Abstract
Background
Walking speed is an important human aging biomarker. Baboons are valuable translational models for aging studies. Establishing if walking speed is a good aging biomarker has value. We hypothesized there would be characteristic age-related decline in baboon walking speed.
Methods
We studied 33 female baboons ages 5–21 yrs. Walking speed was calculated by the time to walk between landmarks separated by known distances. A regression model was developed to describe the relationship between speed, age, and body weight.
Results
Speed negatively associated with age, a relationship enhanced by increased weight (p < 0.0005). For 16 kg animals, speed declined approximately 0.6 cm/sec yearly. For each additional kg of weight, speed declined an additional 0.3 cm/sec yearly.
Conclusions
Baboon walking speed declines with age, an effect modulated by weight. Ease of measurement and strong age association make walking speed a valuable biomarker for aging research with this important experimental species.
Keywords: Gait speed, physical mobility, age-related decline, nonhuman primates
Introduction
Walking speed is a reliable aging biomarker in humans [1, 49]. In healthy humans, walking speed begins to decline significantly around the sixth decade of life [25, 45, 48]. Slower walking speed is associated with higher risk of adverse health outcomes, decline in mobility, and mortality [10, 16, 19, 38].
Much current experimental aging research relies on studies with nonhuman primates, such as baboons (Papio sp.) and macaques (Macaca sp.). To understand how different lifestyle factors influence the aging process, it is necessary to establish measures of aging that can be easily employed in nonhuman primates and parallel those used in humans. Recently, Shively et al. [47] reported age-based decline in walking speed of three species of Old World monkeys: cynomolgus macaques (M. fascicularis), bonnet macaques (M. radiata), and African green monkeys (Chlorocebus aethiops). Older monkeys (mean 20 yrs old, human equivalent 70 yrs) walked 17% more slowly than did younger monkeys (mean 9 yrs old, human equivalent 30 yrs) [47]. This is similar to findings from humans; Shively et al. [47] estimate that if compared in a young versus old dichotomy as they did for monkeys, older humans walk 15–20% more slowly than do younger humans [47]. The system set up by Shively et al. [47] to measure walking speed is relatively easy, inexpensive, and quick. They drew landmarks on the floors and perches of the home cage housing their monkey subjects, and then used a stopwatch to measure how long it took monkeys to walk between landmarks separated by known distances.
Walton et al. [55] also studied age-related decline in movement speed of monkeys, reporting that younger rhesus macaques (M. mulatta) move more quickly than do middle-aged and older rhesus macaques, but their method of videotaping behavior in a dedicated cage is more time consuming and expensive than the simpler landmark drawing method of Shively et al. [47].
The goal of the present study was to test walking speed as an indicator of aging in baboons, using the method of Shively et al. [47]. We are aware of no other studies that have investigated walking speed in baboons. Normative data are necessary to use walking speed as a biomarker in studies on aging of baboons exposed to a variety of maternal nutritional challenges known to affect development of multiple fetal organs: brain [5–7, 32, 33, 35, 41], kidney [17, 40], liver [34, 39], and pancreas [11]. Our aim was to establish that walking speed would be one useful way to measure differences in aging between baboons. Having a known aging biomarker allows for manipulation of the environment and study of subsequent effects on the biomarker. For example, walking speed may be used to test whether early life reduced nutrition affects the aging process, or to test whether a clinical intervention is working. We hypothesized there would be a characteristic decline in baboon walking speed with age.
Methods
Humane Care Guidelines
All procedures were approved by the Texas Biomedical Research Institute Institutional Animal Care and Use Committee and conducted in AAALAC approved facilities [46].
Subjects
Subjects were 33 female baboons (Papio hamadryas), ranging in age from 5.3 to 20.6 years (Table 1), housed at the Southwest National Primate Research Center (SNPRC) in San Antonio, TX. This study period covers the majority of the baboon lifespan. Female baboons reach sexual maturity at about five years of age [4, 50]. For baboons at SNPRC, among individuals that survive the first five years of life, average age at death is 21 years, with maximum recorded lifespan of 33 years [9]. Both obese and normal weight females are included in our sample, with mean weight of 19.6 kg (Table 1). Mean adult weight among non-obese female baboons at SNPRC has been reported as 15.9 kg [20] or 16.9 kg [12]. The baboons are trained to enter a wire mesh chute and walk over a digital scale for body weight measurement. Mean crown-rump length, a measure of baboon body length, is 60 cm among non-obese female SNPRC baboons (crown-rump length is considered a more relevant measure of baboon body size than is body length from crown to heel, since baboons are quadrupedal) [12]. Crown-rump length data were not available for this sample.
