Abstract
Objective
Induction therapy is often recommended for patients with clinical stage IIIA–N2 (cIIIA/pN2) lung cancer. We examined whether postinduction positron emission tomography (PET) scans were associated with ypN2 disease and survival of patients with cIIIA/pN2 disease.
Methods
We performed a retrospective review of a prospectively maintained database to identify patients with cIIIA/pN2 non-small cell lung cancer treated with induction chemotherapy followed by surgery between January 2007 and December 2012. The primary aim was the association between postinduction PET avidity and ypN2 status; the secondary aims were overall survival (OS), disease-free survival (DFS), and recurrence.
Results
Persistent pathologic N2 disease was present in 61% of patients (61/100). PET N2-negative disease increased from 7% (6/92) before induction therapy to 47% (36/77) afterward. The sensitivity, specificity, and accuracy of postinduction PET for identification of ypN2 disease were 59%, 55%, and 57%, respectively. Logistic regression analysis indicated that postinduction PET N2 status was not associated with ypN2 disease. Of the 39 patients with both pre- and postinduction PET N2-avidity, 25 (64%) had ypN2 disease. The 5-year OS was 40% for ypN2 disease versus 38% for N2-persistent disease (p=0.936); the 5-year OS was 43% for postinduction PET N2-negative disease versus 39% for N2-avid disease (p=0.251). The 5-year DFS was 34% for ypN2-negative disease versus 9% for N2-persistent disease (p=0.079).
Conclusions
Postinduction PET avidity for N2 nodes is not associated with ypN2 disease, OS, or DFS in patients undergoing induction chemotherapy for stage IIIA/pN2 disease.
Introduction
Patients with stage IIIA non-small cell lung cancer (NSCLC) have a 5-year overall survival (OS) of 24%, according to the international database reported by Goldstraw and colleagues.1 Induction therapy for locally advanced NSCLC was developed to improve the poor outcomes among patients treated with surgery or radiotherapy alone.2-9 In a phase II study, Martini and colleagues found a 3-year survival of 41% for patients with N2 disease treated with induction therapy.7 They reported a significant survival advantage for patients with a major response to chemotherapy (3-year survival, 34% vs 7%) and for patients who underwent complete resection (3-year survival, 41% vs 5%). Martin and colleagues,10 as well as others,11-13 reported that survival was significantly better among patients with N0/N1 disease than among patients with persistent N2 disease (3-year survival, 43.3% vs 25.5%).
Preoperative prediction of response to therapy in N2 nodes is challenging and often inaccurate.10 Rebollo-Aguirre and colleagues found that the positive predictive value of position emission tomography (PET) scans for persistent N2 disease ranged from 43% to 100%.13 Prior studies showed that PET assessment of N2 disease after induction therapy had a false-negative rate of 25%.10 Given that ypN2 disease may be a predictor of improved OS, we sought to determine whether postinduction PET avidity in N2 nodes was associated with ypN2 disease for patients with stage IIIA/pN2 disease. We also reviewed the outcomes among patients who underwent resection after induction chemotherapy for stage IIIA/pN2 disease to help guide decisions about resection in the presence of persistent nodal disease and to ascertain outcomes and recurrence patterns.
Patients and Methods
Data Collection
We performed a retrospective review of a prospectively maintained database, from the Thoracic Surgery Service at Memorial Sloan Kettering Cancer Center (MSKCC), of patients who underwent surgery for NSCLC between January 1, 2007, and December 31, 2012. As we have previously analyzed patients who underwent induction therapy before 2007,14 we chose this period to avoid any duplication. This study was approved by the MSKCC Institutional Review Board.
Consecutive patients with pathologically confirmed N2 metastatic NSCLC who were treated with induction chemotherapy followed by surgery were included. Prior to induction therapy, N2 disease was confirmed by endobronchial ultrasound (EBUS), diagnostic mediastinoscopy, endoscopic ultrasound (EUS), CT-guided biopsy, or thoracoscopic biopsy. None of the patients had complete mediastinal nodal clearance. EBUS, the current preferred procedure, became more common during this period. In general, patients were considered for surgery if all N2 nodal disease was thought to be surgically resectable following induction therapy, regardless of the size or number of involved N2 nodal stations. Patients treated with induction chemoradiotherapy or who had synchronous primary, superior sulcus, or neuroendocrine lung cancers were excluded. Patients without pathologically confirmed N2 disease were excluded. All consecutive patients were included for determination of the secondary outcomes of survival and recurrence, regardless of whether pre- and postinduction PET scans were performed.
PET scans from other institutions were included in the analysis. Recorded data were SUVmax, nonavid description, and a nonnumerical descriptor of T and N2 status. Typically, “nonavid” reflected SUVmax equivalent to or lower than that in the blood pool. However, the reports came from multiple referring institutions; therefore, a uniform definition did not exist. “Nonnumerical descriptor” refers to studies that reported the SUVmax as “increased,” “decreased,” or “improved” but did not provide a specific SUVmax.
Clinical Assessment
Preoperative assessment included history and physical examination, laboratory assessment, pulmonary function tests, CT of the chest/upper abdomen, MRI of the brain, and PET scan. Staging was performed using the seventh edition of the American Joint Committee on Cancer staging manual (www.cancerstaging.org).
All patients had surgery at MSKCC, although some received induction therapy at other institutions. Complete mediastinal lymph node dissections were performed for all patients before attempted pulmonary resection. If the mediastinal lymph nodes were unresectable, the operation was aborted and patients were not subjected to lobectomy or a larger operation.
Postoperative surveillance was based on National Comprehensive Cancer Network guidelines (www.nccn.org). In general, patients underwent a clinical interview with interval history, physical examination, and CT of the chest/upper abdomen every 6 months for the first 2 to 3 years, then annually thereafter.
Primary and Secondary Study Aims
The primary aim of the study was to determine whether postinduction PET avidity was associated with pathologic persistence of N2 nodes. Secondary aims were 30- and 90-day mortality, OS, disease-free survival (DFS), and the cumulative incidence of recurrence (CIR).
Statistical Analysis
Postinduction PET N2 status was categorized as “PET N2-avid” or “PET N2-negative.” The reference standard was determined by pathologic N2 status and categorized as “pathologic N2-persistent (ypN2)” or “pathologic N2-negative (ypN0-1).”
