Abstract
Background
Transplantation of allogeneic mesenchymal stromal cells (MSCs) is a promising treatment for heart failure. We have shown that epicardial placement of cell sheets markedly increases donor cell survival and augments therapeutic effects compared with the current methods. Although immune rejection of intramyocardially injected allogeneic MSCs have been suggested, allogeneic MSCs transplanted on the heart surface (virtual space) may undergo different courses. This study aimed to elucidate immunologic response against epicardially placed allogeneic MSCs, rejection or acceptance of these cells, and their therapeutic effects for heart failure.
Methods and Results
At 4 weeks after coronary artery ligation, Lewis rats underwent epicardial placement of MSC sheets from syngeneic Lewis or allogeneic Fischer 344 rats or sham treatment. At days 3 and 10 after treatment, similar ratios (≈50% and 30%, respectively) of grafted MSCs survived on the heart surface in both MSC sheet groups. By day 28, survival of syngeneic MSCs was substantially reduced (8.9%); survival of allogeneic MSCs was more extensively reduced (0.2%), suggesting allorejection. Correspondingly, allogeneic MSCs were found to have evoked an immunologic response, albeit low level, as characterized by accumulation of CD4+ T cells and upregulation of interleukin 6. Despite this alloimmune response, the allogeneic MSC sheet achieved myocardial upregulation of reparative factors, enhanced repair of the failing myocardium, and improved cardiac function to the equivalent degree observed for the syngeneic MSC sheet.
Conclusions
Allogeneic MSCs placed on the heart surface evoked an immunologic response; however, this allowed sufficient early phase donor cell survival to induce equivalent therapeutic benefits to syngeneic MSCs. Further development of this approach toward clinical application is warranted.
Keywords: allotransplantation, cell transplantation, heart failure, immunologic response, mesenchymal stem cell
Subject Categories: Cell Therapy, Translational Studies, Heart Failure
Introduction
Recent preclinical and clinical research has suggested that transplantation of bone marrow–derived mesenchymal stromal cells (MSCs) is a promising new approach for the treatment of heart failure.1 Although cardiomyogenic differentiation of these cells in vivo is limited, MSCs are able to induce therapeutic benefits by secreting various biological factors that promote repair of the failing myocardium (ie, paracrine effects). In addition, the potential utility of allogeneic MSCs remarkably increases the value of these cells as an important donor cell type for cell therapy.1
Although immunologically most appropriate, the clinical use of autologous MSCs requires bone marrow aspiration from the patients being treated, prolonged in vitro culture for MSC expansion, and quality control and assurance of MSC products for each treatment, imposing significant logistical, economic, and time constraints. Moreover, MSCs from older patients with comorbidities might undermine therapeutic competencies and reduce the ability to expand in vitro.2 In response to these issues, the use of allogeneic MSCs has been proposed on account of their immune‐modulating ability and their relatively low immunogenic phenotype.1, 3 Previous reports have shown that injection of allogeneic MSCs, without immunosuppressive treatment, improved cardiac function to the same extent as autologous (syngeneic) MSCs in experimental myocardial infarction (MI) models.4, 5, 6 Allogeneic MSCs have already been injected in patients with heart failure, and the safety and feasibility (and preliminary effect) of this treatment has been established.7, 8
Nevertheless, it has also been reported that the immune‐suppressive ability of MSCs observed in in vitro settings is not effective enough for transplanted allogeneic MSCs to establish immune privilege in various disease conditions in vivo.4, 5, 9, 10 Westrich et al observed that ≈1% of total injected allogeneic MSCs survived at day 2 after intramyocardial injection (a similar ratio to syngeneic MSCs, calculated from their data) in a rat subacute MI model, whereas most allogeneic, but not syngeneic, MSCs subsequently disappeared by day 7.4 Huang et al reported that ≈5% of intramyocardially injected allogeneic or syngeneic MSCs similarly existed in the myocardium at day 7 in a rat model of post‐MI heart failure, but only syngeneic MSCs were detectable at day 35.5 Although these data are based on comparisons of quite narrow ranges of donor cell survival (<5% at the initial points studied), they suggest that allogeneic MSCs can survive for a certain early period after transplantation but are eventually rejected.
We recently reported the therapeutic potential of epicardial placement (ie, not injection) of scaffold‐free “MSC sheets,” which were produced using temperature‐responsive polymer‐coated culture dishes in a rat ischemic cardiomyopathy model.11 This cell‐delivery method, compared with intramyocardial injection, achieved markedly increased initial retention and survival of MSCs. Of note, most epicardially placed MSCs remained on the heart surface with quite occasional migration into the myocardium. We considered that donor cells settling on the heart surface (a virtual space) could receive less blood perfusion (and thus less immune cell attack) than MSCs injected into the myocardium by intramyocardial injection. It is also possible that the epicardium, which is an epithelial cell monolayer on the heart, may work as a physical barrier between MSC sheets and the host myocardium; therefore, epicardially placed allogeneic MSCs might have reduced or delayed susceptibility to the host immune reaction. This study thus examined rejection and acceptance, immunologic responses, and therapeutic effects of allogeneic MSC sheets placed on the epicardial heart surface in a rat heart failure model.
Methods
All studies were performed with the approval of the institutional ethics committee and the U.K. Home Office. The investigation conforms to the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals (US National Institutes of Health Publication, 1996). All in vivo and in vitro assessments were carried out in a blinded manner if possible.
Preparation of Rat Bone Marrow–Derived MSCs and Bone Marrow Mononuclear Cells
MSCs were isolated from bone marrow of the tibias and femurs of male Lewis or Fischer 344 rats (100–150 g; Charles River UK, Kent, UK) and expanded, as described previously.11, 12 MSCs were used at passage 3 or 4. Bone marrow mononuclear cells were also collected from the tibias and femurs of male rats, as described previously.13
Flow Cytometry and Differentiation Assay of MSCs
For cell‐surface marker characterization using flow cytometry, 1×106 MSCs were stained with 1:100 dilution of FITC‐conjugated anti‐CD34 (Santa Cruz Biotechnology), CD45 (Chemicon), CD90 (Abcam), major histocompatibility complex (MHC) class II (BD Pharmingen), or Alexa 647–conjugated anti‐CD29 (BioLegend) or phycoerythrin‐conjugated anti–MHC class I (BD Pharmingen) antibodies. Corresponding isotype‐matched control antibodies were used for negative controls. Samples were analyzed using the Dako Cyan flow cytometer (Dako Cytomation).
For in vitro differentiation assays, MSCs were plated on 24‐well plates and subjected to adipogenic or osteogenic differentiation medium, as described previously.11, 12, 14 Adipogenic differentiation medium was α‐minimal essential medium supplemented with 100 μmol/L isobutyl methylxanthine (Sigma‐Aldrich), 60 μmol/L indomethacin (Fluka), 1 μg/mL insulin (Sigma‐Aldrich), and 0.5 μmol/L hydrocortisone (Sigma‐Aldrich), whereas osteogenic differentiation medium was α‐minimal essential medium supplemented with 0.1 μmol/L dexamethasone (Sigma‐Aldrich), 10 mmol/L β‐glycerophosphate (Sigma‐Aldrich), and 0.05 mmol/L ascorbic acid (Sigma‐Aldrich). Medium was changed every 2 to 3 days. After 3‐week incubation, cells were fixed with 4% paraformaldehyde and stained with Oil Red O (Fluka) for detecting adipocytes containing lipid vacuoles or with Alizarin red (Fluka) to detect osteocytes containing calcium deposits.11, 12, 14
Generation of MSC Sheets
To generate an MSC sheet, 4×106 bone marrow–derived MSCs from male Lewis (RT1l) or male Fischer 344 (RT1lvl) rats (150 g; Charles River UK) at passage 3 or 4 were plated onto a 35‐mm temperature‐responsive culture dish (UpCell; CellSeed).11 After 12‐hour incubation at 37°C, the culture dish was placed at 22°C, and the MSC sheet detached as a free membrane from the dish. At the end of 12‐hour culture, the cell numbers included in a cell sheet were unchanged before the culture in both types of MSCs (4.1±0.3×106 for Lewis and 4.0±0.4×106 for Fischer 344). For histological tracking, MSCs were labeled with CM‐DiI (Life Technologies), as described previously.11 There was no difference in readiness of cell‐sheet production between Lewis and Fischer 344 rats.
