Abstract
Since the establishment of mouse embryonic stem cells (mESCs) in the 1980s, a number of important notions on the self-renewal of pluripotent stem cells in vitro have been found. In serum containing conventional culture, an exogenous cytokine, leukemia inhibitory factor (LIF), is absolutely essential for the maintenance of pluripotency. In contrast, in serum-free culture with simultaneous inhibition of Map-kinase and Gsk3 (so called 2i-culture), LIF is no longer required. However, recent findings also suggest that LIF may have a role not covered by the 2i for the maintenance of naïve pluripotency. These suggest that LIF functions for the maintenance of naïve pluripotency in a context dependent manner. We summarize how LIF-signal pathway is converged to maintain the naïve state of pluripotency.
KEYWORDS: Embryonic stem cell (ESC), Lekemia inhibitory factor (LIF) signal, Stat3, MAP kinase, PI3K-Akt, Genetic background, naive state of pluripoetncy, Epigenetics
INTRODUCTION
mESCs are derived from the inner cell mass (ICM) of the blastocyst, and self-renew indefinitely in vitro.1,2 In earlier period, mESCs were maintained in fetal calf serum (FCS)-containing medium with mouse embryonic fibroblast (MEF)-feeder cells. It was technically difficult to retain a fully pluripotent state of mESCs due to the unknown factors of FCS. Later it was found that FCS-medium conditioned by Baffalo-rat liver (BRL) cells counteracted spontaneous differentiation of mESCs.3 This unidentified factor of BRL cells was named differentiation inhibitory activity (DIA). BRL-conditioned FCS-medium maintained pluripotency of mESCs in the absence of MEF-feeder cells. DIA was biochemically purified and turned out that it was identical to the cytokine LIF,4–6 which is now recognized as a critical factor for robust self-renewal of mESCs.7,8 Since then LIF-supplemented FCS-medium (referred to as FCS/LIF-culture hereafter) became a global standard for the culture of mESCs. mESCs rapidly exit from the pluripotent state upon the removal of LIF from the FCS/LIF-culture. The dependency on exogenous LIF for the maintenance of pluripotency is obvious in feeder-free culture but it is somewhat obscure in the presence of MEF-feeder cells, which might be partly due to the secretion of LIF or related ligands by MEF.
LIF shows the beneficial effect only on the mESCs derived from limited types of genetic background such as 129 inbred mouse strain.9,10 Efforts failed to establish mESCs from mouse strains with other genetic backgrounds in FCS/LIF-culture.11–14 From these trials it became evident that there are permissive and non-permissive genetic backgrounds for LIF-dependent self-renewal. In other words, the effect of LIF is not ubiquitously applicable to all types of mESCs derived from various genetic backgrounds.
Because of this limitation of LIF and also the mischievous unknown factors of FCS, more generally stable culture condition was desired. In 2008, Ying et al15 found that the combination of 2 small molecule inhibitors targeting MAPK and glycogen synthase kinase 3b (GSK3b), respectively (2i), supports the self-renewal of mESCs in serum-free culture without LIF. Thus, LIF-dependency is influenced not only by the genetic backgrounds but also by the culture context. However, even in this seemingly LIF-independent 2i-culture condition, addition of LIF further promoted self-renewal of mESCs, suggesting the synergistic effect of 2i and LIF.15 Interestingly, the 2i-culture allows self-renewal of mESCs even from non-permissive mouse strains, which was not allowed in FCS/LIF-culture, indicating the distinct action of 2i from LIF rather than the replacement of LIF.
This raises interesting questions: how 2i cancels LIF-requirement for the self-renewal of mESCs and overcomes the strain dependency, and why LIF exhibits context-dependent activity to support pluripotency. We will summarize our current understanding on LIF-signal that maintains pluripotency of mESCs.
LIF and Its cell-surface receptors
LIF is a member of the interleukine-6 (IL-6) cytokine family which consists of the following members: IL-6, IL-11, IL-27, ciliary neurotrophic factor (CNTF), cardiotropin-1 (CT-1), oncostatin M (OSM) and LIF.16–20 The receptors of the IL-6 family form a complex with the receptor glycoprotein 130 (Gp130, also known as Il6st) (Fig. 1). For example, LIF binds to a hetero-dimer receptor complex that consists of a low-affinity cell surface LIF-receptor (Lifr) and Gp130. None of these receptors possess catalytic domains for enzymatic activity in the cytoplasmic region. The cytoplasmic domains of these receptors are constantly bound by a family of tyrosine kinases, janus kinases (Jaks) that consists of Jak1, Jak2, Jak3 and Tyk2. In the absence of the ligands, these Jaks are inactive by their intra-molecular inhibitory regulation.21 Upon the binding of the ligands to the receptors, Jaks at the cytoplasmic domains become active by auto-phosphorylation of the inhibitory domain. Activated Jaks phosphorylate the tyrosine residues at the Y-x-x-Q motifs of Gp130 and Lifr,22 which allows the binding of the other Jaks-substrate, Stat3, to the cytoplasmic domain of the receptors (Fig. 1). When phosphorylated by Jaks, Stat3 leaves the receptor forming a dimer, then enters into the nucleus where they act as a transcription factor to induce the expressions of the target genes.23,24
Figure 1.
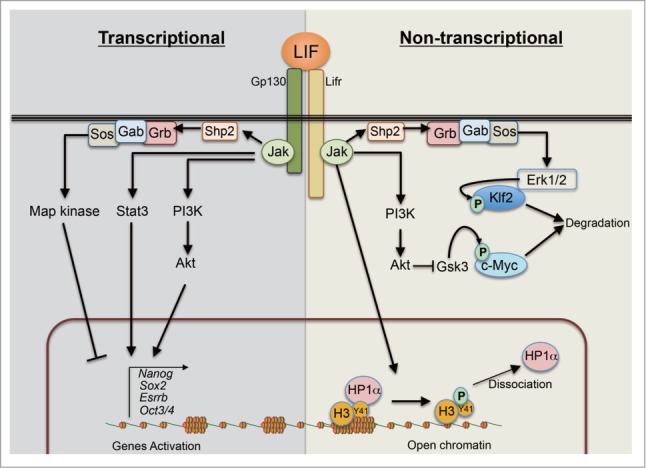
Schematic depiction on exogenous LIF mediated intra-cellular signaling pathways for robust self-renewal in mESCs. LIF activates at least 3 intra-cellular signaling pathways: Jak-Stat3, PI3K-Akt and Shp2-MAPK pathways. Upon LIF stimulation, Jaks (Jak1, Jak2, Jak3 and Tyk2) are activated by auto-phosphorylation. Activated Jaks induce downstream cascades. Stat3 is phosphorylated and activated by Jaks, and subsequently induces the expressions of various pluripotent associated genes. Activated PI3K-Akt by Jaks also positively regulates the genes. In contrast to these 2 pathways, activated MAPK pathway inhibits the expressions of the pluripotency associated genes both at transcriptional and post-transcriptional levels. In addition to signal mediated transcriptional regulation, activated Jak2 tunes the chromatin status through histone modification. Also, activated Erk1 and Erk2 directly interact with Klf2 and phosphorylate it, leading to ubiquitination and degradion of Klf2 via proteasomal pathway. Also, LIF-PI3K-Akt axis stabilizes c-myc via inhibiting Gsk3 activity.
Jak-Stat3 pathway possesses a negative feedback loop: activated Jaks phosphorylate Stat3, which induces the expression of the suppressor of cytokine signaling 3 (Socs3), which then inactivates Jaks in a short period.25–27 Socs3 binds to Jaks through its Src homology 2 (SH2)-domain and blocks the kinase domain of Jak by the kinase inhibitory region (KIR). Therefore, Socs3 functions as a pseudo-substrate of Jaks.28 In addition, activated Stat3 is also inhibited by PIAS3 (protein inhibitor of activated Stat3). PIAS3 directly binds to Stat3 and interferes with its DNA binding activity.29 These negative regulators ensure the temporal activation of the LIF-signaling pathway.
For the culture of mESCs, LIF can be substituted by related IL-6 family cytokines, such as OSM, CT-1 and CNTF, that bind to Lifr.30–32 Also, a soluble form of the IL-6 receptor (sIL-6R) together with IL-6 enables the self-renewal of mESCs in the absence of LIF.33,34 These findings indicate that the signaling mediated by Gp130, one of the components of the receptor complex (Fig. 1), is sufficient for the self-renewal of mESCs. This is further supported by the evidence that a chimeric receptor made of the ligand binding domain of granulocyte colony-stimulating factor receptor (GCSFR) and the cytoplasmic domain of Gp130 can replace the function of LIF when stimulated by the ligand of the chimeric receptor, GCSF.35 In contrast, the results of the functional analyses with the cytoplasmic domain of Lifr using the same system are controvertial.36 Taken together, Gp130-mediated signaling is responsible for the transduction of LIF-signal for the self-renewal of mESCs.
Intra-Cellular signal transduction pathways of LIF
Upon the addition of LIF, 3 intra-cellular signaling pathways are mainly activated: Jak-Stat3, phosphatidylinositol 3-kinase (PI3K)-Akt and mitogen-activated protein kinase (MAPK) pathways. Each of the 3 pathways has its own significant function downstream of LIF in mESCs (Fig. 1).37 Jak-Stat3 and PI3K-Akt pathways facilitate the self-renewal of mESCs, whereas MAPK pathway promotes the differentiation of mESCs. Jak-activation downstream of LIF is not exclusive for the activation of the Jak-Stat3 pathway: it activates the PI3K-Akt pathway, and also via the recruitment and activation of Shp2, it activates the MAPK pathway (Fig. 1).
Jak-Stat3 pathway
Artificial activation of Stat3 is sufficient for the self-renewal of mESCs.38 This was shown by a fusion protein made of Stat3 and a mutated ligand-binding domain of the estrogen receptor (Stat3ER), which translocates into the nuclei upon the activation of the ER by its ligand tamoxifen (Tx), without the phosphorylation of Stat3. mESCs expressing Stat3ER keep self-renewing by the addition of Tx in the FCS-culture without LIF. Another evidence was brought by the chimeric receptor GCSFR-Gp130 with a mutation at the Shp2-docking site of Gp130 (Y118F), which activates the Jak-Stat3 pathway but not the MAPK pathway upon the binding of GCSF. With this receptor, addition of GCSF was sufficient for the self-renewal of mESCs without LIF.35 These data indicated that among the 3 pathways downstream of LIF, the activation of Stat3-pathway is sufficient to support the self-renewal of mESCs. It was also shown that the removal of the Stat3-docking sites from the cytoplasmic domain of the GCSF-Gp130 chimeric receptor abolished its ability to support the self-renewal.35 Moreover, overexpression of a dominant-negative form of Stat3 with a mutation at the Jak-targeted phosphorylation site (Stat3Y705F) strongly induced differentiation of mESCs in FCS/LIF-culture.35 Also Stat3-null mESCs were unable to be derived from Stat3-null blastocysts in FCS/LIF-culture.15 Therefore, the activation of Stat3 is essential for the self-renewal of mESCs.
