Abstract
Background
Evidence from cross-sectional studies has suggested a positive association between moderate alcohol consumption and health-related quality of life but prospective data remain scarce.
Objectives
To examine the bidirectional relationships between alcohol consumption and health-related quality of life using a longitudinal study design.
Methods
A total of 92,448 participants of the Nurses’ Health Study II reported their alcohol consumption (in 1991, 1995, 1999, and 2003) and health-related quality of life (in 1993, 1997, and 2001). Using generalized estimating equations, we modeled the physical and mental component summary (PCS and MCS) scores as a function of alcohol consumption 2 years earlier (n = 88,363), and vice versa (n = 84,621).
Results
Greater alcohol consumption was associated with better PCS scores 2 years later in a dose–response manner up to ~1 serving daily [mean difference (β) = 0.67 ± 0.06 PCS units, for moderate vs. infrequent drinkers]. After adjustment for previous PCS, a similar but attenuated pattern was observed (β = 0.33 ± 0.07). Moderate alcohol consumption was not related to MCS, whereas moderate to heavy alcohol consumption was associated with lower MCS scores (β = -0.34 ± 0.15). Higher PCS scores were associated with greater alcohol consumption 2 years later, also after adjustment for previous alcohol consumption (β = 0.53 ± 0.05 g/day). MCS was not associated with alcohol consumption 2 years later.
Conclusion
Among young and middle-aged women, moderate alcohol intake was associated with a small improvement in physical health-related quality of life 2 years later, and vice versa. Moderate alcohol consumption was not associated with mental health-related quality of life in either direction.
Keywords: alcohol drinking, alcoholic beverages, females, health, longitudinal studies, quality of life
Introduction
Results from cross-sectional studies suggest an association between moderate alcohol consumption and higher health-related quality of life (HRQOL) [1–6]. However, excessive alcohol consumption and binge drinking have been associated with poorer subjective health [7, 8]. Indeed, in longitudinal studies, moderate alcohol consumption has been associated with physical and mental health benefits such as a reduced risk of type 2 diabetes, rheumatoid arthritis, and incident depression [9–11]. Moreover, moderate alcohol consumption has been related to lower psychological distress, increased sociability, and mood enhancement [12]. However, these associations may be biased by reverse causation. For example, moderate drinkers may also engage in more social activities [13]. A few small longitudinal studies have examined the associations between alcohol consumption and HRQOL prospectively. Kaplan et al. [14] and Byles et al. [15] found that persistent moderate alcohol consumption was related to a higher mental and physical HRQOL compared to abstaining or decreasing alcohol consumption. This suggests that alcohol consumption may influence subsequent quality of life. However, the reverse association, in which HRQOL influences subsequent alcohol consumption, may also be true. Bell and Britton suggested that the relationship between mental health and alcohol consumption is driven by mental health [16], meaning that mental health influences change in alcohol but not vice versa. Specifically, they demonstrated that individuals with better mental HRQOL and high alcohol consumption showed a larger decrease in alcohol consumption in the following 5 years [16]. Furthermore, individuals with poorer self-perceived health status tend to be more likely to reduce or stop drinking alcohol than those with excellent health status [17].
These studies provide evidence for a more complex, bidirectional relationship between alcohol consumption and the physical and mental components of HRQOL. However, to our knowledge, these relationships have not been investigated in any large-scale prospective studies with repeated measures of both alcohol consumption and HRQOL. Therefore, the aim of this study was to examine these bidirectional relationships in young and middle-aged women who were followed for 12 years in the Nurses’ Health Study II.
Methods
Study population
The Nurses’ Health Study II was established in 1989, when 116,430 US female nurses aged 25–42 years responded to a mailed questionnaire regarding their diet and medical history. The participants have been followed every 2 years with mailed questionnaires to collect diet, lifestyle, and medical information. We excluded women with missing data on alcohol consumption or HRQOL on every questionnaire throughout the study. Furthermore, we excluded women who were diagnosed with multiple sclerosis or cancer (except those with non-melanoma skin cancer) before 1991, when follow-up for these analyses started, because these diseases have a large negative impact on HRQOL [18, 19]. Additionally, in each cycle, we excluded women who were pregnant in the period from 2 years before exposure until the outcome measurement time, as pregnancy causes most women to stop drinking and reduces quality of life [20]. After these exclusions, 186,845 observations (from 88,363 participants) remained for the analysis of the association between alcohol consumption and subsequent HRQOL and 178,849 observations (from 84,621 participants) remained for the analysis of the reverse association (Supplemental Table 1). The study flow is shown in Supplemental Fig. 1. The study protocol was approved by the institutional review board of Partners Health Care System. The completion and return of the self-administered questionnaires was considered to represent informed consent.
Assessment of alcohol consumption
Alcohol consumption was assessed by a semi-quantitative food frequency questionnaire (FFQ) in 1991 and every 4 years thereafter. The FFQ included separate items for regular beer, light beer, white wine, red wine, and liquor with nine frequency responses ranging from never or less than 1 per month up to 6 or more per day over the previous year. We calculated total alcohol intake by multiplying the average consumption of each beverage by the published alcohol content of the specified portion size based on periodically updated US Department of Agriculture food consumption tables and then summing across beverages [21]. We previously assessed the reproducibility and validity of self-reported alcohol intake with the FFQ against 1-week dietary records completed every 3 months for a year among 173 participants of the Nurses’ Health Study (living in the Boston area), which is a similar cohort of female nurses. The Spearman correlation coefficient between these two measures of alcohol intake was 0.90 [22].
Assessment of HRQOL
HRQOL was measured using the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) in 1993, 1997, and 2001. The SF-36 is a self-administered questionnaire that comprises eight scales of HRQOL: (i) physical functioning, (ii) role limitations due to physical health problems (role physical), (iii) bodily pain, (iv) general health perceptions, (v) vitality, (vi) social functioning, (vii) role limitations due to emotional problems (role emotional), and (viii) mental health. Each scale was scored separately from 0 to 100, with higher scores reflecting better HRQOL [23]. There were two primary outcomes of this study: the physical component summary (PCS) and the mental component summary (MCS) scores. These component summary scores reflect overall physical and mental HRQOL. By design, the PCS and MCS scales represent orthogonal (i.e. uncorrelated) constructs [24]. Summary scores were standardized by using the mean, standard deviation, and factor score coefficients for the SF-36 scales in the US general population, so that a mean PCS and MCS score of 50 (standard deviation of 10) reflects the mean in the US general population [25]. The instrument has been extensively validated, and has good construct validity and high test–retest ability and internal consistency [23, 26, 27].
