Abstract
Context:
Shift work is a risk factor for diabetes. The separate effects of the endogenous circadian system and circadian misalignment (ie, misalignment between the central circadian pacemaker and 24-hour environmental/behavioral rhythms such as the light/dark and feeding/fasting cycles) on glucose tolerance in shift workers are unknown.
Objective:
The objective of the study was to test the hypothesis that the endogenous circadian system and circadian misalignment separately affect glucose tolerance in shift workers, both independently from behavioral cycle effects.
Design:
A randomized, crossover study with two 3-day laboratory visits.
Setting:
Center for Clinical Investigation at Brigham and Women's Hospital.
Patients:
Healthy chronic shift workers.
Intervention:
The intervention included simulated night work comprised of 12-hour inverted behavioral and environmental cycles (circadian misalignment) or simulated day work (circadian alignment).
Main Outcome Measures:
Postprandial glucose and insulin responses to identical meals given at 8:00 am and 8:00 pm in both protocols.
Results:
Postprandial glucose was 6.5% higher at 8:00 pm than 8:00 am (circadian phase effect), independent of behavioral effects (P = .0041). Circadian misalignment increased postprandial glucose by 5.6%, independent of behavioral and circadian effects (P = .0042). These variations in glucose tolerance appeared to be explained, at least in part, by different insulin mechanisms: during the biological evening by decreased pancreatic β-cell function (18% lower early and late phase insulin; both P ≤ .011) and during circadian misalignment presumably by decreased insulin sensitivity (elevated postprandial glucose despite 10% higher late phase insulin; P = .015) without change in early-phase insulin (P = .38).
Conclusions:
Internal circadian time affects glucose tolerance in shift workers. Separately, circadian misalignment reduces glucose tolerance in shift workers, providing a mechanism to help explain the increased diabetes risk in shift workers.
In the United States, almost 15% of the workforce undertakes shift work (1). Epidemiological studies indicate that shift work is a risk factor for type 2 diabetes (2). Shift workers frequently undergo circadian misalignment (ie, misalignment between their endogenous circadian system and 24-hour environmental/behavioral rhythms such as light/dark, wake/sleep, activity/inactivity, and feeding/fasting cycles). This misalignment has been proposed to explain, in part, why shift work is a risk factor for type 2 diabetes (3). The mammalian circadian system is composed of a central pacemaker in the suprachiasmatic nucleus (SCN) of the hypothalamus along with circadian oscillators in virtually all peripheral tissues and organs (4). This system is entrained to the solar day by external photic (ie, the light/dark cycle) and nonphotic (eg, nutrient intake) inputs and optimally times physiology and behaviors relative to the solar day (4). The molecular genesis of circadian rhythms by the SCN and peripheral oscillators involves transcriptional-translation feedback loops (4). In nonshift workers and rodents, internal circadian time has been shown to affect glucose metabolism and circadian misalignment and other forms of circadian disruption (eg, clock gene mutations) have been shown to adversely affect glucose metabolism (5–24). However, the impact of either the endogenous circadian system or circadian misalignment, after accounting for behavioral cycle effects (including the sleep/wake, fasting/feeding, and physical inactivity/activity cycles etc), on glucose tolerance in shift workers is unknown.
Here, we tested the separate effects of circadian phase (biological morning [defined loosely here as the endogenous circadian phase equivalent to approximately 8:00 am] vs biological evening [approximately 8:00 pm]) and of circadian misalignment, both independent of the behavioral cycle (including wake/sleep, activity/inactivity, and feeding/fasting cycles) in shift workers. In order to test these separate effects, we determined glucose tolerance in response to identical mixed meals given at 8:00 am and 8:00 pm when the behavioral cycle of shift workers was aligned or misaligned with their endogenous circadian system using a rapid 12-hour shift of the behavioral cycle (Figure 1). This was tested using a randomized, within-participant, crossover design. Together these protocols (aligned vs misaligned) allow the separate assessment of behavioral and circadian influences by evenly scheduling behavioral and environmental factors (eg, sleep/wake, fasting/feeding, and dark/light) relative to two distinct circadian phases. In addition, the protocols allow the separate assessment of the impact of circadian misalignment by comparing responses to identical test meals when the behavioral and environmental cycles are aligned vs misaligned with the timing of the endogenous circadian system.
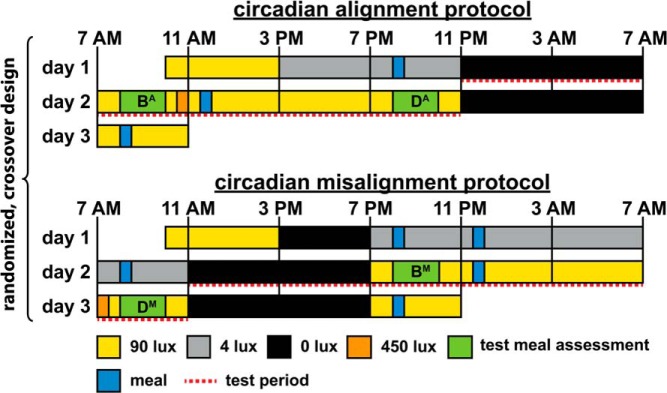
Figure 1.
