Abstract
Background
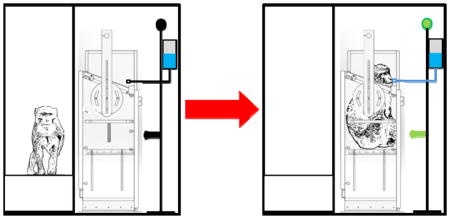
Neuroscience research on non-human primates usually requires the animals to sit in a chair. To do this, typically monkeys are fitted with collars and trained to enter the chairs using either a pole, leash and jump cage. Animals may initially show resistance and risk injury. We have developed an automated chair-training method that minimizes restraints to ease the animals into their chairs.
New method
We developed a method to automatically train animals to enter a primate chair and stick out their heads for neckplate placement. To do this, we fitted the chairs with Arduino microcontrollers coupled to a water-reward system and touch- and proximity sensors.
Results and comparison with existing methods
We found that the animals responded well to the chair, partially entering the chair within hours, sitting inside the chair within days and allowing us to manually introduce a door and neck plate, all within 14–21 sessions. Although each session could last many hours, automation meant that actual training person-hours could be as little as half an hour per day. The biggest advantage was that animals showed little resistance to entering the chair, compared to monkeys trained by leash pulling.
Conclusions
This automated chair-training method can take longer than the standard collar-and-leash approach, but multiple macaques can be trained in parallel with fewer person-hours. It is also a promising method for animal-use refinement and in our case, it was the only effective training approach for an animal suffering from a behavioral pathology.
Graphical Abstract
1. INTRODUCTION
Non-human primates are the best animal model for studying the neural mechanisms of high-level and cognitive behaviors because their brains are so similar to our own. However, this similarity brings its own complications: these social animals can be difficult to persuade into performing unnatural tasks. Monkeys show a wide range of temperaments and may develop pathologic and self-injurious behaviors in response to experimental requirements (Novak et al., 1998; Bellanca and Crockett, 2002). This is a problem because the success of research depends on earning the animals’ cooperation with the least amount of distress. One example of a necessary but counterintuitive task is chair training: requiring an animal to submit itself into an enclosed space, outside the safe familiarity of its home cage. Historically, chair training required using a pole or a leash to transfer the primate into the chair. This process is invariably met with resistance by naïve animals (Mattsson et al., 1976; Robbins et al., 1986). Within our community of non-human primate investigators, the most common way to adapt them is to use a squeeze-cage mechanism to confine the animal, hook the animal’s collar to a pole or leash while providing some reward, then using gentle but firm pressure to get the animal into the chair or jump-cage. Once in the chair, the animal is given abundant liquid and fruit rewards. After some time, the great majority of animals acclimatize to the chair and enter it willingly.
Over time, the chair-training method has been improved in two significant ways: 1) by refining the pole-and-collar method through the use of positive reinforcement training techniques (McMillan et al., 2014) and 2) through the use of desensitization, positive and negative reinforcement training to persuade the animals into a box-style chair, good for animals previously deemed unfit for traditional pole and collar techniques (Bliss-Moreau et al., 2013). Our project advances the latter approach, by using a box-chair design while substituting person-hours with an automated reward system. Our initial motivation was to train an animal with self-biting, stress-coping behaviors using a gentler approach, while still keeping an efficient workflow.
We trained two male macaques, to enter their chairs without poles or leashes, using reinforcement delivered by an automated Arduino-based system. Arduinos are cheap and flexible microcontroller boards, programmed using simple open-source software, which can be interfaced with different types of sensors and motors. This system obviated collars, and the number of automated training sessions required to get the monkey in the chair, with a neckplate on, was not much higher than the number of sessions required by all-human training. Our goals were to reduce the number of person-hours required during initial training and to provide an alternative to monkeys with behavioral problems. Based on the animals’ disposition to approach the chair and investigators during training, the automated system was less stressful to the animals, more precise in its reward timing, and unmatched in its “patience” with anxious animals.
2. MATERIALS AND METHODS
All procedures conformed to USDA and NIH guidelines and were approved by the Harvard Medical School Institutional Animal Care and Use Committee. This article conforms to the Animal Research: Reporting In Vivo Experiments (ARRIVE) Guidelines.