Table 1.
Sample characteristics: age (yrs), walking speed (cm/sec), and weight (kg)
| Individual | Age (yrs) | Mean walking speed (cm/sec) |
Weight (kg) |
|---|---|---|---|
| 1 | 5.3 | 61.3 | 14.0 |
| 2 | 5.3 | 63.7 | 13.3 |
| 3 | 7.2 | 47.6 | 14.7 |
| 4 | 8.6 | 70.7 | 21.3 |
| 5 | 9.0 | 63.0 | 16.6 |
| 6 | 9.2 | 63.6 | 22.9 |
| 7 | 9.2 | 74.3 | 20.6 |
| 8 | 9.3 | 61.9 | 20.1 |
| 9 | 9.4 | 60.2 | 21.7 |
| 10 | 9.4 | 66.6 | 15.7 |
| 11 | 9.4 | 64.3 | 17.3 |
| 12 | 9.4 | 59.0 | 12.4 |
| 13 | 9.4 | 56.4 | 19.7 |
| 14 | 9.4 | 63.4 | 18.1 |
| 15 | 9.5 | 56.3 | 15.8 |
| 16 | 9.7 | 56.6 | 18.6 |
| 17 | 10.9 | 44.2 | 22.2 |
| 18 | 11.0 | 54.9 | 14.1 |
| 19 | 11.7 | 56.6 | 19.7 |
| 20 | 11.8 | 58.2 | 24.5 |
| 21 | 12.1 | 53.1 | 24.0 |
| 22 | 12.5 | 54.3 | 24.9 |
| 23 | 14.4 | 45.1 | 24.5 |
| 24 | 14.8 | 43.6 | 22.0 |
| 25 | 15.3 | 37.6 | 26.4 |
| 26 | 15.4 | 60.0 | 19.0 |
| 27 | 15.7 | 45.4 | 23.2 |
| 28 | 15.7 | 66.1 | 19.2 |
| 29 | 16.0 | 46.5 | 24.2 |
| 30 | 16.1 | 49.4 | 21.5 |
| 31 | 16.5 | 48.7 | 19.9 |
| 32 | 18.4 | 41.6 | 19.8 |
| 33 | 20.6 | 55.7 | 14.2 |
In this initial study, all baboons in the sample were female, as enough males for an adequate male sample were unavailable. All subjects lived in stable social groups consisting of one adult male and 7–15 adult females [46]. Each enclosure has 37 m2 of floor space (roughly 6.1 m by 6.1 m) and is 3.5 m high [46]. Two raised metal platforms run parallel along the full length of the cage, and there are numerous perches. Baboons are provided manipulable enrichment, such as nylon bones (Nylabone™, Neptune, NJ, USA), rubber Kong™ toys (Kong Company, Golden, CO, USA), and plastic Jolly Balls™ (Horseman’s Pride, Inc., Ravenna, OH, USA). All individuals were in good health, not known to be pregnant, had unimpaired locomotion, and had no dependent offspring.
Behavioral observations
Prior to observations, home cages were marked with nontoxic permanent markers. A grid of landmarks was created on the floor and on the perches, and each landmark was assigned a unique identifier (e.g., A, B, C…). Distances between all landmarks were measured. The distance between landmarks ranged from 1.0–3.4 m.
Behavioral observations of walking speed were conducted under ad libitum conditions (i.e., the observer measured instances of walking opportunistically), as described by Altmann in her seminal 1974 paper on methods for observing behavior [3]. Each time a subject crossed a landmark, a single observer (HFH) used a stopwatch to measure the time to reach another landmark. The timer was started as soon as the subject’s foot crossed over one mark on the floor, and was stopped when the foot crossed over another mark on the floor. Only instances of walking straight from one landmark to another were recorded. Walking speed was calculated as distance (cm) / time (sec). The average recorded walking bout was 180 cm. For each subject, 5–15 bouts of walking were timed and recorded (x̄ = 13.2).