Animal Surgery
MI was induced in female Lewis rats (150–200 g; Charles River UK) by ligating the left coronary artery under isoflurane anesthesia and mechanical ventilation, as described previously.11, 13 At 4 weeks after MI, rats were subjected to echocardiography, and those showing appropriate cardiac dysfunction (left ventricular [LV] ejection fraction 25–40%) were selected for further studies. The selected animals were randomly grouped to receive epicardial placement of MSC sheets composed of 4×106 MSCs from syngeneic (Lewis; auto group) or allogeneic (Fischer 344; allo group) male rats. The MSC sheet was placed onto the epicardial surface of the infarcted heart to cover the infarct and border areas.11 For the sham group, sham treatment (open chest only) was performed at 4 weeks after MI induction.
Cardiac Function Measurement
Transthoracic echocardiographic determinations were assessed before treatment (4 weeks after MI) and at 28 days after treatment by the Vevo 770 echocardiography machine (VisualSonics) under 1.5% isoflurane inhalation via a nose cone.11, 13, 14 LV ejection fraction was calculated from the data obtained with 2‐dimensional tracing. LV end‐diastolic and end‐systolic dimensions, LV wall thickness, and heart rate were measured with M mode. Transmitral peak E/A flow ratio was determined by spectral Doppler traces. All data were collected from at least 3 to 5 different measurements in a blinded manner.
Hemodynamic parameters were measured by using cardiac catheterization (SPR‐320 and PVAN3.2; Millar Instruments), as described previously.11, 13, 14 Briefly, under general anesthesia using 1.5% isoflurane inhalation and mechanical ventilation, the catheter was inserted into the LV cavity through the right common carotid artery. Intra‐LV and intra‐aortic pressure signals were measured with a transducer and conditioner (MPVS‐300; Millar Instruments) and digitally recorded with a data acquisition system (PowerLab 8/30; ADInstruments). All data were collected from at least 5 different measurements in a blinded manner.
Quantitative Assessment of Donor Cell Survival
To quantify the presence of grafted male cells in the female heart, the Y chromosome–specific SRY gene was quantitatively assessed by TaqMan real‐time polymerase chain reaction (Prism 7900HT; Applied Biosystems).11, 13, 14 At 3 and 28 days after treatment, the ventricular walls were collected, genomic DNA were extracted using the DNeasy Blood and Tissue Kit (Qiagen), and SRY analysis was performed in technical duplicate. The signal in each sample was normalized to the amount of DNA by measuring the autosomal single‐copy gene SPP1 as an internal standard.11, 13, 14 Ventricular walls from female rats at 56 days after left coronary artery ligation were mixed with 1×107, 1×106, 1×105 or 1×104 of male MSCs and processed for SRY analysis to generate a standard curve (n=3).
Analysis of Gene Expression
Total RNA was extracted from collected cells or from the ventricular walls of rats using the RNeasy Mini Kit (Qiagen) and assessed for myocardial gene expression relevant to immunologic responses and MSC‐mediated myocardial repair/regeneration by quantitative reverse transcription polymerase chain reaction (Prism 7900HT, Applied Biosystems) in technical duplicate, as described previously.11, 13 TaqMan primers and probes for rat TIMP1, IGF1, VCAM1, SDF1, HIF1A, MMP2, IL10, FGF2, and VEGF were purchased from Applied Biosystems, whereas those for MHC class I, MHC class II, and TMSB4 were from Sigma‐Aldrich. Expression was normalized to ubiquitin C. In the figures, expression relative to that of the sham group is presented.
Enzyme‐Linked Immunosorbent Assay for Serum Interleukin 6 Levels
Peripheral blood was collected from rats at day 3 after treatment, and serum was obtained by centrifugation. Serum level of interleukin (IL) 6 was measured by using the Rat IL‐6 Quantikine ELISA Kit (R&D Systems) in technical triplicate, according to the manufacturer's instructions.
Histological Analysis
The hearts were harvested, fixed with 4% paraformaldehyde, and frozen in OCT compound using liquid nitrogen. Cryosections were cut and incubated with polyclonal anti–cardiac troponin‐T antibody (1:200 dilution; HyTest), biotin‐conjugated Griffonia simplicifolia lectin I‐isolectin B4 (1:100; Vector Laboratories), monoclonal anti‐PECAM1 antibody (1:50; AbD Serotec), monoclonal anti‐CD4 and anti‐CD8 antibodies (1:100; BD Pharmingen), or monoclonal anti‐CD68 antibody (1:200; AbD Serotec), followed by visualization using fluorophore‐conjugated secondary antibodies (Life Technologies). Samples were analyzed by fluorescence microscopy (BZ8000; Keyence) with or without nuclear counterstaining using DAPI (4′,6‐diamidino‐2‐phenylindole). For semiquantitative assessments, 10 different fields of the border areas (surrounding the infarct) per heart were randomly selected and assessed. For counting numbers of CD4+, CD8+, or CD68+ cells, only positive cells having clear DAPI‐positive nuclei localized in the MSC sheets were counted.
Another set of sections were stained with 0.1% picrosirius red (Sigma‐Aldrich) to semiquantify extracellular collagen deposition using ImageJ analysis software (National Institutes of Health).11, 13 In addition, for detecting adipogenic and osteogenic differentiation, staining with Oil Red O (Sigma‐Aldrich) and Alizarin red (Sigma‐Aldrich) was performed, as described previously.12, 14
Statistical Methods
Statistical comparison of 2 groups (Figure 4) was performed using the Wilcoxon rank sum test. Comparisons of multiple groups (Figures 7 through 10A and 10B) were performed with the Kruskal–Wallis test followed by the Steel‐Dwass test. These data are presented as box plots showing the median, quartile 1, quartile 3, and maximum/minimum values. Data in Figures 2A and 5 and Table 1 were calculated with the 2‐way repeated measures ANOVA followed by the Bonferroni post hoc test (values are expressed as mean±SEM). A value of P<0.05 was considered statistically significant.
Figure 4.
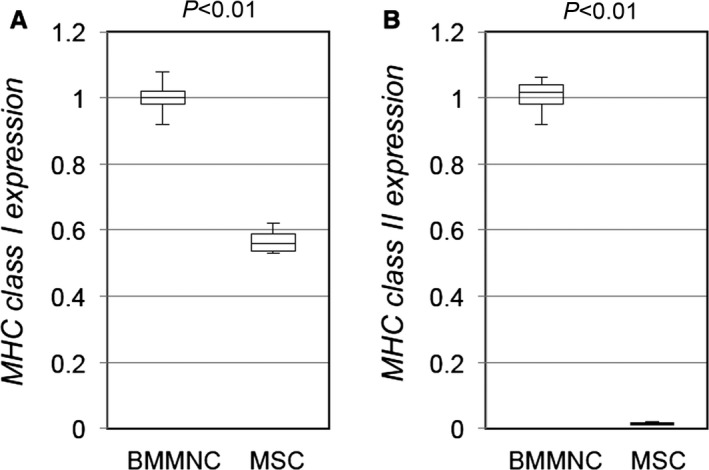
MHC molecule expression of cultured rat MSCs. Quantitative reverse transcription polymerase chain reaction demonstrated that expression of MHC class I (A) in cultured rat MSCs was lower than that in rat BMMNCs and that expression of MHC class II (B) was negligible in rat MSCs. Relative expression to that of BMMNCs is presented. n>3 in each group. BMMNC indicates bone marrow mononuclear cell; MHC, major histocompatibility complex; MSC, mesenchymal stromal cell.
Table 1.
Changes in Cardiac Function Measured by Echocardiography
| LVAWTd (mm) | LVPWTd (mm) | FS (%) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Sham | 0.7±0.1 | 0.7±0.1 | 1.5±0.1 | 1.6±0.1 | 18.2±0.7 | 16.6±0.8a |
| Auto | 0.9±0.1 | 0.9±0.1 | 1.7±0.1 | 1.8±0.1 | 18.2±0.5 | 21.9±0.8a , b |
| Allo | 0.8±0.0 | 0.8±0.1 | 1.7±0.1 | 1.8±0.1 | 18.2±0.3 | 22.1±0.4a , b |
| LVEF (%) | LVDd (mm) | LVDs (mm) | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Sham | 36.1±1.3 | 33.3±1.5 | 8.3±0.1 | 8.8±0.2a | 6.8±0.2 | 7.3±0.2 |
| Auto | 36.3±0.9 | 42.5±1.3a , b | 8.4±0.2 | 8.4±0.3 | 6.9±0.1 | 6.6±0.2 |
| Allo | 36.3±0.6 | 42.9±0.6a , b | 8.4±0.2 | 8.6±0.2 | 6.9±0.2 | 6.7±0.2 |
| MV E/A | HR (bpm) | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Sham | 1.2±0.1 | 1.3±0.1 | 390.5±8.7 | 394.7±5.7 |
| Auto | 1.3±0.1 | 1.2±0.1 | 387.7±6.8 | 399.0±5.8 |
| Allo | 1.4±0.1 | 1.3±0.1 | 395.4±9.8 | 399.8±4.4 |
n=8 to 11 per group. bpm indicates beats per minute; FS, fractional shortening; HR, heart rate; LVAWTd, left ventricular anterior wall thickness at end‐diastole; LVDd, left ventricular end‐diastolic dimension; LVDs, left ventricular end‐systolic dimension; LVEF, left ventricular ejection fraction; LVPWTd, left ventricular posterior wall thickness at end‐diastole; MV E/A, transmitral peak E/A flow ratio; post, day 28 after treatment; pre, before treatment (day 28 after myocardial infarction).
