Abstract
Adolescence is a time of both increased risk taking and increased vulnerability to the neurotoxic effects of alcohol. However, it is unclear whether brain functioning abnormalities in adolescent binge drinkers are a result of alcohol use itself or whether they represent premorbid risk characteristics. The current study addresses this question by using a modified version of the Wheel of Fortune (WOF) task, during functional magnetic resonance imaging (fMRI), at both baseline, while all subjects were alcohol-naïve, and revisit, when half of the subjects had emerged into regular binge drinking (n = 13) and half remained alcohol and substance-naïve (n = 13). Region of interest (ROI) analysis revealed that during decision making, there was a significant binge-drinking related reduction in brain activation in the dorsal striatum, an effect associated with degree of recent use. Furthermore, whole brain analysis revealed a decrease in fronto-parietal brain activation prior to initiation of alcohol use, in adolescents who went on to binge drink. Additionally, there were numerous regions, both cortical and subcortical, in which there was a significant time-related developmental change, across groups. These results demonstrate how abnormalities in decision-making related circuitry might both lead to and perpetuate alcohol drinking behavior. These findings help aid in our ability to disentangle consequences of binge drinking from potential risk markers for future binge drinking, and may help guide future prevention and intervention strategies.
Keywords: Adolescence, Decision making, Alcohol, Binge, Dorsal striatum
1. Introduction
Adolescence is a time of significant neurodevelopment (for review, see Blakemore, 2012) and is also a time of increased risk taking, including experimentation with drugs and alcohol (Eaton et al., 2012). This tendency towards novel exploration and risk taking is believed to stem from continued development of both reward processing and executive control regions during this time (for review, see Geier, 2013). For example, in a cross-sectional analysis, Van Leijenhorst et al. (2010) found from late childhood to early adulthood, during risky decision making, there was a linear decrease in activation in dorsal anterior cingulate cortex (dACC) and an inverted-U shaped trajectory of activation in the ventral medial prefrontal cortex (vmPFC), with a peak during late adolescence. This finding coincides with findings from a recent longitudinal structural neuroimaging study that revealed a mismatch between the development of reward and cognitive control regions, with the nucleus accumbens (NAc) showing relatively earlier maturation than the PFC (Mills et al., 2014). Furthermore, pre-clinical models indicate that during adolescence there is a peak in dopamine receptor levels and binding in the striatum (Seeman et al., 1987), accompanied by an increase in the density of dopaminergic projections to the PFC (Kalsbeek et al., 1988; Rosenberg and Lewis, 1994). Taken together, these findings highlight the dynamic changes taking place in prefrontal and striatal regions of the brain, areas thought to be important for decision making (for review, see Balleine et al., 2007).
Adolescence is also a time of increased vulnerability to the neurotoxic effects of alcohol. Pre-clinical models have found that adolescent rats are more susceptible than adult rats to neuronal cell death as a result of an alcohol binge (Crews et al., 2000). More specifically, neural cells in the PFC are particularly sensitive to binge-like exposure to alcohol during adolescence (Koss et al., 2012). The striatum also responds differently to alcohol in adolescence compared to adulthood. For example, during acute alcohol exposure, increased dopamine release in the striatum is more prominent in adolescents (Pascual et al., 2009; Philpot et al., 2009) and appears to be associated with greater rewarding effects of alcohol (Pautassi et al., 2008; Ristuccia and Spear, 2008). Additionally, alcohol exposure differentially affects cognitive performance, with adolescent, but not adult rodents, showing decreases in learning and memory due to alcohol exposure (Land and Spear, 2004; Markwiese et al., 1998; White et al., 2000). These results suggest that exposure to alcohol, even in seemingly modest doses, may have a greater impact on the developing adolescent brain than on a mature adult brain, and that both prefrontal and striatal regions may be particularly susceptible to these effects. With up to 68% of adolescents having reported drinking alcohol by the 12th grade, and over 22% reporting binge drinking (Johnston et al., 2014), gaining a better understanding of alcohol's effects on these developing regions is extremely relevant, as it may help provide us with better targets for future prevention and intervention strategies.
Numerous cross-sectional studies have been conducted comparing binge-drinking adolescents to their alcohol-naïve peers to assess the effects of alcohol at a structural and functional neurobiological level. Magnetic resonance imaging (MRI) studies have revealed that binge drinking during adolescence is associated with significantly thicker (females) and thinner (males) frontal lobe cortices (Squeglia et al., 2012), reductions in cerebellar volume (Lisdahl et al., 2013), and widespread reductions in white matter integrity (McQueeny et al., 2009). Furthermore, functional MRI studies (fMRI) have revealed that binge-drinking adolescents have decreased brain response in the right superior and inferior frontal gyri during working memory (Squeglia et al., 2011), and numerous regions of differential activation during verbal encoding, including increased posterior parietal cortex activation (Schweinsburg et al., 2010; Schweinsburg et al., 2011). However, to our knowledge, few studies have attempted to look at binge-drinking related effects on adolescent brain response during decision making, despite the likelihood that decision making-related neurobiological alterations, in particular, may be highly relevant for choosing to misuse alcohol. Johnson et al. (2008) found that during affective decision making, binge-drinking adolescents performed significantly worse than their alcohol-naïve counterparts on the decision making portion of the Iowa Gambling Task (IGT), with this result linked to dysfunction in the vmPFC; however, this result was more closely related to a hypersensitivity to reward outcome, as opposed to risky choice selection. Furthermore, Xiao et al. (2013) found that binge-drinking adolescents showed higher activation than non-drinkers in bilateral insula during the IGT; however, this finding was also not specific to the selection phase of risk taking and included other aspects of decision making, such as anticipation and reward processing. Failure to separate decision making from response to outcome makes the interpretation of neuroimaging findings difficult, as there are likely many processes that underlie these complex tasks.
The current study used fMRI and a modified version of the Wheel of Fortune (WOF) Task (Ernst et al., 2004), a reward-based decision-making task, to assess risk-taking behavior and the blood oxygen level-dependent (BOLD) response in binge-drinking adolescents and matched controls. Unlike previous studies (Johnson et al., 2008; Xiao et al., 2013), this task separated the decision making, anticipation, and reward outcome phases of risk-taking behavior, so as to more accurately examine BOLD response during decision making. In fact, brain regions previously shown to have altered BOLD response during decision making among adolescent alcohol users, such as the vmPFC and insula (Johnson et al., 2008; Xiao et al., 2013), have also been shown to be more heavily recruited during reward anticipation using the WOF task (Ernst et al., 2004). Meanwhile, dorsal control regions, including the dorsolateral PFC and dorsal striatum, appear to be more heavily recruited during the selection phase of this task (Ernst et al., 2004), and during decision making in general (Balleine et al., 2007). Based on this, and the increased susceptibility of the PFC and striatum to the neurotoxic effects of alcohol during adolescence (Crews et al., 2000; Koss et al., 2012; Pascual et al., 2009; Philpot et al., 2009), we hypothesized that following emergence into binge drinking, the dorsolateral PFC and dorsal striatum would show less activation in binge-drinking adolescents than controls, above and beyond any premorbid differences seen at baseline in these regions. Additionally, utilization of a longitudinal design allowed us to also explore pre-existing differences, an effect that has yet to be reported on, as well as developmental effects which have previously only been looked at cross-sectionally (e.g. Van Leijenhorst et al., 2010).
