Abstract
Beta-catenin is known to play stage- and cell-specific functions during liver development. However, its role in development of bile ducts has not yet been addressed. Here we used stage-specific in vivo gain- and loss-of-function approaches, as well as lineage tracing experiments in the mouse, to first demonstrate that β-catenin is dispensable for differentiation of liver precursor cells (hepatoblasts) to cholangiocyte precursors. Second, when β-catenin was depleted in the latter, maturation of cholangiocytes, bile duct morphogenesis and differentiation of periportal hepatocytes from cholangiocyte precursors was normal. In contrast, stabilization of β-catenin in cholangiocyte precursors perturbed duct development and cholangiocyte differentiation. We conclude that β-catenin is dispensable for biliary development but that its activity must be kept within tight limits. Our work is expected to significantly impact on in vitro differentiation of stem cells to cholangiocytes for toxicology studies and disease modeling.
Keywords: Liver development, Biliary tract, Cholangiocytes, Hepatocytes, Differentiation, Morphogenesis
1. Introduction
Wnt-β-catenin signaling exerts distinct functions during liver development (Lade and Monga, 2011). Prior to liver formation, namely when the endoderm is being patterned along its antero-posterior axis, Wnt-β-catenin signaling must be repressed in the foregut endoderm to allow expression of transcription factors involved in early liver development (Goessling et al., 2008; Li et al., 2008; McLin et al., 2007). Beyond the stage of endoderm patterning and during the earliest stages of hepatic morphogenesis, Wnt signaling no longer represses, but instead stimulates liver specification and growth (Goessling et al., 2008; McLin et al., 2007; Micsenyi et al., 2004; Ober et al., 2006; Suksaweang et al., 2004). Later, when the liver epithelial cells consist of hepatoblasts, β-catenin depletion is associated with impaired proliferation, increased apoptosis, and deficient differentiation of the hepatoblasts (Tan et al., 2008). However, depletion of adenomatous polyposis coli (APC) in hepatoblasts, which stabilizes β-catenin and increases its activity, unexpectedly exerts similar effects as β-catenin depletion: lack of APC impairs proliferation of hepatoblasts and differentiation to hepatocytes (Decaens et al., 2008). The discrepancy might result from time-dependent effects of β-catenin. Indeed, β-catenin was depleted starting at E9.5, while APC was depleted starting at E11.5. This raises the question of stage-specific effects of β-catenin.
Regarding differentiation of hepatoblasts to the cholangiocyte lineage there is evidence from both loss-of-function and gain-of-function experiments with ex vivo explants that Wnt-β-catenin signaling promotes biliary differentiation (Hussain et al., 2004; Monga et al., 2003). Consistent with this, in vivo activation or inhibition of β-catenin stimulated biliary differentiation of hepatoblasts (Decaens et al., 2008; Tan et al., 2008). At later stages of biliary development, cholangiocyte precursors that are organized as a ductal plate strongly express β-catenin, of which the active non-phosphorylated form is transiently detected around E17.5 (Decaens et al., 2008). However, how β-catenin impacts on maturation of the cholangiocyte precursors and on bile duct morphogenesis has not yet been addressed. This issue is now gaining new and significant importance. Indeed, recent experiments in which stem cells are programmed in vitro to cholangiocytes have recently met with promising success, thereby providing innovative tools for pharmacological studies and biliary disease modeling (Dianat et al., 2014; Ogawa et al., 2015; Sampaziotis et al., 2015). Still, stem cell-derived cholangiocytes are immature, demonstrating the need to fully understand developmental mechanisms driving bile duct development in order to allow optimal recapitulation of developmental processes in cultured stem cells. Here we investigate the in vivo function of β-catenin at several stages of hepatoblast and cholangiocyte differentiation, and bile duct morphogenesis. We show that β-catenin is dispensable for differentiation of hepatoblasts to the biliary lineage, and for development of bile ducts from cholangiocyte precursors, but that overexpression of β-catenin is detrimental for biliary differentiation and morphogenesis.
2. Materials and methods
2.1. Animals
All experimental procedures using animals were approved by the University's Animal Ethics Committee and were conducted in compliance with the animal welfare regulations of Belgium. Animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86–23 revised 1985). Sox9-CreERT2 (Kopp et al., 2011), Alfp-Cre (Kellendonk et al., 2000), Ctnnb1lox (floxed exons 2–6) (Brault et al., 2001), Ctnnb1lox(ex3) (floxed exon 3) (Harada et al., 1999), Apclox (Colnot et al., 2004) and ROSA26ReYFP (Srinivas et al., 2001) mice were maintained in a mixed Bl6/CD1 background. Sections from Foxa3-Cre/βCatlox/lox embryonic livers were from the study of Tan et al. (2008).
