Abstract
Brain-derived neurotrophic factor (BDNF) and STriatal-Enriched protein tyrosine Phosphatase 61 (STEP61) have opposing functions in the brain, with BDNF supporting and STEP61 opposing synaptic strengthening. BDNF and STEP61 also exhibit an inverse pattern of expression in a number of brain disorders, including schizophrenia (SZ). NMDAR antagonists such as phencyclidine (PCP) elicit SZ-like symptoms in rodent models and unaffected individuals, and exacerbate psychotic episodes in SZ. Here we characterize the regulation of BDNF expression by STEP61, utilizing PCP-treated cortical culture and PCP-treated mice. PCP-treated cortical neurons showed both an increase in STEP61 levels and a decrease in BDNF expression. The reduction in BDNF expression was prevented by STEP61 knockdown or use of the STEP inhibitor, TC-2153. The PCP-induced increase in STEP61 expression was associated with the inhibition of CREB-dependent BDNF transcription. Similarly, both genetic and pharmacologic inhibition of STEP prevented the PCP-induced reduction in BDNF expression in vivo and normalized PCP-induced hyperlocomotion and cognitive deficits. These results suggest a mechanism by which STEP61 regulates BDNF expression, with implications for cognitive functioning in CNS disorders.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-015-2057-1) contains supplementary material, which is available to authorized users.
Keywords: BDNF, Phencyclidine, Schizophrenia, STEP inhibitor, Ubiquitination
Introduction
Brain-derived neurotrophic factor (BDNF) and STriatal-Enriched protein tyrosine Phosphatase; STEP, PTPN5) are both implicated in the pathophysiology of schizophrenia (SZ) [1, 2]. BDNF expression is reduced in animal models of SZ [3], and postmortem studies show significant decreases in BDNF and its high-affinity receptor TrkB in the cortex and hippocampus of SZ patients [4, 5]. Moreover, significant reductions in BDNF levels are found in the plasma of SZ patients during their first psychotic episode [6] and are reversed by neuroleptic treatment [7]. BDNF heterozygote and TrkB receptor conditional knock out (KO) mice display SZ-related behaviors that include increased locomotor activity, deficits in sensorimotor gating, and impaired cognitive functions [8, 9]. In addition, the association of a single nucleotide polymorphism (SNP) of the BDNF gene (rs6265; Val66Met) has been found in studies predominantly in Caucasian samples, although contrary reports also exist [10].
The STEP-family of tyrosine phosphatases is alternatively spliced from a single gene to produce several members of which STEP61 is a membrane-associated isoform enriched at post-synaptic compartments and the endoplasmic reticulum [11, 12]. STEP61 is the only isoform expressed in cortex [13]. Substrates of STEP include the GluN2B subunit of the NMDA receptor [14], the GluA2 subunit of the AMPA receptor [15], and the kinases ERK1/2, Fyn, and Pyk2 [16–18]. Dephosphorylation of the glutamate receptors results in internalization of GluN1/GluN2B and GluA1/GluA2, while dephosphorylation of regulatory tyrosines of the kinases leads to their inactivation. The current model of STEP function is that it normally opposes the development of synaptic strengthening [19].
STEP61 is elevated in human postmortem samples from SZ patients and in psychotomimetic mouse models [2]. STEP KO mice are resistant to the locomotor, and cognitive effects of psychotomimetics and neuroleptic treatment of mice result in STEP61 inactivation [2]. Moreover, a case–control study found nominal association between PTPN5 SNP rs4075664 and SZ in all the samples examined and a significant association of two additional SNPs (rs2278732 and rs4757710) in male samples from an Israeli Jewish cohort [20]. These studies indicate that BDNF signaling is generally low, while STEP61 signaling is generally high in SZ patients and in animal models of SZ.
There is crosstalk between BDNF expression and N-methyl-D-aspartate receptor (NMDAR) signaling [21–23], and BDNF potentiates NMDAR function through activation of ERK1/2 and Fyn [24, 25]. On the other hand, NMDAR signaling is known to increase activity-dependent transcription and secretion of BDNF [26–29]. Notably, both ERK1/2 and Fyn are tyrosine dephosphorylated and inactivated by STEP [16, 17, 30]. Mice null for STEP shows increased tyrosine phosphorylation of these substrates [30–32] and increased localization of NMDAR at synaptic membranes [32]. Moreover, pharmacological inhibition of STEP61 by a recently discovered inhibitor, TC-2153, also resulted in increased tyrosine phosphorylation of STEP substrates, showed relative specificity to STEP compared to other PTPs, increased the distribution of NMDAR at synaptic membranes, and reversed cognitive deficits in a mouse model of Alzheimer’s disease [33].
Noncompetitive NMDAR antagonists, such as the psychotomimetics phencyclidine (PCP), ketamine, and MK-801, are used to model SZ-like symptoms in humans, rodents, and nonhuman primates [34–36], supporting aspects of the glutamate hypothesis of SZ [37, 38]. A previous study showed that PCP treatment led to the accumulation of STEP61 [2], while a second study found decreased BDNF expression upon PCP treatment in cultures [39]. However, it remains unclear whether elevated STEP61 contributes to the reduction of BDNF and whether the regulation of BDNF by STEP61 has functional consequence in vivo. Here we examined the relationship of STEP61 activity and BDNF expression, and the functional consequences of their disruption in PCP-treated cortical culture and a mouse model of SZ. STEP61 expression was increased, while BDNF levels were decreased upon PCP administration both in cultures and in mice. Genetic and pharmacological techniques to decrease STEP61 activity in these models normalized BDNF expression and rescued motor and cognitive deficits. These findings suggest that STEP61 regulates BDNF expression and contributes to the observed balance between BDNF and STEP61 signaling that may explain aspects of the pathophysiology of SZ.
Materials and methods
Antibodies and reagents
Antibodies are listed in Supplementary Table 1. PCP was purchased from Sigma (Ronkonkoma, NY); the proteasome inhibitors MG-132 and lactacystin were obtained from Calbiochem (San Diego, CA, USA). The tyrosine kinase inhibitor K252a, the TrkB agonist 7,8-DHF, and the neuroleptic clozapine were purchased from Tocris Biosciences (Ellisville, MO, USA). TC-2153 was synthesized as previously described [33].
Primary cortical cultures
All experimental procedures were approved by the Yale University Institutional Animal Care and Use Committee and were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals. Primary cortical neurons were isolated from rat or mouse E18 embryos as described [33]. Neuronal cultures were maintained in a Neurobasal medium with B27 supplement (Invitrogen, San Diego, CA, USA) for 12–14 days. Cultures were then treated with PCP (10 μM) for 24 h, while in some experiments, the STEP inhibitor (TC-2153, 1 μM) was added 1 h prior to PCP and was present throughout the 24-h PCP treatment. Neurons were lysed in 1× RIPA buffer (Pierce Biotechnology, Rockford, IL, USA) with complete phosphatase and protease inhibitors (Roche, Indianapolis, IN, USA). Lysates were spun at 1000×g for 10 min to isolate homogenates.
Viral infection
Lentivirus-based STEP shRNA (LV-STEP) was validated as described [31, 40, 41]. Rat cortical cultures were infected with LV-STEP or a luciferase control at DIV 7 for 7 days. A recombinant adeno-associated virus of mixed serotype 1/2 (AAV1/2) was custom made (GeneDetect LTD, Auckland, New Zealand), and validated as described [31]. At DIV 5, STEP KO mouse cortical neurons were infected with AAV1/2-STEP61 or AAV1/2-control vector for 7 days prior to PCP exposure.
BDNF ELISA
BDNF levels in rat cortical neuronal cultures (lysates and conditioned media) or frontal cortical tissues were quantified using an Emax ImmunoAssay ELISA kit (Promega, Madison, WI, USA) following the manufacturer’s instructions. Briefly, cells were lysed in RIPA buffer with protease and phosphatase inhibitors. Lysates were centrifuged at 14,000×g for 20 min, and the supernatants were added to microplates pre-coated with a mouse anti-BDNF monoclonal antibody and incubated for 2 h at room temperature (RT). After washes, a rabbit anti-BDNF polyclonal antibody was added for another 2 h at RT. After extensive washes, an anti-IgY HRP conjugate was incubated for 1 h, followed by 3,3′,5,5′-tetramethylbenzidine (TMB) solution. The reaction was stopped with 1 N HCl, and optical density reading was obtained using an ELx800 plate reader (Biotek, Winooski, VT, USA) at 450 nm. To measure BDNF secreted into media, media (1 ml) was collected after treatments and concentrated 10× by using Amicon Ultra Filters with a 3 kDa cutoff (Millipore, Billerica, MA, USA). A standard curve was used to determine BDNF levels in lysates, which were expressed as pg/μg in lysates or pg/ml in media.