The performance of postinduction PET for the detection of persistent mediastinal disease was quantified using the subset of patients with both postinduction PET scans and pathologic N2 status reports. A positive identification was defined as postinduction PET N2-avid, and a negative identification was defined as PET N2-negative. Performance measures (sensitivity, specificity, and positive and negative predictive values) were determined using the standard definitions. Logistic regression was performed to investigate factors associated with persistent mediastinal disease. Other factors included age, sex, smoking status, comorbidities, lung function, histologic profile, method of mediastinal staging, and resection status. Given that the PET scans were performed at multiple institutions, absolute SUVmax and changes in SUVmax were not evaluated.
OS was the duration between surgery and death from all causes. DFS was calculated as the time from surgery to locoregional recurrence, distant metastasis, or death without evidence of recurrence or progression. These analyses were supplemented by analyses of time to recurrence. The Kaplan-Meier method was used to construct OS and DFS curves. CIR curves were generated using a competing risk approach in which death from any cause before recurrence was treated as a competing risk event. Although plots show data through 5 years, all follow-up data were used in the statistical calculations. OS and DFS curves were separated by postinduction PET N2 status (PET N2-avid vs PET N2-negative), pathologic N2 status (pathologic N2-persistent vs pathologic N2-negative), and pathologic staging (≥ypIIIA vs <ypIIIA). The log-rank test was used to compare OS and DFS between groups. Cox proportional hazards models were used to compute hazard ratios (HRs). Comparison of CIRs was performed using the method of Gray.15 Multivariate regression models were constructed, starting with all variables with p<.10 in univariate analyses. All statistical tests were two-tailed, and p<.05 was considered to indicate statistical significance. No multiple comparison adjustment was made. All analyses were performed using Stata 13 (StataCorp, College Station, TX) and R 2.13.1 (R Development Core Team, Vienna, Austria).
Results
Demographic and PET Details
One hundred consecutive patients with stage IIIA/pN2 NSCLC at diagnosis were identified (Table 1). The most common histologic profile was adenocarcinoma (78%; 78/100). The majority of cases of N2 disease (95%; 95/100) were confirmed by EBUS or mediastinoscopy; the other 5 cases were confirmed by EUS, CT-guided biopsy, or thoracoscopic biopsy.
Table 1. Patient demographic, clinical, and operative characteristics.
| N=100 | |
|---|---|
| Sex | |
| Female | 49 (49%) |
| Male | 51 (51%) |
| Age | 65.0 (58.5, 71.0) |
| Smoking Status | |
| Current | 10 (10%) |
| Former | 73 (73%) |
| Never | 17 (17%) |
| Comorbidities | |
| Pulmonary (N=84), yes | 32 (38%) |
| Cardiac (N=85), yes | 53 (62%) |
| NIDDM (N=75), yes | 9 (12%) |
| FEV1 (%) (N=98) | 87.0 (79.0, 99.0) |
| Diffusion (%) (N=94) | 77.0 (63.0, 96.0) |
| Operative Details | |
| Procedure | |
| Wedge | 8 (8.0%) |
| Lobectomy | 75 (75%) |
| Bilobectomy | 7 (7.0%) |
| Pneumonectomy | 8 (8.0%) |
| Segmentectomy | 2 (2.0%) |
| Location of Tumor | |
| Right | 80 (80%) |
| Left | 20 (20%) |
| Operative Approach | |
| Open | 97 (97%) |
| VATS | 3 (3.0%) |
| Resection | |
| R0 | 85 (85%) |
| R1 | 8 (8.0%) |
| R2 | 7 (7.0%) |
| Mortality | |
| 30-day mortality | 2 (2.0%) |
| 90-day mortality | 2 (2.0%) |
| Adverse Events, yes | 27 (27%) |
| Prolonged Air Leak, yes | 5 (5.0%) |
| Respiratory Failure/ARDS, yes | 2 (2.0%) |
| Supraventricular Tachycardia, yes | 7 (7.0%) |
| Clinical and Pathological Details | |
| Method of Pre-induction Mediastinal Staging | |
| Mediastinoscopy-only | 35 (35%) |
| EBUS-only | 52 (52%) |
| Mediastinoscopy + EBUS | 8 (8.0%) |
| Other | 5 (5.0%) |
| Pathology | |
| No Viable Tumor | 2 (2.0%) |
| Adenocarcinoma | 78 (78%) |
| Squamous Cell Carcinoma | 18 (18%) |
| Large Cell Carcinoma | 2 (2.0%) |
| Pathological N Status | |
| N0 | 33 (33%) |
| N1 | 6 (6.0%) |
| N2 | 61 (61%) |
| Post-operative Pathological Stage | |
| 0 | 2 (2.0%) |
| IA | 14 (14%) |
| IB | 11 (11%) |
| IIA | 4 (4.0%) |
| IIB | 5 (5.0%) |
| IIIA | 59 (59%) |
| IIIB | 3 (3.0%) |
| IV | 2 (2.0%) |
| Adjuvant Therapy | |
| None | 39 (39%) |
| PORT-only | 37 (37%) |
| Adjuvant Chemotherapy-only | 16 (16%) |
| PORT + Adjuvant Chemotherapy | 8 (8.0%) |
Data presented as N(%) or median (25th, 75th percentile). VATS = video-assisted thoracic surgery; PORT = post-operative radiation therapy; NIDDM = non-insulin dependent diabetes; EBUS = endobronchial ultrasound; ARDS = Adult respiratory distress syndrome.
Preinduction PET scans were available for 99 patients (98 with reported T and N values; Table 2). Postinduction PET scans were available for 80 patients (79 with reported T and N status). The median SUVmax for N2 nodes was 6.5 for patients with preinduction PET with numerical values and 3.3 for those with postinduction PET with numerical values. Eighty-four percent (65/77) had a decrease in SUVmax T status, and 80% (61/76) had a decrease in SUVmax N status. The percentage of patients with PET N2-negative disease increased from 7% (6/92) before induction therapy to 47% (36/77) after. The median duration between pre- and postinduction PET was 4 months (range, 1.7 to 11.2); the median duration between postinduction PET and surgery was 0.7 months (range, 0.03 to 5.8).
