Abstract
It is estimated that 0.6–1% of the population in the USA and Canada fulfil the Diagnostic and Statistical Manual of Mental Disorders, 5th ed. (DSM-5) criteria for gambling disorders (GD). To date, there are no approved pharmacological treatments for GD. The rat gambling task (rGT) is a recently developed rodent analogue of the Iowa gambling task in which rats are trained to associate four response holes with different magnitudes and probabilities of food pellet rewards and punishing time-out periods. Similar to healthy human volunteers, most rats adopt the optimal strategies (optimal group). However, a subset of animals show preference for the disadvantageous options (suboptimal group), mimicking the choice pattern of patients with GD. Here, we explored for the first time the effects of various cannabinoid ligands (WIN 55,212-2, AM 4113, AM 630 and URB 597) on the rGT. Administration of the cannabinoid agonist CB1/CB2 WIN 55,212-2 improved choice strategy and increased choice latency in the suboptimal group, but only increased perseverative behaviour, when punished, in the optimal group. Blockade of CB1 or CB2 receptors or inhibition of fatty-acid amide hydrolase did not affect rGT performance. These results suggest that stimulation of cannabinoid receptors could affect gambling choice behaviours differentially in some subgroups of subjects.
Keywords: AM 4113; AM 630; choice strategy; gambling; rat; URB 597; WIN 55,212-2
Introduction
It is estimated that 0.6–1% of the population in the USA and Canada fulfil the Diagnostic and Statistical Manual of Mental Disorders, 5th ed. (DSM-5) criteria for gambling disorder (GD) (Kessler et al., 2008; Williams et al., 2011) and neurobiological research in GD supports its characterization as a behavioural addiction (American Psychiatric Association, 2013). Many scientists and clinicians have long believed that patients with GD have common features with patients with substance-use disorders (Dell’Osso et al., 2006; Villella et al., 2011). Dopamine has long been implicated in addiction processes and early articles postulated a similarly important role for dopamine in GD (Pattij and Vanderschuren, 2008; Zeeb et al., 2013). Drugs that are known to increase dopamine levels consistently increase premature responding in the 5-choice serial reaction time task (5-CSRTT) (van Gaalen et al., 2006). Several studies also support the notion that impulsive behaviour is mediated not only by the dopaminergic system but also by other neurotransmitters including serotonin, noradrenaline and glutamate (Pattij and Vanderschuren, 2008). In recent years, there has been increasing interest in the study of the involvement of the endocannabinoid system in executive functions in which dopamine plays a crucial role, such as behavioural flexibility and reversal learning (Egerton et al., 2006).
The endocannabinoid system might be of relevance to impulsivity and decision-making. Administration of high doses of CB1 receptor agonists increases impulsive behaviours, whereas the administration of low doses of CB1 antagonists improves set-shifting performance and reduces the number of impulsive responses (Hill et al., 2006; Pattij et al., 2007). The synthetic CB1/2 cannabinoid agonist WIN 55,212-2, was found to normalize the impulsive profile of adolescent spontaneously hypertensive rats, a strain with lower CB1 receptor density in the prefrontal cortex compared with control rats (Adriani and Laviola, 2004), suggesting that a reduced cortical density of cannabinoid CB1 receptors is associated with enhanced impulsivity. Other studies have also shown the effects of the inhibition of the anandamide degradation system or CB1 antagonism on self-control behaviours (Marco et al., 2007; Pattij et al., 2007). Within the brain, cannabinoid CB1 receptors are located presynaptically and activation of these receptors inhibits synaptic transmission, which enables the endocannabinoid system to modulate neuronal activity of other transmitter systems (Melis and Pistis, 2007). The endocannabinoid system has been implicated in different behaviours, including the reinforcing effects of drugs of abuse (Le Foll and Goldberg, 2005), food intake (Di Marzo and Matias, 2005) and cognitive processing (Takahashi et al., 2005). CB2, initially described as a peripheral receptor (Maldonado et al., 2006, 2011), has been identified in several brain structures including the striatum, hippocampus and thalamus (Gong et al., 2006), and more recently, into ventral tegmental area neurons (Zhang et al., 2014). It has been suggested that the CB2 receptor participates in central functions and growing evidence suggests a role in addictive processes (Gamaleddin et al., 2012b; Navarrete et al., 2013).
Both marijuana and its principal psychoactive ingredient delta-9-tetrahydrocannabinol (THC) are associated with alterations in response inhibition (McDonald et al., 2003), decision-making (Lane et al., 2005) and behavioural flexibility (Ramaekers et al., 2006) in healthy controls. Chronic cannabis use has also been associated with dysfunctions in decision-making [e.g. in the Iowa gambling task (IGT)] compared with nondrug users (Bolla et al., 2005). However, an obstacle to the rigorous study of factors influencing impulsivity is the large variation present in the human population, which is difficult to both categorize and control. As gambling behaviour is a complex phenomenon involving concurrent activation of different systems, the use of animals is required to study its processes. The complexity of these behaviours is mediated by different neurotransmitters and neuromodulators that work together as a network. Animal models therefore play an increasingly important role in the study of GD-related behaviour as this cannot be mimicked by cellular or molecular models. The prototypical task for studying decision-making in rodents is the rodent gambling task (rGT). An analogue of the IGT used in humans, the rGT allows animals to choose between four options, each associated with varying magnitudes and probabilities of gains and losses (Zeeb et al., 2009). Similar to healthy human volunteers, most rats adopt optimal strategies (optimal group). However, a subset of animals show preference for disadvantageous options (suboptimal group), mimicking the choice pattern of patients with GD.
Altogether, the above studies suggest a role of the endocannabinoid system in executive functions. Endocannabinoids appear to have a negative impact on set-shifting and cognitive flexibility, and CB1 receptor antagonists can improve such executive functions (Hill et al., 2006). There are no published data on the impact of the endocannabinoid system on the performance on the rGT in outbred optimal versus suboptimal rats. To this end, in the present study, we hypothesized that the CB1/2 agonist WIN 55,212-2 would promote greater impulse to select the least optimal choice and that the CB2 antagonist/inverse agonist, AM 630, would have no effect on this task. We also hypothesized that the neutral CB1 antagonist AM 4113 and the selective inhibitor of fatty-acid amide hydrolase, URB 597, would increase self-control and therefore promote the most optimal choice (greatest earnable reward per unit time). Therefore, in this study, we tested the effects of the systemic injection of WIN 55,212-2, AM 4113, AM 630 and URB 597 on decision-making in the rGT.
Methods
Subjects
Male Long–Evans rats (N=24; Charles River Laboratories, Lachine, Quebec, Canada) were used in this study. All animals weighed 300–325 g at the start of the experiment. Animals were individually housed in a temperature-controlled colony room under a 12 h reverse light cycle (lights off at 07:00 h). Testing took place between 12:00 and 16:00 h 5 days/week. Water was freely available, except during testing periods. Rats were food restricted (18–20 g of standard rat chow per day) and maintained at 90% of their free-feeding body weight (food was available immediately after behavioural testing). All experiments were conducted in accordance with the Canadian Council of Animal Care and experimental protocols were approved by the Animal Care Committee of the Centre for Addiction and Mental Health.