For induction of Sox9-CreER activity, tamoxifen (Sigma (T5648), Bornem, Belgium) was dissolved in corn oil (Sigma (C8267), Bornem, Belgium) at a concentration of 30 mg/ml and intraperitoneally injected into E14.5 pregnant mothers at 100 mg/kg body weight. Embryos were collected at the indicated times. Quantification of recombination efficiency induced by Sox9CreER in Sox9-CreER/βCatKO/Rosa26ReYFP livers was performed by measuring the percentage of Sox9+ cells that express YFP (YFP+;Sox9+/[YFP+;Sox9+ +YFP– ;Sox9+])*100. At each developmental stage, cells of 6 periportal areas were counted per liver and 2 livers were considered per stage. The total number of counted SOX9+ cells was 996 (E15.5), 925 (E16.5) and 982 (E17.5).
2.2. Immunofluorescence
Mouse liver tissue samples were formalin-fixed and paraffin-embedded. Five μm-thick sections were stained as described (Carpentier et al., 2011) using antibodies and conditions listed in Supplementary Table 1. Pictures were taken with an Axiovert 200 fluorescent microscope using AxioVision system or a Cell Observer Spinning Disk confocal microscope (Carl Zeiss, Zaventem, Belgium).
Quantification of proliferation was performed by measuring the percentage of Sox9+/βcatnormal (wild-type, n=3) and Sox9+/βcathigh cells (Sox9-CreER/Apclox/lox, n=3; Sox9-CreER/beta-catlox(exon 3)lox, n=2) that express phosphohistone H3. The number of cells counted was: 879 Sox9+/βcatnormal cells in wild-type livers, 850 Sox9+/βcathigh cells in Sox9-CreER/Apclox/lox livers, and 812 Sox9+/βcathigh cells in Sox9-CreER/βcatlox(exon 3)lox livers.
2.3. Quantitative real-time PCR analysis
RNA from total liver RNA was extracted from liver using Trizol reagents (Invitrogen, Carlsbad, CA) 2 μg was reverse-transcribed with random hexamers using Moloney Murine Leukemia Virus reverse transcriptase (Invitrogen, Carlsbad, CA). Real-time quantitative PCR was performed by using Kapa SYBR FAST qPCR Master Mix (KapaBiosystems). Primers are listed in Supplementary Table 2. Microsoft Excel was used to calculate standard deviations and evaluate the statistical significance of the results (two-tailed Student's t test). P values <0.05 or lower were considered statistically significant. Gene expression was measured in four wild-type or four mutant embryonic livers. Data were normalized for GAPDH expression.
3. Results
3.1. Beta-catenin is dispensable for differentiation of hepatoblasts to cholangiocyte precursors
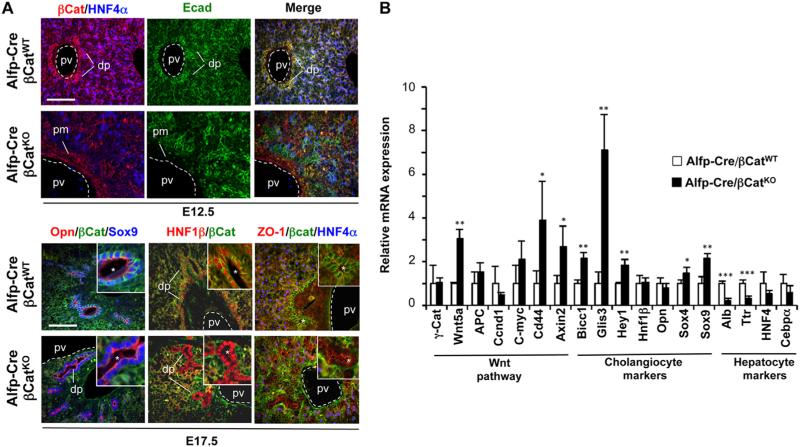
Intrahepatic cholangiocyte precursors derive from hepatoblasts, and the onset of biliary differentiation is marked by expression of SRY-related HMG box transcription factor 9 (Sox9) at E11.5 (Antoniou et al., 2009). The number of Sox9+ cholangiocyte precursors progressively increases from E11.5 to E15.5, and around E15.5 they line up around the branches of the portal vein to form a ductal plate. To address the role of β-catenin during the hepatoblast-to-cholangiocyte precursor transition, we generated embryos that have floxed alleles of β-catenin (Ctnnb1lox/lox) and bear the Albumin-α fetoprotein-Cre (Alfp-Cre) transgene to inactivate β-catenin in the hepatoblasts. Alfp-Cre drives expression of Cre recombinase in hepatoblasts starting at E10.5 (Kellendonk et al., 2000), and time-course analysis revealed that β-catenin expression was lost by E12.5 in almost all liver epithelial cells of Alfp-Cre/Ctnnb1lox/lox embryos (hereafter called Alfp-Cre/βCatKO) (Fig. 1A). The mesenchyme and blood vessels, which do not express Alfp-Cre, retained normal β-catenin levels (Fig. 1A). The regular cord-like organization of hepatoblasts was lost and several epithelial cells throughout the parenchyma were E-cadherin+ HNF4α–, which reflects deficient differentiation toward the hepatocyte lineage (Fig. 1A). These data were consistent with previous observations (Tan et al., 2008).