P<0.05 vs pre data in the same group.
P<0.05 vs sham at the same time point.
Results
We previously published the data showing the efficacy of syngeneic MSC sheet therapy by using a rat model similar to that used in this study11; however, no previously published result is re‐reported in this paper.
MSCs From Lewis and Fischer 344 Rats Showed Similar Phenotypes
We first characterized similarities (and differences) of 2 types of donor cells: bone marrow–derived MSCs from Lewis rats (RT1l; for syngeneic donor) and Fisher 344 rats (RT1lvl; for allogeneic donor). Doubling time between passages 3 and 4 (at which stage MSCs were used for in vivo studies) was similar between these MSCs (35.2±4.8 hours for Lewis‐derived MSCs and 36.9±6.1 for Fischer 344–derived MSCs, n=4 per group). Viability measured by trypan blue was also the same (97.4±1.8% for Lewis‐derived MSCs and 96.8.9±2.1% for Fischer 344‐derived MSCs, n=4 per group). Flow cytometric study and differentiation assay demonstrated that CD marker expression (Figure 1A), adipogenic and osteogenic differentiation (Figure 1B and 1C), and MHC class I and II expression (Figure 1D) were all compatible for the 2 types of MSCs. In addition, quantitative polymerase chain reaction showed that expression of reparative factors (VEGF, IGF1, SDF1, and HIF1A), which are proposed to play a role in MSC‐mediated myocardial repair,1, 11 was not statistically different between Lewis‐ and Fischer 344–derived MSCs (n=4 per group, Wilcoxon rank sum test) (data not shown).
Figure 1.
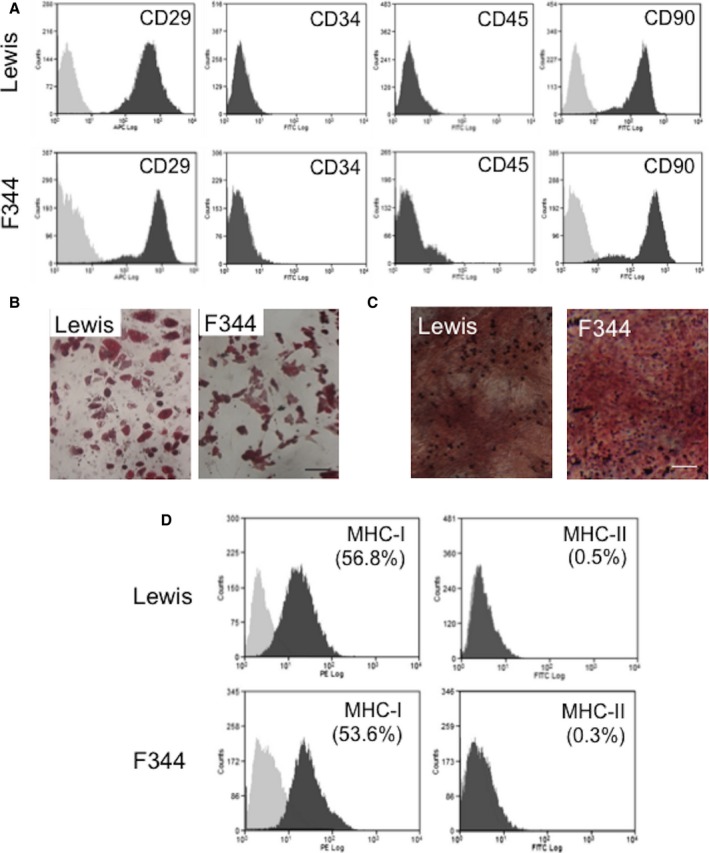
In vitro characterization of bone marrow–derived MSCs from Lewis and Fischer 344 rats. A, Flow‐cytometric analyses showed that cultured MSCs from Lewis and Fischer 344 rats (passage 3 or 4) were positive for CD29 and CD90 and negative for CD34 and CD45. Light gray represents isotype‐matched control data. B and C, After 3‐week chemical stimulation in vitro, MSCs were driven to Oil Red O–positive adipocytes (B) and Alizarin red–positive osteocytes (C), demonstrating similar mesenchymal multipotency between MSCs from Lewis and Fischer 344 rats. Scale bars=50 μm. D, Flow cytometry showed that approximately one‐half of MSCs were positive for MHC class I similarly in Lewis‐ and Fischer 344–derived MSCs. MHC class II was not expressed in MSCs from either strain. F344 indicates Fischer 344 rats; MHC, major histocompatibility complex; MSC, mesenchymal stromal cell; PE, phycoerythrin.
Allogeneic MSC Sheets Achieved Donor Cell Survival Comparable to Syngeneic MSC Sheets in the Early, But Not Late, Phases After Epicardial Placement
We evaluated immunologic rejection (or acceptance) of allogeneic MSCs placed on the epicardial heart surface in a quantitative manner in a rat model of post‐MI heart failure. At 4 weeks after left coronary artery ligation, female Lewis rats (host) underwent epicardial placement of an MSC sheet composed of the same numbers of male allogeneic (Fischer 344) or male syngeneic (Lewis) MSCs. Quantitative polymerase chain reaction for Y chromosome gene sry demonstrated that donor cell survival was equivalent in the auto and allo groups at day 3 (56.0±7.2% versus 51.1±7.6% of the total grafted cell number, respectively) (Figure 2A). This comparable donor cell survival between syngeneic and allogeneic MSCs was continuously observed at day 10, whereas day 10 donor cell survival was reduced from day 3 survival in both groups (33.1±5.1% and 27.2±4.8% in the auto and allo groups). At day 28, donor cell survival of syngeneic MSCs (auto group) was substantially reduced to 8.9±1.7%. This cell loss was considered to be independent of allorejection. Of note, at day 28, survival of allogeneic MSCs (allo group) was more extensively decreased; only 0.2±0.0% of allogeneic MSCs survived at this time point. This amplified donor cell loss was considered to be caused by the immunologic rejection of allogeneic MSCs.
Figure 2.