Covariates
In follow-up biennial questionnaires, we obtained self-reported information on demographic and lifestyle factors [28], social integration [29], diseases, medication, and other characteristics that were included as covariates in multivariable analyses: age (years), race (white or non-white), region of residence (Northeast, Midwest, South, West, or outside the USA), body mass index (BMI) (<18.5, 18.5–22.9, 23–24.9, 25–29.9, 30–34.9, or ≥35 kg/m2), smoking status (never, past, or current smoking), physical activity (metabolic equivalent of task-hours/week), energy intake (kcal/day), marital status (married or not married), living arrangement (alone or with others), parity, employment status (employed or not employed), night shift work [30], arthritis (i.e. osteoarthritis and rheumatoid arthritis), diabetes mellitus, hypertension, hypercholesterolemia, asthma, premenstrual syndrome, regular use (during the past 2 years) of antidepressants (selective serotonin reuptake inhibitors, tricyclic antidepressants, or other), anxiolytics, analgesics (acetaminophen, aspirin, or non-steroidal anti-inflammatory drugs), or oral contraceptives, and menopausal status. Dietary information (in addition to alcohol) was obtained from repeated FFQs. To reflect overall dietary quality, a diet score (without alcohol) was calculated based on the 2010 Alternative Healthy Eating Index (AHEI), where a higher score denotes better overall dietary quality [31]. In addition, the frequencies of sugar-sweetened beverage and candy consumption were added as covariates, because moderate alcohol consumption has been related to lower intake of these food categories [32, 33].
Statistical analysis
Primary analyses
We conducted two sets of analyses to examine the bidirectional associations between alcohol consumption and HRQOL (PCS and MCS scores). In the first analysis, we examined the association between alcohol consumption and HRQOL 2 years later. For example, we used alcohol consumption in 1991 to predict HRQOL scores in 1993, and alcohol consumption in 1995 to predict HRQOL scores in 1997. The following alcohol consumption categories were used: former drinkers (drinking alcohol in the last 8 years, but abstaining during the last year), abstainers (abstaining from alcohol in the last 8 years), and infrequent (0.1–1.24 g/day), light (1.25–4.9 g/day), moderate (5.0–19.9 g/day), and moderate to heavy drinkers (≥20.0 g/day). We defined infrequent drinkers as the reference group. In the second analysis, we examined the association between HRQOL (quintiles of PCS and MCS scores) and alcohol consumption (continuous; g/day) 2 years later. In total, three cycles were analyzed for both directions of the association (Supplemental Fig. 1).
In both sets of primary analyses, the following covariates were included in the model: age, race, region of residence, BMI, smoking status, physical activity, energy intake, marital status, living arrangement, parity, employment status, and rotating night shift work. This was termed the lifestyle-adjusted model. A second model, the morbidity-adjusted model, was further adjusted for arthritis, diabetes mellitus, hypertension, hypercholesterolemia, asthma, premenstrual syndrome, use of antidepressants, anxiolytic agents, regular analgesics, and oral contraceptives, and menopausal status. Finally, a third model, referred to as the diet-adjusted model, was further adjusted for the AHEI score without consumption of alcohol, sugar-sweetened beverages, and candy.
To account for the effect of previous outcomes on current outcome variables, we further adjusted for baseline outcomes (HRQOL in the first analysis and alcohol consumption in the second analysis) in each model. For example, when we used alcohol consumption in 1995 to predict HRQOL scores in 1997, we additionally adjusted for HRQOL scores in 1993. After adjustment for baseline outcome variables, two cycles were available for the association between alcohol consumption and HRQOL 2 years later, and three cycles were available for the reverse direction.
Because each individual contributed repeated measures of HRQOL and alcohol consumption, we used generalized estimating equations (GEEs), using ‘PROC GENMOD’ in SAS software (SAS Institute, Cary, NC, USA), with an identity link (for normal distribution) to estimate β-coefficients of a generalized linear model. We specified subjects as the repeated factor with an exchangeable correlation matrix in the model to account for the correlation of within-person repeated measures [34]. Linear mixed models yielded very similar results.
Secondary analyses
We examined the potential non-linear relation between alcohol consumption and PCS and MCS non-parametrically with restricted cubic splines [35]. Tests for non-linearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms. Results using loess smoothers and fractional polynomials yielded similar findings. We also tested whether results were different if women drinking ≥35 g alcohol/day were excluded from the moderate to heavy alcohol consumption group (≥20 g alcohol/day). In addition, we examined the association between alcohol consumption and the eight HRQOL scales 2 years later with GEE models. The same covariates were used as in the primary analysis.
Because no single summary score for HRQOL exists in the SF-36, we examined the associations between alcohol consumption and ‘overall HRQOL’ using both PCS and MCS scores. In these, we defined ‘good HRQOL’ as having both high PCS and high MCS scores and ‘poor HRQOL’ as having both low PCS and low MCS scores (i.e. above the 60th percentile for good HRQOL and below the 40th percentile for poor HRQOL). These cut-off levels were chosen a priori to yield ~15% of women in the good and poor HRQOL groups. GEE models were used (Poisson distribution and a log link) to calculate the odds ratio (OR) for good and poor overall HRQOL versus ‘intermediate’ HRQOL (i.e. not being in either of the former categories.
In addition to examining total alcohol consumption as a primary outcome variable, we separately determined the likelihood of being a drinker and the amount of alcohol consumed among drinkers. We investigated the association between PCS and MCS scores and the prevalence ratio of any alcohol consumption using GEE models (Poisson distribution and a log link). Former drinkers were excluded from this analysis.