Apparatus
A detailed description of the testing chambers has been reported previously (Zeeb et al., 2009; Di Ciano et al., 2015). Briefly, testing took place in traditional 5-CSRTT chambers (Med Associates, St Albans, Vermont, USA). A house light was located at the top of the chamber. Within the chambers, five holes were positioned 2 cm above a metal bar floor. A stimulus light was located at the back of each hole. Only the outer four of the five holes were utilized in all experimental procedures (i.e. the middle hole, three of five, was unused). A food tray with a tray light at the top of the opening was located on the opposite wall. Nose-poke responses into the response holes or food tray were detected by a horizontal infrared beam. Rewards consisted of non-sweet-enriched pellets (45 mg; Bioserv, Frenchtown, New Jersey, USA) that were delivered to the food tray from an external pellet dispenser. All operant chambers were contained within a ventilated and sound-attenuating box. The chambers were controlled by software written in Med-PC (Med Associates, St. Albans, Vermont, USA) running on a Dell desktop computer.
Pre-rGT Training
Animals were trained according to previously described methods (Zeeb et al., 2009). Briefly, animals were trained in 30 min daily sessions to make nose-poke responses, within 10 s, into an illuminated response hole, which resulted in a non-sweet-enriched pellet (Bioserv) being delivered to the food receptacle. The illuminated hole changed each trial with equal numbers of presentations of each possible hole over the course of the session. Each of the three training stages continued until 30 trials (for the two first sessions) or 100 trials (for the third session) were completed, or 30 min had elapsed, whichever came first. For the third stage of the training phase, animals were trained until they accomplished at least 50 correct responses. The difficulty of the program through the three stages of the training phase was increased by shortening the stimulus presentation and limited-hold periods, increasing the intertrial interval (ITI) and altering criteria for progression (Table 1). The final training program had limited hold and stimulus duration lengths of 10 s and an ITI of 5 s. Animals completing two consecutive sessions (100 trials each), with 80% of trials being correct and less than 20% omissions (i.e. no response made within 10 s), were subjected eight daily sessions (30 min each) of a forced-choice version of the rGT, where only one of the holes was illuminated at each time. During the forced-choice version of the rGT, each of the options was associated only with a particular hole and the probability of reward/punishment (and the reward magnitude) was set at this time and remained unchanged; thus, the rats could learn the association of each hole with the different outcomes. Details of the rewards and punishments delivered at each hole are described below. This procedure ensured that all the animals had an equal training experience in terms of rewards (i.e. 45 mg no-sweet enriched diet pellets), specific duration of punishment (i.e. time-out period) and the respective probabilities of each, for the four different holes, thereby preventing the development of simple biases towards a particular hole. Animals were counterbalanced on two versions of the forced-choice rGT (A or B), which differed only in the hole assignment for each contingency (P1–P4; see Fig. 1 for contingencies).
Table 1.
Training parameters across training stages
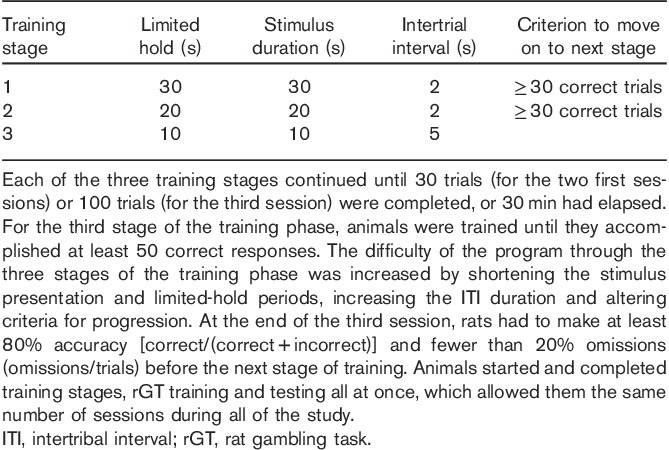
Fig. 1.
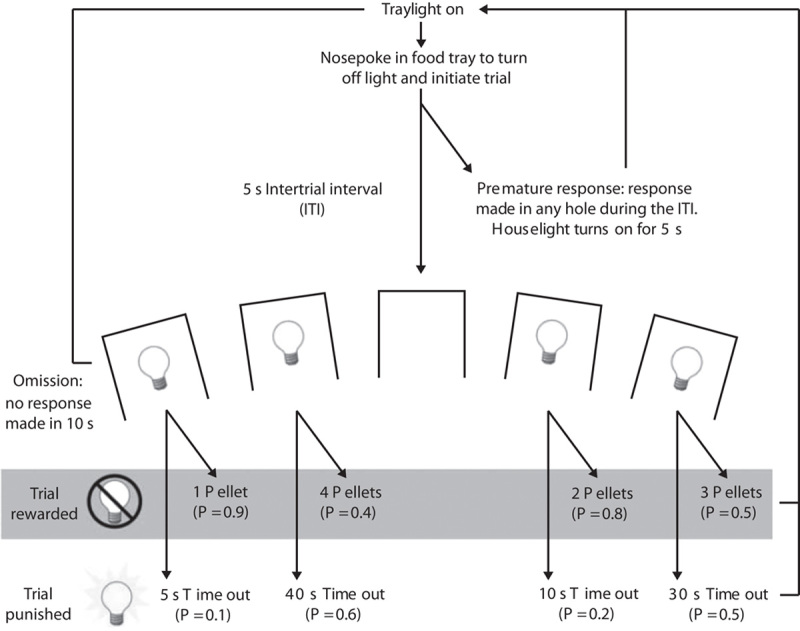
Design of the rat gambling task (rGT). The task began with illumination of the tray light. A nose-poke response in the food tray extinguished the tray light and initiated a new trial. After an intertrial-interval (ITI) of 5 s, four stimulus lights were turned on in holes 1, 2, 4 and 5, and the animal was required to respond in one of these holes within 10 s. This response was then rewarded or punished depending on the reinforcement schedule for that option (indicated by the probability of a win or a loss in brackets for each option). If the animal was rewarded, the stimulus lights were extinguished and the animal received the corresponding number of pellets in the now-illuminated food tray. A response at the food tray then started a new trial. If the animal was punished, the stimulus light in the corresponding hole flashed at a frequency of 0.5 Hz for the duration of the punishing time-out and all other lights were extinguished. At the end of the punishment period, the tray light was turned on and the animal could initiate a new trial. Failure to respond at the illuminated holes resulted in an omission, whereas a response during the ITI was classified as a premature response and punished by a 5-s time-out during which the house light was turned on. Reproduced with permission from Zeeb et al., 2009.
The rGT
The design of the rGT has been described previously (Zeeb et al., 2009) and a diagram of the trial structure and reinforcement schedules is provided in Fig. 1. Briefly, animals were administered 30 daily sessions (30 min each), where they were required to make a nose-poke response into the illuminated food receptacle to start a trial. Once the response was made, the chamber was darkened for a 5 s ITI, during which responses made were recorded as being premature responses and resulted in a 5 s houselight illumination and a time-out period (during which responding had no effect), followed by an illumination of the food receptacle that allowed the animal to begin another trial. If no premature response was made during the ITI, the four response holes were illuminated and the animal had 10 s to nose-poke into any of the four holes. If no response took place during the 10 s, the lights were turned off and the food receptacle light was turned back on, with the trial being counted as an omission. If the animal responded into one of the four holes before 10 s, the four lights were turned off and the trial was either rewarded [i.e. the food receptacle was illuminated and pellet(s) were delivered according to the contingency for the chosen hole; collection of the reward resulted in initiation of a new trial] or punished (the stimulus light of the chosen hole flashed at 0.5 Hz for the duration of the time-out period, according to the contingency of the chosen hole, following which the food receptacle was illuminated, allowing for a new trial to be initiated). The size of reward and punishment probability/duration are shown in Table 2. Perseverative responses made at the array or food tray following a reward or during the time-out periods were recorded, but not punished.
