Abstract
The transcription factors of the myocyte enhancer factor 2 family (MEF2 A-D) are highly expressed in the brain and play a key role in neuronal survival/apoptosis, differentiation and synaptic plasticity. However, the precise genome-wide mapping of different members of the family has not yet been fully elucidated. Here, we report the comparative analysis of MEF2A and MEF2C genome-wide mapping in mouse cortical neurons by ChIP-seq, a powerful approach to elucidate the genomic functions of transcription factors and to identify their transcriptional targets. Our analysis reveals that MEF2A and MEF2C each orchestrate similar epigenomic programs mainly through the binding of enhancer regulatory elements in proximity of target genes involved in neuronal plasticity and calcium signaling. We highlight the differences in the enhancer networks and molecular pathways regulated by MEF2A and MEF2C, which might be determined by the combinatorial action of different transcription factors.
Introduction
The myocyte enhancer factor 2 (MEF2) proteins belong to the MADS (MCM-1-agamous-deficiens-serum response factor) box evolutionarily conserved family of transcription factors (TF). Four vertebrate genes encode for distinct isoforms (A-D) of the MEF2 family, whose expression is detected in a wide range of tissues, but the proteins are most abundant in muscle and brain. In the CNS, MEF2 isoforms exhibit distinct but overlapping patterns throughout the developing brain through adulthood, indicating that they are tightly regulated in multiple processes.1-3 In the mature brain, MEF2 proteins are expressed in brain regions involved in memory formation, including amygdala, hippocampus, cortex and striatum. Each isoform is characterized by a highly conserved N-terminal region including the MADS box, a DNA binding domain that recognizes A/T-rich motif in target genes, and the MEF2 domain that mediates homo- and hetero-dimerization.1,4,5 The C-terminal region of MEF2 is characterized by a divergent transactivation domain that mediates the interaction with numerous co-factors, including co-activators, such as the acetyl-transferases p300 and CBP, or co-repressor, such as class II histone deacetylases (HDACs) and NCoR/SMRT co-repressor complex.6,7 MEF2 factors function as bivalent transcriptional regulators. In the absence of stimulating inputs, MEF2 proteins form a complex with co-repressor complexes to keep their target genes in a repressed state. Upon stimulus, the interaction with HDACs is disrupted to permit MEF2 proteins to recruit co-activator complexes that promote transcription of target genes. The transcriptional activity of MEF2 proteins is tightly regulated by post-translational modifications that include phosphorylation,2 sumoylation,8 acetylation,9 and nitrosylation.10 In the central nervous system, neuronal activity induced by depolarization, neurotrophins or synaptic stimuli, such as Reelin at glutamatergic synapses, triggers calcium signaling cascades involving calmodulin-dependent protein kinases (CaMK) activation that results in phosphorylation of class IIa HDACs. These phosphorylation events lead to HDACs nuclear export and dismissal from MEF2 target genes, permitting recruitment of co-activators.6,11-13 Furthermore, protein phosphatase 2B (PP2B) or calcineurin, a serine/threonine phosphatase, directly dephosphorylates MEF2 influencing the affinity to DNA target sequences and promoting transcriptional activity.14-16 In neuronal cells, MEF2 target genes regulate different aspects of synaptic function such as presynaptic vesicle release, excitatory and inhibitory postsynaptic strengthening; with many target genes linked to increased genetic susceptibility to neurological disorders, including autism, epilepsy and intellectual disabilities.17-20 Recent efforts in elucidating the distinct function of MEF2 family members using in vitro and in vivo systems strongly suggest important roles for specific MEF2 isoforms in brain plasticity.21 While MEF2C specific KO clearly impairs hippocampal-dependent learning and memory by increasing synapses number and potentiating synaptic transmission,22 manipulation of MEF2A and MEF2D results in deficits in motor coordination and enhanced hippocampal short-term synaptic plasticity without impairments in learning and memory behaviors.23 Consistently, an increase in MEF2A suppresses synapses number15 and inhibits dendritic spine growth in vitro.8 Collectively, these findings indicate the sophisticated and complex regulation of diverse neuronal processes by distinct MEF2 isoforms that is likely affected by other factors, such as environmental cues or other signaling pathways. However, the precise transcriptional programs regulated by individual MEF2 isoforms remain to be defined. Here, we report the genome-wide epigenetic analysis of MEF2A and MEF2C cistromes in primary cortical neurons by ChIP-seq, which identifies the unique and overlapping genomic loci occupied by each isoform. Our analysis reveals a widespread localization of MEF2 transcription factors to enhancer regulatory elements in the genome of neuronal cells, suggesting a function of these factors in directing neuronal lineage specification. Both transcription factors orchestrate overlapping but unique programs correlated to a variety of neuronal functions, such as glutamatergic synaptic transmission, drug addiction and MAPK signaling pathway. Although, both isoforms orchestrate a similar epigenomic program, the motif discovery reveals some key differences indicating that the combinatorial action of different transcription factors might determine the regulation of distinct enhancer-driven transcriptional programs.