Stat3 is phosphorylated at Y705 by Jaks and S727 by MAPK/JNKs (c-Jun N-terminal kinases)/ PKCδ (protein kinase Cδ), respectively.39–41 Phosphorylation of Stat3 at Y705 is critical for the self-renewal of mESCs as mentioned above. On the other hand, phosphorylation at S727 is dispensable for self-renewal but promotes proliferation.42 After differentiated into the neural lineage, phosphorylation of Stat3 at S727 becomes essential,42 suggesting the cell-type-specific role of Stat3-phosphorylation.
Upon the stimulation by LIF, Stat3 and MAPKs ERK1/2 are rapidly phosphorylated and then dephosphorylated in a short time period (∼30 mins). Deletion of Socs3, the negative-regulator of Jaks, leads to the activation of both Stat3 and MAPK downstream of LIF at the maximum level for a long period (at least for 4 hours) without significant reduction.43,44 Probably this prolonged activation of MAPK causes endodermal differentiation in Socs3-null mESCs because this propensity of the Socs3-null mESCs is partially suppressed by Mek (Map kinase kinase) inhibitor (MEKi).44 This result highlights the role of Jak-Stat3-Socs3 axis as a negative-feedback loop for the tight regulation of LIF-induced intra-cellular signaling that should be maintained within an adequate range.
LIF-signaling possesses a positive-feedback loop in which transcriptions of Gp130, Lifr and Stat3 are enhanced downstream of LIF, further activating the LIF-signal itself.45 Positive-feedbacks are efficient to maintain highly active status, which was first found during neural development: according to He et al.,46 when gliogenesis starts after neurogenesis, the positive-feedback loop of LIF-signaling becomes active to provide highly active status of the LIF signal in a stage-specific manner.
In like wise, the negative and positive feedback systems of LIF-signaling in mESCs may underlie the precise and sustained control of pluripotency in mESCs.
MAPK pathway
LIF activates MAPK pathway via Shp2-mediated Grb/Sos pathway (Fig. 1). Shp2 is known as a non-receptor protein tyrosine phosphatase (PTP) with a SH2-domain on its N-terminus.17 When the activity of Shp2 is blocked by a Jak-inhibitor, MAPK is not activated. Shp2 binds to Gp130 at the tyrosine 118 residue (Y118) upon the phosphorylation by Jaks. The chimeric receptor GCSF-Gp130 with a mutation at the Shp2 docking site of Gp130 (Y118F) activates the Jak-Stat3 pathway but not the MAPK pathway and its activation by GCSF is sufficient for the self-renewal of mESCs in FCS-culture without LIF,38 indicating that the activation of MAPK pathway is not required for the self-renewal in mESCs.
The role of MAPK pathway in mESCs is suggested by a series of loss-of-function experiments. Addition of MEKi PD098059 promotes self-renewal of mESCs in FCS/LIF-culture47 and inhibits neural differentiation in serum-free culture,48 suggesting the involvement of MAPK pathway in differentiation. mESCs lacking Grb2, a component of Grb/Sos pathway upstream of MAPK cascade, were resistant to the induction of differentiation by the withdrawal of LIF in FCS-culture.49 Erk2-null mESCs could be passaged without LIF in serum-free medium and failed to differentiate into either neural or mesodermal lineage with sustained expression of Oct3/4, Nanog and Rex1.50 Taken together, LIF-induced activation of MAPK pathway is involved in the differentiation of mESCs.
PI3K-Akt pathway
Upon the activation by LIF, PI3K is activated via Jaks, then it activates the downstream Akt and mTOR pathways.17 LY294002, a potent PI3K inhibitor, made mESCs less proliferative with prolonged G1-phase of the cell cycle.51 mTOR (mammalian target of rapamycin)-null mESCs also show defects in proliferation and a similar result was obtained from chemical inhibition of mTOR with rapamycin.52 Genetic ablation of Eras, a small GTPase specifically expressed in mESCs, also made mESCs less proliferative, with reduced PI3K-activity without affecting pluripotency, which was rescued by the overexpression of constitutively-active form of p110α, a catalytic subunit of PI3K.53 These data indicated that PI3K-Akt pathway contributes to the proliferation of mESCs. Isoforms of PI3K have distinct roles on the self-renewal of mESCs. Class-IA PI3Ks consist of a 110-kDa catalytic subunit and a regulatory subunit, and there are 3 isoforms with different 110-kDa catalytic subunits. Among them, inhibition of p110α-activity reduces proliferation without affecting pluripotency, whereas inhibition of p110β reduces Nanog expression and leads to differentiation, suggesting their differential roles.48
Pten (phosphatase and tensin homolog) antagonizes the PI3K-activity.17 Pten-null mESCs have high proliferation-rates accompanied by high G1/S-phase transition and low p27kip1 expression,54 consistent with the loss-of-function of PI3K described above that resulted in less proliferation.51–53 Expression of constitutively active form of Akt, one of downstream effectors of PI3K, cancels the requirement of LIF for the self-renewal of mESCs in FCS/LIF-culture.55 These data suggest that PI3K-Akt signaling pathways are involved in both proliferation and the maintenance of pluripotency in mESCs downstream of LIF.
Integration of LIF-signal into the transcription factor network system
Downstream of the intracellular signal transduction, transcription factor networks are formed. It was shown that 3 pathways under the LIF-signal integrate into the pluripotency-associated transcription factor network that forms a robust parallel pathway.37
Jak-Stat3 pathway
Stat3 is a pivotal molecule for the integration of the LIF-signal into the pluripotency-associated transcription factor network, in which Stat3 itself works as a transcription factor after translocating into the nuclei.24,56–58 However, among the genes whose expressions rapidly response to LIF, only a subset is directly regulated by Stat3. For example, both Klf4 and Tbx3, whose transgenic expressions support self-renewal of mESCs in FCS-culture without LIF,37 are induced rapidly upon the stimulation by LIF.37,59,60 However the analyses using pathway-specific inhibitors revealed that Klf4 is a direct target of Stat337,59 while Tbx3 is regulated by the PI3K-Akt pathway.37,61 Several reports identified multiple transcription factors regulated by Stat3: such as Zfp57,62 Nr0b1/Dax1,63 Gbx264 and Tfcp2l1.60,65 Among them, Gbx2 and Tfcp2l1 are interesting because the transgenic expressions of Gbx2 or Tfcp2l1 support LIF-independent self-renewal in FCS-culture,60,64,65 in a similar manner as shown by the exogenous expressions of Klf4 or Tbx3.37 Further comprehensive analysis of Stat3-dependent transcription revealed 58 direct target genes including several transcription factors other than the ones mentioned above: such as Junb, Klf5, Fos, Sp5, Myc and Sall4.59 Among them, the transgenic expressions of Klf5 and Myc support LIF-independent self-renewal in FCS-culture.66,67 These data indicate that multiple transcription factors that function for the self-renewal of mESCs are regulated by Stat3.
Stat3 cooperates with epigenetic regulators. Brg1 (also know as Smarca4) is a component of the ES-specific ATP-dependent chromatin-remodelling complex (esBAF), which establishes chromatin accessibility at Stat3-target sites across the genome. On the other hand, artificial activation of Stat3 partially rescued the defect of the self-renewability in Brg1-null mESCs, elucidating their mutual functional link.68,69 Interestingly, genome-wide chromatin-immunoprecipitation-sequencing (ChIP-seq) revealed that Stat3 co-occupies a huge number of target sites with Brg1, which include the sites at Oct3/4, Sox2 and Nanog loci, suggesting a global transcriptional regulation by Stat3.68 These transcription factors may not solely be targeted by Stat3.37,59 It is shown that in addition to Stat3, other transcription factors regulate these genes cooperatively.37 Taken together, distinct responsiveness to Stat3 might reflect quantitative (rather than qualitative) differences in their dependency on Stat3.37
Interestingly, Jaks are implicated in epigenetic modifications apart from Stat3. In 2009, Dawson et al70 reported that Jak2 is localized in the nuclei of hematopietic cells and directly phosphorylates the Tyr41 residue of histone H3 (H3Y41) and prevents the recruitment of the heterochromatin protein 1α (HP1α). The release from HP1α results in de-repression of the genes which are otherwise suppressed by heterochromatin-formation. When a constitutively-active form of Jak2 (V617F) was expressed in mESCs, they showed self-renewal without any growth factors in serum-free N2B27 medium, independent of Stat3 and PI3K-Akt pathways, with increased levels of H3Y41-phosphorylation and decreased HP1α-recruitment to the chromatin.71 Nanog is activated by epigenetic modification of the promoter, which might occur via the Jak2-mediated HP1α-release mentioned above, as suggested by its requirement for the factor-independency downstream of constitutively active Jak2-V617F.71 Jak2-mediated HP1α-release that supports self-renewal might work in parallel with the canonical Jak-Stat3 pathway in physiological context but is not sufficient to compensate the loss of Stat3, since Stat3-null mESCs cannot be established in FCS/LIF-culture.
MAPK pathway
MAPK pathway, with its negative impact on self-renewal as mentioned earlier, could be a negative regulator of the pluripotency-associated transcription factor network. Overexpression of either Nanog or Tfcp2l1 that cancels the requirement of MEKi in serum-free culture supports this idea.60,65 Expression of Nanog is repressed by a tyrosine phosphatase-inhibitor and is restored by MEKi, suggesting the negative transcriptional regulation of Nanog by the MAPK pathway.72 In addition, while Nanog protein expression is heterogeneous in mESCs in FCS/LIF-culture, Nanog is up-regulated with decreased heterogeneity in Erk2-null mESCs.73 Also it was recently reported that Nanog is directly phosphorylated and destabilized by Erk1.74,75 These data suggested that Nanog could be a functional target that is upregulated by one of the components of the 2i-culture, MEKi.