Circadian alignment protocol (top panel) and circadian misalignment protocol (bottom panel). On day 1 in both protocols, participants received an ad libitum lunch at approximately 12:00 pm. The letters B and D indicate the test meals at breakfast (first meal of the scheduled wake episode) and dinner (last meal of the scheduled wake episode), respectively. Letters following B or D indicate whether the test meals were consumed during the circadian alignment (A) or circadian misalignment (M) protocol. To graphically represent the independent effects of the behavioral cycle, circadian phase, and circadian misalignment in Figure 3, we did the following: 1) averaged breakfast time (BA and BM) and dinner time (DA and DM) test meal values separately across both protocols (behavioral cycle effect); 2) averaged 8:00 am (BA and DM) and 8:00 pm (DA and BM) test meal values separately across both protocols (circadian phase effect); and 3) averaged alignment (BA and DA) and misalignment (BM and DM) test meal values within each protocol (circadian misalignment effect). Light levels (in the horizontal angle of gaze); approximately 90 lux to simulate typical room light intensity, 30-minute periods of approximately 450 lux to simulate the morning commute preceding the simulated day shift and following the simulated night shift, approximately 4 lux to permit assessment of dim-light melatonin levels, 0 lux during scheduled sleep opportunities. Light levels were also 90 lux during test meal assessments.
Materials and Methods
Experimental design
Each participant underwent two 3-day laboratory protocols, according to a crossover design, to test the separate effects of the behavioral cycle, circadian phase, and circadian misalignment on glucose metabolism (Figure 1). One protocol included a simulated day shift (circadian alignment protocol) and the other a simulated night shift (circadian misalignment protocol). The visits were separated by 3–8 weeks (mean ± SD: 5 ± 2 wk). Minimization was used to minimize imbalance, according to age, gender, and body mass index (BMI), in the order of laboratory visits (five participants undertook the circadian alignment first and four participants undertook the circadian misalignment first).
Participants
Nine, healthy, nonsmoking, drug- and medication-free (except for oral contraceptives) adults completed this study (mean ± SD [range] age 34 ± 8 y [24–48 y]; BMI 24.2 ± 3.4 kg/m2 [19.3–29.3 kg/m2]; three males). Health status was established by medical history, physical examination, electrocardiography, standard blood and urine analysis, and interview by a clinical psychologist. All participants were currently employed shift workers (≥ 12 mo of consecutive shift work), who had five or more night shifts per month (6 h between 10:00 pm and 8:00 am, with no shift duration > 12 h). Participants' mean ± SD consecutive shift work experience was 4.5 ± 7.7 years (range 1.1–25.1 y). Participants' mean ± SD lifetime cumulative shift work experience was 5.3 ± 7.7 years (range 1.1–25.1 y). Two participants were rotating shift workers and seven participants were permanent night shift workers. Participants' cumulative nap duration while working a night shift was less than 1 hour. Participants were excluded if they maintained their night shift sleep/wake schedule (ie, sleeping during the day and awake at night) while working day shifts and/or on days off from work. The Partners Human Research Committee approved this research, which was conducted in the Center for Clinical Investigation at Brigham and Women's Hospital (Boston, Massachusetts). All participants provided written informed consent.
Preinpatient study conditions
For 14 days or longer (mean ± SD: 19 ± 5 d) before each laboratory visit, participants wore an Actiwatch Spectrum (Philips-Respironics) and recorded their bedtimes, wake times, and work schedules in a diary and by reporting the information to a time-stamped voicemail system (Supplemental Figure 1). Participants were instructed to sleep between 11:00 pm and 7:00 am on the night preceding each inpatient admission to reduce possible sleep debt before entering the laboratory.
Inpatient study conditions
On the first day of each 3-day laboratory protocol, participants were admitted to the Center for Clinical Investigation at approximately 10:00 am to undertake either the circadian alignment protocol or circadian misalignment protocol in a crossover design (Figure 1). Participants remained in a private laboratory room throughout each laboratory protocol to allow strict control of environmental conditions. In the circadian alignment protocol, participants' sleep opportunity occurred from 11:00 pm until 7:00 am for days 1–3. In the circadian misalignment protocol, on day 1 the participants' sleep/wake cycle was inverted by 12 hours by including an 8-hour wake episode plus a 4-hour sleep opportunity between 3:00 pm and 7:00 pm. The participants then stayed awake for 16 hours until their next sleep opportunity, which occurred from 11:00 am until 7:00 pm. This sleep/wake cycle was maintained until the end of the protocol (d 3). Metabolic responses to test meals were assessed on day 2 in the circadian alignment protocol and across days 2–3 in the circadian misalignment protocol (see below for details). Light levels during both protocols are shown in Figure 1.
Diet
Participants were given an ad libitum lunch around noon on the first day of each laboratory protocol, after which they received an isocaloric diet throughout the rest of the protocol, calculated according to the Harris-Benedict equation with an activity factor of 1.4. The diet consisted of 45%–50% carbohydrate, 30%–35% fat, and 15%–20% protein, 150 mEq Na+ (±20%), 100 mEq K+ (±20%), and at least 2.5 L of water per 24 hours. Participants were instructed to consume all food provided (verified by checking their food trays). Because the circadian misalignment protocol was 12 hours longer (to enable a 12 h slam shift of the behavioral cycle) than the circadian alignment protocol, participants were given additional prorated food and water in the circadian misalignment protocol (50% of calculated 24 h calorie and water requirements). This additional food and water was distributed across breakfast (8:00 pm) and lunch (11:30 pm) on day 1 (wake period 2) of the circadian misalignment protocol, such that premeals and test meals were identical in both protocols (see below for details). Other than the above-mentioned additional food and water, the diet was identical within each participant between laboratory visits. In each protocol, following the final sleep opportunity (d 3), participants consumed an ad libitum breakfast before leaving the laboratory.