2.1. Subjects and training history
Both animals were adult male rhesus macaques (Macaca mulatta), monkey R (8 year-old, 13 kg) and monkey G (4 year-old, 5 kg). They were each pair-housed with other monkeys in standard primate caging at Harvard Medical School, under a 12-hour light/dark cycle with ad lib access to chow. Water and fruits were available during experimental sessions.
Monkey R arrived with a history of abnormal and self-injurious behaviors, including hair-pulling and arm-biting. Both animals were acclimatized for two weeks. Monkey R was collared with a rubber-lined chain and a five-inch extension with an O-loop at the end for the leash. Monkey R began under our standard training, which is as follows: first, we teach them clicker lessons (associating the sound of a click with reward), and then use the clicker to reinforce desired behaviors, such as moving to the front of the cage. At this point, we familiarize the animals to the leash, using the cage’s squeeze apparatus to bring them closer to the front and touching their collar loops with a small hook, all while providing reward. Animals are acclimatized to the squeeze by confining them to the front for several minutes. After they learn to sit calmly while being confined, we get them used to being touched with the small hook by putting it close to their collar loop. Usually, animals resist to being touched with the hook, batting it away or showing fear grimaces. However, they gradually show less resistance as this action is followed by fruit and liquid rewards. The great majority of monkeys “graduate” to leash training, where we attach the leash and let them move around their cage while providing gentle but firm resistance to guide them within the cage and into the chair. However, before graduating to leash training, monkey R reactivated his self-injurious behavior, biting himself at the sight of the hook, and we transferred him to the new training protocol. Monkey G was never collared or leash-trained, only clicker-trained.
2.2. Primate chairs
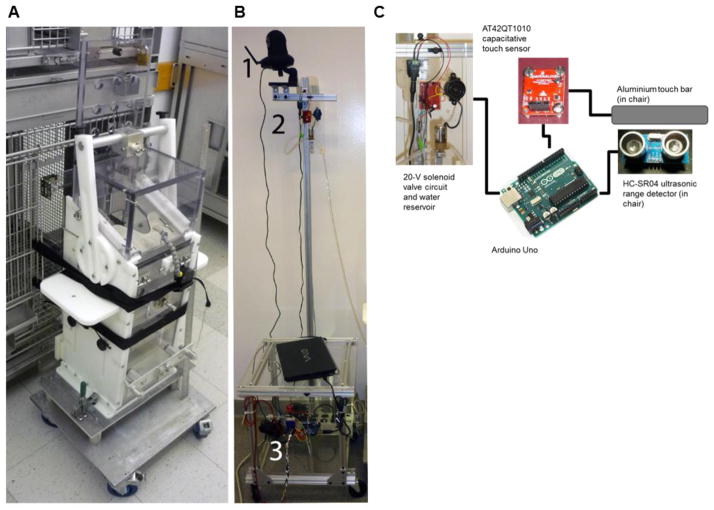
The primate chairs consisted of a polycarbonate primary box enclosure (13 × 13 × 36 inches, width x length x height) mounted on a wheeled aluminium platform (17 × 19 × 9 inches, w x l x h, Fig. 1A). This is the same type of chair used for all training approaches (leash, pole and automated). The top of the primary box had a fixed front neck plate and a removable back neck plate. The back of the chair had a guillotine-style door, and the floor of the chair was an aluminium perch that permitted the animal to achieve solid footing while letting waste pass onto a bottom tray. The top of the chair had a removable clear cover that could be snapped into place using metal pins, to prevent escape before the neck plate was inserted. The chairs were built on-site. The chairs were attached securely to the housing cage using 1-inch-wide nylon webbing straps with feed-through buckles (McMaster-Carr). To permit free movement of the chair door, we attached acrylic spacers to the back of the chair, forming a 5-inch-long “tunnel” between the cage door and chair entrance. We cut 4–5 holes in the chair to permit attachment of our touch sensor bar, the motion-sensor, and ultrasonic range sensors. These devices were framed by acrylic plates that could be screwed into the chair or attached with heavy-duty Velcro.
Figure 1.
Chair and Arduino workstation setup. A. The chair was attached to the home cage using two nylon-web straps. The guillotine door is shown in the secured open position. We attached a plastic frame between the cage and chair, to provide a passageway for the monkey while still permitting the back door to be closed. B. Arduino workstation. Made of T-slotted framing, the wheeled station comprised a wireless IP camera (1), a reward-circuit and water bottle holder (2), and a boxed platform to hold the Arduino and laptop. C. Major components of the electronic circuit.