However, since Nanog-null mESCs can be maintained in 2i-culture,76 it seems not to be a sole target of MAPK-inhibition. Tbx3 should also be a possible target that is negatively regulated by the MAPK pathway, because its heterogeneous expression becomes homogeneous in FCS/LIF-culture by the addition of MEKi,37 and also because it is up-regulated and expressed homogeneously in Erk2-null mESCs.73 Klf2 could also be a target that is negatively regulated by the MAPK pathway. Klf2 protein is destabilized by direct phosphorylation by Erk2.77 While Klf2-null mESCs cannot self-renew in 2i-culture, over-expression of Klf2 allows it.63 Moreover, the overexpression of Klf2 cancels the requirement of MEKi in 2i-culture,68 which means as long as Klf2 is stablely expressed against the distabilization by Erk2, inhibition of the MAPK-pathway is no longer required for the maintenance of pluripotency in mESCs. Taken together, MAPK pathway exhibits negative impacts on the self-renewal of mESCs through inhibition of the transcription factor network at both transcription- and protein stability/localization-levels.
PI3K-Akt pathway
Pharmacological inhibition of PI3K results in the loss of self-renewability with repression of Nanog, which is rescued by transgenic expression of Nanog, suggesting the role of Nanog under PI3K.78 Global gene expression profiling identified genes repressed after the inhibition of PI3K, which include Nanog, Esrrb, Tbx3 and Tcl1.61 These transcription factors are known as the downstream targets of LIF-signal and they might have higher dependency on PI3K-Akt pathway although they may also have parallel regulations by the other 2 pathways.
Zscan4c, a member of the zinc finger and SCAN domain containing 4, is also identified as a putative PI3K target.61 In vivo, Zscan4 and its related genes are expressed specifically at the 2-cell stage in mouse embryos, while in vitro they are expressed in a minor population (∼5%) of mESCs.79 Interestingly, its function is essential for continuous self-renewal of mESCs and its knock-down causes crisis of mESCs after 1 month of continuous culture due to genomic instability.80 Zscan4c interacts with the machinery that mediates homologous recombination and facilitates telomere elongation independent of canonical telomerase activity.80 Nr0b1/Dax1 is a negative regulator of Zscan4c, and Nr0b1-null mESCs show increased population of Zscan4c-positive cells accompanied by higher incidence of cell death, suggesting the importance of the repression of Zscan4c for proper self-renewal in mESCs.81 Since Nr0b1 is a target of Stat3,63 Zscan4c is regulated by multiple pathways under the LIF-signal.
Interaction of LIF-signal with other signals
LIF-signal shows multiple interactions with other signals. This is partly because the intracellular signaling pathways downstream of LIF are not exclusively regulated by LIF but shared by others. Due to these interactions, LIF and other signals show synergistic effects.
Wnt signal
2i-culture supports robust self-renewal of mESCs. One of the components of 2i, the MEKi PD0325901, suppresses the differentiation of mESCs but does not support proliferation.15,82 The other component, Gsk3-inhibitor (Gsk3i) CHIR99021, restores the proliferation of mESCs in the presence of MEKi. Gsk3, at the downstream of canonical Wnt-signal, destabilizes β-catenin. Activated Wnt-signal suppresses the Gsk3-activity, which prevents the destabilization of β-catenin. Thus GSK3i mimics the activation of Wnt, which stabilizes β-catenin. Indeed, 2i-culture does not support the self-renewal of β-catenin-null mESCs,83 and ectopic expression of β-catenin recapitulates the effect of Gsk3i on the self-renewal of mESCs in 2i-culture.84–86 These lines of evidence further suggest Gsk3i functions via stabilization of β-catenin downstream of canonical Wnt-signal.
The role of Wnt-signal on the self-renewal of mESCs was first suggested by the analysis using the Gsk3i BIO. In this experiment, BIO was able to replace the requirement of LIF in FCS/LIF-culture.87 However, it might be an artifact due to the broad spectrum of this inhibitor on many kinases. Either the addition of recombinant Wnt3a or artificial activation of β-catenin could not replace the requirement of LIF in FCS/LIF-culture although they had synergistic effect with a sub-threshold level of LIF.88 CHIR99021 is highly specific to Gsk3 and it alone is not sufficient to support the self-renewal of mESCs in serum-free culture.15 Moreover, the addition of BIO in FCS culture causes moderate activation of Stat3,88 suggesting that Wnt-signal alone is not sufficient to support self-renewal of mESCs.
How does Wnt-signal show synergistic effects with LIF-signal to support the self-renewal of mESCs? Inhibition of the Gsk3-activity stabilizes β-catenin. One of the functions of β-catenin is to translocate into the nuclei and repress the Tcf3 repressor.89–91 As a consequence of Gsk3-inhibition, the repressive function of the Tcf3 repressor becomes abrogated, and the downstream target genes of Tcf3, including Nanog, Tfcp2l1, Esrrb, Klf2 and Nr0b1,76 becomes active. Overexpression of Tcf3 causes the differentiation of mESCs,92 whereas Tcf3-null mESCs stably self-renew with increased expressions of pluripotency-associated genes.93 Invention of the 2i-culture tells us that mESCs are able to self-renew by simply blocking 2 pathways with MAPKi and Gsk3i. Why Gsk3i that up-regulates Tcf3-targeted genes has such an impact? Gain- and loss-of-function studies showed that among the target genes of Tcf3, Esrrb is necessary and sufficient to mediate the effects of Gsk3i: i.e. with attenuated expression of Esrrb, the response to Gsk3i was eliminated, and by transgenic expression of Esrrb, the requirement of GSK3i was canceled in 2i-culture.76 Esrrb is also a target gene of the LIF-signal. It could be speculated that Esrrb, at the crossroad of LIF- and Wnt-signals, might mediate the synergistic action of the 2 pathways.
Gsk3 also destabilizes c-myc at the downstream of LIF in mESCs.67 Gsk3 phosphorylates c-myc at the threonine 58 residue, leading to its rapid degradation. Expression of phosphorylation-resistant form of c-myc (T58A) replaces the requirement of LIF in FCS/LIF-culture. Although it is still unclear how Gsk3 is regulated by the LIF-signal, there is one suggestive report showing that prolonged inhibition of PI3K results in reduced phosphorylation of β-catenin.94
Fgf signal
It is known that mESCs secrete fibroblast growth factor 4 (Fgf4) that promotes differentiation.50 Downstream of the Fgf-signal, MAPK pathway is the major pathway. Although to a lesser extent than the Fgf-signal, LIF-signal also activates the MAPK pathway. This was shown by Fgf4-null mESCs50: stimulation by LIF increased Erk1/2 phosphorylation however in a lower magnitude compared to wild-type mESCs. In serum-free culture, addition of Fgf-inhibitor enhances the effect of MEKi but cannot replace it, which again suggests the existence of multiple inputs into the MAPK pathway.15 Fgf signal may also contribute to the activation of PI3K-Akt pathway95 but whether that is the case in mESCs is unclear.
BMP signal
Bone morphogenetic protein (BMP) is implicated in the self-renewal of mESCs in concert with LIF in serum-free culture.48 Canonical BMP signal is mediated by Smad1/5/8 with Smad4 and induces the expression of Id (inhibitor of differentiation) gene.96,97 Overexpression of Id replaces the function of BMP but not LIF, which suggests LIF- and BMP-signals have, at least partially, different target genes.48 The identification of Dusp9 explains how BMP supports the self-renewal of mESCs.98 Dusp9 is one of the target genes downstream of BMP-signal, and encodes the Erk-specific dual-specificity phosphatase that dephosphorylates Erk1/2 and represses the Erk1/2-activity. BMP4 up-regulates Dusp9 via canonical Smad pathway, resulting in the repression of Erk-activity. Thus as well as MEKi together with LIF,60 BMP together with LIF also supports the self-renewal of mESCs in serum-free culture.47
An alternative pathway that mediates the synergy of BMP-signal with LIF-signal was reported. In non-canonical BMP-signaling pathway, BMP suppresses Erk1/2 and p38 MAPK pathways simultaneously,99 which could confer its synergistic action with LIF. Alk3 encodes a component of BMP receptor and Alk3-null mESCs were unable to be established from Alk3-null blastocysts in FCS/LIF-culture but the inhibition of p38 MAPK by a specific inhibitor enabled it.99 This suggests a negative role of the p38MAPK-pathway that destabilize the self-renewability of mESCs as well as the canonical MAPK pathway, which are both suppressed downstream of BMP.
E-cadherin
The characteristic compact colonies of mESCs are formed by homophilic adhesion of E-cadherin at the cell surface. Genetic ablation of E-cadherin in mESCs converts them to an epiblast stem cell (EpiSC)-like state, suggesting the requirement of E-cadherin to support naïve pluripotent state (see below).100–102 E-cadherin-coated culture surface instead of gelatin reduces the required dose of LIF to support self-renewal.103,104 Interestingly, E-cadherin is shown to interact directly with Lifr and Gp130 at the cell membrane of mESCs, and its over-expression reduces the requirement of LIF for self-renewal, which led to the speculation that E-cadherin might enhance LIF-signaling by stabilizing the receptor complex at the membrane.105 It is also possible that E-cadherin-mediated signal may affect the intra-cellular LIF-signaling pathway, or it may interfere with the Wnt-signal by competing for β-catenin, although the existence of such indirect interactions between E-cadherin and LIF-signal is still unknown.
Roles of the LIF-signaling in development
Since mESCs are derived from the ICM of the blastocyst-stage embryos, the requirement of LIF in mESCs could reflect the character of the pluripotent stem cells in vivo. Recent comprehensive transcriptome analysis revealed that mESCs show closest similarity to epiblast cells in the late blastocyst-stage embryos at E4.5.106 LIF-signal components Lif, Lifr and Gp130 are not transcribed at detectable level in 1- or 2-cell stage embryos but become detectable in the blastocysts.107 In the blastocysts, LIF-components show complementary expression patterns: Lifr and Gp130 in the ICM, and Lif in the trophectoderm, suggesting the function of LIF as a paracrine factor to support pluripotency of the ICM cells.107 However, Lif, Lifr and Gp130-null embryos are apparently normal at least until the mid-gestation stage,108–110 suggesting that LIF-signal is not exclusively necessary for the maintenance of pluripotent cells in normal development. Alternatively, it is possible that there may be compensatory effects by other related cytokines such as OSM, CT-1 and CNTF.
