Abstract
The objective of the study was to compare nasal, pharyngeal, and sputum eosinophil peroxidase (EPX) levels with induced sputum eosinophil percentage in 10 adults with poorly controlled asthma and 10 normal controls. EPX was measured using an ELISA and normalized for grams of protein for nasal and pharynx specimens and for mL-gram of protein for sputum. Sputum EPX levels were statistically different between asthma and control subjects (p=0.024). EPX levels measured in the nasal and pharyngeal swab samples derived from the same patients were also different between asthma and control subjects, each displaying a high degree of significance (p=0.002). Spearman’s correlation coefficients for nasal EPX and pharyngeal EPX levels compared to induced sputum eosinophil percentage were 0.81 (p=0.0007) and 0.78 (p=0.0017), respectively. There is a strong association in a given patient between both nasal and pharyngeal EPX levels and the eosinophil percentage of induced sputum.
Keywords: asthma, diagnostics, eosinophil granule proteins, eosinophils, sputum
Systematic review and meta-analyses of available studies suggest that titrating inhaled corticosteroids using sputum eosinophil percentage is an effective strategy to reduce the frequency of asthma exacerbations.(1) However, the required technical expertise and other confounding factors (e.g., labor-intensive character) associated with sputum eosinophil measurements have limited their use in clinical practice. Several proposed surrogate biomarkers of airway eosinophilia, including the fractional exhaled nitric oxide (FeNO), circulating blood eosinophil levels, and serum Immunoglobulin E, were compared in a systematic diagnostic testing accuracy review/meta-analysis and were found to have moderate sensitivity and specificity for predicting the presence of eosinophils in induced sputum (i.e., lower airway eosinophilia).(2) Therefore, a less invasive, technically simpler, and more precise surrogate biomarker would represent an un-met need to facilitate widespread implementation of management algorithms that incorporate eosinophil biomarkers.
A surprisingly high correlation between lower airway eosinophilia and the presence of eosinophils in nasal lavage was previously demonstrated with a receiver operating characteristic (ROC) curve whose area was 0.84 (p<0.001) using induced sputum as the comparative measure.(3) In contrast, the relationship between sputum eosinophilia and the presence of pharyngeal eosinophils has not been extensively studied. Eosinophil peroxidase (EPX) is a secondary granule protein uniquely secreted by eosinophils that we have shown is easily detected and quantified using a novel enzyme-linked immunosorbent assay (ELISA).(4) In particular, our previous studies of respiratory patients have shown that sputum EPX levels strongly correlate with eosinophil cell counts in sputum (r= 0.84).(5) The objective of this current study was to expand our assessments of asthma patients and determine if EPX was detectable in topical swabs of the mucosal surfaces from the nasal and pharyngeal cavities of asthma patients as a means of providing a minimally invasive and technically simpler point-of-care diagnostic assay.
Adult participants were identified from pulmonary and allergy-immunology subspecialty clinics from April 1, 2014 through March 31, 2015. Inclusion criteria for the case group of poorly-controlled asthma patients were provider-diagnosed asthma, FEV1 >1.0 Liter, Asthma Control Test (6) (ACT)™ <20, and FeNO (7) >50 ppb; exclusion criteria were a diagnosis of COPD or evidence of bronchiectasis. Control participants were age-matched to asthma participants (within 10 years) and were excluded if they had a history of rhinitis, sinusitis, asthma, or any eosinophil disorder. Three specimen types were collected from each participant: (i) Initially, a nasal specimen was collected using a sterile polyester fiber-tipped swab passing 5 times along the nasal floor and below the inferior turbinate bilaterally. (ii) A sterile polyester fiber-tipped swab was rubbed across the oropharynx 5 times. (iii) Participants underwent a sputum induction as previously described.(5) The measurement of EPX levels has been previously described (4, 5) and details regarding our current protocols and methods are included as an online supplement. EPX levels between asthma and control subjects were compared using the Wilcoxon signed-rank test; Spearman’s test was used to assess correlations between EPX levels and sputum eosinophil percentages. This study was approved by the Mayo Clinic IRB (14-001168).