We assessed participants' metabolic responses to identical test meals (33.3% of calculated daily calorie intake) given both 1 hour and 13 hours after the scheduled wake time in the circadian alignment protocol (wake period 2) and circadian misalignment protocol (wake period 3). Participants chose one of two test meals: 1) Glucola (0.45 g/kg), a bagel with butter, cereal with milk and sugar, egg, and peanuts; or 2) Glucola (0.45 g/kg), a bagel with butter, cereal with milk and sugar, turkey sausage, and almonds. Glucola was consumed within the first minute and other food items were consumed subsequently, in the order listed above. Test meals were consumed within 20 minutes and were identical within each participant across both protocols. The test meal given 1 hour after scheduled wake time was preceded by the same meal (ie, a test meal), consumed in the prior wake period (8:00 pm in the circadian alignment protocol and 8:00 am in the circadian misalignment protocol). Thus, the meal preceding the breakfast test meal was standardized. The test meal given 13 hours after the scheduled wake was preceded by a lunch premeal (33.3% of calculated daily calorie intake), consumed at 11:30 am in the circadian alignment protocol and 11:30 pm in the circadian misalignment protocol. For the lunch premeal that preceded the dinner test meal, participants preselected one of two meals, which were identical within each participant across both protocols. The above-mentioned lunch meal had the same macronutrient ratios as the test meals.
Blood sampling
In the circadian alignment protocol, dim light melatonin samples were collected hourly from the middle of wake period 1 until the end of sleep period 1, covering a 16-hour span. In the circadian misalignment protocol, dim light melatonin samples were collected hourly from the start of wake period 2 until the end of sleep period 2, covering a 24-hour span (see Figure 2).
Figure 2.
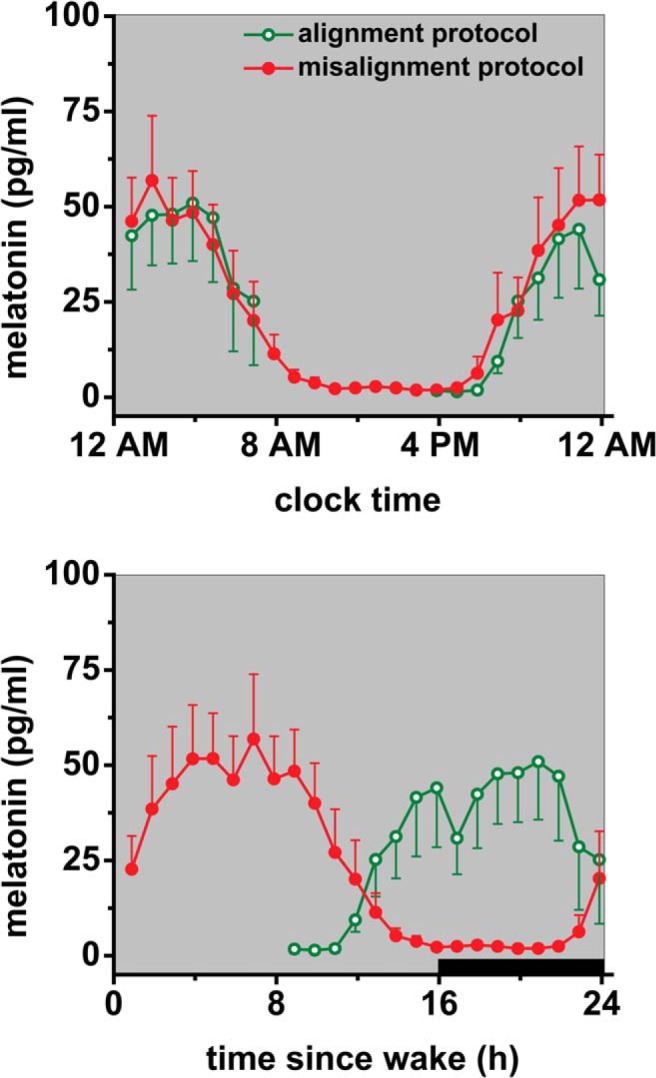
Dim light melatonin levels in the circadian alignment and misalignment protocols. Central circadian phase, as estimated by dim light melatonin profile, was similar for the circadian alignment and circadian misalignment protocol (top panel), resulting in an approximately 12-hour difference in circadian alignment relative to the behavioral cycle (bottom panel). Bottom panel, data are plotted according to scheduled wake time; in the circadian alignment protocol, data are plotted relative to a scheduled 7:00 am wake time on day 1, whereas in the circadian misalignment protocol, data are plotted relative to a scheduled 7:00 pm wake time on day 1 (start of wake period 2). Black bar represents sleep opportunity. In the circadian alignment protocol, no melatonin samples were obtained from 7:53 am to 2:53 pm, whereas in the circadian misalignment protocol, melatonin samples were obtained hourly for 24 hours. Samples for melatonin measurement were collected under dim light conditions (∼<4 lux in the horizontal angle of gaze). Data are presented as mean ± SEM.
For metabolic test periods, 24-hour blood drawing for metabolite and hormone assessment started shortly after bedtime until bedtime 24 hours later, ie, between 11:00 pm and 11:00 pm in the circadian alignment protocol (sleep opportunity 1 and wake period 2) and between 11:00 am and 11:00 am in the circadian misalignment protocol (sleep opportunity 2 and wake period 3). For each of the four test meals per participant, fasting blood was drawn 7 minutes before each test meal, and postprandial blood was drawn every 10 minutes for 90 minutes, starting 10 minutes after the participant began eating the test meal, and every 30 minutes for the next 90 minutes, totaling 3 hours (see Supplemental Information for assay details).