Results and Discussion
MEF2A and MEF2C genome-wide profiles in cortical neurons
The finding that MEF2A/D restrain memory formation, whereas MEF2C promotes associative learning and memory, raises the question of whether their divergent actions are mediated via regulation of distinctive or common cohorts of target genes. To identify the direct target genes of different isoforms of the MEF2 family, we used genome-wide readout of MEF2A and MEF2C binding sites by chromatin immuno-precipitation coupled with deep sequencing (ChIP-seq) in mouse primary cortical neurons. As previously reported, MEF2A and MEF2C are the most abundant isoforms expressed in cultured neurons.7 Using HOMER ChIP-seq analysis program (http://homer.salk.edu/homer/motif/), we identified a total of 75,072 MEF2A or 35,710 MEF2C binding sites with FDR threshold <0.001 using input control ChIP-seq data as background to filter out false positive peaks. Examination of MEF2A/MEF2C genome-wide occupancy revealed a similar distribution of the 2 factors across different functional regions of the genome, including a very limited occupancy of promoters and transcriptional start sites (TSS) but widespread distribution of inter- and intragenic sites that total >90% of identified peaks for both MEF2A and MEF2C (Fig. 1A). The median distance from the TSS is 56 kb for MEF2A and 55kb for MEF2C enriched regions. As expected the most represented sequence element by de novo motif analysis was the MEF2 motif for both factors, which was enriched in roughly 40% of ChIP-seq peaks compared to the background sequences (Fig. 1B). To gain insight in the common or isoform-specific occupied loci, peaks were considered co-bound when they were found within 200bp of each other. We observed that the majority (66.7%) of MEF2C binding regions were co-bound by MEF2A, while only a minor fraction of MEF2A were detected as common regions (Fig. 1C), suggesting that the 2 isoforms might control diverse epigenomic programs.
Figure 1.
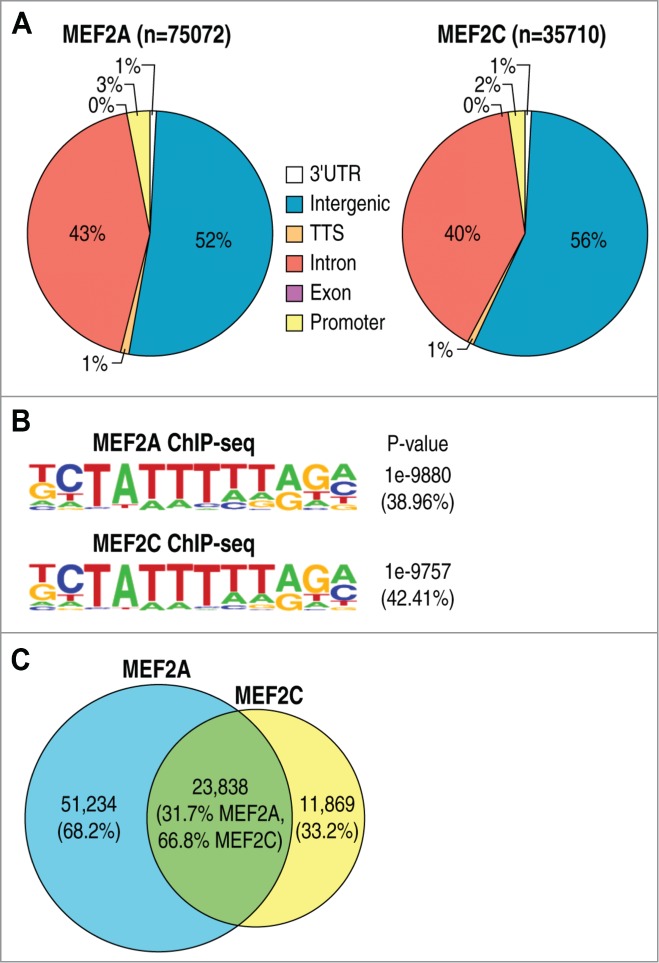
Analysis of MEF2A and MEF2C ChIP-Seq experiments in mouse cortical neurons. (A) Pie charts showing the genomic distribution of MEF2A binding sites in the genome. (B) Pie charts showing the genomic distribution of MEF2C binding sites in the genome. (C) De novo motif analysis of MEF2A or MEF2C-bound genomic regions showing the top enriched sequence motifs. P-values and frequencies of motifs are indicated.