This discrepancy between embryos and mESCs was first explained in a particular context. It is known that embryonic diapause occurs when implantation is perturbed in mice, and blastocysts become dormant in the uteri. In these dormant blastocysts, the condition of pluripotent stem cells are sustained, as shown by the ability to undergo normal development when their implantation is allowed and also by the establishment of mESCs from them.11 When Gp130-null blastocysts were kept in diapause, they survived but were unable to resume development due to increased number of apoptotic cells, suggesting the role of LIF-signal to sustain the pluripotent cell-population during diapause. Embryonic diapause is observed in almost 100 mammals in 7 different mammalian orders and might have been evolved independently in many occasions,111 so the function of LIF-signal in embryonic diapause might be a unique system evolutionally acquired in rodents.
The puzzle was solved by the recent revisit to Stat3-null embryos. Pioneer study showed that Stat3-null embryos develop to the egg-cylinder stage (E6.0) then undergo a rapid degeneration,112 indicating that the function of Stat3 is unnecessary to maintain the pluripotent cell-population during pre-implantation development. However, it did not rule out the possibility that the maternally expressed Stat3 compensates the loss of zygotic Stat3. Do et al113 addressed this point and found that maternally expressed Stat3 protein was presented in the oocytes, and ablation of both maternal and zygotic Stat3 resulted in the loss of epiblasts and primitive endodermal cells in the late blastocysts. This result clearly indicates that Stat3 is essential to maintain the pluripotent cell-population during pre-implantation development, and maternal Stat3 is sufficient to support it.113 They also revealed that in Lif-null blastocysts, active form of Stat3 with phosphorylation at the Y705 residue was decreased, although not completely eliminated, suggesting the involvement of LIF-signal in activating Stat3.113 These data clarified that LIF-signal is implicated in the maintenance of the pluripotent cell-population in mouse pre-implantation embryos in physiological context.
Impact of the genetic background
The mESCs so far we described are derived from the 129 mouse strain. This particular inbred strain is preferred because the derivative mESCs can stably be cultured in conventional FCS/LIF-culture. The efforts to establish mESCs from other genetic backgrounds resulted in 2 categories: permissive strains that allow the establishment of mESCs in conventional FCS/LIF-culture, and non-permissive strains that do not allow it. 129 is an exceptional strain that delivers mESCs with robust self-renewability, full pluripotency, the capability of giving rise to germ-line chimeras, and the endurance for long-term culture as well as gene manipulation. In contrast, non-obese diabetic (NOD) mouse strain, a model of type-I diabetes, did not allow the establishment of mESCs in conventional FCS/LIF-culture. Since the addition of a huge amount of recombinant LIF could not solve this problem, it seemed like either the receptor or the downstream pathway had differences, but still why the responsiveness to LIF-signal differed among the genetic backgrounds was unknown.
Serum-free 2i-culture changed the paradigm. This culture allows the establishment of mESCs from any genetic backgrounds.114,115 It even allowed the establishment of ESCs from rats,116,117 which was impossible in conventional FCS/LIF-culture.118 Establishment of mESCs from both permissive and non-permissive strains using 2i-culture made it possible to compare their LIF-responsiveness. mESCs established in 2i-culture showed distinct characters depending on their genetic backgrounds when transferred into FCS/LIF-culture: only mESCs with permissive genetic backgrounds continued self-renewal, indicating the inherited capability of maintenaning pluripotency in FCS/LIF-culture. When the mode of LIF-signal integration was assessed, there were quantitative differences in the activation of the intracellular signal pathways downstream of LIF115(Fig. 2). In permissive strains such as 129, Jak-Stat3 pathway was strongly activated and the activation of MAPK pathway was moderate. In contrast, in non-permissive strains such as NOD, MAPK pathway was highly activated whereas the activation of Jak-Stat3 pathway was weaker than that in permissive strains. As mentioned earlier, Jak-Stat3 pathway is obligatory to maintain pluripotency whereas MAPK pathway counteracts it, and the differential balances of the quantitative activation of these pathways fit to the genetic background-dependent permissiveness. Artificial activation of Stat3 partially restored the self-renewability in mESCs derived from non-permissive strains in FCS/LIF-culture, suggesting that the weakness in the activation of obligatory Jak-Stat3 pathway partly confers the non-permissiveness.
Figure 2.
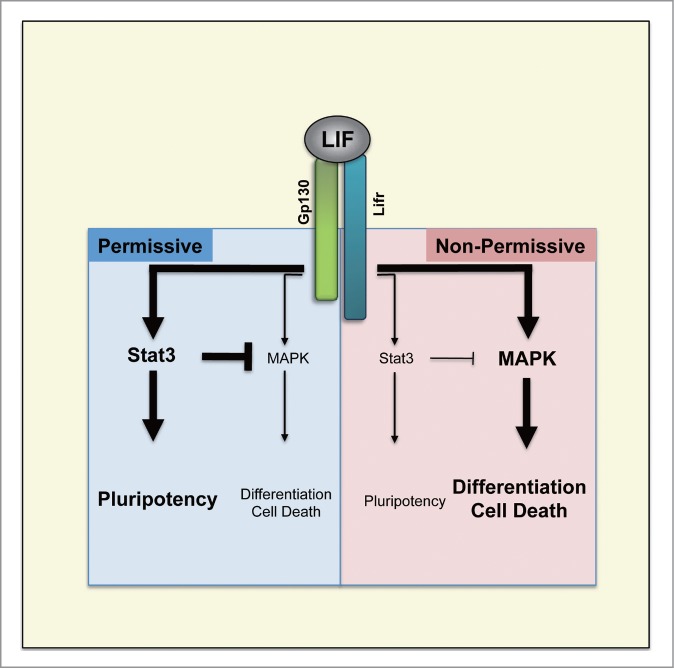
Comparison of LIF intra-cellular signaling activity in mESCs derived from permissive and non-permissive genetic backgrounds. Permissiveness for derivation and maintenance of mESCs is determined by the balance between Stat3 and MAPK pathways downstream of LIF.
In FCS/LIF-culture, it is unable to establish ESCs from any mammals except for mouse. Human ESCs are established and maintained in a different culture condition without LIF (see below). Even by the application of 2i-culture, the problem has not been solved except in the case of rat. Interestingly, the quantitative balance of Jak-Stat3 and MAPK pathways in rat ESCs was similar to that in non-permissive mESCs,115 suggesting that the genetic regulation of the components downstream of LIF might be involved in the different responsiveness to LIF-signal in different mammalian species.
The function of LIF in distinct pluripotent state
Human ESCs were established in the culture containing Activin A and Fgf2 with MEF-feeder cells without LIF.119 The difference in the factor requirements between human and mouse ESCs might reflect the different mechanisms to maintain pluripotency among species. However, establishment of mouse EpiSCs gave different interpretation. Mouse EpiSCs were established from post-implantation embryos around E6.0 and were maintained in the culture containing Activin A and Fgf2 with MEF-feeder cells without LIF as human ESCs,120,121 suggesting that the different factor requirements of human and mouse ESCs may be due to the difference in the developmental stages rather than the difference in the species. Indeed, in addition to the factor requirement, there are several similarities between human ESCs and mouse EpiSCs, which are distinct from mESCs.120,121 Based on these observations, it was proposed that there are 2 types of pluripotent stem cells, designated as naïve and primed states, which resembles the characteristics of pluripotent stem cells at early and late developmental stages, respectively.122,123 The comparison between these pluripotent stem cells identified several genes specifically expressed in mESCs at naïve state but not at primed state. These naïve state-specific genes include Klf2, Klf4, Klf5, Tbx3, Esrrb and Nr0b1 that are known as transcription factors regulated downstream of LIF. These findings suggest that LIF-signal may function particularly to maintain the naïve state of pluripotency.
Mouse EpiSCs are gradually reprogrammed into naïve state when they are maintained in FSC/LIF-culture.124 When they are transferred into 2i-culture, they cannot proliferate. However transgenic expressions of Nanog, Klf2, Klf4, Tbx3 or Esrrb allow their propagation in 2i-culture.125–127 Artificial activation of LIF-signal with the chimeric receptor or Stat3ER is also sufficient to reprogram EpiSCs into the naïve state, indicating that LIF-signal is instructive to establish and maintain naïve pluripotency. Recently, establishment of naïve human pluripotent stem cells were reported from several groups.128–133 Interestingly, most of them (5 out of 6, except for Ware et al131) used the combination of MEKi and human-LIF. Chen et al134 recently reported that reinforcement of Stat3 in human ESCs that are naturally at primed state, promoted their reprogramming into naïve-like state. These findings suggested that the function of LIF-signal to maintain naïve pluripotency might be conserved across mammals but just the balance between Stat3 versus MAPK pathways downstream of LIF is not ideal in the species except for permissive mouse strains. As in the case of non-permissive mouse strains, most of the mammalian species (except for permissive mouse strains) may have dominant MAPK pathway downstream of LIF. And thus supplementation of MEKi125–127,129,130 or forced activation of Stat3131 may have enabled naïve pluripotency in human ESCs.
CLOSING REMARKS
Here we described the molecular mechanisms how LIF-signal functions to maintain pluripotency. Since most of the findings were obtained using mESCs derived from 129, the most dominant strain for LIF-responsiveness, the interpretation for now may not be generally applicable. However, recent results suggested that the function of LIF-signal for the maintenance of naïve pluripotent stem cells is shared among the species, at least to some degree. Further analysis on the mechanisms of genetic differences downstream of LIF should open the door for ubiquitous establishment of naïve pluripotency.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
FUNDING
This project was supported by a RIKEN grant to H.N.