Total RNA extraction and quantitative reverse transcription (RT)-PCR
Total RNA was extracted from cortical cultures using RNeasy kit (Qiagen, Valencia, CA, USA) and from cortical tissues using RNeasy lipid tissue kit (Qiagen), following the manufacturer’s instructions. RNA quantification was done by a nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE, USA), and 1 μg of total RNA was used in reverse transcription reactions to obtain complimentary DNA (cDNA) using a Quanti Tech reverse transcription kit (Qiagen). Real-time quantitative PCR was performed in 96-well plates using a StepOnePlus Real-Time PCR Systems (v2.3, Applied Biosystems) kit and Syber Green PCR master mix (Applied Biosystems, Carlsbad, CA, USA). Levels of target mRNAs were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. The forward primers used for BDNF: Exon I (5′-CTCAAAGGGAAACGTGTCTCT-3′), Exon IV (5′-TGCGAGTATTACCTCCGCCAT-3′), Exon VI (5′-TTGGGGCAGACGAGAAAGCGC-3′) and a common reverse primer: (5′-TCACGTGCTCAAAAGTGTCAG-3′), GAPDH forward primer: (5′-TCCATGACAACTTTGGCATTGTGG-3′), and reverse primer: (5′-GTTGCTGTTGAAGTCGCAGGAGAC-3′) were synthesized at the Yale Keck Center as described [42].
Drug administration for biochemical analysis
Male C57BL/6J mice (3–4-month old) were obtained from the Jackson Laboratory (Bar Harbor, Maine). Mice were injected with vehicle (2 % DMSO in saline) or TC-2153 (10 mg/kg, i.p.) followed by PCP administration (7.5 mg/kg, i.p. for 1 h). The effective doses of TC-2153 and PCP were chosen based on previous findings [2, 33]. Frontal cortices were collected after PCP administration and processed by differential centrifugation to get crude synaptosomal membrane fractions (P2) as described [2]. Briefly, brain tissue was homogenized in TEVP (in mM): 10 Tris–HCl, pH 7.4, 1 EDTA, 1 EGTA, and 320 sucrose with protease and phosphatase inhibitors (Roche, Indianapolis, IN, USA). Homogenates were spun at 1000×g for 10 min to obtain supernatant (S1), and S1 was further spun at 12,000×g for 15 min to obtain the crude synaptosomal membrane fraction (P2). P2 was solubilized in the TEVP buffer with brief sonication and saved for later analysis.
Measurement of ubiquitinated STEP after PCP treatment
Ubiquitinated proteins were pulled down as described [2]. Briefly, lysates of PCP-treated (10 μM, 24 h) neurons or P2 fractions from PCP-administrated (7.5 mg/kg, i.p. for 1 h) mouse frontal cortices were incubated with agarose-TUBE2 beads (Tandem Ubiquitin Binding Entity, LifeSensors, Malvern, PA, USA) overnight at 4 °C. Ubiquitinated STEP species were visualized using anti-STEP antibody.
Immunoblot analysis
Samples were resolved on 8 or 12 % SDS-PAGE, transferred onto nitrocellulose membranes (Bio-Rad, Richmond, CA, USA), and incubated with primary antibodies (Supplementary Table 1) overnight at 4 °C, and peroxidase-conjugated secondary antibodies (1:5000; Pierce) for 2 h at RT. Immunoreactivity was developed with a Chemiluminescent Substrate kit (Pierce) and visualized by a G:BOX with the GeneSnap software (Syngene, Cambridge, UK). All densitometric bands were quantified with the Genetools program (Syngene).
Behavioral assessments
Locomotor activity
Male WT C57BL/6 mice (3–5-month old) were used for behavioral tests. Mice were injected i.p. with vehicle (2 % DMSO in saline), TC-2153 (10 mg/kg in 2 % DMSO), 7,8-DHF (5 mg/kg in 2 % DMSO), or clozapine (2 mg/kg in 2 % DMSO). Thirty min after drug pretreatment, mice were placed in an activity chamber (MED Associates, St. Albans, VT, USA) for 30 min of baseline recording, followed by Veh or PCP injection (7.5 mg/kg, i.p.), and monitored for an additional 1 h. Horizontal activity and stereotypies were measured using the Activity Monitor version 5 software (MED Associates, St. Albans, VT, USA).
Novel object recognition (NOR)
Male C57BL/6 mice were administrated PCP (5 mg/kg, i.p. twice daily) for 5 days, followed by a 1-week break. Some groups of mice were administrated i.p. with vehicle (Veh, 3 h prior to the first PCP injection, daily), TC-2153 (10 mg/kg, 3 h prior to the first PCP injection, daily), or 7,8-DHF (5 mg/kg, 1 h prior to the first PCP injection, daily). Mice were off treatment during the 1-week break. We used a three-day paradigm for the NOR test as described [33]. On the first day, mice were habituated to an open-field box for 10 min. On the second day, mice were injected i.p. with Veh (2 % DMSO in saline, 3 h prior to training), TC-2153 (10 mg/kg, 3 h prior to training), or 7,8-DHF (5 mg/kg, 1 h prior to training). Mice were returned to the same box with two identical objects and allowed to explore both for a total of 30 s. A cohort of mice was sacrificed for biochemical analysis 9 h after the training session, and another cohort was run through the behavioral test. Twenty-four hours after training (3rd day), mice were subjected to another trial (3 min), this time with one familiar and one novel object. The location of objects was counter balanced to minimize bias. Mouse activity was recorded throughout the NOR test with an ANY-maze video system (Stoelting, Wood Dale, IL, USA). Time spent with the novel or familiar object was used to calculate a discrimination index (DI) = (novel time − familiar time)/(novel time + familiar time).
Data analysis
Data are expressed as mean ± SEM, and statistical analysis were performed with IBM SPSS Statistics 19 and Prism 5.0 (GraphPad Software, La Jolla, CA, USA). Significance (p < 0.05) was determined by Student’s t test, one-way analysis of variance (ANOVA,) or two-way ANOVA with Bonferroni’s post hoc test. For locomotion, data were analyzed with two-way ANOVA with repeated measures, with Bonferroni’s post hoc or paired t test.
Results
Elevated STEP61 contributes to PCP-induced reduction of BDNF in cortical neurons
To address the relationship between the expression of STEP61 and BDNF, to identify underlying mechanisms, and to establish reagents and conditions that could be extended to a PCP mouse model of SZ, we initially made use of cortical cultures. Our previous studies established that PCP treatment of cortical cultures leads to increased expression of STEP61 [2]. We confirmed that PCP treatment (10 μM, 24 h) led to a significant increase in STEP61 level and decreased the tyrosine phosphorylation of its substrate pERK1/2 in cortical cultures (p < 0.05, Figure S1A). Similar to previous findings [2], PCP treatment resulted in increased ubiquitination of STEP, suggesting that disruption of STEP degradation contributed to its accumulation (p < 0.01, Figure S1B). We also observed a decrease in the phosphorylation of CREB (p < 0.05, Figure S1A), a downstream target of ERK1/2 that regulates several BDNF transcripts [28, 43, 44]. Consistent with previous studies [39], PCP reduced BDNF protein level in cortical neurons as determined by Western blotting (p < 0.05, Figure S1A) and ELISA (p < 0.01, Figure S1C). ELISA analysis of conditioned medium also showed a decreased secretion of BDNF after PCP treatment (p < 0.05, Fig. 1d). Several BDNF transcripts were measured with quantitative RT-PCR (qRT-PCR). PCP decreased CREB-dependent (Exon I: p < 0.01 and IV: p < 0.001, Figure S1E) and CREB-independent (Exon VI: p < 0.05, Figure S1E) transcripts, consistent with a previous report [45].