2. Methods
2.1 Participants
2.1.1 Recruitment and exclusionary criterion
Healthy adolescent participants (13 to 16 years old) were recruited from the local community as part of an ongoing longitudinal study on adolescent neurodevelopment. After a telephone pre-screen to determine initial eligibility, written consent and assent were obtained from parents and youth, respectively, followed by separate comprehensive screening interviews of participants and their parents. Exclusionary criteria during this screening interview included left-handedness [Edinburgh Handedness Inventory (Oldfield, 1971)], diagnosis of a DSM-IV psychiatric disorder [Diagnostic Interview Schedule for Children Predictive Scales (Lucas et al., 2001)], inability to obtain family history information, serious medical problems (including head trauma), mental retardation or learning disability, psychotic illness in a biological parent, known prenatal drug or alcohol exposure, MRI contradictions, and pregnancy. Furthermore, at baseline, adolescents were excluded for prior drug or alcohol use that exceeded >10 lifetime alcohol drinks, >2 drinks on any one occasion, >5 uses of marijuana, >4 cigarettes per day, or any other drug use [Brief Lifetime version of the Customary Drinking and Drug Use Record (CDDR) (Brown et al., 1998)]. The study was approved by the Oregon Health & Science University (OHSU) Institutional Review Board.
2.1.2 Participant characteristics
To assess socioeconomic status (SES), parents were administered the Hollingshead Index of Social Position, a measure based on the educational attainment and occupation of each parent (Hollingshead and Redlich, 1958). To provide an estimate of overall intellectual functioning, youth were administered the 2-subtest version of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). To measure pubertal development, self-assessment of puberty was obtained using a modified line drawing version of the Tanner's Sexual Maturation Scale (Taylor et al., 2001), with drawings ranging from stage 1 (pre-adolescent) through stage 5 (adult-like maturation). To evaluate family history of alcohol use disorders (AUD), a known risk factor for alcoholism shown to be associated with unique neurobiological features (Cservenka et al., 2014a; Cservenka et al., 2014b; Cservenka and Nagel, 2012), a family history density (FHD) score was calculated for each participant using the Family History Assessment Module (Rice et al., 1995). FHD was based on how many and how closely related an adolescent was to the relative(s) with an AUD; parents contributed 0.5, grandparents 0.25, and aunts and uncles a weighted ratio of 0.25 divided by the number of their siblings, with higher scores indicating greater prevalence of familial AUDs.
2.1.3 Follow-ups and binge-drinking criterion
Following initial recruitment and collection of baseline neuropsychological and neuroimaging measures, follow-up phone interviews were conducted with youth approximately every 90 days, during which the CDDR and 90-day Timeline Followback (Sobell et al., 1996) were administered to assess substance use. Once a participant reported binge drinking (≥5 drinks for males or ≥4 drinks for females, in one occasion), as well as had ≥3 total occasions of ≥4 drinks within the last 90 days, they were brought in for re-assessment with neuropsychological and neuroimaging measures analogous to those conducted at baseline. For every binge-drinking adolescent that was re-assessed after initiation of alcohol use, a time-since-baseline and developmentally (based on sex, age and pubertal stage) matched non-using control was also brought in for re-assessment. This procedure resulted in a total of 13 binge-drinking youth and 13 non-using controls. Youth were instructed to refrain from any drug and alcohol use for 72 hours prior to their revisit, and a negative breathalyzer prior to their imaging session was used to confirm absence of acute alcohol intoxication.
2.2 Wheel of Fortune Task
During their imaging sessions, adolescents performed a modified version of the WOF task (Cservenka et al., 2012; Cservenka and Nagel, 2012) adapted from the original WOF paradigm (Ernst et al., 2004), to assess risk-taking behavior and associated BOLD response. Each trial of this two-choice decision-making task consisted of a selection (3 seconds), anticipation (3.5 seconds), and feedback (4 seconds) phase, with intertrial fixation intervals jittered between 1 and 11 seconds. During the selection phase, participants chose between varying probabilities of winning various monetary amounts which were depicted as portions of a wheel. Selecting a low-probability option (10% or 30% of a wheel) with a high monetary reward value ($7.00 or $2.00, respectively) was considered a risky choice, while selecting a high-probability option (70% or 90%) with a low monetary reward value ($1.00 for both) was considered a safe choice. Selecting either 50% portion of a wheel ($2.00) was considered a chance choice. Participants were instructed to select their choices in an effort to win as much money as possible, because they would receive a portion of their earnings after the task. A participant's choice that matched the computer's choice, based on the predefined probabilities, was considered a winning trial (win), whereas trials in which the participant's choice did not match that of the computer were non-winning trials (no win). Furthermore, trials in which the participant chose the safe option, but did not win (safe no win), were categorized separately from no win trials, as these safe no win trials may represent the highest expectancy violations during the task. While in the scanner, adolescents performed 72 trials, presented during two 10-minute runs of 36 trials each. Each run included twelve 90/10 wheels, fourteen 70/30 wheels, and ten 50/50 wheels. A greater number of 90/10 and 70/30 wheels were included to increase power to model risky and safe selections. Following choice selection was the anticipation phase, in which participants responded on a scale of 1-3 as to how sure they were of winning. During the final phase, the feedback phase, the screen indicated whether the participant won or did not win the amount of money for the choice they selected, alongside their cumulative total dollar amount won up through that trial. To ensure subjects were paying attention during the feedback phase, they were asked to respond if they had won or did not win each trial. All participants received a practice session prior to entering the scanner to familiarize them with the task. In the scanner, all responses were recorded on a 4-button optical button box. Following scanning, participants completed a questionnaire that assessed effort and response strategies used during the task. Since the aim of this study was to understand BOLD response during risk taking, only the selection phase of each trial was examined for the imaging analysis. Further, BOLD response during reward processing, using the feedback phase of this task, has been previously examined by our laboratory in a subset of individuals involved in the current study (Cservenka et al., 2015).
2.3 Imaging
2.3.1 Acquisition
Participants were scanned at OHSU's Advanced Imaging Research Center on a 3.0-Tesla Siemens Magnetom Tim Trio (Siemens Medical Solutions, Erlangen, Germany). High-resolution, anatomical T1-weighted MPRAGE structural scans were collected in the sagittal plane (time to repetition [TR] = 2300 ms, time to echo [TE] = 3.58 ms, inversion time [TI] = 900 ms, flip angle = 10°, field of view [FOV] = 240 × 256 mm, voxel size = 1 × 1 × 1.1 mm, 160 slices, acquisition time = 9:14). Functional T2* weighted gradient echo-planer images were collected axially, parallel to the anterior commissure-posterior commissure line (TR = 2000 ms, TE = 30 ms, flip angle = 90°, FOV = 240 mm2, voxel size = 3.75 × 3.75 × 3.8 mm, 33 slices, acquisition time = 2 runs of 300 TRs, lasting 10:00 minutes each).
2.3.2 Pre-processing
Analysis of Functional NeuroImages (AFNI) was used for preprocessing. Following image reconstruction, anatomical masks were skull-stripped to remove non-brain skull and tissue. Functional data preprocessing included slice-timing correction, visual inspection for and removal of artifacts, and realignment of individual TRs to the volume requiring the least amount of adjustment based on a least squares algorithm (Cox and Jesmanowicz, 1999). During this process, TRs with movement values greater than 2.5 mm or 2.5° in any of the three displacement or rotational parameters were censored prior to further analysis. For this study, only one subject had any TRs censored, all three of which occurred outside of the decision-making phase of the task. Furthermore, functional data were spatially smoothed with a 6.0 mm full-width half-maximum Gaussian kernel, co-registered to anatomical images (Cox, 1996), and normalized to a whole brain signal to obtain relative percent signal changes. Additionally, all participants’ runs were checked to ensure only runs with less than 1.5 mm root mean squared (RMS) of within-run motion, across six motion parameters, were included in analysis. No group differences were found in mean RMS at baseline [t(24) = 0.38, p > 0.05] or re-assessment [t(24) = 0.13, p > 0.05].