References
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981; 292:154-6; PMID:7242681; http://dx.doi.org/ 10.1038/292154a0 [DOI] [PubMed] [Google Scholar]
- 2.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A 1981; 78:7634-8; PMID:6950406; http://dx.doi.org/ 10.1073/pnas.78.12.7634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith AG, Hooper ML. Buffalo rat liver cells produce a diffusible activity which inhibits the differentiation of murine embryonal carcinoma and embryonic stem cells. Dev Biol 1987; 121:1-9; PMID:3569655; http://dx.doi.org/ 10.1016/0012-1606(87)90132-1 [DOI] [PubMed] [Google Scholar]
- 4.Moreau JF, Donaldson DD, Bennett F, Witek-Giannotti J, Clark SC, Wong GG. Leukaemia inhibitory factor is identical to the myeloid growth factor human interleukin for DA cells. Nature 1988; 336:690-2; PMID:3143918; http://dx.doi.org/ 10.1038/336690a0 [DOI] [PubMed] [Google Scholar]
- 5.Hilton DJ, Nicola NA, Gough NM, Metcalf D. Resolution and purification of three distinct factors produced by Krebs ascites cells which have differentiation-inducing activity on murine myeloid leukemic cell lines. J Biol Chem 1988; 263:9238-43; PMID:2837482 [PubMed] [Google Scholar]
- 6.Godard A, Gascan H, Naulet J, Peyrat MA, Jacques Y, Soulillou JP, Moreau JF. Biochemical characterization and purification of HILDA, a human lymphokine active on eosinophils and bone marrow cells. Blood 1988; 71:1618-23; PMID:3130906 [PubMed] [Google Scholar]
- 7.Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 1988; 336:684-7; PMID:3143916; http://dx.doi.org/ 10.1038/336684a0 [DOI] [PubMed] [Google Scholar]
- 8.Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature 1988; 336:688-90; PMID:3143917; http://dx.doi.org/ 10.1038/336688a0 [DOI] [PubMed] [Google Scholar]
- 9.Batlle-Morera L, Smith A, Nichols J. Parameters influencing derivation of embryonic stem cells from murine embryos. Genesis 2008; 46:758-67; PMID:18837461; http://dx.doi.org/ 10.1002/dvg.20442 [DOI] [PubMed] [Google Scholar]
- 10.Brook FA, Evans EP, Lord CJ, Lyons PA, Rainbow DB, Howlett SK, Wicker LS, Todd JA, Gardner RL. The derivation of highly germline-competent embryonic stem cells containing NOD-derived genome. Diabetes 2003; 52:205-8; PMID:12502514; http://dx.doi.org/ 10.2337/diabetes.52.1.205 [DOI] [PubMed] [Google Scholar]
- 11.Brook FA, Gardner RL. The origin and efficient derivation of embryonic stem cells in the mouse. Proc Natl Acad Sci U S A 1997; 94:5709-12; PMID:9159137; http://dx.doi.org/ 10.1073/pnas.94.11.5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawase E, Suemori H, Takahashi N, Okazaki K, Hashimoto K, Nakatsuji N. Strain difference in establishment of mouse embryonic stem (ES) cell lines. Int J Dev Biol 1994; 38:385-90; PMID:7981049 [PubMed] [Google Scholar]
- 13.Nagafuchi S, Katsuta H, Kogawa K, Akashi T, Kondo S, Sakai Y, Tsukiyama T, Kitamura D, Niho Y, Watanabe T. Establishment of an embryonic stem (ES) cell line derived from a non-obese diabetic (NOD) mouse: in vivo differentiation into lymphocytes and potential for germ line transmission. FEBS Lett 1999; 455:101-4; PMID:10428481; http://dx.doi.org/ 10.1016/S0014-5793(99)00801-7 [DOI] [PubMed] [Google Scholar]
- 14.Cinelli P, Casanova EA, Uhlig S, Lochmatter P, Matsuda T, Yokota T, Rülicke T, Ledermann B, Bürki K. Expression profiling in transgenic FVB/N embryonic stem cells overexpressing STAT3. BMC Dev Biol 2008; 8:57; PMID:18500982; http://dx.doi.org/ 10.1186/1471-213X-8-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ying Q-L, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature 2008; 453:519-23; PMID:18497825; http://dx.doi.org/ 10.1038/nature06968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Y, Tian XC. JAK-STAT3 and somatic cell reprogramming. Jak-Stat 2013; 2:1-10; http://dx.doi.org/ 10.4161/jkst.24935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirai H, Karian P, Kikyo N. Regulation of embryonic stem cell self-renewal and pluripotency by leukaemia inhibitory factor. Biochem J 2011; 438:11-23; PMID:21793804; http://dx.doi.org/ 10.1042/BJ20102152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eulenfeld R, Dittrich A, Khouri C, Müller PJ, Mütze B, Wolf A, Schaper F. Interleukin-6 signalling: more than Jaks and STATs. Eur J Cell Biol 2012; 91:486-95; PMID:22138086; http://dx.doi.org/ 10.1016/j.ejcb.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 19.Gearing DP, Gough NM, King JA, Hilton DJ, Nicola NA, Simpson RJ, Nice EC, Kelso A, Metcalf D. Molecular cloning and expression of cDNA encoding a murine myeloid leukaemia inhibitory factor (LIF). EMBO J 1987; 6:3995-4002; PMID:3127201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirano T, Nakajima K, Hibi M. Signaling mechanisms through gp130: A model of the cytokine system. Cytokine & Growth Factor Reviews 1997; 8:241-52; PMID:9620640; http://dx.doi.org/ 10.1016/S1359-6101(98)80005-1 [DOI] [PubMed] [Google Scholar]
- 21.Yasukawa H, Misawa H, Sakamoto H, Masuhara M, Sasaki A, Wakioka T, Ohtsuka S, Imaizumi T, Matsuda T, Ihle JN, et al.. The JAK-binding protein JAB inhibits Janus tyrosine kinase activity through binding in the activation loop. EMBO J 1999; 18:1309-20; PMID:10064597; http://dx.doi.org/ 10.1093/emboj/18.5.1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stahl N, Farruggella TJ, Boulton TG, Zhong Z, Darnell JE, Yancopoulos GD. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science 1995; 267:1349-53; PMID:7871433; http://dx.doi.org/ 10.1126/science.7871433 [DOI] [PubMed] [Google Scholar]
- 23.Wegenka UM, Buschmann J, Lütticken C, Heinrich PC, Horn F. Acute-phase response factor, a nuclear factor binding to acute-phase response elements, is rapidly activated by interleukin-6 at the posttranslational level. Mol Cell Biol 1993; 13:276-88; PMID:7678052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukada T, Hibi M, Yamanaka Y, Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, Nakajima K, Hirano T. Two Signals Are Necessary for Cell Proliferation Induced by a Cytokine Receptor gp130: Involvement of STAT3 in Anti-Apoptosis. Immunity 1996; 5:449-60; PMID:8934572; http://dx.doi.org/ 10.1016/S1074-7613(00)80501-4 [DOI] [PubMed] [Google Scholar]
- 25.Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, et al.. A new protein containing an SH2 domain that inhibits JAK kinases. Nature 1997; 387:921-4; PMID:9202126; http://dx.doi.org/ 10.1038/43213 [DOI] [PubMed] [Google Scholar]
- 26.Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, et al.. A family of cytokine-inducible inhibitors of signalling. Nature 1997; 387:917-21; PMID:9202125; http://dx.doi.org/ 10.1038/43206 [DOI] [PubMed] [Google Scholar]
- 27.Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, et al.. Structure and function of a new STAT-induced STAT inhibitor. Nature 1997; 387:924-9; PMID:9202127; http://dx.doi.org/ 10.1038/43219 [DOI] [PubMed] [Google Scholar]
- 28.Sasaki A, Yasukawa H, Suzuki A, Kamizono S, Syoda T, Kinjyo I, Sasaki M, Johnston JA, Yoshimura A. Cytokine-inducible SH2 protein-3 (CIS3/SOCS3) inhibits Janus tyrosine kinase by binding through the N-terminal kinase inhibitory region as well as SH2 domain. Genes to Cells 1999; 4:339-51; PMID:10421843; http://dx.doi.org/ 10.1046/j.1365-2443.1999.00263.x [DOI] [PubMed] [Google Scholar]
- 29.Chung CD, Liao J, Liu B, Rao X, Jay P, Berta P, Shuai K. Specific inhibition of Stat3 signal transduction by PIAS3. Science 1997; 278:1803-5; PMID:9388184; http://dx.doi.org/ 10.1126/science.278.5344.1803 [DOI] [PubMed] [Google Scholar]
- 30.Conover JC, Ip NY, Poueymirou WT, Bates B, Goldfarb MP, DeChiara TM, Yancopoulos GD. Ciliary neurotrophic factor maintains the pluripotentiality of embryonic stem cells. Development 1993; 119:559-65; PMID:8187629 [DOI] [PubMed] [Google Scholar]
- 31.Pennica D, Shaw KJ, Swanson TA, Moore MW, Shelton DL, Zioncheck KA, Rosenthal A, Taga T, Paoni NF, Wood WI. Cardiotrophin-1: Biological activities and binding to the leukemia inhibitory factor receptor/gp130 signaling complex. J Biol Chem 1995; 270:10915-22; PMID:7738033; http://dx.doi.org/ 10.1074/jbc.270.18.10915 [DOI] [PubMed] [Google Scholar]
- 32.Rose TM, Weiford DM, Gunderson NL, Bruce AG. Oncostatin M (OSM) inhibits the differentiation of pluripotent embryonic stem cells in vitro. Cytokine 1994; 6:48-54; PMID:8003633; http://dx.doi.org/ 10.1016/1043-4666(94)90007-8 [DOI] [PubMed] [Google Scholar]
- 33.Yoshida K, Chambers I, Nichols J, Smith A, Saito M, Yasukawa K, Shoyab M, Taga T, Kishimoto T. Maintenance of the pluripotential phenotype of embryonic stem cells through direct activation of gp130 signalling pathways. Mech Dev 1994; 45:163-71; PMID:8199053; http://dx.doi.org/ 10.1016/0925-4773(94)90030-2 [DOI] [PubMed] [Google Scholar]
- 34.Nichols J, Chambers I, Smith A. Derivation of germline competent embryonic stem cells with a combination of interleukin-6 and soluble interleukin-6 receptor. Exp Cell Res 1994; 215:237-9; PMID:7957676; http://dx.doi.org/ 10.1006/excr.1994.1338 [DOI] [PubMed] [Google Scholar]
- 35.Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev 1998; 12:2048-60; PMID:9649508; http://dx.doi.org/ 10.1101/gad.12.13.2048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starr R, Novak U, Willson TA, Inglese M, Murphy V, Alexander WS, Metcalf D, Nicola NA, Hilton DJ, Ernst M. Distinct roles for leukemia inhibitory factor receptor alpha-chain and gp130 in cell type-specific signal transduction. J Biol Chem 1997; 272:19982-6; PMID:9242667; http://dx.doi.org/ 10.1074/jbc.272.32.19982 [DOI] [PubMed] [Google Scholar]