Table 2.
Reward and punishment received for the various response options in the rat gambling task
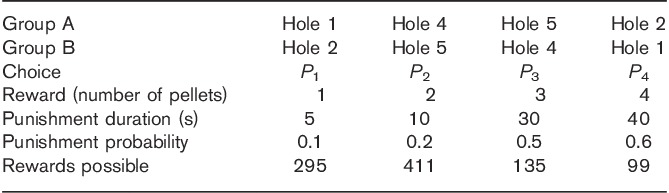
Responses in the holes were rewarded such that P1 resulted in one pellet with a probability of 0.9 and the punishment was a 5-s time-out with a 0.1 probability. P2 was rewarded with two pellets with a probability of 0.8 and the punishment was a 10-s time-out with a 0.2 probability. P3 was rewarded with three pellets with a probability of 0.5 and a 30-s time-out signalled the punishment with a 0.5 probability. P4 was rewarded with four pellets with a probability of 0.4 and the punishment was a 40-s time-out with a 0.6 probability. Reinforcement schedules were designed such that the two-pellet choice (P2) was optimal (i.e. this option resulted in the most rewards earned per unit time). Selection from any other option (one-pellet, three-pellet or four-pellet options) yielded fewer rewards per unit of time as a consequence of the probability of winning or losing and the duration of the punishing time-out periods associated with each option (i.e. the maximum numbers of pellets were P1=295, P2=411, P3=135 and P4=99). Thus, P3 and P4 would be considered the risky/disadvantageous choices, where the greatest reward would be associated with the highest probability and size of punishment. Selection of P1 and P2 represented a more advantageous strategy (Table 2). The location of the response holes remained stable across sessions. A high rGT impulsive choice behaviour reflects persistent choice of high-risk options, which are linked to larger rewards, but ultimately result in fewer pellets earned.
Pharmacological treatment
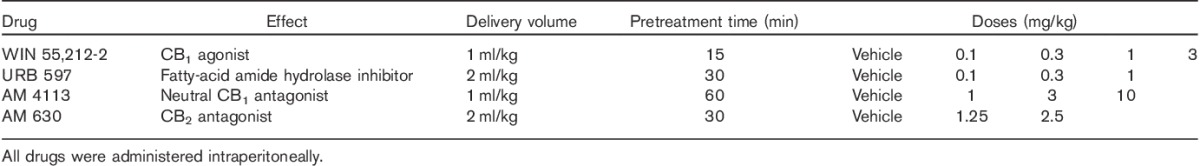
All drugs were obtained from Cayman Chemical (Ann Arbor, Michigan, USA), with the exception of AM 4113, which was obtained through Dr Alexandros Makriyannis’ laboratory (Center for Drug Discovery, Northeastern University, Boston, Massachusetts, USA). The neutral CB1 antagonist AM 4113 was dissolved in Tween-80 (10%), DMSO (10%) and saline (80%). The CB2 antagonist AM 630 was dissolved in Tween-80 (10%), DMSO (10%) and distilled water (80%). WIN 55,212-2, a CB1/2 agonist was dissolved in Tween-80 (0.3%) and saline (99.7%). URB 597, a selective inhibitor of fatty-acid amide hydrolase, was dissolved in DMSO (20%) and saline (80%). All drugs were administered intraperitoneally at a volume of 1 ml/kg body weight, except for URB 597 and AM 630, which were administered at 2 ml/kg (Table 3). Drug injections were administered on a 3-day cycle, starting initially with a baseline session (rats went into the session, but received no drugs). The following day, rats received a drug or vehicle injection before testing. On the third day, animals were not tested. Treatments commenced with URB 597 (30 min before behavioural testing), followed by WIN 55,212-2 (15 min before behavioural testing), AM 630 (30 min before behavioural testing) and AM 4113 (60 min before behavioural testing) (Table 3). Animals were tested drug free for a minimum of 1 week between compounds to prevent carryover effects. All drugs were prepared fresh daily.
Table 3.
Doses, delivery volume and pretreatment time for the cannabinoid drugs used in the study
Data analysis
The following formula was used to calculate the percentage of trials on which an animal chose a particular option: number of choices of a particular option/number of total trials made×100. To determine the percentage of advantageous choices made, the percentages from P1 and P2 were summed; disadvantageous choices were calculated as the percentage sum of P3 and P4 choices. Rats were divided into optimal and suboptimal groups on the basis of their performance under the vehicle condition; those that made more advantageous choices under vehicle were assigned to the optimal group, whereas rats that made more (P3+P4) choices than (P1+P2) were then assigned to the suboptimal group. The numbers of animals for the optimal and suboptimal groups (respectively) were as follows: URB 597, n=15 and n=9; WIN 55,212-2, n=13 and n=11; AM 630, n=12 and n=12; and AM 4113, n=13 and n=11.
The percentage of premature responses made was calculated as the number of premature responses made/total number of trials initiated×100. Perseverative responses made during the punishment period were analysed as a fraction of the total punishment duration experienced. Similarly, perseverative responses (i.e. made after a reward was received) were analysed as a fraction of the total number of trials rewarded (Di Ciano et al., 2015). In addition, the latency to respond at the array and to collect the reward for each choice option was analysed.
During the acquisition phase, the main variables analysed were percentage choice for each option (P1–P4) and percentage of optimal choices (P1+P2). Data were analysed using two-way repeated-measures analysis of variance (ANOVA) (session×choice) or (dose×choice) separately for each of the optimal and suboptimal groups during the acquisition phase, or three-way ANOVA (dose×choice×group) during the test phase, followed by post-hoc Fisher least significant difference tests. Comparisons for the average percentage of advantageous choice during the total 30 sessions of acquisition were performed using Student’s t-test. Differences were considered statistically significant when P is less than 0.05. All statistical analyses were carried out using statistical package Stat Soft, version 10 (Stat soft, Inc. Tulsa, OKA, USA).
Results
Acquisition of the rGT in the optimal and suboptimal groups of rats
The learning curve for the acquisition of choice behaviour by the optimal group of rats (n=15) significantly differed from that of the suboptimal rats (n=9) (Fig. 2). Two-way ANOVA showed no significant session×group interaction [F(29,64)=1.89, NS], suggesting that the overall advantageous or disadvantageous choices remained constant for the duration of the training in both groups (Fig. 2a and b). A significant session×choice×group interaction was observed [F(87,19)=1.32, P<0.05], indicating the progression of learning rate along sessions, with the optimal group of rats showing a significantly greater preference for the advantageous choice (P1 and P2) during the acquisition phase (Fig. 2c), compared with the suboptimal group [t(58)=50.44 P<0.001], as expected. The low choice for P1 was fully compensated by increased P2 choice strategy in the optimal group; thus, the advantageous choice strategy (P1+P2) remained high and constant in that group. In contrast, in the suboptimal group, the low P1 choice strategy was not compensated by an increased P2 choice strategy. The suboptimal group showed a preference for P3 and P4 choices (disadvantageous) during the rGT acquisition.
Fig. 2.