Polysomnography
Sleep was recorded by polysomnography (Vitaport; TEMEC Instruments), in accordance with the American Academy of Sleep Medicine recommendations (25), during sleep opportunity 1 in the circadian alignment protocol and during sleep opportunity 2 in the circadian misalignment protocol (see Supplemental Information for details).
Data analysis and statistics
Postprandial glucose was based on samples obtained from 10 to 90 minutes relative to the start of the meal. For test meal analysis of insulin, early-phase response was based on samples collected 10–30 minutes after the start of the meal, and the late-phase response was based on samples obtained 40–90 minutes relative to the start of the meal. The central circadian phase was estimated from dim light melatonin onset and offset (see Supplemental Information for details).
Statistical tests were performed with linear mixed models (with participant included as a random factor) and with Spearman's rank correlation coefficients, unless otherwise indicated. Statistical significance was accepted as P < .05. Data are presented as mean ± SEM, unless otherwise indicated. See Supplemental Information for details.
Results
Phase of the central circadian pacemaker in the circadian alignment and misalignment protocols as assessed under dim light conditions
Two participants' dim light melatonin onset (DLMOn) or dim light melatonin offset (DLMOff) showed a phase difference greater than 4 hours between alignment protocols. Consequently, these two participants (females, both aged 24 y with a BMI of 19.3 or 27.5 kg/m2) were excluded from all subsequent data analysis because they had an unstable timing of their central circadian clock, which would otherwise have interfered with assessing the effect of the scheduled circadian alignment vs misalignment. This criterion regarding the phase difference limit has been used previously (24). The dim light melatonin levels for the remaining seven participants (mean ± SD [range], age, 37 ± 7 y [30–48 y]; BMI 24.4 ± 3.1 kg/m2 [21.0–29.3 kg/m2]; three males) are shown in Figure 2. There was no significant difference in DLMOn between alignment conditions (−0.80 ± 0.47 h; range −2.82 to +0.80 h; P = .14, paired samples t test). For the alignment condition, DLMOn was at 8:11 pm ± 54 minutes (range 6:34 pm to 1:22 am), and for the misalignment condition, DLMOn was at 7:23 pm ± 34 minutes (range 5:54 pm to 10:33 pm).
Behavioral cycle effects, independent of circadian effects: glucose tolerance was lower at dinner time than at breakfast time
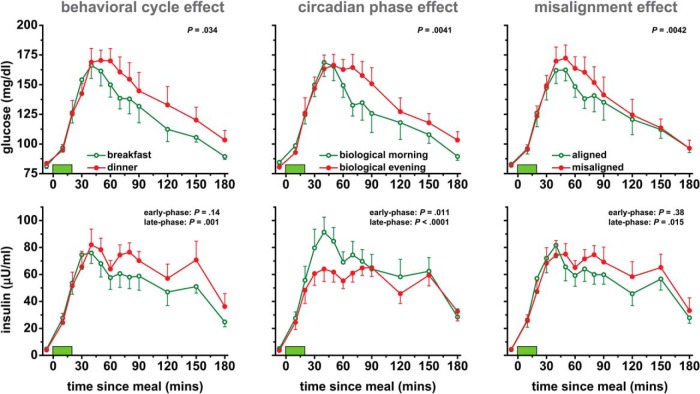
Postprandial glucose was 5% higher at dinner time than breakfast time (P = .034; Figure 3), reflecting relatively reduced glucose tolerance at dinner time. There was no significant effect of the behavioral cycle on early-phase postprandial insulin (P = .14; Figure 3), indicating similar β-cell function at breakfast time and dinner time. Late-phase postprandial insulin was 18% higher at dinner time than breakfast time (P = .001; Figure 3), suggesting decreased insulin sensitivity at dinner time (as evidenced by dinner time postprandial glucose being higher despite late-phase postprandial insulin also being higher). There were no significant effects of the behavioral cycle on fasting glucose or insulin (both P ≥ .12; Supplemental Figure 2).
Figure 3.
Effects of the behavioral cycle (left panels), circadian phase (middle panels), and circadian misalignment (right panels) on postprandial glucose and insulin profiles. Data are derived from identical test meals given at 8:00 am and 8:00 pm in both the circadian alignment and misalignment protocols. Data are derived as described in the legend of Figure 1. Green bars represent 20-minute test meals. Probability values: behavioral cycle effect, breakfast vs dinner; circadian-phase effect, biological morning vs biological evening; misalignment effect, circadian alignment vs circadian misalignment. Data are presented as mean ± SEM.
Circadian effects, independent of behavioral effects: glucose tolerance and early- and late-phase insulin were lower in the biological evening than in the biological morning
Postprandial glucose was 6.5% higher in the biological evening than morning (P = .0041; Figure 3), reflecting relatively reduced glucose tolerance in the biological evening. Early-phase postprandial insulin was 18% lower in the biological evening than morning (P = .011; Figure 3), indicating lower β-cell function in the biological evening. Late-phase postprandial insulin was also 18% lower in the biological evening than morning (P < .0001; Figure 3), further suggesting insufficient β-cell function in the biological evening (as evidenced by late-phase postprandial insulin being lower despite postprandial glucose being higher). Fasting glucose was 5% lower in the biological evening than morning (P = .040; Supplemental Figure 2). Fasting insulin was 28% lower in the biological evening than morning (P = .005; Supplemental Figure 2).
