrontal, entorhinal, and piriform and olfactory cortex, which is engaged in olfactory and gustatory processing (Augustine 1996; Mesulam and Mufson 1985). Interestingly, the mid-dorsal insula also receives input from the thalamic taste area (Craig 2002; Pritchard et al. 1986), which matches well with the present findings of gustatory activation in this part. The mid-posterior regions, finally, are densely connected to primary and secondary sensory and motor areas, while the anterior dorsal areas are preferably connected to frontal regions (Augustine 1996; Friedman et al. 1986; Mesulam and Mufson 1985).
This separation into an anterior-basal region, which is closely linked to the social-emotional and the olfacto-gustatory system, a mid-posterior insular region, which is closely connected to sensorimotor areas and an anterior dorsal part, which is more closely connected to frontal association areas matches well with the present results. The mid-posterior insula, which is connected to somatosensory and motor cortices in monkeys, was found to process the sensorimotor domain in the present study. Equally, the anterior-basal region, which in monkeys is connected to limbic areas like the amygdala, periamygdaloid, and entorhinal cortices, was observed to process the socio-emotional domain and the anterior-dorsal insula with its connections to frontal association areas was activated by the cognitive domain. Finally, the reported connectivity between anterior-dorsal insula and frontal regions matches well the identification of a cognitive insular region, as frontal areas are regularly reported to serve working memory, attention, and other cognitive tasks (Cabeza and Nyberg 2000). However, it must be taken into account that cross-species comparisons are problematic, as they disregard the obvious and considerable differences between the brains. A comparison to non-human tracing data is unfortunately the best reference for insular connectivity available at the present, as there is currently no technique which allows a reliable delineation the connections of insular regions in the human brain. In future, more recent imaging and analysis techniques will hopefully help to unravel connectivity patterns of the human insula and allow a comparison with that of other species.
In good accordance with the anatomical findings in non-human primates, the anterior insula and in particular its anterior-basal parts have been discussed in the processing of emotion and empathy (Adolphs 2002; Dolan 2002; Frith and Singer 2008; Lamm and Singer 2010; Phillips et al. 2003; Singer and Lamm 2009). In particular, it has been proposed that this region is involved in the generation and mediation of feeling states as a response to environmental stimuli and affective states (Dolan 2002; Phillips et al. 2003). In empathy-related processing it is involved in the recognition of emotions in faces (Phillips et al. 2003) and the feeling of pain in others (Frith and Singer 2008; Singer et al. 2006). Finally, the anterior basal insula was hypothesized to provide a link between the mirror neuron system and emotional processing, thus enabling a matching between own and observed emotions (Iacoboni and Dapretto 2006). This focus on the anterior basal part is supported by electrophysiological findings. Preoperative direct recordings in patients suffering from drug-refractory epilepsy revealed that a role in the categorization of facial expressions is confined to this region (Krolak-Salmon et al. 2003). The findings from the present study corroborate these results, as they show an involvement of the anterior insula and in particular the anterior-basal part in the processing of emotions.
Different functional studies reported insular activation in olfactory and gustatory tasks (Kringelbach et al. 2004; Poellinger et al. 2001; Royet and Plailly 2004; Small et al. 1999). These studies show that the anterior insula processes interactions between emotion, memory and olfaction (Poellinger et al. 2001), habituation to odorants (Royet and Plailly 2004), and several tasks testing taste processing (Kringelbach et al. 2004; Small et al. 1999). Most interestingly, the insula was reported to be part of a network involved, especially in integration of taste and flavor (Rolls 2006; Small and Prescott 2005), supporting an insular role in olfacto-gustatory processing. Intraoperative direct stimulations of the anterior insula also led to gustatory and olfactory sensations (Penfield and Faulk 1955). It is interesting to note though, that these sensations were also elicited as well near the basal part of the precentral gyrus and on the anterior part. However, on the anterior part, insular stimulation was accompanied by descriptions like “bad taste” or feelings of fear, while more posterior rather sensations in the mouth, salivation or increased gastric motility were observed. This matches well the hypothesis that the insular taste area is located rather in the more posterior parts in humans (Small 2010, Craig 2010), while the more anterior parts will be more engaged in an integration with odors and emotions.