- 37.Niwa H, Ogawa K, Shimosato D, Adachi K. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature 2009; 460:118-22; PMID:19571885; http://dx.doi.org/ 10.1038/nature08113 [DOI] [PubMed] [Google Scholar]
- 38.Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, Yokota T. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J 1999; 18:4261-9; PMID:10428964; http://dx.doi.org/ 10.1093/emboj/18.15.4261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene 2000; 19:2628-37; PMID:10851062; http://dx.doi.org/ 10.1038/sj.onc.1203481 [DOI] [PubMed] [Google Scholar]
- 40.Jain N, Zhang T, Kee WH. Protein Kinase C δ Associates with and Phosphorylates Stat3 in an Protein Kinase C δ Associates with and Phosphorylates Stat3 in an Interleukin-6-dependent Manner. J Biol Chem 1999; 274:24392-400; PMID:10446219; http://dx.doi.org/ 10.1074/jbc.274.34.24392 [DOI] [PubMed] [Google Scholar]
- 41.Schuringa JJ, Jonk LJ, Dokter WH, Vellenga E, Kruijer W. Interleukin-6-induced STAT3 transactivation and Ser727 phosphorylation involves Vav, Rac-1 and the kinase SEK-1/MKK-4 as signal transduction components. Biochem J; Internet 2000; cited 2015 Aug 6; 347 Pt 1:89-96. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1220935&tool=pmcentrez&rendertype=abstract; PMID:10727406; http://dx.doi.org/ 10.1042/bj3470089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang G, Yan H, Ye S, Tong C, Ying QL. STAT3 phosphorylation at tyrosine 705 and serine 727 differentially regulates mouse esc fates. Stem Cells 2014; 32:1149-60; PMID:24302476; http://dx.doi.org/ 10.1002/stem.1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyle K, Zhang J-G, Nicholson SE, Trounson E, Babon JJ, McManus EJ, Nicola NA, Robb L. Deletion of the SOCS box of suppressor of cytokine signaling 3 (SOCS3) in embryonic stem cells reveals SOCS box-dependent regulation of JAK but not STAT phosphorylation. Cell Signall 2009; 21:394-404; PMID:19056487; http://dx.doi.org/ 10.1016/j.cellsig.2008.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forrai A, Boyle K, Hart AH, Hartley L, Rakar S, Willson TA, Simpson KM, Roberts AW, Alexander WS, Voss AK, et al.. Absence of suppressor of cytokine signalling 3 reduces self-renewal and promotes differentiation in murine embryonic stem cells. Stem Cells 2006; 24:604-14; PMID:16123385; http://dx.doi.org/ 10.1634/stemcells.2005-0323 [DOI] [PubMed] [Google Scholar]
- 45.Davey RE, Onishi K, Mahdavi A, Zandstra PW. LIF-mediated control of embryonic stem cell self-renewal emerges due to an autoregulatory loop. FASEB J 2007; 21:2020-32; PMID:17356004; http://dx.doi.org/ 10.1096/fj.06-7852com [DOI] [PubMed] [Google Scholar]
- 46.He F, Ge W, Martinowich K, Becker-Catania S, Coskun V, Zhu W, Wu H, Castro D, Guillemot F, Fan G, et al.. A positive autoregulatory loop of Jak-STAT signaling controls the onset of astrogliogenesis. Nat Neurosci 2005; 8:616-25; PMID:15852015; http://dx.doi.org/ 10.1038/nn1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burdon T, Stracey C, Chambers I, Nichols J, Smith A. Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev Biol 1999; 210:30-43; PMID:10364425; http://dx.doi.org/ 10.1006/dbio.1999.9265 [DOI] [PubMed] [Google Scholar]
- 48.Ying Q-LL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 2003; 115:281-92; PMID:14636556; http://dx.doi.org/ 10.1016/S0092-8674(03)00847-X [DOI] [PubMed] [Google Scholar]
- 49.Cheng AM, Saxton TM, Sakai R, Kulkarni S, Mbamalu G, Vogel W, Tortorice CG, Cardiff RD, Cross JC, Muller WJ, et al.. Mammalian Grb2 Regulates Multiple Steps in Embryonic Development and Malignant Transformation. Cell 1998; 95:793-803; PMID:9865697; http://dx.doi.org/ 10.1016/S0092-8674(00)81702-X [DOI] [PubMed] [Google Scholar]
- 50.Kunath T, Saba-El-Leil MK, Almousailleakh M, Wray J, Meloche S, Smith A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development 2007; 134:2895-902; PMID:17660198; http://dx.doi.org/ 10.1242/dev.02880 [DOI] [PubMed] [Google Scholar]
- 51.Welham MJ, Storm MP, Kingham E, Bone HK. Phosphoinositide 3-kinases and regulation of embryonic stem cell fate. Biochem Soc Trans 2007; 35:225-8; PMID:17371244; http://dx.doi.org/ 10.1042/BST0350225 [DOI] [PubMed] [Google Scholar]
- 52.Murakami M, Ichisaka T. mTOR is essential for growth and proliferation in early mouse embryos and embryonic stem cells. Mol Cell Biol 2004; 24:6710-8; PMID:15254238; http://dx.doi.org/ 10.1128/MCB.24.15.6710-6718.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi K, Mitsui K, Yamanaka S. Role of ERas in promoting tumour-like properties in mouse embryonic stem cells. 2003; 423:541-5; PMID:12774123 [DOI] [PubMed] [Google Scholar]
- 54.Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J, Gavrilova N, Mueller B, Liu X, Wu H. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci U S A 1999; 96:6199-204; PMID:10339565; http://dx.doi.org/ 10.1073/pnas.96.11.6199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, Nakano T. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene 2006; 25:2697-707; PMID:16407845; http://dx.doi.org/ 10.1038/sj.onc.1209307 [DOI] [PubMed] [Google Scholar]
- 56.Akira S, Nishio Y, Inoue M, Wang X-J, We S, Matsusaka T, Yoshida K, Sudo T, Naruto M, Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell 1994; 77:63-71; PMID:7512451; http://dx.doi.org/ 10.1016/0092-8674(94)90235-6 [DOI] [PubMed] [Google Scholar]
- 57.Minami M, Inoue M, Wei S, Takeda K, Matsumoto M, Kishimoto T, Akira S. STAT3 activation is a critical step in gp130-mediated terminal differentiation and growth arrest of a myeloid cell line. Proc Natl Acad Sci U S A 1996; 93:3963-6; PMID:8632998; http://dx.doi.org/ 10.1073/pnas.93.9.3963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakajima K, Yamanaka Y, Nakae K, Kojima H, Ichiba M, Kiuchi N, Kitaoka T, Fukada T, Hibi M, Hirano T. A central role for Stat3 in IL-6-induced regulation of growth and differentiation in M1 leukemia cells. EMBO J 1996; 15:3651-8; PMID:8670868 [PMC free article] [PubMed] [Google Scholar]
- 59.Bourillot PY, Aksoy I, Schreiber V, Wianny F, Schulz H, Hummel O, Hubner N, Savatier P. Novel STAT3 target genes exert distinct roles in the inhibition of mesoderm and endoderm differentiation in cooperation with Nanog. Stem Cells 2009; 27:1760-71; PMID:19544440; http://dx.doi.org/ 10.1002/stem.110 [DOI] [PubMed] [Google Scholar]
- 60.Martello G, Bertone P, Smith A. Identification of the missing pluripotency mediator downstream of leukaemia inhibitory factor. EMBO J 2013; 32:2561-74; PMID:23942233; http://dx.doi.org/ 10.1038/emboj.2013.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Storm MP, Kumpfmueller B, Thompson B, Kolde R, Vilo J, Hummel O, Schulz H, Welham MJ. Characterization of the phosphoinositide 3-kinase-dependent transcriptome in murine embryonic stem cells: Identification of novel regulators of pluripotency. Stem Cells 2009; 27:764-75; PMID:19350676; http://dx.doi.org/ 10.1002/stem.3 [DOI] [PubMed] [Google Scholar]
- 62.Akagi T, Usuda M, Matsuda T, Ko MSH, Niwa H, Asano M, Koide H, Yokota T. Identification of Zfp-57 as a downstream molecule of STAT3 and Oct-3/4 in embryonic stem cells. Biochem Biophy Res Commun 2005; 331:23-30; PMID:15845352; http://dx.doi.org/ 10.1016/j.bbrc.2005.03.118 [DOI] [PubMed] [Google Scholar]
- 63.Sun C, Nakatake Y, Ura H, Akagi T, Niwa H, Koide H, Yokota T. Stem cell-specific expression of Dax1 is conferred by STAT3 and Oct3/4 in embryonic stem cells. Biochem Biophy Res Commun 2008; 372:91-6; PMID:18471437; http://dx.doi.org/ 10.1016/j.bbrc.2008.04.154 [DOI] [PubMed] [Google Scholar]
- 64.Tai C-I, Ying Q-L. Gbx2, a LIF/Stat3 target, promotes reprogramming to and retention of the pluripotent ground state. - PubMed - NCBI. J Cell Sci 2013; 126:1093-8; PMID:23345404; http://dx.doi.org/ 10.1242/jcs.118273 [DOI] [PubMed] [Google Scholar]
- 65.Ye S, Li P, Tong C, Ying Q-L. Embryonic stem cell self-renewal pathways converge on the transcription factor Tfcp2l1. EMBO J 2013; 32:2548-60; PMID:23942238; http://dx.doi.org/ 10.1038/emboj.2013.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ema M, Mori D, Niwa H, Hasegawa Y, Yamanaka Y, Hitoshi S, Mimura J, Kawabe YI, Hosoya T, Morita M, et al.. Krüppel-like factor 5 Is Essential for Blastocyst Development and the Normal Self-Renewal of Mouse ESCs. Cell Stem Cell 2008; 3:555-67; PMID:18983969; http://dx.doi.org/ 10.1016/j.stem.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 67.Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, Dalton S. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development 2005; 132:885-96; PMID:15673569; http://dx.doi.org/ 10.1242/dev.01670 [DOI] [PubMed] [Google Scholar]