2.3. Mechanical frame for electronics
The electronic reward system was mounted on a 16 × 17 × 60-inch (w x l x h) wheeled platform built using T-slotted aluminium extrusions (80/20 Inc., Fig. 1B). These frames were designed to hold a 1-L water bottle on top, to allow gravity dispensation of the liquid, and a small plate for a solenoid activation circuit triggered by the Arduino. The water bottle was connected via plastic tubing into the animal’s regular cage bottle or to the chair’s water tube (“lixit”).
2.4. Electronics
2.4.1. Arduino and sensors
We monitored the animals’ behavior using off-the-shelf microcontrollers (Arduino Uno, https://www.arduino.cc/), a capacitive touch sensor (AT42QT1010, www.sparkfun.com), pyroelectric (motion) (www.adafruit.com) and ultrasonic range detectors (HC-SR04, www.sunfounder.com). The touch sensor transmitted binary information about touch. The ultrasonic range sensors returned a voltage step with a duration proportional to the proximity of the animal. We used two ultrasonic range sensors to detect the position of the animals’ lower bodies. The reward circuit used a 24-V power source to activate a two-way solenoid valve (www.parker.com). The same power source was connected to a voltage divider (5-V output) to power the Arduino and a small speaker as a reinforcement signal. The sensors and Arduino were connected on 1.5 × 2-inch breadboards. Code and schematics are available at https://github.com/crponce/chairTraining.git.
2.4.2. Data acquisition
We used Matlab to record the reward delivery times as marked by the Arduino microcontroller and the proximity sensor readings. The Arduino was programmed using slightly modified example scripts (such as https://www.arduino.cc/en/Tutorial/Blink). The Matlab script checked for serial port communications via a USB connected to the Arduino. The program opened serial communication once, and then entered an infinite while-loop to scan the serial port every few milliseconds. We only collected this fine temporal precision data on monkey G.
2.5. Behavioral paradigms and monitoring
The animals were scheduled to receive all their water during training, and the minimum duration of the session was determined by how long the animal took to get its water, up to six hours per day. The training regimen included three stages: 1) training them to associate the touch bar with reward; 2) training them to reach into the chair to the touch bar, and 3) training them to move their entire bodies into the chair using proximity sensors. The animals were monitored using a wireless IP camera (Foscam FI8910W) and periodic animal room visits.
2.5.1. Touch-bar training
The animals were first trained to touch a short, metal bar connected to the capacitive touch sensor (2.4.1). The touch bar was fixed onto a non-conductive acrylic plate that could be attached to the bars of the cage. If the animals touched the bar, they were rewarded with a 400 ms-long pulse of water. After they learned to touch the bar, we fixed the touch bar inside the front plate of the chair. The chair was then attached to the home cage and water was released via the chair lixit. This required the animal to reach into the chair to touch the bar. Because the chair width was 13″ and the chair back was open, the animals were free to keep their lower bodies outside the chair. The training criterion for this phase was the animal getting at least 40 mL/kg body weight; the animals usually drank 50–60 mL/kg).
2.5.2. Touch-bar training, bar in chair, with proximity monitoring
To encourage the animal to sit entirely inside the chair, we attached proximity sensors to the chair lower front plate to monitor the position of the animal’s lower body. We programmed the Arduino to dispense larger rewards upon bar touch when the monkey was closer to the distance sensors. We defined three zones within the chair: zone 1 was between 0–11 cm from the front, zone two 12–24 cm, and zone three > 25 cm away from the sensors. The Arduino was programmed to deliver higher rewards in the closer zones: open-solenoid times were 200, 400 or 800 ms. We looked for changes in the animal’s tendency to enter the chair by gross behavioral observation and through analysis of distance data collected in MATLAB. Upon improvement, we reduced the reward magnitude scale to 50 ms, 150 ms and 500 ms. This training lasted until the animal sat entirely within the chair while being rewarded for bar touch, and did not return to the cage if the experimenter walked into the room. We entered the room periodically to monitor performance, desensitize them to our presence, and adjust the reward scale based on the animal’s motivation. This encouraged work and prevented extinction.