The anterior-dorsal insula has repeatedly been implicated in several cognitive tasks. For example, it processes both working memory and attention tasks with additive BOLD effects for both tasks (Mayer et al. 2007; Soros et al. 2007). It was furthermore assigned to be a part of the inferior frontoparietal network, which responds to behavioral relevant rather than to expected stimuli (Corbetta et al. 2008) and reported to play a role in language processing (McCarthy et al. 1993; Price 2000; Riecker et al. 2000). These findings might implicate an abstract role in extracting and processing task-relevant and salient information.
The mid-posterior insula, finally, has been repeatedly demonstrated to be involved in different somato- and viscerosensory stimuli. In particular it responds to painful, visceral and somatosensory stimulation (Chen 2007; Dupont et al. 2003; Ostrowsky et al. 2002; Peyron et al. 2000; Peyron et al. 2002; Shelley and Trimble 2004). In addition to this sensory processing, movement was elicited by electrical stimulation of this region in humans (Showers and Lauer 1961), which indicates a role in sensorimotor processing of this region. Other reports of direct stimulation of this region reported interoceptive and somatic sensations, changes in gastric motility, breathing or heart rate as well as the sensation or urge of movement (Penfield and Faulk 1955). Interestingly, these findings also match reports from frontotemporal dementia (Seeley et al. 2008). Seeley et al. showed that early changes in this disease, which present with cognitive and emotional impairments, appear in the anterior insula among other regions. Only at later stages of this disease, when the patients show impairments in executive functions, these changes will comprise the posterior parts.
It is important to note though that all results from the current analysis reflect the convergence across a large number of studies. Along with this they map the common neuronal substrates between different experiments on the same topic, for example, the shared neural substrate of different emotions in various experimental setups. The inference one can draw from these results is that observed regions are involved in the process, which underlies all -or at least most-investigated experiments. While we can thus say that the anterior insula is involved in the processing of emotions in general, any inference from these results on a specific emotion in a specific context must be regarded with extreme caution. The fact that in any given individual study only a sub-category of all emotions is investigated in a particular experimental context (this might be “rating a disgusting taste in comparison to a memorized reference”) will lead to conclusions which may diverge from the current results. Therefore, although this analysis can reliably identify a general functional differentiation of the insula, it cannot predict or reflect effects observed in a specific experimental task.
The described converging evidence for a functional segregation of the insula was confirmed and extended in the current meta-analysis, which showed a reliable differentiation of the human insula into functional specific regions. Still, in spite of this ample evidence for functional specification within the insula, this region has also repeatedly been implicated as the anatomical substrate for the behaviorally essential integration of information from different functional systems. Sensory stimuli, for example, have an immediate effect on emotions (Chen 2007; Craig 2002; Williams and Bargh 2008), which is in particular reflected by the James-Lange theory and Damasio’s “somatic marker” hypothesis (Damasio 1994; James 1894; Lange 1885). These hypotheses state that interoceptive information and emotion are directly depending on another and even cause each other. Recently, the human insula has emerged as the prime candidate for sustaining this integration, as it was reported to be directly involved in processing the reciprocal influence of interoception and emotion (Craig 2002; Critchley et al. 2004; Critchley 2005). Moreover, not only integration between interoception and emotion, but also between cognitive tasks and sensation as well as emotion was described to involve this region. For example, the insula has been reported to be involved in processing attention to sensory stimuli like attention to touch or comparing the own heartbeat to an external rhythm (Critchley et al. 2004; Johansen-Berg and Matthews 2002). Also, it is involved in distinguishing between self and other, which was assigned to this region using the rubber hand illusion (Ehrsson et al. 2004; Karnath and Baier 2010; Tsakiris et al. 2007). Finally, a convincing theory of how interoception, emotion/empathy and the cognitive evaluation of risk and uncertainty may be linked within the anterior insula cortex was recently brought up (Singer et al. 2009). In this theory the insula was described to process current feeling states that are closely linked to interoception, predicted feeling states and a prediction error, which allows for an evaluation and adaption of the own prediction. This combination of different modalities makes the insula a highly integrative region.