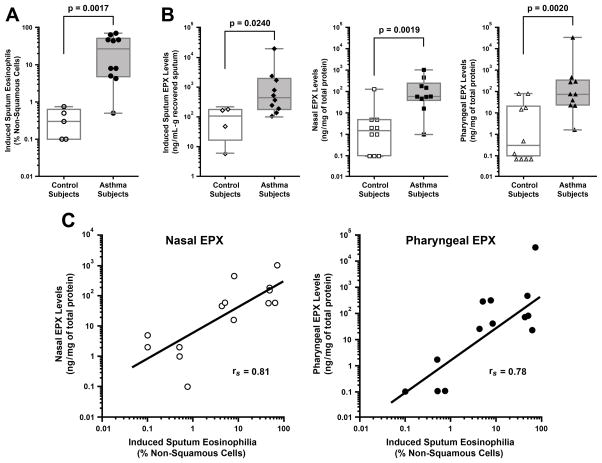
A summary of the participant demographics, clinical information, and laboratory data are displayed in Table 1, with equal numbers of females and males in the asthma and control subject groups. The mean age (±SD) of the asthma subjects was 48 years old (±8.7) and for controls was 43 (±13.6). Three of the subjects with asthma were using intranasal corticosteroids and 6 were using inhaled corticosteroids at the time samples were collected. All 10 of the asthma subjects had a diagnosis of rhinitis or sinusitis, while these diagnoses were excluded in the control subjects. None of the subjects smoked cigarettes. The mean ACT score (±SD) in the asthma group was 10.2 (±3.2) with <20 considered to be poorly controlled asthma. The mean FeNO measurements (±SD) in the asthma group was 93ppb (±40.3) with >39ppb considered to be abnormal. Mean pre-bronchodilator FEV1 % predicted (±SD) was 64% (±17.3%) for the asthma subjects and 101% (±9.8%) for the control group. Induced sputum eosinophilia was a highly significant metric (p=0.0017) stratifying asthma vs. control subjects with all but one of the enrolled subjects in the asthma group having an induced sputum eosinophil percentage >3% (Figure 1(A)). It is noteworthy that even in this structured setting, recovery and assessment of induced sputum from patients (particularly control subjects) was difficult with only 4 of 10 subjects capable of producing the necessary sample. As we have previously demonstrated, sputum EPX levels measured by our novel ELISA were statistically different between asthma and control subjects (p=0.024) (Figure 1(B)). More importantly, EPX levels measured in the nasal and pharyngeal swab samples derived from the same patients were also different between asthma and control subjects, each displaying a high degree of significance (p=0.002) (Figure 1(B)). Further statistical assessments of the data showed that Spearman’s correlation coefficients for nasal EPX and pharyngeal EPX levels compared to induced sputum eosinophil percentage were 0.81 (p=0.0007) and 0.78 (p=0.0017), respectively (Figure 1(C)). Indeed, the areas under ROC curves of these sample sources relative to induced sputum eosinophil percentage were each 0.89 (p=0.003).
Table 1.
Clinical Characterization of Participating Control and Asthma Patients
| Age | Subject Type | Gender | Nasal Steroid | Inhaled Steroid | ACT™ score | FEV1 (% predicted) | FENO (ppb) | Sputum Eosinophil (% non-squamous cells) | Sputum EPX (ng/mL-g protein) | Nasal EPX (ng/mg protein) | Pharyngeal EPX (ng/mg protein) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 61 | Asthma | Male | Y | Y | 17 | 72 | 53 | 4.3 | 532 | 46 | 24 |
| 53 | Asthma | Male | Y | Y | 5 | 57 | 141 | 45.5 | 806 | 57 | 74 |
| 52 | Asthma | Female | - | - | 11 | 57 | 77 | 62.7 | 369 | 58 | 22 |
| 50 | Asthma | Female | Y | Y | 10 | 69 | 73 | 48 | 20196 | 151 | 76 |
| 46 | Asthma | Male | - | - | 8 | 75 | 175 | 8 | 191 | 446 | 39 |
| 56 | Asthma | Male | - | Y | 12 | 37 | 104 | 5 | 247 | 59 | 274 |
| 38 | Asthma | Male | - | - | 9 | 43 | 70 | 0.5 | 1735 | 1 | 1.63 |
| 42 | Asthma | Female | - | Y | 10 | 66 | 70 | 7.8 | 106 | 16 | 303 |
| 47 | Asthma | Female | - | - | 12 | 99 | 115 | 48 | 139 | 182 | 469 |
| 32 | Asthma | Female | - | Y | 8 | 65 | 52 | 70.8 | 2374 | 1026 | 33487 |
| 25 | Control | Female | - | - | NP | 124 | NP | 0.75 | 6 | 0 | 0 |
| 36 | Control | Female | - | - | NP | 105 | NP | 0.3 | NA | 0 | 15 |
| 37 | Control | Female | - | - | NP | 99 | NP | NA | NA | 5 | 80 |
| 36 | Control | Female | - | - | NP | 97 | NP | NA | NA | 1 | 0.49 |
| 63 | Control | Male | - | - | NP | 91 | NP | NA | NA | 5 | 80 |
| 44 | Control | Male | - | - | NP | 107 | NP | NA | NA | 127 | 17 |
| 58 | Control | Male | - | - | NP | 100 | NP | 0 | 167 | 5 | 0 |
| 56 | Control | Male | - | - | NP | 104 | NP | NA | NA | 1 | 0.13 |
| 25 | Control | Male | - | - | NP | 89 | NP | 0.5 | 183 | 2 | 0 |
| 52 | Control | Female | - | - | NP | 96 | NP | 0 | 48 | 2 | 0 |
NA = Not Available
NP = Not Performed
Y = Yes
-= No
Figure 1. Nasal and pharyngeal EPX levels represent diagnostic metrics that significantly correlate with the lower airway eosinophilia observed in poorly controlled asthmatics.