Fig. 1.
β-catenin controls hepatic morphogenesis but is not required for differentiation of hepatoblasts to the biliary lineage. (A) Embryos with inactivation of β-catenin starting around E12.5 (Alfp-Cre/βCatKO) showed perturbed liver architecture, abnormal differentiation of hepatocytes, and ductal plates composed of well-differentiated cholangiocytes organized around abnormally shaped luminal structures. (B) qRT-PCR analysis of E17.5 Alfp-Cre/βCatKO livers showed that cholangiocyte markers were normal or upregulated while hepatocyte markers were normal or downregulated. Data are means ±SD, n=4, ***p < 0.001, **p < 0.01, *p < 0.05. Alb, albumin; APC, adenomatous polyposis coli; Bicc1, bicaudal-c1; βCat, β-catenin; dp, ductal plate; Ecad, E-cadherin; γCat, γ-catenin; Opn, osteopontin; pv, portal vein; Ttr, transthyretin; ZO-1, zona occludens-1; *, lumen of ductal structure; scale bar, 100 μm.
Alfp-Cre/βCatKO embryos were present in a Mendelian ratio until E17.5 but none survived beyond birth (data not shown). Analysis of the livers at E17.5 further showed perturbed liver architecture (Fig. 1A). Consistent with previous reports (Tan et al., 2008), hepatocyte differentiation was inhibited as shown by the low levels of Albumin and Transthyretin (Fig. 1B). We also detected Sox9+ biliary cells in the ductal plate and developing ducts of Alfp-Cre/βCatKO livers, and these cells did not express β-catenin. They expressed the transcription factor Hepatocyte Nuclear Factor (HNF)1β but not the hepatocyte marker HNF4α (Fig. 1A), indicating that they were correctly specified and differentiated. At E17.5 polarity markers Osteopontin (OPN)) and Zonula Occludens-1 (ZO1) were expressed at the apical pole of these ducts, indicating that β-catenin-deficient biliary cells were well-polarized (Fig. 1A). qRT-PCR analyses at E17.5 revealed that the expression of biliary markers was normal or increased (Fig. 1B). Interestingly, markers of Wnt-β-catenin signaling were also normal or increased. Gamma-catenin expression was unaffected, and may contribute to compensate for the loss of β-catenin (Wickline et al., 2011), thereby potentially explaining why several markers of the Wnt-β-catenin pathway remain in the normal range. Wnt5a is increased, revealing a negative feedback between β-catenin and Wnt5a. The morphology of the ductal plate and developing ducts was abnormal: the number of luminal duct-like structures was increased and the latter were often irregularly shaped. Sox9+ β-catenin– cholangiocytes were detected at the hilum, center and periphery of the liver lobes in Alfp-Cre/βCatKO livers at E17.5, indicating that differentiation of biliary cells along the hilum-to-periphery axis occurs independently of β-catenin (Supplementary Fig. 1). We concluded that β-catenin is dispensable for differentiation of hepatoblasts to the cholangiocyte lineage but that β-catenin inactivation in hepatoblasts induces subsequent perturbed duct morphogenesis, most likely as a result of perturbed liver architecture.
The above results contrasted with previous in vivo β-catenin depletion experiments which instead suggested that β-catenin was required for biliary differentiation (Tan et al., 2008). However, in the present work, β-catenin was depleted by Alfp-Cre around E12–E13, while Tan and coworkers studied Foxa3-Cre/βCatKO livers in which β-catenin was suppressed earlier, namely at E9.5. At least a subset of Sox9+ β-catenin– cholangiocyte precursors in Alfp-Cre/βCatKO livers could have been specified before β-catenin was completely suppressed. Alternatively, the two-day window that differs among the two studies might reveal a restricted time period during which β-catenin is necessary to prime hepatoblasts for subsequent β-catenin-independent biliary specification. To clarify the issue, we reanalyzed biliary differentiation in Foxa3-Cre/βCatKO embryonic livers. These livers showed the previously described parenchymal disorganization, but to our surprise, biliary cells were detected: Sox9+ β-catenin– cells were present and formed duct-like structures that expressed HNF1β and OPN, but not HNF4α, which is indicative of their biliary maturation (Fig. 2). Therefore, we conclude that β-catenin is not required to initiate differentiation of biliary cells from hepatoblasts.
Fig. 2.