On the background of the described functional differentiation of the insula, the question of how this functional integration is implemented arises. We would argue that there may be two complementary answers to this: On the one hand the different areas or zones are extremely well interconnected as it was shown in tracing studies in non-human primates (Augustine 1996). These dense connections may represent a structural basis for a rapid flow of information between the functional systems. On the other hand, the present study also revealed an overlap of all categories (except for basic somatosensory and motor processing) on the anterior-dorsal insula. This overlap probably reflects a shared role in information processing between the systems. This shared role might reflect a multimodal integration between the different categories relaying information between the different functional systems on the one hand and on the other hand a basic functional role that all categories have in common. In the following, we will discuss this shared role and its implications for this region. Still, in particular, the combination of this shared functional role and the close connectivity between insular domains gives this region a unique position for regulating flow and integration of information between the systems.
It must be mentioned here that an interpretation of the observed overlap is subject to a limitation that cannot be solved within the scope of this paper, namely the question of what constitutes an independent cognitive process. It is well known that, for example, pain is not a solely sensory process, but has also an important emotional component (Chen 2007). The same will go for interoception, which may be regarded a sensory category but is an important component in the generation and interpretation of emotions and empathy (Damasio 1994; James 1894; Lange 1885). The same goes in some way for nearly all categories, i.e., they always have some potential aspect of ambiguity. Unambiguous classifications, however, are probably impossible to define without a full knowledge of the intrinsic ontology of cognitive processes, i.e., the building blocks of brain organization. The question will now be, if this potential ambiguity of the categories led to the mapped overlap in the insula, or if this mapped overlap in the insula caused the possible ambiguity of the categories. That is, is the overlap spurious or does it reflect more basic processes that are shared among categories. By a careful selection of studies, which avoided ambiguity between different categories as much as possible, we attempted to avoid this possibly artificial overlap. Although a definite answer to this problem cannot be given here, a review of literature points the anterior dorsal insula to be involved in a multitude of different studies (e.g. Craig 2009).
In the following, we will assume that there is an overlap in the mapping of the categories in the anterior-dorsal insula, as observed in this study. This region was recently proposed to integrate momentary images of own feelings, the sensory environment, and the motivational, hedonic and social interaction between these into one whole representation of the sentient self (Craig 2009). In this concept, the anterior-dorsal insula was proposed to constitute the final stage of a hierarchical processing of information in the insular cortex––starting with pure sensory information posterior and after integrating emotional and cognitive valuation ending with a full representation of the sentient self in the anterior parts. This representation, which thus constitutes an integration of salient information from all functional systems led subsequently to the suggestion that the anterior-dorsal insula might be a potential neural correlate of awareness (Craig 2009). This described hierarchy of processing also explains the finding that the functional overlap between different functional systems on the insular cortex did not include basic somatosensory and motor tasks. The pure somatosensory and motor tasks that were analyzed in this study do not have the need for cognitive or social-emotional evaluation. Hence, they do not have a need for integration. Thus, it can be expected that they do not elicit activation in the anterior-dorsal multimodal integration region, but rather in the posterior part of the insula.
However, the shared role which probably underlies the observed overlap between different functional categories can also be due to a basic functional role that all categories have in common. This basic functional role might be a general role in task processing, like starting, updating, and ending of a given task. This was indicated by an fMRI study investigating ten different functional paradigms, including different language, sensorimotor and cognitive tasks, in the same set of subjects (Dosenbach et al. 2006). Using a conjunction analysis, the authors found only the dACC/msFC and the bilateral anterior-dorsal insula to be active across all different paradigms. This result, which is confirmed and extended by our current data, was interpreted as evidence for a central role of the anterior-dorsal insula in initiating, updating, and maintaining tasks. That is, as an alternative to the theory outlined above, this view regards the anterior-dorsal insula not primarily as a region integrating information from different functional systems into a putative correlate of awareness (Craig 2009). Rather, it regards this overlap between the different processing as a task-set region responsible for maintaining the task-set which is necessary to perform any task in the scanner, or––to put it to the extreme––as the smallest common denominator. These two hypotheses may be regarded as two potential interpretations of the observed overlap across many functional domains in this region, which, however, cannot be finally differentiated, from the current data.