(A) The presence of sputum eosinophils stratifies asthma from control subjects. (B) EPX levels in sputum as well as the mucosal surfaces of the nasal and pharyngeal cavities of asthma patients are each statistically different between asthma and control subjects. (C) Spearman correlation demonstrates that both nasal and pharyngeal EPX assessments increase as a function of induced sputum eosinophilia.
We recognize that there are several limitations to keep in mind when considering the results of this study. For example, poorly controlled asthma patients were selected for study inclusion using elevated FeNO measurements so our findings may not apply to the broader asthma population, which includes patients with non-eosinophilic asthma. In addition, some of the patients had very high sputum eosinophil percentages and could possibly have diagnoses in addition to asthma such as allergic bronchopulmonary aspergillosis or eosinophilic granulomatosis with polyangiitis. Finally, the ability to report the best cut-off levels for EPX measurements and analyze potentially confounding factors such as nasal steroid medications was limited by sample size.
The primary findings of this study are two-fold: (1) Nasal and pharyngeal EPX levels are elevated in poorly controlled asthma patients with elevated FeNO levels compared to normal control subjects and (2) There is a strong association in a given patient between both nasal and pharyngeal EPX levels and the eosinophil percentage of induced sputum. The demonstration that EPX levels in these minimally invasive and easily accessible samples are surrogate biomarkers for lower airway eosinophilia is significant. That is, eosinophil percentage of induced sputum, albeit a technically difficult measurement not widely adopted into clinical practice, is nonetheless considered a “gold standard” metric for adjusting asthma therapy. Thus, the use of nasal or pharyngeal swabs may represent a clinically relevant diagnostic metric whose simplicity of use would provide a novel point-of-care assay in the management of asthma patients. This is particularly true for the management of pediatric patients where the diagnostic tools available to clinicians (e.g., questionnaires and the use of pulmonary inflammatory assessments) are limited relative to adults.
Supplementary Material
Acknowledgments
The authors wish to thank members of Lee Laboratories who reviewed various drafts of this manuscript. We also wish to acknowledge the invaluable assistance of the Mayo Clinic in Arizona Study Coordinator Vardhini Mohan, and the excellent administrative support provided to Lee Laboratories by Linda Mardel and Shirley (“Charlie”) Kern. This work was supported by Mayo Foundation for Medical Education and Research and grants from NIH (HL065228 and HL058723)
Footnotes
All authors meet conditions 1, 2, and 3 according to the ICMJE authorship criteria.
None of the authors has a financial conflict of interest related to the publication of this study.
References
- 1.Petsky HL, Kynaston JA, Turner C, Li AM, Cates CJ, Lasserson TJ, et al. Tailored interventions based on sputum eosinophils versus clinical symptoms for asthma in children and adults. The Cochrane database of systematic reviews. 2007;(2):CD005603. doi: 10.1002/14651858.CD005603.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Korevaar DA, Westerhof GA, Wang J, Cohen JF, Spijker R, Sterk PJ, et al. Diagnostic accuracy of minimally invasive markers for detection of airway eosinophilia in asthma: a systematic review and meta-analysis. The Lancet Respiratory medicine. 2015;3(4):290–300. doi: 10.1016/S2213-2600(15)00050-8. [DOI] [PubMed] [Google Scholar]
- 3.Amorim MM, Araruna A, Caetano LB, Cruz AC, Santoro LL, Fernandes AL. Nasal eosinophilia: an indicator of eosinophilic inflammation in asthma. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2010;40(6):867–74. doi: 10.1111/j.1365-2222.2009.03439.x. [DOI] [PubMed] [Google Scholar]
- 4.Ochkur SI, Kim JD, Protheroe CA, Colbert D, Moqbel R, Lacy P, et al. The Development of a Sensitive and Specific ELISA for Mouse Eosinophil Peroxidase: Assessment of Eosinophil Degranulation Ex Vivo and in Models of Human Disease. Journal of Immunological Methods. 2012;375(1–2):138–47. doi: 10.1016/j.jim.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair P, Ochkur SI, Protheroe CA, Radford K, Efthimiadis A, Lee NA, et al. Eosinophil Peroxidase in Sputum Represents a Unique Biomarker of Airway Eosinophilia. Allergy. 2013;68:1177–1184. doi: 10.1111/all.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. The Journal of allergy and clinical immunology. 2004;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–15. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.