Advances in treatments for multiple myeloma (MM) have resulted in improved survival. However, patients who have become refractory to novel agents have a poor prognosis, with a median overall survival (OS) of nine months, or as low as three months in the absence of further treatment after failure of regimens including bortezomib (BORT), thalidomide (THAL) or lenalidomide (LEN).1 After multiple lines of therapy, the health-related quality of life (HRQoL) of patients with relapsed and/or refractory MM (RRMM) is often compromised,2 with impairments in physical, emotional, social and cognitive functioning.3
Pomalidomide (POM) was recently approved in the US and Europe in combination with dexamethasone for use in patients with RRMM who have received at least two prior treatment regimens, including both LEN and BORT, and have demonstrated disease progression on their last therapy. In the pivotal MM-003 trial, POM+LoDEX provided significant and clinically meaningful improvements in progression-free survival (PFS) and OS compared with high-dose dexamethasone (HiDEX), a commonly used salvage treatment for heavily pre-treated patients at the time the study was initiated.4 MM-003 was the first study to investigate HRQoL in RRMM patients receiving POM. Here, we present HRQoL results of cross-sectional and longitudinal analyses that were included as pre-specified secondary end points.
The design of MM-003 has been reported previously4 and is summarized in Figure 1. Enrolled patients were refractory to their last treatment and had failed BORT and LEN after at least two cycles of each (alone or in combination). Patients progressing on HiDEX were allowed to receive POM in a companion MM-003C trial (Figure 1); these patients were not included in the HRQoL analysis after progression from their trial regimen.
Figure 1.
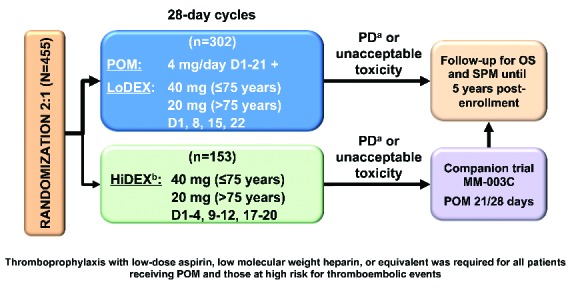
Trial design. aProgression of disease was independently adjudicated in real time. bHiDEX was chosen as comparator because at the time of trial design it was the standard salvage therapy for heavily pre-treated patients. D: days; HiDEX: high-dose dexamethasone; LoDEX: low-dose dexamethasone; OS: overall survival; PD: progressive disease; POM: pomalidomide; SPM: second primary malignancy.
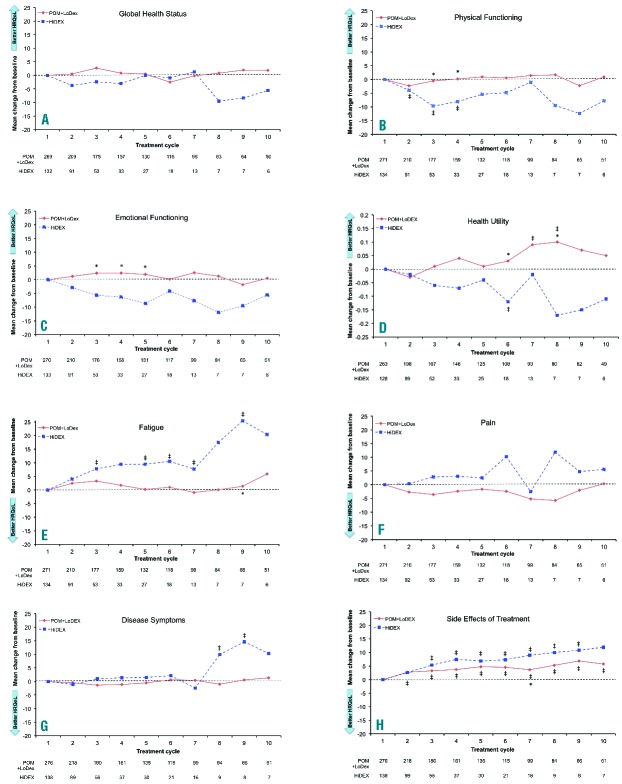
Three HRQoL instruments that have been validated previously in MM patients5 were included as pre-defined end points for non-progressing patients and scored according to their user manuals: the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30, EORTC QLQ-MY20, and EQ-5D. The analysis focused on eight pre-selected domains chosen following a workshop discussion with specialists on perceived clinical relevance and on results of multivariate regression analysis comparing domains with the EQ-5D utility index.6 The domains included five from EORTC QLQ-C30 (Global Health Status, Physical Functioning, Emotional Functioning, Fatigue, and Pain), two from EORTC QLQ-MY20 (Disease Symptoms, Side Effects of Treatment), and the EQ-5D Health Utility value (mapped to a scale with 1 = best health state and 0 = death using a time trade-off methodology).