- 68.Ho L, Miller EL, Ronan JL, Ho WQ, Jothi R, Crabtree GR. esBAF facilitates pluripotency by conditioning the genome for LIF/STAT3 signalling and by regulating polycomb function. Nat Cell Biol 2011; 13:903-13; PMID:21785422; http://dx.doi.org/ 10.1038/ncb2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ho L, Jothi R, Ronan JL, Cui K, Zhao K, Crabtree GR. An embryonic stem cell chromatin remodeling complex, esBAF, is an essential component of the core pluripotency transcriptional network. Proc Natl Acad Sci U S A 2009; 106:5187-91; PMID:19279218; http://dx.doi.org/ 10.1073/pnas.0812888106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dawson MA, Bannister AJ, Göttgens B, Foster SD, Bartke T, Green AR, Kouzarides T. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature 2009; 461:819-22; PMID:19783980; http://dx.doi.org/ 10.1038/nature08448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Griffiths DS, Li J, Dawson MA, Trotter MWB, Cheng Y-H, Smith AM, Mansfield W, Liu P, Kouzarides T, Nichols J, et al.. LIF-independent JAK signalling to chromatin in embryonic stem cells uncovered from an adult stem cell disease. Nat Cell Biol 2011; 13:13-21; PMID:21151131; http://dx.doi.org/ 10.1038/ncb2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamazaki T, Kehoe SM, Nakano T, Terada N. The Grb2/Mek pathway represses Nanog in murine embryonic stem cells. Mol Cell Biol 2006; 26:7539-49; PMID:16908534; http://dx.doi.org/ 10.1128/MCB.00508-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hamilton WB, Kaji K, Kunath T. ERK2 Suppresses Self-Renewal Capacity of Embryonic Stem Cells, but Is Not Required for Multi-Lineage Commitment. PLoS ONE 2013; 8:e60907; PMID:23613754; http://dx.doi.org/ 10.1371/journal.pone.0060907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brumbaugh J, Russell JD, Yu P, Westphall MS, Coon JJ, Thomson JA. NANOG is multiply phosphorylated and directly modified by ERK2 and CDK1 in vitro. Stem Cell Rep 2014; 2:18-25; PMID:24678451; http://dx.doi.org/ 10.1016/j.stemcr.2013.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim S-H, Kim MO, Cho Y-Y, Yao K, Kim DJ, Jeong C-H, Yu DH, Bae KB, Cho EJ, Jung SK, et al.. ERK1 phosphorylates Nanog to regulate protein stability and stem cell self-renewal. Stem Cell Res 2014; 13:1-11; PMID:24793005; http://dx.doi.org/ 10.1016/j.scr.2014.04.001 [DOI] [PubMed] [Google Scholar]
- 76.Martello G, Sugimoto T, Diamanti E, Joshi A, Hannah R, Ohtsuka S, Göttgens B, Niwa H, Smith A. Esrrb is a pivotal target of the Gsk3/Tcf3 axis regulating embryonic stem cell self-renewal. Cell Stem Cell 2012; 11:491-504; PMID:23040478; http://dx.doi.org/ 10.1016/j.stem.2012.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yeo J-CC, Jiang J, Tan Z-YY, Yim G-RR, Ng J-HH, Göke J, Kraus P, Liang H, Gonzales KAU, Chong H-CC, et al.. Klf2 is an essential factor that sustains ground state pluripotency. Cell Stem Cell 2014; 14:864-72; PMID:24905170; http://dx.doi.org/ 10.1016/j.stem.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 78.Storm MP, Bone HK, Beck CG, Bourillot PY, Schreiber V, Damiano T, Nelson A, Savatier P, Welham MJ. Regulation of nanog expression by phosphoinositide 3-kinase-dependent signaling in murine embryonic stem cells. J Biol Chem 2007; 282:6265-73; PMID:17204467; http://dx.doi.org/ 10.1074/jbc.M610906200 [DOI] [PubMed] [Google Scholar]
- 79.Falco G, Lee S-L, Stanghellini I, Bassey UC, Hamatani T, Ko MSH. Zscan4: a novel gene expressed exclusively in late 2-cell embryos and embryonic stem cells. Dev Biol 2007; 307:539-50; PMID:17553482; http://dx.doi.org/ 10.1016/j.ydbio.2007.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zalzman M, Falco G, Sharova LV, Nishiyama A, Thomas M, Lee S-L, Stagg CA, Hoang HG, Yang H-T, Indig FE, et al.. Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature 2010; 464:858-63; PMID:20336070; http://dx.doi.org/ 10.1038/nature08882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fujii S, Nishikawa-Torikai S, Futatsugi Y, Toyooka Y, Yamane M, Ohtsuka S, Niwa H. Nr0b1 is a negative regulator of Zscan4c in mouse embryonic stem cells. Sci Rep 2015; 5:9146; PMID:25772165; http://dx.doi.org/ 10.1038/srep09146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang G, Ye S, Zhou X, Liu D, Ying Q-L. Molecular basis of embryonic stem cell self-renewal: from signaling pathways to pluripotency network. Cell Mol Life Sci 2015; 72(9):1741-57; PMID:25595304; http://dx.doi.org/ 10.1007/s00018-015-1833-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lyashenko N, Winter M, Migliorini D, Biechele T, Moon RT, Hartmann C. Differential requirement for the dual functions of β-catenin in embryonic stem cell self-renewal and germ layer formation. Nat Cell Biol 2011; 13:753-61; PMID:21685890; http://dx.doi.org/ 10.1038/ncb2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wray J, Kalkan T, Gomez-Lopez S, Eckardt D, Cook A, Kemler R, Smith A. Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nat Cell Biol 2011; 13:838-45; PMID:21685889; http://dx.doi.org/ 10.1038/ncb2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ye S, Tan L, Yang R, Fang B, Qu S, Schulze EN, Song H, Ying Q, Li P. Pleiotropy of glycogen synthase kinase-3 inhibition by CHIR99021 promotes self-renewal of embryonic stem cells from refractory mouse strains. PLoS ONE 2012; 7:e35892; PMID: 22540008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meek S, Wei J, Sutherland L, Nilges B, Buehr M, Tomlinson SR, Thomson AJ, Burdon T. Tuning of b-Catenin activity is required to stabilize self-renewal of rat embryonic stem cells. Stem Cells 2013; 31:2104-15; PMID:23843312; http://dx.doi.org/ 10.1002/stem.1466 [DOI] [PubMed] [Google Scholar]
- 87.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med 2004; 10:55-63; PMID:14702635; http://dx.doi.org/ 10.1038/nm979 [DOI] [PubMed] [Google Scholar]
- 88.Ogawa K, Nishinakamura R, Iwamatsu Y, Shimosato D, Niwa H. Synergistic action of Wnt and LIF in maintaining pluripotency of mouse ES cells. Biochem Biophys Res Commun 2006; 343:159-66; PMID:16530170; http://dx.doi.org/ 10.1016/j.bbrc.2006.02.127 [DOI] [PubMed] [Google Scholar]
- 89.Atlasi Y, Noori R, Gaspar C, Franken P, Sacchetti A, Rafati H, Mahmoudi T, Decraene C, Calin GA, Merrill BJ, et al.. Wnt Signaling Regulates the Lineage Differentiation Potential of Mouse Embryonic Stem Cells through Tcf3 Down-Regulation. PLoS Genet 2013; 9:e1003424; PMID:23658527; http://dx.doi.org/ 10.1371/journal.pgen.1003424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hikasa H, Ezan J, Itoh K, Li X, Klymkowsky MW, Sokol SY. Regulation of TCF3 by Wnt-dependent phosphorylation during vertebrate axis specification. Dev Cell 2010; 19:521-32; PMID:20951344; http://dx.doi.org/ 10.1016/j.devcel.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shy BR, Wu C-I, Khramtsova GF, Zhang JY, Olopade OI, Goss KH, Merrill BJ. Regulation of Tcf7l1 DNA binding and protein stability as principal mechanisms of Wnt/β-catenin signaling. Cell Rep 2013; 4:1-9; PMID:23810553; http://dx.doi.org/ 10.1016/j.celrep.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pereira L, Yi F, Merrill BJ. Repression of Nanog gene transcription by Tcf3 limits embryonic stem cell self-renewal. Mol Cell Biol 2006; 26:7479-91; PMID:16894029; http://dx.doi.org/ 10.1128/MCB.00368-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo G, Huang Y, Humphreys P, Wang X, Smith A. A piggybac-based recessive screening method to identify pluripotency regulators. PLoS ONE 2011; 6:e18189; PMID: 21533166; http://dx.doi.org/ 10.1371/journal.pone.0018189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Paling NRD, Wheadon H, Bone HK, Welham MJ. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J Biol Chem 2004; 279:48063-70; PMID:15328362; http://dx.doi.org/ 10.1074/jbc.M406467200 [DOI] [PubMed] [Google Scholar]
- 95.Ornitz DM, Itoh N. The Fibroblast Growth Factor signaling pathway. WIREs Dev Biol 2015; 4:215-66; PMID:25772309; http://dx.doi.org/ 10.1002/wdev.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hollnagel A, Oehlmann V, Heymer J, Rüther U, Nordheim A. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem 1999; 274:19838-45; PMID:10391928; http://dx.doi.org/ 10.1074/jbc.274.28.19838 [DOI] [PubMed] [Google Scholar]
- 97.López-Rovira T, Chalaux E, Massagué J, Rosa JL, Ventura F. Direct binding of Smad1 and Smad4 to two distinct motifs mediates bone morphogenetic protein-specific transcriptional activation of Id1 gene. J Biol Chem 2002; 277:3176-85; http://dx.doi.org/ 10.1074/jbc.M106826200 [DOI] [PubMed] [Google Scholar]
- 98.Li Z, Fei T, Zhang J, Zhu G, Wang L, Lu D, Chi X, Teng Y, Hou N, Yang X, et al.. BMP4 signaling acts via dual-specificity phosphatase 9 to control ERK activity in mouse embryonic stem cells. Cell Stem Cell 2012; 10:171-82; PMID:22305567; http://dx.doi.org/ 10.1016/j.stem.2011.12.016 [DOI] [PubMed] [Google Scholar]
- 99.Qi X, Li T-G, Hao J, Hu J, Wang J, Simmons H, Miura S, Mishina Y, Zhao G-Q. BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways. Proc Natl Acad Sci U S A 2004; 101:6027-32; PMID:15075392; http://dx.doi.org/ 10.1073/pnas.0401367101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Soncin F, Mohamet L, Eckardt D, Ritson S, Eastham AM, Bobola N, Russell A, Davies S, Kemler R, Merry CLR, et al.. Abrogation of E-cadherin-mediated cell-cell contact in mouse embryonic stem cells results in reversible LIF-independent self-renewal. Stem Cells 2009; 27:2069-80; PMID:19544408; http://dx.doi.org/ 10.1002/stem.134 [DOI] [PubMed] [Google Scholar]
- 101.Pieters T, van Roy F. Role of cell-cell adhesion complexes in embryonic stem cell biology. J Cell Sci 2014; 127:2603-13; PMID:24931943; http://dx.doi.org/ 10.1242/jcs.146720 [DOI] [PubMed] [Google Scholar]