Table 2. Pre- and Post-induction PET Results.
| Pre-induction PET (N=99) | Post-induction PET (N=80) | |
|---|---|---|
| T Status | ||
| Non-avid | 1 (1.0%) | 10 (13%) |
| No value reported | 1 (1.0%) | -- |
| Non-numerical value reported | 4 (4.0%) | 1 (1.3%) |
| Numerical value reported | 93 (94%) | 69 (86%) |
| SUVmax N among numerical values | 9.8 (6.2, 14.7) | 5.0 (3.0, 9.8) |
| N Status | ||
| Non-avid | 6 (6.1%) | 36 (45%) |
| No value reported | 1 (1.0%) | 1 (1.3%) |
| Non-numerical value reported | 6 (6.1%) | 2 (2.5%) |
| Numerical value reported | 86 (87%) | 41 (51%) |
| SUVmax T among numerical values | 6.5 (3.8, 9.5) | 3.3 (2.7, 4.5) |
| N2 Status | ||
| N2-Negative | 6/92 (6.5%) | 36/77 (47%) |
| N2-Avid | 85/92 (93%) | 41/77 (53%) |
| PET Response to Induction Therapy: Change from Pre- to Post-induction values* | SUVmax T (N=77) | SUVmax N (N=76) |
| Pre- to Post-induction | ||
| No change | 1 (1.3%) | 6 (7.9%) |
| Decreased | 65 (84%) | 61 (80%) |
| Increased | 11 (14%) | 9 (12%) |
| Not interpretable | 2 | 3 |
Data presented as N(%) or median (25th, 75th percentile).
Pre- and post-PET scans available for 79 patients; 77 with interpretable change in SUVmax T and 76 with interpretable change in SUVmax N2. Non-numeric descriptions in post-induction PET cannot be categorized as a change unless pre-induction PET is also a non-numeric description.
PET = positron emission scan; SUVmax = Maximum standardized uptake values
Operative Management and Adjuvant Therapy
All patients received doublet induction therapy, with the majority (99/100; 99%) receiving a platin-based regimen. The most common operation was lobectomy (75%; 75/100). Eighty-five patients had R0 resections, 8 had R1, and 7 had R2. Eight percent of patients (8/100) underwent a nonanatomic wedge resection. Resection of N2 nodal disease was attempted before pulmonary resection. If the mediastinal disease was unable to be completely resected (R2), the planned pulmonary resection was aborted. In such patients, if possible, the primary tumor was resected by a wedge resection to limit the subsequent radiation field. Thirty- and 90-day mortalities were 2% (2/100); both patients experienced adult respiratory distress syndrome after a segmentectomy and a lobectomy. There were no deaths following pneumonectomy (Table 1).
Sixty-one patients (61%) received adjuvant therapy, 37 received postoperative radiotherapy (PORT) only, 16 received adjuvant chemotherapy only, and 8 received both PORT and adjuvant chemotherapy (Table 1 and Supplemental Table 1).
Pathologic Staging and N2 Status
Postoperative pathologic staging revealed that 36% of patients had <ypIIIA disease (36/100) and 64% had ≥ypIIIA. Two patients had complete pathologic responses (Table 1). Pathologic staging found ypN2 disease in 61% of patients (pN2, 61/100) and ypN0-1 disease in 39% (pN0, 33/100; pN1, 6/100). Of the 39 patients with N2-avid disease on both pre- and postinduction PET, 25 (64%) had ypN2 disease.
Accuracy and Performance of Postinduction PET N2 Staging
We quantified the ability of postinduction PET N2 nodal assessment to identify persistent pathologic N2 disease. Postinduction PET scans and corresponding ypN2 status reports were available for 77 patients (Supplemental Table 2 and 3). Postinduction PET scan indicated N2-negative disease in 36 patients (47%) and N2-avid disease in 41 (53%). Pathologic N2-staging confirmed ypN0-1 disease in 31 patients (40%) and ypN2 disease in 46 (60%). There were 27 true-positives [PET(+)/Path(+)], 14 false-positives [PET(+)/Path(-)], 17 true-negatives [PET(-)/Path(-)], and 19 false-negatives [PET(-)/Path(+)]. The sensitivity, specificity, and accuracy of postinduction PET for the detection of ypN2 disease were 59%, 55%, and 57%, respectively; positive and negative predictive values were 66% and 47%.
Factors Associated with ypN2 Disease
We investigated the association between postinduction PET N2 staging and ypN2 disease among 77 patients with known postinduction PET N2 status. The results of logistic regression indicated that the odds of ypN2 disease were 1.73-times higher for patients with PET N2-avid disease than for patients with PET N2-negative disease, although this relationship was not statistically significant (95% CI, 0.69–4.33; p=0.2). Having a postinduction PET SUVmax of N2 nodes that was higher than the median value of 3.3 was significantly associated with greater odds of ypN2 disease (HR=4.6; p = 0.045), although the 95% CI was large secondary to a small sample size. Expanding to the entire cohort of 100 patients, the only factor that was statistically significant in univariable models was adenocarcinoma (OR 2.9; 95% CI, 1.09–7.63; p=0.032). Age, lung function, and method of preinduction N2 staging were not statistically significant (Supplementary Table 4).
Factors Associated with OS
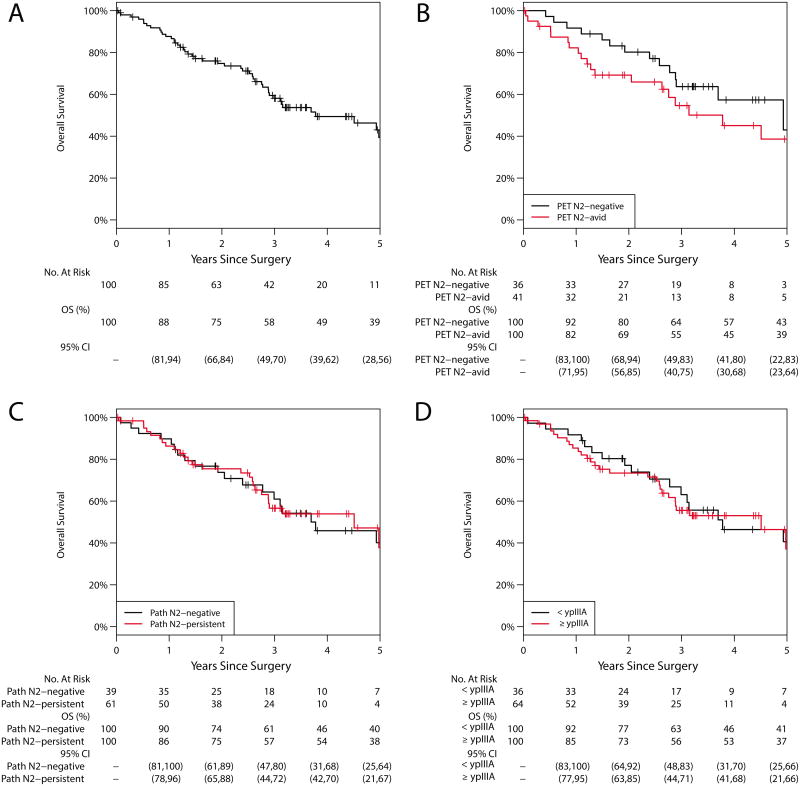
The median duration of follow-up was 2.7 years (range, 0.02 to 7.2). Forty-five patients died during follow-up. The majority (41/45; 92%) died of disease; 4 died of other causes. The median time to death was 1.6 years (range, 0.04–5.0). The 5-year OS was 39% (95% CI, 28%–56%; Figure 1); median survival was 3.78 years. Median survival for ypN0, ypN1, and ypN2 disease was 3.8, 2.0, and 4.5 years, respectively. The 5-year OS was 40% (95% CI, 25%–64%) for patients with ypN0-1 disease versus 38% (95% CI, 21%–67%) for patients with ypN2 disease (p=0.936); median survival was 3.7 years and 4.5 years. The 5-year OS was 41% (95% CI, 25%–66%) for patients with <ypIIIA disease versus 37% (95% CI, 21%–66%) for patients with ≥ypIIIA disease (p=0.791). Among patients with valid postinduction PET N2 status, the 5-year OS was 43% (95% CI, 22%–83%) for PET N2-negative disease versus 39% (95% CI, 23%–64%) for PET N2-avid disease (p=0.251). Univariable analyses revealed that no factors, among those evaluated, were significantly associated with hazard of any death (Table 3). Pathologic T stage ≥IIA had a HR of 1.80 (95% CI, 0.98–3.31; p=0.058), suggesting a potential worse prognosis with higher T stage. No multivariable analysis was performed.