Patients completed HRQoL questionnaires at baseline, on day 1 of each treatment cycle, and at discontinuation. HRQoL scores were calculated from baseline through to cycle (C) 10 in the cross-sectional analyses. Later cycle data were limited in value due to few remaining patients. Intention-to-treat (ITT) subjects with at least one HRQoL measurement in this period were included in the analyses.
We compared changes in HRQoL scores from baseline and between treatment arms at each cycle by cross-sectional and longitudinal analyses. Cross-sectional descriptive and comparative (between and within treatment arms) analyses used domain scores analyzed as continuous variables. Longitudinal repeated measure mixed effects models were generated to estimate the treatment effect on HRQoL over time and between treatment arms, and adjusted means of score differences from baseline were calculated.
Of the 455 ITT subjects, 433 completed HRQoL measurements and were included in this analysis (POM+LoDEX n=289; HiDEX n=144). Median patient age was 64 years, and over 94% (410/433) had received more than 2 prior therapies; 82% (355 of 433) were refractory to LEN and BORT. Median follow up was ten months. There was no significant difference in patients’ base-line demographics and disease-related characteristics between arms.4
Of the 289 patients randomized to receive POM+LoDEX, 17.6% (51 of 289) had EORTC QLQ-C30 and QLQ-MY20 available data at C10 versus 5.5% (8 of 144) of HiDEX patients; 17.3% (50 of 289) of POM+LoDEX patients had EQ-5D data available at C10 versus 4.8% (7 of 144) of HiDEX patients.
Compliance rates up to C10 were generally high (≥77.8% of presenting patients across cycles) and were consistent between treatment groups. Due to the progressive nature of MM, the number of participating patients dropped as the study progressed. Patients in the HiDEX group discontinued from the study earlier than those in the POM+LoDEX group.4 Reasons for discontinuation were comparable between treatment arms.
In the cross-sectional analysis, mean score improved significantly from baseline (P<0.05, paired t-test) in the POM+LoDEX arm for the Health Utility domain, and deteriorated significantly from baseline for Side Effects of Treatment. In the HiDEX arm, no domains showed improvement, and 5 showed deterioration from baseline: Physical Functioning, Health Utility, Fatigue, Disease Symptoms, and Side Effects of Treatment (Figure 2, indicated by ‡).
Figure 2.
Cross-sectional analysis of mean HRQoL score changes from baseline per treatment cycle. *P<0.05 unpaired t-test comparing the two treatment groups from baseline; ‡P<0.05 paired t-test (within group mean change from baseline).
Between treatment groups, significant differences (P<0.05, unpaired t-test) in favor of POM+LoDEX were observed in five of the eight pre-selected domains (Physical Functioning, Emotional Functioning, Health Utility, Fatigue, and Side Effects of Treatment) at specific treatment cycles (Figure 2, indicated by *). Trends (P<0.1, unpaired t-test) in favor of POM+LoDEX were observed at specific time points for the Health Utility, Fatigue, and Disease Symptoms domains. HiDEX treatment was not superior to POM+LoDEX in any domain. Results of subgroup analyses in patients aged over 75 years and 75 years or under were consistent with the overall findings.