All analyses were performed using SAS software, version 9.2. A two-tailed P-value of 0.05 was considered statistically significant. All values shown are mean difference (β-coefficient) ± SE or OR and 95% confidence interval.
Results
Alcohol consumption and subsequent HRQOL
Baseline characteristics
The characteristics of participants in the Nurses’ Health Study II by baseline alcohol consumption in 1991 are summarized in Supplemental Table 2. Women drinking higher amounts of alcohol had a lower BMI and reported higher physical activity. They were less often unemployed or married, and less often had children. The prevalence of hypertension, hypercholesterolemia, osteoarthritis, diabetes, and asthma was lower among women drinking alcohol up to a moderate amount (5.0–19.9 g/day). However, regular use of analgesics was more common in higher alcohol consumers.
Alcohol consumption and subsequent PCS score
The associations between alcohol consumption and subsequent PCS and MCS scores are shown in Table 1. The diet-adjusted model showed that alcohol consumption was associated with higher PCS scores in a dose–response manner, with women consuming ≥20 g/day having the highest PCS scores compared to the infrequent drinkers reference group (β = 0.83 ± 0.11). After further adjustment for previous PCS scores, the association between alcohol consumption and PCS was attenuated. The dose–response relationship was only evident up to a moderate consumption of 5.0–19.9 g/day (β = 0.33 ± 0.07, for moderate vs. infrequent drinkers). For comparison, in the lifestyle-adjusted model (also adjusted for previous PCS score), a 1-unit increment in BMI was associated with a decrease of 0.20 in PCS score, and a 1-year increment in age was associated with a 0.10 decrease. Furthermore, smoking was associated with a decrease of 0.93 in PCS score (current vs. never smokers). The non-linear dose–response relationship between alcohol intake and PCS score was confirmed in a restricted cubic spline (Fig. 1; test for non-linear relationship: P < 0.001). Exclusion of women drinking at least 35 g alcohol/day in the moderate to heavy alcohol consumption category did not change the results (β = 0.43 ± 0.12 and β = 0.48 ± 0.13 for the ≥20 g/day category and 20–34.9 g/day category in the diet-adjusted model, respectively).
Table 1.
Association between alcohol consumption and physical (PCS) and mental component summary (MCS) scores 2 years later in the Nurses’ Health Study II
| Outcome | Alcohol consumption category
|
|||||
|---|---|---|---|---|---|---|
| Abstainer 0 g/day | Former drinker 0 g/day | Infrequent drinker 0.1–1.24 g/day | Light Drinker 1.25–4.9 g/day | Moderate drinker 5.0–19.9 g/day | Moderate/heavy drinker ≥20 g/day | |
| PCS | ||||||
| Unadjusted for previous PCS (nobs = 186,845; nwomen = 88,363) | ||||||
| Lifestyle-adjusted modela | -0.84 (0.07)* | -0.53 (0.06)* | 0.00 (referent) | 0.36 (0.06)* | 0.78 (0.06)* | 0.91 (0.12)* |
| Morbidity-adjusted modelb | -0.81 (0.06)* | -0.48 (0.06)* | 0.00 (referent) | 0.31 (0.06)* | 0.69 (0.06)* | 0.86 (0.11)* |
| Diet-adjusted modelc | -0.79 (0.06)* | -0.47 (0.06)* | 0.00 (referent) | 0.30 (0.06)* | 0.67 (0.06)* | 0.83 (0.11)* |
| Adjusted for previous PCS (nobs = 114,404; nwomen = 69,001) | ||||||
| Lifestyle-adjusted modela | -0.53 (0.07)* | -0.45 (0.08)* | 0.00 (referent) | 0.20 (0.07)† | 0.34 (0.07)* | 0.39 (0.12)† |
| Morbidity-adjusted modelb | -0.54 (0.07)* | -0.36 (0.08)* | 0.00 (referent) | 0.19 (0.07)† | 0.35 (0.07)* | 0.45 (0.12)* |
| Diet-adjusted modelc | -0.52 (0.07)* | -0.36 (0.08)* | 0.00 (referent) | 0.17 (0.07)‡ | 0.33 (0.07)* | 0.43 (0.12)* |
| MCS | ||||||
| Unadjusted for previous MCS (nobs = 186,845, nwomen = 88,363) | ||||||
| Lifestyle-adjusted modela | 0.28 (0.08)* | -0.17 (0.07)‡ | 0.00 (referent) | 0.12 (0.07) | 0.16 (0.08)‡ | -0.24 (0.15) |
| Morbidity-adjusted modelb | 0.31 (0.08)* | -0.12 (0.07) | 0.00 (referent) | 0.09 (0.07) | 0.12 (0.08) | -0.26 (0.15) |
| Diet-adjusted modelc | 0.34 (0.08)* | -0.10 (0.07) | 0.00 (referent) | 0.06 (0.07) | 0.06 (0.08) | -0.36 (0.15)‡ |
| Adjusted for previous MCS (nobs = 114,404; nwomen = 69,001) | ||||||
| Lifestyle-adjusted modela | 0.11 (0.08) | -0.21 (0.09)‡ | 0.00 (referent) | 0.06 (0.08) | 0.08 (0.08) | -0.25 (0.15) |
| Morbidity-adjusted modelb | 0.15 (0.08) | -0.14 (0.09) | 0.00 (referent) | 0.04 (0.08) | 0.06 (0.08) | -0.25 (0.15) |
| Diet-adjusted modelc | 0.17 (0.08)‡ | -0.12(0.09) | 0.00 (referent) | 0.01 (0.08) | 0.00 (0.08) | -0.34 (0.15)‡ |
Mean difference, i.e. β-coefficient (SE), in PCS and MCS score between the alcohol consumption category and the reference category.
P < 0.001;
P < 0.01;
P < 0.05.
Lifestyle-adjusted model: adjusted for time, age, physical activity, energy intake, body mass index, smoking, marital status, employment status, night shift work, race, region, living arrangement, and parity.
Morbidity-adjusted model: lifestyle-adjusted model and additionally adjusted for arthritis (osteoarthritis and rheumatoid arthritis), diabetes mellitus, hypertension, hypercholesterolemia, asthma, premenstrual syndrome, use of antidepressants, anxiolytics, analgesics, and oral contraceptives, and menopausal status.