Figure 1.
Overall survival (OS). A, Entire cohort: the 5-year OS was 39% (95% CI, 28%–56%). B, Postinduction PET N2 status: the 5-year OS for N2-negative was 43% (95% CI, 22%–83%) vs 39% (95% CI, 23%–64%) for N2-avid disease (p=0.251). C, Pathologic N2 status: the 5-year OS for N2-negative was 40% (95% CI, 25%–64%) vs 38% (95% CI, 21%–67%) for N2-persistent disease (p=0.936). D, Pathologic staging: the 5-year OS for <ypIIIA was 41% (95% CI, 25%–66%) vs 37% (95% CI, 21%–66%) for ≥ypIIIA (p = 0.791).
Table 3. Univariable Cox models for Overall Survival and Disease-Free Survival Analyses.
| Overall Survival | Disease-Free Survival | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Post-induction PET (N2-avid v N2-negative) | 1.50 | 0.75, 3.00 | 0.3 | 1.05 | 0.59, 1.86 | 0.9 |
| Pathological N2 Status (N2-persistent v N2-negative) | 0.98 | 0.54, 1.77 | 0.9 | 1.58 | 0.94, 2.63 | 0.082 |
| Pathologic Staging (≥ypIIIA v <ypIIIA) | 1.09 | 0.59, 1.99 | 0.8 | 1.67 | 0.99, 2.82 | 0.056 |
| Age at Surgery | 0.99 | 0.96, 1.03 | 0.6 | 0.97 | 0.94, 1.00 | 0.044 |
| Male | 1.49 | 0.82, 2.70 | 0.2 | 1.07 | 0.66, 1.75 | 0.8 |
| Smoking, yes | 1.02 | 0.48, 2.20 | 0.9 | 0.93 | 0.48, 1.78 | 0.8 |
| Smoking Pack-years >30, yes | 0.98 | 0.54, 1.75 | 0.9 | 0.71 | 0.43, 1.16 | 0.2 |
| Pulmonary comorbidity, yes | 1.07 | 0.55, 2.10 | 0.8 | 0.77 | 0.44, 1.35 | 0.4 |
| Cardiac comorbidity, yes | 0.95 | 0.48, 1.88 | 0.9 | 0.69 | 0.40, 1.21 | 0.2 |
| NIDDM, yes | 1.43 | 0.50, 4.09 | 0.5 | 1.73 | 0.68, 4.41 | 0.3 |
| FEV1 (%) | 0.99 | 0.97, 1.01 | 0.2 | 0.99 | 0.97, 1.00 | 0.10 |
| Diffusion (%) | 1.00 | 0.99, 1.02 | 0.6 | 1.01 | 1.00, 1.02 | 0.2 |
| Adenocarcinoma, yes | 1.07 | 0.52, 2.23 | 0.9 | 1.65 | 0.88, 3.10 | 0.12 |
| EGFR, present | 0.74 | 0.26, 2.12 | 0.6 | 0.79 | 0.35, 1.75 | 0.6 |
| KRAS, present | 1.66 | 0.81, 3.40 | 0.2 | 1.18 | 0.64, 2.20 | 0.6 |
| Adjuvant Therapy | ||||||
| PORT-only only vs None | 1.19 | 0.61, 2.33 | 0.6 | 1.14 | 0.65, 2.01 | 0.6 |
| Adjuvant Ch-only vs None | 0.64 | 0.26, 1.58 | 0.3 | 0.78 | 0.37, 1.63 | 0.5 |
| PORT+Adjuvant Ch vs None | 0.60 | 0.17, 2.08 | 0.4 | 0.94 | 0.38, 2.31 | 0.9 |
| Resection: R1 vs R0 | 2.08 | 0.87, 4.97 | 0.10 | 1.46 | 0.66, 3.22 | 0.3 |
| Resection: R2 vs R0 | 1.33 | 0.41, 4.34 | 0.6 | 2.32 | 0.92, 5.88 | 0.075 |
| Pathologic T-Stage (≥IIA v <IIA)a | 1.80 | 0.98, 3.31 | 0.058 | 2.22 | 1.33, 3.73 | 0.002 |
Pathologic T-stage: 45 had <IIA (0, IA, IB) and 55 had ≥IIA (includes IIA, IIB, IIIA, III and IV). NIDDM = non-insulin dependent diabetes; PORT = post-operative radiation therapy; Ch = chemotherapy
PET = positron emission scan; NIDDM = non-insulin dependent diabetes; FEV1 = Forced expiratory volume in one second; EGFR = Endothelial growth factor receptor; KRAS = Kirsten rat sarcoma viral oncogene homolog; PORT = post-operative radiation therapy.