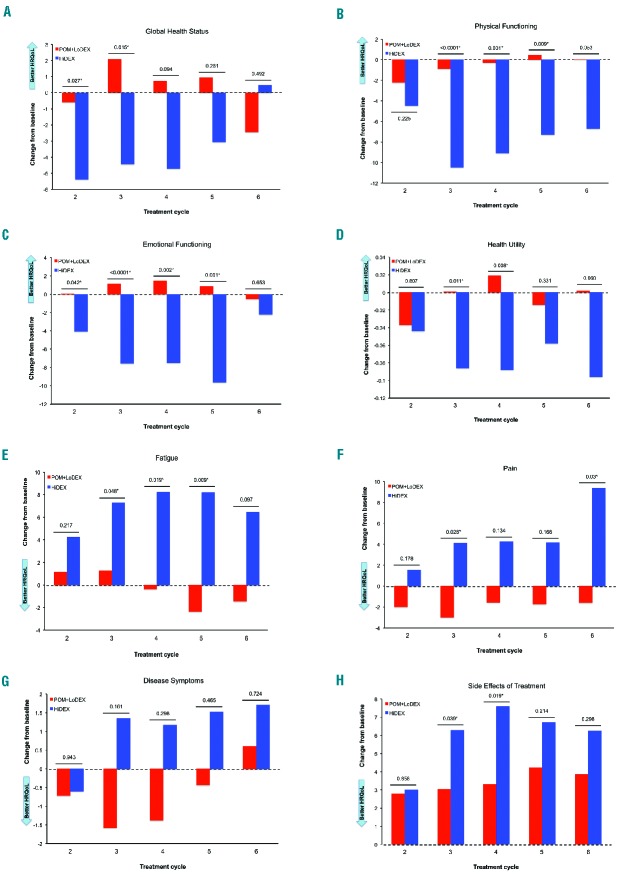
To understand how the HRQoL progressed with time we performed longitudinal analyses using repeated measure mixed-effect models. There were significant (P<0.05) overall treatment differences between POM+LoDEX and HiDEX over the course of treatment in seven of the eight pre-selected clinically relevant domains: Global Health Status (P=0.0451), Physical Functioning (P<0.0001), Emotional Functioning (P=0.0003), Health Utility (P=0.005), Fatigue (P=0.0008), Pain (P=0.0049), and Side Effects of Treatment (P=0.0253). Only Disease Symptoms did not demonstrate a significant difference. Comparisons of adjusted means of score differences from baseline between treatment arms at each cycle confirmed the superiority of POM+LoDEX treatment arm in these seven domains (Figure 3).
Figure 3.
Repeated measure mixed model score changes from baseline (adjusted means). P values indicate unpaired t-test comparing treatment groups. *Significant P value (P<0.05).
We show that in heavily pre-treated RRMM patients who failed BORT and LEN, POM+LoDEX not only results in significantly better clinical outcomes, but also in significantly better HRQoL compared with HiDEX treatment.4
The cross-sectional analyses show that across key clinically relevant HRQoL domains associated with MM, patients on POM+LoDEX had better HRQoL during the first 10 treatment cycles compared with those receiving HiDEX. The significant within-group improvement from baseline over the course of treatment in Health Utility for POM+LoDEX patients and the significant deteriorations from baseline observed in the HiDEX arm in the Physical Functioning, Health Utility, Fatigue, Disease Symptoms, and Side Effects of Treatment domains, as well as significant between-group differences in favor of POM+LoDEX in the Physical Functioning, Emotional Functioning, Health Utility, Fatigue, and Side Effects of Treatment domains at given cycles indicate better HRQoL among patients on POM+LoDEX versus HiDEX. There was no significant worsening of scores from baseline in the POM+LoDEX arm for 7 of the 8 pre-selected domains, indicating that POM+LoDEX does not adversely affect HRQoL. Maintenance of HRQoL is a benefit in this late line population with limited treatment options. There was no demonstration of worsening with POM, while treatment with HiDEX did show worsening of HRQoL.
The repeated measure mixed-model longitudinal analysis across time points confirmed the significant difference in score changes from baseline favoring the POM+LoDEX versus the HiDEX arm over the course of treatment for seven of the eight evaluated domains, despite cross-sectional results at individual cycles.
These findings, together with the primary efficacy results of the MM-003 trial, demonstrate that POM+LoDEX is a safe and effective treatment option for RRMM that does not negatively affect HRQoL, and offers improved HRQoL in key domains versus HiDEX. Given that even minor changes in patient-related outcomes may have long-term implications for RRMM patients, these results are meaningful.
Our approach to evaluate HRQoL is consistent with prior studies of novel oral anti-myeloma therapies and dexamethasone in RRMM,7–9 and is strengthened by considering HRQoL changes over time, the regular timing of assessments, and the randomized study design. It should, however, be borne in mind that the MM-003 study was powered to detect differences in PFS rather than pre-specified HRQoL end points.
There are adverse events associated with HiDEX that can negatively affect HRQoL. POM+LoDEX did not show a deterioration of HRQoL for 7 of 8 analyzed domains despite this regimen containing two agents compared to single agent HiDEX.
In heavily pre-treated RRMM patients with end-stage disease, maintaining HRQoL may be an important clinical decision factor. The HRQoL domains evaluated here are directly related to quality of survival, and may be helpful in making treatment decisions.10–12 Response in MM patients should not be the unique outcome; other end points, such as safety and HRQoL must also be considered, as some patients may derive greater benefit from a lower level of response while achieving prolonged survival without facing unnecessary risk.13
POM+LoDEX has become a standard of care treatment in the US for relapsing patients who have received LEN and BORT, and has seen rapid uptake in European countries since its introduction.14 We show that this treatment combination leads to significant HRQoL improvements that could potentially enhance the lives of patients with RRMM.