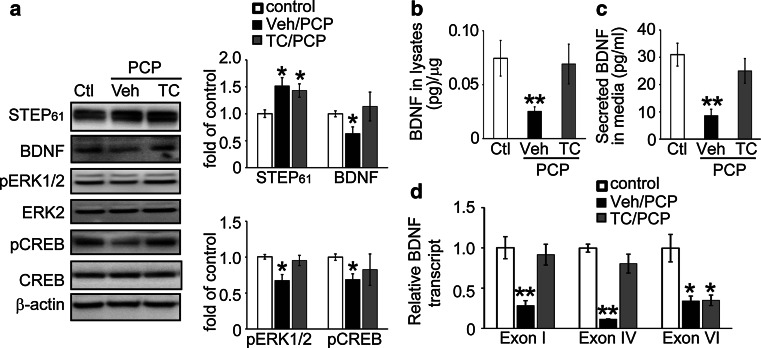
Fig. 1.
The STEP inhibitor TC-2153 prevents PCP-induced decrease in BDNF signaling. a Cortical cultures were pretreated with vehicle or TC-2153 (1 μM) 1 h prior to PCP treatment (10 μM for 24 h). Cells were lysed and subjected to Western blotting. Proteins were probed with phospho-specific- or pan-antibodies, and phospho-levels were normalized to total protein levels, and then to β-actin as loading control. BDNF levels in cell lysates (b) and culture medium (c) were assayed with ELISA. d mRNA levels of 3 BDNF transcripts (Exon I, IV, and VI) were measured with quantitative real-time PCR. Target expression levels were normalized to GAPDH as internal control. Experimental data were compared to controls and expressed as mean ± SEM. Statistical significance was determined with one-way ANOVA with post hoc Bonferroni’s test (*p < 0.05, **p < 0.01, n = 6 independent batches of cultures for a–d)
If elevated STEP61 levels and activity contribute to the decrease in BDNF expression after PCP treatment, then STEP61 inhibition should prevent the decrease. TC-2153, a recently characterized STEP inhibitor [33], potently inhibits STEP activity by forming a covalent bond with the active site Cys472 required for activity, without changing total or phosphorylation levels of STEP. Treatment with TC-2153 prevented PCP-induced decreases in ERK1/2 and CREB phosphorylation, and prevented the decrease in BDNF protein expression in cortical cultures (Fig. 1a). We confirmed the Western blot data with ELISA, again showing that STEP inhibition restored BDNF protein levels in cells (Fig. 1b) as well as in media (Fig. 1c).
Analysis with qRT-PCR revealed that upon PCP treatment, TC-2153 normalized levels of the two CREB-dependent BDNF transcripts (Exon I and IV, Fig. 1d), but not the CREB-independent BDNF transcript (Exon VI, Fig. 1d). Incubation with TC-2153 alone (1 μM, 1–24 h) induced a transient increase in ERK and CREB phosphorylation levels (p < 0.05 at 1 and 3 h, Figure S2A and S2B), but did not alter STEP61 (Figure S2C), BDNF protein (Figure S2D and S2E), or mRNA (Exon I, IV and VI; Figure S1F) levels. These results demonstrate that STEP61 inhibition is sufficient to block PCP-induced decreases in BDNF levels.
As a complementary approach, we used a lentivirus (LV)-based knockdown technique to determine whether reduction of STEP61 expression would normalize BDNF expression after PCP treatment. shRNA virus targeting STEP61 was added to cultured neurons at DIV7 for 7 days, followed by PCP treatment (10 μM for 24 h). A two-way ANOVA analysis showed virus infection (LV-STEP61 shRNA) resulted in a significant decrease in STEP61 (F(1, 20) = 78.01, p < 0.001, Fig. 2a). PCP induced an increase in STEP61 levels in control LV-luciferase-treated cultures (p < 0.05) and a decrease in BDNF expression (p < 0.05, Fig. 2a lanes 1 and 2; representative blots in Figure S3A). In contrast, LV-STEP61 shRNA-treated neurons showed a significant reduction in STEP61 (p < 0.01) and a concomitant increase in pERK1/2 and pCREB levels (p < 0.05, Fig. 2a lanes 1 and 3). Importantly, PCP failed to induce changes in pERK, pCREB, or BDNF levels in the presence of STEP61 shRNA virus (p > 0.05, Fig. 2a lanes 3 and 4).
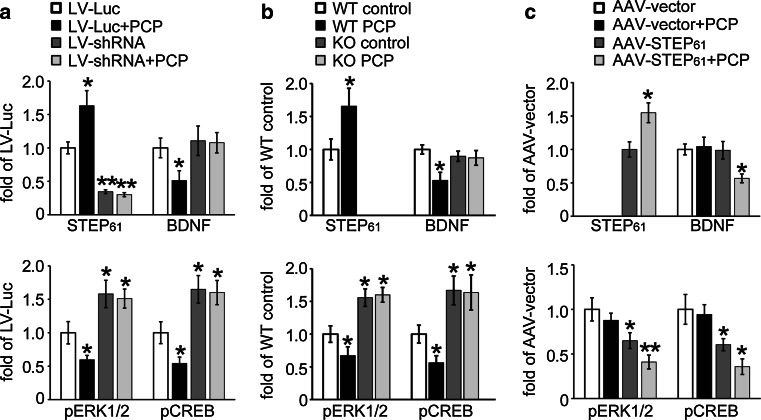
Fig. 2.
STEP61 contributes to the PCP-induced decrease in BDNF expression in cortical cultures. a Cortical neurons were infected with lentivirus containing luciferase vector (LV-Luc) or STEP shRNA (LV-shRNA) for 7 days, followed by control or PCP treatment (10 μM) for 24 h. b Cortical neurons from WT or STEP KO mice were treated with control or PCP (10 μM) for 24 h. c STEP61 was added back to cortical neurons derived from STEP KO mice by infecting with AAV1/2 STEP61 (AAV-STEP61) for 7 days, followed by control or PCP treatment. Neurons were lysed after treatment and subjected to Western blotting. STEP61 and its substrates were probed with phospho-specific- or pan-antibodies, and phospho-levels were normalized to total protein levels, and then to β-actin as loading control. Data were expressed as mean ± SEM, and statistical significance was determined using one-way ANOVA with post hoc Bonferroni’s test (*p < 0.05, **p < 0.01, n = 6 independent batches of cultures for a–c)
The direct involvement of STEP61 in the regulation of BDNF by PCP was evaluated in cultures derived from STEP KO mice, combined with rescue experiments, where STEP61 was added back to the KO cultures. Wild-type (WT) mouse cortical cultures treated with PCP showed similar changes in STEP61 (p < 0.05), BDNF, pERK1/2, and pCREB (p < 0.05) levels compared to rat cortical cultures (Fig. 2b; representative blots in Figure S3B). Cultures derived from STEP KO mice did not show PCP-induced decreases in BDNF, pERK1/2, and pCREB levels (p > 0.05, Fig. 2b; representative blots in Figure S3B). In the absence of PCP administration, STEP KO cultures had elevated basal levels of pERK1/2 and pCREB (p < 0.05) as previously shown [30]. Reintroduction of STEP61 into STEP KO cultures led to a significant decrease in basal pERK1/2 and pCREB (p < 0.05, Fig. 2c; representative blots in Figure S3C). After restoring STEP61 to KO cultures, PCP once again increased STEP61 (p < 0.05), decreased BDNF level (p < 0.05), and down-regulated ERK1/2 and CREB phosphorylation (p < 0.05, Fig. 2c; representative blots in Figure S3C). AAV1/2-STEP61 alone did not alter BDNF expression in the absence of PCP administration. Taken together, these results demonstrate that STEP61 is necessary and sufficient for PCP-induced alterations in BDNF expression in cultures.