A few last points may be presented here, which will still need further investigation. We have compared our findings to invasive tracing studies in non-human primates. While there are analogies between the results from this study and these tracing studies, any comparison assumes that human and non-human insula are matchable. This assumption can be regarded critically, as the insula differs considerably between species (Craig 2009; Craig 2010; Rose 1928). A cytoarchitectonic mapping study of the posterior human insula supported that notion, as this region proved to be far more complex than studies from non-human primates report (Kurth et al. 2009). Unfortunately, the areas mapped in this study comprise just a very limited part of the insular cortex, limiting all comparisons to imaging data. As there are currently ongoing further mapping studies, a comprehensive comparison of insular structure and function will become feasible in the future.
Another important point may be the finding that variable occurrences of lateralization and localization were found on the insula with respect to the current study. To give some examples, Jabbi et al. (2007) reported a representation of disgust on the left anterior central region, Royet et al. (1999) did not report activation from a complex olfactory task on the insula, and Qureshy et al. (2000) reported activation from another complex olfactory task on the left insula. Partly, these differences in localization can be explained by the fact that these studies did not only investigate basic emotional or olfactory processing. However, if we extrapolate the results from the cytoarchitectonic mapping of the posterior insula (Kurth et al. 2009), we will expect a multitude of different areas in this region. Thus, the here presented functional differentiation might be rather a framework for a more generalized concept of the insular role. Its detailed functioning and structure–function relationships will certainly remain topics of further research.
Conclusion
Using a large-scale meta-analysis, we revealed a functional differentiation as well as a functional integration within the human insula. The identified regions are involved in the processing of different functional systems. This differentiation is also reflected in anatomical connectivity studies in monkeys, thus corroborating the present observations. The observed functional integration in the anterior-dorsal insula might constitute a link between these different functional systems and a correlate for the relaying of information between them. Indeed, this might be interpreted as a representation of a sentient self and even as a neural correlate of self-awareness. However, the observed overlap of activations by multiple different tasks might also represent a smallest common denominator, which is a task-set region for initiating, updating, and maintaining a function. Still, as these interpretations do not totally disqualify each other––a task set region can also be a multimodal integration region and vice versa––they rather describe extreme interpretations. To characterize this intriguing role of the anterior-dorsal insula subsequent studies will be necessary.
Supplementary Material
Acknowledgments
This research was funded by the National Institute of Biomedical Imaging and Bioengineering, the National Institute of Neurological Disorders and Stroke, and the National Institute of Mental Health. K.Z. acknowledges further funding by the Helmholtz Alliance “Systembiologie” the “Human Brain Model”. A.R.L. and P.T.F. were supported by the Human Brain Project of the NIMH (R01-MH074457-01A1).
Footnotes
Electronic supplementary material: The online version of this article (doi:10.1007/s00429-010-0255-z) contains supplementary material, which is available to authorized users.
Conflict of interest statement: None.
Contributor Information
Florian Kurth, Email: f.kurth@fz-juelich.de, C. & O. Vogt Institute of Brain Research, University Düsseldorf, Düsseldorf, Germany. Institute for Neuroscience and Medicine (INM-2), Research Center Jülich, Jülich, Germany.
Karl Zilles, C. & O. Vogt Institute of Brain Research, University Düsseldorf, Düsseldorf, Germany. Institute for Neuroscience and Medicine (INM-2), Research Center Jülich, Jülich, Germany. JARA - Translational Brain Medicine, Jülich, Germany.