Circadian misalignment reduced glucose tolerance, independent of behavioral or circadian phase effects
Postprandial glucose was 5.6% higher in the circadian misalignment than alignment condition (P = .0042; Figure 3), reflecting relatively lower glucose tolerance during misalignment. Early-phase postprandial insulin was not significantly affected by circadian misalignment (P = .38; Figure 3). Late-phase postprandial insulin was 10% higher in the circadian misalignment than alignment condition (P = .015; Figure 3), suggesting decreased insulin sensitivity during misalignment (as evidenced by misalignment postprandial glucose being higher despite late phase postprandial insulin also being higher). There were no significant effects of circadian misalignment on fasting glucose or insulin or on 24-hour glucose or insulin area under the curves (all P ≥ .061; Figure 4 and Supplemental Figure 2).
Figure 4.
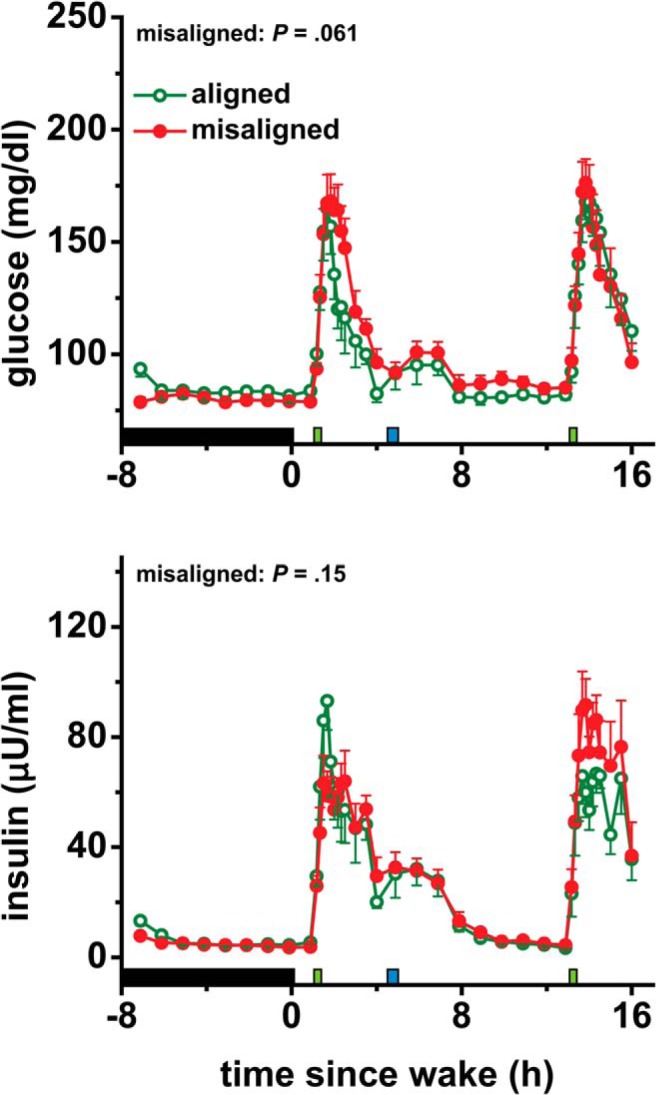
Effects of circadian misalignment on 24-hour glucose and insulin levels. Black bar represents sleep opportunity; narrow green bar represents a test meal; narrow blue bar represents a lunch meal. Probability values from 24-hour area under the curve analyses are shown. Data are presented as mean ± SEM.
Effect of circadian misalignment on 24-hour cortisol levels
Circadian misalignment did not significantly affect 24-hour mean cortisol levels (P = .079; Figure 5). Circadian misalignment changed the timing of the cortisol profile relative to the behavioral cycle (P < .0001; Figure 5). When misaligned, cortisol levels peaked toward the end of the wake period, rather than at the start of the wake period in the aligned condition.
Figure 5.
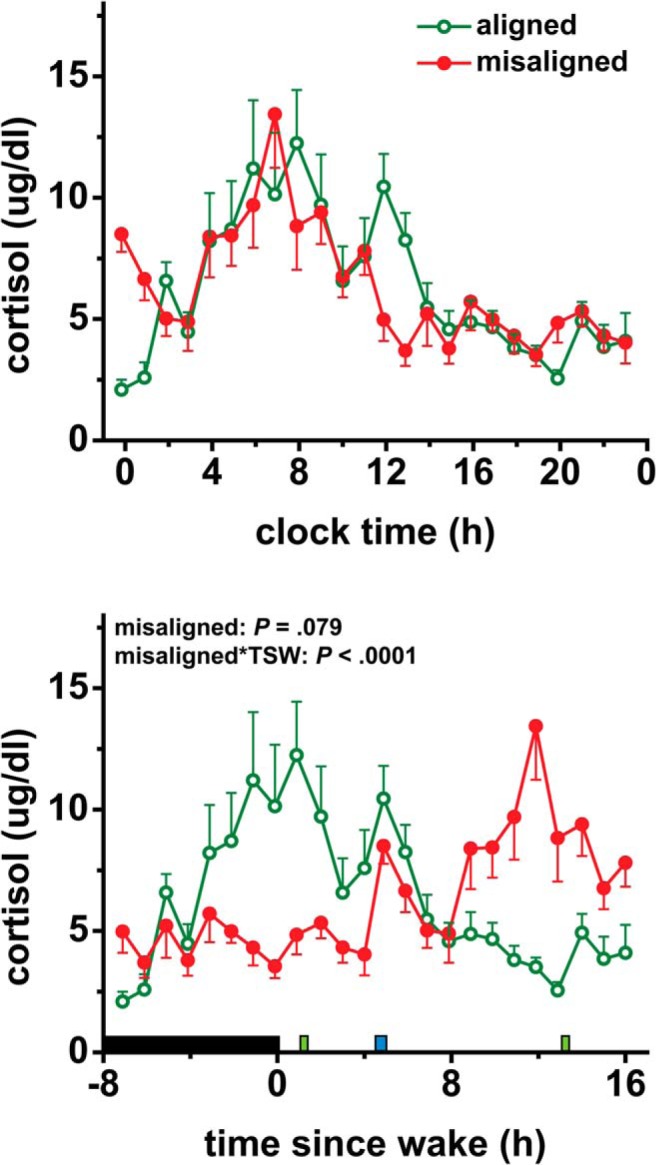
Effects of circadian misalignment on 24-hour cortisol levels. Upper panel, data are plotted according to clock time. Bottom panel, data are plotted relative to scheduled wake time. TSW, time since wake; black bar represents sleep opportunity; narrow green bar represents a test meal; narrow blue bar represents a lunch meal. Data are represented as mean ± SEM.
