Abstract
Attention-deficit/hyperactivity disorder (ADHD) is a common, highly heritable neuropsychiatric disorder with hyperactivity as one of the hallmarks. Aberrant dopamine signaling is thought to be a major theme in ADHD, but how this relates to the vast majority of ADHD candidate genes is illusive. Here we report a Drosophila dopamine-related locomotor endophenotype that is shared by pan-neuronal knockdown of orthologs of the ADHD-associated genes Dopamine transporter (DAT1) and Latrophilin (LPHN3), and of a gene causing a monogenic disorder with frequent ADHD comorbidity: Neurofibromin (NF1). The locomotor signature was not found in control models and could be ameliorated by methylphenidate, validating its relevance to symptoms of the disorder. The Drosophila ADHD endophenotype can be further exploited in high throughput to characterize the growing number of candidate genes. It represents an equally useful outcome measure for testing chemical compounds to define novel treatment options.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a common neuropsychiatric disorder characterized by age inappropriate, sustained hyperactivity and impulsivity and/or problems in focusing attention.1 The disorder affects 5–6% of children worldwide, and is present in 2.5% of the adult population.2, 3 Comorbidities like depressive episodes, anxiety and substance use disorders are often seen in ADHD, and 60–80% of adults show symptoms of sleep disorders and circadian rhythm defects.4, 5, 6
With a heritability of 76%, ADHD is amongst the most heritable neuropsychiatric disorders.7 The genetic basis underlying the majority of ADHD cases is thought to be complex and involve multiple common variants of moderate individual effect.8 Through candidate gene-based and genome-wide genetic studies several chromosomal regions and genes associated with ADHD have been suggested.9 Most studies have investigated genes regulating dopamine homeostasis for their role in the disorder.10, 11, 12 Especially, the gene encoding the dopamine transporter, SLC6A3 (also known as DAT1), was associated with ADHD in several meta-analyses.7, 10, 11 The Latrophilin gene (LPHN3) was identified more recently; it was observed in an ADHD linkage region in a large study of multigenerational families from a genetic isolate.13 Association with ADHD was subsequently confirmed in US, German, Spanish and Norwegian samples of children and adults with the disorder.13 However, these genes have not been detected in ADHD genome-wide association studies so far. As the molecular genetics landscape of ADHD is poorly understood, there is significant value in addressing the role of candidate genes in the biology of specific ADHD-associated behavioral abnormalities. In addition to genes contributing to ADHD risk through common genetic variants also rare gene variants with larger effects may contribute to this disorder. Whereas this remains to be established for ADHD itself, a number of rare genetic syndromes show frequent comorbidity with ADHD. One of these disorders is neurofibromatosis type I (NF-I), a disorder characterized by benign nerve sheath tumors, which is caused by heterozygous loss of function of the NF1 gene. Children with NF-I frequently show hyperactivity, impaired attention and impulse control; 38–49% of children meet diagnostic criteria for ADHD, which is a strong increase compared with the population prevalence.14, 15, 16, 17 Children with NF-I–ADHD significantly improve on methylphenidate (MPH) treatment.14 Besides these identified genes, the genetic factors causing ADHD symptoms in nearly all patients remain unexplained. The still limited number of identified ADHD-associated genes and the lack of disease-relevant functional information for them form a major bottleneck for clinical research and the development of novel therapeutic strategies. To overcome this, an efficient model is required that permits investigation of relevant functional information in a short time frame.
Although animal models are an excellent way to study the in vivo effects of altered gene functioning, only a few models for ADHD have been generated. The most studied are Slc6a3 and Snap25 mutant mice.18, 19 A number of phenotypic models for which the genetic origin is unknown also exist, including the hyperactive wheel-running mouse and the spontaneously hypertensive rat.20, 21 A zebrafish model of lphn3.1 downregulation shows a hyperactive/impulsive locomotor phenotype, accompanied by severe reduction and misplacement of dopamine-positive neurons in the ventral diencephalon.22 Mouse Lphn3 null mutants also have a hyperactive phenotype, accompanied by increased levels of dopamine and serotonin in the dorsal striatum.23 A mouse model of Nf1 demonstrated that reduced dopamine signaling is responsible for cAMP-dependent defects in neuron function, attention and learning.24, 25 The models appear to show inconsistent changes in dopamine levels, and also in humans the direction of dopamine regulation alterations is still debated.26 Thus, the molecular pathology of ADHD needs to be further elucidated. Application of a genetic model with efficient tools and comprehensive resources holds the potential to significantly advance the understanding of the molecular, cellular and developmental basis of ADHD.
The fruit fly Drosophila melanogaster is a cost efficient and powerful genetic model with a large repertoire of behaviors and resources to manipulate any gene of interest.27, 28 The common ancestor of insects and vertebrates, the urbilaterian, already had a complex nervous system, resulting in a strong conservation in the mechanisms of neuronal development and signaling.29 This makes Drosophila a valuable tool for studying human brain diseases.30, 31 Successful studies of such disorders in Drosophila include numerous neurodegenerative disorders, but also early-onset cognitive disorders such as Fragile X syndrome and other forms of intellectual disability.32, 33, 34 Work on the Fragile X fly model identified chemical compounds that rescued phenotypes including cognitive defects,35, 36 and a number of related compounds are currently being tested in clinical trials.37
The amenability of Drosophila to modeling ADHD is strongly supported by studies on the role of dopamine signaling in the behavioral output of the fly. A hyperactive mutant was described with a lesion in the homolog of the human ADHD-associated dopamine transporter gene SLC6A3.38 More recently, a Drosophila memory mutant (radish) was reported to display attention deficits and hyperactivity, and these phenotypes could be rescued by the ADHD-medication MPH.39 However, the radish gene is not conserved in humans and thus ADHD-relevant Drosophila phenotypes and their druggability remain to be elucidated. Here we use Drosophila to model hyperactivity, one of the hallmarks of the disorder, and reveal a dopamine-related signature of locomotor activity and sleep that is common to the orthologs of ADHD-associated genes, DAT, latrophilin and Nf1.
Materials and methods
Drosophila genetics and breeding
Conditional knockdown of Drosophila genes was achieved with the UAS-GAL4 system,40 using pan-neuronal drivers (w; UAS-Dcr-2; elav-GAL4 or yw; UAS-Dcr-2 hs(X); n-syb-GAL4) and UAS-RNAi lines.41 A copy of UAS-Dicer-2 was included to improve the efficiency of knockdown.41 UAS-RNAi lines (DAT v106961; Cirl v100749; Nf1 v109637) and lines targeting a set of random control genes (Supplementary Table 1), their genetic background control (v60100) and UAS-Dcr-2 (v60009) were obtained from the Vienna Drosophila RNAi Centre (VDRC).41 UAS-RNAi line (Cirl 27524) and its genetic background control (36303) were obtained from the Bloomington Drosophila stock center (Indiana University).42 All RNA-mediated interference (RNAi) constructs used in this study are predicted to have no off-targets (http://www.flyrnai.org/RNAi_find_frag_free.html and www.vdrc.at). The pKC43 insertion sites of the three KK RNAi lines (DAT v106961; Cirl v100749; Nf1 v109637) used in this paper were verified using a PCR-based diagnostic assay, according to Green et al. methods.43 Our results indicate that all three hairpin constructs are inserted in the preferred landing site and are thus positioned so that they do not interrupt endogenous genes. The isogenic host strains into which the P-elements were integrated, namely: v60100) y,w[1118] P{attP,y[+],w[3′]} (VDRC KK library) and 36303) y[1] v[1] P{y[+t7.7]=CaryP}attP2 (TRIP collection) served to generate the genetic background controls. These were crossed to the driver lines, in parallel to RNAi lines from the respective collections. Crosses were cultured according to standard procedures at 28 °C.