2.5.3. Back door and neckplate closing
Once the animals learned to put their legs and bottom inside the chair, we familiarized them to the chair door closing behind them. We started this training using two alternative strategies, gradual desensitization or flooding. In gradual desensitization, we lowered the door one inch at a time and offered clicks and rewards if the animal stayed in place (water, grape and apple pieces). The animal was always free to duck under the door and return to his home cage, and rewarding resumed upon return to the chair. In the flooding approach, the door was quickly lowered into place after the animal entered the chair, followed by abundant rewards. Our approach to neckplate insertion took place in the laboratory and consisted of two steps: shaping the environment to encourage the monkey to stick its head out of the chair body and rewarding whenever it permitted the neckplate to make contact with its fur. First, we encouraged the monkey to keep its neck out of the box by raising the chair’s bottom perch; we also moved the chair lixit such that the monkey could only drink if it put its head out. We then let the monkey sit with the Arduino touch bar reward system active, while in the lab, while we worked on other tasks. Second, we got him used to the neck plate by successive approximation: we touched the plate to the chair and rewarded with clicks and small bits of fruit if he did not crouch; then we lined it up for insertion and again rewarded if he kept his head up. Finally, we began sliding the neckplate in small intervals with rewards at each step. Once the monkey let the neckplate make contact with his fur, we could try one of two approaches: desensitization and flooding. Desensitization meant that we kept going with the small step approach until the neckplate could prevent crouching. Flooding meant that we slid the neckplate quickly enough to prevent the animal from crouching. Because monkey R had self-injurious behaviors, we only used sensitization in this case. We experimented with flooding with monkey G to determine whether this would accelerate the process (it did not, see Results).
Finally, we calculated a rough approximation of the number of person-hours spent actively working with the monkeys every session above, based on our daily notes, but these should be seen as approximate.
3. RESULTS
Monkey R and monkey G learned to sit inside their chairs, and tolerate closing the door and inserting the neck plate, within 21 and 14 days. The animals maintained their weights during training.
3.1. Touch bar training, bar in cage and chair
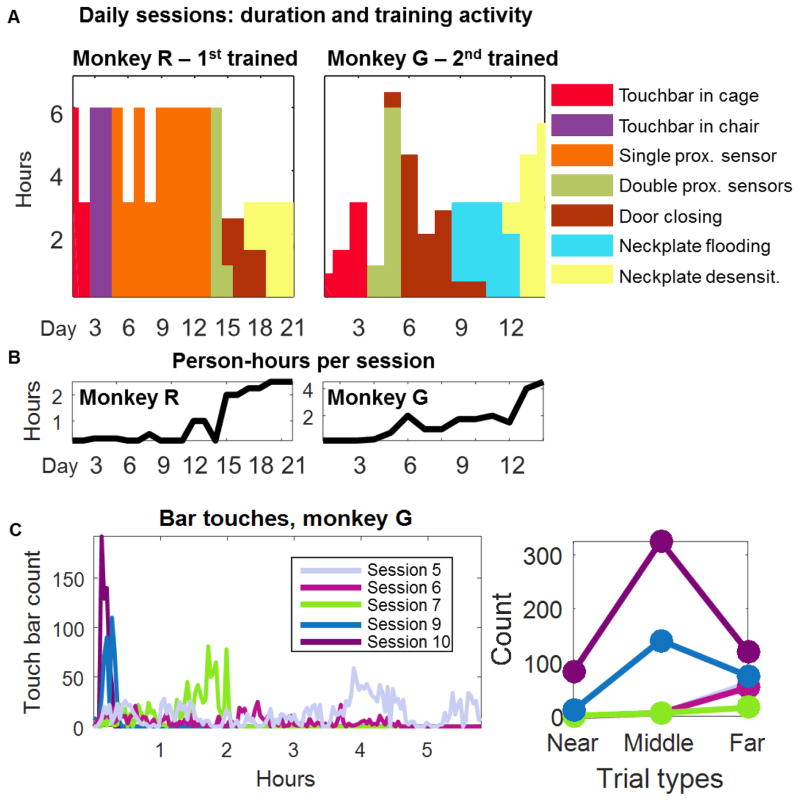
Both animals learned to touch the bar in their cage within three hours. The animals were allowed to continue in-cage touch training for up to three sessions (monkey R, 9 hours in two sessions; monkey G, 5.25 h in 3 sessions). We then attached the chair to the cage, with the door open, and moved the touch bar to the front of the chair. Monkey R was our first subject, and we let him reach into the chair without a proximity sensor; he did so for 12 h (2 sessions) without much inclination to get into the chair. Next we attached a single proximity sensor to give increasing reward sizes as the animal sat closer to the front of the chair when touching the bar. We observed him for 10 sessions (54 h total) but the animal again showed no progress moving fully into the chair (the animal learned to “fool” the sensor using his free hand). Finally we attached two proximity sensors, and then the animal was persuaded to sit calmly inside the chair to drink water. This occurred within 7 hours (two sessions). Consequently, we introduced the two-sensor system immediately with monkey G, and this animal learned to bar touch while sitting entirely inside the chair in two sessions (7 h total). The time to entry and amount of time spent in the chair improved throughout subsequent sessions (Fig. 2A,C). Monkey R developed a habit of leaving at least one leg out of the chair. To complete his training, we used the cage squeeze mechanism to nudge his leg into the chair; shortly thereafter he learned to move into the chair completely.