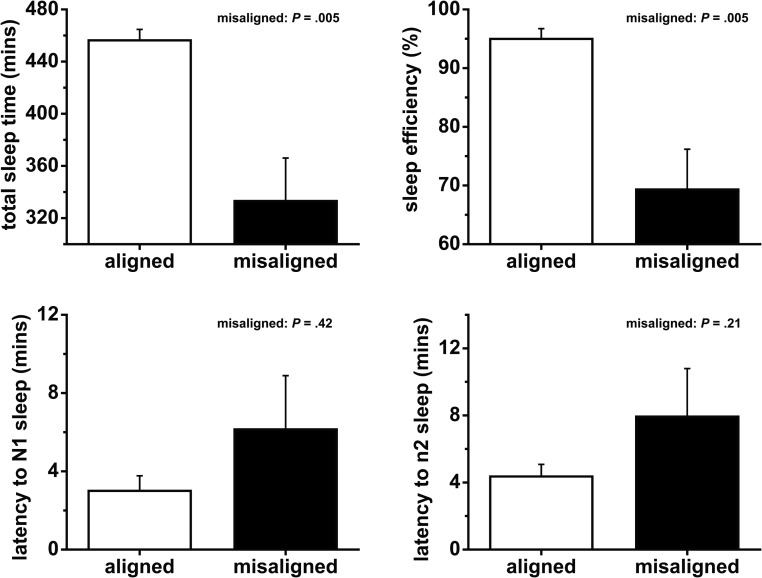
Circadian misalignment decreased total sleep time (TST)
Circadian misalignment decreased TST during the sleep opportunities preceding the test meals by 123 minutes (P = .005; Figure 6). Circadian misalignment decreased durations of N1 (stage 1), N2 (stage 2), and rapid eye movement sleep by 5, 70, and 40 minutes, respectively (all P ≤ .040; Supplemental Figure 3). There was no significant effect of circadian misalignment on N3 sleep (slow wave sleep, or deep sleep) or latencies to either N1 or N2 sleep (all P ≥ .14; Figure 6 and Supplemental Figure 3).
Figure 6.
Effects of circadian misalignment on sleep duration and latency. Data are represented as mean ± SEM.
No correlation between circadian misalignment-mediated changes in glucose metabolism and in sleep measured by PSG
There were no significant correlations between circadian misalignment-mediated changes in postprandial glucose, early- or late-phase postprandial insulin and circadian misalignment-mediated changes in TST or any sleep stage (all P ≥ .094).
Discussion
Our results revealed separate effects of the endogenous circadian system and of circadian misalignment, independent from effects of the behavioral cycle, on glucose tolerance in chronic shift workers. Glucose tolerance was lower in the biological evening than morning, independent of the behavioral cycle; thus, the internal circadian time of food intake may be an important factor to consider in shift workers and not just in people with a normal behavioral cycle (ie, sleeping at night and awake during the day), as we have shown before (10). Separately, circadian misalignment, typical in shift workers, decreased glucose tolerance, and this may help explain why shift work is a risk factor for type 2 diabetes (2). Third, glucose tolerance was also lower at dinner time than at breakfast time, independent of circadian phase. In nonshift workers and rodents, we and others have previously shown that the circadian phase affects glucose metabolism and that circadian misalignment and other forms of circadian disruption (eg, light at night and clock gene mutations) adversely impacts glucose metabolism (5–10, 12, 13, 18, 20, 21, 23). Here, for the first time, we show that circadian phase and circadian misalignment both affect glucose tolerance in shift workers, whose circadian alignment with the behavioral and environmental cycle is chronically and repeatedly disrupted. This further suggests that chronic shift workers do not become “immune” or are a self-selected population that is resistant, against the adverse metabolic effects of circadian misalignment. We found that the effect of the circadian system and circadian misalignment on glucose tolerance could be mediated, at least in part, by two different insulin mechanisms: 1) lower glucose tolerance in the biological evening was related to a 18% lower early-phase insulin response and a 18% lower late-phase insulin response, both implying reduced β-cell function; and 2) lower glucose tolerance during circadian misalignment that was associated with a 10% higher late-phase insulin response despite elevated postprandial glucose concentrations, suggesting decreased insulin sensitivity. Our results may also be due to circadian phase and alignment condition effects on factors such as gastrointestinal absorption, hepatic glucose output suppression, and noninsulin-dependent glucose metabolic pathways.