DFS and Factors Associated with DFS
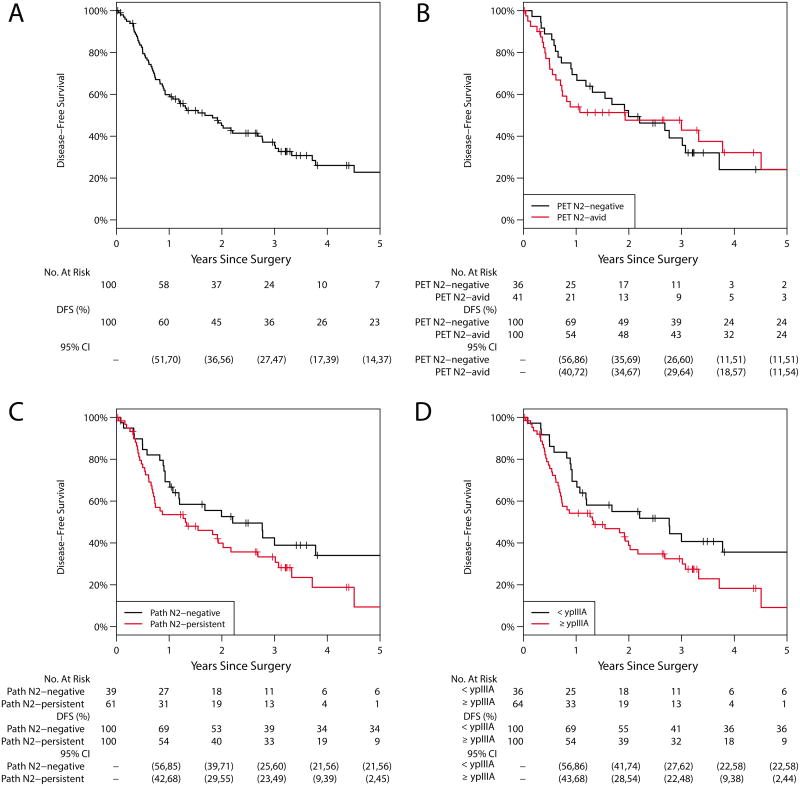
Sixty-five patients experienced recurrence or progression or died (Figure 2). Sixty-one patients (61%) experienced recurrence or progression: 7 locoregional only, 28 distant only, and 26 locoregional and distant (Table 4). Five patients died without a reported recurrence or progression. The 5-year DFS was 23% (95% CI, 14%–37%); median DFS was 1.68 years. Median DFS for pN0, pN1, and pN2 disease was 2.8, 1.5, and 1.3 years, respectively. The 5-year DFS was 34% (95% CI, 21%–56%) for ypN0-1 disease versus 9% (95% CI, 2%–45%) for ypN2 disease (p=0.079); median survival was 2.2 years and 1.3 years. The 5-year DFS was 36% (95% CI, 22%–58%) for patients with <ypIIIA disease versus 9% (95% CI, 2%–44%) for patients with ≥ypIIIA disease (p=0.054). Although not statistically significant, the HR for ypN2 disease was 1.58 (95% CI, 0.94–2.63; p=0.082), and the HR for ≥ypIIIA disease was 1.67 (95% CI, 0.99–2.85; p=0.056), suggesting a worse prognosis for ypN2 disease or higher pathologic stage. Among patients with postinduction PET N2 status, the 5-year DFS was 24% (95% CI, 11%–51%) for patients with PET N2-negative disease versus 24% (95% CI, 11%–54%) for patients with N2-avid disease (p=0.867). R2 resection, pathologic T stage ≥IIA, and younger age at surgery were also significantly associated with worse prognosis (Table 3). With acknowledgment of the limitations in terms of power, we present the final multivariable Cox model stratified by resection status with only two variables: age (HR=0.97; 95% CI, 0.94–1.00; p=0.059) and pathologic T stage ≥IIA (HR=1.92; 95% CI, 1.12–3.31; p=0.018). Postinduction PET N2 status showed evidence of violating the proportionality assumption, but adjusting for this assumption did not affect the significance of the other factors. We did not include postinduction N2 nodal avidity in the final multivariable model.
Figure 2.
Disease-free survival (DFS). A, Entire cohort: the 5-year DFS was 23% (95% CI, 14%–37%). B, Postinduction PET N2 status: the 5-year DFS for N2-negative was 24% (95% CI, 11%–51%) vs 24% (95% CI, 11%–54%) for N2-avid disease (p=0.867). C, Pathologic N2 status: the 5-year DFS for N2-negative was 34% (95% CI, 21%–56%) vs 9% (95% CI, 2%–45%) for N2-persistent disease (p=0.079). D, Pathologic staging: the 5-year DFS for <ypIIIA was 36% (95% CI, 22%–58%) vs 9% (95% CI, 2%–44%) for ≥ypIIIA (p=0.054).
Table 4. Patient Outcomes and Recurrence and Progression Patterns by Resection Status.
| Resection Status | ||||
|---|---|---|---|---|
| Overall (N=100) | R0 (N=85; 85%) | R1 (N=8; 8.0%) | R2 (N=7; 7.0%) | |
| Pathologic N2-Status | ||||
| N2-negative | 39 (39%) | 33 (39%) | 4 (50%) | 2 (29%) |
| N2-persistent | 61 (61%) | 52 (61%) | 4 (50%) | 5 (71%) |
| Status | ||||
| Alive with Disease | 18 (18%) | 14 (16%) | 1 (13%) | 3 (43%) |
| Died of other causes | 4 (4.0%) | 3 (3.5%) | 1 (13%) | 0 (0%) |
| Died of disease | 41 (41%) | 33 (39%) | 5 (63%) | 3 (43%) |
| No evidence of recurrence | 37 (37%) | 35 (41%) | 1 (13%) | 1 (14%) |
| Recurrence or progression (N=61) | ||||
| Locoregional only | 7 (11%) | 7 (14%) | 0 (0%) | 0 (0%) |
| Distant only | 28 (46%) | 26 (52%) | 2 (33%) | 0 (0%) |
| Both locoregional and distant | 26 (43%) | 17 (34%) | 4 (67%) | 5 (100%) |
CIR
Sixty-one patients experienced recurrence postoperatively (Table 4 and Supplemental Table 5). The 3-year CIR for the entire cohort was 70% (95% CI, 60%–81%; Supplemental Figure 1). The 5-year CIR was 76% (95% CI, 58%–99%) for postinduction PET N2-negative disease versus 58% (95% CI, 41%–80%) for N2-avid disease. The 5-year CIR was 59% (95% CI, 44%–79%) for ypN0-1 disease versus 80% (95% CI, 66%–96%) for ypN2 disease. The 5-year CIR was 57% (95% CI, 41%–78%) for <ypIIIA disease versus 80% (95% CI, 67%–96%) for ≥ypIIIA disease (Gray's test, p=0.049), indicating a worse prognosis for recurrence for patients with ≥ypIIIA disease. The CIRs were not significantly different by postinduction PET N2 status (p=0.571) or ypN2 status (p=0.077).