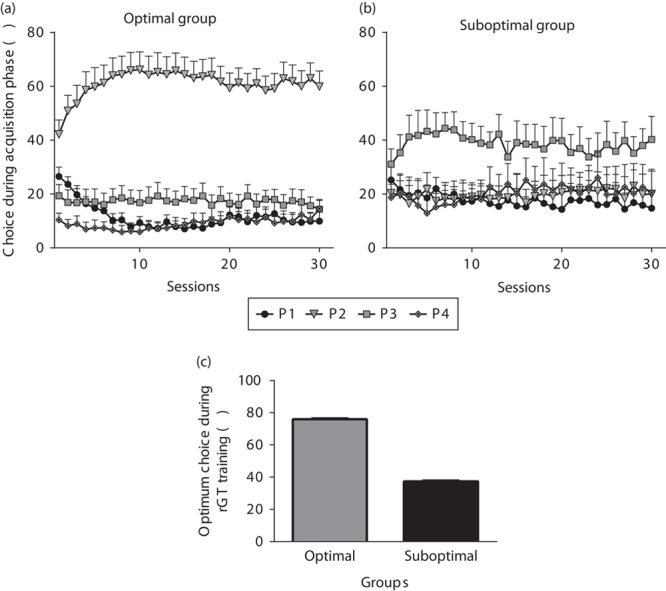
Acquisition of the rat gambling task (rGT) in optimal and suboptimal groups of rats during the training phase. In (a) and (b), rats in the optimal group (n=15) showed a different learning acquisition curve for the four choice options compared with the suboptimal group (n=9) during the 30 sessions (of 30 min) corresponding to the training phase. In (c), the optimal group of rats showed a significantly greater preference for optimal choices (P1+P2) compared with the suboptimal group of animals. ***P<0.001, Student’s t-test.
Effects of cannabinoid ligands on the rGT
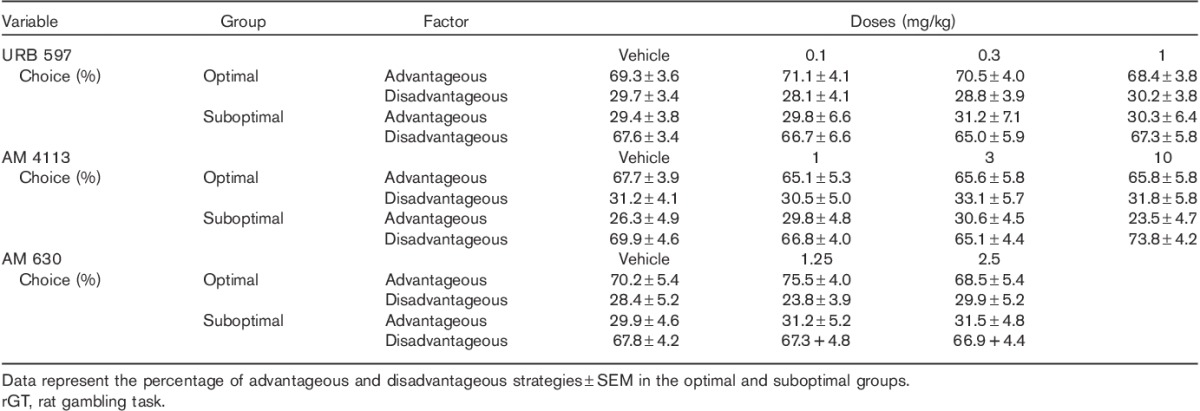
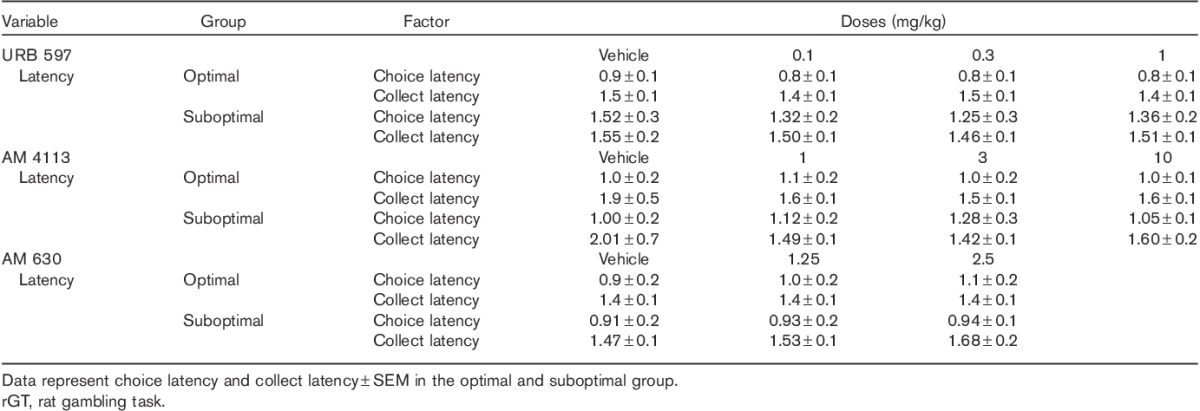
Administration of the CB1/2 agonist WIN 55,212-2 did not modify the number of trials completed. Two-way repeated-measure ANOVA (dose×group) indicated an overall significant effect of group [F(1,22)=12.40, P<0.01], but no significant effect of dose [F(4,88)=1.39, NS] or dose×group interaction [F(4,88)=1.86, NS] (Table 4). Administration of WIN 55,212-2 dose dependently increased the advantageous choices (in detriment of disadvantageous choices) in the suboptimal group (Fig. 3a), but had no significant effects in the optimal group of rats (Fig. 3b), with significant choice×group [F(1,22)=47.70, P<0.001] and dose×choice×group [F(4,88)=3.51, P<0.05] interactions. Fisher least significant difference post-hoc analysis showed a significant increase in the preference for the advantageous strategy and a decrease for the disadvantageous strategy (P<0.005–0.01) in the Suboptimal group of animals pretreated with WIN 55,212-2 (3 mg/kg) compared with vehicle. No significant effects on choice strategy were observed in the optimal group following the administration of WIN 55,212-2. Pretreatment with URB 597, AM 4113 and AM 630 did not modify the choice strategy [dose×choice×group interaction: F(3,66)=0.12, NS, F(3,66)=1.42, NS, F(2,44)=1.37, NS, respectively] (Table 5).
Table 4.
Effects of the CB1 agonist (WIN 55,212-2) on the number of trials completed during the 30 min period of rGT
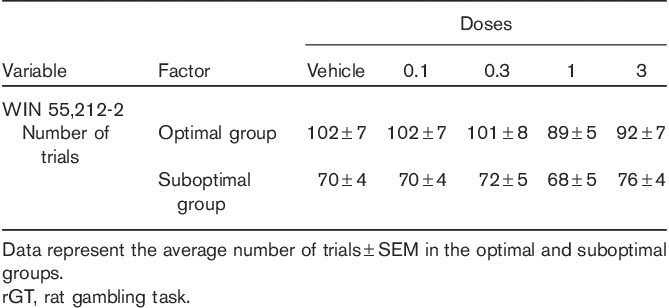
Fig. 3.