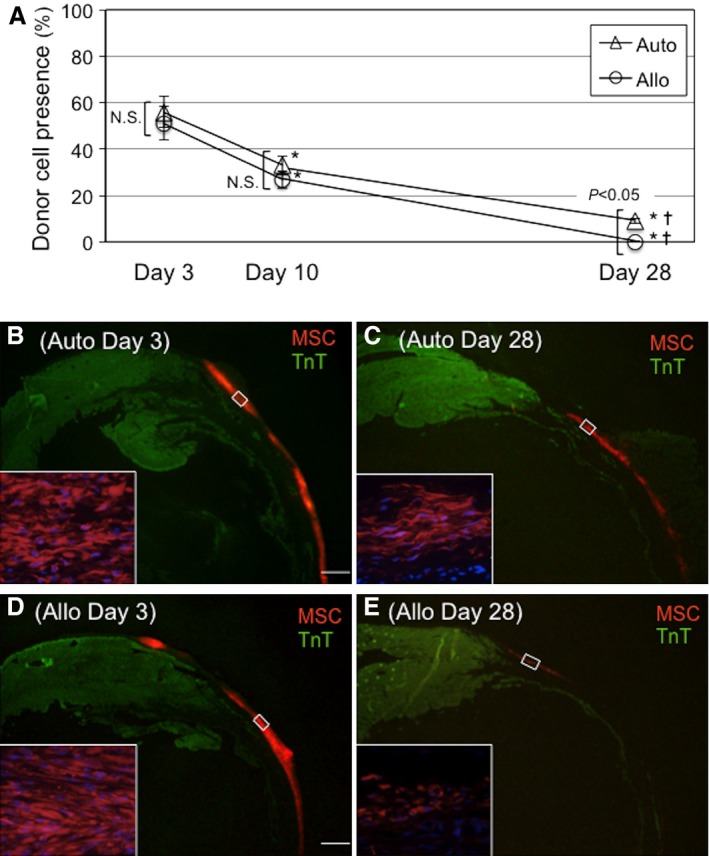
Allogeneic MSC sheet therapy achieved donor cell survival similar to syngeneic MSC sheet therapy in the early, but not late, phase. A, Male rat MSCs, syngeneic (auto group) or allogeneic (allo group), were epicardially placed in the form of cell sheets onto female rat hearts with post‐MI heart failure. Quantitative polymerase chain reaction for the male specific SRY gene showed that donor cell survival was equivalent at days 3 and 10 between the groups but was more extensively reduced in the allo group by day 28. n=5 per group at each point, *P<0.05 vs day 3 data in each corresponding group, ✝ P<0.05 vs day 10 data in each corresponding group. B through E, Immunohistostaining showed that the majority of donor MSCs labeled with CM‐DiI were retained at the surface of the left ventricular wall similarly between groups at day 3 after epicardial placement (B and D). Thin ventricular walls represent infarct areas. By day 28, donor cell survival was more extensively reduced in the allo group compared with the auto group (C and E). Scale bar=1 mm. MSC indicates mesenchymal stromal cell; N.S., not significant; TnT, troponin T.
These time courses of donor cell survival after epicardial placement were confirmed by immunohistofluorescence studies. It was demonstrated that similar quantities of CM‐DiI–labeled donor MSCs existed with the same epicardial localization in both allo and auto groups at day 3 (Figure 2B and 2D). Subsequently, the numbers of CM‐DiI–labeled donor cells reduced in both groups by day 28; however, the reduction of donor MSCs in the allo group appeared to be more drastic compared with the auto group (Figure 2C and 2E). The majority of surviving MSCs remained on the heart surface in both groups, and very few donor MSCs migrated into the myocardium throughout the course studied.
These data suggest that epicardially placed allogeneic MSCs survived to an extent similar to syngeneic MSCs in the early phase after transplantation. In the later phase, even syngeneic MSCs were largely lost (for reasons other than allorejection), but the loss of allogeneic MSCs was more extensive because of the addition of alloimmune rejection.
Allogeneic and Syngeneic MSCs Underwent Similar Differentiation in the Heart
Regarding differentiation of donor MSCs after epicardial placement, immunohistolabeling studies demonstrated that there were no donor cells clearly expressing cardiac troponin‐T in either the auto or allo group, suggesting limited occurrence of transdifferentiation of donor MSCs to cardiomyocytes. In addition, we did not observe any donor MSCs differentiated to adipocytes or osteocytes in the cell sheet or in the heart (Figure 3A and 3B), supporting safety of transplantation of MSCs into the heart. In contrast, detectable numbers of cells were double‐positive for CM‐DiI and PECAM1 similarly in both allo and auto groups (Figure 3C). This indicates that similar endothelial differentiation of donor MSCs (or fusion with host endothelial cells) occurred in both groups. In addition, host‐derived (CM‐DiI–negative) endothelial cells migrated into the MSC sheets in both groups, suggesting communication between the MSC sheets and host myocardium.
Figure 3.
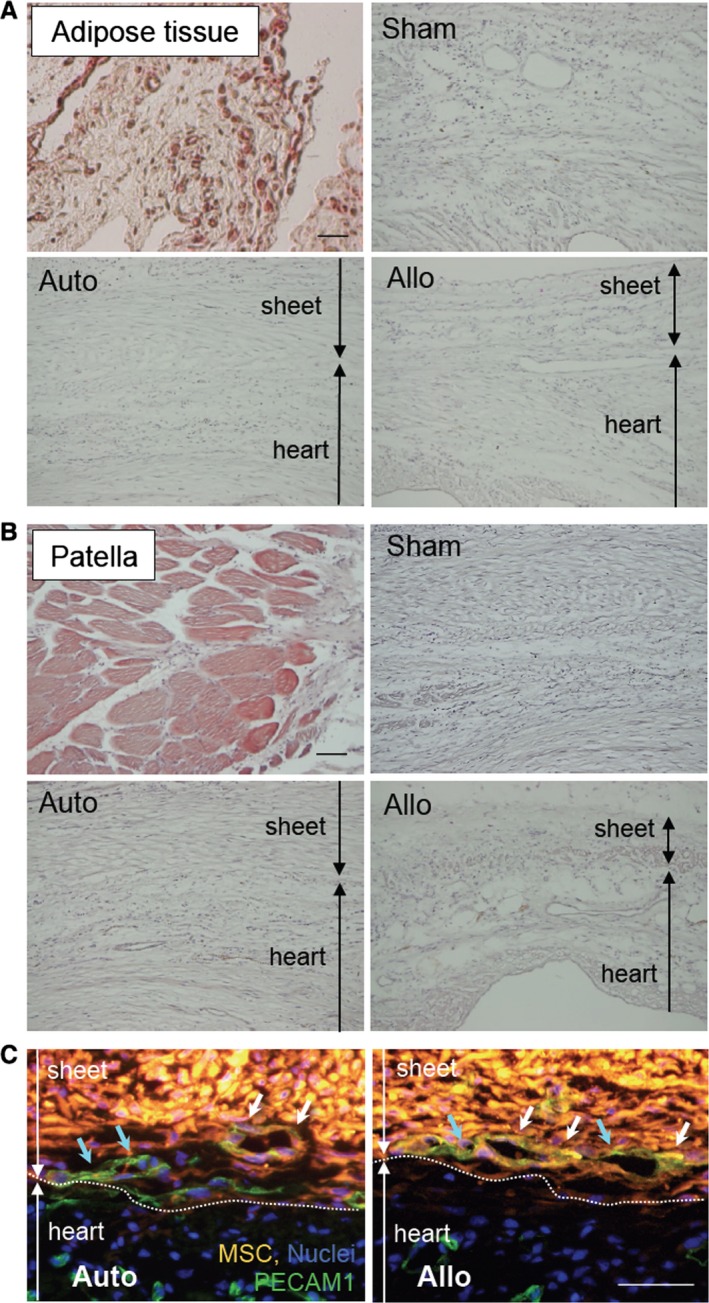
Donor MSC differentiation in vivo. A, Oil Red O staining detected the existence of adipocytes (dark red signal) in the peritoneal adipose tissue (positive control). No such Oil Red O–positive adipocytes were observed in the heart or in the MSC sheets at day 28 in any groups (5 hearts in each group). B, Alizarin red staining showed the calcium deposition in patella (positive control; red signal). No such signal was observed throughout the hearts or in the sheets at day 28. C, Immunohistostaining showed that there were DiI+ PECAM1+ donor‐derived endothelial cells (white arrows) as well as DiI− PECAM1+ host‐derived endothelial cells (blue arrows) in the sheets at day 3 similarly in the auto and allo groups. Green indicates PECAM1; orange indicates donor MSCs (CM‐DiI); blue indicates nuclei (DAPI). For (A through C), 5 hearts were studied in each group. Scale bars=100 μm (A and B) and 50 μm (C). Allo indicates allogeneic MSC sheet placement at 4 weeks after myocardial infarction; Auto, syngeneic MSC sheet placement at 4 weeks after myocardial infarction; MSC, mesenchymal stromal cell; Sham, sham treatment (open chest only) at 4 weeks after myocardial infarction.
Allogeneic MSC Sheets Evoked a Low‐Level Immunologic Response
We found that, consistent with previous reports,1, 3 in vitro cultured MSCs showed minimal expression of MHC class II molecules and relatively less expression of MHC class I molecules than unfractionated bone marrow mononuclear cells (Figure 4). In addition, immunosuppressive abilities of MSCs in vitro have been reported.1, 3 Despite these findings, our results for donor cell survival (Figure 2A) indicated that allogeneic MSCs were rejected in the long term, suggesting that the above properties were not effective enough for allogeneic MSCs to be immunologically privileged in vivo.