Discussion
We found that postinduction N2 nodal PET activity was not associated with ypN2 disease following induction chemotherapy for known pN2 disease. Unsurprisingly, a difference in 5-year OS, with or without residual PET avidity in N2 nodes, was not detected.
Candela and Detterbeck reviewed the literature on restaging after induction therapy.10 Across the 10 studies they analyzed, the aggregate false-positive rate was 33%, and the false-negative rate was 25%, with no subset in which PET performed sufficiently well. However, only 3 of the studies reported N2 data for PET restaging after induction chemotherapy only. These 3 studies had false-positive rates of 25% to 40% and false-negative rates of 23% to 36%.11,12,16 These studies were published from 2004 to 2006 and reported the outcomes of only 25-30 patients, whereas we reported 100 patients included consecutively from 2006 to 2012. Only De Leyn and colleagues reported that all patients had preinduction pathologic confirmation of N2 disease.11 By including only patients with pathologically confirmed N2 disease, and as a result of our institution's routinely performing systematic, complete mediastinal lymph node dissections, we believe that our data represent a reliable assessment of postinduction PET scans. We still report positive and negative predictive values of 66% and 47%, which do not support the reliability of postinduction PET assessment of N2 nodal disease. We also found that the 5-year OS was the same with or without residual PET avidity in N2 nodes. This strongly suggests that N2 PET avidity is not reliably associated with ypN2 node disease following induction therapy. Furthermore, an important and incremental advance of this study is that postinduction PET assessment of N2 nodes is not associated with OS.
The previously reported 5-year OS after induction chemotherapy and surgical resection ranges from 17% to 36%; the 5-year OS in our series was 39% (Figure 1A).14 Albain and colleagues reported the results of Intergroup Trial 0139, in which patients randomized to receive induction chemoradiotherapy had a 5-year OS of 27%.6 Our 5-year OS is higher, which we believe may be secondary to several factors. We postulate that the emphasis on adequate preresection DLCO, the avoidance of surgery in patients with severe comorbid conditions, the increase in adenocarcinoma relative to squamous cell carcinoma histologic profile, the lack of radiotherapy in the induction regimens, the low rate of pneumonectomy, and evolution of chemotherapy are all likely contributors to more favorable outcomes.
N2 nodal downstaging has been associated with improved survival,17-20 yet it was not a significant prognostic factor in our series. This finding is in contrast with older reports from our institution and others.5,7,17,19,21 Betticher and colleagues reported that clearance of N2 lymph node involvement was a significant predictor of OS; 3-year OS was 11% with ypN2 disease versus 61% for those without it.17 In contrast, OS in our series was not different with regard to pathological staging (<ypIIIA) or ypN2 disease (Figure 1C-D). Despite the lack of difference in OS for ypN2 disease, we cannot conclude ypN2 disease is equivalent to no residual N2 disease; rather, we believe this finding reflects the lack of statistical power. In all likelihood, even though this series had a relatively large number of patients with stage IIIA disease, 100 patients is sufficiently small that differences that cannot be detected may exist (type II error). Some improvement in OS may be secondary to the use of adjuvant therapy, given that 72% of patients with N2 disease (44/61) received adjuvant therapy; 61% (37/61) received PORT. Owing to the small numbers and the heterogeneity of adjuvant therapies, we cannot determine the efficacy of adjuvant therapies with statistical confidence in this series.
Complete resection has been a significant predictor of survival.3,7,22 Thomas and colleagues, from the German Lung Cancer Cooperative Group, reported that 55% of the patients in the surgical control group underwent complete resection.22 Van Meerbeeck and colleagues, from the European Organization for the Research and Treatment of Cancer, reported that only approximately half of the patients underwent complete resection, which was a significant predictor of survival on multivariate analysis.23 We believe that complete removal of all N2 nodes contributed to the favorable outcomes in our series. However, R1-R2 resections were not associated with decreased survival in our series. Since 85% of patients (85/100) underwent an R0 resection, the numbers of R1-R2 resections are most certainly too small to detect a difference.
The limitations of our study include a selection bias on the basis of the inability to identify the number of patients with cIIIA/pN2 disease who were not offered resection. The biases that influenced the decision of definitive chemoradiotherapy versus induction chemotherapy followed by resection cannot be evaluated. However, a recent prospective, randomized trial suggests the addition of radiation to chemotherapy induction regimens followed by surgery for stage IIIA NSCLC offers no benefit to chemotherapy alone.24 Although we could speculate that patients with poor performance status or marginal lung function were excluded from surgery, we do not have the data to know with certainty. Second, whether some patients had progression or newly detected metastatic disease on postinduction PET scan and never underwent resection is unknown. This limitation prevents us from recommending not performing postinduction PET scans. Third, although we report all consecutive patients, to determine secondary endpoints of survival and recurrence, only 80% had postinduction PET scans. However, we believe that reporting consecutive patients is most reflective of the overall experience at our institution. Last, on the basis of the review of PET scan documentation from multiple institutions, we were unable to accurately discern the extent (single vs multistation) of N2 disease, and we were unable to evaluate changes in SUVmax accurately. To accurately compare PET scans, the pre- and postinduction scans would need to be performed on the same scanners, with clearly defined protocols. In addition, assessment of single versus multistation disease would need to be similarly investigated. We chose to include all scans regardless of the institution from which they were performed, because that most accurately reflects the referral practice of our group. We do not routinely repeat pre- or postinduction PET scans if those studies are deemed to be of high quality.
Conclusions
Our experience indicates that the persistence of postinduction PET avidity in N2 nodes is not associated with ypN2 disease or decreased OS. We offer surgical resection to all postinduction patients in whom an R0 resection is thought to be possible, regardless of postinduction PET imaging. We do not perform mediastinal surgical restaging procedures to assess for N2 nodal response to induction therapy. Finally, we believe a complete R0 N2 nodal dissection is mandatory in these patients.
Supplementary Material
Supplemental Figure 1. Cumulative incidence of recurrence (CIR). A, Entire cohort: the 5-year CIR was 70% (95% CI, 60%–81%). B, Postinduction PET N2 status: the 5-year CIR for N2-negative was 76% (95% CI, 58%–99%) vs 58% (95% CI, 41%–80%) for N2-avid disease (Gray's test, p=0.571). C, Pathologic N2 status: the 5-year CIR for N2-negative was 59% (95% CI, 44%–79%) vs 80% (95% CI, 66%–96%) for N2-persistent disease (Gray's test, p=0.077). D, Pathologic staging: the 5-year CIR for <ypIIIA was 57% (95% CI, 41%–78%) vs 80% (95% CI, 67%–96%) for ≥ypIIIA (Gray's test, p=0.049).