Inhibition of STEP in vivo prevents PCP-induced down regulation of BDNF expression
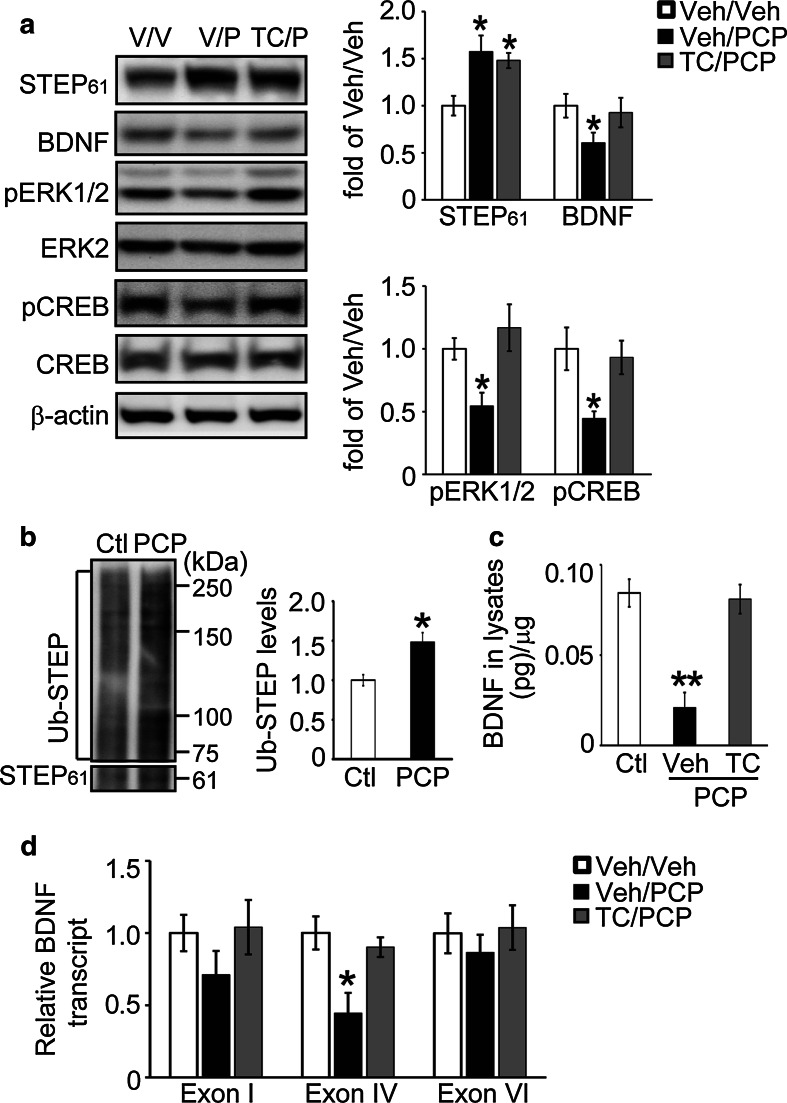
We next examined the effects of TC-2153 on BDNF expression after acute PCP administration in mice (7.5 mg/kg, i.p). PCP administration resulted in elevated STEP61 in the frontal cortex of mice (Fig. 3a), in agreement with previous findings [2]. We also found increased ubiquitination of STEP61 upon PCP treatment (p < 0.05, Fig. 3b), suggesting the disruption of STEP61 degradation might contribute to its accumulation upon PCP administration in mice. There was a decrease in the phosphorylation of ERK1/2 and CREB, as well as a decrease in BDNF levels, as measured by Western blot (Fig. 3a) or ELISA (Fig. 3c). TC-2153 administration alone (10 mg/kg, i.p.) did not change total STEP61 level (Figure S4A) and, although it evoked a transient increase in pERK1/2 and pCREB levels (Figure S4B and S4C, p < 0.05 at 3 h), it did not alter BDNF protein (Figure S4D and S4E) or mRNAs levels (Figure S4F). However, when given prior to PCP administration, TC-2153 blocked PCP-induced reductions in pERK1/2, pCREB, and BDNF (Fig. 3a,c). We also found a correlated change in Exon IV of BDNF mRNA, but not of Exon I or Exon VI (Fig. 3d). These findings indicate that STEP inhibition with TC-2153 does not change baseline BDNF expression, but is sufficient to prevent PCP-induced reduction of BDNF expression in vivo.
Fig. 3.
TC-2153 prevents PCP-induced decreases in BDNF signaling in vivo. a WT (C57BL/6) mice were treated with TC-2153 (10 mg/kg, i.p.) for 3 h, followed by control or PCP administration (7.5 mg/kg, i.p.) for 1 h. Total homogenates from frontal cortex were processed for Western blot analysis. Proteins were probed with phospho-specific- or pan-antibodies, and phospho-levels normalized to total protein levels, and then to β-actin as loading control. b PCP led to increased ubiquitination of STEP61 in mice. c An ELISA assay was used to measure BDNF levels. d Total RNA was extracted from mouse frontal cortices, and mRNA levels of 3 BDNF transcripts (Exon I, IV, and VI) were measured using quantitative real-time PCR. Target expression levels were normalized to GAPDH as internal control. All data were compared to Veh/Veh, and were expressed as mean ± SEM, and statistical significance was determined using one-way ANOVA with post hoc Bonferroni’s test (for a, c, d) or Student’s t test (for b) (*p < 0.05, **p < 0.01, n = 4 C57BL/6 mice for a, c, d; n = 6 C57BL/6 mice for b)
A complementary analysis was carried out using STEP KO mice. WT and STEP KO mice were acutely injected with PCP (7.5 mg/kg, i.p.), and biochemical changes were examined in frontal cortex 1 h later. PCP administration in WT mice increased STEP61 levels, decreased ERK1/2 and CREB phosphorylation (p < 0.05, Figure S5A), and decreased BDNF protein levels (p < 0.05, Figure S5A and S5B). Administration of PCP to STEP KO mice failed to change ERK1/2 and CREB phosphorylation levels (Figure S5A) or reduce BDNF protein levels (Figure S5A and S5B).
STEP inhibition reverses hyperlocomotion in PCP-treated mice
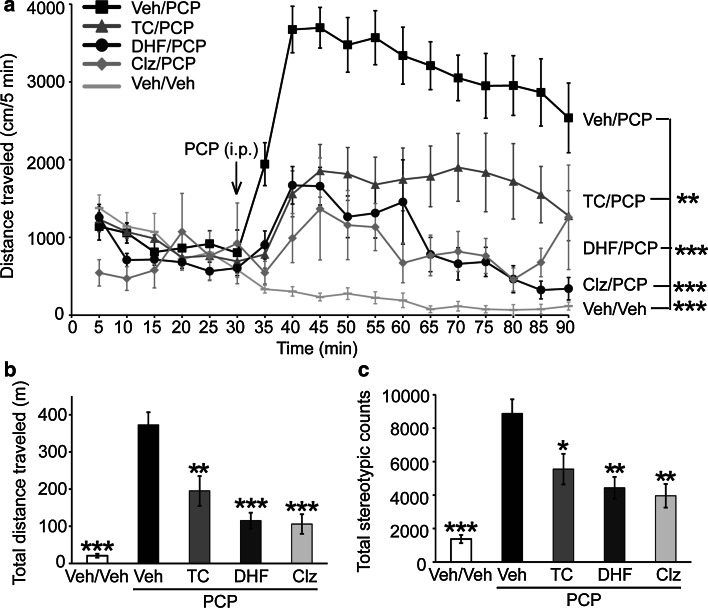
The previous results demonstrate that genetic reduction or pharmacological inhibition of STEP61 reversed the effects of PCP in the biochemical analyses. Since acute administration of PCP results in hyperactive behavior [46], we next tested whether this behavior could be reversed directly with TC-2153. Acute administration of PCP (7.5 mg/kg, i.p.) led to the expected robust increase in locomotion. Two-way ANOVA with repeated measures analysis demonstrated a significant Time × Treatment interaction [F(51, 544) = 7.85, p < 0.001]. There was a significant attenuation of PCP-induced hyperlocomotion by TC-2153 (p < 0.01, compared to PCP alone) (Fig. 4a). Two-way ANOVA analysis of total distance traveled showed that there was a significant effect of pretreatment (Veh or TC-2153, F(2, 48) = 14.66, p < 0.001) and interaction with treatment (Veh or PCP, F(2, 48) = 16.40, p < 0.001). Bonferroni’s post hoc test also revealed that TC-2153 led to a significant attenuation of PCP-induced hyperactivity compared to the Veh/PCP group (p < 0.01) (Fig. 4b). TC-2153 alone did not alter locomotor activity (data not shown). In addition, pretreatment of Veh or TC-2153 had a significant effect on stereotypic grooming (F(2, 48) = 8.45, p < 0.001), and there was a significant interaction between pretreatment and treatment (Veh or PCP, F(2, 48) = 8.03, p < 0.001). Bonferroni’s post hoc test revealed TC-2153 (p < 0.05) also attenuated PCP-induced stereotypic grooming (Fig. 4c). Consistent with previous findings [46], clozapine also reversed PCP-induced hyperlocomotion and stereotypic grooming (Fig. 4).