Acknowledgments
The authors would like to thank the patients who participated in this study, the staff members at the study sites who cared for them, and the representatives of the sponsors who were involved in data gathering and analyses. We thank Vanessa Gray-Schopfer and Ron Hogg, OmniScience SA, Adam Hutchings, Global Market Access Solutions (GMAS), and Craig Gibson, Celgene, who provided medical writing services and assistance funded by Celgene Corporation. The authors were fully responsible for contents and editorial decisions for this manuscript.
Footnotes
Funding: this research was funded by the Celgene Corporation.
Trial registration: clinicaltrials.gov identifier NCT01311687.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26(1):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boland E, Eiser C, Ezaydi Y, Greenfield DM, Ahmedzai SH, Snowden JA. Living with advanced but stable multiple myeloma: a study of the symptom burden and cumulative effects of disease and intensive (hematopoietic stem cell transplant-based) treatment on health-related quality of life. J Pain Symptom Manage. 2013; 46(5):671–680. [DOI] [PubMed] [Google Scholar]
- 3.Molassiotis A, Wilson B, Blair S, Howe T, Cavet J. Unmet supportive care needs, psychological well-being and quality of life in patients living with multiple myeloma and their partners. Psychooncology. 2011;20(1):88–97. [DOI] [PubMed] [Google Scholar]
- 4.Miguel JS, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(11):1055–1066. [DOI] [PubMed] [Google Scholar]
- 5.Kvam AK, Fayers PM, Wisloff F. Responsiveness and minimal important score differences in quality-of-life questionnaires: a comparison of the EORTC QLQ-C30 cancer-specific questionnaire to the generic utility questionnaires EQ-5D and 15D in patients with multiple myeloma. Eur J Haematol. 2011;87(4):330–337. [DOI] [PubMed] [Google Scholar]
- 6.Proskorovsky I, Lewis P, Williams CD, et al. Mapping EORTC QLQ-C30 and QLQ-MY20 to EQ-5D in patients with multiple myeloma. Health Qual Life Outcomes. 2014;12(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SJ, Richardson PG, Sonneveld P, et al. Bortezomib is associated with better health-related quality of life than high-dose dexamethasone in patients with relapsed multiple myeloma: results from the APEX study. Br J Haematol. 2008;143(4):511–519. [DOI] [PubMed] [Google Scholar]
- 8.Alegre A, Oriol-Rocafiguera A, Garcia-Larana J, et al. Efficacy, safety and quality-of-life associated with lenalidomide plus dexamethasone for the treatment of relapsed or refractory multiple myeloma: the Spanish experience. Leuk Lymphoma. 2012;53(9):1714–1721. [DOI] [PubMed] [Google Scholar]
- 9.Yong K, Alegre Amor A, Browne P, et al. A multicenter, single-arm, open-label safety and quality of life study of lenalidomide plus dexamethasone in previously treated patients with multiple myeloma. Proceedings of the 15th Congress of the European Hematology Association (EHA), Barcelona, Spain Haematologica 2010;95(S2):392 (Abstract 0944). [Google Scholar]
- 10.Strasser-Weippl K, Ludwig H. Psychosocial QOL is an independent predictor of overall survival in newly diagnosed patients with multiple myeloma. Eur J Haematol. 2008;81(5):374–379. [DOI] [PubMed] [Google Scholar]
- 11.Mols F, Oerlemans S, Vos AH, et al. Health-related quality of life and disease-specific complaints among multiple myeloma patients up to 10 yr after diagnosis: results from a population-based study using the PROFILES registry. Eur J Haematol. 2012;89(4):311–319. [DOI] [PubMed] [Google Scholar]
- 12.Terpos E, Berenson J, Cook RJ, Lipton A, Coleman RE. Prognostic variables for survival and skeletal complications in patients with multiple myeloma osteolytic bone disease. Leukemia. 2010;24(5):1043–1049. [DOI] [PubMed] [Google Scholar]
- 13.Lonial S, Anderson KC. Association of response endpoints with survival outcomes in multiple myeloma. Leukemia. 2014;28(2):258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimopoulos MA, Leleu X, Palumbo A, et al. Expert panel consensus statement on the optimal use of pomalidomide in relapsed and refractory multiple myeloma. Leukemia. 2014;28(8):1573–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]