Supplemental Table 1: Adjuvant Therapy Based on Nodal Status
Supplemental Table 2: Results of Post-Induction N2-Avidity in Detecting Pathological Mediastinal (N2-Persistent) disease.
Supplemental Table 3: Pre-induction and Post-induction PET N2-status for those with both pre-and post-induction PET scans.*
Supplemental Table 4: Univariable logistic regression models to investigate association between factors and odds of pathologic N2-persistent disease.
Supplemental Table 5: Locations of Distant Recurrences
Central Picture Figure 1B.
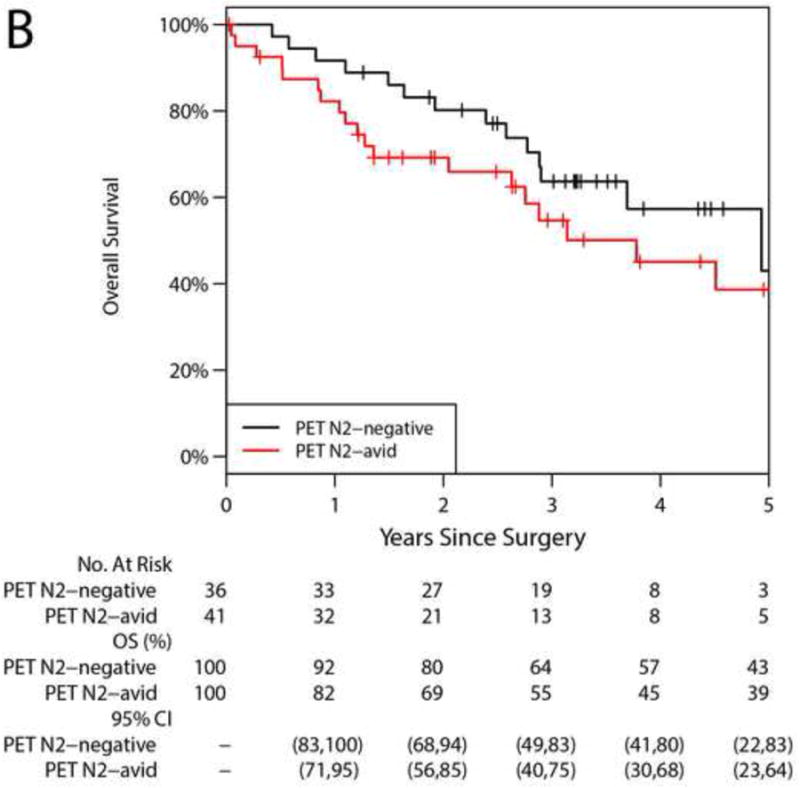
5-year overall survival was 43% for postinduction PET N2-negative disease versus 39% for N2-avid disease.
Perspective Statement.
For patients with stage IIIA non-small cell lung cancer with pN2 disease treated with induction chemotherapy, persistence of postinduction PET avidity in N2 nodes is not associated with ypN2 disease. The 5-year overall survival of 39% in this series highlights the steadily improving outcomes of multimodality therapy including surgery.
Acknowledgments
Financial support: NIH/NCI Cancer Center Support Grant P30 CA008748
Abbreviations
- ARDS
adult respiratory distress syndrome
- CI
confidence interval
- cIIIA/pN2
clinical stage IIIA–N2
- CIR
cumulative incidence of recurrence
- DFS
disease-free survival (DFS)
- EBUS
endobronchial ultrasound
- EGFR
endothelial growth factor receptor
- EUS
endoscopic ultrasound
- FEV1
forced expiratory volume in one second
- HR
hazard ratio
- KRAS
Kirsten rat sarcoma viral oncogene homolog
- NIDDM
non-insulin dependent diabetes
- OR
odds ratio
- OS
overall survival
- PET
positron emission tomography
- PORT
postoperative radiotherapy
- MSKCC
Memorial Sloan Kettering Cancer Center
- NSCLC
non-small cell lung cancer
- SUVmax
maximum standardized uptake values
- VATS
video-assisted thoracic surgery
- ypN2
pathologic N2-persistent
- ypN0-1
pathologic N2-negative
Footnotes
Conflicts of interest: All authors have no conflicts of interest.
Central Message: For stage IIIA/pN2 non-small cell lung cancer, postinduction PET avidity of N2 nodes is not associated with ypN2 disease or overall survival.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2007 Aug;2(8):706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 2.Roth JA, Atkinson EN, Fossella F, et al. Long-term follow-up of patients enrolled in a randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Lung cancer. 1998 Jul;21(1):1–6. doi: 10.1016/s0169-5002(98)00046-4. [DOI] [PubMed] [Google Scholar]
- 3.Roth JA, Fossella F, Komaki R, et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. Journal of the National Cancer Institute. 1994 May 4;86(9):673–680. doi: 10.1093/jnci/86.9.673. [DOI] [PubMed] [Google Scholar]
- 4.Rosell R, Gomez-Codina J, Camps C, et al. A randomized trial comparing preoperative chemotherapy plus surgery with surgery alone in patients with non-small-cell lung cancer. The New England journal of medicine. 1994 Jan 20;330(3):153–158. doi: 10.1056/NEJM199401203300301. [DOI] [PubMed] [Google Scholar]
- 5.Albain KS, Rusch VW, Crowley JJ, et al. Concurrent cisplatin/etoposide plus chest radiotherapy followed by surgery for stages IIIA (N2) and IIIB non-small-cell lung cancer: mature results of Southwest Oncology Group phase II study 8805. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1995 Aug;13(8):1880–1892. doi: 10.1200/JCO.1995.13.8.1880. [DOI] [PubMed] [Google Scholar]
- 6.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet. 2009 Aug 1;374(9687):379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martini N, Kris MG, Flehinger BJ, et al. Preoperative chemotherapy for stage IIIa (N2) lung cancer: the Sloan-Kettering experience with 136 patients. The Annals of thoracic surgery. 1993 Jun;55(6):1365–1373. doi: 10.1016/0003-4975(93)91072-u. discussion 1373-1364. [DOI] [PubMed] [Google Scholar]
- 8.Sugarbaker DJ, Herndon J, Kohman LJ, Krasna MJ, Green MR. Results of cancer and leukemia group B protocol 8935. A multiinstitutional phase II trimodality trial for stage IIIA (N2) non-small-cell lung cancer. Cancer and Leukemia Group B Thoracic Surgery Group. The Journal of thoracic and cardiovascular surgery. 1995 Mar;109(3):473–483. doi: 10.1016/s0022-5223(95)70278-4. discussion 483-475. [DOI] [PubMed] [Google Scholar]
- 9.Song WA, Zhou NK, Wang W, et al. Survival benefit of neoadjuvant chemotherapy in non-small cell lung cancer: an updated meta-analysis of 13 randomized control trials. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2010 Apr;5(4):510–516. doi: 10.1097/JTO.0b013e3181cd3345. [DOI] [PubMed] [Google Scholar]
- 10.de Cabanyes Candela S, Detterbeck FC. A systematic review of restaging after induction therapy for stage IIIa lung cancer: prediction of pathologic stage. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2010 Mar;5(3):389–398. doi: 10.1097/JTO.0b013e3181ce3e5e. [DOI] [PubMed] [Google Scholar]