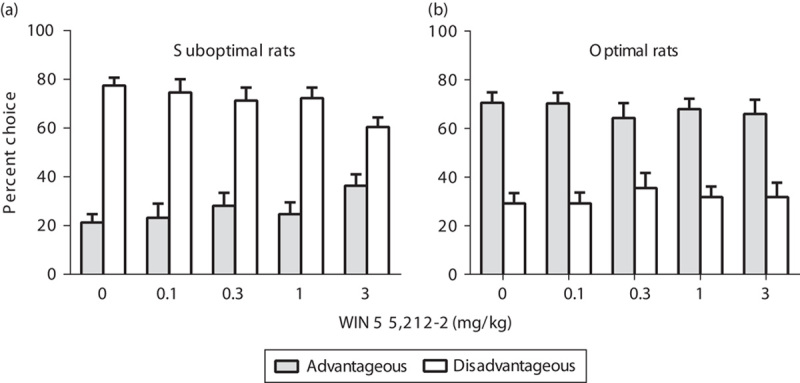
Effects of the CB1/2 agonist WIN 55,212-2 on choice behaviour in optimal and suboptimal groups of rats. The figure shows behaviour in the group of rats showing a preference for P3 and P4 (disadvantageous choices) under vehicle (suboptimal group) or in the group of rats showing a preference for P1 and P2 (advantageous choices) under vehicle (optimal group). Bars represent the average (±SEM) percentage of choice for the advantageous strategy (gray bars) and for the disadvantageous strategy (white bars) following the administration of the CB1/2 agonist WIN 55,212-2 in suboptimal rats (a) and optimal rats (b). *P<0.05, **P<0.01, versus vehicle, Fisher LSD post-hoc tests. The maximum number of pellets for each option is as follows: P1=295, P2=411, P3=135 and P4=99. The advantageous choice consisted of the percentage sum of responses on P1 and P2, whereas the percentage sum of responses on P3 and P4 options was considered the disadvantageous choice. LSD, least significant difference.
Table 5.
Effects of the FAAH inhibitor (URB 597), the CB1 antagonist (AM 4113) and the CB2 antagonist (AM 630) on decision-making during the rGT
Effects of cannabinoid ligands on choice and collect latencies
The administration of the highest dose of WIN 55,212-2 tested in this study (3 mg/kg) increased the latency to make a choice on the suboptimal group of rats (Fig. 4a). Two-way ANOVA analysis indicated a significant effect of latency type [F(1,20)=15.23, P<0.001] and dose [F(4,80)=10.49, P<0.001] and a significant dose×latency type interaction [F(4,80)=3.25, P<0.05]. Post-hoc analyses showed that WIN 55,212-2, at the dose of 3 mg/kg, increased choice latency (advantageous and disadvantageous) compared with the vehicle condition (Fig. 4a). The effects of WIN 55,212-2 in the optimal group of rats were indicated by two-way ANOVA showing significant main effects of latency type [F(1,24)=17.06, P<0.001] and dose [F(4,96)=6.28, P<0.001], but no significant interactions [F(4,96)=1.09, NS] (Fig. 4b).
Fig. 4.
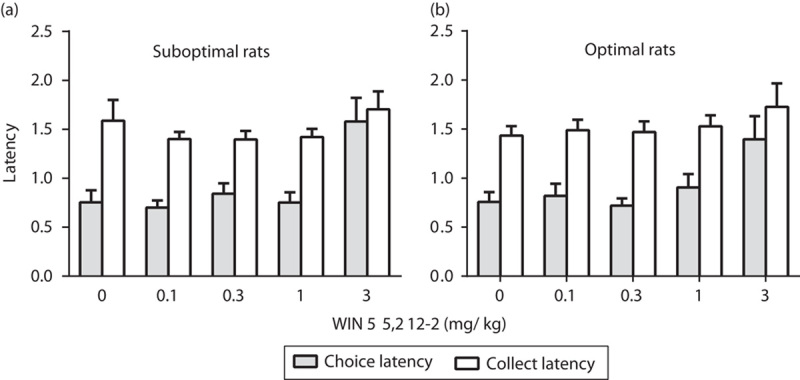
Effects of the CB1/2 agonist WIN 55,212-2 on the latency to make a choice and collect latency in optimal and suboptimal groups of rats. Graphs show mean±SEM for the latency to make a choice (gray bars) and the latency to collect the food reward (white bars) during the rGT following the administration of the CB1/2 agonist WIN 55,212-2 in the suboptimal group (a) (rats showing a preference for the disadvantageous strategy under vehicle treatment) and the optimal group (b) (rats showing a preference for the advantageous strategy under vehicle treatment). ***P<0.001 versus vehicle, Fisher LSD post-hoc tests. The advantageous choice consisted of the percentage sum of responses on P1 and P2, whereas the percentage sum of responses on P3 and P4 options was considered the disadvantageous choice. LSD, least significant difference.
Pretreatments with URB 597, AM 4113 or AM 630 had no significant effects on choice or collect latencies in either optimal or suboptimal groups of rats (Table 6).
Table 6.
Effects of the FAAH inhibitor (URB 597), the CB1 antagonist (AM 4113) and the CB2 antagonist (AM 630) on choice and collect latency during the rGT
Effects of cannabinoid ligands on perseverative behaviour
Perseverative responses made during the punishment period were analysed as a fraction of the total punishment duration experienced in the suboptimal group. WIN 55,212-2 pretreatment exerted no significant effects on punishment and reward perseverative behaviour [F(4,80)=1.95, NS] (Fig. 5a). The CB1/2 agonist WIN 55,212-2 dose dependently increased punishment perseveration in the optimal group. Two-way ANOVA analysis showed a significant interaction between dose and perseverative response type [F(4,96)=1.89, P<0.05]. Post-hoc analyses showed significant differences between vehicle and the two higher doses of WIN 55,212-2 in the optimal group: under vehicle treatment, rats showed fewer punishment perseverative responses compared with WIN 55,212-2 treatment at the doses of 1 and 3 mg/kg (P<0.5 and P<0.01, respectively, Fig. 5b).
Fig. 5.
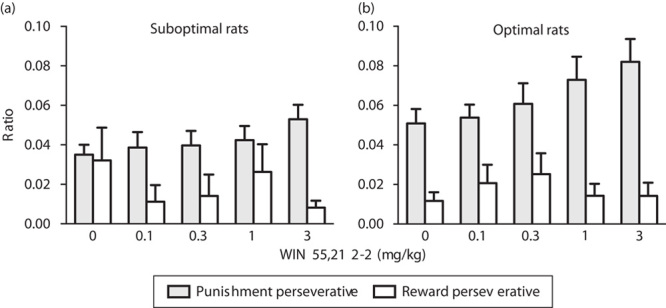
Effects of the CB1/2 agonist WIN 55,212-2 on punishment and reward perseverative behaviour in optimal and suboptimal groups of rats. Graphs represent mean (±SEM) number of punishment perseverative responses (gray bars) and reward perseverative responses (white bars), (a) in the group of rats showing a preference for the disadvantageous strategy under vehicle treatment (suboptimal group) and (b) in the group of rats showing a preference for the advantageous strategy under vehicle treatment (optimal group) following administration of the CB1/2 agonist WIN 55,212-2. *P<0.05, **P<0.01 versus vehicle, Fisher LSD post-hoc tests. The advantageous choice consisted of the percentage sum of responses on P1 and P2, whereas the percentage sum of responses on P3 and P4 options was considered the disadvantageous choice. LSD, least significant difference.
Perseverative responses (i.e. responses made after a reward was received) were analysed as a fraction of the total number of trials rewarded. No significant differences were observed in either the suboptimal or the optimal group (Fig. 5a and b).