Figure 2.
A. Training activities per daily session. Each histogram shows the duration of the session (height) and the type of training procedures used that day (colors). B. Estimated person-time hours per session. C. Occurences of bar touches by monkey G over five sessions, after proximity sensors were introduced into the chair. C. Counts of bar touches ocurring when the animal’s legs were less than 11 cm (“near”) from the proximity sensors inside the chair, 12–24 cm (“middle”) away, and over 25 cm away (“far”). Each color represents the same session as in B. Each point shows the count for each specific trial type, within the first 15 minutes of chair access.
3.2. Back door and neckplate placement
Once the animals sat in the chair for minutes at a time, we trained them to allow us to close the back door and the neckplate. With monkey R, we used gradual desensitization, lowering the door slowly and rewarding after each movement if the animal stayed still. This took a collective time of 7 hours of training, 5.5 during which the trainer was actively involved (person-hours), before the door could be closed (divided into four sessions, Fig. 2B). By the end of training, monkey R was comfortable in the chair with the trainer present, showed little resistance to the closing of the door. This gradual desensitization approach was important for this monkey because of his behavioral pathology history and relatively large size; moving out of the chair was possible but energetically costly. This was in contrast with monkey G, a younger, smaller, less patient animal. Because this animal did not have any behavioral pathologies, we tried using flooding to see if we could finish neckplate training faster and with fewer person hours, lowering the door briskly and confining the animal to the chair for short periods. Once inside, we offered abundant rewards. This approach was less optimal: it took 5 person-hours and 10.75 total hours to achieve door closing (divided into six sessions). Although it did require a smaller ratio of person-hours to total hours, the person-hours were only marginally decreased while the total hours were substantially increased. Additionally, monkey G became reluctant to approach the chair and continued proximity sensor training concurrently. Eventually, the monkey became desensitized, showing little reluctance to door closing.
We performed training for neckplate insertion in the laboratory instead of the animal housing room. Within each session, the animals were permitted to take breaks playing with the Arduino touch bar circuit while we worked at the computer. For neckplate insertion, we also compared gradual desensitization vs. flooding. Desensitization involved moving the neckplate in 1-cm steps and while reinforcing; flooding involved briskly moving the neckplate into position followed by reinforcement. Using desensitization (monkey R), we needed 10 person-hours out of 12 total hours (five sessions). With the flooding approach, monkey G required 6 person-hours out of 10 total hours (four sessions) before we could reliably fix the neckplate, but we were still “surprising” the animal, not earning his cooperation. Thus we switched to a more gradual desensitization for an additional three sessions (9 person-hours out of 11 total hours). Thus we emphasize that flooding was a weak training approach in both door-closing and neckplate insertion.