Data were collected from late August to late October 2014. To standardize observations, they were conducted between 8:00–11:00am. Subjects were fed at 7:30am. Food was present in the cages during data collection. Data collection during this time ensured observations were conducted during morning daylight hours when it was cooler outside and the baboons were most active. These procedures avoided variable behaviors among the subjects, such as nervous pacing while waiting for food. To ensure that the study data represented normal walking speed, data were recorded only for instances of walking that were not toward a food source and did not involve fleeing, chasing, or displacement. These approaches minimized the amount of time it took to collect the data as well as the potential influence of circadian rhythms. Social hierarchy, seasonality, and reproductive cycle timing were not considered; these factors are not known to be related to normal walking speed in humans or baboons. It was assumed that the observer’s presence did not have an effect on the speed of the animals. The animals are habituated to the presence of observing humans, since they have been the subject of long-term study and are attended to throughout every day by multiple human workers. They never appeared to flee from the observer, only from other baboons.
Statistical analysis
A regression model was developed with walking speed (as measured by the average of all timed bouts for each individual) as the response variable and age (years) and weight (kg) as predictors. Since the relationship of walking speed with age was of primary interest, the model with age only, with age and weight, and with both plus an interaction between age and weight were investigated. The usual regression assumptions (goodness of fit, homoscedasticity, and adequate normality in the distribution of residuals) were considered. Statistical significance was set at p ≤ 0.05.
Results
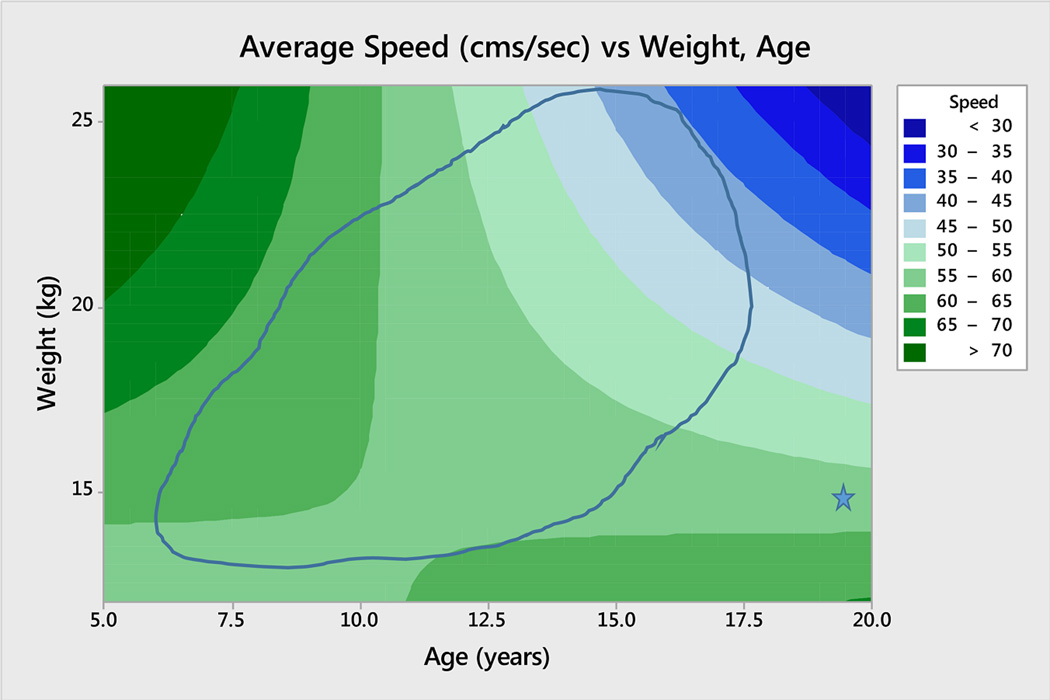
The best model included age, weight, and their interaction. The model equation was S = 15.7 + 3.16×W + 4.20×A − 0.3012×A×W (R2 = 54.7%, up from 34.1% for the model that excluded the interaction term). The overall model was highly statistically significant (F3,29 = 11.68; p < 0.0005, and p = 0.001 for the interaction term, indicating its statistical relevance). Some care should be taken in interpreting this equation, as a casual glance would suggest a positive effect of age (coefficient is 4.20). The inclusion of the interaction term (coefficient is −0.3012) implies that the effect of age changes with weight. The particular value 4.20 becomes operational only when weight is 0, clearly not a meaningful result. In our data, age ranges from 5 years to 20. For 5-year-old animals, the average weight was about 16 kg. Let W = 16 in the regression equation, and we see the coefficient for age for such an animal is 4.20 − .3012*16 = −0.6, indicating a slightly negative effect of age. As they age (and on average get heavier), the coefficient for age becomes more and more negative. Had a 5-year-old not gained weight, the effect of age would have stayed at about −0.6 cms/sec with each advancing year, a slow decline in speed with age. It is perhaps easier to grasp the nature of the relationship visually for such a model; mean values for a suite of ages and weights are depicted in Figure 1. For direct comparison with the results of Shively et al. [47], we used our model to estimate mean walking speeds for 9-year-old and for 20-year-old baboons. Among our animals, 9-year-old baboons weighed approximately 18 kg; for them, mean walking speed was 63.5 cm/sec. For 20-year-old animals (for whom mean weight was 25 kg), mean speed was 29.7 cm/sec. The speed of the younger animals was more than twice that of older. That said, many of the animals in our study were obese, and heavier as they aged; those in Shively et al. [47] were not. If we take 16 kg as a more typical weight for adult female baboons (and assume no gain in weight with age), then the relevant mean speeds for 9-year-olds and 20-year-olds are 62.5 cm/sec and 55.7 cm/sec, respectively. In this case, the speed of younger animals is approximately 12% faster than the older.