Using AFNI's BLOCK option, regressors representing selection (risky, safe, or chance), anticipation (following risky, safe, or chance choices), and feedback (wins, no wins, or safe no wins) phases of the task were modeled; however, brain response during anticipation and feedback phases were treated as regressors of no interest for this analysis. Stimulus times corresponded to the onset of each phase, with duration of the event coded as the length of each phase convolved with a gamma-variate hemodynamic response function, while modeling delays in the hemodynamic response (Cohen, 1997). The estimated baseline, AFNI's intrinsic baseline, included the mean BOLD signal from the entire timecourse of the task, linear drift, unmodeled fixation periods between trials, and regressors of no interest (e.g. motion parameters) (Cox, 1996). Contrast images for average percent signal change of risky selections versus baseline, safe selections versus baseline, and risky selections versus safe selections were analyzed. A minimum of 8 TRs, of both risky and safe selections, were required to create these contrasts. Finally, functional data were transformed into standard Talairach coordinates (Talairach and Tournoux, 1988) and resampled into 3 mm3 voxels prior to group-level analysis.
2.4 Group-level analysis
2.4.1 Demographics and behavioral data
Statistical analyses for demographic and behavioral data were performed in SPSS (SPSS for Windows, release 20.0, 2011; SPSS Inc., Chicago IL). Demographic data were examined for outliers (>2.5 SD from the mean), as well as normal distribution using Shapiro-Wilk tests. These data were then analyzed using independent-samples t-tests or Mann-Whitney tests when data were ordinal or non-normally distributed. Reaction time on risky and safe selections was analyzed using a 2 (group: binge and control) by 2 (selection type: risky and safe) by 2 (time: baseline and revisit) mixed factorial analysis of variance (ANOVA). Furthermore, the number of risky choice selections (by percentage) for each group was analyzed using a 2 (group) by 2 (time) mixed factorial ANOVA. All significant interactions were followed up post-hoc using Fisher's LSD correction for multiple comparisons.
2.4.2 Imaging data
To best represent task-related activity for both binge-drinking and control groups at both baseline and revisit, four separate one-sample t-test maps for the risky vs. safe selection contrast were created and voxel thresholded at p < 0.05. These unclustered, voxel thresholded maps were then combined to form a single task-related activity map for the entire sample at both time points (Figure 1), which was applied to further analyses to examine group differences. Visual inspection of this voxel thresholded task-related activity map found patterns of positive (risky selection > safe selection) activation throughout the brain similar to previous studies (Cservenka and Nagel, 2012; Ernst et al., 2004).
Figure 1. Task activation map.
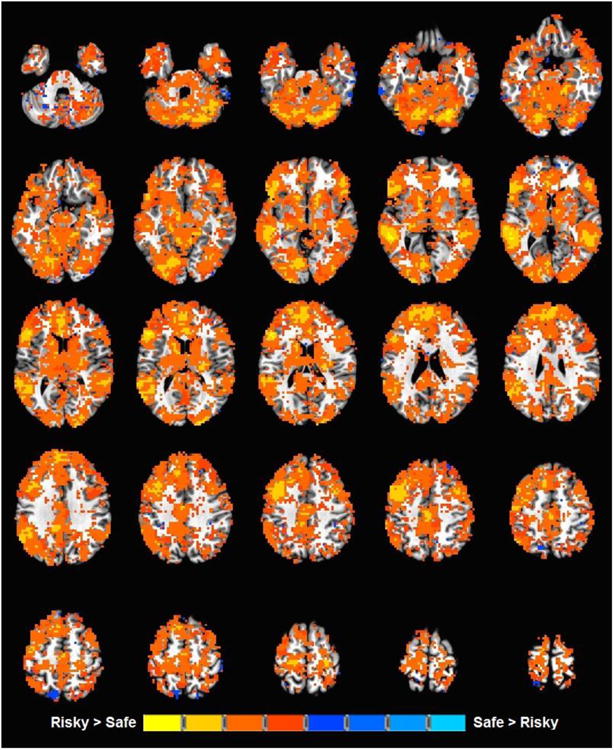
Regions of the brain in which there is a significant risky vs. safe contrast, in binge-drinking adolescents or controls at either baseline or revisit.
To investigate alcohol-related effects in the dorsal frontal and striatal regions, a priori region of interest (ROI) masks were made for the right and left dorsal striatum by creating a 3.5 mm radius mask of the left and right dorsal caudate (in Talairach space) at x = ± 13, y = 15, z = 8 as described by (Di Martino et al., 2008), based on a meta-analysis of striatal functional connectivity (Postuma and Dagher, 2006). Furthermore, 10 mm radius masks of the left and right dorsolateral prefrontal cortex at peak coordinates x =-43, y = 25, z = 26 and x = 36, y = 31, z = 30, respectively, were made based on their functional connectivity to the dorsal caudate at these specific coordinates (Postuma and Dagher, 2006). These masks were then resampled into 3 mm3 voxels to align with the functional data. Percent signal change for all three contrasts (risky vs. safe selection, risky selection vs. baseline, and safe selection vs. baseline) was extracted from all four ROIs for each participant and a 2 (group) by 2 (time) mixed factorial ANOVA conducted in SPSS was used to examine effects of group, time and group-by-time interactions.
To explore the nature of premorbid and developmental effects, a whole-brain analysis, using a 2 (group) by 2 (time) mixed factorial ANOVA was conducted using AFNI's 3dANOVA3 to examine between-group and within-subject differences in risky vs. safe selection BOLD response. To correct for multiple comparisons, AFNI's AlphaSim Monte Carlo was performed using both a voxel (p < 0.05) and cluster threshold (α < 0.05, ≥ 92 voxels) (Forman et al., 1995). For all significant clusters, AFNI's 3dROIstats was used to extract percent signal change values for the risky vs. safe selection contrast, as well as the risky selection vs. baseline and safe selection vs. baseline contrasts, to examine the simple effects using SPSS. To explore the contribution of risky and safe selections in clusters with a significant risky vs. safe selection contrast, a 2 (group) by 2 (selection type) by 2 (time) mixed factorial ANOVA was run for each cluster, and all significant interactions were followed up post-hoc using Fisher's LSD correction for multiple comparisons.
2.4.3 Drinking measures
Following group-level analyses, alcohol and drug use variables from the CDDR and TLFB were correlated with imaging results in the binge-drinking youth to relate the neuroimaging findings to drinking or drug use measures. To investigate the effect of alcohol and drug use over time in this group, the percent signal change in the risky vs. safe selection contrast at baseline was subtracted from this same contrast at revisit to create a difference in percent signal change value. This difference value was then correlated with drinking and drug use variables of interest, with correlations of p < 0.05 considered significant. Furthermore, to investigate if brain activation in regions of group differences at baseline were associated with future substance use, risky vs. safe selection brain activation was also correlated with drinking and drug use variables. A false discovery rate (FDR) correction for multiple comparisons was implemented (Benjamini and Hochberg, 1995) with a false discovery rate of 0.2 due to the preliminary nature of this study and the cost of a false negative (McDonald, 2014).
3. Results
3.1 Participant characteristics and task behavior
Participant demographics and substance use characteristics, at baseline and revisit, are presented in Table 1.