Peter T. Fox, Research Imaging Center, University of Texas Health Science Center at San Antonio, Texas, USA
Angela R. Laird, Research Imaging Center, University of Texas Health Science Center at San Antonio, Texas, USA
Simon B. Eickhoff, Institute for Neuroscience and Medicine (INM-2), Research Center Jülich, Jülich, Germany. JARA - Translational Brain Medicine, Jülich, Germany. Department of Psychiatry and Psychotherapy, RWTH Aachen University, Aachen, Germany
References
- Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Chen LM. Imaging of pain. Int Anesthesiol Clin. 2007;45:39–57. doi: 10.1097/AIA.0b013e31803419d3. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel––now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD. The sentient self. Brain Struc Func. 2010;214(5–6) doi: 10.1007/s00429-010-0248-y. this issue. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes’ error: emotion, reason and the human brain. Putnam’s Sons; New York: 1994. [Google Scholar]
- Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298:1191–1194. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Bouilleret V, Hasboun D, Semah F, Baulac M. Functional anatomy of the insula: new insights from imaging. Surg Radiol Anat. 2003;25:113–119. doi: 10.1007/s00276-003-0103-4. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Spence C, Passingham RE. That’s my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science. 2004;305:875–877. doi: 10.1126/science.1097011. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Laird AR, Fox SP, Fox PM, Uecker AM, Crank M, Koenig SF, Lancaster JL. BrainMap taxonomy of experimental design: description and evaluation. Hum Brain Mapp. 2005;25:185–198. doi: 10.1002/hbm.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DP, Murray EA, O’Neill JB, Mishkin M. Cortical connections of the somatosensory fields of the lateral sulcus of macaques: evidence for a corticolimbic pathway for touch. J Comp Neurol. 1986;252:323–347. doi: 10.1002/cne.902520304. [DOI] [PubMed] [Google Scholar]
- Frith CD, Singer T. The role of social cognition in decision making. Philos Trans R Soc Lond B Biol Sci. 2008;363:3875–3886. doi: 10.1098/rstb.2008.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Breitbart MA, Danish M, Pannoni V. Insular and gustatory inputs to the caudal ventral striatum in primates. J Comp Neurol. 2005;490:101–118. doi: 10.1002/cne.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoistad M, Barbas H. Sequence of information processing for emotions through pathways linking temporal and insular cortices with the amygdala. Neuroimage. 2008;40:1016–1033. doi: 10.1016/j.neuroimage.2007.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nat Rev Neurosci. 2006;7:942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34:1744–1753. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- James W. Physical basis of emotion. Psychol Rev. 1894;1:516–529. doi: 10.1037/0033-295x.101.2.205. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Matthews PM. Attention to movement modulates activity in sensory-motor areas, including primary motor cortex. Exp Brain Res. 2002;142:13–24. doi: 10.1007/s00221-001-0905-8. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of neural science. Appleton & Lange; East Norwalk: 1991. [Google Scholar]
- Karnath HO, Baier B. Right insula for our sense of limb ownership and self-awareness of actions. Brain Struc Func. 2010;214(5–6) doi: 10.1007/s00429-010-0250-4. this issue. [DOI] [PubMed] [Google Scholar]
- King-Casas B, Sharp C, Lomax-Bream L, Lohrenz T, Fonagy P, Montague PR. The rupture and repair of cooperation in borderline personality disorder. Science. 2008;321:806–810. doi: 10.1126/science.1156902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, de Araujo I, Rolls ET. Taste-related activity in the human dorsolateral prefrontal cortex. Neuroimage. 2004;21:781–788. doi: 10.1016/j.neuroimage.2003.09.063. [DOI] [PubMed] [Google Scholar]