Diet-adjusted model: morbidity-adjusted model and additionally adjusted for Alternative Healthy Eating Index-2010 without alcohol and sweetened beverage and candy consumption.
Fig. 1.
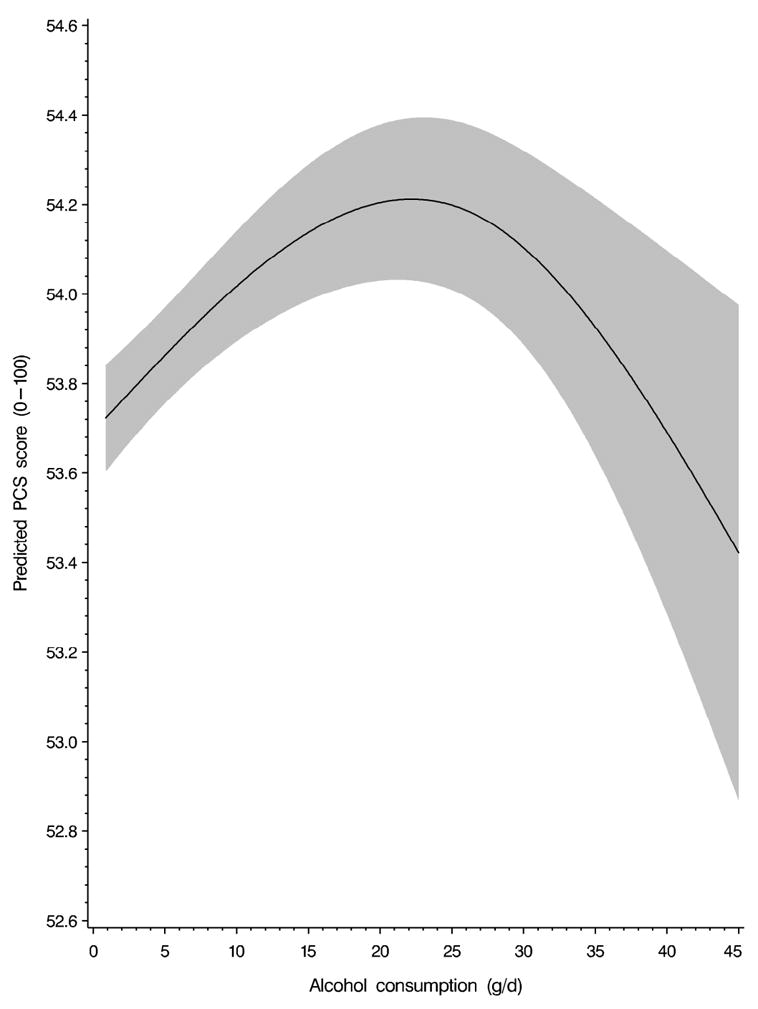
Non-linear dose–response relationship between alcohol consumption and subsequent physical component summary (PCS) scores. Data are derived from a restricted cubic spline generalized estimating equation model (lifestyle-adjusted model) in alcohol consumers. The model is adjusted for previous PCS score, age, physical activity, energy intake, body mass index, smoking, marital status, employment status, night shift work, race, region, living arrangement, and parity. Alcohol consumption is winsorized at the 99.5th percentile. The 95% confidence interval is indicated by the gray area. P < 0.001, test for non-linear relationship.
Alcohol consumption and subsequent MCS score
The diet-adjusted model showed that light to moderate alcohol consumption was not associated with higher MCS scores compared to infrequent drinking. However, compared to infrequent drinkers, MCS score was lower among women drinking ≥20 g/day (β = −0.36 ± 0.15) and higher among abstainers (β = 0.34 ± 0.08). After additional adjustment for previous MCS score, moderate to heavy alcohol consumption remained associated with a lower MCS score (β = −0.34 ± 0.15). This was further confirmed by results from a restricted cubic spline, which showed an inverse linear relation between alcohol consumption and MCS score (P = 0.032). However, after excluding women consuming at least 35 g alcohol/day in the moderate to heavy alcohol consumption category, no difference in MCS score was observed between the moderate to heavy drinkers and the reference group (β = −0.25 ± 0.17 in the diet-adjusted model).
Alcohol consumption and subsequent HRQOL scales
The associations between alcohol consumption and eight scales of the SF-36 are shown in Supplemental Fig. 2. Compared to infrequent drinkers, abstainers and former drinkers scored lower on all scales whereas moderate drinkers (5.0–19.9 g/day) scored higher on all scales, except for role-emotional and mental health. Moderate to heavy drinkers (≥20 g/day) scored higher on role-physical and vitality, but lower on role-emotional and mental health.
Alcohol consumption and subsequent overall HRQOL
Moderate drinkers had a lower likelihood of poor overall HRQOL (OR 0.90, 95% CI 0.84–0.97) and a higher likelihood of good overall HRQOL (OR 1.09, 95% CI 1.02–1.16), compared to infrequent drinkers (diet-adjusted model, additionally adjusted for previous PCS and MCS scores).
HRQOL and subsequent alcohol consumption
Baseline characteristics
The characteristics of the participants in the Nurses’ Health Study II by baseline PCS and MCS scores are summarized in Supplemental Tables 3 and 4. Women with lower PCS and MCS scores had higher BMI and energy intake and reported lower physical activity. In addition, they more often worked rotating night shifts and used antidepressants. Women with lower PCS scores were older, whereas women with lower MCS scores were younger.
PCS score and subsequent alcohol consumption
The associations between PCS and MCS scores and subsequent alcohol consumption are shown in Table 2. Subsequent alcohol consumption was higher in women with higher initial PCS scores. Compared with the lowest quintile of PCS score, women in the highest quintile consumed 0.58 ± 0.06 g alcohol per day more (diet-adjusted model). After further adjustment for previous alcohol consumption, the association was attenuated; the difference in alcohol consumption between the highest and the lowest quintile was 0.53 ± 0.05 g/day.
Table 2.