Table 1. Demographic and substance use characteristics.
| Bingers (n = 13) M (SD) | Control (n = 13) M (SD) | |
|---|---|---|
| Baseline | ||
| Gender (male/female) | 8/5 | 8/5 |
| Family history density | 0.30 (0.32) | 0.29 (0.25) |
| Age | 14.89 (1.22) | 14.94 (1.07) |
| Pubertal stage | 4.00 (1.15) | 3.85 (1.07) |
| IQ | 112.69 (8.71) | 114.46 (6.31) |
| Hollingshead SES | 23.23 (11.77) | 27.62 (10.73) |
| Lifetime drinks | 0.85 (1.82) | 0.00 (0.00) |
| Lifetime marijuana use | 0.00 (0.00) | 0.00 (0.00) |
| Revisit | ||
| Age | 17.70 (1.16) | 17.02 (1.11) |
| Pubertal stage | 4.77 (0.44) | 4.58 (0.67)a |
| IQ | 113.00 (15.85) | 115.15 (9.92) |
| Hollingshead SES | 24.31 (11.34) | 27.23 (12.42) |
| Lifetime drinks* | 55.35 (32.28) | 0.00 (0.00) |
| Age first drank | 15.38 (1.98) | - |
| Drinking days (past 3 months)* | 6.45 (3.84) | 0.00 (0.00) |
| Drinks per drinking day (past 3 months)* | 4.74 (1.20) | 0.00 (0.00) |
| Heavy drinking days (≥4 drinks, past 3 months)* | 4.92 (3.35) | 0.00 (0.00) |
| Largest amount drank (past 3 months)* | 6.69 (2.01) | 0.00 (0.00) |
| Lifetime marijuana use* | 67.38 (110.18) | 0.00 (0.00) |
| Age first smoked marijuana | 14.90 (1.79)b | - |
p < 0.05
n = 12 because of missing data
n = 10 because 3 subjects did not report marijuana use
In addition to the substance use characteristics presented, there were also six binge-drinking adolescents who reported cigarette use at revisit; however, only one of these youth reported >10 lifetime uses (∼600 uses). Furthermore, two binge-drinking adolescents also reported other substance use at revisit, including five uses of lysergic acid diethylamide (LSD or “acid”) and/or psilocybin mushrooms by one participant and four uses of 3,4 methylenedioxymethamphetamine (“ecstasy”), LSD, and/or psilocybin mushrooms by another participant. There were no significant differences between binge-drinking adolescents and controls for any demographic variables; however, as expected, binge-drinking adolescents differed significantly from controls on all substance use variables at revisit (all p's < 0.05).
Furthermore, during the WOF task, a three-way mixed factorial ANOVA for reaction time revealed that youth had faster reaction times at revisit than baseline, regardless of group or selection type [F(1,24) = 11.96, p < 0.01]; however, there was not a significant main effect of group, selection type, or any significant interactions (all p's > 0.05). Additionally, a two-way mixed factorial ANOVA for risky selections (by percentage) revealed there was no main effect of group [F(1,24) = 0.67, p = 0.43], such that binge-drinking adolescents and controls did not different in their number of risky choice selections (by percentage) at either baseline (Bingers: M = 51.22, SD = 16.58; Controls: M = 52.23, SD = 20.57) or revisit (Bingers: M = 63.19, SD = 12.30; Controls: M = 54.66, SD = 14.87), no main effect of time [F(1,24) = 2.61, p = 0.12], and no group-by-time interaction [F(1,24) = 1.15, p = 0.30].
3.2 A priori ROI analyses
The a priori ROI analysis of the risky vs. safe selection brain response in the dorsal caudate revealed no main effects of group or time; however, there was a significant group-by-time interaction in the left dorsal caudate [F(1,24) = 5.06, p < 0.05]. Pairwise comparisons indicated that binge-drinking adolescents showed significantly decreased risky vs. safe selection brain activation at revisit compared to baseline (p < 0.05), and at revisit, risky vs. safe selection brain activation was significantly lower in binge-drinking adolescents than controls (p < 0.05), who remained alcohol and drug-naïve at revisit (Figure 2). Due to an increase in risky choice selection, between baseline and revisit, in binge-drinking youth, albeit not statistically significant, the previous analysis was repeated covarying for the change in risky choice selection (by percentage) between baseline and revisit. This reduced the group-by-time interaction in the left dorsal caudate to trend levels [F(1,23) = 4.02, p = 0.06].
Figure 2. Group-by-time interaction in the left dorsal caudate.
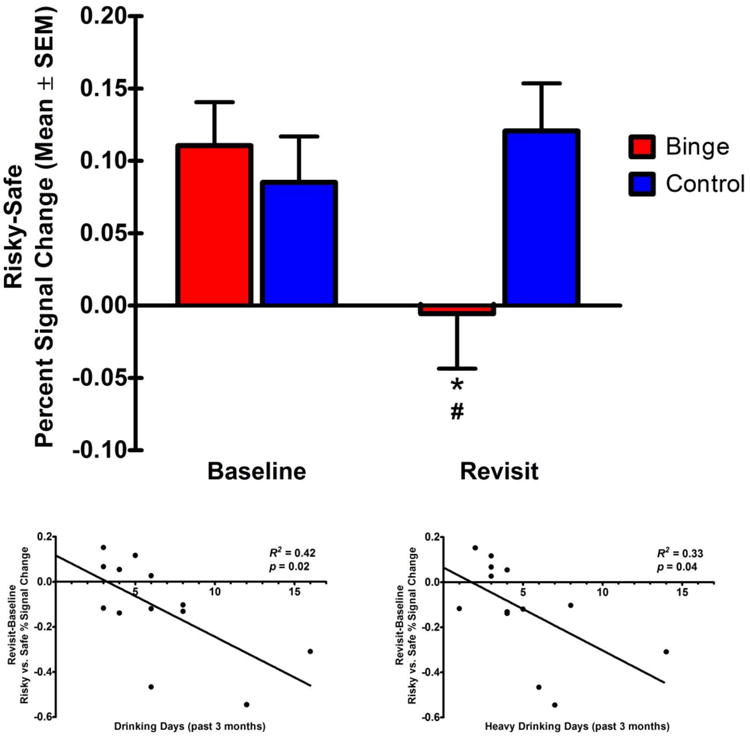
(a) Percent signal change (mean ± SEM) in risky vs. safe brain activation at baseline and revisit. *Binge-drinking adolescents showed decreased risky vs. safe brain activation at revisit compared to baseline. #At revisit, binge-drinking adolescents showed decreased risky vs. safe brain activation compared to controls. (b) Drinking days in the past 3 months, and (c) heavy drinking days in the past 3 months1, were negatively related to the change in risky vs. safe percent signal change, between revisit and baseline, in binge-drinking adolescents.
To better understand whether this risky vs. safe selection brain activation contrast interaction was driven by risky or safe selections, the contrast was separated into brain activation during risky selection vs. baseline and safe selection vs. baseline. A three-way mixed factorial ANOVA revealed that, after collapsing across group and time, risky selection brain activation was significantly greater than safe selection brain activation [F(1,24) = 22.27, p < 0.001], and there was a significant group-by-time-by-selection type interaction [F(1,24) = 5.06, p < 0.05]. Pairwise comparisons revealed that this three-way interaction was driven by a significant decrease in risky selection brain activation, from baseline to revisit, in binge-drinking adolescents (p < 0.01) but not controls (p > 0.05), while there was no significant change in safe selection brain activation for either group (all p's > 0.05). Furthermore, correlation of this change (revisit – baseline) in risky vs. safe selection brain activation in binge-drinking adolescents with the alcohol and drug use variables in Table 1 yielded two significant correlations. Both, total number of drinking days and number of heavy drinking days (≥ 4 drinks), in the past 3 months, were found to be significantly negatively correlated with the change in risky vs. safe selection brain activation (Figure 2). No significant correlations were found with any other drug use variables (e.g., marijuana) (all p's > 0.05).