We characterized local immunologic responses induced by epicardially placed allogeneic MSC sheets. Immunohistostaining demonstrated that the number of CD4+ T cells accumulated in the MSC sheet at days 3 and 10 was increased in the allo group compared with the auto group (Figures 5A and 6A). In contrast, the number of CD8+ T cells or CD68+ macrophages accumulated in the MSC sheet was not significantly different between the allo and auto groups (Figures 5B, 5C and 6B, 6C). Quantitative reverse transcription polymerase chain reaction revealed that myocardial expression of IL‐6 was upregulated in the allo group compared with the auto group (Figure 7A). IL‐6 has been reported to be involved in promoting allograft rejection.15, 16 Serum IL‐6 levels, however, were unchanged at the minimum detectable levels between groups (Figure 7B). In contrast, there was no statistically significant change in expression of IFNG, CCL2, IL2RA, IL1B, or TNF between the auto and allo groups. In addition, upregulation of NOS2, IDO1, TGFB1, and HGF, which reportedly play a role in MSC‐mediated suppression of allogeneic responsiveness,1, 3 in the MSC sheet groups was not statistically significant compared with the sham group (Figure 8).
Figure 5.
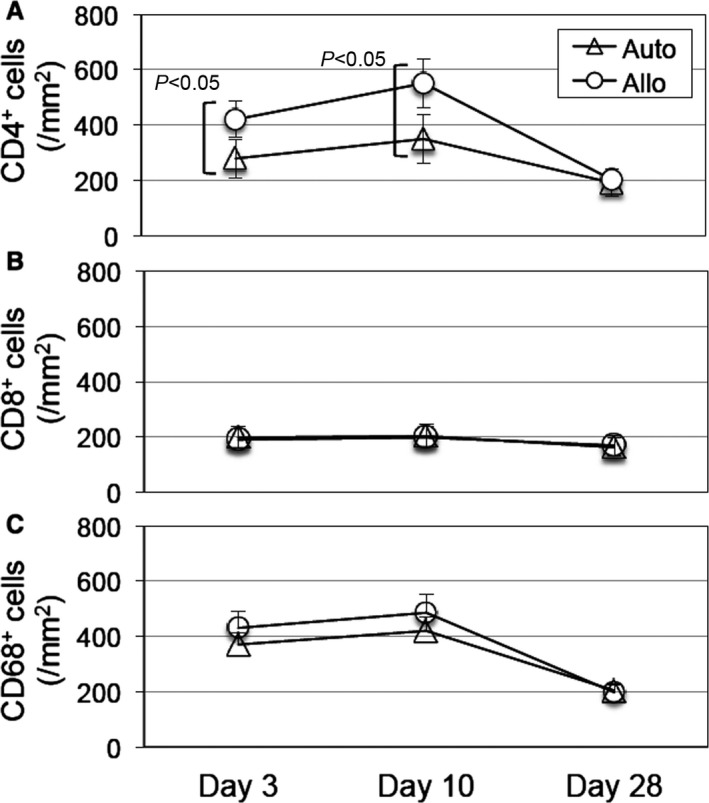
Allogeneic MSC sheets induced accumulation of CD4+ cells but not CD8+ cells or CD68+ cells. Immunohistostaining showed that accumulation of CD4+ cells (A), but not CD8+ cells (B) or CD68+ cells (C), within the MSC sheets was increased in the allo group at days 3 and 10 compared with the auto group. Representative images are shown in Figure 6. n=5 per group. MSC indicates mesenchymal stromal cell.
Figure 6.
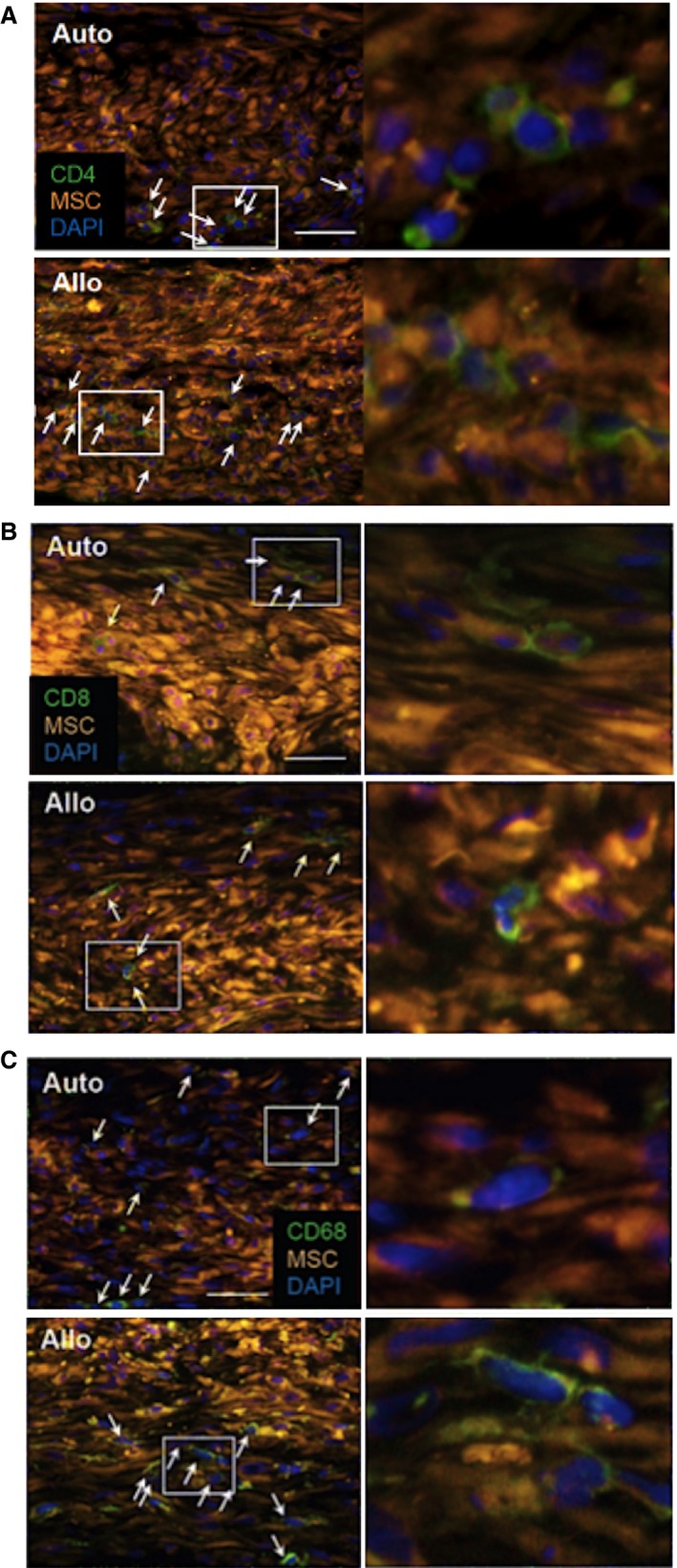
Accumulation of CD4+ cells but not CD8+ cells or CD68+ cells after allogeneic MSC sheet placement. Representative images of immunohistostaining for accumulation of CD4+, CD8+, and CD68+ cells in the MSC sheets at day 3 after treatment (Figure 5A through 5C, respectively) are shown (A through C). Right panels are higher magnification images of white rectangle areas in the left panels. Only positive cells with nuclei (white arrows) were counted for comparison. Scale bars=50 μm. MSC indicates mesenchymal stromal cell.
Figure 7.
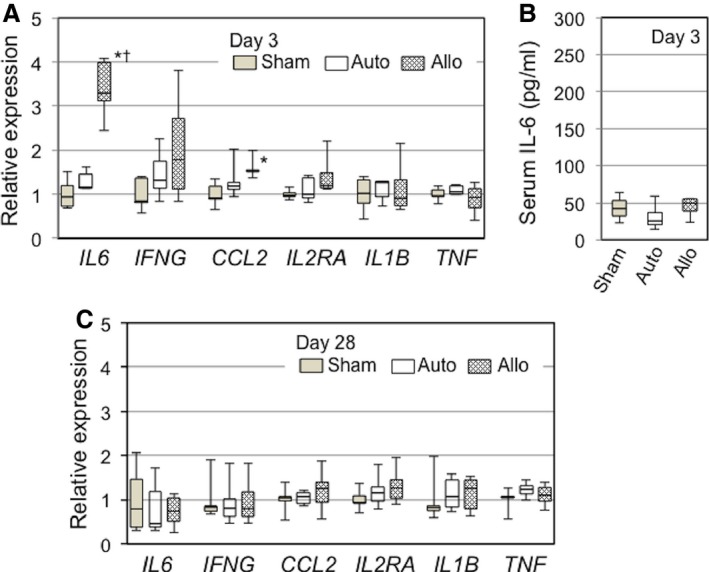
Allogeneic MSC sheets evoked a low‐level immunologic response at early phase after epicardial placement. A, Quantitative RT‐PCR detected that IL6, but not many other key genes involved in immunologic responses, was upregulated in the left ventricular myocardium (including the MSC sheets) at day 3 in the allo group compared with the auto groups. B, Enzyme‐linked immunosorbent assay demonstrated that serum levels of IL‐6 were similarly low (detection limit) at day 3 after treatment in all groups. C, Quantitative RT‐PCR showed that myocardial upregulation of molecules relevant to immunologic responses observed at day 3 in the allo group (A) was diminished by day 28 after treatment. *P<0.05 vs sham. † P<0.05 vs auto. n=5 per group (A through C). Allo indicates allogeneic MSC sheet placement at 4 weeks after myocardial infarction; Auto, syngeneic MSC sheet placement at 4 weeks after myocardial infarction; MSC, mesenchymal stromal cell; RT‐PCR, reverse transcription polymerase chain reaction; Sham, sham treatment (open chest only) at 4 weeks after myocardial infarction.