Early deletion of β-catenin in developing liver impairs hepatic morphogenesis but is compatible with biliary differentiation. Embryos with inactivation of β-catenin starting around E10.5 (Foxa3-Cre/βCatKO) showed perturbed liver architecture and differentiation of hepatocytes at E17.5; the number of HNF4α+ cells (arrowheads) was reduced. Biliary cells expressed the expected biliary markers but formed an increased number or irregularly-shaped luminal structures. βCat, β-catenin; Ecad, E-cadherin; hd, hepatic duct; OPN, osteopontin; pv, portal vein; *, lumen of ductal structure; scale bar, 100 μm.
3.2. Beta-catenin expression in cholangiocyte precursors is dispensable for bile duct morphogenesis
The study of Alfp-Cre/βCatKO and Foxa3-Cre/βCatKO livers does not enable us to specifically address the role of β-catenin in duct morphogenesis. Indeed, this process starts around E15.5 (Antoniou et al., 2009; Takashima et al., 2015), and the morphogenic anomalies in Alfp-Cre/βCatKO and Foxa3-Cre/βCatKO may result from the globally perturbed organization of the liver parenchyma. Therefore, we inactivated β-catenin specifically in the ductal plate by generating embryos that have floxed alleles of β-catenin (Ctnnb1lox/lox) and bear the tamoxifen-inducible Sox9-CreER transgene (Kopp et al., 2012). An additional ROSA26ReYFP reporter allele was included to allow tracing of the cells in which CreER has been activated.
A single intraperitoneal injection of tamoxifen in pregnant females at E14.5 resulted in near total recombination efficiency in the ductal plate after 48 h. Indeed, in Sox9-CreER/βCatKO/Rosa26ReYFP embryos, β-catenin was still expressed in most Sox9+ cells at E15.5, but it was no longer detected in the vast majority of Sox9+ cells at E16.5 and E17.5 (Fig. 3A). Sox9+ eYFP+ cells were devoid of β-catenin, as expected. Analysis of livers at E18.5 revealed that β-catenin-deficient cholangiocytes were phenotypically indistinguishable from their wild-type counterparts (Fig. 3B): the differentiation markers HNF6, HNF1β and Sox9, were expressed at normal levels, while the hepatocyte marker HNF4α was absent as expected. Apical polarity markers OPN, Mucin-1 (Muc1) and Zonula occludens-1 (ZO1), as well as the basolateral and basal markers E-cadherin and αSMA were also expressed normally (Fig. 3B).
Fig. 3.
Beta-catenin is dispensable for bile duct morphogenesis. (A) After injection of tamoxifen at E14.5, inactivation of β-catenin in developing ducts (Sox9-CreER/βCatKO/Rosa26ReYFP) was detectable at E15.5 and near complete at E16.5 and E17.5. Recombination efficiency, expressed as percentage of Sox9+ cells that express YFP ((YFP+;Sox9+/[YFP+;Sox9+ +YFP–;Sox9+])*100) is indicated. (B) Inactivation of β-catenin in developing bile ducts was associated with normal cholangiocyte differentiation and polarity, and normal development of bile ducts at E18.5. αSMA, α smooth muscle actin; βCat, β-catenin; dp, ductal plate; Muc1, mucin-1; OPN, osteopontin; pv, portal vein; ZO-1, zona occludens-1; *, lumen of ductal structure; scale bar, 100 μm.
3.3. Beta-catenin is not required for cell fate determination of cholangiocyte precurors
Cholangiocyte precursors in the ductal plate were shown earlier to give rise to the adult biliary tract but also to a subset of periportal hepatocytes (Carpentier et al., 2011). To verify if this cell fate allocation depends on β-catenin, we injected tamoxifen in mothers bearing Sox9-CreER/βCatKO/Rosa26ReYFP embryos to label the cholangiocyte precursors, and analyzed the livers of the off-spring at postnatal day (P) 20. Mothers injected with tamoxifen devoured their pups immediately after delivery (data not shown). To circumvent that problem we obtained lineage-traced Sox9-CreER/βCatKO/Rosa26ReYFP pups by cesarian birth at E19.0. These pups were adopted by foster mothers and survived with no overt phenotype. Analysis of Sox9-CreER/βCatKO/Rosa26ReYFP livers at P20 revealed that β-catenin-deficient bile ducts were normal (Fig. 4). β-catenin-deficient cholangiocytes expressed the biliary marker Sox9, and cytokeratin 19 (CK19) and did not express the hepatocyte markers HNF4α, carbamoyl phosphate synthetase-1 (CPS1) or glutamine synthetase (GS) (Fig. 4; Supplementary Fig. 2). Importantly, a subset of periportal hepatocytes were YFP+ (Fig. 4), indicating that they derived from the β-catenin-deficient ductal plate, and demonstrating that fate determination of cholangiocyte precursors does not critically depend on β-catenin.
Fig. 4.