4. DISCUSSION
We conduct a visual neuroscience research program using rhesus macaques, and occasionally we get animals that show significant behavioral pathologies. These animals present us with a dilemma: is it better to exclude them from the research program, or is it possible to train them using alternative methods? In this project, we responded to a behaviorally abnormal animal by introducing a patient and cheap training tutor: Arduino microcontrollers. Arduinos cost $30 and can be combined with $10 sensors to shape the animals’ behavior while the experimenter works elsewhere. This saves time and reduces much of the stress associated with chair training: our animals were actively engaged by the touch sensor tasks. Considering the chair training as a whole, the animals responded better to the automatic trainer than to us. At the end, was this approach faster than sticking to our traditional pole/leash method? Under our standard regime, when training a new monkey, we usually allow 7–10 days to “chair train” before moving onto other behavioral tasks. By that description, this process is longer. However, the truth is that even after two weeks of leash-based chairing, most monkeys are hardly willing to enter the chair: the process of acclimatizing the animal to enter the chair on its own could last weeks or months and we simply let that training happen in parallel to other experimental goals. In this view, the use of automated training does not prolong the process as much as it preserves the focus on achieving a good foundational behavior early on. Again, while we loved automation and believe the monkeys preferred it too, this is going to be most helpful with self-injurious monkeys and when training more than one animal in parallel. We would like to expand this automation to other stages, such as closing the chair door and neckplate insertion, but this will require a motorized door and pressure or trip-wire sensors to avoid injury. More recent iterations of our system have replaced the Arduino/laptop setup with miniaturized computers like the Raspberry Pi, communicating data via Wi-Fi to a Matlab session outside the monkey housing area. One final motivation for implementing automated systems is that it addresses the refinement goals of animal care and use programs.
Supplementary Material
Highlights.
Working with macaques requires that they be trained to enter a primate chair.
We developed a “smart chair” that responds to the animal and trains it to enter without humans in the room.
The animals learned to enter the chairs within a few sessions, and this method was best for an animal with a behavioral pathology.
Acknowledgments
This work was supported by US National Institutes of Health grants EY 16187 (MSL), the Burroughs Wellcome Fund (CRP), the Harvard College Research Program (MPG) and the Harvard Medical School Office for Diversity Inclusion and Community Outreach (GPM). We would like to thank Tim LaFratta, John LeBlanc, Ofer Mazor and Pavel Gorelik of the Research Core facilities.
Footnotes
AUTHOR CONTRIBUTIONS
CRP designed the prototype Arduino setup and developed it with MPG and MSL. MPG, GPM and CRP conducted the animal training. MPG, CRP and MSL wrote and edited the manuscript. CRP and MPG are co-first authors.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bellanca RU, Crockett CM. Factors predicting increased incidence of abnormal behavior in male pigtailed macaques. [Accessed November 18, 2015];Am J Primatol. 2002 58:57–69. doi: 10.1002/ajp.10052. Available at: http://www.ncbi.nlm.nih.gov/pubmed/12386914. [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E, Theil JH, Moadab G. Efficient cooperative restraint training with rhesus macaques. [Accessed November 5, 2015];J Appl Anim Welf Sci. 2013 16:98–117. doi: 10.1080/10888705.2013.768897. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3692558&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson JL, Alligood JP, Robertson LF. A chest harness and pole-leash for routine transfer of rhesus monkeys from home cage to behavioral test apparatus and back. [Accessed January 11, 2016];Lab Anim Sci. 1976 26:626–629. Available at: http://www.ncbi.nlm.nih.gov/pubmed/823373. [PubMed] [Google Scholar]
- McMillan JL, Perlman JE, Galvan A, Wichmann T, Bloomsmith MA. Refining the pole-and-collar method of restraint: emphasizing the use of positive training techniques with rhesus macaques (Macaca mulatta) [Accessed November 5, 2015];J Am Assoc Lab Anim Sci. 2014 53:61–68. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3894649&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- Novak MA, Kinsey JH, Jorgensen MJ, Hazen TJ. Effects of puzzle feeders on pathological behavior in individually housed rhesus monkeys. [Accessed November 18, 2015];Am J Primatol. 1998 46:213–227. doi: 10.1002/(SICI)1098-2345(1998)46:3<213::AID-AJP3>3.0.CO;2-L. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9802512. [DOI] [PubMed] [Google Scholar]
- Robbins DO, Zwick H, Leedy M, Stearns G. Acute restraint device for rhesus monkeys. [Accessed January 11, 2016];Lab Anim Sci. 1986 36:68–70. Available at: http://www.ncbi.nlm.nih.gov/pubmed/3959538. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.