Association between physical (PCS) and mental component summary (MCS) scores and alcohol consumption 2 years later in the Nurses’ Health Study II
| Outcome | Quintiles of PCS score
|
||||
|---|---|---|---|---|---|
| Q1 (8–46) | Q2 (47–52) | Q3 (53–55) | Q4 (56–58) | Q5 (59–75) | |
| Alcohol intake (g/day) | |||||
| Unadjusted for previous alcohol intake (nobs = 178,849; nwomen = 84,621) | |||||
| Lifestyle-adjusted modela | 0.00 (referent) | 0.32 (0.05)* | 0.53 (0.05)* | 0.63 (0.05)* | 0.61 (0.06)* |
| Morbidity-adjusted modelb | 0.00 (referent) | 0.31 (0.05)* | 0.51 (0.05)* | 0.62 (0.05)* | 0.61 (0.06)* |
| Diet-adjusted modelc | 0.00 (referent) | 0.30 (0.05)* | 0.50 (0.05)* | 0.60 (0.05)* | 0.58 (0.06)* |
| Adjusted for previous alcohol intake (nobs = 165,693; nwomen = 77,479) | |||||
| Lifestyle-adjusted modela | 0.00 (referent) | 0.23 (0.04)* | 0.38 (0.04)* | 0.48 (0.04)* | 0.55 (0.05)* |
| Morbidity-adjusted modelb | 0.00 (referent) | 0.23 (0.04)* | 0.38 (0.04)* | 0.49 (0.05)* | 0.57 (0.05)* |
| Diet-adjusted modelc | 0.00 (referent) | 0.22 (0.04)* | 0.37 (0.04)* | 0.47 (0.05)* | 0.53 (0.05)* |
|
| |||||
|
Quintiles of MCS score
|
|||||
| Outcome | Q1 (0–42) | Q2 (43–49) | Q3 (50–53) | Q4 (54–56) | Q5 (57–71) |
|
| |||||
| Alcohol intake (g/day) | |||||
| Unadjusted for previous alcohol intake (nobs = 178,849; nwomen = 84,621) | |||||
| Lifestyle-adjusted modela | 0.00 (referent) | 0.00 (0.05) | -0.02 (0.05) | -0.05 (0.05) | -0.03 (0.06) |
| Morbidity-adjusted modelb | 0.00 (referent) | 0.01 (0.05) | -0.01 (0.05) | -0.03 (0.05) | 0.00 (0.06) |
| Diet-adjusted modelc | 0.00 (referent) | 0.00 (0.05) | -0.02 (0.05) | -0.05 (0.05) | -0.03 (0.06) |
| Adjusted for previous alcohol intake (nobs = 165,693; nwomen = 77,479) | |||||
| Lifestyle-adjusted modela | 0.00 (referent) | 0.02 (0.05) | 0.01 (0.04) | 0.04 (0.04) | 0.04 (0.05) |
| Morbidity-adjusted modelb | 0.00 (referent) | 0.03 (0.05) | 0.03 (0.05) | 0.07 (0.05) | 0.07 (0.05) |
| Diet-adjusted modelc | 0.00 (referent) | 0.02 (0.05) | 0.02 (0.05) | 0.04 (0.05) | 0.03 (0.05) |
Mean difference, i.e.β-coefficient (SE), in PCS and MCS between the alcohol consumption category and the reference category.
P < 0.001.
Lifestyle-adjusted model: adjusted for time, age, physical activity, energy intake, body mass index, smoking, marital status, employment status, night shift work, race, region, living arrangement, and parity.
Morbidity-adjusted model: lifestyle-adjusted model and additionally adjusted for arthritis (osteoarthritis and rheumatoid arthritis), diabetes mellitus, hypertension, hypercholesterolemia, asthma, premenstrual syndrome, use of antidepressants, anxiolytics, analgesics, and oral contraceptives, and menopausal status.
Diet-adjusted model: morbidity-adjusted model and additionally adjusted for Alternative Healthy Eating Index-2010 without alcohol and consumption of sweetened beverages and candy.
To further investigate this positive association between PCS score and subsequent alcohol consumption, we analyzed whether the association was also evident among alcohol drinkers only. In addition, we analyzed the association between PCS score and the likelihood of drinking alcohol.
Among alcohol drinkers, women in the highest quintile of PCS score consumed 0.40 ± 0.08 g alcohol per day more than women in the lowest quintile. The association between PCS scores and subsequent alcohol consumption was linear, with higher scores associated with higher consumption in a dose–response manner (Fig. 2).
Fig. 2.
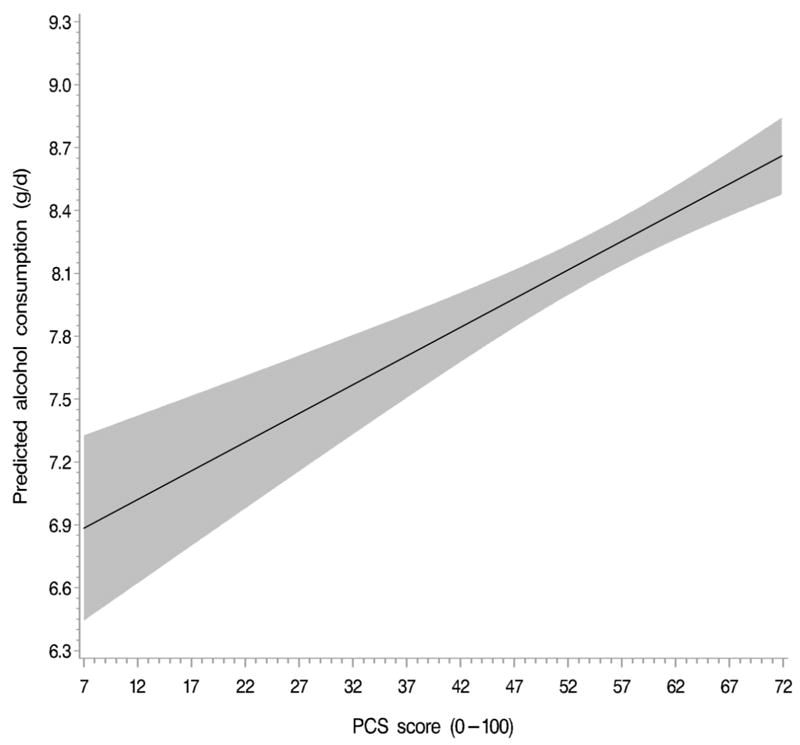
Linear dose–response relationship between physical component summary (PCS) scores and subsequent alcohol consumption. Data are derived from a restricted cubic spline generalized estimating equation model (lifestyle-adjusted model) in alcohol consumers. The model is adjusted for previous alcohol consumption, age, physical activity, energy intake, body mass index, smoking, marital status, employment status, night shift work, race, region, living arrangement, and parity. PCS scores are winsorized at the 99.5th percentile. The 95% confidence interval is indicated by the gray area. P < 0.001, test for linear relationship.