Two of our findings suggest that the circadian system, in part, controls glucose tolerance by affecting β-cell function in shift workers. First, early-phase insulin response was lower in the biological evening than morning. Second, late-phase insulin response was also lower in the biological evening than morning, despite glucose levels being higher. The circadian system could control β-cell function via multiple pathways. First, there are multisynaptic projections from the SCN to the pancreas (26, 27). Second, there are circadian clocks in the pancreas, and their specific disruption impairs insulin secretion and reduces glucose tolerance (14, 16). Third, circadian rhythms in hormones such as cortisol and melatonin may help entrain circadian clocks in the pancreas (28, 29). Fourth, some circadian-controlled hormones such as melatonin can acutely affect β-cell function (30, 31). The circadian-phase effect on glucose tolerance is probably not only a result of circadian modulation of β-cell function. For example, it is known that the circadian system controls insulin sensitivity in rodents (12, 13, 32). In this study, we have no evidence for or against a circadian phase effect on insulin sensitivity in shift workers. Future studies should use techniques that directly assess insulin sensitivity (eg, hyperinsulinemic-euglycemic clamps).
For the first time, we show that circadian misalignment reduces glucose tolerance in shift workers. This may be partly explained by circadian misalignment decreasing insulin sensitivity, as evidenced by glucose levels being higher despite insulin levels also being higher in the circadian misalignment condition. Reduced insulin sensitivity is an early defect in the development of type 2 diabetes (33). Thus, the circadian misalignment-mediated decrease in insulin sensitivity we observed in shift workers may help explain why epidemiological studies report that shift work is a risk factor for type 2 diabetes (2). In this study, we could not determine why circadian misalignment decreased insulin sensitivity. In rodents, peripheral clocks in metabolic tissues such as the liver and pancreas are rapidly entrained to a reversed feeding schedule, whereas the central circadian clock is not (34, 35). This results in internal desynchrony between central and peripheral clocks, which could cause mistimed/conflicting signals between different circadian clocks controlling metabolism. Thus, internal desynchrony may underlie the adverse metabolic effects of circadian misalignment (3). Currently there is no evidence for or against internal desynchrony occurring in humans. We found no evidence for short-term circadian misalignment affecting β-cell function. However, various forms of chronic circadian disruption (eg, clock gene mutations or repeated desynchrony between the circadian systems and 24-hour behavioral and environmental cycles) have been shown to impair β-cell function in rodents (11, 14, 20, 24, 36).
Sleep restriction and N3 sleep suppression reduce glucose tolerance, β-cell function, and insulin sensitivity (37–39). In this study, circadian misalignment decreased total sleep time by 123 minutes, and this reduction could help explain why we found circadian misalignment reduced glucose tolerance. However, we found no significant correlation between circadian misalignment-mediated changes in glucose metabolism and circadian misalignment-mediated changes in any of our sleep parameters. This may be a result of insufficient statistical power. Circadian misalignment can reduce insulin sensitivity, independent of sleep loss in nonshift workers (23).
Strengths of our study include studying chronic shift workers in highly-controlled, laboratory conditions. Limitations of our study also need to be considered. First, our sample size was small, owing to the arduous task of recruiting currently employed chronic shift workers to undertake two 3-day in-laboratory stays. Thus, our study was probably statistically underpowered to detect some effects. Second, we tested the effect of only short-term circadian misalignment on glucose metabolism; longer-term studies are needed. Third, we assessed glucose and insulin responses to meals consumed at only two phases of the behavioral cycle and circadian cycle. Thus, we may be underestimating the impact of the behavioral cycle and/or circadian cycle if we missed the peak and/or trough of the behavioral and/or circadian effect on glucose metabolism.
Summary
We have tested the separate effects of the behavioral cycle, circadian phase, and circadian misalignment on glucose and insulin responses to test meals in shift workers. We found that glucose tolerance is lower in the biological evening than morning, independent of behavioral cycle effects. This suggests that the internal circadian time of food intake may be an important factor to consider in shift workers. The circadian effect on glucose tolerance seemed to be partly explained by lower β-cell function in the biological evening than biological morning. We also found that circadian misalignment decreases glucose tolerance in shift workers, independent of behavioral or circadian effects. This effect appeared to be partly explained by a circadian misalignment-mediated decrease in insulin sensitivity. These adverse metabolic effects of circadian misalignment we observed in shift workers may help explain why shift work is a risk factor for type 2 diabetes.
Acknowledgments
We thank the research volunteers and Center for Clinical Investigation's dietetic, nursing, and technical staff.
This work was supported by National Heart, Lung, and Blood Institute (NHLBI) Grant R01 HL094806 (to F.A.J.L.S.). C.J.M. was partly supported by the National Space Biomedical Research Institute through National Aeronautics and Space Administration Grant NCC 9-58. F.A.J.L.S. was supported in part by NHLBI Grant R01 HL094806, the Fund to Sustain Research Excellence by the Brigham and Women's Hospital Biomedical Research Institute, National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK099512, and NHLBI Grant R01 HL118601. This project was also supported by Clinical Translational Science Award UL1RR025758 from the National Center for Research Resources (to Harvard University and Brigham and Women's Hospital).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- SCN
- suprachiasmatic nucleus
- TST
- total sleep time.
References
- 1. Bureau of Labor Statistics. US workers on flexible and shift schedules in May 2004. 2005. http://www.bls.gov/news.release/pdf/flex.pdf Accessed May 27, 2015.