- Krolak-Salmon P, Henaff MA, Isnard J, Tallon-Baudry C, Guenot M, Vighetto A, Bertrand O, Mauguiere F. An attention modulated response to disgust in human ventral anterior insula. Ann Neurol. 2003;53:446–453. doi: 10.1002/ana.10502. [DOI] [PubMed] [Google Scholar]
- Kurth F, Eickhoff SB, Schleicher A, Hoemke L, Zilles K, Amunts K. Cytoarchitecture and probabilistic maps of the human posterior insular cortex. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005a;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Lancaster JL, Fox PT. BrainMap: the social evolution of a human brain mapping database. Neuroinformatics. 2005b;3:65–78. doi: 10.1385/ni:3:1:065. [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT. ALE meta-analysis workflows via the Brainmap database: progress towards a probabilistic functional brain atlas. Front Neuroinform. 2009;3:23. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Singer T. The role of anterior insular cortex in social emotions. Brain Struc Func. 2010;214(5–6) doi: 10.1007/s00429-010-0251-3. this issue. [DOI] [PubMed] [Google Scholar]
- Lange CG. The mechanism of the emotion. In: Rand B, editor. The classical psychologist. Houghton Mifflin; Boston: 1885. pp. 672–685. [Google Scholar]
- Mayer JS, Bittner RA, Nikolic D, Bledowski C, Goebel R, Linden DE. Common neural substrates for visual working memory and attention. Neuroimage. 2007;36:441–453. doi: 10.1016/j.neuroimage.2007.03.007. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Blamire AM, Rothman DL, Gruetter R, Shulman RG. Echo-planar magnetic resonance imaging studies of frontal cortex activation during word generation in humans. Proc Natl Acad Sci USA. 1993;90:4952–4956. doi: 10.1073/pnas.90.11.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. The insula of Reil in man and monkey. Architectonics, connectivity and function. In: Peters A, Jones EG, editors. Cerebral cortex. Plenum Press; New York: 1985. pp. 179–226. [Google Scholar]
- Morosan P, Rademacher J, Schleicher A, Amunts K, Schormann T, Zilles K. Human primary auditory cortex: cytoarchitectonic subdivisions and mapping into a spatial reference system. Neuroimage. 2001;13:684–701. doi: 10.1006/nimg.2000.0715. [DOI] [PubMed] [Google Scholar]
- Mutschler I, Wieckhorst B, Kowalevski S, Derix J, Wentlandt J, Schulze-Bonhage A, Ball T. Functional organization of the human anterior insular cortex. Neurosci Lett. 2009;457:66–70. doi: 10.1016/j.neulet.2009.03.101. [DOI] [PubMed] [Google Scholar]
- Ostrowsky K, Magnin M, Ryvlin P, Isnard J, Guenot M, Mauguiere F. Representation of pain and somatic sensation in the human insula: a study of responses to direct electrical cortical stimulation. Cereb Cortex. 2002;12:376–385. doi: 10.1093/cercor/12.4.376. [DOI] [PubMed] [Google Scholar]
- Penfield W, Faulk ME., Jr The insula; further observations on its function. Brain. 1955;78:445–470. doi: 10.1093/brain/78.4.445. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Peyron R, Frot M, Schneider F, Garcia-Larrea L, Mertens P, Barral FG, Sindou M, Laurent B, Mauguiere F. Role of operculoinsular cortices in human pain processing: converging evidence from PET, fMRI, dipole modeling, and intracerebral recordings of evoked potentials. Neuroimage. 2002;17:1336–1346. doi: 10.1006/nimg.2002.1315. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Poellinger A, Thomas R, Lio P, Lee A, Makris N, Rosen BR, Kwong KK. Activation and habituation in olfaction––an fMRI study. Neuroimage. 2001;13:547–560. doi: 10.1006/nimg.2000.0713. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. J Anat. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard TC, Hamilton RB, Morse JR, Norgren R. Projections of thalamic gustatory and lingual areas in the monkey, Macaca fascicularis. J Comp Neurol. 1986;244:213–228. doi: 10.1002/cne.902440208. [DOI] [PubMed] [Google Scholar]