Enrichment of ADHD-associated genes among Drosophila hyperactive genes
We derived 91 ADHD candidate genes from (1) association meta-analyses in candidate gene studies (n=6)10, 11 and (2) from our earlier work which had retrieved ADHD candidates from genome-wide association studies (GWAS) based on a single-nucleotide polymorphism (SNP) associated to ADHD at a P-value <0.0001 (n=85).44 These candidate genes (Supplementary Table 2) were defined irrespective of evolutionary conservation and do not include GWAS published after the inventory was made.45, 46, 47, 48
Drosophila mutants displaying aspects of hyperactive behavior were identified by keyword analysis in the FlyBase resource.49 The keywords Hyperactiv*, Startle*, Distract*, Attention*, Hyperexci*, Locomotor*, Bang*, Hyper-respons*, Ethanol-sens* identified 78 genes (fly candidate genes), excluding motor-impaired conditions. Of note, all alleles and phenotypes were identified by ADHD-unrelated research, excluding acquisition biases. Sixty-nine human orthologs were recovered for the 78 Drosophila proteins (88%, Supplementary Table 3) by accessing the Ensembl Compara database50 using a BioPerl script (Supplementary Method 1). As background for the enrichment analysis of ADHD-associated genes among Drosophila hyperactive genes, 20 sets of genes were randomly selected from the Drosophila genome using a Php script and translated to human genes as above (Supplementary Method 2). Representation of ADHD candidates among human orthologs of fly hyperactivity genes was compared with representation among 20 equally sized random gene sets. Significance of the difference between comparisons was tested with the Wilcoxon signed-rank test.
Phylogenetic analysis
A phylogenetic tree for each of the three candidates analyzed in the paper (DAT, LPHN3 and NF1) was constructed using MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0.51 The relevant protein families were retrieved from Ensembl, namely: SODIUM DEPENDENT TRANSPORTER, TRANSPORTER SOLUTE CARRIER FAMILY 6 MEMBER ENSFM00730001521312 (Homo sapiens (Hs): SLC6A2, SLC6A3, SLC6A4; Mus musculus (Mm): Slc6a2, Slc6a3, Slc6a4; Danio rerio (Dr): slc6a2-like, slc6a3, slc6a4b; Drosophila melanogaster (Dm): DAT, SerT), LATROPHILIN ENSFM00260000050328 (Hs LPHN1, LPHN2, LPHN3, ELTD1; Mm: Lphn1, Lphn2, Lphn3, Eltd1; Dr: lphn1-like, lphn2a, lphn3.1, eltd1; Dm: Cirl), NEUROFIBROMIN NEUROFIBROMATOSIS RELATED NF 1 ENSFM00250000001252 (Hs: NF1; Mm: Nf1; Dr: nf1a, nf1b; Dm: Nf1). The best-fitting isoform was retrieved using NCBI protein DELTA blast and multiple alignment was performed using MUSCLE with the UPGMB clustering method. The phylogenetic trees were constructed with maximum likelihood Jones–Taylor–Thornton (JTT) G model using all sites.
Pairwise protein sequence alignment
Using EMBOSS needle the percentage of amino-acid identity, similarity and gaps between proteins was determined using optimal global alignment of pairwise sequences.52
Locomotor activity profiling
Locomotor activity of individual male flies was recorded with the Drosophila Activity Monitor (DAM) system, in which motion is detected by infrared light beams (Trikinetics, Waltham, MA, USA). Activity of 3–5 days old flies was recorded over 4 days on a 12-h light:dark cycle, followed by 2 days in constant darkness (DD) during which the effect of darkness on activity was measured. Adult flies were transferred into activity monitors at an age of 3–5 days after eclosion. Here they were exposed to normal food or, for the first time, to food containing 1 mg ml−1 MPH), according to the literature. Initially, 0.5 mg ml−1 and 1 mg ml−1 MPH (Brocacef, Maarssen) were tested; 1 mg ml−1 had the stronger effect and was chosen as standard concentration for subsequent experiments. Flies were allowed to acclimatize to activity monitors and food for 24 h before data acquisition. They remain on normal or MPH-supplemented food during the data acquisition. Raw locomotor activity data were collected in 10-s bins, activity and sleep were both analyzed in 1-min bins, whereby 5-min of inactivity is defined as sleep. Activity is plotted in 10-min bins, sleep is plotted in 30-min bins. Analysis was performed in pySolo,53 modified to analyze activity and sleep between 120–540-min relative day (RD) and 840–1260-min relative night (RN) to reflect the stable locomotor activity in those intervals. RNE (early) refers to the first half and RNL (late) to the second half of this interval. Statistical analysis was performed in GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego California USA, www.graphpad.com). T-tests were performed with a Welch's correction when variances were unequal. To compare day and night activity, the delta activity of RNAi vs control was calculated: deltaRD=(RNAiRD−controlRD) and deltaRN=(RNAiRN−controlRN). The fold change was calculated as (deltaRN/deltaRD). Arrhythmicity in DD was evaluated using the χ2-method of the ActogramJ Fiji plugin with a P-value threshold of 0.05.
Quantification of relative gene expression
Flies carrying UAS-RNAi and their genetic background control were crossed with w; UAS-Dcr-2; elav-GAL4 or yw; UAS-Dcr-2 hs(X); n-syb-GAL4 driver and raised at 28 °C. Per condition 10 1-day-old fly heads were collected in three biological replicates and snap frozen. RNA was extracted using the ARCTURUS PicoPure RNA Isolation Kit (Applied Biosystems, Waltham, MA, USA), the concentration was measured with Qubit Fluorometer (Life Technologies, Carlsbad, CA, USA). cDNA was synthesized using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Relative gene expression was quantified in technical duplicates using a qPCR kit (Promega, Madison, WI, USA) on 7500 Real-Time PCR Systems (Applied Biosystems).
Analysis of Tyrosine hydroxylase expressing neurons
Five-day and 11-day old adult brains were dissected and fixed in 3.7% paraformaldehyde for 30 min, washed with PBS-T (PBS (phosphate-buffered saline) containing 0.3% Triton X-100), and blocked in 2% normal goat serum for 30 min. Brains were incubated overnight with rabbit anti-TH antibody in 1% normal goat serum (ab152, Millipore, Billerica, MA, USA, 1:100 dilution) at 4 °C, washed five times in PBS-T and incubated 2 h with goat anti-rabbit Alexa Fluor 488 antibody (A11008, Molecular Probes, Waltham, MA, USA, 1:500 dilution in PBS-T) at room temperature. Finally, brains were washed five times in PBS-T and mounted in Vectashield Mounting Medium (Vector Labs, Burlingame, CA, USA).
Results
Drosophila hyperactivity genes predict human ADHD association
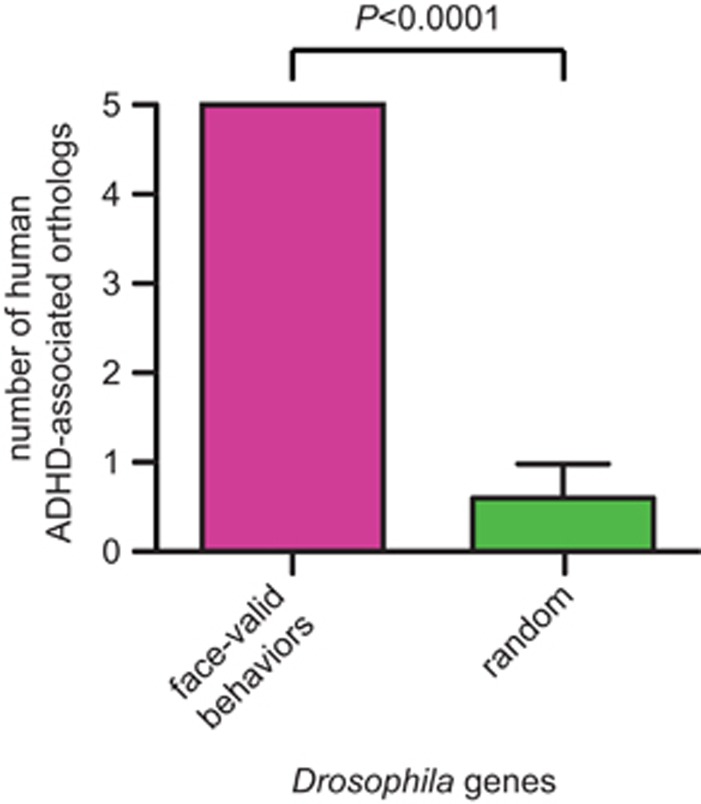
The core symptoms of ADHD include inattention, hyperactivity and impulsivity. If Drosophila can serve as an informative system for studying biological aspects of ADHD, its phenotypes may predict, to a certain extent, human genes associated with ADHD. We therefore mined the Drosophila Genes and Genomes database49 for mutants that display altered attention, hyperactivity or hyperexcitability (see Materials and Methods). Seventy-eight mutants were identified and 69 of the implicated genes are conserved in humans (Supplementary Table 3a). When comparing this list to a catalog of 91 ADHD-associated human candidate genes from meta-analyses and GWAS, five of them overlapped: FLNC, IL16, KCNC1, PRKG1 and SLC6A3 (DAT; Supplementary Table 2). In 20 equally sized random gene sets of human homologs of Drosophila proteins (Supplementary Table 3b) significantly less human ADHD candidate genes were identified: 0.60±0.82 (P<0.0001, Wilcoxon signed-rank testl; Figure 1). These data show that ADHD-associated human candidate genes occur more frequent among genes known to cause face-valid ADHD behaviors in Drosophila. We sought to (1) replicate the behavioral phenotype of the most robust ADHD candidate of the five genes using pan-neuronal knockdown and (2) identify a similar behavioral phenotype for a well-established ADHD gene that has not previously been tested in the assay.