MEF2A and MEF2C enhancer programs
To gain further insight in the putative functional role of the genomic regions bound by MEF2 transcriptional regulators, we used histone 3 lysine 27 acetylation (H3K27Ac) profiling by ChIP-seq, which marks both promoters and enhancers cis-regulatory elements of the mammalian genome. Enhancer elements are defined as DNA sequences that function far away from the promoter or TSS of target genes independently of their orientation, and have the capability to promote basal transcriptional activity in a cell-type specific manner and in response to external stimuli.24,25 A large body of evidence has shown that active enhancers are characterized by defined properties of the chromatin, including a nucleosomal free region flanked by nucleosomes containing histones with specific posttranslational modifications.26 The histone 3 lysine 27 acetylation (H3K27ac) mark has been extensively reported to mark cell-type specific and active enhancers.27,28 Therefore, we used available H3K27Ac ChIP-seq data in cortical neurons as an enhancer mark.7 First, we overlapped the H3K27Ac ChIP-seq peaks with those identified for each transcription factor, and we identified 17,290 and 9,314 co-bound peaks for MEF2A and MEF2C, respectively. We observed that H3K27Ac mark labels 30–35% distal regions for MEF2A or MEF2C ChIP-seqs. Only the distal peaks away from the TSS (>1 ,000 bp) were considered as enhancers (14,593 for MEF2A and 8,518 for MEF2C) (Fig. 2A). Scatter plots of the tag counts around peak coordinates of TFs enable comparative analysis of MEF2A- and MEF2C-dependent enhancer programs. Based on this analysis, we identified 6,554 common H3K27Ac-enhancers (Fig. 2B). To examine functional pathways associated to the genes in proximity of MEF2 enhancers, we used gene ontology analysis for 3 datasets of MEF2-enhancers (MEF2A-specific, MEF2C-specific and MEF2A/C-common). Based on the KEGG pathway analysis, the most enriched functional annotations were nearly identical for the common or isoform-specific subsets of genes and they included signaling pathways known to be regulated by MEF2 factors, such as glutamatergic synaptic transmission, drug addiction, axon guidance, and MAPK signaling pathways (Fig. 2C). This result suggests that MEF2A and MEF2C regulate common or distinct transcriptional targets that are involved in similar neuronal processes. To explore whether specific sequence determinants may account for distinct MEF2 binding patterns, we examined the MEF2-enhancer subsets for enrichment of distinctive sequence elements by de novo motif analysis (Fig. 2D). We observed that in the top 10 most enriched sequences each subset of enhancers contains a core combination of consensus motifs, including the MEF2, AP1 and NeuroD1. We believe that MEF2 TFs together with NeuroD1 and AP1 constitute the factors that determine neuronal-lineage specific enhancers. However, each class is associated with different transcription factors, such as SRF for the common enhancers, CREB/ATF for the MEF2A enhancers, CEBP/A and forkhead box factors for MEF2C. How different combinations of transcription factors cooperate to activate cis-active regulatory elements regulated by MEF2 isoforms remains an interesting question to address in the future. We speculate that the activation of diverse signaling pathways might differentially regulate specific isoforms of MEF2 family by posttranslational modifications or activation of other transcription factors. Recently, we have shown that the secreted protein Reelin triggers a synapse-to-nucleus pathway involving the γ-secretase-dependent cleavage of its receptor LRP8 that is required for proper hippocampal-dependent associative learning. This signaling pathway relies on the induction of transcriptional programs activated by a specific subset of enhancers, known as LRP8-Reelin-Regulated Neuronal enhancers (LRN). These enhancers are characterized by the occupancy of both MEF2A and MEF2C factors before stimulation, but only MEF2C isoform exhibits an increased recruitment upon Reelin treatment, suggesting a specific role for MEF2C in mediating learning and memory processes. Other synaptic inputs might be involved in the fine-tuned regulation of subsets of enhancers bound by MEF2 factors. Characterizing the MEF2 transcriptional programs has the potential not only to shed light on underlying mechanisms of transcriptional regulation of memory, but also might provide key insights into the disease mechanisms involved in the development of many neurological disorders linked to dysfunction of MEF2 transcription factors and to the misregulation of their specific target genes.