- Qureshy A, Kawashima R, Imran MB, Sugiura M, Goto R, Okada K, Inoue K, Itoh M, Schormann T, Zilles K, Fukuda H. Functional mapping of human brain in olfactory processing: a PET study. J Neurophysiol. 2000;84:1656–1666. doi: 10.1152/jn.2000.84.3.1656. [DOI] [PubMed] [Google Scholar]
- Riecker A, Ackermann H, Wildgruber D, Dogil G, Grodd W. Opposite hemispheric lateralization effects during speaking and singing at motor cortex, insula and cerebellum. Neuroreport. 2000;11:1997–2000. doi: 10.1097/00001756-200006260-00038. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Brain mechanisms underlying flavour and appetite. Philos Trans R Soc Lond B Biol Sci. 2006;361:1123–1136. doi: 10.1098/rstb.2006.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. Die Inselrinde des Menschen und der Tiere. J Psychol Neurol. 1928;37:467–624. [Google Scholar]
- Royet JP, Plailly J. Lateralization of olfactory processes. Chem Senses. 2004;29:731–745. doi: 10.1093/chemse/bjh067. [DOI] [PubMed] [Google Scholar]
- Royet JP, Koenig O, Gregoire MC, Cinotti L, Lavenne F, Le BD, Costes N, Vigouroux M, Farget V, Sicard G, Holley A, Mauguiere F, Comar D, Froment JC. Functional anatomy of perceptual and semantic processing for odors. J Cogn Neurosci. 1999;11:94–109. doi: 10.1162/089892999563166. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Crawford R, Rascovsky K, Kramer JH, Weiner M, Miller BL, Gorno-Tempini ML. Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol. 2008;65:249–255. doi: 10.1001/archneurol.2007.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley BP, Trimble MR. The insular lobe of Reil––its anatamico-functional, behavioural and neuropsychiatric attributes in humans––a review. World J Biol Psychiatry. 2004;5:176–200. doi: 10.1080/15622970410029933. [DOI] [PubMed] [Google Scholar]
- Showers MJ, Lauer EW. Somatovisceral motor patterns in the insula. J Comp Neurol. 1961;117:107–115. doi: 10.1002/cne.901170109. [DOI] [PubMed] [Google Scholar]
- Singer T, Lamm C. The social neuroscience of empathy. Ann N Y Acad Sci. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439:466–469. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Small DM. Taste representation in the human insula. Brain Struc Func. 2010;214(5–6) doi: 10.1007/s00429-010-0266-9. this issue. [DOI] [PubMed] [Google Scholar]
- Small DM, Prescott J. Odor/taste integration and the perception of flavor. Exp Brain Res. 2005;166:345–357. doi: 10.1007/s00221-005-2376-9. [DOI] [PubMed] [Google Scholar]
- Small DM, Zald DH, Jones-Gotman M, Zatorre RJ, Pardo JV, Frey S, Petrides M. Human cortical gustatory areas: a review of functional neuroimaging data. Neuroreport. 1999;10:7–14. doi: 10.1097/00001756-199901180-00002. [DOI] [PubMed] [Google Scholar]
- Soros P, Marmurek J, Tam F, Baker N, Staines WR, Graham SJ. Functional MRI of working memory and selective attention in vibrotactile frequency discrimination. BMC Neurosci. 2007;8:48. doi: 10.1186/1471-2202-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J Comp Neurol. 2002;451:301–323. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- Tsakiris M, Hesse MD, Boy C, Haggard P, Fink GR. Neural signatures of body ownership: a sensory network for bodily self-consciousness. Cereb Cortex. 2007;17:2235–2244. doi: 10.1093/cercor/bhl131. [DOI] [PubMed] [Google Scholar]
- von Economo C, Koskinas GN. Die Cytoarchitectonik der Hirnrinde des erwachsenen Menschen. Springer; Berlin: 1925. [Google Scholar]
- Wager TD, Barrett LF. From affect to control: functional specialization of the insula in motivation and regulation. 2004 Available online via: PsycExtra http://www.columbia.edu/cu/psychology/tor/
- Williams LE, Bargh JA. Experiencing physical warmth promotes interpersonal warmth. Science. 2008;322:606–607. doi: 10.1126/science.1162548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K. Architecture of the human cerebral cortex. Regional and laminar organization. In: Paxinos G, editor. The human nervous system. 2. Elsevier; San Diego: 2004. pp. 997–1055. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.