We next tested the association between PCS and the likelihood of any alcohol intake. In the diet-adjusted model we observed a linear association for PCS: women with a higher PCS score had a higher likelihood of drinking (OR 1.11, 95% CI 1.10–1.13, for highest vs. lowest quintile). Further adjustment for previous alcohol intake did not change the results.
MCS score and subsequent alcohol consumption
No association was found between MCS scores and subsequent alcohol consumption in both diet-adjusted models, with and without adjustment for previous alcohol consumption (highest vs. lowest quintile of MCS score: −0.03 ± 0.06 and 0.03 ± 0.05 g/day, respectively).
Discussion
In this large, longitudinal cohort study we have provided evidence that the association between alcohol consumption and HRQOL is bidirectional. Moderate alcohol consumption was associated with better subsequent PCS, but not with subsequent MCS. In the reverse direction, we observed an association between higher PCS score and higher subsequent alcohol consumption. MCS was not related to subsequent alcohol consumption.
Comparison with other studies
Our findings confirm those of previous studies that have demonstrated a positive association between moderate alcohol consumption and physical HRQOL. We observed a non-linear dose–response relationship between alcohol consumption and PCS score 2 years later, with the highest PCS scores in women drinking moderately. A similar association was observed between alcohol consumption and scales of PCS, i.e. physical functioning, role-physical, bodily pain, and general health. Previous studies also demonstrated higher scores for PCS and its scales in moderate drinkers as compared to abstainers or light drinkers [1, 2, 5, 15]. Although the association found in this study is consistent with the findings of previous studies, persisted after multivariate adjustment, and remained consistent across PCS scales, the strength of the association was modest. The 0.33-unit higher PCS score observed in moderate alcohol drinkers was comparable to the change in score for women with a 1.7-unit lower BMI or for women 3.5 years younger in our sample.
We observed no association between moderate alcohol consumption and MCS score. This has been investigated in two other (cross-sectional) studies. Valencia-Martín et al. [5] did not find an association in male and female adults, whereas Chan et al. [3] reported higher MCS scores in moderate drinkers among men only. However, several studies have shown a positive association between moderate alcohol consumption and scales of MCS, such as mental health [1, 15], vitality [1, 2], and social functioning [1, 15]. This parallels the higher vitality and social functioning scores in moderate drinkers observed in the present study.
Overall, the likelihood of having a good HRQOL in both physical and mental domains was highest among moderate drinkers. A similar positive association between moderate alcohol consumption and quality of life [3, 14], subjective wellbeing [36], subjective health [7], and life satisfaction [3] has been reported. As there is no measure available for SF-36 in which PCS and MCS sores are integrated, we created this overall score to explore the association between alcohol and overall HRQOL. However, this overall score has limitations, because PCS and MCS are designed to be uncorrelated.
To our knowledge, this is the first study showing a positive association between PCS per se and subsequent alcohol consumption. However, previous studies have reported an association between subjective health status and alcohol use. Specifically, suboptimal health has been related to quitting or reducing alcohol consumption [17, 37]. Additionally, abstainers have more characteristics related to a poor health status compared to light or moderate drinkers [38]. However, after excluding abstainers and former drinkers from the analysis, the positive relationship between PCS and subsequent alcohol consumption persisted.
MCS was neither associated with subsequent alcohol consumption nor with the likelihood of drinking alcohol. This finding contrasts with the results of Bell and Britton, showing an inverse association between MCS score and change in alcohol consumption in 6330 mainly male British civil servants [16]. Participants with good mental health appeared to reduce their alcohol consumption, whereas individuals with poor mental health either increased or maintained a high level of consumption. We cannot directly compare our results to theirs, as in the present study most women (~70%) did not change their alcohol consumption between measurements performed 4 years apart.
Limitations
Some limitations of our study warrant consideration. First, alcohol intake was self-reported by a semi-quantitative FFQ, which generally causes underreporting of alcohol intake, especially at higher levels of alcohol [39]. However, a validation study in nurses showed a high correlation between alcohol consumption from the FFQ and four 1-week dietary recalls and HDL cholesterol, a strong biomarker of alcohol intake [22].
Secondly, the time lag of 2 years between alcohol consumption and HRQOL measurements may have introduced misclassification bias, as changes in alcohol intake or HRQOL could have occurred during that period. However, alcohol intake and HRQOL both tended to be stable over 4 years, as most women did not change their alcohol intake and average changes in PCS and MCS scores were small. Therefore, we do not expect this to have a large influence on our results.
Thirdly, the distribution of the PCS and MCS scales was not as widespread as the distribution in the US general population; in particular, the standard deviations were somewhat smaller (~9 vs. 10, respectively). However, average PCS and MCS scores were comparable to those of the US population for women of 35–44 years (51.9 and 49.1 in our sample vs. 51.7 and 47.8 in the US population, respectively), indicating that the women in the cohort had HRQOL that was comparable to that of other American women of their age [40]. We excluded women who were pregnant in a given cycle, but the fact that pregnant women could re-enter the cohort in the next cycle was an important advantage of our longitudinal study design.