Figure 1.
Eightfold enrichment of human ADHD genes among Drosophila genes unbiasedly reported with ADHD face-valid behaviors. Candidate ADHD genes (n=91) were collected from candidate gene meta-analyses and GWAS. Random gene sets (n=20) were picked with a random number generator script. Genes annotated to induce face-valid ADHD behaviors were retrieved from the Drosophila Genes and Genomes database (n=78). The random sets contained significantly less hits (0.60±0.82 (n=20)) compared with five hits among the candidate ADHD genes (P<0.0001; Wilcoxon signed-rank test).
DAT or latrophilin knockdown causes increased hyperactivity in the dark
The more than eightfold enrichment of ADHD-associated genes among genes linked to ADHD-like Drosophila behaviors motivated our further experimental study, in which we chose to investigate one of the major hallmarks of ADHD, hyperactivity. One of the overlapping genes in our enrichment analysis was DAT, the ortholog of the ADHD-associated dopamine transporter SLC6A3. The mutant was identified in a forward genetic screen with hyperactive, sleep- and locomotor behavior-defective phenotypes. Accordingly, it was named fumin, Japanese for sleepless.38 As sleep problems are a common feature of ADHD in addition to hyperactivity,54 we tested ADHD genes in an activity paradigm in which both behaviors can simultaneously be assessed. Locomotor activity of individual age-controlled Drosophila was recorded in activity monitors (Trikinetics) and analyzed in 10-min bins. Sleep was defined as 5-min bins of inactivity, according to the standards in the field.55 Gene activity was reduced using pan-neuronal promoters and established tools for conditional RNAi.41
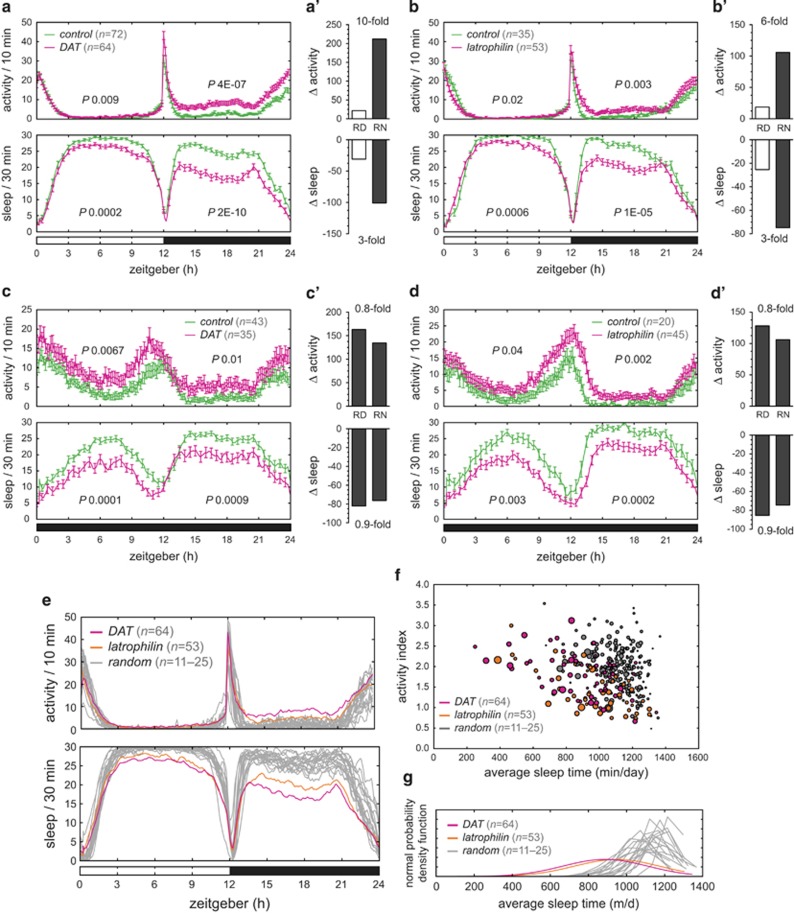
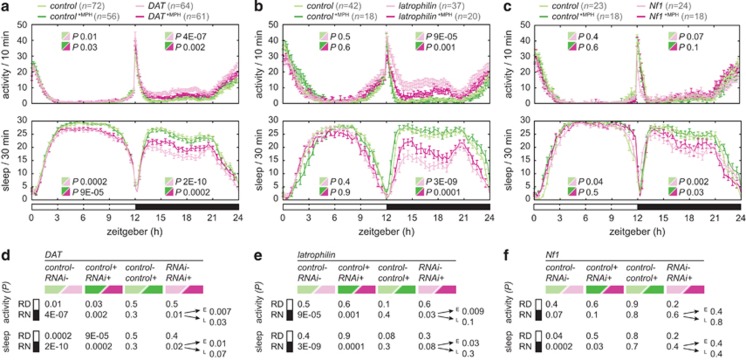
Pan-neuronal knockdown of the dopamine transporter resulted in hyperactivity and sleep loss (Figure 2a), in agreement with the previous report describing the fumin mutant.38 Comparing activity and sleep profiles of the DAT knockdown to its genetic background control revealed that hyperactivity was present during the relative day and relative night (RD P=0.009, RN P=4E-07; Figure 2a, Supplementary Table 4). Sleep was significantly decreased (RD P=0.0002, RN P=2E-10; Figure 2a, Supplementary Table 4). It was noticeable that activity and sleep were much less disturbed during the day, compared with the night. Quantitative evaluation of these differences (Δactivity, Δsleep) in the DAT RNAi versus the control condition revealed a 10-fold increase of activity and a 3-fold decrease of sleep during the relative night compared with the relative day (Figure 2a').
Figure 2.
Dopamine transporter and latrophilin pan-neuronal knockdown give rise to hyperactivity in the dark, which is not observed in random controls. (a and b) Locomotor activity and sleep after knockdown in 12-h light:dark cycle. (a) DAT knockdown (v106961/UAS-Dcr-2; +/elav-GAL4, n=64), (b) latrophilin knockdown (v100749/UAS-Dcr-2; +/elav-GAL4, n=53), both plotted together with their genetic background controls (v60100/UAS-Dcr-2; +/elav-GAL4, n=72 and 35, respectively). (a' and b') ΔActivity and Δsleep bar graphs reveal that activity and sleep are most severely affected during darkness; the fold change is indicated. (c–g) DAT and latrophilin genotypes as indicated in (a and b). (c and d) Locomotor activity and sleep after knockdown in 24-h dark:dark cycle. Hyperactivity and sleep defects during daytime are more severe when lights are switched off. (c) DAT (n=35) and (d) latrophilin (n=45) flies, both plotted together with their genetic background controls (n=43 and 20, respectively). (c' and d') ΔActivity and Δsleep is now severely affected during constant darkness at both the relative day and night. (e) Hyperactivity in the dark is specific to DAT (n=64) and latrophilin (n=53) knockdown. Locomotor activity of 18 random control gene knockdowns (UAS-RNAi/UAS-Dcr-2; +/elav-GAL4, n=11–25) do not display hyperactivity or abnormal sleep. (f) Activity index plotted against average sleep time reveals DAT (n=64) and latrophilin knockdown flies to cluster separately from the control genes. (g) DAT (n=64) and latrophilin (n=53) have a distinct normal probability density function from the random controls (n=11–25). The data is consistent with nighttime hyperactivity representing a dopamine signature, as dopamine signaling is repressed by light in Drosophila.