Figure 8.
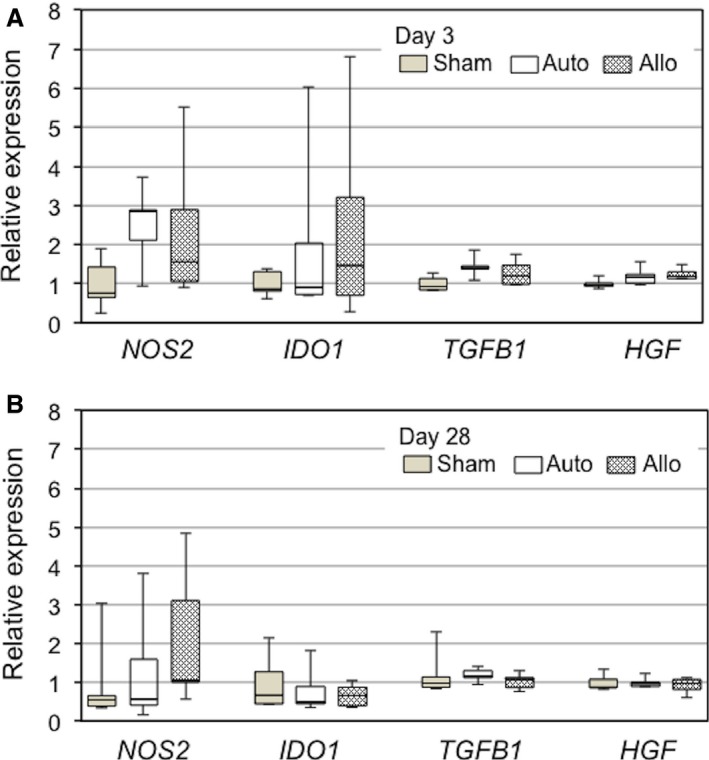
Myocardial upregulation of immunosuppressive genes after MSC sheet placement. Quantitative reverse transcription polymerase chain reaction demonstrated that expression of immunosuppressive genes (regulating NOS2, IDO1, TGFB1, and HGF) in the whole left ventricular walls (including the MSC sheets) was not statistically different among the sham (sham treatment at 4 weeks after MI), auto (syngeneic MSC sheet placement at 4 weeks after MI), and allo (allogeneic MSC sheet placement at 4 weeks after MI) groups at day 3 (A) and day 28 (B). n=5 per group. MI indicates myocardial infarction; MSC, mesenchymal stromal cell.
These data collectively suggest that epicardial placement of allogeneic MSC sheets evoked a local immunologic response, albeit low level; however, such responses, characterized with accumulation of CD4+ cells (Figure 5A) and upregulation of IL6 (Figure 7A), were mostly eliminated by day 28, corresponding to the observation that allogeneic MSCs had been mostly rejected and disappeared by that time (Figure 2A).
Allogeneic MSC Sheets Induced Myocardial Upregulation of Factors Related to Myocardial Repair Comparable to Syngeneic MSC Sheets
We next investigated whether epicardially placed allogeneic MSCs, which settled on the heart surface and survived only in the early phase (although at least until day 10), could contribute to a therapeutic effect for heart failure in comparison with syngeneic MSCs. Because the major mechanism underlying MSC‐based therapy is considered to be the paracrine effect,1, 11 we screened for expression of molecules that potentially play a role in this mechanism by quantitative reverse transcription polymerase chain reaction. The results demonstrated that comparable profiles and degrees of myocardial upregulation of factors relevant to tissue repair, anti‐inflammation, and neovascular formation, including TIMP1, IGF1, VCAM1, SDF1A, HIF1A, and MMP2, in the allo and auto groups at day 3 after treatment compared with the sham group (Figure 9A). It was also noted that upregulation of these genes was reduced to the baseline (values in the sham group) by day 28 (Figure 9B).
Figure 9.
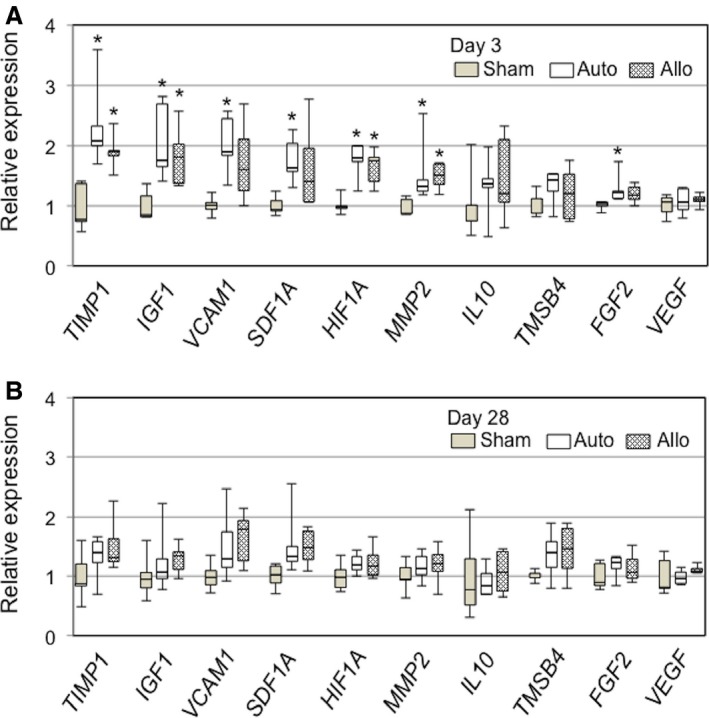
Allogeneic MSC sheets induced analogous upregulation of reparative factors to syngeneic MSC sheets. Quantitative reverse transcription polymerase chain reaction detected similar myocardial upregulation of genes possibly underpinning MSC‐mediated myocardial repair in the auto and allo groups at day 3 (A). All this upregulation was eliminated at day 28 (B). *P<0.05 vs sham, n=5 per group. MSC indicates mesenchymal stromal cell.
Epicardially Placed Allogeneic MSC Sheets Induced Tissue Repair of Chronically Failing Myocardium After MI, Similar to Syngeneic MSC Sheets
We examined whether the above‐identified early phase upregulation of paracrine factors correlated to histological repair of the post‐MI failing myocardium (paracrine effects). At day 28 after MSC sheet therapy, picrosirius red staining showed that post‐MI extracellular collagen deposition was reduced in the peri‐infarct viable myocardium in both auto and allo groups compared with the sham group (Figure 10A and 10C). Isolectin B4 staining showed that the capillary density in the peri‐infarct areas was increased similarly in the auto and allo groups compared with the sham group (Figure 10B and 10D). These histological results indicate that both syngeneic and allogeneic MSC sheets were able to similarly induce myocardial repair of the injured myocardium in post‐MI heart failure, regardless of the different donor cell survival at day 28 (both <10%) (Figure 2A).
Figure 10.