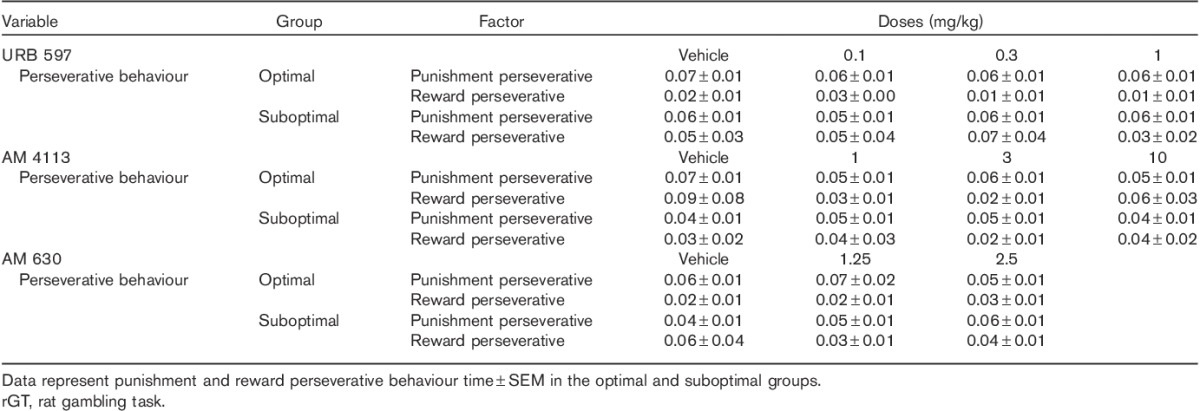
Two-way ANOVA analysis showed no significant effects of URB 597, AM 4113 and AM 630 pretreatments on punishment and reward perseverative behaviour in either group [suboptimal: F(3,48)=0.47, NS; F(3,60)=1.07, NS; F(2,44)=1.05, NS; optimal: F(3,84)=1.61, NS; F(3,72)=0.39, NS; F(2,44)=3.03, NS] (Table 7).
Table 7.
Effects of the FAAH inhibitor (URB 597), the CB1 antagonist (AM 4113) and AM 630 on perseverative behaviours during the rGT
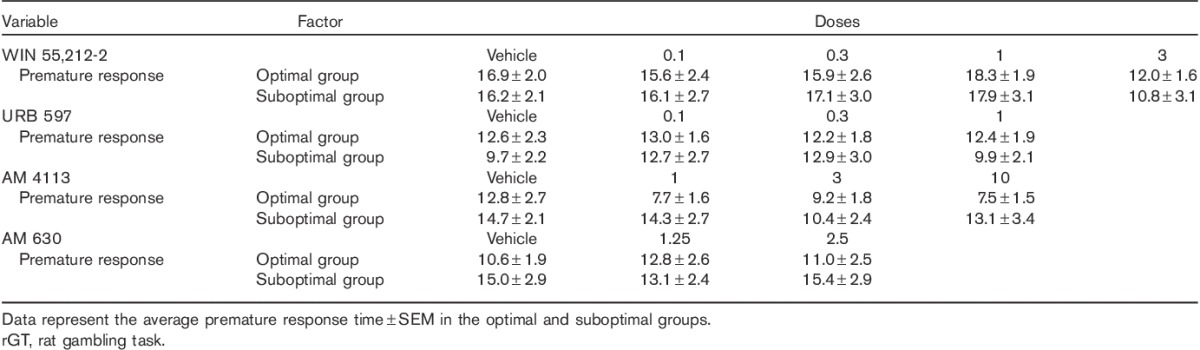
The cannabinoid drugs tested in this study had no significant effects on the premature response behaviour in either the optimal or the suboptimal group. The mean response times across treatments with WIN 55,212-2, URB 597, AM 4113 and AM 630 and vehicle control are shown in Table 8. Two-way ANOVA analysis comparing optimal and suboptimal groups showed no significant dose×group interactions.
Table 8.
Effects of the CB1 agonist (WIN 55,212-2), the FAAH inhibitor (URB 597), the CB1 antagonist (AM 4113) and the CB2 antagonist (AM 630) on premature response during the rGT
Discussion
In this study, we evaluated the effects of various drugs affecting the endocannabinoid system in a rodent model of decision-making under risk, the rGT, which is similar to the IGT, a human model of decision-making under risk (Zeeb et al., 2009). The results show that the CB1/2 receptor agonist WIN 55,212-2 increased the preference for advantageous choices and decreased disadvantageous choices in rats showing Suboptimal response strategies at baseline. However, WIN 55,212-2 had no effect on choice preference in rats formerly showing optimal choice preferences. Administration of WIN 55,212-2 also increased the latency to make a choice in the suboptimal group of rats, and dose dependently increased punishment perseverance behaviour in the optimal group. The CB2 receptor antagonist tested (AM 630), the CB1 antagonist (AM 4113) and the endocannabinoid hydrolysis inhibitor (URB 597) had no effects on choice performance and impulsive behaviour, as evaluated on rGT.
The logic behind the rGT procedure predicts that the advantageous choice produces the highest reward delivery (i.e. most number of pellets in a given time), despite the fact that each rewarded trial produces relatively few pellets. However, the disadvantageous option may appear to be more ‘tempting’ as it results in a higher number of pellets per trial, but overall, the number of pellets available in a session is fewer. Previous studies using the IGT have observed that pathological gamblers show a greater preference for these risky options (Petry, 2001; Linnet et al., 2006). In the present study, we could identify a subset of animals as a suboptimal group on the basis of their preference for disadvantageous versus advantageous choices. These animals may represent a phenotype akin to those at risk for GD.
Pretreatment with WIN 55,212-2 could improve choice strategy during the rGT in the formerly suboptimal group of animals. The improvement consisted of increased choice of smaller immediate gains, despite this suboptimal group of animals still showing a preference for disadvantageous strategies. This suggests that acute WIN 55,212-2 administration may improve the advantageous strategy in the group of rats that are prone to poor decision-making. Previous studies have shown that the effects of cannabinoids on impulsive decision-making are complex and even contradictory: the CB1 antagonists rimonabant and AM 251 increase impulsive choice (Hernandez et al., 2014), whereas no effects or decreased impulsive choice were observed with THC (McDonald et al., 2003) and WIN 55,212-2 (Pattij et al., 2007; Wiskerke et al., 2011), respectively. Our results strengthen the notion that the acute administration of WIN 55,212-2 in rats prone to poor decision-making had specific benefits in the ability to balance rewards and punishments, which might contribute towards improvement of risky behaviours. These results are consistent with findings from other studies showing that pharmacological manipulations, aimed to enhance the endocannabinoid tone, resulted in increased self-control and reduced novelty-seeking behaviour (Marco et al., 2007).
In humans, chronic marijuana use is associated with deficits in decision-making that impair the ability to make advantageous decisions over time (Whitlow et al., 2004; Hermann et al., 2009). Acute and long-term effects of CB1 receptor agonists (such as THC) are associated positively with deficits in cognitive flexibility (Pope et al., 2001; Bolla et al., 2002; Lundqvist, 2005; Crean et al., 2011). Specifically, deficits in cognitive flexibility were reported in chronic cannabis users (Pope et al., 2001; Bolla et al., 2002) and seem to be persistent after 28 days of cannabis abstinence (Bolla et al., 2002). Our results provide a new insight in that the acute administration of a CB1 agonist could improve the performance on the rGT task in rats prone to poor decision-making.
It should be noted that WIN 55,212-2 is a potent synthetic agonist that has some broader effects that could be relevant to these findings. Thus, it has been shown that WIN 55,212-2 has reinforcing effects of its own (Martellotta et al., 1998; Fattore et al., 2001; Lefever et al., 2014) and can reinstate nicotine-seeking behaviour and enhance motivation for nicotine (Gamaleddin et al., 2012a). Therefore, the present results should be interpreted with caution. The effects of WIN 55,212-2 on reward and motivation may contribute towards the findings observed in this study. Indeed, both dopaminergic and endocannabinoid systems have been implicated independently in the temporal control of behaviour (Pattij et al., 2008; Narayanan et al., 2012). The impulsive choice might be influenced by altered sensitivity to reinforcer magnitude (i.e. perceived rewarding properties), inappropriate perception of time (i.e. delay) or both (Ho et al., 1999). Cannabinoid agonists have also been shown to alter time perception in humans (McDonald et al., 2003) and in rats (Crystal et al., 2003), and to enhance an aversive motivational state (Arguello and Jentsch, 2004).