We asked whether Drosophila hyperactive phenotypes can also be found for ADHD-associated genes whose Drosophila orthologs have not previously been shown to be hyperactive. We set out to test Latrophilin, one of the few well-established (replicated) ADHD candidate gene. A single ortholog exists in Drosophila that represents the latrophilin protein family (Supplementary Figure 2). The LPHN3 Drosophila ortholog Cirl (here further referred to as latrophilin) is highly expressed in the larval CNS and adult brain (Supplementary Figure 1, www.flyatlas.org).56
Pan-neuronal latrophilin knockdown induced hyperactivity and loss of sleep in a pattern closely resembling DAT knockdown (Figure 2b). Activity was significantly increased (RD P=0.02; RN P=0.003; Figure 2a, Supplementary Table 4) and sleep was significantly reduced (RD P=0.0006; RN P=1E-05; Figure 2a, Supplementary Table 4). The phenotype was most prominent during the night, with the Δactivity being sixfold increased and Δsleep threefold decreased (Figure 2b'). The hyperactivity was replicated with an independent pan-neuronal driver (UAS-Dcr-2; n-syb-GAL4; data not shown) and an independent RNAi stock (27524; see Figure 5b).
DAT and latrophilin locomotor hyperactivity is negatively regulated by light
The night-predominated hyperactivity in DAT and latrophilin knockdown flies raised the question whether activity was being repressed by an external signal, such as light. Indeed, it has recently been demonstrated that dopamine signaling is repressed by light in Drosophila.57 We tested this hypothesis by monitoring DAT and latrophilin flies in constant darkness. In this condition hyperactivity and reduced sleep associated with both genes was increased during the relative day, now dark, reaching similar level as in the night period (Figures 2c, c' and d, d'). This demonstrated that the identified activity and sleep defects were light dependent, suggesting that not only DAT but also latrophilin knockdown alters dopamine-mediated signaling.
To determine the prevalence/specificity of the night-hyperactive phenotype, a panel of 18 random control genes (Supplementary Table 1) was tested for activity and sleep parameters. Knockdown of none of these 18 genes gave rise to hyperactivity or defects in sleep; DAT and latrophilin phenotypes clearly stood out from the control group (Figure 2e). On a single-fly level the activity and sleep distribution of DAT and latrophilin knockdown was distinct from the random mutants, showing reduced average sleep time (Figure 2f). When the sleep data was fitted to a normal probability density function, DAT and latrophilin had a characteristic overlapping function that was distinct from the 18 random controls (Figure 2g). In conclusion, DAT and latrophilin sleep and activity parameters overlap, but are distinct from a larger randomly selected set of controls. The night hyperactivity thus shows considerable specificity for (dopamine-related) ADHD candidate genes.
The dopamine signature is not caused by abnormal DA neuron count
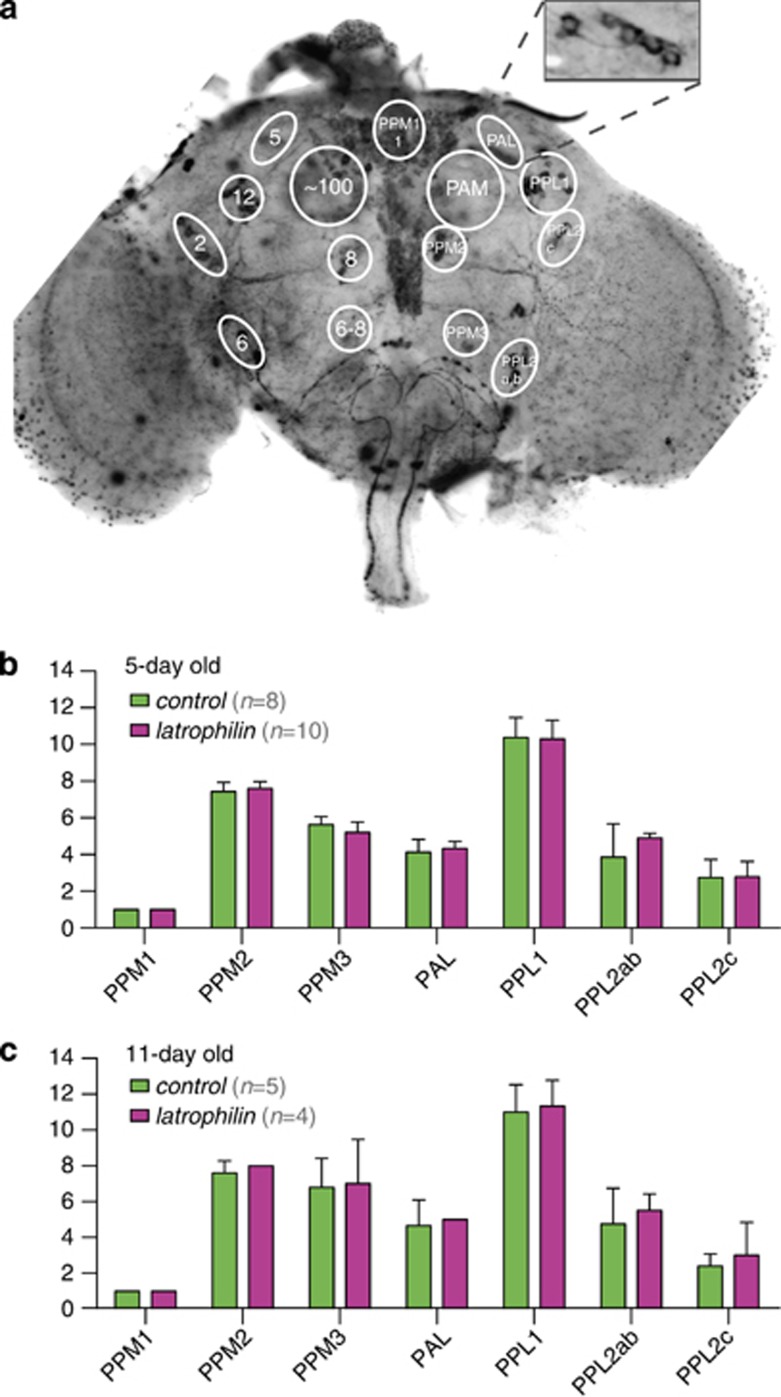
Disturbed locomotor and sleep patterns in DAT and latrophilin knockdown are consistent with altered functionality of dopaminergic circuits. This might be caused by altered specification or survival of dopamine-expressing neurons, or by defects in signaling cascades. To distinguish these possibilities, dopaminergic neurons were visualized by staining for tyrosine hydroxylase, a key enzyme in dopamine biosynthesis (Figure 3a). The Drosophila brain contains distinct clusters of characterized dopaminergic neurons.58 These include neurons previously implicated in motor control and arousal (PPL1, PPM3).59, 60 Latrophilin knockdown flies at day 5 and 11 (corresponding to start and end of monitoring their activity) exhibited the normal number of neurons (Figures 3b and c). These data demonstrate that latrophilin mutant phenotypes are not caused by altered specification or death of dopaminergic neurons, suggesting a direct role of latrophilin in dopamine signaling.
Figure 3.
The dopamine-signature hyperactivity does not result from abnormal count of dopaminergic neurons. (a) Wild-type adult brain. Dopamine neurons were visualized by tyrosine hydroxylase staining and neurons of clusters (PPM1, PPM2, PPM3, PAL, PPL1, PPL2ab, PPL2c) were counted. (b and c) The number of dopaminergic neurons was unaltered in all clusters of the brain after strong pan-neuronal latrophilin knockdown (27524/UAS-Dcr-2; +/n-syb-GAL4). (b) 5-day old (n=10) and (c) 11-day old (n=4) adult brains, both compared to their genetic background controls of the same age (36303/UAS-Dcr-2; +/n-syb-GAL4; n=8 and 5, respectively).