The a priori ROI analysis of the risky vs. safe selection brain response in the DLPFC found that regardless of group, risky vs. safe selection brain activation was greater at baseline than revisit [F(1,24) = 5.79, p < 0.05]; however, there was not a significant main effect of group or group-by-time interaction. Investigation of risky selection vs. baseline and safe selection vs. baseline effects revealed a significant time-by-selection type interaction [F(1,24) = 5.79, p < 0.05], and pairwise comparisons indicated that risky selection brain activation was significantly greater than safe selection brain activation at baseline (p < 0.01) but not at revisit (p > 0.05). Furthermore, there was a significant decrease in risky selection brain activation over time, such that risky selection brain response was lower at revisit than baseline (p < 0.05).
3.3 Whole-brain analyses
Results of AFNI's 3dANOVA3 investigating significant differences in risky vs. safe selection BOLD response revealed no additional regions demonstrating a significant group-by-time interaction; however, there were numerous regions where a significant main effect of group or time was observed (Table 2). Findings from these main effects are discussed in turn below.
Table 2. Whole-Brain Analysis of BOLD response in Binge-Drinking and Alcohol-Naïve Youth.
| Anatomical Location | Side | x | y | z | Volume (voxels) | F-value | Partial Eta Squared |
|---|---|---|---|---|---|---|---|
| Group by Time Interaction | |||||||
| No significant clusters found | |||||||
| Main Effect of Group | |||||||
| Cluster 1: IPL, SMG, STG | L | -58 | -46 | 41 | 251 | 6.41 | 0.21 |
| Cluster 2: IFG, STG | L | -28 | 22 | -27 | 215 | 7.69 | 0.24 |
| Cluster 3: MTG, STG | L | -67 | -25 | -9 | 149 | 7.78 | 0.24 |
| Cluster 4: STG, MTG | R | 67 | -31 | 2 | 125 | 6.59 | 0.22 |
| Main Effect of Time | |||||||
| Cluster 1: Cerebellum, FG, MOG, IOG | L | -28 | -76 | -15 | 555 | 6.52 | 0.21 |
| Cluster 2: SFG, MFG | R | 28 | 46 | 38 | 306 | 8.60 | 0.26 |
| Cluster 3: PostCG, IPL, PreCG | L | -46 | -34 | 56 | 305 | 7.58 | 0.24 |
| Cluster 4: ACG, MeFG, SFG | R/L | -1 | 22 | 38 | 218 | 6.82 | 0.22 |
| Cluster 5: MOG, Cuneus | R | 46 | -73 | -3 | 198 | 7.40 | 0.24 |
| Cluster 6: Thalamus, LN | L | -1 | -10 | -9 | 131 | 6.81 | 0.22 |
Clusters with significant interactions or main effects in whole-brain analysis of risky vs. safe selection brain activation. Presented for each cluster is the anatomical location of significant voxels, the peak voxel coordinates in Talairach space, the size of the cluster, the corresponding F-value and effect size.
R, right; L, left; ACG, anterior cingulate gyrus; FG, fusiform gyrus; IFG, inferior frontal gyrus; IOG inferior occipital gyrus; IPL, inferior parietal lobule; LN, lentiform nucleus; MeFG, medial frontal gyrus; MFG, middle frontal gyrus; MOG, middle occipital gyrus, MTG, middle temporal gyrus; PostCG, postcentral gyrus; PreCG, precentral gyrus; SFG, superior frontal gyrus; SMG, supramarginal gyrus; STG, superior temporal gyrus.
3.3.1 Pre-existing group differences (main effect of group)
As seen in Table 2, a significant main effect of group in risky vs. safe selection brain response was found in four clusters, including IPL, IFG, MTG, and superior temporal gyrus (STG). A two-way mixed factorial ANOVA, covarying for percent risky selection at baseline and revisit, was conducted and confirmed that, across time, controls had significantly greater risky vs. safe selection brain response than binge drinkers in all four clusters [all F(1,24) ≥ 12.81, p < 0.01], as seen in Figure 3.
Figure 3. Main effect of group.
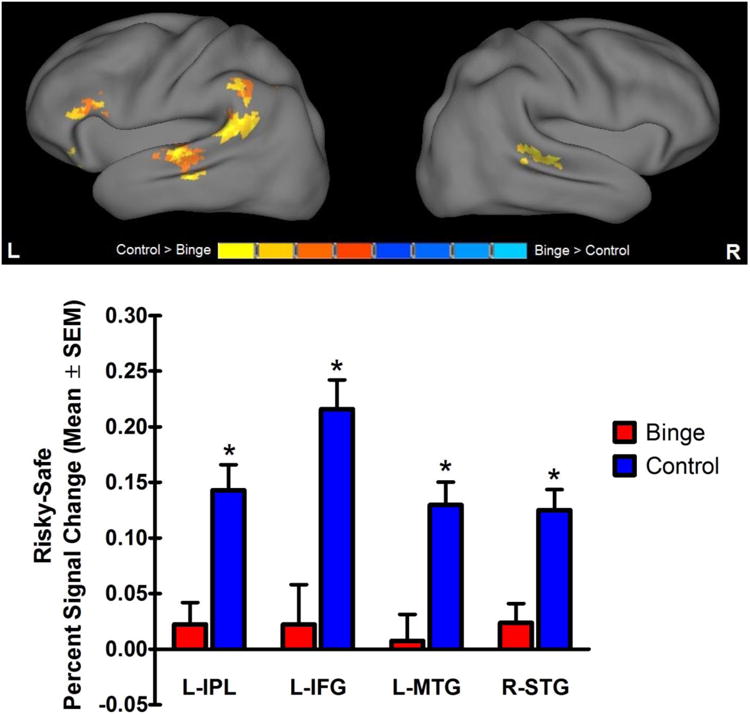
Significant main effect of group in risky vs safe brain activation in the left inferior parietal lobule, left inferior frontal gyrus, left middle temporal gyrus and right superior temporal gyrus. Percent signal change (mean ± SEM) during risky vs. safe selection in both groups is collapsed across time. *Controls had higher risky vs. safe selection response than binge-drinking adolescents.
To better understand whether these risky vs. safe selection contrast main effects were driven by risky or safe selections, the contrast was separated, as previously described, into brain activation during risky selection vs. baseline and safe selection vs. baseline. A three-way mixed factorial ANOVA found that when collapsing across group and time, in all four clusters, risky selection brain response appeared to be greater than safe selection brain response [all F(1,24) ≥ 28.85, p < 0.001]. However, there was a significant group-by-selection type interaction [all F(1,24) ≥ 14.48, p < 0.01], and pairwise comparisons indicated that risky selections resulted in significantly greater brain activation than safe selections only in controls (all p's < 0.001), not in binge drinkers (all p's > 0.05). Correlation of baseline risky vs. safe selection brain activation with revisit drinking and drug use variables revealed no significant effects (all p's > 0.05).
3.3.2 Developmental effects (main effect of time)
A significant main effect of time in risky vs. safe selection brain response between baseline and revisit was found in six clusters, including regions in the cerebellum, superior frontal gyrus, postcentral gyrus, anterior cingulate gyrus, middle occipital gyrus and thalamus (Table 2). As depicted in Figure 4, separate two-way mixed factorial ANOVAs confirmed that for all clusters, there was significantly greater risky vs. safe selection brain response at baseline than at revisit [all F(1,24) ≥ 11.44, p < 0.01].
Figure 4. Main effect of time.