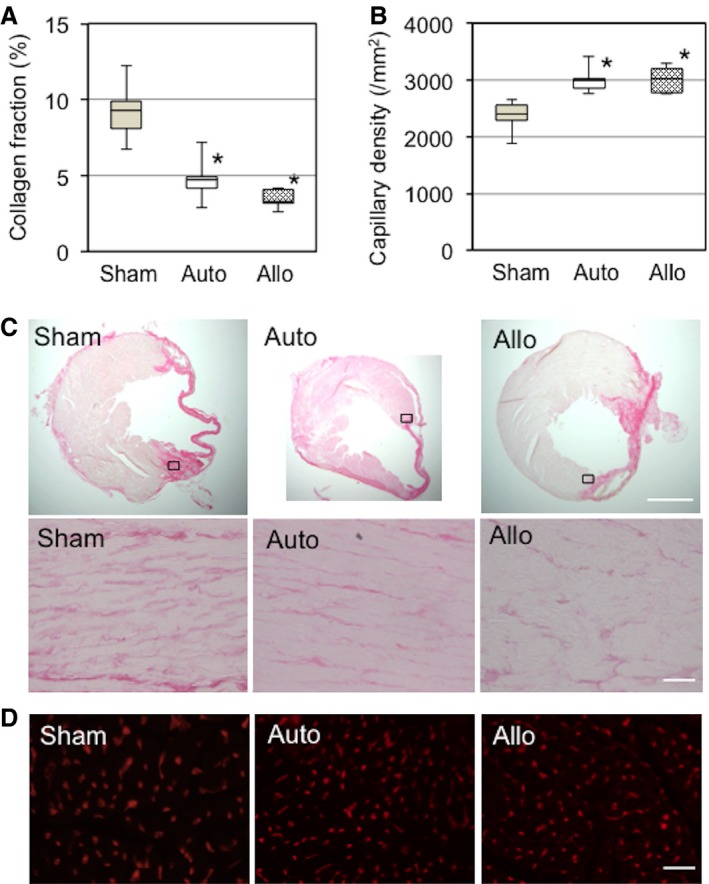
Allogeneic MSC sheets induced myocardial repair comparable to syngeneic MSC sheets. A, Extracellular collagen deposition in the peri‐infarct area, assessed by picrosirius red staining at day 28, was reduced in the auto and allo groups compared with the sham group. B, Isolectin B4 staining detected an increase in capillary density in the peri‐infarct area in the auto and allo groups compared with the sham group at day 28. C, Representative images for (A). Upper panels are whole heart images, whereas lower panels are higher magnification images of chosen areas (black squares) in the border area. Collagen deposition is shown in deep pink. Scale bar=300 μm (upper panels) and 50 μm (lower panels). D, Representative images for (B). Capillaries are stained in red. n=5 hearts per group. *P<0.05 vs sham. Scale bar=50 μm. MSC indicates mesenchymal stromal cell.
Epicardial Placement of Allogeneic MSC Sheets Improved Global Cardiac Function to the Same Extent as Syngeneic MSC Sheets
We evaluated overall therapeutic effects of allogeneic MSC sheet therapy by measuring cardiac function and structure, in comparison to syngeneic MSC sheet therapy, in the rat model of post‐MI chronic heart failure. All pretreatment data on cardiac function measured by echocardiography (at 4 weeks after MI) were comparable between groups (Table 1). At day 28 after treatment (8 weeks after MI), echocardiography showed that LV ejection fraction was similarly improved in the auto and allo groups compared with the sham group (Table 1). LV end‐diastolic and end‐systolic dimensions had a decreasing trend (although not statistically significant) in the auto and allo groups compared with the sham group (Table 1). Consistent with these, cardiac catheterization demonstrated that maximum/minimum pressure and maximum and minimum dP/dt in the allo group were all improved to the same extent as in the auto group (Table 2). Both MSC sheet groups had a tendency toward reduced LV end‐diastolic pressure compared with the sham group (Table 2). These results confirmed that allogeneic MSC sheet therapy was able to achieve therapeutic effects for post‐MI heart failure comparable to syngeneic MSC sheet therapy.
Table 2.
Cardiac Catheterization Analysis at Day 28 After Treatment
| Heart Rate (bpm) | Maximum Pressure (mm Hg) | EDP (mm Hg) | Mean Pressure (mm Hg) | Maximum‐Minimum Pressure (mm Hg) | |
|---|---|---|---|---|---|
| Sham | 365.8±9.0 | 114.4±4.3 | 10.4±2.0 | 54.5±2.1 | 109.4±3.3 |
| Auto | 372.0±6.5 | 139.6±4.1a | 8.8±0.5 | 65.0±2.3a | 135.1±4.1a |
| Allo | 375.0±7.0 | 141.7±3.9a | 7.9±0.9 | 64.7±2.4a | 137.8±4.1a |
| Maximum dP/dt (mm Hg/s) | Contractility Index (1/s) | Minimum dP/dt (mm Hg/s) | IRP Average dP/dt (mm Hg/s) | Pressure Time Index (mm Hg/s) | |
|---|---|---|---|---|---|
| Sham | 6542.5±532.9 | 96.9±4.4 | −6594.8±580.0 | −3249.3±208.7 | 7.6±0.3 |
| Auto | 8823.2±222.5a | 103.5±2.4 | −8967.8±453.0a | −4053.7±168.5a | 8.8±0.3a |
| Allo | 9171.6±388.4a | 103.7±3.9 | −9185.8±936.1a | −4130.0±164.0a | 8.9±0.4a |
n=8 to 11 per group. bpm indicates beats per minute; EDP, left ventricular end‐diastolic pressure; IRP, isovolumic relaxation period.
P<0.05 vs sham.
Discussion
This study demonstrated that epicardial placement of allogeneic MSCs in the form of cell sheets achieved early phase donor cell survival equivalent to that of syngeneic MSC sheets (≈50% at day 3 and ≈30% at day 10) in a rat model of post‐MI chronic heart failure. Toward day 28, donor cell survival was significantly decreased to <10% even in syngeneic MSCs (independent of immunologic rejection), whereas additional cell loss in allogeneic MSCs (survival rate 0.2%) suggested immunologic rejection of these cells. Despite this rejection, which was underpinned by a low‐level immunologic response, surviving allogeneic MSCs in the early phase were able to achieve a range and degree of myocardial upregulation of reparative factors equivalent to those of the syngeneic MSCs. Importantly, this was sufficient to accomplish structural repair of the post‐MI failing myocardium, including increased neovascular formation and reduced fibrosis, to the same extent as the syngeneic MSC sheet therapy. Consequently, overall therapeutic effects (global cardiac function improvement) were equivalent between allogeneic and syngeneic MSC sheets. These data shed light on the potential of allogeneic MSC sheet therapy as an advanced form of MSC‐based therapy for heart failure.
Our results accumulate important information regarding immunologic response after allogeneic MSC transplantation to the heart. To date, all such information has been from studies using intramyocardial injection as the cell delivery method, for which the numbers of retained and surviving donor MSCs at the initial assessment point (days 34 and 75) were notably and similarly small (only ≤5% of the total injected cells) in both allogeneic and syngeneic MSCs.4, 5 This means that >95% of injected MSCs were lost or died early after transplantation independent of alloimmunologic responses. In contrast, the cell sheet technique achieved much greater early survival of MSCs (>50% at day 3; comparable to the syngeneic MSCs). By examining the fate of such larger numbers of initial surviving donor cells, we were able to conclude with more confidence that allogeneic MSCs could afford to survive in the early phase of transplantation (at least for 10 days) but eventually underwent allorejection. The cell sheet technique offers unique epicardial distribution of donor allogeneic MSCs; in contrast, the cells grafted by intramyocardial injection localize within the myocardium.14 This difference, however, did not appear to significantly affect the majority of immunologic responses by allogeneic MSCs. There appeared to be substantial communication between MSCs between the sheets and the host myocardium, given our results of migration of host immune cells and endothelial cells into the MSC sheets (Figures 3C and 5).
We observed that rat MSCs exhibit relatively low expression of MHC molecules in vitro, consistent with the previous results.1, 3 In addition, even if in vivo upregulation of immunosuppressive factors by MSC sheets was not statistically significant in the whole LV samples (presumably due to dilution of the lesion with healthy tissues), immunomodulating secretion by MSCs in vitro is widely known.1, 3 These properties, however, were not extensive or persistent enough for the allogeneic MSCs to establish immunologically privileged status in our in vivo model; increased accumulation of CD4+ T cells occurred within the sheets. This immunologic response evoked by epicardially settled allogeneic MSCs appeared to be low level, considering insignificant changes in other major immunologic genes and unchanged accumulation of macrophages and CD8+ T cells. Nonetheless, such responses were sufficient to reject most allogeneic MSCs by day 28 (although only 9% of transplanted syngeneic MSCs survived at this stage). Our results highlighted that IL6 was particularly upregulated in the heart after allogeneic MSC sheet placement compared with both syngeneic MSC sheet placement and sham treatment. Given the knowledge that IL‐6 plays an important role in promoting allograft rejection by modulating T‐cell immunity15, 16 and our finding that other known factors responsible for immunologic responses were not upregulated, it is likely that IL‐6 may have a key role in regulating the acceptance and rejection of allogeneic MSCs in the heart. Dhingra et al demonstrated a role for prostaglandin E2 in the survival of allogeneic MSCs after intramyocardial injection in a rat model of subacute MI.6 Further studies are needed to fully elucidate the mechanism underlying immunologic responses induced by allogeneic MSCs.