- 11.De Leyn P, Stroobants S, De Wever W, et al. Prospective comparative study of integrated positron emission tomography-computed tomography scan compared with remediastinoscopy in the assessment of residual mediastinal lymph node disease after induction chemotherapy for mediastinoscopy-proven stage IIIA-N2 Non-small-cell lung cancer: a Leuven Lung Cancer Group Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006 Jul 20;24(21):3333–3339. doi: 10.1200/JCO.2006.05.6341. [DOI] [PubMed] [Google Scholar]
- 12.Hoekstra CJ, Stroobants SG, Smit EF, et al. Prognostic relevance of response evaluation using [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography in patients with locally advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005 Nov 20;23(33):8362–8370. doi: 10.1200/JCO.2005.01.1189. [DOI] [PubMed] [Google Scholar]
- 13.Rebollo-Aguirre AC, Ramos-Font C, Villegas Portero R, Cook GJ, Llamas Elvira JM, Romero Tabares A. Is FDG-PET suitable for evaluating neoadjuvant therapy in non-small cell lung cancer? Evidence with systematic review of the literature. Journal of surgical oncology. 2010 May 1;101(6):486–494. doi: 10.1002/jso.21525. [DOI] [PubMed] [Google Scholar]
- 14.Ripley RT, Rusch VW. Role of induction therapy: surgical resection of non-small cell lung cancer after induction therapy. Thoracic surgery clinics. 2013 Aug;23(3):273–285. doi: 10.1016/j.thorsurg.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 16.Port JL, Kent MS, Korst RJ, Keresztes R, Levin MA, Altorki NK. Positron emission tomography scanning poorly predicts response to preoperative chemotherapy in non-small cell lung cancer. The Annals of thoracic surgery. 2004 Jan;77(1):254–259. doi: 10.1016/s0003-4975(03)01457-7. discussion 259. [DOI] [PubMed] [Google Scholar]
- 17.Betticher DC, Hsu Schmitz SF, Totsch M, et al. Prognostic factors affecting long-term outcomes in patients with resected stage IIIA pN2 non-small-cell lung cancer: 5-year follow-up of a phase II study. British journal of cancer. 2006 Apr 24;94(8):1099–1106. doi: 10.1038/sj.bjc.6603075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaklitsch MT, Herndon JE, 2nd, DeCamp MM, Jr, et al. Nodal downstaging predicts survival following induction chemotherapy for stage IIIA (N2) non-small cell lung cancer in CALGB protocol #8935. Journal of surgical oncology. 2006 Dec 1;94(7):599–606. doi: 10.1002/jso.20644. [DOI] [PubMed] [Google Scholar]
- 19.Martin J, Ginsberg RJ, Venkatraman ES, et al. Long-term results of combined-modality therapy in resectable non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002 Apr 15;20(8):1989–1995. doi: 10.1200/JCO.2002.08.092. [DOI] [PubMed] [Google Scholar]
- 20.Paul S, Mirza F, Port JL, et al. Survival of patients with clinical stage IIIA non-small cell lung cancer after induction therapy: age, mediastinal downstaging, and extent of pulmonary resection as independent predictors. The Journal of thoracic and cardiovascular surgery. 2011 Jan;141(1):48–58. doi: 10.1016/j.jtcvs.2010.07.092. [DOI] [PubMed] [Google Scholar]
- 21.Van Meerbeeck JP, Van Schil PE, Senan S Group EO-LC. Reply: Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small cell lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2007 Dec;2(12):1138–1139. doi: 10.1097/JTO.0b013e31815ba815. [DOI] [PubMed] [Google Scholar]
- 22.Thomas M, Rube C, Hoffknecht P, et al. Effect of preoperative chemoradiation in addition to preoperative chemotherapy: a randomised trial in stage III non-small-cell lung cancer. The Lancet Oncology. 2008 Jul;9(7):636–648. doi: 10.1016/S1470-2045(08)70156-6. [DOI] [PubMed] [Google Scholar]
- 23.van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. Journal of the National Cancer Institute. 2007 Mar 21;99(6):442–450. doi: 10.1093/jnci/djk093. [DOI] [PubMed] [Google Scholar]
- 24.Pless M, Stupp R, Ris HB, et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: a phase 3 randomised trial. Lancet. 2015 Aug 11; doi: 10.1016/S0140-6736(15)60294-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Cumulative incidence of recurrence (CIR). A, Entire cohort: the 5-year CIR was 70% (95% CI, 60%–81%). B, Postinduction PET N2 status: the 5-year CIR for N2-negative was 76% (95% CI, 58%–99%) vs 58% (95% CI, 41%–80%) for N2-avid disease (Gray's test, p=0.571). C, Pathologic N2 status: the 5-year CIR for N2-negative was 59% (95% CI, 44%–79%) vs 80% (95% CI, 66%–96%) for N2-persistent disease (Gray's test, p=0.077). D, Pathologic staging: the 5-year CIR for <ypIIIA was 57% (95% CI, 41%–78%) vs 80% (95% CI, 67%–96%) for ≥ypIIIA (Gray's test, p=0.049).
Supplemental Table 1: Adjuvant Therapy Based on Nodal Status
Supplemental Table 2: Results of Post-Induction N2-Avidity in Detecting Pathological Mediastinal (N2-Persistent) disease.
Supplemental Table 3: Pre-induction and Post-induction PET N2-status for those with both pre-and post-induction PET scans.*
Supplemental Table 4: Univariable logistic regression models to investigate association between factors and odds of pathologic N2-persistent disease.
Supplemental Table 5: Locations of Distant Recurrences