Figure 2.

Analysis of H3K27Ac-MEF2 enhancer subsets. (A) Venn diagram showing H3K27Ac-MEF2A or H3K27Ac-MEF2C overlapping peaks identified using a false discovery threshold of 0.001. Peaks were considered co-bounded when they were found within 200 bp of each other. (B) On the right, the scatter plot of MEF2A and MEF2C ChIP-Seq peaks in cortical neurons is represented by log2 of normalized ChIP-seq tag counts; on the left, the Venn diagram shows the fractions of common or isoform-specific H3K27Ac-enhancers. (C) Functional gene ontology annotations associated with common or isoform-specific H3K27Ac-enhancers. (D) De novo motif analysis of common or isoform-specific H3K27Ac-enhancers showing the top enriched sequence motifs, with associated p values as indicated.
Methods
Deep-sequencing analysis
For ChIP-seq, the 50 bp sequence tag was aligned to the mm9 assembly by using Bowtie2. During alignment to the reference genome, we allowed a mismatch of only two base pairs. The data were visualized by UCSC genome browser using HOMER. The total number of mapped reads was normalized to 10^7 for each experiment presented in this study. Using HOMER and applying the same criteria as in our previously published methods (Telese et. al, 2015), we identified the MEF2A/MEF2C or H3K27ac ChIP-seq peaks. First, we used input sequencing run as a control to filter out false positive peaks. Second, we used different parameters to define the width of peaks by applying a distinct sliding window for searching the enrichment regions, which is 200 bp for transcription factors and 500bp for histone modifications. Third, redundant peaks detection was applied by merging peaks when the distance between each other was less than 500 bp. Furthermore, to reduce clonal amplification only single tags per each genomic position are used for the identification of ChIP-seq peaks. Finally, a threshold of false discovery rate (FDR) < 0.001 was applied to call peaks by comparing randomized tag positions with an effective genomic size of 2×109 bp. All the peaks identified as described above are assigned to genes by cross-referencing the NCBI Reference Sequence Database (RefSeq). Peaks from individual experiments were considered overlapping if they were located within 200 bp from each other. The motif finding and enrichment p-values were computed by using HOMER algorithm as described before (Heinz, 2010). The scatter plots were then generated by plotting as (x,y) coordinates the normalized tag counts (log2) within 1000bp around the center of the peaks identified for each experiments as describe above.
The NCBI Gene Expression Omnibus accession number for the sequencing data is reported in previously published work.7
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Janet Hightower for assistance with figure preparation and Rachel Pardee for assistance with editing of the manuscript. This work was supported by Roche Extending Innovation Network Program to F.T.