The night hyperactivity and sleep defect signatures represent an ADHD-relevant endophenotype in Drosophila that is rescued by MPH
To demonstrate the relevance of the night-specific hyperactivity and sleep-defective signatures to ADHD, we applied two strategies. First, we asked whether we can phenocopy the behavioral signatures with manipulation of a gene associated to a monogenic disorder characterized by ADHD. Heterozygous mutations in human NF1 give rise to neurofibromatosis type I with increased prevalence of ADHD symptoms.
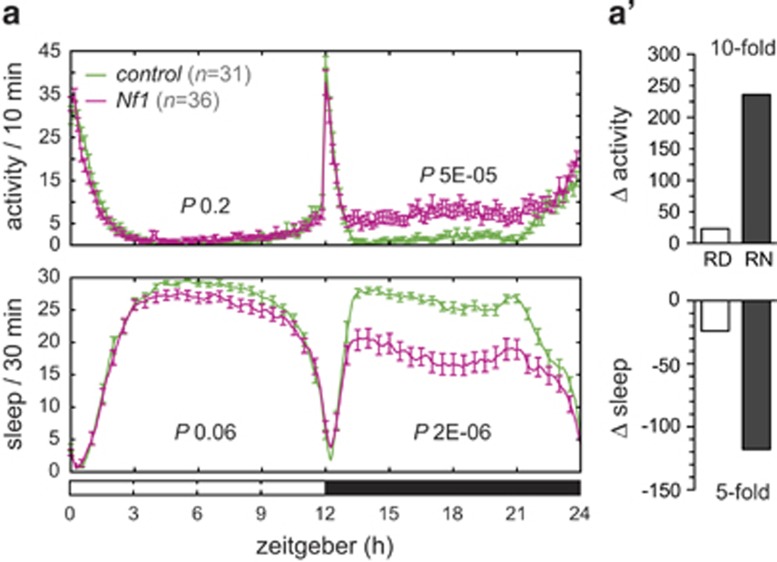
Pan-neuronal knockdown of Drosophila Nf1 resulted in 0.63 fold relative gene expression in whole heads (P=0.01). Arrhythmicity in 25/36 Nf1 RNAi flies (69%) was observed, compared to 2/31 genetic background controls (6.5%), recapitulating previous findings in the mutant.61 Knockdown also resulted in a significant night-specific hyperactivity phenotype (RN P=5E-05; Figure 4a, Supplementary Table 4) and significantly disturbed sleep (RN P=2E-06; Figure 4a, Supplementary Table 4). The activity profile (Figure 4a) strongly resembled the behavioral signatures exhibited by DAT and latrophilin mutant flies: the phenotype was most prominent during the night, where Δactivity was 10-fold increased and Δsleep fivefold decreased (Figure 4a'). Thus the behavioral signature is present in a monogenic model with increased prevalence of ADHD, strengthening the relevance of the observed Drosophila phenotypes to hallmark behaviors associated with the human condition.
Figure 4.
Knockdown of Nf1 causes night hyperactivity, resembling DAT and latrophilin knockdown phenotypes. (a) Pan-neuronal knockdown of Nf1 (v109637/UAS-Dcr-2; +/elav-GAL4, n=36) gives rise to hyperactivity in the dark, compared to its genetic background control (v60100/UAS-Dcr-2; +/elav-GAL4, n=31). (a') ΔActivity and Δsleep is most severely affected during darkness, like in DAT and latrophilin knockdowns (see Figures 2a' and b').
Second, we set out to address whether night hyperactivity and sleep loss in the Drosophila models can be ameliorated by medication used to treat ADHD. We subjected adult DAT, latrophilin and Nf1 models and their genetic background controls to acute pharmacological intervention with the most commonly prescribed medication for ADHD, MPH. Upon supplementation of fly food with 1 mg ml−1 MPH, the DAT-, latrophilin- and Nf1-related hyperactivity and sleep phenotypes were normalized (Figures 5a–c), whereas the same concentration of MPH had no effect on wild-type flies (Figures 5a–f). The difference in activity between DAT RNAi −/+ MPH and latrophilin RNAi −/+ MPH was significantly different (RN activity P=0.01 and P=0.03, respectively). Consistent with the previously reported repressive effect of GABAergic signaling on activity in the late night,62, 63 the rescue was more pronounced during the early (E) versus the late (L) part of the night (DAT RNE P=0.007, RNL P=0.03; latrophilin RNE P=0.009, RNL P=0.1), with a minimum around zeitgeber 21 h (Figures 5a and b, see discussion).
Figure 5.
Hyperactivity in Drosophila ADHD models is ameliorated by methylphenidate. (a–c) Activity and sleep graphs for (a) DAT knockdown (v106961/UAS-Dcr-2; +/elav-GAL4), (b) latrophilin knockdown (27524/UAS-Dcr-2; +/elav-GAL4) and (c) Nf1 knockdown (v109637/UAS-Dcr-2; +/elav-GAL4) models, treated and untreated with MPH. DAT and Nf1 -MPH graphs are replotted from Figures 2 and 4 for direct comparison. No effect of MPH on control (v60100/UAS-Dcr-2; +/elav-GAL4 and 36303/UAS-Dcr-2; +/elav-GAL4) activity is seen. (d–f) P-values for control versus RNAi and treated versus untreated conditions in relative day (RD) and relative night (RN). At zeitgeber 21 h a peak is seen where hyperactivity is endogenously repressed without the addition of MPH. P-values for the RNAi−MPH versus RNAi+MPH were therefore calculated for RN split into RNE (early) and RNL (late) to reflect this change of behavior. DAT hyperactivity and sleep defects, latrophilin hyperactivity, and latrophilin sleep defects at RNE are significantly rescued by MPH supplementation. Hyperactivity and sleep induced by Nf1 knockdown are also quantitatively reduced. The pharmacologic rescue with MPH supports the relevance of the behavioral signature to ADHD.
We conclude that the DAT-, latrophilin- and Nf1-associated light-dependent hyperactivity and sleep signatures suggest disrupted dopamine signaling and identify a Drosophila ADHD endophenotype.
Discussion
ADHD is a common neuropsychiatric disorder of major socioeconomic importance.64, 65 Its etiology and neurobiology are poorly understood, but potential genetic risk factors and candidate genes are being reported at increasing pace through statistical association and probably soon through exome sequencing approaches. However, their relevance for the disease remains to be proven and the biological consequences remain to be discovered. The limited availability of animal models represents a major bottleneck for these endeavors. Here we introduce a novel organism for ADHD research, the fruit fly Drosophila, an organism that has made seminal contributions to our understanding of human biology and disease.28
Our in silico analysis shows that Drosophila genes associated with ADHD-like behaviors are significantly enriched among human ADHD-associated genes. Notably, without exception these fly phenotypes were found by unbiased, non-disease driven approaches, illustrating the power of Drosophila behavioral genetics and the comprehensiveness of information available in this organism. We therefore set out to experimentally investigate the potentially overlapping locomotor behavior through neuronal knockdown of ADHD candidate genes with strong genetic evidence: SLC6A3, and LPHN3. To validate the phenotype we investigated a monogenic disorder that shows high ADHD comorbidity. Among several genes causing monogenic disorder with co-occurrence of ADHD we selected neurofibromatosis type I, caused by mutations in NF1. Although there are no association studies that link common variations in NF1 to ADHD in the population, there is a high and quantitatively well-documented comorbidity with ADHD in carriers of rare neurofibromatosis-causing mutations. The prevalence of ADHD among children with NF-I is highly increased: from 5–6% in children of the general population to 38–49%.14, 15, 16, 17 Thus, NF1 mutations increase ADHD risk by approximately eightfold, and ADHD is diagnosed in nearly every second child with NF-I. None of the ADHD candidate genes studied for association of common variants reaches an effect size of this magnitude (maximal effect sizes around 1.4 have been described).7 The fact that NF-I patients are successfully treated with MPH is a further argument for the existence of a molecular link between NF1 and ADHD-associated genes characterized by common variants.14, 15, 16, 17 Indeed, we found all three models to exhibit hyperactive features. This hyperactivity could be reduced by MPH, supporting the relevance of the observed phenotype for human ADHD. Sleep, widely affected in ADHD patients,4, 54 was also defective in the Drosophila models and improved by MPH. We found the behavioral defects to manifest in a characteristic pattern: increased activity and reduced sleep was most pronounced in the absence of light. Thus, we identified a behavioral signature associated with three ADHD genes that we propose can serve as a Drosophila ADHD endophenotype. The genetic overlap between monogenic disorders and ADHD may be greater than currently appreciated. It is of interest to systematically investigate genes related to monogenic disorders with co-occurring ADHD symptoms in the future.