Fourthly, although we controlled for a large number of health behaviors and sociodemographic factors, residual confounding remains possible. In particular, limited information was available on socioeconomic status, sleep quality, and disorders such as depression and anxiety. However, related covariates such as antidepressant and anxiolytic medication use and sociodemographic factors (race, region, marital status, employment status, and night shift work) were examined in the model. In addition, our study population primarily consisted of white educated US women with high and fairly homogeneous socioeconomic status. Therefore, we expect that the influence of residual confounding on our results will be limited. However, this may limit generalizability to other ethnic and socioeconomic groups (as well as men).
Finally, a limited number of participants were heavy alcohol consumers (>40 g/day). Therefore, we could not examine the association between heavy drinking and HRQOL, and the results are not generalizable to heavy alcohol consumers.
Conclusion
In summary, the results of this study support the presence of a bidirectional relationship between alcohol consumption and HRQOL. Among young and middle-aged women, greater alcohol intake (up to ~1 serving daily) was associated with a small improvement in physical HRQOL 2 years later, and vice versa. No significant relationship was observed between moderate alcohol consumption and mental HRQOL in either direction.
These results indicate the importance of exploring bidirectional associations in studies concerning alcohol consumption. Additionally, the current US guidelines on alcohol consumption recommend consuming alcohol in moderation (up to 1 serving per day for women) if alcohol is consumed at all [41]. Our results are in agreement with these guidelines: moderate alcohol consumption may be beneficial for physical HRQOL, whereas drinking more than 1 drink per day may be harmful for mental HRQOL.
Supplementary Material
Acknowledgments
Funding sources This work was supported by the Nurses’ Health Study II grant UM1 CA176726 from the National Institutes of Health. ICS and HFJH were supported both by the Dutch Ministry of Economic Affairs, Agriculture and Innovation and by the Dutch Foundation for Alcohol Research (SAR) representing Dutch producers of and traders in beer, wine, and spirits and The Netherlands Organization for Applied Scientific Research (TNO) (Grant EZ1503). Their joint aim is to independently investigate the health effects of moderate alcohol consumption. The funding sources had no role in conducting, analyzing, or interpreting the study results or in the decision to submit the manuscript for publication.
Abbreviations
- PCS
physical component summary
- MCS
mental component summary
- HRQOL
health-related quality of life
- SF-36
Medical Outcomes Study 36-Item Short-Form Health Survey
- FFQ
food frequency questionnaire
- AHEI
Alternative Healthy Eating Index
- BMI
body mass index
- GEE
generalized estimating equation
Footnotes
Conflict of interest statement No potential conflicts of interest relevant to this article were reported.
References
- 1.Van Dijk AP, Toet J, Verdurmen J. The relationship between health-related quality of life and two measures of alcohol consumption. J Stud Alcohol Drugs. 2004;65:241. doi: 10.15288/jsa.2004.65.241. [DOI] [PubMed] [Google Scholar]
- 2.Saito I, Okamura T, Fukuhara S, et al. A cross-sectional study of alcohol drinking and health-related quality of life among male workers in Japan. J Occup Health. 2005;47:496–503. doi: 10.1539/joh.47.496. [DOI] [PubMed] [Google Scholar]
- 3.Chan AM, von Mühlen D, Kritz-Silverstein D, Barrett-Connor E. Regular alcohol consumption is associated with increasing quality of life and mood in older men and women: the Rancho Bernardo Study. Maturitas. 2009;62:294–300. doi: 10.1016/j.maturitas.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saarni SI, Joutsenniemi K, Koskinen S, et al. Alcohol consumption, abstaining, health utility, and quality of life--a general population survey in Finland. Alcohol Alcohol. 2008;43:376–86. doi: 10.1093/alcalc/agn003. [DOI] [PubMed] [Google Scholar]
- 5.Valencia-Martín JL, Galan I, Guallar-Castillón P, Rodriguez-Artalejo F. Alcohol drinking patterns and health-related quality of life reported in the Spanish adult population. Prev Med. 2013;57:703–7. doi: 10.1016/j.ypmed.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Stranges S, Notaro J, Freudenheim JL, et al. Alcohol drinking pattern and subjective health in a population-based study. Addiction. 2006;101:1265–76. doi: 10.1111/j.1360-0443.2006.01517.x. [DOI] [PubMed] [Google Scholar]
- 7.Poikolainen K, Vartiainen E, Korhonen HJ. Alcohol intake and subjective health. Am J Epidemiol. 1996;144:346–50. doi: 10.1093/oxfordjournals.aje.a008935. [DOI] [PubMed] [Google Scholar]
- 8.Okoro CA, Brewer RD, Naimi TS, Moriarty DG, Giles WH, Mokdad AH. Binge drinking and health-related quality of life: do popular perceptions match reality? Am J Prev Med. 2004;26:230–3. doi: 10.1016/j.amepre.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 9.Baliunas DO, Taylor BJ, Irving H, et al. Alcohol as a Risk Factor for Type 2 Diabetes: A systematic review and meta-analysis. Diabetes Care. 2009;32:2123–32. doi: 10.2337/dc09-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gea A, Martinez-Gonzalez MA, Toledo E, et al. A longitudinal assessment of alcohol intake and incident depression: the SUN project. BMC Public Health. 2012;12:954–2458. 12–954. doi: 10.1186/1471-2458-12-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin Z, Xiang C, Cai Q, Wei X, He J. Alcohol consumption as a preventive factor for developing rheumatoid arthritis: a dose-response meta-analysis of prospective studies. Ann Rheum Dis. 2014;73:1962–7. doi: 10.1136/annrheumdis-2013-203323. [DOI] [PubMed] [Google Scholar]
- 12.Peele S, Brodsky A. Exploring psychological benefits associated with moderate alcohol use: a necessary corrective to assessments of drinking outcomes? Drug Alcohol Depend. 2000;60:221–47. doi: 10.1016/s0376-8716(00)00112-5. [DOI] [PubMed] [Google Scholar]