References
- 1.McKinsey TA, Zhang CL, Olson EN, MEF2 : a calcium-dependent regulator of cell division, differentiation and death. Trends Biochem Sci 2002; 27(1):p. 40-7; http://dx.doi.org/ 10.1016/S0968-0004(01)02031-X [DOI] [PubMed] [Google Scholar]
- 2.Shalizi AK, A Bonni, brawn for brains: the role of MEF2 proteins in the developing nervous system. Curr Top Dev Biol 2005; 69:p. 239-66; http://dx.doi.org/ 10.1016/S0070-2153(05)69009-6 [DOI] [PubMed] [Google Scholar]
- 3.Lyons GE, Micales BK, Schwarz J, Martin JF, Olson EN. Expression of Mef2 Genes in the Mouse Central-Nervous-System Suggests a Role in Neuronal Maturation. J Neurosci 1995; 15(8):p. 5727-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.West AG, Shore P, Sharrocks AD, DNA binding by MADS-box transcription factors: a molecular mechanism for differential DNA bending. Mol Cell Biol 1997; 17(5):p. 2876-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molkentin JD, Li L, Olson EN, Phosphorylation of the MADS-box transcription factor MEF2C enhances its DNA binding activity. J Biol Chem 1996; 271(29):p. 17199-204.; PMID:8663403; PMID:8663403; http://dx.doi.org/ 10.1074/jbc.271.29.17199 [DOI] [PubMed] [Google Scholar]
- 6.McKinsey TA, Zhang CL, Lu J, Olson EN. Signal-dependent nuclear export of a histone deacetylase regulates muscle differentiation. Nature 2000; 408(6808):p. 106-11; http://dx.doi.org/ 10.1038/35040593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Telese F, Ma Q, Perez PM, Notani D, Oh S, Li W, Comoletti D, Ohgi KA, Taylor H, Rosenfeld MG. LRP8-Reelin-Regulated Neuronal Enhancer Signature Underlying Learning and Memory Formation. Neuron 2015; 86(3):p. 696-710; http://dx.doi.org/ 10.1016/j.neuron.2015.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shalizi A, Gaudillière B, Yuan Z, Stegmüller J, Shirogane T, Ge Q, Tan Y, Schulman B, Harper JW, Bonni A. A calcium-regulated MEF2 surnoylation switch controls postsynaptic differentiation. Science 2006; 311(5763):p. 1012-7; http://dx.doi.org/ 10.1126/science.1122513 [DOI] [PubMed] [Google Scholar]
- 9.Ma KW, Chan JK, Zhu G, Wu Z. Myocyte enhancer factor 2 acetylation by p300 enhances its DNA binding activity, transcriptional activity, and myogenic differentiation. Mol Cell Biol 2005; 25(9):p. 3575-82; http://dx.doi.org/ 10.1128/MCB.25.9.3575-3582.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okamoto S, Nakamura T, Cieplak P, Chan SF, Kalashnikova E, Liao L, Saleem S, Han X, Clemente A, Nutter A, et al., S-Nitrosylation-Mediated Redox Transcriptional Switch Modulates Neurogenesis and Neuronal Cell Death. Cell Rep 2014; 8(1):p. 217-28; http://dx.doi.org/ 10.1016/j.celrep.2014.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gregoire S, Tremblay AM, Xiao L, Yang Q, Ma K, Nie J, Mao Z, Wu Z, Giguère V, Yang XJ. Control of MEF2 transcriptional activity by coordinated phosphorylation and sumoylation (vol 281, pg 4423, 2006). J Biol Chem 2006; 281(13):p. 8996-6 [DOI] [PubMed] [Google Scholar]
- 12.McKinsey TA, Zhang CL, Olson EN, Identification of a signal-responsive nuclear export sequence in class II histone deacetylases. Mol Cell Bio 2001; 21(18):p. 6312-21; PMID:11509672; http://dx.doi.org/ 10.1128/MCB.21.18.6312-6321.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grozinger CM, Schreiber SL, Regulation of histone deacetylase 4 and 5 and transcriptional activity by 14-3-3-dependent cellular localization. Proc Natl Acad Sci U S A 2000; 97(14):p. 7835-40; PMID:10869435; http://dx.doi.org/ 10.1073/pnas.140199597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li JL, Vargas MA, Kapiloff MS, Dodge-Kafka KL. Regulation of MEF2 transcriptional activity by calcineurin/mAKAP complexes. Exp Cell Res 2013; 319(4):p. 447-54; http://dx.doi.org/ 10.1016/j.yexcr.2012.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science 2006; 311(5763):p. 1008-12; http://dx.doi.org/ 10.1126/science.1122511 [DOI] [PubMed] [Google Scholar]