Beta-catenin is dispensable for cell fate determination of cholangiocyte precurors. Lineage tracing of the cholangiocyte precusors labeled at E14.5 (Sox9-CreER/βCatKO/Rosa26ReYFP) showed that these cells generate bile ducts and periportal hepatocytes in postnatal livers, in the presence (Sox9-CreER/βCatWT/Rosa26ReYFP) or absence (Sox9-CreER/βCatKO/Rosa26ReYFP) of β-catenin. Periportal hepatocytes derived from the wild-type or β-catenin-deficient cholangiocyte precursors expressed carbamoyl-phosphate-synthetase-1 (CPS1), but not glutamine synthetase (GS). βCat, β-catenin; cv, central vein; Ecad, E-cadherin; pv, portal vein; YFP, yellow fluorescent protein; *, lumen of bile duct, scale bar (200 μm).
3.4. Overexpression of β-catenin in developing cholangiocytes perturbs bile duct morphogenesis and cholangiocyte differentiation
Beta-catenin is not required for bile duct development, but we next wished to determine if it can promote bile duct morphogenesis. To address this question, embryos from two transgenic lines with activation of β-catenin were investigated, namely embryos that have inducible and bile duct-specific inactivation of APC (Sox9-CreER/Apclox/lox), or deletion of the third exon of the β-catenin gene (Sox9-CreER/Ctnnb1lox(ex3)/lox(ex3)). Removal of this exon prevents phosphorylation-induced degradation of β-catenin (Harada et al., 1999). Inactivation of APC or removal of β-catenin exon 3 was induced by injection of tamoxifen at E14.5, followed by phenotypic analysis at E18.5.
Compared to their wild-type littermates, mutant embryos showed striking bile duct dysplasia (Fig. 5; Supplementary Fig. 2). We observed patches of pseudo-tubular structures with strong induction of β-catenin. Beta-catenin-overexpressing cells were Sox9+, CK19+, HNF4α–, as expected for biliary cells. However, these cells failed to express HNF1β, Osteopontin or Mucin-1, indicating that differentiation and polarity were affected. Embryos with APC inactivation showed the same phenotype as those with deletion of β-catenin's exon 3, demonstrating that this phenotype is indeed caused by activation of β-catenin. Tamoxifen treatment at E14.5 induces Sox9-CreER activity around E15.5 (Carpentier et al., 2011), a stage where HNF1β, Muc1 and Opn are already expressed in developing ductal structures; this implies that β-catenin not only blocks cholangiocyte maturation but also reverts their phenotype to a less well differentiated state. Beta-catenin did not impact on proliferation: expression of phosphohistone H3 was similar in Sox9+/βcatnormal (wild-type) and Sox9+/βcathigh (Sox9-CreER/Apclox/lox and Sox9-CreER/beta-catlox(exon 3)lox) cells (Fig. 5B). We concluded that β-catenin is dispensable for bile duct morphogenesis but that accurate regulation of β-catenin concentration and function is necessary for proper biliary development.
Fig. 5.
Beta-catenin overexpression in developing bile ducts induces biliary dysplasia and perturbs cholangiocyte differentiation and polarity. (A) Beta-catenin was induced by inactivation of APC or deletion of exon 3 from the β-catenin gene (Ctnnb1) in developing bile ducts using tamoxifen-inducible Sox9-CreER. Tamoxifen was injected at E14.5 and embryos were collected at E18.5, prior immunostaing analysis with the indicated markers. (B) Proliferation was quantified by measuring the percentage (7SD) of Sox9+/βcatnormal (wild-type) and Sox9+/βcathigh cells (Sox9-CreER/Apclox/lox and Sox9-CreER/beta-catlox(exon 3)lox) that express phosphohistone H3 (see materials and methods). βCat, β-catenin; Muc1, mucin-1; OPN, osteopontin; pv, portal vein; *, lumen of ductal structure; scale bar, 100 μm.
4. Discussion
Beta-catenin plays essential roles in adult and developing liver (Gougelet and Colnot, 2012; Lade and Monga, 2011; Monga, 2015; Torre et al., 2011). During liver development, cell- and stage-specific effects have been identified, but β-catenin function has not been addressed during bile duct morphogenesis. Here, using gain-and loss-of-function mouse models we show that β-catenin is dispensable for differentiation of hepatoblasts to the biliary line-age and for bile duct morphogenesis, but that overexpression of β-catenin is detrimental for duct morphogenesis.