Figure 1.
Contour plot of mean speed as it changes with weight and age. The blue closed shape shows where most of the data points lie, on the two-dimensional weight and age continuum (the star is an older animal who is quite lighter than her peers). As animals age, they tend to gain weight (at least those in this study, many of whom are obese); that combination leads to the reduction in walking speed.
Discussion
Baboons and macaques are the most common primates studied in experimental biomedical investigations [14]. Ancestors of baboons and macaques split with hominoids when the Hominoidea split from the Cercopithecoidea, making them the phylogenetically closest living relatives to humans of any biomedical model besides chimpanzees [22, 57]. A major advantage of the baboon model, in addition to their similarity to humans, is the behavior and biology of baboons have been studied extensively in captivity and in the wild [8, 30, 50]. Given the prevalence and utility of baboons as biomedical models, identifying baboon aging biomarkers is of great importance to research on the aging process. Baboon aging biomarkers already identified include bone mineral density [23, 31], cellular senescence [24, 29], hepatic pseudocapillarization [13], and levels of adrenal hormones like cortisol and didehydroepiandrosterone [36]. All of these biomarkers require sedation and/or invasive procedures for measurement, such as biopsy, blood draw, or euthanasia. We sought to determine whether walking speed, a noninvasive behavioral measure, can serve as an aging biomarker in baboons, since walking speed is a useful aging biomarker in humans [1, 10, 15, 16, 19, 38, 49] and several other Old World monkey species [47, 55].
Walking speed declined with age, as predicted by our hypothesis. This effect was modulated by weight; the decrease in speed was greater when associated with heavier animals. Predictions generated by our model indicate that for obese female baboons, walking speed is less than half as fast in 20-year-olds as it is in 9-year-olds. Our model predicts that the difference is less pronounced in non-obese individuals, with a decrease in speed between young and old of 12%. These findings correspond with those from the other two studies of walking speed in Old World monkeys. However, to our knowledge, consideration of weight has not been included in previous analyses. Walton et al. [55] found that a linear regression model robustly describes the relationship between speed and age in rhesus monkeys; in their model, speed of movement is predicted to decline by 0.2 cm/sec per year. In our baboon model, for a lighter animal (e.g., 16 kg), speed declines by approximately 0.6 cm/sec with each advancing year. For each additional kg of weight, speed declined an additional 0.3 cm/sec each year. Shively et al. [47] studied a dichotomous sample of two groups, young (x̄ = 9 yrs) and old (x̄ = 20 yrs) female monkeys, finding young monkeys to be 17% faster; they did not include middle-aged monkeys in their sample of cynomolgus macaques, bonnet macaques, and African green monkeys. There is a need for continuous data to develop precise models of age-related decline in walking speed and determine whether there are any specific break points in the changes.
These data demonstrate that walking speed is a useful aging biomarker in baboons, as it is in humans. In humans, walking speed declines by a small amount with every adult decade (~1–2%), until the beginning to middle of the sixth decade of life (ages 60–65), when it declines significantly with each decade (~12–16%) [25, 45, 48]. Human walking speed is highly associated with morbidity and mortality [1, 10, 15, 16, 19, 38, 49]. Specifically, walking speed is a significant predictor of cardiovascular disease [37, 38], mobility limitation [38], disability [1, 21, 38], cognitive impairment [1, 27, 42–44, 56], institutionalization [1], and falls [1, 18]. Because of this strong relationship between walking speed and adverse health outcomes, one meta-analysis concluded that walking speed as a single-item tool is just as reliable as composite tools in predicting poor health outcomes [1].