- 2. Gan Y, Yang C, Tong X, et al. Shift work and diabetes mellitus: a meta-analysis of observational studies. Occup Environ Med. 2015;72:72–78. [DOI] [PubMed] [Google Scholar]
- 3. Morris CJ, Yang JN, Scheer FA. The impact of the circadian timing system on cardiovascular and metabolic function. Prog Brain Res. 2012;199:337–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Mol Cell Endocrinol. 2012;349:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106:4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab. 2005;90:2537–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morgan L, Arendt J, Owens D, et al. Effects of the endogenous clock and sleep time on melatonin, insulin, glucose and lipid metabolism. J Endocrinol. 1998;157:443–451. [DOI] [PubMed] [Google Scholar]
- 8. Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. 1991;88:934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frank SA, Roland DC, Sturis J, et al. Effects of aging on glucose regulation during wakefulness and sleep. Am J Physiol. 1995;269:E1006–E1016. [DOI] [PubMed] [Google Scholar]
- 10. Morris CJ, Yang JN, Garcia JI, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci USA. 2015;112:E2225–E2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science. 2005;308:1043–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coomans CP, van den Berg SAA, Lucassen EA, et al. The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes. 2013;62:1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shi S-q, Ansari TS, McGuinness Owen P, Wasserman David H, Johnson Carl H. Circadian disruption leads to insulin resistance and obesity. Curr Biol. 2013;23:372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marcheva B, Ramsey KM, Buhr ED, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA. 2008;105:15172–15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sadacca LA, Lamia KA, deLemos AS, Blum B, Weitz CJ. An intrinsic circadian clock of the pancreas is required for normal insulin release and glucose homeostasis in mice. Diabetologia. 2011;54:120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci USA. 2011;108:1657–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fonken LK, Workman JL, Walton JC, et al. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA. 2010;107:18664–18669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salgado-Delgado RC, Saderi N, Basualdo MdC, Guerrero-Vargas NN, Escobar C, Buijs RM. Shift work or food intake during the rest phase promotes metabolic disruption and desynchrony of liver genes in male rats. PLoS One. 2013;8:e60052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qian J, Block GD, Colwell CS, Matveyenko AV. Consequences of exposure to light at night on the pancreatic islet circadian clock and function in rats. Diabetes. 2013;62:3469–3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hampton SM, Morgan LM, Lawrence N, et al. Postprandial hormone and metabolic responses in simulated shift work. J Endocrinol. 1996;151:259–267. [DOI] [PubMed] [Google Scholar]
- 22. Lund J, Arendt J, Hampton SM, English J, Morgan LM. Postprandial hormone and metabolic responses amongst shift workers in Antarctica. J Endocrinol. 2001;171:557–564. [DOI] [PubMed] [Google Scholar]
- 23. Leproult R, Holmbäck U, Van Cauter E. Circadian misalignment augments markers of insulin resistance and inflammation, independently of sleep loss. Diabetes. 2014;63:1860–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buxton OM, Cain SW, O'Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Vol 1 Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 26. Ueyama T, Krout KE, Nguyen XV, et al. Suprachiasmatic nucleus: a central autonomic clock. Nat Neurosci. 1999;2:1051–1053. [DOI] [PubMed] [Google Scholar]
- 27. Buijs RM, Chun SJ, Niijima A, Romijn HJ, Nagai K. Parasympathetic and sympathetic control of the pancreas: a role for the suprachiasmatic nucleus and other hypothalamic centers that are involved in the regulation of food intake. J Comp Neurol. 2001;431:405–423. [DOI] [PubMed] [Google Scholar]
- 28. Mühlbauer E, Gross E, Labucay K, Wolgast S, Peschke E. Loss of melatonin signalling and its impact on circadian rhythms in mouse organs regulating blood glucose. Eur J Pharmacol. 2009;606:61–71. [DOI] [PubMed] [Google Scholar]
- 29. Balsalobre A, Brown SA, Marcacci L, et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2348. [DOI] [PubMed] [Google Scholar]
- 30. Mühlbauer E, Albrecht E, Bazwinsky-Wutschke I, Peschke E. Melatonin influences insulin secretion primarily via MT1 receptors in rat insulinoma cells (INS-1) and mouse pancreatic islets. J Pineal Res. 2012;52:446–459. [DOI] [PubMed] [Google Scholar]
- 31. Rubio-Sastre P, Scheer FA, Gomez-Abellan P, Madrid JA, Garaulet M. Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep. 2014;37:1715–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. la Fleur SE, Kalsbeek A, Wortel J, Fekkes ML, Buijs RM. A daily rhythm in glucose tolerance: a role for the suprachiasmatic nucleus. Diabetes. 2001;50:1237–1243. [DOI] [PubMed] [Google Scholar]
- 33. Lillioja S, Mott DM, Howard BV, et al. Impaired glucose tolerance as a disorder of insulin action, longitudinal and cross-sectional studies in Pima Indians. N Engl J Med. 1988;318:1217–1225. [DOI] [PubMed] [Google Scholar]
- 34. Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. [DOI] [PubMed] [Google Scholar]
- 36. Gale JE, Cox HI, Qian J, Block GD, Colwell CS, Matveyenko AV. Disruption of circadian rhythms accelerates development of diabetes through pancreatic β-cell loss and dysfunction. J Biol Rhythms. 2011;26:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. [DOI] [PubMed] [Google Scholar]
- 38. Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tasali E, Leproult R, Ehrmann DA, Van Cauter E. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci USA. 2008;105:1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]