Canonical Wnt-β-catenin signaling is tightly dependent on the cellular context. An appropriate balance between time- and tissue-specific regulators (ligands, ligand traps, receptors) that turn signaling on or off determines the eventual level of signaling activity during early liver development (McLin et al., 2007). Evaluating such balance at the experimental level is exceedingly difficult considering the number of Wnt ligands and receptors functional in liver (Zeng et al., 2007). Cross-talks between Wnt-β-catenin and other pathways add another layer of complexity, as illustrated by the modulation of Wnt signaling in liver by Fibroblast Growth Factor 10 (Berg et al., 2007; Shin et al., 2012). In addition, several processes are controlled by an equilibrium between canonical and non-canonical Wnt signaling, as shown by the non-canonical Wnt5a-dependent inhibition of β-catenin that results in hepatocyte proliferation arrest at the end of liver regeneration (Yang et al., 2015). Along the same lines, the Wnt5a-calcium/calmodulin-dependent protein kinase II pathway has been shown to repress biliary differentiation (Kiyohashi et al., 2013), while Wnt-β-catenin signaling is usually considered to promote biliary differentiation.
The complex regulation of Wnt-β-catenin signaling outlined above is susceptible to generate conflicting results when studying the function of signaling mediators. Indeed, despite that β-catenin depletion is expected to generate an opposite phenotype to that resulting from β-catenin stabilization by APC depletion, both approaches generated similar effects on hepatoblast proliferation and survival, and on hepatocyte differentiation (Decaens et al., 2008; Tan et al., 2008). This apparent discrepancy might result from the fact that β-catenin was partially depleted starting at E9.5 (Tan et al., 2008) while APC was inactivated by E11.5 (Decaens et al., 2008), potentially revealing time-dependent effects of β-catenin in hepatoblasts. However, our present results combined with those of Decaens and co-workers show that inactivation or stabilization of β-catenin at the same developmental stage similarly perturb liver architecture and hepatocyte differentiation. Therefore, time-dependent effects of β-catenin are unlikely to explain the discrepancy. Consequently, we suggest that compensatory mechanism appearing in conditions of β-catenin depletion might not have a counterpart when β-catenin is stabilized. In this context, β-catenin depletion is known to be associated with compensatory effects of γ-catenin (Wickline et al., 2011), a gene whose expression was maintained in β-catenin-deficient embronic liver. The normal expression of γ-catenin in the absence of β-catenin may contribute to maintain the expression of β-catenin target genes, as observed here. Along the same lines, APC-independent effects of β-catenin have been described (Xiao et al., 2003), leading to the possibility that APC depletion may lead to a phenotype that is not a mirror image of β-catenin depletion. Surprisingly, β-catenin-deficient livers develop cholangiocytes but express higher levels of Wnt5a, a repressor of biliary differentiation. Our interpretation is that the rise in Wnt5a is not sufficient to inhibit differentiation.
Earlier work, either by APC depletion or by β-catenin inactivation, indicated that β-catenin is both necessary and sufficient to promote differentiation of hepatoblasts toward a biliary phenotype (Decaens et al., 2008; Hussain et al., 2004; Monga et al., 2003; Tan et al., 2008). Here we update this conclusion by demonstrating that β-catenin is actually not required for biliary differentiation. Foxa3-Cre dependent deletion of β-catenin in developing liver led to a substantial distortion of hepatic architecture, which led to the previous deductions of an important and primary role of β-catenin in early biliary development. However, with more timely markers of biliary development, such as Sox9 and OPN, we were able to directly and more conclusively show that β-catenin is dispensable for biliary differention.
Our present work investigates for the first time the developmental role of β-catenin beyond the stage of hepatoblast-biliary precursor transition, namely during bile duct morphogenesis and cholangiocyte maturation. Using an in vivo loss-of-function approach, we show that β-catenin is not required for cholangiocyte maturation and duct formation. It remains an open-ended question though, if loss of β-catenin in Sox9 cells could be compensated by another mechanisms such as+ γ-catenin and future studies would be necessary to address that directly. In parallel, using two independent gain-of-function approaches we demonstrated that excessive β-catenin activity in developing ducts stimulates biliary development. However, the latter was characterized by aberrant morphology of ducts and perturbed differentiation of the cholangiocytes. We note that excessive β-catenin activity in hepatoblasts stimulated cholangiocyte differentiation, but in that case also, differentiation was abnormal, as illustrated by low HNF1β levels (Decaens et al., 2008).
We conclude that β-catenin is dispensable for differentiation of hepatoblasts to cholangiocyte precursors, but that β-catenin activity must be kept within tight limits to allow normal maturation of the precursors to mature cholangiocytes, and to permit normal bile duct formation. These data need to be taken into account when programming stem cells in vitro to cholangiocytes for toxicology studies and disease modeling experiments.
Supplementary Material
Acknowledgments
The authors thank C. Pierreux, J.-B. Beaudry and members of the Lemaigre laboratory for help, and R. Kemler and M. Taketo for mice. The work of FPL was supported by the Interuniversity Attraction Pole Programme (Belgian Science Policy (BELSPO), Grant PVII-47), the D.G. Higher Education and Scientific Research of the French Community of Belgium (Grant 10/15-029), the Alphonse and Jean Forton Fund, and the Fonds de la Recherche Scientifique Médicale (Grant 3.4536.10 F.S. Colnot was supported by the Ligue Nationale Contre le Cancer (France). PJ is senior research associate at the FRS-FNRS (Belgium).