Fig. 4.
TC-2153 reverses hyperlocomotor activity in acute PCP-injected mice. Male C57BL/6 mice (4–6-month old) were administered with vehicle (Veh), TC-2153 (TC, 10 mg/kg, i.p.), or clozapine (Clz, 2 mg/kg, i.p.) followed by control (Veh/Veh) or PCP challenge (7.5 mg/kg, i.p.) for 1 h. Distance traveled every 5 min during the 90-min test period (a), total distance traveled (b), and total stereotypic counts (c) were recorded and analyzed using Activity Monitor (MED Associates). Data were expressed as mean ± SEM, and statistical significance was determined using two-way ANOVA with repeated measures followed by post hoc Bonferroni’s test (for a) or one-way ANOVA with post hoc Bonferroni’s test (for b, c) (*p < 0.05, **p < 0.01; ***p < 0.001, n = 9 C57BL/6 mice per group for a–c)
To explore the role of BDNF signaling in the observed effects of PCP in mice, we pretreated mice with a selective TrkB agonist, 7,8-dihydroxyflavone (7,8-DHF) prior to PCP administration. 7,8-DHF has a good bioavailability after peripheral administration [47–49]. We proposed that activation of BDNF/TrkB signaling by 7,8-DHF could attenuate PCP-induced hyperlocomotion. Indeed, 7,8-DHF significantly reduced hyperactivity (p < 0.001, compared to PCP alone) (Fig. 4a, b). In addition, pretreatment of 7,8-DHF also had a significant effect on PCP-induced stereotypic grooming (p < 0.01, compared to PCP alone) (Fig. 4c).
STEP inhibition prevents subchronic PCP-induced cognitive deficits
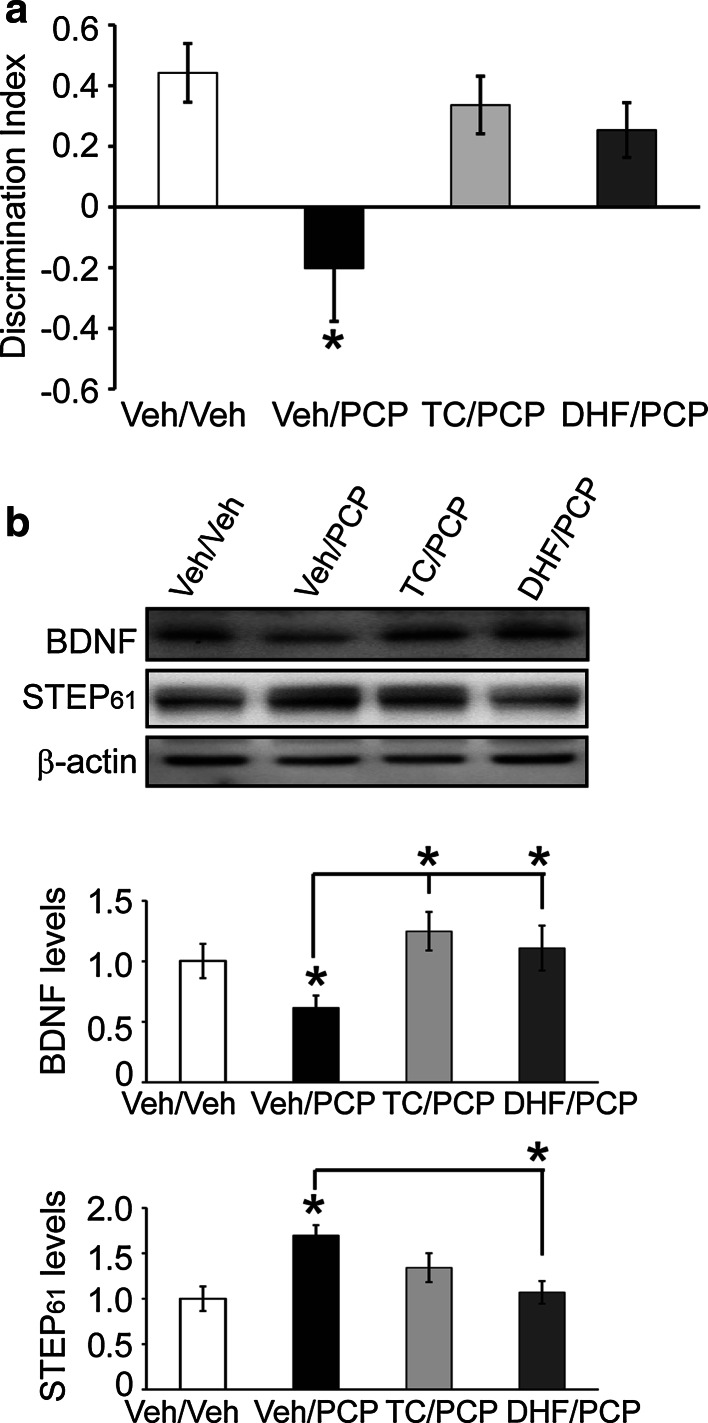
Subchronic administration of PCP in mice also disrupts cognitive function [2, 35]. We, therefore, examined whether TC-2153 might prevent these effects by using the novel object recognition (NOR) test. TC-2153 (10 mg/kg, i.p) or vehicle was administrated 3 h prior to PCP administration (5 mg/kg, i.p., twice daily) over 5 days, at which point animals were trained in the NOR task and were tested 24 h later for memory retention. A discrimination index was calculated to compare memory retention. Vehicle-treated mice spent more time exploring the novel object, whereas mice injected with PCP did not (treatment effect: (F(2, 23) = 7.001), p < 0.01). In contrast, the PCP/TC-2153-treated group performed similarly to the control group not given PCP (p > 0.05, Fig. 5a). TC-2153 alone did not change recognition memory (data not shown). Similarly, 7,8-DHF (5 mg/kg, i.p.; given 1 h before PCP) reversed PCP-induced cognitive deficits (Fig. 5a).
Fig. 5.
TC-2153 prevents PCP-induced decrease of BDNF expression during memory consolidation and memory deficits in the novel object recognition (NOR) test. a Male C57BL/6 mice (4–6-month old) were pretreated with vehicle (Veh) or TC-2153 (TC, 10 mg/kg, i.p.) followed by vehicle or subchronic PCP administration (5 mg/kg, i.p. twice daily) for 5 days, followed by a 1-week drug-free break. A discrimination index was used to evaluate memory retention in different treatment groups. b A second cohort of mice was sacrificed 9 h post-training in the NOR test. BDNF and STEP61 levels were measured in synaptosomal fraction (P2) from hippocampus. Data were expressed as mean ± SEM. One-way ANOVA with post hoc Bonferroni’s test was performed to determine statistical significance (*p < 0.05, n = 9 C57BL/6 mice per group for a; n = 6 C57BL/6 mice per group for b)
BDNF signaling is also implicated in memory consolidation in several behavioral paradigms, including the NOR task [50]. We, therefore, analyzed the effects of STEP61 inhibition on BDNF levels after training in the NOR test. Mice were subjected to subchronic PCP administration (5 mg/kg, i.p., twice daily for 5 days followed by 1 week break) and 9 h post-training, at a time point within the critical period for memory consolidation [50], and hippocampi were collected for biochemical analysis. Subchronic PCP administration reduced BDNF and increased STEP61 levels at this time point (Fig. 5b). Administration of TC-2153 or 7,8-DHF normalized BDNF levels in PCP-treated mice (TC/PCP vs. Veh/PCP or DHF/PCP vs. Veh/PCP, p < 0.05, Fig. 5b).
We also treated STEP KO mice with the same protocol and found that these mice did not show a reduction in BDNF upon PCP challenge (Figure S6). These findings, together with the prior results, indicate that lowering STEP61 activity directly with TC-2153 prevents PCP-induced reduction in BDNF and has beneficial effects on cognitive function.
Discussion
One of the more interesting aspects of recent studies on the neurobiology of STEP is the finding that STEP activity is disrupted in multiple disorders. Both high [2, 32, 51–53] and low [54–56] levels of STEP activity are implicated in a growing number of neuropsychiatric and neurodegenerative disorders. Dysfunctional BDNF/TrkB signaling is implicated in many of the same neurodegenerative and neuropsychiatric disorders [9], but in an opposite direction. The present study provides a possible explanation by demonstrating the regulation of BDNF expression by STEP61 activity. These results suggest that STEP61 contributes to the regulation of BDNF expression patterns, with implications for cognitive functioning in CNS disorders [19].