The ease of measuring walking speed combined with its reliability as a health predictor has garnered praise for its value in human clinical evaluations [10]. Walking speed is also easy to measure in monkeys [47], and is a very inexpensive way to obtain robust, reliable data, requiring only nontoxic indelible markers, a tape measure, and a stopwatch. All individuals in a social group of 7–15 can be sampled simultaneously within a single three-hour period. Depending on the design of the housing, cleaning the floors, waiting for them to dry, and then drawing the landmarks can be accomplished in just a few hours (depending on weather) within the week prior to data collection.
Identifying aging biomarkers is important to understanding biological factors contributing to healthy aging. Rodents have been the primary models of age-related physical mobility studied thus far [2, 26, 53]. However, data from primates are essential because they are large animals whose central nervous system is similar to that of humans [47]. The central nervous system is thought to be important to age-related decline in physical mobility, but this relationship is poorly studied [44]. Moreover, Old World monkeys are important for skeletal research because their skeletal structure and composition are more similar to those of humans than are rats in important ways [2, 26, 31, 53]. Baboons have a similar age-related decline in bone mineral density to humans, making them a particularly good model for aging studies [23, 31]. Bone mass in baboons is affected by many of the same genetic and environmental factors as in humans, and heritability of bone mass is similar in baboons and humans [31]. These characteristics make baboons a good model for osteoporosis and osteoarthritis [23, 28, 31].
Since walking speed is a good indicator of physical mobility in humans and is highly associated with morbidity and mortality, having a similar measure in nonhuman primates to associate to other age-related changes, such as metabolism and endocrine function, enhances the translational value of studies in these species. Having a standard biomarker allows for manipulations of the environment and subsequent correlation of observed changes with the changes that occur in the biomarker, assisting in the identification of biological systems potentially contributing to decline in walking speed [47]. Additionally, characterization of walking speed as an aging biomarker in baboons has the potential to lead to assessment of value of interventions to enhance physical mobility late in life, and it allows for assessment of whether clinical interventions are working.
A limitation of this study is that it only included females. Due to the composition of breeding groups of captive baboons, which consist of one male and many females, there were too few old age males available to study. Although obtaining data for males should be a goal, female data are required because among humans, there are more aged females as well as more frail females than males [51, 54].
In conclusion, walking speed declines with age in humans and in baboons. Walking speed is a reliable predictor of morbidity and mortality, and is easy and inexpensive to measure. These features make walking speed a highly useful biomarker for aging research. Establishing walking speed as an aging biomarker in baboons opens the door for studying how different life experiences contribute to the aging process.
Acknowledgments
Funding
This work was supported by funding from a European Union F7 grant, 5R24 RR021367 (Nutrient Restriction and Developmental Programming), and R21HD057480 (Effects of Prenatal Nutrition on Developmental Outcomes in the Juvenile Baboon).
Special thanks are due to Steve Rios for his support in study preparation and animal handling. We also thank Martha Avila and Sam Vega for their skilled maintenance of the animals, and Sue Jenkins for data archiving and analysis.
Footnotes
Institution at Which Work Was Performed
This work was performed at the Southwest National Primate Research Center, Texas Biomedical Research Institute, San Antonio, TX, USA.