Abbreviations
- APC
adenomatous polyposis coli
- CK19
cytokeratin 19
- E
embryonic day
- HNF
Hepatocyte Nuclear Factor
- OPN
Osteopontin
- Muc1
Mucin-1
- P
postnatal day
- Sox9
SRY-related HMG box transcription factor 9
- ZO1
Zonula Occludens-1
Footnotes
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.diff.2016.02.001.
References
- Antoniou A, Raynaud P, Cordi S, Zong Y, Tronche F, Stanger B, Jacquemin P, Pierreux CE, Clotman F, Lemaigre FP. Intrahepatic bile ducts develop according to a new mode of tubulogenesis regulated by the transcription factor SOX9. Gastroenterology. 2009;136:2325–2333. doi: 10.1053/j.gastro.2009.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg T, Rountree CB, Lee L, Estrada J, Sala FG, Choe A, Veltmaat JM, De Langhe S, Lee R, Tsukamoto H, Crooks GM, Bellusci S, Wang KS. Fibroblast growth factor 10 is critical for liver growth during embryogenesis and controls hepatoblast survival via beta-catenin activation. Hepatology. 2007;46:1187–1197. doi: 10.1002/hep.21814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Carpentier R, Suner RE, Van Hul N, Kopp JL, Beaudry JB, Cordi S, Antoniou A, Raynaud P, Lepreux S, Jacquemin P, Leclercq IA, Sander M, Lemaigre FP. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology. 2011;141:1432–1438. doi: 10.1053/j.gastro.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colnot S, Decaens T, Niwa-Kawakita M, Godard C, Hamard G, Kahn A, Giovannini M, Perret C. Liver-targeted disruption of APC in mice activates beta-catenin signaling and leads to hepatocellular carcinomas. Proc. Natl. Acad. Sci. USA. 2004;101:17216–17221. doi: 10.1073/pnas.0404761101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaens T, Godard C, de Reynies A, Rickman DS, Tronche F, Couty JP, Perret C, Colnot S. Stabilization of beta-catenin affects mouse embryonic liver growth and hepatoblast fate. Hepatology. 2008;47:247–258. doi: 10.1002/hep.21952. [DOI] [PubMed] [Google Scholar]
- Dianat N, Dubois-Pot-Schneider H, Steichen C, Desterke C, Leclerc P, Raveux A, Combettes L, Weber A, Corlu A, Dubart-Kupperschmitt A. Generation of functional cholangiocyte-like cells from human pluripotent stem cells and HepaRG cells. Hepatology. 2014;60:700–714. doi: 10.1002/hep.27165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling W, North TE, Lord AM, Ceol C, Lee S, Weidinger G, Bourque C, Strijbosch R, Haramis AP, Puder M, Clevers H, Moon RT, Zon LI. APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Dev. Biol. 2008;320:161–174. doi: 10.1016/j.ydbio.2008.05.526. [DOI] [PubMed] [Google Scholar]
- Gougelet A, Colnot S. A complex interplay between Wnt/beta-catenin signalling and the cell cycle in the adult liver. Int. J. Hepatol. 2012;2012:816125. doi: 10.1155/2012/816125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain SZ, Sneddon T, Tan X, Micsenyi A, Michalopoulos GK, Monga SP. Wnt impacts growth and differentiation in ex vivo liver development. Exp. Cell Res. 2004;292:157–169. doi: 10.1016/j.yexcr.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Kellendonk C, Opherk C, Anlag K, Schutz G, Tronche F. Hepatocyte-specific expression of Cre recombinase. Genesis. 2000;26:151–153. doi: 10.1002/(sici)1526-968x(200002)26:2<151::aid-gene17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Kiyohashi K, Kakinuma S, Kamiya A, Sakamoto N, Nitta S, Yamanaka H, Yoshino K, Fujiki J, Murakawa M, Kusano-Kitazume A, Shimizu H, Okamoto R, Azuma S, Nakagawa M, Asahina Y, Tanimizu N, Kikuchi A, Nakauchi H, Watanabe M. Wnt5a signaling mediates biliary differentiation of fetal hepatic stem/progenitor cells in mice. Hepatology. 2013;57:2502–2513. doi: 10.1002/hep.26293. [DOI] [PubMed] [Google Scholar]
- Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, Ma J, Sander M. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development. 2011;138:653–665. doi: 10.1242/dev.056499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp JL, von Figura G, Mayes E, Liu FF, Dubois CL, Morris J, Pt, Pan FC, Akiyama H, Wright CV, Jensen K, Hebrok M, Sander M. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:737–750. doi: 10.1016/j.ccr.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lade AG, Monga SP. Beta-catenin signaling in hepatic development and progenitors: which way does the WNT blow? Dev. Dyn. 2011;240:486–500. doi: 10.1002/dvdy.22522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Rankin SA, Sinner D, Kenny AP, Krieg PA, Zorn AM. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 2008;22:3050–3063. doi: 10.1101/gad.1687308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–2217. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- Micsenyi A, Tan X, Sneddon T, Luo JH, Michalopoulos GK, Monga SP. Beta-catenin is temporally regulated during normal liver development. Gastroenterology. 2004;126:1134–1146. doi: 10.1053/j.gastro.2003.12.047. [DOI] [PubMed] [Google Scholar]