It is now widely agreed that the major mechanism by which transplanted MSCs improve function of damaged hearts is the paracrine effect rather than cardiomyogenic differentiation of donor MSCs.1, 11 We demonstrated that even though donor cell survival was significantly reduced by day 28, allogeneic MSC sheets achieved the paracrine effect, comparable to syngeneic MSC sheets. Donor cell survival at days 3 and 10 after allogeneic MSC sheet placement was equivalent to the syngeneic MSC sheet, which enabled similar introduction of reparative signals in the myocardium, including TIMP1, IGF1, VCAM1, SDF1, and HIF1A, during the early phase after treatment. This early phase upregulation was likely sufficient to induce histological repair of the failing myocardium after MI (ie, improved microvasculature formation and reduced pathological fibrosis). Such structural repair by the paracrine effects would, once established, be persistent for a long time, resulting in long‐term improvement (at least for 28 days) of cardiac function. These data validate the potential of allogeneic MSC sheet therapy for the treatment of heart failure.
Allogeneic MSCs have been administered to patients with various diseases, including graft‐versus‐host disease, neurological diseases, acute MI, and heart failure.3, 17 These clinical trials have shown safety (and preliminary efficacy) of allogeneic MSCs,3, 7, 8, 17 encouraging further development of this strategy. The practicability of allogeneic MSCs validates development of an MSC bank that will enable an “off‐the‐shelf” supply of quality‐controlled, quality‐assured, most appropriate MSCs with reduced costs on request (no waiting time for MSC expansion). This will greatly support allogeneic MSC sheet therapy in becoming a widely adopted standard treatment for heart failure.1, 3, 17 Because the cell sheet technique requires the heart to be exposed, a practical strategy would be to combine allogeneic MSC sheet therapy with routine cardiac surgery such as coronary artery bypass grafting; the addition of cell therapy is known to improve the effect of bypass surgery18 while lowering the shared costs. That said, how long such therapeutic effects of allogeneic MSCs last is a remaining issue and is controversial in small animal models. Lopez et al showed that intramyocardial injection of allogeneic MSCs improves LV ejection fraction for 32 weeks,19 whereas Huang et al and Dai et al reported negative LV ejection fraction improvement by intramyocardial injection of allogeneic MSCs at 24 weeks.5, 20 Further studies are needed, probably in a more appropriate setting for investigating a long‐term effect of allogeneic MSC sheets, including large animal models or clinical trials.
In conclusion, our data suggested that epicardial placement of allogeneic MSC sheets was not entirely privileged immunologically, but substantial numbers of allogenic MSCs managed to survive for a certain early period. This resulted in significant improvement of cardiac function through enhanced myocardial repair comparable to the syngeneic MSC sheet without causing complications. Further development of this approach (allogeneic MSC sheet therapy), which has potential to be an established treatment for heart failure, is warranted for clinical application.
Sources of Funding
This project was funded by the British Heart Foundation (PG/12/10/29389) and Heart Research UK (RG2618/12/13), and supported by the National Institute for Health Research‐funded Barts Cardiovascular Biomedical Research Unit.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e002815 doi: 10.1161/JAHA.115.002815)
References
- 1. Williams AR, Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109:923–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jiang S, Kh Haider H, Ahmed RPH, Idris NM, Salim A, Ashraf M. Transcriptional profiling of young and old mesenchymal stem cells in response to oxygen deprivation and reparability of the infarcted myocardium. J Mol Cell Cardiol. 2008;44:582–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kode JA, Mukherjee S, Joglekar MV, Hardikar AA. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 2009;11:377–391. [DOI] [PubMed] [Google Scholar]
- 4. Westrich J, Yaeger P, He C, Stewart J, Chen R, Seleznik G, Larson S, Wentworth B, O'Callaghan M, Wadsworth S, Akita G, Molnar G. Factors affecting residence time of mesenchymal stromal cells (MSC) injected into the myocardium. Cell Transplant. 2010;19:937–948. [DOI] [PubMed] [Google Scholar]
- 5. Huang X‐P, Sun Z, Miyagi Y, McDonald Kinkaid H, Zhang L, Weisel RD, Li RK. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long‐term benefits for myocardial repair. Circulation. 2010;122:2419–2429. [DOI] [PubMed] [Google Scholar]
- 6. Dhingra S, Li P, Huang X‐P, Guo J, Wu J, Mihic A, Li SH, Zang WF, Shen D, Weisel RD, Singal PK, Li RK. Preserving prostaglandin E2 level prevents rejection of implanted allogeneic mesenchymal stem cells and restores postinfarction ventricular function. Circulation. 2013;128:S69–S78. [DOI] [PubMed] [Google Scholar]
- 7. Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB Jr, Reisman MA, Schaer GL, Sherman W. A randomized, double‐blind, placebo‐controlled, dose‐escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis‐Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Prigozhina TB, Khitrin S, Elkin G, Eizik O, Morecki S, Slavin S. Mesenchymal stromal cells lose their immunosuppressive potential after allotransplantation. Exp Hematol. 2008;36:1370–1376. [DOI] [PubMed] [Google Scholar]
- 10. Poncelet AJ, Vercruysse J, Saliez A, Gianello P. Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro, intracardiac injection elicits an immune response in vivo. Transplantation. 2007;83:783–790. [DOI] [PubMed] [Google Scholar]
- 11. Tano N, Narita T, Kaneko M, Ikebe C, Coppen SR, Campbell NG, Shiraishi M, Shintani Y, Suzuki K. Epicardial placement of mesenchymal stromal cell‐sheets for the treatment of ischaemic cardiomyopathy; in vivo proof‐of‐concept study. Mol Ther. 2014;22:1864–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hayashi Y, Tsuji S, Tsujii M, Nishida T, Ishii S, Iijima H, Nakamura T, Eguchi H, Miyoshi E, Hayashi N, Kawano S. Topical transplantation of mesenchymal stem cells accelerates gastric ulcer healing in rats. Am J Physiol Gastrointest Liver Physiol. 2008;294:778–786. [DOI] [PubMed] [Google Scholar]
- 13. Kaneko M, Shintani Y, Narita T, Ikebe C, Tano N, Yamahara K, Fukushima S, Coppen SR, Suzuki K. Extracellular high mobility group box 1 plays a role in the effect of bone marrow mononuclear cell transplantation for heart failure. PLoS One. 2013;8:e76908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Narita T, Shintani Y, Ikebe C, Kaneko M, Campbell NG, Coppen SR, Uppal R, Sawa Y, Yashiro K, Suzuki K. The use of scaffold‐free cell sheet technique to refine mesenchymal stromal cell‐based therapy for heart failure. Mol Ther. 2013;21:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Booth AJ, Grabauskiene S, Wood SC, Lu G, Burrell BE, Bishop DK. IL‐6 promotes cardiac graft rejection mediated by CD4+ cells. J Immunol. 2011;187:5764–5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shen H, Goldstein DR. IL‐6 and TNF‐alpha synergistically inhibit allograft acceptance. J Am Soc Nephrol. 2009;20:1032–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Donndorf P, Kundt G, Kaminski A, Yerebakan C, Liebold A, Steinhoff G, Glass A. Intramyocardial bone marrow stem cell transplantation during coronary artery bypass surgery: a meta‐analysis. J Thorac Cardiovasc Surg. 2011;142:911–920. [DOI] [PubMed] [Google Scholar]
- 19. López Y, Lutjemeier B, Seshareddy K, Trevino EM, Hageman KS, Musch TI, Borgarelli M, Weiss ML. Wharton's jelly or bone marrow mesenchymal stromal cells improve cardiac function following myocardial infarction for more than 32 weeks in a rat model: a preliminary report. Curr Stem Cell Res Ther. 2013;8:46–59. [DOI] [PubMed] [Google Scholar]
- 20. Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short‐ and long‐term effects. Circulation. 2005;112:214–223. [DOI] [PubMed] [Google Scholar]