The results help clarify the mechanisms involved in the regulation of STEP61 and BDNF by PCP. PCP is a noncompetitive NMDAR antagonist that recapitulates in rodents some of the behavioral symptoms observed in humans affected by SZ, including disrupted sensorimotor gating, impaired cognitive functions, and increased social isolation and stereotypic behaviors [2, 38]. In general, PCP rodent models are considered to have good predictable validity as they recapitulate many endophenotypes present in SZ [57, 58]. Previous findings show that acute and subchronic PCP treatments result in increased STEP61 levels, dephosphorylation of the STEP61 substrate GluN2B at Tyr1472, and internalization of NMDAR from synaptosomal membranes [2]. PCP increases STEP61 activity [2] and decreases BDNF gene transcription and protein expression [39, 45]; however, whether these observations were causally related was not fully appreciated. The present study demonstrates that either genetic or pharmacological reduction of STEP activity reversed PCP-induced disruption of BDNF expression and reversed cognitive and motor abnormalities in PCP-treatment mice. In addition, small molecule TrkB agonists (BDNF mimetics) are emerging as new therapeutic agents because of their superior pharmaceutical properties. Indeed, 7,8-DHF and its analogs confer neuroprotection and improve cognitive functions in a variety of rodent models of neuropsychiatric and neurodegenerative disorders [33, 47–49, 59]. However, the efficacy of 7,8-DHF in PCP animal models has not been evaluated. Here, we showed direct activation of BDNF/TrkB signaling by 7,8-DHF achieved comparable efficacy to STEP inhibition by TC-2153, consistent with the hypothesis that STEP and BDNF signaling have opposing functions.
NMDA receptor antagonists, including PCP, induce hyperlocomotion in humans and animal models of SZ [34–36]. Here we found that, in addition to reversing subchronic PCP-induced cognitive deficits, STEP inhibition also attenuated acute PCP-induced hyperactivity. Previous findings have demonstrated that neuroleptics block the cognitive and hyperlocomotion induced by PCP [46]. This result is explained in part by the finding that neuroleptics function through dopamine D2 receptor blockade and activation of protein kinase A (PKA). PKA phosphorylates STEP at the inhibitory Ser221 within the STEP substrate-binding domain, which prevents STEP from binding to and dephosphorylating its substrates [2, 16]. Because both clozapine and TC-2153 are effective in reducing PCP-induced deficits, future studies are necessary to determine whether combining neuroleptics and TC-2153 might allow the use of lower dosages of each to reduce PCP-induced effects. At the present time, TC-2153 is a useful tool for laboratory studies, and additional efforts are underway to discover novel STEP inhibitor platforms that might prove useful therapeutically.
The results also show that STEP61 modulates BDNF expression pattern by regulating BDNF transcription, possibly through ERK1/2-CREB signaling. Distinct promoters regulate the temporal and spatial expression of BDNF transcripts. In particular, transcriptions of Exon I and IV of the BDNF gene are regulated by CREB in a neuronal activity-dependent manner through NMDAR or voltage-gated calcium channels [28, 29, 44]. STEP gates ERK1/2 activity by direct dephosphorylation of a regulatory Tyr residue within the activation loop [16], and STEP KO mice display elevated basal pERK1/2 and pCREB levels [30]. At the same time, STEP dampens NMDAR signaling by dephosphorylation at Tyr1472, thus facilitating its internalization. Consistent with these findings, we demonstrate that direct inhibition of STEP61 by TC-2153 restored BDNF expression, possibly through Exon IV transcription, which was reestablished upon STEP inhibition both in vitro and in vivo. Importantly, STEP61 inhibition did not rescue the expression of BDNF Exon VI, a CREB-independent transcript. We found a different pattern of regulation of Exon I and Exon IV in cultures and in vivo, which may be due to different responses of each promoter to distinct temporal responses in the cell and in vivo models. In accordance with this hypothesis, it has been shown that distinct neuronal activity results in changes in selective transcripts (either Exon I or Exon IV), despite similar changes of CREB phosphorylation levels [60, 61].
STEP inhibition by TC-2153 did not alter BDNF expression in the absence of PCP treatment. TC-2153 administration in neuronal cultures and in mice did lead to a transient increase in pERK1/2 and pS133 CREB levels. However, it is possible that more sustained activation of ERK1/2/CREB or phosphorylation of CREB by additional kinases at other sites is required to stimulate target transcription including BDNF [62–64]. Moreover, modification of CREB by post-translational changes other than phosphorylation may be needed for full activation [65]. Thus, STEP61 inhibition may be required but is not sufficient to alter BDNF transcription under baseline conditions. In agreement with this, STEP KO mice did not show increased BDNF levels.
The ubiquitin proteasome system (UPS) is a major degradation pathway that regulates STEP61 levels [51], and dysfunction of this pathway in Alzheimer’s disease patients and animal models results in the accumulation of STEP61 [32, 51]. Recent studies have shown that disruptions of the UPS contribute to the accumulation of STEP61 in MK-801-treated neuronal cultures and mice [2] and Parkinson’s disease patients [53]. Here we demonstrate that disruption of STEP61 ubiquitination also may contribute to its accumulation in PCP-treated cultures and in mice. Consistent with this finding in SZ, several studies now report alterations of several UPS components both in pyramidal neurons from SZ patients [66] and after PCP administration to rats [67]. Moreover, NMDAR signaling (inhibited by PCP) is essential for the activation and translocation of the proteasome into spines [68], and the blockade of NMDARs leads to a disruption of the UPS and accumulation of synaptic proteins [69].
In summary, our data indicate that increased STEP61 levels lead to the reduction of BDNF levels in a PCP mouse model of SZ. Inhibition of STEP61 could have beneficial effects by restoring BDNF expression. A better understanding of the interaction between STEP and BDNF will extend our understanding of the molecular basis of a number of neuropsychiatric disorders characterized by disruptions in BDNF or STEP61 expression patterns.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We thank Dr. Marilee Ogren for helpful discussion, and Drs. Thomas Lanz and Thao Nguyen (Pfizer Research and Development, Cambridge, MA, USA) for providing lentivirus-STEP shRNA. This work was supported by the National Institute of Health grants MH091037 and MH52711 (P.J.L.), MH090963 (A.C.N.), and GM054051 (J.A.E.).
Abbreviations
- AAV1/2
Adeno-associated virus of mixed serotype ½
- BDNF
Brain-derived neurotrophic factor
- DIV
Days in vitro
- ERK
Extracellular-signal regulated kinase
- HRP
Horseradish peroxidase
- i.p.