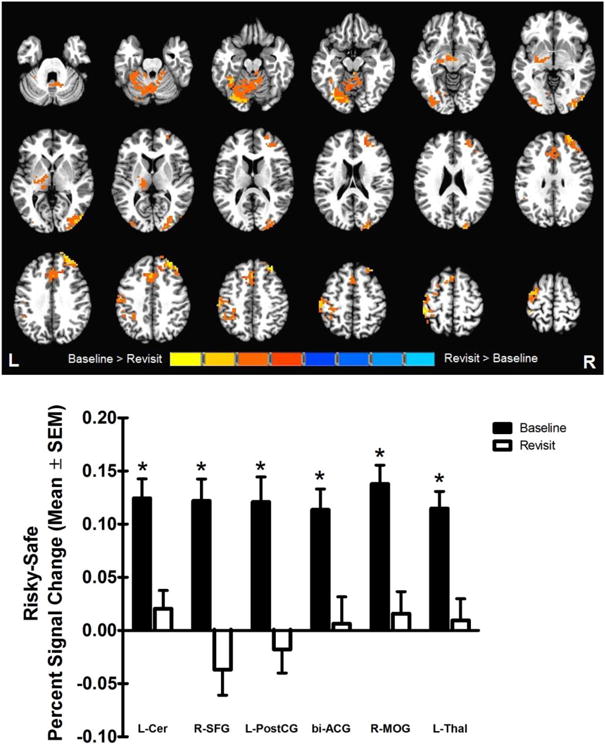
Significant main effect of time in risky vs safe brain activation in the left cerebellum, right superior frontal gyrus, left postcentral gyrus, bilateral anterior cingulate, right middle occipital gyrus, and left thalamus. Percent signal change (mean ± SEM) during risky vs. safe selection at baseline and revisit is collapsed across group. *Risky vs. safe selection response was higher at baseline than revisit.
To further investigate the contribution of risky and safe selection BOLD response to this effect, the risky vs. safe selection contrast was separated, as mentioned previously. A three-way mixed factorial ANOVA revealed that when collapsing across group and time, there was significantly greater risky selection brain response than safe selection BOLD response for all clusters [all F(1,24) ≥ 7.29, p < 0.05]. However, there was a significant time-by-selection type interaction [all F(1,24) ≥ 11.44, p < 0.01], and pairwise comparisons revealed that brain activation during risky selections was greater than during safe selections only at baseline (all p's < 0.001), not revisit (all p's > 0.05). Furthermore, in all clusters except for cluster 4, there was a decrease in risky selection brain response, such that risky selection brain activation was significantly lower at revisit than baseline (all p's < 0.05).
To follow-up these significant differences between baseline and revisit, and to assess test-retest reliability of this task, intra-class correlation coefficients (ICC) were calculated, in SPSS, using brain response during risky selection vs. baseline and safe selection vs. baseline, during baseline and revisit. For the six clusters in which there was a main effect of time, the mean ICC for risky selection vs. baseline activation was 0.65, while the mean ICC for safe selection vs. baseline activation was 0.64. These ICC values correspond to good (0.60-0.74) reliability (Cicchetti, 2001; Cicchetti and Sparrow, 1981; Fleiss, 1986), and fall within accepted range for neuroimaging analyses (Aron et al., 2006).
4. Discussion
The goal of the current study was to investigate differences in risky decision making and associated brain response in adolescents who emerge into binge drinking compared to those who do not. To our knowledge, this is the first study that has investigated brain activation associated with risky decision making in a longitudinal manner, in an effort to disentangle potential risk markers for future binge drinking from consequences of alcohol use. Although no significant group-by-time interactions occurred in the DLPFC, there was a significant alcohol-related effect in the left dorsal caudate, as hypothesized. This result was driven by a decrease in risky selection brain response in binge-drinking adolescents and was related to both total number of drinking episodes, as well as the number of heavy drinking episodes in the 90 days prior to follow-up.
Previous cross-sectional studies in alcohol-dependent adults have found altered striatal brain response during reward anticipation (Beck et al., 2009) and receipt (Bjork et al., 2008); however, this study is the first to present alcohol-related differences in striatal response during the selection phase of a reward-based decision-making task. In contrast to published findings in the ventral portion of the striatum, in alcohol-dependent individuals (Alba-Ferrara et al., 2015; Gilman et al., 2015), alcohol-related differences in our sample were prevalent in the dorsal striatum and are supported by previous studies suggesting the involvement of more dorsal regions of the striatum during this phase of the task (for review, see Balleine et al., 2007). Additionally, these findings are in accordance with a recent study which found dysregulation of functional connectivity between the dorsal striatum and prefrontal regions during decision making in alcohol-dependent patients (Lee et al., 2013). Furthermore, pre-clinical studies also support the notion of binge-drinking-related changes in the dorsal striatum. Wilcox et al. (2014) found that in the dorsal striatum of binge-drinking rodents, there was a decrease in the frequency of GABA(A) receptor mediated inhibitory postsynaptic currents in medium spiny neurons. This is accompanied by findings of decreased behavioral control and resistance to extinction during adulthood in adolescent rodents exposed to binge levels of alcohol (Gass et al., 2014). These findings may help explain how a decrease in risky selection brain activation in the dorsal striatum could lead to increases in future risk taking, and continued alcohol consumption. Furthermore, these findings compliment related research from our laboratory which demonstrated binge-drinking related alterations in cerebellar BOLD response during reward processing, a construct of risk-taking separate from, but related to, decision making (Cservenka et al., 2015). Taken together these findings suggest that binge drinking during adolescence results in functional changes in the brain that may differ across the components of risk-taking behavior.
In addition to a significant group-by-time interaction, suggesting the possibility of alcohol use-related effects, we also observed pre-existing group differences in the left IPL and IFG, as well as in bilateral MTG and STG, suggesting potential neural risk markers, prior to the emergence into binge drinking. Results indicated that unlike controls, who had significantly greater brain response to risky than safe selection, binge-drinking youth showed no differences between risky and safe selection brain activation, an effect that was present at baseline, when both groups were alcohol-naïve, and persisted at revisit. This finding of significant differences in risky and safe selection brain response in alcohol-naïve controls replicates previous studies which found significant IPL and MTG activation during risky choice conditions, with IPL activation during risky choice conditions being significantly greater than certain choice conditions (Guo et al., 2013). Furthermore, these results replicate those found in the original version of this task, which found greater activation during risky than safe choice selection in the IPL and IFG of healthy adolescents (Ernst et al., 2004). More importantly, this finding of decreased activation in the IPL and IFG supports our hypothesis, and is in agreement with findings by Norman et al. (2011), which showed that decreased IPL, IFG and MTG activation during inhibition (arguably a critical component of risky decision making) predicted whether an adolescent would later emerge into heavy drinking. Taken together, these findings support the role of fronto-parietal brain regions in risky decision making and suggest that adolescents who exhibit hypoactivity of this network during decision making, may be more prone to exhibit risk-taking behavior, such as experimentation with drugs and alcohol.
Another benefit of the longitudinal design of this study is that it allowed for exploration of normal neurodevelopmental processes related to decision making. Our analysis found that across groups, there were numerous brain regions in which there was greater risky than safe selection brain response at baseline, but no difference in brain response between risky and safe selections at revisit, primarily due to a decrease in risky selection brain response. As hypothesized, the dACC, along with other cognitive control regions (including the lateral PFC), showed significant decreases in brain activation over time during decision making. This finding is in agreement with previous studies examining brain activation during the selection phase of decision making (Van Leijenhorst et al., 2010), and may reflect a more mature, less robust pattern of activation in these regions as they continue to develop throughout adolescence. In fact, studies investigating other tasks involving executive functioning have found similar decreases in activation with age, across adolescence (e.g. Tamm et al., 2002). In regards to risky decision making, a widespread decrease in risky vs. safe selection activation may suggest an overall decrease in the salience of risky choices compared to safe choices, such that the cognitive demand required to make a risky or safe choice becomes more similar across development.