Four lines of arguments indicate that the locomotor endophenotype characterizes a dopamine signature. First, it is caused by knockdown of DAT, a key transporter that regulates synaptic dopamine homeostasis. Second, it results from knockdown of latrophilin and Nf1 genes, both of which have previously been linked to dopamine signaling in other organisms.22, 25, 66 Third, the light-dependence of our identified signature perfectly matches the previous finding that light suppresses the wake-promoting effect of dopamine.57 Fourth, the phenotype is partially rescued by the ADHD drug MPH, targeting the dopaminergic and noradrenaline system.67 Behavioral and imaging analyses in Drosophila suggested that dopamine is a stronger arousal signal than octopamine, the equivalent of noradrenalin in Drosophila.57 Based on the four lines of arguments we suggest that hyperactivity and reduced sleep in Drosophila locomotor profiles occurring predominantly at night represent a valuable ADHD endophenotype that can identify novel players in dopamine circuits from among novel candidate ADHD genes. Whereas an absence of a dopamine signature cannot disprove a gene's contribution to ADHD, the relative specificity of the night-hyperactive phenotype for ADHD-implicated genes is illustrated by its absence in a random control gene set. Interestingly, the hyperactivity is repressed in the three investigated models with a peak around zeitgeber 21 h. This is likely due to GABAergic inhibition of wake-promoting l-LNv clock neurons, dominating over dopamine-dependent activation in the late night.62, 63
The light-dependence of the hyperactivity in Drosophila is noticeably different from the human ADHD characteristics, where hyperactivity manifests prominently during the day as well. What is causing these species-specific differences? The mechanism underlying light-dependent changes in Drosophila activity was recently identified. Light was shown to buffer the wake-promoting effect of dopamine.57 Shortly after, it was reported that dopamine acts through the circadian photoreceptor cryptochrome.68 Of note, Drosophila cryptochrome is sensitive to light, causing it to be rapidly degraded.69 Mammalian cryptochrome has a light-independent role in the circadian clock and –contrary to Drosophila– does not function as a diurnal regulator of activity.70 This difference between the mammalian and Drosophila molecular build-up does not decrease the impact and applicability of the discovered night-hyperactive behavioral signature as a dopamine-related ADHD endophenotype in Drosophila. Instead, it increases phenotype specificity, as it allows distinguishing dopamine dysregulation through cryptochrome from other activity-promoting signaling pathways. Moreover, despite lacking evidence that mechanisms paralleling those found in Drosophila operate in humans, it is worth noting that light therapy is applied to alleviate ADHD symptoms.71 Light-mediated regulation of circadian rhythm may thus play a yet underappreciated role in the etiology of ADHD.
The mechanisms of dopamine-related pathology in the human ADHD brain are still poorly understood due to contradicting findings related to dopamine and DAT levels. The consequences of MPH application are also not completely understood, possibly due to opposing acute vs long-term effects.67, 72 Different effects of genes on the dopaminergic system are also seen in different models. Whereas in zebrafish it was found that latrophilin knockdown mutants show severe disorganization of the dopaminergic system,22 its development was left intact in the Drosophila knockdown model (Figure 3). This allowed us to address gene function independent of compromised circuits. In the Drosophila brain, loss of DAT, latrophilin, and Nf1 caused hyperactivity and reduced sleep in a light-dependent manner, phenocopying acute activation of dopaminergic neurons.57 That MPH, a drug that prolongs residence of secreted dopamine in the synaptic cleft and is thought to increase dopamine signaling, can improve ADHD-like phenotypes seems paradoxical but is a known phenomenon. It was found that expression of DAT carrying a mutation found in ADHD causes anomalous dopamine efflux, leading to elevated synaptic dopamine levels that could be rescued with MPH.73 Locomotor hyperactive mice lacking DAT (DAT-KO) show a marked reduction of locomotor activity in response to MPH administration, demonstrating the method of action is more complex than just DAT antagonism.74 This can include cross-talk between the D2 dopamine receptor (D2R), the rate-limiting enzyme for the biosynthesis of dopamine (TH) and the dopamine transporter (DAT).75, 76, 77, 78 In Drosophila, MPH rescued defective optomotor response caused by activated but not by inhibited dopaminergic neurons.39 Further research is needed to understand the mechanisms linking LPHN3 and NF1 with dopamine signaling.
We would like to note that the Drosophila models presented here should not be viewed as an attempt to model human ADHD in its complexity. We here focused on hyperactivity as a start into using Drosophila as a model to advance our understanding of genetics and neurobiology of this (complex) disorder. Investigating specific aspects of diseases has strongly advanced our understanding of disease pathologies. At the most extreme, findings in the baker's yeast Saccharomyces cerevisiae have provided many insights into cancer biology, despite the fact that this organism is not able to form tissues and recapitulate processes like angiogenesis and metastasis. Nonetheless the simple single-cell organism has had a major impact in the cancer field.79, 80, 81 The same applies to studying specific forms of learning and memory in animal models of intellectual disability82, even though patients are often affected in numerous cognitive domains. Future studies exploring additional hallmarks of ADHD, most importantly defects in attention, for example by using an optomotor maze or other attention-like performance assays39, would be highly useful complements to our study.
In summary, our study introducing Drosophila as a model to study ADHD-associated hyperactivity has shown that manipulation of three ADHD-associated genes in Drosophila yields an ADHD-relevant, specific and readily recognizable locomotor phenotype that is indicative of dysregulated signaling in a dopaminergic circuit. Our analysis suggests, that at least a subset of ADHD-associated genes is characterized by night-predominant hyperactivity. We propose that (1) Drosophila is a versatile and fast, cheap organism to test novel candidates emerging from large-scale genetics studies in humans; (2) Drosophila is a good model to dissect the mechanisms and pathways from gene to disease, in particular those associated with dopamine-related genes and that (3) drug sensitivity makes Drosophila models a suitable tool in lead identification for novel treatments.
Acknowledgments
We thank the TRiP at Harvard Medical School (NIH/NIGMS R01-GM084947), the Bloomington Drosophila Stock Center at Indiana University, and Vienna Drosophila RNAi center for providing transgenic RNAi fly stocks used in this study. We acknowledge Tom Koemans for verifying the KK RNAi library landing sites. This research is supported by the Netherlands Organization for Scientific Research (NWO): a VENI grant (91.614.084) to MV, a VICI grant (016.130.669) to BF, a Brain and Cognition Excellence Program grant (433-09-229) to BF and AS, VIDI and TOP grants (917-96-346, 912-12-109) to AS, and by the European Union's FP7 large scale integrated network Gencodys (HEALTH-241995) to AS.