However, we did not find significant changes in the choice preferences of the optimal and suboptimal groups following the blockade of CB1 receptors with AM 4113. This finding is in agreement with the previously reported absence of effects of the CB1 inverse agonist rimonabant in the 5-CSRTT (Pattij et al., 2007). Similarly, the blockade of CB2 receptors with AM 630 exerted no effects on choice preference in either optimal or suboptimal groups of rats.
It is interesting to note that the change in preference (in the suboptimal group) for more advantageous choice strategies, at the highest dose of WIN 55,212-2 tested, was also associated with higher latencies to make the choice. These findings are in agreement with previous studies showing that THC and WIN 55,212-2 increased response and collection latencies (Pattij et al., 2007; Wiskerke et al., 2011) as evaluated using 5-CSRTT. The increase in latency induced by cannabinoid agonists could be produced by effects on the locomotor response. In fact, exogenous cannabinoid receptors agonists such as THC have consistently been found to decrease locomotor activity (Herkenham, 1992). Similarly, increasing the neural levels of the endocannabinoid anandamide decreases locomotor activity, similar to exogenous cannabinoids (Romero et al., 1995; de Lago et al., 2004). It is possible that high doses of WIN 55,212-2 might produce some nonspecific motor impairment. Previous studies have shown that WIN 55,212-2 at the doses of 1 and 3 mg/kg significantly decreased response rates of rats trained under the nicotine discrimination task (Gamaleddin et al., 2012a). However, WIN 55,212-2 at the lower range of doses (i.e. 1 mg/kg) did not disrupt responding during reinstatement of nicotine-seeking behaviours (Gamaleddin et al., 2012a). The numbers of trials completed for the suboptimal group at the different doses of WIN 55,212-2 tested in this study (70±4; 70±4; 72±5; 68±5 and 76±4 for the doses of 0, 0.1, 0.3, 1 and 3 mg/kg of, respectively) suggest that the effects of WIN 55,212-2 observed in the rGT latencies are not attributable to an impairment in locomotor activity. This notion is supported by the absence of changes in the number of omissions through all doses during the rGT (data not shown). Furthermore, in the present study, the effect of WIN 55,212-2 (3 mg/kg) to increase choice latency in the suboptimal group was correlated positively with speed of decision-making. With respect to the latency data, the fact that animals showed the same reward-collection latency and that they were uniformly faster to collect larger rewards, both under vehicle and under WIN 55,212-2 (3 mg/kg) treatments, suggests that rats were not hypoactive in the presence of reward or differentially sensitive to changes in reward magnitude.
The absence of effects of URB 597 on choice and collection latencies is in agreement with previous studies showing no effects of URB 597 on locomotor activity (Jayamanne et al., 2006; Adamczyk et al., 2009). None of the cannabinoid drugs tested in this study could modify premature responding.
There are some limitations to the interpretation of the present results. We cannot rule out the possible existence of differences in the expression of CB1/2 receptors in the optimal versus suboptimal groups that could explain the differential effects of the drugs tested. Another limitation is the observation of increased latencies, particularly in the suboptimal group, which might be caused by a decreased motivation to obtain this particular reinforcer. A study of the motivation for food (i.e. food self-administration under a progressive ratio schedule in optimal vs. Suboptimal groups) may help to elucidate whether differences in motivation between groups could account for the observed increase in latency. An additional limitation of the present study, in terms of therapeutic relevance, is the use of a potent synthetic cannabinoid (WIN 55,212-2), which has different pharmacokinetic properties compared with THC.
In conclusion, the administration of a CB1/2 agonist improves choice performance in a suboptimal group of rats, as evaluated using the rGT. It is premature to propose that the stimulation of CB1/2 receptors may provide a treatment for gambling individuals prone to poor decision-making. Our study implicates the cannabinoid system in the processing of cost-benefit decision-making. As we used a systemic administration of cannabinoid agents, the observed effects might be because of the net result of the cannabinoid action in several brain regions expressing cannabinoid receptors. Future studies targeting discrete brain areas might help to further clarify the specific role of these on the effects of cannabinoids on decision-making. Our preliminary findings suggest that such a possibility should be explored further as it could result in a novel therapeutic strategy for GD.
Acknowledgements
This study was funded through a grant provided by the Canadian Institutes of Health Research (CIHR).
Conflicts of interest
There are no conflicts of interest.
References
- Adamczyk P, McCreary AC, Przegalinski E, Mierzejewski P, Bienkowski P, Filip M. (2009). The effects of fatty acid amide hydrolase inhibitors on maintenance of cocaine and food self-administration and on reinstatement of cocaine-seeking and food-taking behavior in rats. J Physiol Pharmacol 60:119–125. [PubMed] [Google Scholar]
- Adriani W, Laviola G. (2004). Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol 15:341–352. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association [DSM 5] (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM 5. 5th ed Washington, DC: American Psychiatric Publishing. [Google Scholar]
- Arguello PA, Jentsch JD. (2004). Cannabinoid CB1 receptor-mediated impairment of visuospatial attention in the rat. Psychopharmacology (Berl) 177:141–150. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. (2002). Dose-related neurocognitive effects of marijuana use. Neurology 59:1337–1343. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. (2005). Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage 26:480–492. [DOI] [PubMed] [Google Scholar]
- Crean RD, Crane NA, Mason BJ. (2011). An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med 5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal JD, Maxwell KW, Hohmann AG. (2003). Cannabinoid modulation of sensitivity to time. Behav Brain Res 144:57–66. [DOI] [PubMed] [Google Scholar]
- de Lago E, de Miguel R, Lastres-Becker I, Ramos JA, Fernández-Ruiz J. (2004). Involvement of vanilloid-like receptors in the effects of anandamide on motor behavior and nigrostriatal dopaminergic activity: in vivo and in vitro evidence. Brain Res 1007:152–159. [DOI] [PubMed] [Google Scholar]
- Dell’Osso B, Altamura AC, Allen A, Marazziti D, Hollander E. (2006). Epidemiologic and clinical updates on impulse control disorders: a critical review. Eur Arch Psychiatry Clin Neurosci 256:464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Pushparaj A, Kim A, Hatch J, Masood T, Ramzi A, et al. (2015). The impact of selective dopamine D2, D3 and D4 ligands on the rat gambling task. PLoS One 10:e0136267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo V, Matias I. (2005). Endocannabinoid control of food intake and energy balance. Nat Neurosci 8:585–589. [DOI] [PubMed] [Google Scholar]
- Egerton A, Allison C, Brett RR, Pratt JA. (2006). Cannabinoids and prefrontal cortical function: insights from preclinical studies. Neurosci Biobehav Rev 30:680–695. [DOI] [PubMed] [Google Scholar]
- Fattore L, Cossu G, Martellotta CM, Fratta W. (2001). Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212-2 in rats. Psychopharmacology (Berl) 156:410–416. [DOI] [PubMed] [Google Scholar]
- Gamaleddin I, Wertheim C, Zhu AZ, Coen KM, Vemuri K, Makryannis A, et al. (2012a). Cannabinoid receptor stimulation increases motivation for nicotine and nicotine seeking. Addict Biol 17:47–61. [DOI] [PubMed] [Google Scholar]