Intraperitoneal
- KO
Knock out
- NMDAR
N-methyl-d-aspartate receptor
- NOR
Novel object recognition
- PAGE
Polyacrylamide electrophoresis
- Pyk2
Proline-rich tyrosine kinase 2
- RIPA buffer
Radio-immunoprecipitation assay buffer
- SDS
Sodium dodecyl sulfate
- SEM
Standard error of the mean
- shRNA
Short hairpin RNA
- SNP
Single nucleotide polymorphism
- STEP61
STriatal-Enriched protein tyrosine Phosphatase, 61 kDa
- WT
Wild-type
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol. 2010;70:304–322. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carty NC, Xu J, Kurup P, Brouillette J, Goebel-Goody SM, Austin DR, Yuan P, Chen G, Correa PR, Haroutunian V, Pittenger C, Lombroso PJ. The tyrosine phosphatase STEP: implications in schizophrenia and the molecular mechanism underlying antipsychotic medications. Transl Psychiatry. 2012;2:e137. doi: 10.1038/tp.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molteni R, Lipska BK, Weinberger DR, Racagni G, Riva MA. Developmental and stress-related changes of neurotrophic factor gene expression in an animal model of schizophrenia. Mol Psychiatry. 2001;6:285–292. doi: 10.1038/sj.mp.4000865. [DOI] [PubMed] [Google Scholar]
- 4.Weickert CS, Ligons DL, Romanczyk T, Ungaro G, Hyde TM, Herman MM, Weinberger DR, Kleinman JE. Reductions in neurotrophin receptor mRNAs in the prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2005;10:637–650. doi: 10.1038/sj.mp.4001678. [DOI] [PubMed] [Google Scholar]
- 5.Issa G, Wilson C, Terry AV, Jr, Pillai A. An inverse relationship between cortisol and BDNF levels in schizophrenia: data from human postmortem and animal studies. Neurobiol Dis. 2010;39:327–333. doi: 10.1016/j.nbd.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Jindal RD, Pillai AK, Mahadik SP, Eklund K, Montrose DM, Keshavan MS. Decreased BDNF in patients with antipsychotic naive first episode schizophrenia. Schizophr Res. 2010;119:47–51. doi: 10.1016/j.schres.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Pinto A, Mosquera F, Palomino A, Alberich S, Gutierrez A, Haidar K, Vega P, Barbeito S, Ortiz A, Matute C. Increase in brain-derived neurotrophic factor in first episode psychotic patients after treatment with atypical antipsychotics. Int Clin Psychopharmacol. 2010;25:241–245. doi: 10.1097/YIC.0b013e328338bc5a. [DOI] [PubMed] [Google Scholar]
- 8.Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Notaras M, Hill R, van den Buuse M. A role for the BDNF gene Val66Met polymorphism in schizophrenia? A comprehensive review. Neurosci Biobehav Rev. 2015;51:15–30. doi: 10.1016/j.neubiorev.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Lombroso PJ, Naegele JR, Sharma E, Lerner M. A protein tyrosine phosphatase expressed within dopaminoceptive neurons of the basal ganglia and related structures. J Neurosci. 1993;13:3064–3074. doi: 10.1523/JNEUROSCI.13-07-03064.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bult A, Zhao F, Dirkx R, Jr, Sharma E, Lukacsi E, Solimena M, Naegele JR, Lombroso PJ. STEP61: a member of a family of brain-enriched PTPs is localized to the endoplasmic reticulum. J Neurosci. 1996;16:7821–7831. doi: 10.1523/JNEUROSCI.16-24-07821.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulanger LM, Lombroso PJ, Raghunathan A, During MJ, Wahle P, Naegele JR. Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci. 1995;15:1532–1544. doi: 10.1523/JNEUROSCI.15-02-01532.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, Nairn AC, Salter MW, Lombroso PJ, Gouras GK, Greengard P. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Venkitaramani DV, Gladding CM, Zhang Y, Kurup P, Molnar E, Collingridge GL, Lombroso PJ. The tyrosine phosphatase STEP mediates AMPA receptor endocytosis after metabotropic glutamate receptor stimulation. J Neurosci. 2008;28:10561–10566. doi: 10.1523/JNEUROSCI.2666-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen TH, Liu J, Lombroso PJ. Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem. 2002;277:24274–24279. doi: 10.1074/jbc.M111683200. [DOI] [PubMed] [Google Scholar]
- 18.Xu J, Kurup P, Bartos JA, Patriarchi T, Hell JW, Lombroso PJ. STriatal-enriched protein tyrosine phosphatase (STEP) regulates Pyk2 activity. J Biol Chem. 2012;287:20942–20956. doi: 10.1074/jbc.M112.368654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goebel-Goody SM, Baum M, Paspalas CD, Fernandez SM, Carty NC, Kurup P, Lombroso PJ. Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol Rev. 2012;64:65–87. doi: 10.1124/pr.110.003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelov I, Teltsh O, Greenbaum L, Rigbi A, Kanyas-Sarner K, Lerer B, Lombroso P, Kohn Y. Involvement of PTPN5, the gene encoding the striatal-enriched protein tyrosine phosphatase, in schizophrenia and cognition. Psychiatr Genet. 2012;22:168–176. doi: 10.1097/YPG.0b013e3283518586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohara K, Kitamura A, Morishima M, Tsumoto T. Activity-dependent transfer of brain-derived neurotrophic factor to postsynaptic neurons. Science. 2001;291:2419–2423. doi: 10.1126/science.1057415. [DOI] [PubMed] [Google Scholar]
- 22.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 23.Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci. 2009;10:850–860. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- 24.Li W, Keifer J. BDNF-induced synaptic delivery of AMPAR subunits is differentially dependent on NMDA receptors and requires ERK. Neurobiol Learn Mem. 2009;91:243–249. doi: 10.1016/j.nlm.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu F, Plummer MR, Len GW, Nakazawa T, Yamamoto T, Black IB, Wu K. Brain-derived neurotrophic factor rapidly increases NMDA receptor channel activity through Fyn-mediated phosphorylation. Brain Res. 2006;1121:22–34. doi: 10.1016/j.brainres.2006.08.129. [DOI] [PubMed] [Google Scholar]
- 26.Hartmann M, Heumann R, Lessmann V. Synaptic secretion of BDNF after high-frequency stimulation of glutamatergic synapses. EMBO J. 2001;20:5887–5897. doi: 10.1093/emboj/20.21.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park H, Popescu A, Poo MM. Essential role of presynaptic NMDA receptors in activity-dependent BDNF secretion and corticostriatal LTP. Neuron. 2014;84:1009–1022. doi: 10.1016/j.neuron.2014.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/S0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 29.Shieh PB, Ghosh A. Molecular mechanisms underlying activity-dependent regulation of BDNF expression. J Neurobiol. 1999;41:127–134. doi: 10.1002/(SICI)1097-4695(199910)41:1<127::AID-NEU16>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 30.Venkitaramani DV, Paul S, Zhang Y, Kurup P, Ding L, Tressler L, Allen M, Sacca R, Picciotto MR, Lombroso PJ. Knockout of striatal enriched protein tyrosine phosphatase in mice results in increased ERK1/2 phosphorylation. Synapse. 2009;63:69–81. doi: 10.1002/syn.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, Kurup P, Foscue E, Lombroso PJ. Striatal-enriched protein tyrosine phosphatase regulates the PTPalpha/Fyn signaling pathway. J Neurochem. 2015;134:629–641. doi: 10.1111/jnc.13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Kurup P, Xu J, Carty N, Fernandez SM, Nygaard HB, Pittenger C, Greengard P, Strittmatter SM, Nairn AC, Lombroso PJ. Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer’s disease mouse model. Proc Natl Acad Sci USA. 2010;107:19014–19019. doi: 10.1073/pnas.1013543107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu J, Chatterjee M, Baguley TD, Brouillette J, Kurup P, et al. Inhibitor of the tyrosine phosphatase STEP reverses cognitive deficits in a mouse model of Alzheimer’s disease. PLoS Biol. 2014;12:e1001923. doi: 10.1371/journal.pbio.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 35.Jentsch JD, Redmond DE, Jr, Elsworth JD, Taylor JR, Youngren KD, Roth RH. Enduring cognitive deficits and cortical dopamine dysfunction in monkeys after long-term administration of phencyclidine. Science. 1997;277:953–955. doi: 10.1126/science.277.5328.953. [DOI] [PubMed] [Google Scholar]
- 36.Jentsch JD, Elsworth JD, Taylor JR, Redmond DE, Jr, Roth RH. Dysregulation of mesoprefrontal dopamine neurons induced by acute and repeated phencyclidine administration in the nonhuman primate: implications for schizophrenia. Adv Pharmacol. 1998;42:810–814. doi: 10.1016/S1054-3589(08)60870-4. [DOI] [PubMed] [Google Scholar]
- 37.Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 38.Krystal JH, Anand A, Moghaddam B. Effects of NMDA receptor antagonists: implications for the pathophysiology of schizophrenia. Arch Gen Psychiatry. 2002;59:663–664. doi: 10.1001/archpsyc.59.7.663. [DOI] [PubMed] [Google Scholar]