- 13.Graham K. Alcohol abstention among older adults: reasons for abstaining and characteristics of abstainers. Addiction Research & Theory. 1998;6:473–87. [Google Scholar]
- 14.Kaplan MS, Huguet N, Feeny D, et al. Alcohol use patterns and trajectories of health-related quality of life in middle-aged and older adults: a 14-year population-based study. J Stud Alcohol Drugs. 2012;73:581–90. doi: 10.15288/jsad.2012.73.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byles J, Young A, Furuya H, Parkinson L. A Drink to Healthy Aging: The Association Between Older Women’s Use of Alcohol and Their Health-Related Quality of Life. J Am Geriatr Soc. 2006;54:1341–7. doi: 10.1111/j.1532-5415.2006.00837.x. [DOI] [PubMed] [Google Scholar]
- 16.Bell S, Britton A. An exploration of the dynamic longitudinal relationship between mental health and alcohol consumption: a prospective cohort study. BMC medicine. 2014;12:91. doi: 10.1186/1741-7015-12-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang W, Chikritzhs T. Reduction in alcohol consumption and health status. Addiction. 2011;106:75–81. doi: 10.1111/j.1360-0443.2010.03164.x. [DOI] [PubMed] [Google Scholar]
- 18.Nortvedt MW, Riise T, Myhr KM, Nyland HI. Quality of life in multiple sclerosis: measuring the disease effects more broadly. Neurology. 1999;53:1098–103. doi: 10.1212/wnl.53.5.1098. [DOI] [PubMed] [Google Scholar]
- 19.Trentham-Dietz A, Sprague BL, Klein R, et al. Health-related quality of life before and after a breast cancer diagnosis. Breast Cancer Res Treat. 2008;109:379–87. doi: 10.1007/s10549-007-9653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otchet F, Carey MS, Adam L. General health and psychological symptom status in pregnancy and the puerperium: what is normal? Obstet Gynecol. 1999;94:935–41. doi: 10.1016/s0029-7844(99)00439-1. [DOI] [PubMed] [Google Scholar]
- 21.U.S. Department of Agriculture ARS. USDA Nutrient Database for Standard Reference. Washington, DC: U.S Government Printing Office; 1999. [Google Scholar]
- 22.Giovannucci E, Colditz G, Stampfer MJ, et al. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991;133:810–7. doi: 10.1093/oxfordjournals.aje.a115960. [DOI] [PubMed] [Google Scholar]
- 23.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992:473–83. [PubMed] [Google Scholar]
- 24.Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995:AS264–79. [PubMed] [Google Scholar]
- 25.Ware J, Kosinski M, Keller S. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston, MA: The Health Institute; 1994. [Google Scholar]
- 26.Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–4. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McHorney CA, Ware JE, Rogers W, Raczek AE, Lu JFR. The validity and relative precision of MOS short-and long-form health status scales and Dartmouth COOP charts: results from the Medical Outcomes Study. Med Care. 1992:MS253–65. doi: 10.1097/00005650-199205001-00025. [DOI] [PubMed] [Google Scholar]
- 28.Duncan MJ, Kline CE, Vandelanotte C, Sargent C, Rogers NL, Di Milia L. Cross-Sectional Associations between Multiple Lifestyle Behaviors and Health-Related Quality of Life in the 10,000 Steps Cohort. PloS one. 2014;9:e94184. doi: 10.1371/journal.pone.0094184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Achat H, Kawachi I, Levine S, Berkey C, Coakley E, Colditz G. Social networks, stress and health-related quality of life. Quality of life research. 1998;7:735–50. doi: 10.1023/a:1008837002431. [DOI] [PubMed] [Google Scholar]
- 30.Kaliterna LL, Prizmic LZ, Zganec N. Quality of life, life satisfaction and happiness in shift-and non-shiftworkers. Revista de Saúde Pública. 2004;38:3–10. doi: 10.1590/s0034-89102004000700002. [DOI] [PubMed] [Google Scholar]
- 31.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–18. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colditz GA, Giovannucci E, Rimm EB, et al. Alcohol intake in relation to diet and obesity in women and men. Am J Clin Nutr. 1991;54:49–55. doi: 10.1093/ajcn/54.1.49. [DOI] [PubMed] [Google Scholar]
- 33.Joosten MM, Grobbee DE, van der ADL, Verschuren WM, Hendriks HF, Beulens JW. Combined effect of alcohol consumption and lifestyle behaviors on risk of type 2 diabetes. Am J Clin Nutr. 2010;91:1777–83. doi: 10.3945/ajcn.2010.29170. [DOI] [PubMed] [Google Scholar]
- 34.Hanley JA, Negassa A, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol. 2003;157:364–75. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 35.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 36.Lang I, Wallace RB, Huppert FA, Melzer D. Moderate alcohol consumption in older adults is associated with better cognition and well-being than abstinence. Age Ageing. 2007;36:256–61. doi: 10.1093/ageing/afm001. [DOI] [PubMed] [Google Scholar]
- 37.Powers JR, Young AF. Longitudinal analysis of alcohol consumption and health of middle - aged women in Australia. Addiction. 2008;103:424–32. doi: 10.1111/j.1360-0443.2007.02101.x. [DOI] [PubMed] [Google Scholar]
- 38.Hansel B, Thomas F, Pannier B, et al. Relationship between alcohol intake, health and social status and cardiovascular risk factors in the urban Paris-Ile-De-France Cohort: is the cardioprotective action of alcohol a myth? Eur J Clin Nutr. 2010;64:561–8. doi: 10.1038/ejcn.2010.61. [DOI] [PubMed] [Google Scholar]
- 39.Feunekes GI, van ’t Veer P, van Staveren WA, Kok FJ. Alcohol intake assessment: the sober facts. Am J Epidemiol. 1999;150:105–12. doi: 10.1093/oxfordjournals.aje.a009909. [DOI] [PubMed] [Google Scholar]
- 40.Ware J, Kosinski M, Bjorner J, Turner-Bowker D, Gandek B, Maruish M. User’s Manual for the SF-36v2® Health Survey. QualityMetric Incorporated; Lincoln: 2007. [Google Scholar]
- 41.U.S. Department of Agriculture, U.S. Department of Health and Human Services. Dietary guidelines for Americans. Washington D.C.: 2010. available from: http://www.health.gov/dietaryguidelines/2010.asp. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