Author Contributions
MV performed the experiments and analyzed the data. BH performed anti-TH staining, neuron quantification and RT-qPCR experiments. MV, BF and AS conceived the study and wrote the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders. 4th edn American Psychiatric Association: Arlington, VA, 2000. [Google Scholar]
- Simon V, Czobor P, Bálint S, Mészáros A, Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry 2009; 194: 204–211. [DOI] [PubMed] [Google Scholar]
- Franke B, Faraone SV, Asherson P, Buitelaar J, Bau CHD, Ramos-Quiroga JA et al. The genetics of attention deficit/hyperactivity disorder in adults, a review. Mol Psychiatry 2012; 17: 960–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SYR, Jain U, Shapiro C. Sleep in attention-deficit/hyperactivity disorder in children and adults: past, present, and future. Sleep Med Rev 2012; 16: 371–388. [DOI] [PubMed] [Google Scholar]
- Van Veen MM, Kooij JJS, Boonstra AM, Gordijn MCM, Van Someren EJW. Delayed circadian rhythm in adults with attention-deficit/hyperactivity disorder and chronic sleep-onset insomnia. Biol Psychiatry 2010; 67: 1091–1096. [DOI] [PubMed] [Google Scholar]
- Baird AL, Coogan AN, Siddiqui A, Donev RM, Thome J. Adult attention-deficit hyperactivity disorder is associated with alterations in circadian rhythms at the behavioural, endocrine and molecular levels. Molecular Psychiatry 2012; 17: 988–995. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA et al. Molecular genetics of attention-deficit/hyperactivity disorder. Biological Psychiatry 2005; 57: 1313–1323. [DOI] [PubMed] [Google Scholar]
- Acosta MT, Arcos-Burgos M, Muenke M. Attention deficit/hyperactivity disorder (ADHD): complex phenotype, simple genotype? Gene Med 2004; 6: 1–15. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chang S, Li Z, Zhang K, Du Y, Ott J et al. ADHDgene: a genetic database for attention deficit hyperactivity disorder. Nucleic Acids Res 2012; 40: D1003–D1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizer IR, Ficks C, Waldman ID. Candidate gene studies of ADHD: a meta-analytic review. Hum Genet 2009; 126: 51–90. [DOI] [PubMed] [Google Scholar]
- Li D, Sham PC, Owen MJ, He L. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD). Hum Mol Genet 2006; 15: 2276–2284. [DOI] [PubMed] [Google Scholar]
- Bralten J, Franke B, Waldman I, Rommelse N, Hartman C, Asherson P et al. Candidate genetic pathways for attention-deficit/hyperactivity disorder (ADHD) show association to hyperactive/impulsive symptoms in children with ADHD. J Am Acad Child Adolesc Psychiatry 2013; 52: 1204–1212. [DOI] [PubMed] [Google Scholar]
- Arcos-Burgos M, Jain M, Acosta MT, Shively S, Stanescu H, Wallis D et al. A common variant of the latrophilin 3 gene, LPHN3, confers susceptibility to ADHD and predicts effectiveness of stimulant medication. Mol Psychiatry 2010; 15: 1053–1066. [DOI] [PubMed] [Google Scholar]
- Mautner V-F, Kluwe L, Thakker SD, Leark RA. Treatment of ADHD in neurofibromatosis type 1. Dev Med Child Neurol 2002; 44: 164–170. [DOI] [PubMed] [Google Scholar]
- Millan MJ. An epigenetic framework for neurodevelopmental disorders: from pathogenesis to potential therapy. Neuropharmacology 2013; 68: 2–82. [DOI] [PubMed] [Google Scholar]
- Barton B, North K. Social skills of children with neurofibromatosis type 1. Dev Med Child Neurol 2004; 46: 553–563. [DOI] [PubMed] [Google Scholar]
- Pride NA, Payne JM, North KN. The impact of ADHD on the cognitive and academic functioning of children with NF1. Dev Neuropsychol 2012; 37: 590–600. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Caron MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science 1999; 283: 397–401. [DOI] [PubMed] [Google Scholar]
- Wilson MC. Coloboma mouse mutant as an animal model of hyperkinesis and attention deficit hyperactivity disorder. Neurosci Biobehav Rev 2000; 24: 51–57. [DOI] [PubMed] [Google Scholar]
- Swallow JG, Carter PA, Garland T. Artificial selection for increased wheel-running behavior in house mice. Behav Genet 1998; 28: 227–237. [DOI] [PubMed] [Google Scholar]
- Tsai CF, Lin MT. Locomotor hyperactivity in hypertensive rats. Pharmacology 1988; 36: 27–34. [DOI] [PubMed] [Google Scholar]
- Lange M, Norton W, Coolen M, Chaminade M, Merker S, Proft F et al. The ADHD-susceptibility gene lphn3.1 modulates dopaminergic neuron formation and locomotor activity during zebrafish development. Mol Psychiatry 2012; 17: 946–954. [DOI] [PubMed] [Google Scholar]
- Wallis D, Hill DS, Mendez IA, Abbott LC, Finnell RH, Wellman PJ et al. Initial characterization of mice null for Lphn3, a gene implicated in ADHD and addiction. Brain Res 2012; 1463: 85–92. [DOI] [PubMed] [Google Scholar]
- Diggs-Andrews KA, Tokuda K, Izumi Y, Zorumski CF, Wozniak DF, Gutmann DH. Dopamine deficiency underlies learning deficits in neurofibromatosis-1 mice. Ann Neurol 2013; 73: 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Emnett RJ, White CR, Yuede CM, Conyers SB, O'Malley KL et al. Reduced striatal dopamine underlies the attention system dysfunction in neurofibromatosis-1 mutant mice. Hum Mol Genet 2010; 19: 4515–4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy F. Dopamine vs noradrenaline: inverted-U effects and ADHD theories. Aust N Z J Psychiatry 2009; 43: 101–108. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Kaufman TC, Gelbart WM. Research resources for Drosophila: the expanding universe. Nat Rev Genet 2005; 6: 179–193. [DOI] [PubMed] [Google Scholar]
- van der Voet M, Nijhof B, Oortveld MAW, Schenck A. Drosophila models of early onset cognitive disorders and their clinical applications. Neurosci Biobehav Rev 2014; 46: 326–342. [DOI] [PubMed] [Google Scholar]
- Lichtneckert R, Reichert H. Insights into the urbilaterian brain: conserved genetic patterning mechanisms in insect and vertebrate brain development. Heredity (Edinb) 2005; 94: 465–477. [DOI] [PubMed] [Google Scholar]
- Bier E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet 2005; 6: 9–23. [DOI] [PubMed] [Google Scholar]
- O'Kane CJ. Drosophila as a model organism for the study of neuropsychiatric disorders. In: Molecular and functional models of neuropsychiatry. Curr Topic Behav Neurosci 2011; 7, p 37–60. [DOI] [PubMed] [Google Scholar]
- van Alphen B, van Swinderen B. Drosophila strategies to study psychiatric disorders. Brain Res Bull 2013; 92: 1–11. [DOI] [PubMed] [Google Scholar]
- Schenck A, Bardoni B, Langmann C, Harden N, Mandel J-L, Giangrande A. CYFIP/Sra-1 controls neuronal connectivity in Drosophila and links the Rac1 GTPase pathway to the fragile X protein. Neuron 2003; 38: 887–898. [DOI] [PubMed] [Google Scholar]
- Kramer JM, Kochinke K, Oortveld MAW, Marks H, Kramer D, de Jong EK et al. Epigenetic regulation of learning and memory by Drosophila EHMT/G9a. PLoS Biol 2011; 9: e1000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Bray SM, Li Z, Zarnescu DC, He C, Jin P et al. Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nat Chem Biol 2008; 4: 256–263. [DOI] [PubMed] [Google Scholar]
- McBride SMJ, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D et al. Pharmacological rescue of synaptic plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron 2005; 45: 753–764. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E. Mechanism-based treatments in neurodevelopmental disorders: fragile X syndrome. Pediatr Neurol 2014; 50: 297–302. [DOI] [PubMed] [Google Scholar]
- Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. J Neurosci 2005; 25: 7377–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Swinderen B, Brembs B. Attention-like deficit and hyperactivity in a Drosophila memory mutant. J Neurosci 2010; 30: 1003–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993; 118: 401–415. [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su K-C, Barinova Y, Fellner M et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 2007; 448: 151–156. [DOI] [PubMed] [Google Scholar]
- Ni J-Q, Liu L-P, Binari R, Hardy R, Shim H-S, Cavallaro A et al. A Drosophila resource of transgenic RNAi lines for neurogenetics. Genetics 2009; 182: 1089–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green EW, Fedele G, Giorgini F, Kyriacou CP. A Drosophila RNAi collection is subject to dominant phenotypic effects. Nat Meth 2014; 11: 222–223. [DOI] [PubMed] [Google Scholar]