While this study is the first to use a longitudinal design to examine the effects of binge drinking on brain activation during risky decision making, it is not without limitations. First, the imaging analysis in this study is directly tied to subjects' behavior on the task, such that if there was not an adequate distribution of risky and safe choices chosen, then a valid risky vs. safe selection contrast could not be constructed. Future studies using this task may benefit from considering analytic strategies to examine decision making, regardless of choice selection. Such an analysis would allow for the inclusion of subjects that made all, or almost all, risky selections, which may represent a subset of individuals who are at the highest risk for engaging in risky behavior, such as experimentation with alcohol. In line with the previous limitation, it is important to note that the statistically significant group-by-time interaction, reported in the dorsal caudate, only reached trend level significance, when covarying for the non-significant change in percent risky choice selection between baseline and revisit. While this may suggest that a different number of risky selection trials per group resulted in different power to identify risky vs. safe differences per group, it is equally likely that a change in brain response resulted in a change in behavior, or vice versa. Furthermore, due to the small sample size, we were unable to examine sex-related differences associated with binge drinking and decision making; however, this is an important future direction, considering documented sex differences in alcohol's effects on the adolescent brain (Medina et al., 2008; Squeglia et al., 2011). Also, it is also important to note that many of the youth in the binge-drinking sample also reported marijuana and cigarette use by the time of revisit. Therefore, despite a lack of a dose-response association between other drug use and our neuroimaging findings, it is difficult to definitively claim these results solely represent alcohol-related effects. Additionally, while mean ICC values, in regions of developmental effects, fell within the acceptable range, it is important to note that more modest values may be impacted by individual variability in development change in these regions, rendering reliability over time difficult to assess. Finally, with regard to the group differences in brain activation prior to the initiation of alcohol consumption found in the current study, it cannot be ruled out that control participants, currently abstaining for alcohol use, will not initiate binge drinking at a later time. Therefore, caution must be taken when considering these findings as risk markers of future alcohol consumption.
5. Conclusions
To our knowledge, this is the first study to use a longitudinal design to assess brain activity during decision making in adolescent binge drinkers. At revisit, we found reduced activation during risky decisions in the left dorsal caudate of binge-drinking adolescents, which may reflect functional consequences of alcohol intake, and is congruent with the role of the dorsal striatum in decision making, as well as its vulnerability to alcohol induced effects during adolescence. Furthermore, we found pre-existing differences between binge-drinking youth and controls at baseline in the IPL, IFG, STG and MTG that persisted over time, which may reflect a potential risk marker for future binge-drinking and suggests that hypoactivation of fronto-parietal regions during decision making may lead to an increase in drug and alcohol experimentation. There were also many task-relevant regions of the brain that showed a decrease in brain activation over time in both groups, representing replication of developmental effects seen in other executive functioning tasks (Tamm et al., 2002; Van Leijenhorst et al., 2010). These findings aid in better differentiating functional abnormalities due to alcohol use from those that occur prior to experimentation with alcohol. Understanding which regions of cognitive control and reward-related circuitry are disrupted prior to initiation of alcohol use, and which are disrupted by alcohol use itself, potentially further perpetuating intake, is crucial for identifying targets for future prevention and intervention strategies. In fact, recent studies have begun to use such findings, alongside behavioral intervention strategies, to elicit functional changes in the brain of substance users (Feldstein Ewing et al., 2013), highlighting the importance of these neuroimaging findings for future translational research.
Highlights.
Adolescent binge drinking is associated with decreased dorsal striatal response during decision making.
Dorsal striatal decision making-related brain response is associated with degree of recent alcohol use.
Lower task-related fronto-parietal activation precedes initiation of binge drinking.
Widespread developmental decreases in decision making-related brain response exist.
Acknowledgments
Dr. Suzanne Mitchell is thanked for her feedback on preliminary drafts of this manuscript. Past and current members of the Developmental Brain Imaging Lab are thanked for assisting in participant scheduling and data collection. This research was supported by the National Institute on Alcohol Abuse and Alcoholism [T32 AA007468 (Phillips); R01 AA017664 (Nagel)].
Footnotes
Two subjects had less than 3 heavy drinking episodes in the last 90 days, however, they still met binge-drinking criterion due to having 3 heavy drinking episodes in a 90 day window that included the overlap of the 3 and 6 month follow-up interviews.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alba-Ferrara L, Muller-Oehring EM, Sullivan EV, Pfefferbaum A, Schulte T. Brain responses to emotional salience and reward in alcohol use disorder. Brain Imaging Behav. 2015 doi: 10.1007/s11682-015-9374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron AR, Gluck MA, Poldrack RA. Long-term test–retest reliability of functional MRI in a classification learning task. Neuroimage. 2006;29:1000–1006. doi: 10.1016/j.neuroimage.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hagele C, Knutson B, Heinz A, Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B (Methodological) 1995l:289–300. [Google Scholar]
- Bjork JM, Smith AR, Hommer DW. Striatal sensitivity to reward deliveries and omissions in substance dependent patients. Neuroimage. 2008;42:1609–1621. doi: 10.1016/j.neuroimage.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ. Imaging brain development: the adolescent brain. Neuroimage. 2012;61:397–406. doi: 10.1016/j.neuroimage.2011.11.080. [DOI] [PubMed] [Google Scholar]
- Brown SA, Myers MG, Lippke L, Tapert SF, Stewart DG, Vik PW. Psychometric evaluation of the Customary Drinking and Drug Use Record (CDDR): a measure of adolescent alcohol and drug involvement. J Stud Alcohol. 1998;59:427–438. doi: 10.15288/jsa.1998.59.427. [DOI] [PubMed] [Google Scholar]
- Cicchetti DV. Methodological commentary the precision of reliability and validity estimates re-visited: distinguishing between clinical and statistical significance of sample size requirements. Journal of Clinical and Experimental Neuropsychology. 2001;23:695–700. doi: 10.1076/jcen.23.5.695.1249. [DOI] [PubMed] [Google Scholar]
- Cicchetti DV, Sparrow SA. Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. American journal of mental deficiency. 1981 [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Cservenka A, Casimo K, Fair DA, Nagel BJ. Resting state functional connectivity of the nucleus accumbens in youth with a family history of alcoholism. Psychiatry Res. 2014a;221:210–219. doi: 10.1016/j.pscychresns.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Fair DA, Nagel BJ. Emotional processing and brain activity in youth at high risk for alcoholism. Alcohol Clin Exp Res. 2014b;38:1912–1923. doi: 10.1111/acer.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Herting MM, Seghete KL, Hudson KA, Nagel BJ. High and low sensation seeking adolescents show distinct patterns of brain activity during reward processing. Neuroimage. 2012;66C:184–193. doi: 10.1016/j.neuroimage.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Jones SA, Nagel BJ. Reduced cerebellar brain activity during reward processing in adolescent binge drinkers. Dev Cogn Neurosci. 2015;16:110–120. doi: 10.1016/j.dcn.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Nagel BJ. Risky decision-making: an FMRI study of youth at high risk for alcoholism. Alcohol Clin Exp Res. 2012;36:604–615. doi: 10.1111/j.1530-0277.2011.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Shanklin S, Flint KH, Hawkins J, Harris WA, Lowry R, McManus T, Chyen D, Whittle L, Lim C, Wechsler H Centers for Disease, C., Prevention. Youth risk behavior surveillance - United States, 2011. MMWR Surveill Summ. 2012;61:1–162. [PubMed] [Google Scholar]