- 16.Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, et al., Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron 2008; 59(4):p. 621-33; http://dx.doi.org/ 10.1016/j.neuron.2008.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flavell SW, Kim TK, Gray JM, Harmin DA, Hemberg M, Hong EJ, Markenscoff-Papadimitriou E, Bear DM, Greenberg ME. Genome-Wide Analysis of MEF2 Transcriptional Program Reveals Synaptic Target Genes and Neuronal Activity-Dependent Polyadenylation Site Selection. Neuron 2008; 60(6):p. 1022-38; http://dx.doi.org/ 10.1016/j.neuron.2008.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Meur N, Holder-Espinasse M, Jaillard S, Goldenberg A, Joriot S, Amati-Bonneau P, Guichet A, Barth M, Charollais A, Journel H, et al., MEF2C haploinsufficiency caused by either microdeletion of the 5q14.3 region or mutation is responsible for severe mental retardation with stereotypic movements, epilepsy and/or cerebral malformations. J Med Gen 2010; 47(1):p. 22-9; http://dx.doi.org/ 10.1136/jmg.2009.069732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalachikov S, Evgrafov O, Ross B, Winawer M, Barker-Cummings C, Martinelli Boneschi F, Choi C, Morozov P, Das K, Teplitskaya E, et al., Mutations in LGI1 cause autosomal-dominant partial epilepsy with auditory features. Nat Gen 2002; 30(3):p. 335-41; http://dx.doi.org/ 10.1038/ng832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greer PL, Hanayama R, Bloodgood BL, Mardinly AR, Lipton DM, Flavell SW, Kim TK, Griffith EC, Waldon Z, Maehr R, et al., The Angelman Syndrome Protein Ube3A Regulates Synapse Development by Ubiquitinating Arc. Cell 2010; 140(5):p. 704-16; http://dx.doi.org/ 10.1016/j.cell.2010.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rashid AJ, Cole CJ, Josselyn SA, Emerging roles for MEF2 transcription factors in memory. Gen Brain Behav 2014; 13(1):p. 118-25; http://dx.doi.org/ 10.1111/gbb.12058 [DOI] [PubMed] [Google Scholar]
- 22.Barbosa AC, Kim MS, Ertunc M, Adachi M, Nelson ED, McAnally J, Richardson JA, Kavalali ET, Monteggia LM, Bassel-Duby R, et al., MEF2C, a transcription factor that facilitates learning and memory by negative regulation of synapse numbers and function. Proc Natl Acad Sci U S A 2008; 105(27):p. 9391-6; PMID:18599438; http://dx.doi.org/ 10.1073/pnas.0802679105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akhtar MW, Kim MS, Adachi M, Morris MJ, Qi X, Richardson JA, Bassel-Duby R, Olson EN, Kavalali ET, Monteggia LM. In Vivo Analysis of MEF2 Transcription Factors in Synapse Regulation and Neuronal Survival. Plos One 2012; 7(4):e34863; http://dx.doi.org/ 10.1371/journal.pone.0034863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinz S, Romanoski CE, Benner C, Glass CK. The selection and function of cell type-specific enhancers. Nat Rev Mol Cell Biol 2015; 16(3):p. 144-54; http://dx.doi.org/ 10.1038/nrm3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li WB, Notani D, Ma Q, Tanasa B, Nunez E, Chen AY, Merkurjev D, Zhang J, Ohgi K, Song X, et al., Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature 2013; 498(7455):p. 516; http://dx.doi.org/ 10.1038/nature12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shlyueva D, Stampfel G, Stark A, Transcriptional enhancers: from properties to genome-wide predictions. Nat Rev Genet 2014; 15(4):p. 272-86; http://dx.doi.org/ 10.1038/nrg3682 [DOI] [PubMed] [Google Scholar]
- 27.Creyghton MP, Cheng AW, Welstead GG, Kooistra T, Carey BW, Steine EJ, Hanna J, Lodato MA, Frampton GM, Sharp PA, et al., Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci U S A 2010; 107(50):p. 21931-6; PMID:21106759; http://dx.doi.org/ 10.1073/pnas.1016071107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ernst J, Kellis M, Discovery and characterization of chromatin states for systematic annotation of the human genome. Res Computational Mol Biol 2011; 6577:p. 53-3; http://dx.doi.org/ 10.1007/978-3-642-20036-6_6 [DOI] [PMC free article] [PubMed] [Google Scholar]