- Poelmans G, Pauls DL, Buitelaar JK, Franke B. Integrated genome-wide association study findings: identification of a neurodevelopmental network for attention deficit hyperactivity disorder. Am J Psychiatry 2011; 168: 365–377. [DOI] [PubMed] [Google Scholar]
- Hinney A, Scherag A, Jarick I, Albayrak Ö, Pütter C, Pechlivanis S et al. Genome-wide association study in German patients with attention deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet 2011; 156B: 888–897. [DOI] [PubMed] [Google Scholar]
- Stergiakouli E, Hamshere M, Holmans P, Langley K, Zaharieva I, deCODE Genetics et al. Investigating the contribution of common genetic variants to the risk and pathogenesis of ADHD. Am J Psychiatry 2012; 169: 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Neale BM, Liu L, Lee SH, Wray NR, Ji N et al. Polygenic transmission and complex neuro developmental network for attention deficit hyperactivity disorder: genome-wide association study of both common and rare variants. Am J Med Genet B Neuropsychiatr Genet 2013; 162B: 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Mora C, Ramos-Quiroga JA, Bosch R, Corrales M, Garcia-Martínez I, Nogueira M et al. Case-control genome-wide association study of persistent attention-deficit hyperactivity disorder identifies FBXO33 as a novel susceptibility gene for the disorder. Neuropsychopharmacology 2015; 40: 915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marygold SJ, Leyland PC, Seal RL, Goodman JL, Thurmond J, Strelets VB et al. FlyBase: improvements to the bibliography. Nucleic Acids Res 2013; 41: D751–D757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilella AJ, Severin J, Ureta-Vidal A, Heng L, Durbin R, Birney E. EnsemblCompara GeneTrees: Complete, duplication-aware phylogenetic trees in vertebrates. Genome Res 2009; 19: 327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 2013; 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWilliam H, Li W, Uludag M, Squizzato S, Park YM, Buso N et al. Analysis Tool Web Services from the EMBL-EBI. Nucleic Acids Res 2013; 41: W597–W600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilestro GF, Cirelli C. pySolo: a complete suite for sleep analysis in Drosophila. Bioinformatics 2009; 25: 1466–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miano S, Parisi P, Villa MP. The sleep phenotypes of attention deficit hyperactivity disorder: the role of arousal during sleep and implications for treatment. Med Hypotheses 2012; 79: 147–153. [DOI] [PubMed] [Google Scholar]
- Rosato E, Kyriacou CP. Analysis of locomotor activity rhythms in Drosophila. Nat Protoc 2006; 1: 559–568. [DOI] [PubMed] [Google Scholar]
- Chintapalli VR, Wang J, Dow JAT. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet 2007; 39: 715–720. [DOI] [PubMed] [Google Scholar]
- Shang Y, Haynes P, Pírez N, Harrington KI, Guo F, Pollack J et al. Imaging analysis of clock neurons reveals light buffers the wake-promoting effect of dopamine. Nat Neurosci 2011; 14: 889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Z, Davis RL. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits 2009; 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Liu S, Kodama L, Driscoll MR, Wu MN. Two dopaminergic neurons signal to the dorsal fan-shaped body to promote wakefulness in Drosophila. Curr Biol 2012; 22: 2114–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Tomita J, Tanimoto H, Endo K, Ito K, Kume S et al. Identification of a dopamine pathway that regulates sleep and arousal in Drosophila. Nat Neurosci 2012; 15: 1516–1523. [DOI] [PubMed] [Google Scholar]
- Williams JA, Su HS, Bernards A, Field J, Sehgal A. A circadian output in Drosophila mediated by neurofibromatosis-1 and Ras/MAPK. Science 2001; 293: 2251–2256. [DOI] [PubMed] [Google Scholar]
- Chung BY, Kilman VL, Keath JR, Pitman JL, Allada R. The GABA(A) receptor RDL acts in peptidergic PDF neurons to promote sleep in Drosophila. Curr Biol 2009; 19: 386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmeiner F, Kołodziejczyk A, Yoshii T, Rieger D, Nässel DR, Helfrich-Förster C. GABA(B) receptors play an essential role in maintaining sleep during the second half of the night in Drosophila melanogaster. J Exp Biol 2013; 216: 3837–3843. [DOI] [PubMed] [Google Scholar]
- Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jönsson B et alCDBE2010 study group. The economic cost of brain disorders in Europe. Eur J Neurol 2012; 19: 155–162. [DOI] [PubMed] [Google Scholar]
- Swensen AR, Birnbaum HG, Secnik K, Marynchenko M, Greenberg P, Claxton A. Attention-deficit/hyperactivity disorder: increased costs for patients and their families. J Am Acad Child Adolesc Psychiatry 2003; 42: 1415–1423. [DOI] [PubMed] [Google Scholar]
- Kaufmann D, Wiandt S, Veser J, Krone W. Increased melanogenesis in cultured epidermal melanocytes from patients with neurofibromatosis 1 (NF 1). Hum Genet 1991; 87: 144–150. [DOI] [PubMed] [Google Scholar]
- Volz TJ. Neuropharmacological mechanisms underlying the neuroprotective effects of methylphenidate. Curr Neuropharmacol 2008; 6: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Chen D, Sehgal A. Dopamine acts through Cryptochrome to promote acute arousal in Drosophila. Genes Dev 2012; 26: 1224–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinayak P, Coupar J, Hughes SE, Fozdar P, Kilby J, Garren E et al. Exquisite light sensitivity of Drosophila melanogaster cryptochrome. PLoS Genet 2013; 9: e1003615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J, Jones AR, Danon A, Sakuma M, Hoang N, Robles D et al. Human cryptochrome-1 confers light independent biological activity in transgenic Drosophila correlated with flavin radical stability. PLoS One 2012; 7: e31867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij JJS, Bijlenga D. The circadian rhythm in adult attention-deficit/hyperactivity disorder: current state of affairs. Expert Rev Neurother 2013; 13: 1107–1116. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Salahpour A, CARON MG, Jones SR. Methylphenidate amplifies the potency and reinforcing effects of amphetamines by increasing dopamine transporter expression. Nat Commun 2013; 4: 2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowton E, Saunders C, Erreger K, Sakrikar D, Matthies HJ, Sen N et al. Dysregulation of dopamine transporters via dopamine D2 autoreceptors triggers anomalous dopamine efflux associated with attention-deficit hyperactivity disorder. J Neurosci 2010; 30: 6048–6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J-M, Sotnikova TD, Gainetdinov RR, Caron MG. Paradoxical striatal cellular signaling responses to psychostimulants in hyperactive mice. J Biol Chem 2006; 281: 32072–32080. [DOI] [PubMed] [Google Scholar]
- O'Hara CM, Uhland-Smith A, O'Malley KL, Todd RD. Inhibition of dopamine synthesis by dopamine D2 and D3 but not D4 receptors. J Pharmacol Exp Ther 1996; 277: 186–192. [PubMed] [Google Scholar]
- Tissari AH, Atzori L, Galdieri MT. Inhibition of dopamine synthesis in striatal synaptosomes by lisuride: stereospecific reversal by (-)-sulpiride. Naunyn Schmiedebergs Arch Pharmacol 1983; 322: 89–91. [DOI] [PubMed] [Google Scholar]
- Mayfield RD, Zahniser NR. Dopamine D2 receptor regulation of the dopamine transporter expressed in Xenopus laevis oocytes is voltage-independent. Mol Pharmacol 2001; 59: 113–121. [DOI] [PubMed] [Google Scholar]
- Meiergerd SM, Patterson TA, Schenk JO. D2 receptors may modulate the function of the striatal transporter for dopamine: kinetic evidence from studies in vitro and in vivo. J Neurochem 1993; 61: 764–767. [DOI] [PubMed] [Google Scholar]
- Bjornsti M-A. Cancer therapeutics in yeast. Cancer Cell 2002; 2: 267–273. [DOI] [PubMed] [Google Scholar]
- Wassmann K, Benezra R. Mitotic checkpoints: from yeast to cancer. Curr Opin Genet Dev 2001; 11: 83–90. [DOI] [PubMed] [Google Scholar]
- Pfau SJ, Amon A. Chromosomal instability and aneuploidy in cancer: from yeast to man. EMBO reports 2012; 13: 515–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto CL, Broadie K. Drosophila modeling of heritable neurodevelopmental disorders. Curr Opin Neurobiol 2011; 21: 834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.