- Ernst M, Nelson EE, McClure EB, Monk CS, Munson S, Eshel N, Zarahn E, Leibenluft E, Zametkin A, Towbin K, Blair J, Charney D, Pine DS. Choice selection and reward anticipation: an fMRI study. Neuropsychologia. 2004;42:1585–1597. doi: 10.1016/j.neuropsychologia.2004.05.011. [DOI] [PubMed] [Google Scholar]
- Feldstein Ewing SW, McEachern AD, Yezhuvath U, Bryan AD, Hutchison KE, Filbey FM. Integrating brain and behavior: evaluating adolescents' response to a cannabis intervention. Psychol Addict Behav. 2013;27:510–525. doi: 10.1037/a0029767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiss JL. Reliability of measurement. The design and analysis of clinical experiments. 1986:1–32. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gass JT, Glen WB, Jr, McGonigal JT, Trantham-Davidson H, Lopez MF, Randall PK, Yaxley R, Floresco SB, Chandler LJ. Adolescent alcohol exposure reduces behavioral flexibility, promotes disinhibition, and increases resistance to extinction of ethanol self-administration in adulthood. Neuropsychopharmacology. 2014;39:2570–2583. doi: 10.1038/npp.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF. Adolescent cognitive control and reward processing: implications for risk taking and substance use. Horm Behav. 2013;64:333–342. doi: 10.1016/j.yhbeh.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Smith AR, Bjork JM, Ramchandani VA, Momenan R, Hommer DW. Cumulative gains enhance striatal response to reward opportunities in alcohol-dependent patients. Addict Biol. 2015;20:580–593. doi: 10.1111/adb.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Chen J, Liu S, Li Y, Sun B, Gao Z. Brain areas activated by uncertain reward-based decision-making in healthy volunteers. Neural Regen Res. 2013;8:3344–3352. doi: 10.3969/j.issn.1673-5374.2013.35.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AB, Redlich FC. Social class and mental illness: Community study. 1958 doi: 10.2105/ajph.97.10.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CA, Xiao L, Palmer P, Sun P, Wang Q, Wei Y, Jia Y, Grenard JL, Stacy AW, Bechara A. Affective decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in 10th grade Chinese adolescent binge drinkers. Neuropsychologia. 2008;46:714–726. doi: 10.1016/j.neuropsychologia.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future: National Results on Adolescent Drug Use: 1975-2013: Overview, Key Findings on Adolescent Drug Use. Ann Arbor: Institute for Social Research, The University of Michigan; 2014. [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Koss WA, Sadowski RN, Sherrill LK, Gulley JM, Juraska JM. Effects of ethanol during adolescence on the number of neurons and glia in the medial prefrontal cortex and basolateral amygdala of adult male and female rats. Brain Res. 2012;1466:24–32. doi: 10.1016/j.brainres.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land C, Spear NE. Ethanol impairs memory of a simple discrimination in adolescent rats at doses that leave adult memory unaffected. Neurobiol Learn Mem. 2004;81:75–81. doi: 10.1016/j.nlm.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Lee S, Lee E, Ku J, Yoon KJ, Namkoong K, Jung YC. Disruption of orbitofrontostriatal functional connectivity underlies maladaptive persistent behaviors in alcohol-dependent patients. Psychiatry Investig. 2013;10:266–272. doi: 10.4306/pi.2013.10.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Thayer R, Squeglia LM, McQueeny TM, Tapert SF. Recent binge drinking predicts smaller cerebellar volumes in adolescents. Psychiatry Res. 2013;211:17–23. doi: 10.1016/j.pscychresns.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio-Stipec M, Lahey BB, Friman P. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry. 2001;40:443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- McDonald JH. Handbook of biological statistics 2014 [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, Tapert SF. Altered white matter integrity in adolescent binge drinkers. Alcohol Clin Exp Res. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Goddings AL, Clasen LS, Giedd JN, Blakemore SJ. The developmental mismatch in structural brain maturation during adolescence. Dev Neurosci. 2014;36:147–160. doi: 10.1159/000362328. [DOI] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Adolescent but not adult rats exhibit ethanol-mediated appetitive second-order conditioning. Alcohol Clin Exp Res. 2008;32:2016–2027. doi: 10.1111/j.1530-0277.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot RM, Wecker L, Kirstein CL. Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi. Int J Dev Neurosci. 2009;27:805–815. doi: 10.1016/j.ijdevneu.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb Cortex. 2006;16:1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Hesselbrock VM, Nurnberger JI, Jr, Schuckit MA, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP. Adolescent and adult heart rate responses to self-administered ethanol. Alcohol Clin Exp Res. 2008;32:1807–1815. doi: 10.1111/j.1530-0277.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: a tyrosine hydroxylase immunohistochemical study. Biol Psychiatry. 1994;36:272–277. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, McQueeny T, Nagel BJ, Eyler LT, Tapert SF. A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol. 2010;44:111–117. doi: 10.1016/j.alcohol.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction. 2011;106:564–573. doi: 10.1111/j.1360-0443.2010.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Bzowej NH, Guan HC, Bergeron C, Becker LE, Reynolds GP, Bird ED, Riederer P, Jellinger K, Watanabe S, et al. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1:399–404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Brown J, Leo GI, Sobell MB. The reliability of the Alcohol Timeline Followback when administered by telephone and by computer. Drug Alcohol Depend. 1996;42:49–54. doi: 10.1016/0376-8716(96)01263-x. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Schweinsburg AD, Pulido C, Tapert SF. Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcohol Clin Exp Res. 2011;35:1831–1841. doi: 10.1111/j.1530-0277.2011.01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF. Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology (Berl) 2012;220:529–539. doi: 10.1007/s00213-011-2500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Coplanar stereotaxic atlas of the human brain: threedimensional proportional system: an approach to cerebral imaging 1988. Thieme Medical Publishers; New York: 1988. [Google Scholar]
- Tamm L, Menon V, Reiss AL. Maturation of brain function associated with response inhibition. J Am Acad Child Adolesc Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Taylor SJ, Whincup PH, Hindmarsh PC, Lampe F, Odoki K, Cook DG. Performance of a new pubertal self-assessment questionnaire: a preliminary study. Paediatr Perinat Epidemiol. 2001;15:88–94. doi: 10.1046/j.1365-3016.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Gunther Moor B, Op de Macks ZA, Rombouts SA, Westenberg PM, Crone EA. Adolescent risky decision-making: neurocognitive development of reward and control regions. Neuroimage. 2010;51:345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler abbreviated scale of intelligence. Psychological Corporation; 1999. [Google Scholar]
- White AM, Ghia AJ, Levin ED, Swartzwelder HS. Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 2000;24:1251–1256. [PubMed] [Google Scholar]
- Wilcox MV, Cuzon Carlson VC, Sherazee N, Sprow GM, Bock R, Thiele TE, Lovinger DM, Alvarez VA. Repeated binge-like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission. Neuropsychopharmacology. 2014;39:579–594. doi: 10.1038/npp.2013.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Bechara A, Gong Q, Huang X, Li X, Xue G, Wong S, Lu ZL, Palmer P, Wei Y, Jia Y, Johnson CA. Abnormal affective decision making revealed in adolescent binge drinkers using a functional magnetic resonance imaging study. Psychol Addict Behav. 2013;27:443–454. doi: 10.1037/a0027892. [DOI] [PubMed] [Google Scholar]