- 102.Eastham AM, Spencer H, Soncin F, Ritson S, Merry CLR, Stern PL, Ward CM. Epithelial-mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res 2007; 67:11254-62; PMID:18056451; http://dx.doi.org/ 10.1158/0008-5472.CAN-07-2253 [DOI] [PubMed] [Google Scholar]
- 103.Nagaoka M, Koshimizu U, Yuasa S, Hattori F, Chen H, Tanaka T, Okabe M, Fukuda K, Akaike T. E-cadherin-coated plates maintain pluripotent ES cells without colony formation. PLoS ONE 2006; 1:1-7; http://dx.doi.org/ 10.1371/journal.pone.0000015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nagaoka M, Hagiwara Y, Takemura K, Murakami Y, Li J, Duncan SA, Akaike T. Design of the artificial acellular feeder layer for the efficient propagation of mouse embryonic stem cells. J Biol Chem 2008; 283:26468-76; PMID:18614540; http://dx.doi.org/ 10.1074/jbc.M805037200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Del Valle I, Rudloff S, Carles A, Li Y, Liszewska E, Vogt R, Kemler R. E-cadherin is required for the proper activation of the Lifr/Gp130 signaling pathway in mouse embryonic stem cells. Development 2013; 140:1684-92; PMID:23487312; http://dx.doi.org/ 10.1242/dev.088690 [DOI] [PubMed] [Google Scholar]
- 106.Boroviak T, Loos R, Bertone P, Smith A, Nichols J. The ability of inner-cell-mass cells to self-renew as embryonic stem cells is acquired following epiblast specification. Nat Cell Biol 2014; 16:516-28; PMID:24859004; http://dx.doi.org/ 10.1038/ncb2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nichols J, Chambers I, Taga T, Smith A. Physiological rationale for responsiveness of mouse embryonic stem cells to gp130 cytokines. Development 2001; 128:2333-9; PMID:11493552 [DOI] [PubMed] [Google Scholar]
- 108.Li M, Sendtner M, Smith A. Essential function of LIF receptor in motor neurons. Nature 1995; 378:724-7; PMID:7501019; http://dx.doi.org/ 10.1038/378724a0 [DOI] [PubMed] [Google Scholar]
- 109.Ware CB, Horowitz MC, Renshaw BR, Hunt JS, Liggitt D, Koblar SA, Gliniak BC, McKenna HJ, Papayannopoulou T, Thoma B. Targeted disruption of the low-affinity leukemia inhibitory factor receptor gene causes placental, skeletal, neural and metabolic defects and results in perinatal death. Development 1995; 121:1283-99; PMID:7789261 [DOI] [PubMed] [Google Scholar]
- 110.Nakashima K, Wiese S, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Yoshida K, Kishimoto T, Sendtner M, Taga T. Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation. J Neurosci 1999; 19:5429-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Renfree MB, Shaw G. Diapause. Ann Rev Physiol 2000; 62:353-75; PMID:10845095; http://dx.doi.org/ 10.1146/annurev.physiol.62.1.353 [DOI] [PubMed] [Google Scholar]
- 112.Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci U S A 1997; 94:3801-4; PMID:9108058; http://dx.doi.org/ 10.1073/pnas.94.8.3801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Do DV, Ueda J, Messerschmidt DM, Lorthongpanich C, Zhou Y, Feng B, Guo G, Lin PJ, Hossain MZ, Zhang W, et al.. A genetic and developmental pathway from STAT3 to the OCT4-NANOG circuit is essential for maintenance of ICM lineages in vivo. Genes Dev 2013; 27:1378-90; PMID:23788624; http://dx.doi.org/ 10.1101/gad.221176.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nichols J, Jones K, Phillips JM, Newland SA, Roode M, Mansfield W, Smith A, Cooke A. Validated germline-competent embryonic stem cell lines from nonobese diabetic mice. Nat Med 2009; 15:814-8; PMID:19491843; http://dx.doi.org/ 10.1038/nm.1996 [DOI] [PubMed] [Google Scholar]
- 115.Ohtsuka S, Niwa H. The differential activation of intracellular signaling pathways confers the permissiveness of embryonic stem cell derivation from different mouse strains. Development 2015; 142:431-7; PMID:25564647; http://dx.doi.org/ 10.1242/dev.112375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Buehr M, Meek S, Blair K, Yang J, Ure J, Silva J, McLay R, Hall J, Ying Q-LL, Smith A. Capture of Authentic Embryonic Stem Cells from Rat Blastocysts. Cell 2008; 135:1287-98; PMID:19109897; http://dx.doi.org/ 10.1016/j.cell.2008.12.007 [DOI] [PubMed] [Google Scholar]
- 117.Li P, Tong C, Mehrian-Shai R, Jia L, Wu N, Yan Y, Maxson RE, Schulze EN, Song H, Hsieh CL, et al.. Germline Competent Embryonic Stem Cells Derived from Rat Blastocysts. Cell 2008; 135:1299-310; PMID:19109898; http://dx.doi.org/ 10.1016/j.cell.2008.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Buehr M, Nichols J, Stenhouse F, Mountford P, Greenhalgh CJ, Kantachuvesiri S, Brooker G, Mullins J, Smith G. Rapid loss of Oct-4 and pluripotency in cultured rodent blastocysts and derivative cell lines. Biol Reprod 2003; 68:222-9; PMID:12493717; http://dx.doi.org/ 10.1095/biolreprod.102.006197 [DOI] [PubMed] [Google Scholar]
- 119.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science 1998; 282:1145-7; PMID:9804556; http://dx.doi.org/ 10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- 120.Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RDG. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 2007; 448:196-9; PMID:17597760; http://dx.doi.org/ 10.1038/nature05972 [DOI] [PubMed] [Google Scholar]
- 121.Brons IGM, Smithers LE, Trotter MWB, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, et al.. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 2007; 448:191-5; PMID:17597762; http://dx.doi.org/ 10.1038/nature05950 [DOI] [PubMed] [Google Scholar]
- 122.De Los Angeles A, Loh Y-HH, Tesar PJ, Daley GQ. Accessing naïve human pluripotency. Curr Opin Genet Dev 2012; 22:272-82; PMID:22463982; http://dx.doi.org/ 10.1016/j.gde.2012.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nichols J, Smith A. Naive and Primed Pluripotent States. Cell Stem Cell 2009; 4:487-92; PMID:19497275; http://dx.doi.org/ 10.1016/j.stem.2009.05.015 [DOI] [PubMed] [Google Scholar]
- 124.Bao S, Tang F, Li X, Hayashi K, Gillich A, Lao K, Surani MA. Epigenetic reversion of post-implantation epiblast to pluripotent embryonic stem cells. Nature 2009; 461:1292-5; PMID:19816418; http://dx.doi.org/ 10.1038/nature08534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Festuccia N, Osorno R, Halbritter F, Karwacki-Neisius V, Navarro P, Colby D, Wong F, Yates A, Tomlinson SR, Chambers I. Esrrb is a direct Nanog target gene that can substitute for Nanog function in pluripotent cells. Cell Stem Cell 2012; 11:477-90; PMID:23040477; http://dx.doi.org/ 10.1016/j.stem.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guo G, Yang J, Nichols J, Hall JS, Eyres I, Mansfield W, Smith A. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development 2009; 136:1063-9; PMID:19224983; http://dx.doi.org/ 10.1242/dev.030957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gillich A, Bao S, Grabole N, Hayashi K, Trotter MWB, Pasque V, Magnúsdóttir E, Surani MA. Epiblast stem cell-based system reveals reprogramming synergy of germline factors. Cell Stem Cell 2012; 10:425-39; PMID:22482507; http://dx.doi.org/ 10.1016/j.stem.2012.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, Kalma Y, Viukov S, Maza I, Zviran A, et al.. Derivation of novel human ground state naive pluripotent stem cells. Nature 2013; 504:282-6; PMID:24172903; http://dx.doi.org/ 10.1038/nature12745 [DOI] [PubMed] [Google Scholar]
- 129.Chan Y-S, Göke J, Ng J-H, Lu X, Gonzales KAU, Tan C-P, Tng W-Q, Hong Z-Z, Lim Y-S, Ng H-H. Induction of a human pluripotent state with distinct regulatory circuitry that resembles preimplantation epiblast. Cell Stem Cell 2013; 13:663-75; PMID:24315441; http://dx.doi.org/ 10.1016/j.stem.2013.11.015 [DOI] [PubMed] [Google Scholar]
- 130.Valamehr B, Robinson M, Abujarour R, Rezner B, Vranceanu F, Le T, Medcalf A, Lee TT, Fitch M, Robbins D, et al.. Platform for Induction and Maintenance of Transgene-free hiPSCs Resembling Ground State Pluripotent Stem Cells. Stem Cell Rep 2014; 2:366-81; PMID:24672758; http://dx.doi.org/ 10.1016/j.stemcr.2014.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ware CB, Nelson AM, Mecham B, Hesson J, Zhou W, Jonlin EC, Jimenez-Caliani AJ, Deng X, Cavanaugh C, Cook S, et al.. Derivation of naive human embryonic stem cells. Proc Natl Acad Sci U S A 2014; 111:4484-9; PMID:24623855; http://dx.doi.org/ 10.1073/pnas.1319738111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Theunissen TW, Powell BE, Wang H, Mitalipova M, Faddah DA, Reddy J, Fan ZP, Maetzel D, Ganz K, Shi L, et al.. Systematic Identification of Defined Conditions for Induction and Maintenance of Naive Human Pluripotency. Cell Stem Cell 2014; 15:471-87; PMID:25090446; http://dx.doi.org/ 10.1016/j.stem.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Takashima Y, Guo G, Loos R, Nichols J, Ficz G, Krueger F, Oxley D, Santos F, Clarke J, Mansfield W, et al.. Resetting Transcription Factor Control Circuitry toward Ground-State Pluripotency in Human. Cell 2014; 158:1254-69; PMID:25215486; http://dx.doi.org/ 10.1016/j.cell.2014.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen H, Aksoy I, Gonnot F, Osteil P, Aubry M, Hamela C, Rognard C, Hochard A, Voisin S, Fontaine E, et al.. Reinforcement of STAT3 activity reprogrammes human embryonic stem cells to naive-like pluripotency. Nat Commun 2015; 6:7095; PMID:25968054; http://dx.doi.org/ 10.1038/ncomms8095 [DOI] [PMC free article] [PubMed] [Google Scholar]