References
- 1.Abellan van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging. 2009;13(10):881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 2.Aerssens J, Boonen S, Lowet G, Dequeker J. Interspecies differences in bone composition, density, and quality: potential implications for in vivo bone research. Endocrinology. 1998;139(2):663–670. doi: 10.1210/endo.139.2.5751. [DOI] [PubMed] [Google Scholar]
- 3.Altmann J. Observational study of behavior: Sampling methods. Behaviour. 1974;49(3/4):227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- 4.Altmann J, Gesquiere L, Galbany J, Onyango PO, Alberts SC. Life history context of reproductive aging in a wild primate model. Ann. N. Y. Acad. Sci. 2010;1204(1):127–138. doi: 10.1111/j.1749-6632.2010.05531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonow-Schlorke I, Ebert M, Li C, et al. Lack of effect of antenatal glucocorticoid therapy in the fetal baboon on cerebral cortical glucose transporter proteins. J. Med. Primatol. 2007;36(1):17–20. doi: 10.1111/j.1600-0684.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- 6.Antonow-Schlorke I, Schwab M, Cox LA, et al. Vulnerability of the fetal primate brain to moderate reduction in maternal global nutrient availability. Proc. Natl. Acad. Sci. 2011;108(7):3011–3016. doi: 10.1073/pnas.1009838108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonow-Schlorke I, Schwab M, Li C, Nathanielsz PW. Glucocorticoid exposure at the dose used clinically alters cytoskeletal proteins and presynaptic terminals in the fetal baboon brain. J. Physiol. 2003;547(1):117–123. doi: 10.1113/jphysiol.2002.025700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brent L. The study of captive baboon behavior. In: VandeBerg JL, Williams-Blangero S, Tardif SD, editors. The Baboon in Biomedical Research. New York: Springer; 2009. pp. 21–34. [Google Scholar]
- 9.Bronikowski AM, Alberts SC, Altmann J, Packer C, Carey KD, Tatar M. The aging baboon: Comparative demography in a non-human primate. Proc. Natl. Acad. Sci. 2002;99(14):9591–9595. doi: 10.1073/pnas.142675599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cesari M, Kritchevsky SB, Penninx BWHJ, et al. Prognostic value of usual gait speed in well-functioning older people—Results from the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2005;53(10):1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 11.Choi J, Li C, McDonald TJ, Comuzzie A, Mattern V, Nathanielsz PW. Emergence of insulin resistance in juvenile baboon offspring of mothers exposed to moderate maternal nutrient reduction. Am. J. Physiol. - Regul. Integr. Comp. Physiol. 2011;301(3):R757–R762. doi: 10.1152/ajpregu.00051.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coelho AM. Baboon dimorphism: growth in weight, length and adiposity from birth to 8 years of age. In: Watts ES, editor. Nonhuman Primate Models for Human Growth and Development. New York: Alan R. Liss; 1985. pp. 125–159. [Google Scholar]
- 13.Cogger VC, Warren A, Fraser R, Ngu M, McLean AJ, Le Couteur DG. Hepatic sinusoidal pseudocapillarization with aging in the non-human primate. Exp. Gerontol. 2003;38(10):1101–1107. doi: 10.1016/j.exger.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Conlee KM, Hoffeld EH, Stephens ML. A demographic analysis of primate research in the United States. Altern. Lab. Anim. ATLA. 2004;32(Suppl 1A):315–322. doi: 10.1177/026119290403201s52. [DOI] [PubMed] [Google Scholar]
- 15.Cooper R, Kuh D, Cooper C, et al. Objective measures of physical capability and subsequent health: a systematic review. Age Ageing. 2011;40(1):14–23. doi: 10.1093/ageing/afq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper R, Kuh D, Hardy R Mortality Review Group, on behalf of the FALCon and HALCyon study teams. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467–c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cox LA, Nijland MJ, Gilbert JS, et al. Effect of 30 per cent maternal nutrient restriction from 0.16 to 0.5 gestation on fetal baboon kidney gene expression. J. Physiol. 2006;572(1):67–85. doi: 10.1113/jphysiol.2006.106872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dargent-Molina P, Schott AM, Hans D, et al. Separate and combined value of bone mass and gait speed measurements in screening for hip fracture risk: results from the EPIDOS study. Osteoporos. Int. 1999;9(2):188–192. doi: 10.1007/s001980050134. [DOI] [PubMed] [Google Scholar]