- Gamaleddin I, Zvonok A, Makriyannis A, Goldberg SR, Le Foll B. (2012b). Effects of a selective cannabinoid CB2 agonist and antagonist on intravenous nicotine self administration and reinstatement of nicotine seeking. PLoS One 7:e29900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. (2006). Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res 1071:10–23. [DOI] [PubMed] [Google Scholar]
- Herkenham M. (1992). Cannabinoid receptor localization in brain: relationship to motor and reward systems. Ann N Y Acad Sci 654:19–32. [DOI] [PubMed] [Google Scholar]
- Hermann D, Leménager T, Gelbke J, Welzel H, Skopp G, Mann K. (2009). Decision making of heavy cannabis users on the Iowa gambling task: stronger association with THC of hair analysis than with personality traits of the Tridimensional Personality Questionnaire. Eur Addict Res 15:94–98. [DOI] [PubMed] [Google Scholar]
- Hernandez G, Oleson EB, Gentry RN, Abbas Z, Bernstein DL, Arvanitogiannis A, Cheer JF. (2014). Endocannabinoids promote cocaine-induced impulsivity and its rapid dopaminergic correlates. Biol Psychiatry 75:487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Froese LM, Morrish AC, Sun JC, Floresco SB. (2006). Alterations in behavioral flexibility by cannabinoid CB1 receptor agonists and antagonists. Psychopharmacology (Berl) 187:245–259. [DOI] [PubMed] [Google Scholar]
- Ho MY, Mobini S, Chiang TJ, Bradshaw CM, Szabadi E. (1999). Theory and method in the quantitative analysis of "impulsive choice" behaviour: implications for psychopharmacology. Psychopharmacology (Berl) 146:362–372. [DOI] [PubMed] [Google Scholar]
- Jayamanne A, Greenwood R, Mitchell VA, Aslan S, Piomelli D, Vaughan CW. (2006). Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. Br J Pharmacol 147:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Hwang I, LaBrie R, Petukhova M, Sampson NA, Winters KC, Shaffer HJ. (2008). DSM-IV pathological gambling in the National Comorbidity Survey Replication. Psychol Med 38:1351–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Cherek DR, Tcheremissine OV, Lieving LM, Pietras CJ. (2005). Acute marijuana effects on human risk taking. Neuropsychopharmacology 30:800–809. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. (2005). Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther 312:875–883. [DOI] [PubMed] [Google Scholar]
- Lefever TW, Marusich JA, Antonazzo KR, Wiley JL. (2014). Evaluation of WIN 55,212-2 self-administration in rats as a potential cannabinoid abuse liability model. Pharmacol Biochem Behav 118:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnet J, Røjskjaer S, Nygaard J, Maher BA. (2006). Episodic chasing in pathological gamblers using the Iowa gambling task. Scand J Psychol 47:43–49. [DOI] [PubMed] [Google Scholar]
- Lundqvist T. (2005). Cognitive consequences of cannabis use: comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol Biochem Behav 81:319–330. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. (2006). Involvement of the endocannabinoid system in drug addiction. Trends Neurosci 29:225–232. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Berrendero F, Ozaita A, Robledo P. (2011). Neurochemical basis of cannabis addiction. Neuroscience 181:1–17. [DOI] [PubMed] [Google Scholar]
- Marco EM, Adriani W, Canese R, Podo F, Viveros MP, Laviola G. (2007). Enhancement of endocannabinoid signalling during adolescence: Modulation of impulsivity and long-term consequences on metabolic brain parameters in early maternally deprived rats. Pharmacol Biochem Behav 86:334–345. [DOI] [PubMed] [Google Scholar]
- Martellotta MC, Cossu G, Fattore L, Gessa GL, Fratta W. (1998). Self-administration of the cannabinoid receptor agonist WIN 55,212-2 in drug-naive mice. Neuroscience 85:327–330. [DOI] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H. (2003). Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology 28:1356–1365. [DOI] [PubMed] [Google Scholar]
- Melis M, Pistis M. (2007). Endocannabinoid signaling in midbrain dopamine neurons: more than physiology? Curr Neuropharmacol 5:268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Land BB, Solder JE, Deisseroth K, DiLeone RJ. (2012). Prefrontal D1 dopamine signaling is required for temporal control. Proc Natl Acad Sci USA 109:20726–20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete F, Rodríguez-Arias M, Martín-García E, Navarro D, García-Gutiérrez MS, Aguilar MA, et al. (2013). Role of CB2 cannabinoid receptors in the rewarding, reinforcing, and physical effects of nicotine. Neuropsychopharmacology 38:2515–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Vanderschuren LJ. (2008). The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci 29:192–199. [DOI] [PubMed] [Google Scholar]
- Pattij T, Janssen MC, Schepers I, González-Cuevas G, de Vries TJ, Schoffelmeer AN. (2007). Effects of the cannabinoid CB1 receptor antagonist rimonabant on distinct measures of impulsive behavior in rats. Psychopharmacology (Berl) 193:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattij T, Wiskerke J, Schoffelmeer AN. (2008). Cannabinoid modulation of executive functions. Eur J Pharmacol 585:458–463. [DOI] [PubMed] [Google Scholar]
- Petry NM. (2001). Substance abuse, pathological gambling, and impulsiveness. Drug Alcohol Depend 63:29–38. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. (2001). Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry 58:909–915. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. (2006). High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology 31:2296–2303. [DOI] [PubMed] [Google Scholar]
- Romero J, García L, Cebeira M, Zadrozny D, Fernández-Ruiz JJ, Ramos JA. (1995). The endogenous cannabinoid receptor ligand, anandamide, inhibits the motor behavior: role of nigrostriatal dopaminergic neurons. Life Sci 56:2033–2040. [DOI] [PubMed] [Google Scholar]
- Takahashi RN, Pamplona FA, Fernandes MS. (2005). The cannabinoid antagonist SR141716A facilitates memory acquisition and consolidation in the mouse elevated T-maze. Neurosci Lett 380:270–275. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, Brueggeman RJ, Bronius PF, Schoffelmeer AN, Vanderschuren LJ. (2006). Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology (Berl) 187:73–85. [DOI] [PubMed] [Google Scholar]
- Villella C, Martinotti G, Di Nicola M, Cassano M, La Torre G, Gliubizzi MD, et al. (2011). Behavioural addictions in adolescents and young adults: results from a prevalence study. J Gambl Stud 27:203–214. [DOI] [PubMed] [Google Scholar]
- Whitlow CT, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn CM, et al. (2004). Long-term heavy marijuana users make costly decisions on a gambling task. Drug Alcohol Depend 76:107–111. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Rehm J, Stevens RMG. (2011). The social and economic impacts of gambling: final report: Canadian Consortium for Gambling Research; 11 March 2011.
- Wiskerke J, Stoop N, Schetters D, Schoffelmeer AN, Pattij T. (2011). Cannabinoid CB1 receptor activation mediates the opposing effects of amphetamine on impulsive action and impulsive choice. PLoS One 6:e25856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Robbins TW, Winstanley CA. (2009). Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology 34:2329–2343. [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Wong AC, Winstanley CA. (2013). Differential effects of environmental enrichment, social-housing, and isolation-rearing on a rat gambling task: dissociations between impulsive action and risky decision-making. Psychopharmacology (Berl) 225:381–395. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Gao M, Liu QR, Bi GH, Li X, Yang HJ, et al. (2014). Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci USA 111:E5007–E5015. [DOI] [PMC free article] [PubMed] [Google Scholar]