- Monga SP. Beta-catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology. 2015;148:1294–1310. doi: 10.1053/j.gastro.2015.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monga SP, Monga HK, Tan X, Mule K, Pediaditakis P, Michalopoulos GK. Beta-catenin antisense studies in embryonic liver cultures: role in proliferation, apoptosis, and lineage specification. Gastroenterology. 2003;124:202–216. doi: 10.1053/gast.2003.50000. [DOI] [PubMed] [Google Scholar]
- Ober EA, Verkade H, Field HA, Stainier DY. Mesodermal Wnt2b signalling positively regulates liver specification. Nature. 2006;442:688–691. doi: 10.1038/nature04888. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Ogawa S, Bear CE, Ahmadi S, Chin S, Li B, Grompe M, Keller G, Kamath BM, Ghanekar A. Directed differentiation of cholangiocytes from human pluripotent stem cells. Nat. Biotechnol. 2015;33:853–861. doi: 10.1038/nbt.3294. [DOI] [PubMed] [Google Scholar]
- Sampaziotis F, Cardoso de Brito M, Madrigal P, Bertero A, Saeb-Parsy K, Soares FA, Schrumpf E, Melum E, Karlsen TH, Bradley JA, Gelson WT, Davies S, Baker A, Kaser A, Alexander GJ, Hannan NR, Vallier L. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat. Biotechnol. 2015;33:845–852. doi: 10.1038/nbt.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D, Weidinger G, Moon RT, Stainier DY. Intrinsic and extrinsic modifiers of the regulative capacity of the developing liver. Mech. Dev. 2012;128:525–535. doi: 10.1016/j.mod.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suksaweang S, Lin CM, Jiang TX, Hughes MW, Widelitz RB, Chuong CM. Morphogenesis of chicken liver: identification of localized growth zones and the role of beta-catenin/Wnt in size regulation. Dev. Biol. 2004;266:109–122. doi: 10.1016/j.ydbio.2003.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima Y, Terada M, Kawabata M, Suzuki A. Dynamic three-dimensional morphogenesis of intrahepatic bile ducts in mouse liver development. Hepatology. 2015;61:1003–1011. doi: 10.1002/hep.27436. [DOI] [PubMed] [Google Scholar]
- Tan X, Yuan Y, Zeng G, Apte U, Thompson MD, Cieply B, Stolz DB, Michalopoulos GK, Kaestner KH, Monga SP. Beta-catenin deletion in hepatoblasts disrupts hepatic morphogenesis and survival during mouse development. Hepatology. 2008;47:1667–1679. doi: 10.1002/hep.22225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre C, Perret C, Colnot S. Transcription dynamics in a physiological process: beta-catenin signaling directs liver metabolic zonation. Int. J. Biochem. Cell Biol. 2011;43:271–278. doi: 10.1016/j.biocel.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Wickline ED, Awuah PK, Behari J, Ross M, Stolz DB, Monga SP. Hepatocyte gamma-catenin compensates for conditionally deleted beta-catenin at adherens junctions. J. Hepatol. 2011;55:1256–1262. doi: 10.1016/j.jhep.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao JH, Ghosn C, Hinchman C, Forbes C, Wang J, Snider N, Cordrey A, Zhao Y, Chandraratna RA. Adenomatous polyposis coli (APC)-independent regulation of beta-catenin degradation via a retinoid X receptor-mediated pathway. J. Biol. Chem. 2003;278:29954–29962. doi: 10.1074/jbc.M304761200. [DOI] [PubMed] [Google Scholar]
- Yang J, Cusimano A, Monga JK, Preziosi ME, Pullara F, Calero G, Lang R, Yamaguchi TP, Nejak-Bowen KN, Monga SP. WNT5A inhibits hepatocyte proliferation and concludes beta-catenin signaling in liver regeneration. Am. J. Pathol. 2015 doi: 10.1016/j.ajpath.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng G, Awan F, Otruba W, Muller P, Apte U, Tan X, Gandhi C, Demetris AJ, Monga SP. Wnt'er in liver: expression of Wnt and frizzled genes in mouse. Hepatolog. 2007;45:195–204. doi: 10.1002/hep.21473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.