- 19.Ferrucci L, Penninx BW, Leveille SG, et al. Characteristics of nondisabled older persons who perform poorly in objective tests of lower extremity function. J. Am. Geriatr. Soc. 2000;48(9):1102–1110. doi: 10.1111/j.1532-5415.2000.tb04787.x. [DOI] [PubMed] [Google Scholar]
- 20.Glassman DM, Coelho AM, Carey KD, Bramblett CA. Weight growth in savannah baboons: a longitudinal study from birth to adulthood. Growth. 1984;48(4):425–433. [PubMed] [Google Scholar]
- 21.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower Extremity Function and Subsequent Disability Consistency Across Studies, Predictive Models, and Value of Gait Speed Alone Compared With the Short Physical Performance Battery. J. Gerontol. A. Biol. Sci. Med. Sci. 2000;55(4):M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartwig W. Primate evolution. In: Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Bearder SK, editors. Primates in Perspective. New York: Oxford University Press; 2007. pp. 11–22. [Google Scholar]
- 23.Havill LM, Mahaney MC, Czerwinski SA, Carey KD, Rice K, Rogers J. Bone mineral density reference standards in adult baboons (Papio hamadryas) by sex and age. Bone. 2003;33(6):877–888. doi: 10.1016/s8756-3282(03)00231-x. [DOI] [PubMed] [Google Scholar]
- 24.Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311(5765):1257. doi: 10.1126/science.1122446. [DOI] [PubMed] [Google Scholar]
- 25.Himann JE, Cunningham DA, Rechnitzer PA, Paterson DH. Age-related changes in speed of walking. Med. Sci. Sports Exerc. 1988;20(2):161–166. doi: 10.1249/00005768-198820020-00010. [DOI] [PubMed] [Google Scholar]
- 26.Ingram DK. Age-related decline in physical activity: generalization to nonhumans. Med. Sci. Sports Exerc. 2000;32(9):1623–1629. doi: 10.1097/00005768-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Inzitari M, Newman AB, Yaffe K, et al. Gait speed predicts decline in attention and psychomotor speed in older adults: The Health Aging and Body Composition Study. Neuroepidemiology. 2008;29(3–4):156–162. doi: 10.1159/000111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jerome CP, Peterson PE. Nonhuman primate models in skeletal research. Bone. 2001;29(1):1–6. doi: 10.1016/s8756-3282(01)00477-x. [DOI] [PubMed] [Google Scholar]
- 29.Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U. Accumulation of senescent cells in mitotic tissue of aging primates. Mech. Ageing Dev. 2007;128(1):36–44. doi: 10.1016/j.mad.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jolly CJ. Baboons, mandrills, and mangabeys: Afro-Papionin socioecology in a phylogenetic perspective. In: Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Bearder SK, editors. Primates in Perspective. New York: Oxford University Press; 2007. pp. 240–251. [Google Scholar]
- 31.Kammerer CM, Sparks ML, Rogers J. Effects of age, sex, and heredity on measures of bone mass in baboons (Papio hamadryas) J. Med. Primatol. 1995;24(4):236–242. doi: 10.1111/j.1600-0684.1995.tb00176.x. [DOI] [PubMed] [Google Scholar]
- 32.Li C, McDonald TJ, Wu G, Nijland MJ, Nathanielsz PW. Intrauterine growth restriction alters term fetal baboon hypothalamic appetitive peptide balance. J. Endocrinol. 2013;217(3):275–282. doi: 10.1530/JOE-13-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li C, Ramahi E, Nijland MJ, et al. Up-regulation of the fetal baboon hypothalamo-pituitary-adrenal axis in intrauterine growth restriction: Coincidence with hypothalamic glucocorticoid receptor insensitivity and leptin receptor down-regulation. Endocrinology. 2013;154(7):2365–2373. doi: 10.1210/en.2012-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li C, Schlabritz-Loutsevitch NE, Hubbard GB, et al. Effects of maternal global nutrient restriction on fetal baboon hepatic insulin-like growth factor system genes and gene products. Endocrinology. 2009;150(10):4634–4642. doi: 10.1210/en.2008-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDonald TJ, Nijland MJ, Nathanielsz PW. The insulin-like growth factor system and the fetal brain: Effects of poor maternal nutrition. Rev. Endocr. Metab. Disord. 2007;8(2):71–84. doi: 10.1007/s11154-007-9044-2. [DOI] [PubMed] [Google Scholar]