- 39.Adachi N, Numakawa T, Kumamaru E, Itami C, Chiba S, Iijima Y, Richards M, Katoh-Semba R, Kunugi H. Phencyclidine-induced decrease of synaptic connectivity via inhibition of BDNF secretion in cultured cortical neurons. Cereb Cortex. 2013;23:847–858. doi: 10.1093/cercor/bhs074. [DOI] [PubMed] [Google Scholar]
- 40.Reinhart VL, Nguyen T, Gerwien R, Jr, Kuhn M, Yates PD, Lanz TA. Downstream effects of striatal-enriched protein tyrosine phosphatase reduction on RNA expression in vivo and in vitro. Neuroscience. 2014;278:62–69. doi: 10.1016/j.neuroscience.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Lanz TA, Guilmette E, Gosink MM, Fischer JE, Fitzgerald LW, Stephenson DT, Pletcher MT. Transcriptomic analysis of genetically defined autism candidate genes reveals common mechanisms of action. Mol Autism. 2013;4:45. doi: 10.1186/2040-2392-4-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng F, Zhou X, Luo Y, Xiao H, Wayman G, Wang H. Regulation of brain-derived neurotrophic factor exon IV transcription through calcium responsive elements in cortical neurons. PLoS One. 2011;6:e28441. doi: 10.1371/journal.pone.0028441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pruunsild P, Sepp M, Orav E, Koppel I, Timmusk T. Identification of cis-elements and transcription factors regulating neuronal activity-dependent transcription of human BDNF gene. J Neurosci. 2011;31:3295–3308. doi: 10.1523/JNEUROSCI.4540-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-O. [DOI] [PubMed] [Google Scholar]
- 45.Katanuma Y, Numakawa T, Adachi N, Yamamoto N, Ooshima Y, Odaka H, Inoue T, Kunugi H. Phencyclidine rapidly decreases neuronal mRNA of brain-derived neurotrophic factor. Synapse. 2014;68:257–265. doi: 10.1002/syn.21735. [DOI] [PubMed] [Google Scholar]
- 46.Sun T, Hu G, Li M. Repeated antipsychotic treatment progressively potentiates inhibition on phencyclidine-induced hyperlocomotion, but attenuates inhibition on amphetamine-induced hyperlocomotion: relevance to animal models of antipsychotic drugs. Eur J Pharmacol. 2009;602:334–342. doi: 10.1016/j.ejphar.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 47.Jiang M, Peng Q, Liu X, Jin J, Hou Z, Zhang J, Mori S, Ross CA, Ye K, Duan W. Small-molecule TrkB receptor agonists improve motor function and extend survival in a mouse model of Huntington’s disease. Hum Mol Genet. 2013;22:2462–2470. doi: 10.1093/hmg/ddt098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng Y, Lv F, Li L, Yu H, Dong M, Fu Q. 7,8-dihydroxyflavone rescues spatial memory and synaptic plasticity in cognitively impaired aged rats. J Neurochem. 2012;122:800–811. doi: 10.1111/j.1471-4159.2012.07830.x. [DOI] [PubMed] [Google Scholar]
- 49.Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci USA. 2010;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim DH, Kim JM, Park SJ, Cai M, Liu X, Lee S, Shin CY, Ryu JH. GABA(A) receptor blockade enhances memory consolidation by increasing hippocampal BDNF levels. Neuropsychopharmacology. 2012;37:422–433. doi: 10.1038/npp.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurup P, Zhang Y, Xu J, Venkitaramani DV, Haroutunian V, Greengard P, Nairn AC, Lombroso PJ. Abeta-mediated NMDA receptor endocytosis in Alzheimer’s disease involves ubiquitination of the tyrosine phosphatase STEP61. J Neurosci. 2010;30:5948–5957. doi: 10.1523/JNEUROSCI.0157-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goebel-Goody SM, Wilson-Wallis ED, Royston S, Tagliatela SM, Naegele JR, Lombroso PJ. Genetic manipulation of STEP reverses behavioral abnormalities in a fragile X syndrome mouse model. Genes Brain Behav. 2012;11:586–600. doi: 10.1111/j.1601-183X.2012.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kurup PK, Xu J, Videira RA, Ononenyi C, Baltazar G, Lombroso PJ, Nairn AC. STEP61 is a substrate of the E3 ligase parkin and is upregulated in Parkinson’s disease. Proc Natl Acad Sci USA. 2015;112:1202–1207. doi: 10.1073/pnas.1417423112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saavedra A, Giralt A, Rue L, Xifro X, Xu J, Ortega Z, Lucas JJ, Lombroso PJ, Alberch J, Pérez-Navarro E. Striatal-enriched protein tyrosine phosphatase expression and activity in Huntington’s disease: a STEP in the resistance to excitotoxicity. J Neurosci. 2011;31:8150–8162. doi: 10.1523/JNEUROSCI.3446-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dabrowska J, Hazra R, Guo JD, Li C, Dewitt S, Xu J, Lombroso PJ, Rainnie DG. Striatal-enriched protein tyrosine phosphatase-STEPs toward understanding chronic stress-induced activation of corticotrophin releasing factor neurons in the rat bed nucleus of the stria terminalis. Biol Psychiatry. 2013;74:817–826. doi: 10.1016/j.biopsych.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gladding CM, Fan J, Zhang LY, Wang L, Xu J, Li EH, Lombroso PJ, Raymond LA. Alterations in STriatal-Enriched protein tyrosine Phosphatase expression, activation, and downstream signaling in early and late stages of the YAC128 Huntington’s disease mouse model. J Neurochem. 2014;130:145–159. doi: 10.1111/jnc.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neill JC, Barnes S, Cook S, Grayson B, Idris NF, McLean SL, Snigdha S, Rajagopal L, Harte MK. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: focus on NMDA receptor antagonism. Pharmacol Ther. 2010;128:419–432. doi: 10.1016/j.pharmthera.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 58.Jones CA, Watson DJ, Fone KC. Animal models of schizophrenia. Br J Pharmacol. 2011;164:1162–1194. doi: 10.1111/j.1476-5381.2011.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang YJ, Li YK, Wang W, Wan JG, Yu B, Wang MZ, Hu B. Small-molecule TrkB agonist 7,8-dihydroxyflavone reverses cognitive and synaptic plasticity deficits in a rat model of schizophrenia. Pharmacol Biochem Behav. 2014;122:30–36. doi: 10.1016/j.pbb.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 60.Peregud DI, Panchenko LF, Gulyaeva NV. Elevation of BDNF Exon I-specific transcripts in the frontal cortex and midbrain of rat during spontaneous morphine withdrawal is accompanied by enhanced pCreb1 occupancy at the corresponding promoter. Neurochem Res. 2014;40:130–138. doi: 10.1007/s11064-014-1476-y. [DOI] [PubMed] [Google Scholar]
- 61.Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, et al. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. J Neurosci. 2010;30:11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun P, Enslen H, Myung PS, Maurer RA. Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev. 1994;8:2527–2539. doi: 10.1101/gad.8.21.2527. [DOI] [PubMed] [Google Scholar]
- 63.Deisseroth K, Tsien RW. Dynamic multiphosphorylation passwords for activity-dependent gene expression. Neuron. 2002;34:179–182. doi: 10.1016/S0896-6273(02)00664-5. [DOI] [PubMed] [Google Scholar]
- 64.Mayr BM, Canettieri G, Montminy MR. Distinct effects of cAMP and mitogenic signals on CREB-binding protein recruitment impart specificity to target gene activation via CREB. Proc Natl Acad Sci USA. 2001;98:10936–10941. doi: 10.1073/pnas.191152098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rexach JE, Clark PM, Mason DE, Neve RL, Peters EC, Hsieh-Wilson LC. Dynamic O-GlcNAc modification regulates CREB-mediated gene expression and memory formation. Nat Chem Biol. 2012;8:253–261. doi: 10.1038/nchembio.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arion D, Corradi JP, Tang S, Datta D, Boothe F, et al. Distinctive transcriptome alterations of prefrontal pyramidal neurons in schizophrenia and schizoaffective disorder. Mol Psychiatry. 2015 doi: 10.1038/mp.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ernst A, Ma D, Garcia-Perez I, Tsang TM, Kluge W, Schwarz E, Guest PC, Holmes E, Sarnyai Z, Bahn S. Molecular validation of the acute phencyclidine rat model for schizophrenia: identification of translational changes in energy metabolism and neurotransmission. J Proteome Res. 2012;11:3704–3714. doi: 10.1021/pr300197d. [DOI] [PubMed] [Google Scholar]
- 68.Bingol B, Wang CF, Arnott D, Cheng D, Peng J, Sheng M. Autophosphorylated CaMKIIalpha acts as a scaffold to recruit proteasomes to dendritic spines. Cell. 2010;140:567–578. doi: 10.1016/j.cell.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 69.Guo L, Wang Y. Glutamate stimulates glutamate receptor interacting protein 1 degradation by ubiquitin-proteasome system to regulate surface expression of GluR2. Neuroscience. 2007;145:100–109. doi: 10.1016/j.neuroscience.2006.11.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.