- 36.Muehlenbein MP, Campbell BC, Richards RJ, et al. Dehydroepiandrosterone-sulfate as a biomarker of senescence in male non-human primates. Exp. Gerontol. 2003;38(10):1077–1085. doi: 10.1016/j.exger.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J. Gerontol. A. Biol. Sci. Med. Sci. 2001;56(3):M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 38.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA J. Am. Med. Assoc. 2006;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 39.Nijland MJ, Mitsuya K, Li C, et al. Epigenetic modification of fetal baboon hepatic phosphoenolpyruvate carboxykinase following exposure to moderately reduced nutrient availability. J. Physiol. 2010;588(8):1349–1359. doi: 10.1113/jphysiol.2009.184168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nijland MJ, Schlabritz-Loutsevitch NE, Hubbard GB, Nathanielsz PW, Cox LA. Non-human primate fetal kidney transcriptome analysis indicates mammalian target of rapamycin (mTOR) is a central nutrient-responsive pathway. J. Physiol. 2007;579(3):643–656. doi: 10.1113/jphysiol.2006.122101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez JS, Bartlett TQ, Keenan KE, Nathanielsz PW, Nijland MJ. Sex-dependent cognitive performance in baboon offspring following maternal caloric restriction in pregnancy and lactation. Reprod. Sci. 2012;19(5):493–504. doi: 10.1177/1933719111424439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosano C, Newman AB, Katz R, Hirsch CH, Kuller LH. Association between lower digit symbol substitution test score and slower gait and greater risk of mortality and of developing incident disability in well-functioning older adults. J. Am. Geriatr. Soc. 2008;56(9):1618–1625. doi: 10.1111/j.1532-5415.2008.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosano C, Studenski SA, Aizenstein HJ, Boudreau RM, Longstreth WT, Newman AB. Slower gait, slower information processing and smaller prefrontal area in older adults. Age Ageing. 2012;41(1):58–64. doi: 10.1093/ageing/afr113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosso AL, Studenski SA, Chen WG, et al. Aging, the central nervous system, and mobility. J. Gerontol. A. Biol. Sci. Med. Sci. 2013;68(11):1379–1386. doi: 10.1093/gerona/glt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samson MM, Crowe A, de Vreede PL, Dessens JA, Duursma SA, Verhaar HJ. Differences in gait parameters at a preferred walking speed in healthy subjects due to age, height and body weight. Aging Milan Italy. 2001;13(1):16–21. doi: 10.1007/BF03351489. [DOI] [PubMed] [Google Scholar]
- 46.Schlabritz-Loutsevitch NE, Howell K, Rice K, et al. Development of a system for individual feeding of baboons maintained in an outdoor group social environment. J. Med. Primatol. 2004;33(3):117–126. doi: 10.1111/j.1600-0684.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 47.Shively CA, Willard SL, Register TC, et al. Aging and physical mobility in group-housed Old World monkeys. AGE. 2012;34(5):1123–1131. doi: 10.1007/s11357-011-9350-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shumway-Cook A, Guralnik JM, Phillips CL, et al. Age-Associated Declines in Complex Walking Task Performance: The Walking InCHIANTI Toolkit. J. Am. Geriatr. Soc. 2007;55(1):58–65. doi: 10.1111/j.1532-5415.2006.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA J. Am. Med. Assoc. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Swedell L. African papionins: diversity of social organization and ecological flexibility. In: Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Bearder SK, editors. Primates in Perspective. New York: Oxford University Press; 2011. pp. 241–277. [Google Scholar]
- 51.Syddall H, Roberts HC, Evandrou M, Cooper C, Bergman H, Sayer AA. Prevalence and correlates of frailty among community-dwelling older men and women: findings from the Hertfordshire Cohort Study. Age Ageing. 2009;39(2):197–203. doi: 10.1093/ageing/afp204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson DD, Simmons HA, Pirie CM, Ke HZ. FDA guidelines and animal models for osteoporosis. Bone. 1995;17(4, Supplement):S125–S133. doi: 10.1016/8756-3282(95)00285-l. [DOI] [PubMed] [Google Scholar]
- 53.Walston J, Fried LP. Frailty and the older man. Med. Clin. North Am. 1999;83(5):1173–1194. doi: 10.1016/s0025-7125(05)70157-7. [DOI] [PubMed] [Google Scholar]
- 54.Walton A, Branham A, Gash DM, Grondin R. Automated video analysis of age-related motor deficits in monkeys using EthoVision. Neurobiol. Aging. 2006;27(10):1477–1483. doi: 10.1016/j.neurobiolaging.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 55.Watson NL, Rosano C, Boudreau RM, et al. Executive function, memory, and gait speed decline in well-functioning older adults. J. Gerontol. A. Biol. Sci. Med. Sci. 2010;65A(10):1093–1100. doi: 10.1093/gerona/glq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xing J, Wang H, Han K, et al. A mobile element based phylogeny of Old World monkeys. Mol. Phylogenet. Evol. 2005;37(3):872–880. doi: 10.1016/j.ympev.2005.04.015. [DOI] [PubMed] [Google Scholar]